Summary
Background
Paediatric scalp nevi may represent a source of anxiety for practitioners and parents, as the clinical and dermoscopic features of typical nevi have yet to be defined. Prompted by concern about the large size, irregular borders, and colour variation of scalp nevi, clinicians and parents may request unnecessary excision of these nevi.
Objective
The purpose of this study is to establish the typical clinical and dermoscopic patterns of scalp nevi in children younger than 18 years old to help optimize clinical care and management.
Methods
Scalp nevi were imaged with a camera (Canon Rebel, XSi) and dermoscopic attachment (3Gen, Dermlite Foto, 30mm lens) to the camera. The clinical and dermoscopic images were reviewed and analyzed. Both acquired and congenital scalp nevi were included but were not further differentiated from each other.
Results
We obtained clinical and dermoscopic images of 88 scalp nevi in 39 Caucasian children. Two subjects had received chronic immunosuppressive medication. Nineteen children have had a family history of melanoma. Males (18/39 subjects, 46%) possessed 68% (60 nevi) of scalp nevi imaged. Younger (<10 years old) subjects (24/39 subjects, 62%) possessed 42% (37 nevi) of scalp nevi. The main clinical patterns included eclipse (n=18), cockade (n=3), solid brown (n=42), and solid pink (n=25) nevi. Solid-coloured nevi showed the following dermoscopic patterns: globular (57%), complex (reticular-globular) (27%), reticular (9%), homogenous (6%), and fibrillar (1%). The majority of nevi had a unifying feature—perifollicular hypopigmentation, which caused the appearance of scalloped, irregular borders if occurring on the periphery, or variegation in pigmentation, if occurring within the nevi.
Conclusions
Older subjects and males tend to harbour a larger proportion of scalp nevi. The main clinical patterns include solid-coloured and eclipse nevi. The most common dermoscopic pattern of scalp nevi is the globular pattern. Perifollicular hypopigmentation is a hallmark feature of signature scalp nevi. Dermoscopy is a non-invasive tool in the evaluation of cutaneous melanocytic lesions in children and may decrease the number of unnecessary excisions.
Introduction
Dermoscopy is widely used by dermatologists in the adult population and has helped improve accuracy in the diagnosis of pigmented skin lesions1,2. Identification of characteristic structures (pigmented network, globules, scar-like areas, milia-like cysts), their symmetry, and their arrangement provides additional information for the experienced user.
Publications on the dermoscopic patterns of pigmented lesions in paediatric patients, especially dermoscopic features of nevi on the scalp in children, have been scarce. Paediatric scalp nevi may represent a source of anxiety for practitioners and parents, as the clinical and dermoscopic features of typical nevi have yet to be defined. When excised, most nevi of the scalp in children demonstrate some degree of atypia3, although the role of atypical nevi as precursor lesions for melanoma is far from established. Melanoma on the scalp in children is associated with a worse prognosis compared to lesions located on the extremities or torso4, and a higher proportion of melanoma is found on the scalp compared to other body sites in children5. Therefore, prompt detection of nevi that demonstrate concerning features is important. Gathering more information on these common pigmented lesions will further our understanding of typical scalp nevi and help optimize clinical care and management. The purpose of this study is to establish the clinical and dermoscopic patterns of signature scalp nevi in children younger than 18 years old.
Methods
The Institutional Review Board at Duke University Medical Center approved this study. Subjects under the age of 18 years old with scalp nevi were eligible to participate and were consented prior to enrolment. Specific information was solicited from each subject and included the following: hair colour, eye colour, family history of melanoma, past medical history (i.e. history of cancer or genetic syndromes), and medications (i.e. immunosuppressive medications). Therefore, hair and eye colour were self-described. As the dermoscopic patterns of scalp nevi from subjects with a history of immunosuppression and family history of melanoma were similar to other subjects, they were included in the statistical analysis with all other subjects.
To facilitate adequate visualization of the images, immediately surrounding hairs were trimmed. Scalp nevi were imaged with a standard camera (Canon Rebel, XSi) and dermoscopic attachment (3Gen, Dermlite Foto with 30mm lens) to the camera. The dermoscopic attachment utilized a polarized light source and an index fluid (rubbing alcohol or surgical lubricant) to minimize refraction from the epidermis and to optimize visualization and interpretation of underlying structures. The clinical images (global to assist in localization of the lesion) and dermoscopic images (closer magnification for lesion detail) were then reviewed and analyzed by two of the authors (W.J.T. and pigmented lesion specialist, K.C.N.). Both acquired and congenital scalp nevi were included but were not further differentiated from each other. Scalp nevi were divided into the following anatomic locations—frontal, temporal, vertex, occipital, and temporal areas according to the classification by Katz, et al6. We also catalogued nevi into clinical (solid brown, solid pink, eclipse, and cockade) and dermoscopic groups (homogenous, globular, reticular, reticular-globular, and fibrillar). We analyzed these patterns for younger (less than 10 years old), older (greater than or equal to 10 years old), and all subjects collectively.
Descriptive statistics were used unless otherwise indicated. The Wilcoxon rank-sum test (WRST) was used to test for differences in the number of scalp nevi between gender and age groups. Pearson’s chi-square (χ2) test or Fisher’s exact test were used when testing for association between dermoscopic patterns. A p-value of less than 0.05 (95% confidence) was considered significant. All analyses were performed using the statistical software R version 2.10.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
We obtained clinical and dermoscopic images of 88 scalp nevi in 39 healthy Caucasian children—22 blondes (42/88 nevi), 16 brunettes (40/88 nevi), and one red head (6/88 nevi)). (Table 1) The number of moles per subject ranged from one to 8 nevi with a mean of 2.2 nevi per subject. Males comprised less than half of the subjects (18 males, 46%) but possessed the majority of scalp nevi (60 nevi, 68%)—therefore, averaging 3.3 nevi per male (60 nevi/18males) compared to 1.3 nevi per female (28 nevi/21 females). We found a strong statistical association between gender and the number of scalp nevi as males tended to produce more scalp nevi compared to females (p=0.0005, WRST). Mean age was 8.79 years (range 3 years to 17 years) with 24 younger subjects (less than 10 years old). Three children had a history of cancer including two with melanoma and one with astrocytoma—all three were free of disease at the time of examination. Two subjects, with 9 scalp nevi, had received chronic immunosuppressive medication. Nineteen children, possessing 50 scalp nevi, have had a family history of melanoma. (Table 1)
Table 1.
Patient Characteristics
| Patient Characteristics, n = 39 | ||
|---|---|---|
| Gender | 18 m / 21 f | |
| Ethnicity | 37 Caucasian/2 Caucasian-Asian | |
| Hair Colour, n | ||
| Blonde | 22 | |
| Brown | 16 | |
| Red | 1 | |
| Eye Colour, n | ||
| Blue | 20 | |
| Green | 4 | |
| Brown | 13 | |
| Hazel | 2 | |
| Family History of Melanoma, n | 19 | |
| Age | ||
| Mean | 8.79 years | |
| <10 years old, n | 24 | |
| # of nevi | 18 m / 19 f | |
| >=10 years old, n | 15 | |
| # of nevi | 42 m / 9 f | |
| History of Cancer, n | 3 | |
| History of Immunosuppressive Medications, n | 2 | |
The anatomic locations that harboured the largest number of nevi, with 48 nevi combined (55%), included the vertex and parietal scalp. The occipital scalp exhibited the fewest scalp nevi. (Fig 1)
Figure 1. Anatomic Distribution of Scalp Nevi.
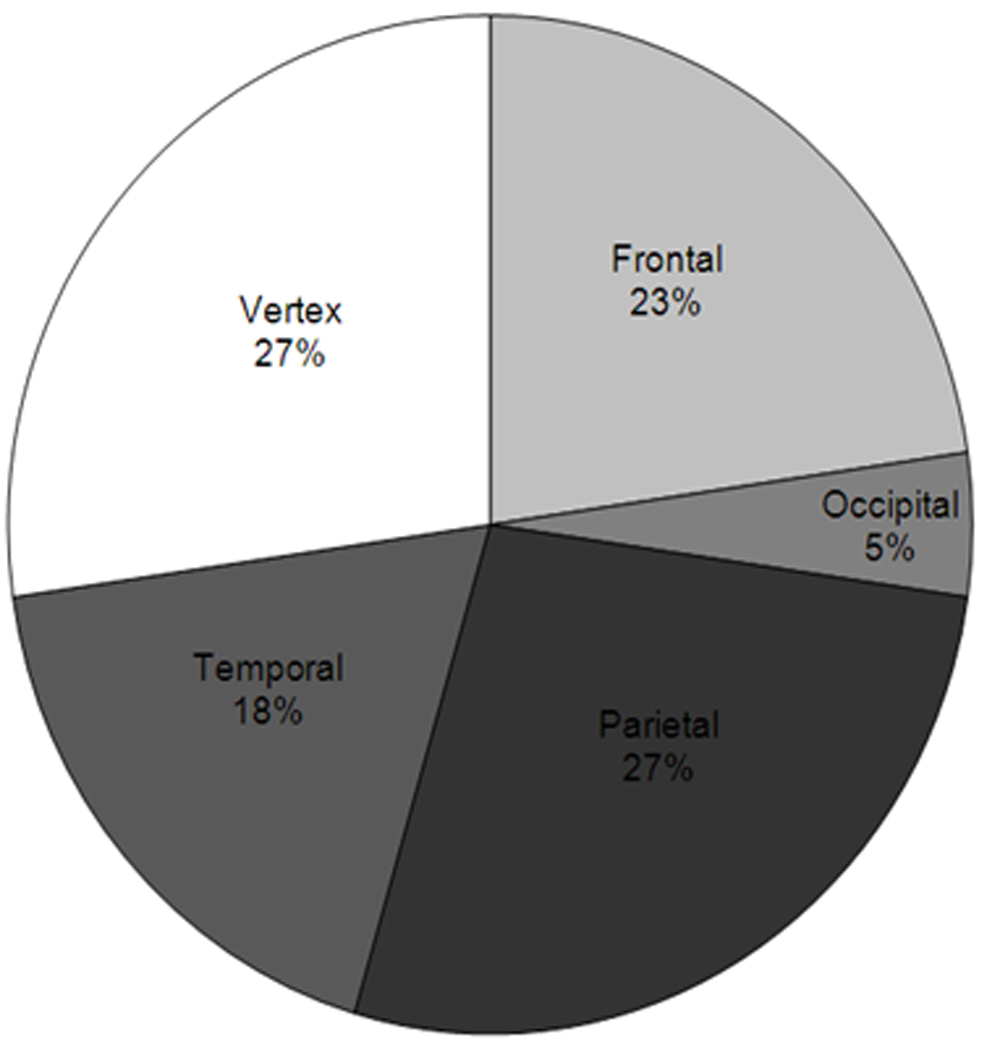
Proportion of each anatomic region of the scalp populated by scalp nevi. The parietal and vertex scalp possess the most number of scalp nevi while the occipital scalp has the least.
The main clinical patterns included solid brown (n= 42), solid pink (n=25), eclipse (n=18), and cockade (n=3) nevi. (Fig. 2) Clinically, eclipse nevi were composed of a central tan circular area with a darker surrounding ring; cockade nevi had a target-like appearance, with a dark brown centre, tan inner ring, and a dark brown outer ring. (Fig. 3) On dermoscopy, eclipse nevi demonstrated a tan homogenous centre with a darker, reticulated peripheral ring. (Fig 4) Cockade nevi demonstrated a darker, central globular pattern, lighter homogenous inner ring, and a peripheral darker reticular ring. (Fig. 4) Twenty-four younger subjects (mean age of 5.6 years) contributed 37 nevi (37/88, 42%) with 28 solid-coloured, 6 eclipse, and 3 cockade nevi. Fifteen older subjects (mean age of 13.9 years) contributed 51 nevi (51/88, 58%) with 39 solid-coloured and 12 eclipse nevi. Older subjects demonstrated significantly more nevi per subject compared to younger subjects, with 3.4 nevi (51nevi/15 older subjects) per former subject and 1.5 nevi (37 nevi/24 younger subjects) per latter subject, respectively (p=0.0004, WRST). The number of scalp nevi in self-reported fair-haired (blondes and red-haired subjects) and dark-haired subjects (brunettes) was not significantly different (p=0.328, WRST).
Figure 2. Percentage of Subjects with each Clinical Pattern of Scalp Nevi.
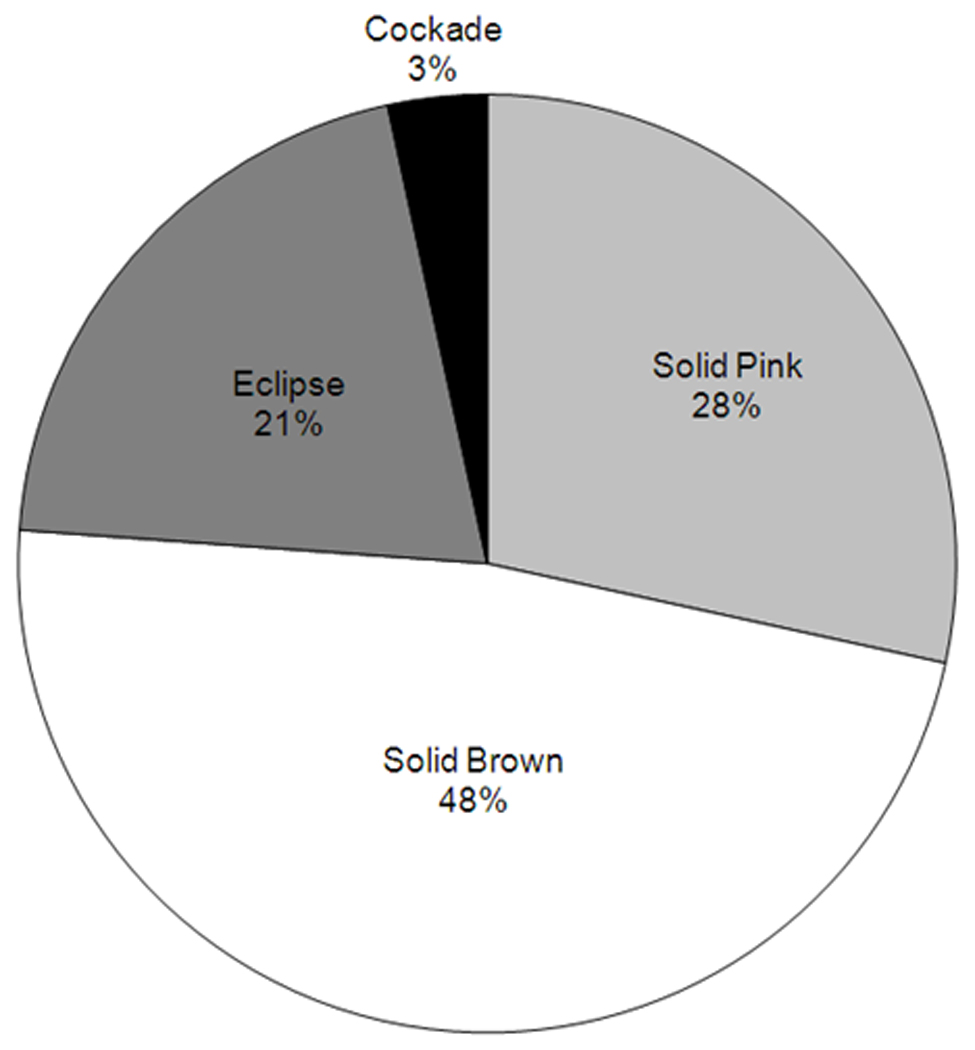
Distribution of clinical patterns of scalp nevi.
Figure 3. Clinical Patterns of Scalp Nevi.
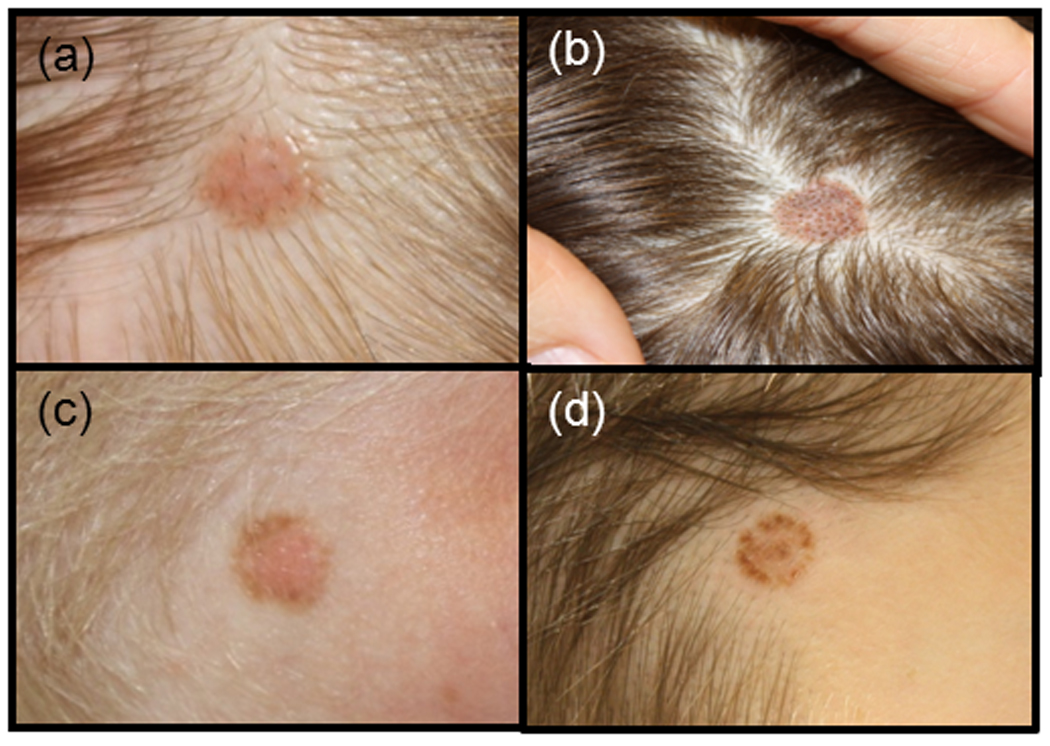
Common clinical patterns of scalp nevi. a) solid pink nevus, b) solid brown nevus, c) eclipse nevus with tan centre and darker outer ring, and d) cockade nevus with darker centre, tan inner ring, and darker outer ring.
Figure 4. Dermoscopic Patterns of Scalp Nevi.
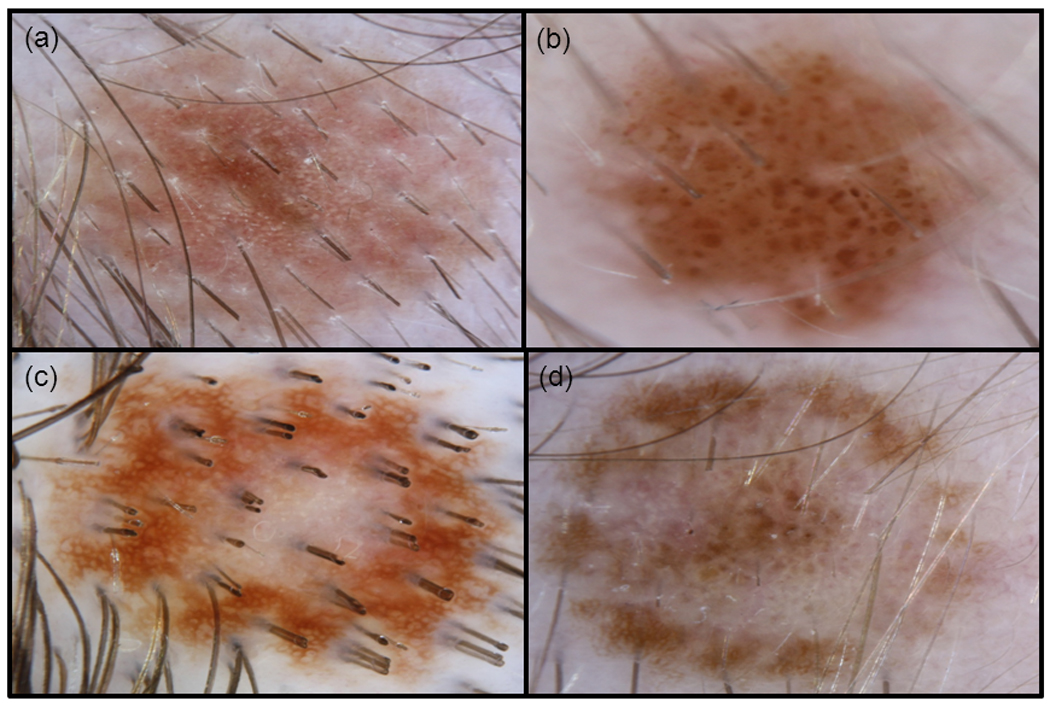
Common dermoscopic patterns of scalp nevi. a) solid pink nevus with a reticulated network, b) solid brown nevus with a globular pattern, c) eclipse nevus with a light brown homogenous centre and a darker, reticulated outer ring, d) cockade nevus with a target-like appearance with a darker, globular centre, tan homogenous inner ring, and darker reticulated outer ring.
Solid brown (42/88) and pink (25/88) nevi (67 solid-coloured nevi) showed the following dermoscopic patterns: globular (38/67, 57%), complex (reticular-globular) (18/67, 27%), reticular (6/67, 9%), homogenous (4/67, 6%), and fibrillar (1/67, 1%). (Table 2 and Fig. 4) These dermoscopic trends were similar for younger and older subjects; no significant differences were found when comparing each pattern’s prevalence between the two age groups using chi-square and Fisher’s exact test analysis. (Fig. 5) We also observed specific structured components within certain globular and reticulated nevi; these included 21 nevi with haloed globules, 15 nevi with target globules, and 9 nevi with target networks. (Fig. 6)
Table 2.
Clinical and Corresponding Dermoscopic Patterns
| Clinical Patterns |
Solid Brown and Pink Nevi (76%) |
Eclipse (20%) |
Cockade (3%) |
|---|---|---|---|
|
Corresponding Dermoscopic Patterns |
57% Globular 27 % Reticular-Globular 9% Reticular 6 % Homogenous 1% Fibrillar |
tan homogenous centre with a darker, reticulated peripheral ring |
darker, central globular pattern with a lighter homogenous inner ring and a peripheral darker reticular ring |
Figure 5. Dermoscopic Patterns of Solid-Coloured Nevi.
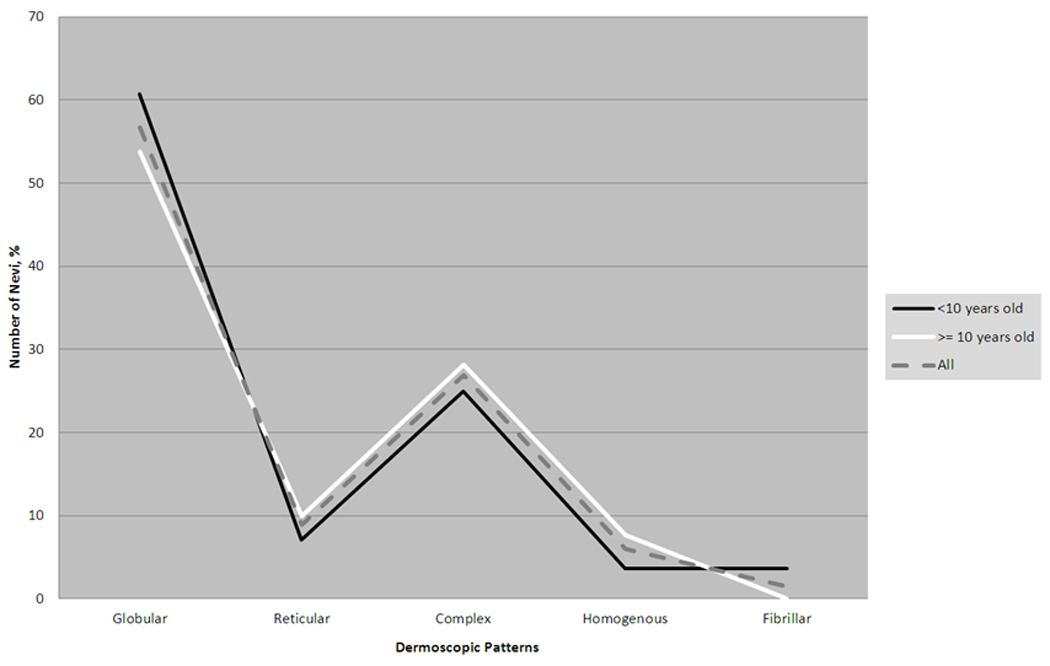
Distribution of each dermoscopic pattern of scalp nevi in those <10 years old, >=10 years old, and for all subjects collectively.
Figure 6. Dermoscopic Patterns of Ultra Structures within Reticulated and Globular Nevi.
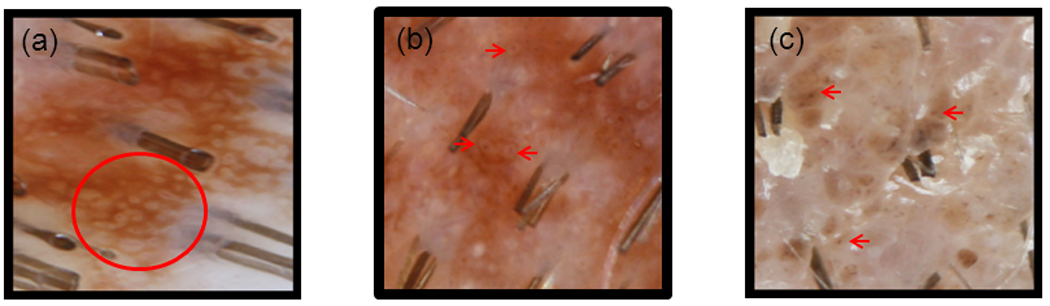
Dermoscopic images of ultra-structures. a) target network which consists of a reticulated network with interspersed pigmented dots and blood vessels, b) haloed globules which comprises of a darkly pigmented globule or dot with a hypopigmented surrounding ring , c) target globules which consists of a pigmented globule with a central darker pigmented dot.
The majority of scalp nevi (87/88) had a unifying feature—perifollicular hypopigmentation, which caused the appearance of scalloped, irregular borders if occurring on the periphery, or variation in pigmentation, if occurring within the nevi. (Fig. 7) One nevus was so light in colour that perifollicular hypopigmentation was difficult to detect. In addition, a predominant dermoscopic vascular pattern was discerned on the majority of scalp nevi—dotted vessels (60/88). (Fig. 8) Several lesions also demonstrated other vascular patterns: 10 hairpin, 5 linear, and 4 looped vessels. Twenty-five nevi did not have a visible vascular pattern. All nevi were organized and benign in appearance.
Figure 7. Perifollicular Hypopigmentation on Dermoscopy.
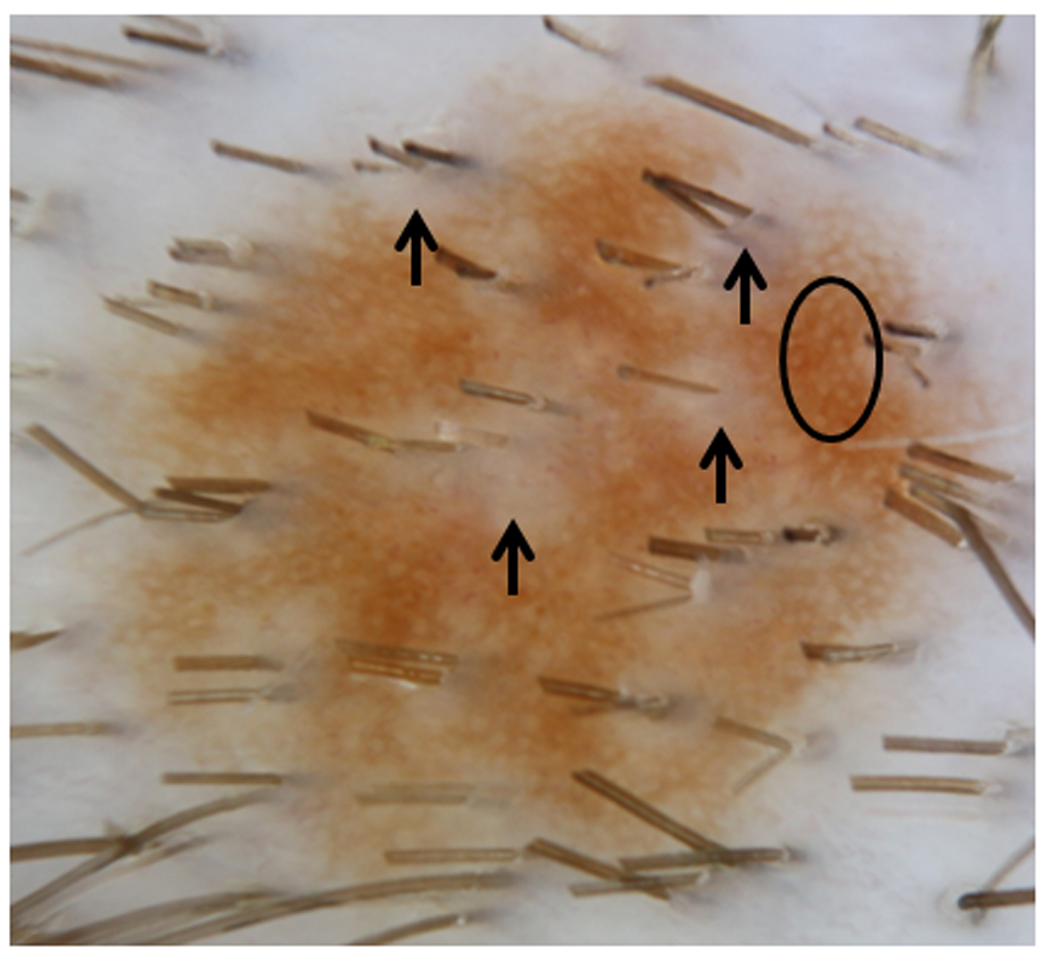
Dermoscopic pattern of perifollicular clearing within the lesion as well as peripherally (arrows). Reticular network (circle) is the main pattern.
Figure 8. Dotted Vascular Pattern on Dermoscopy.
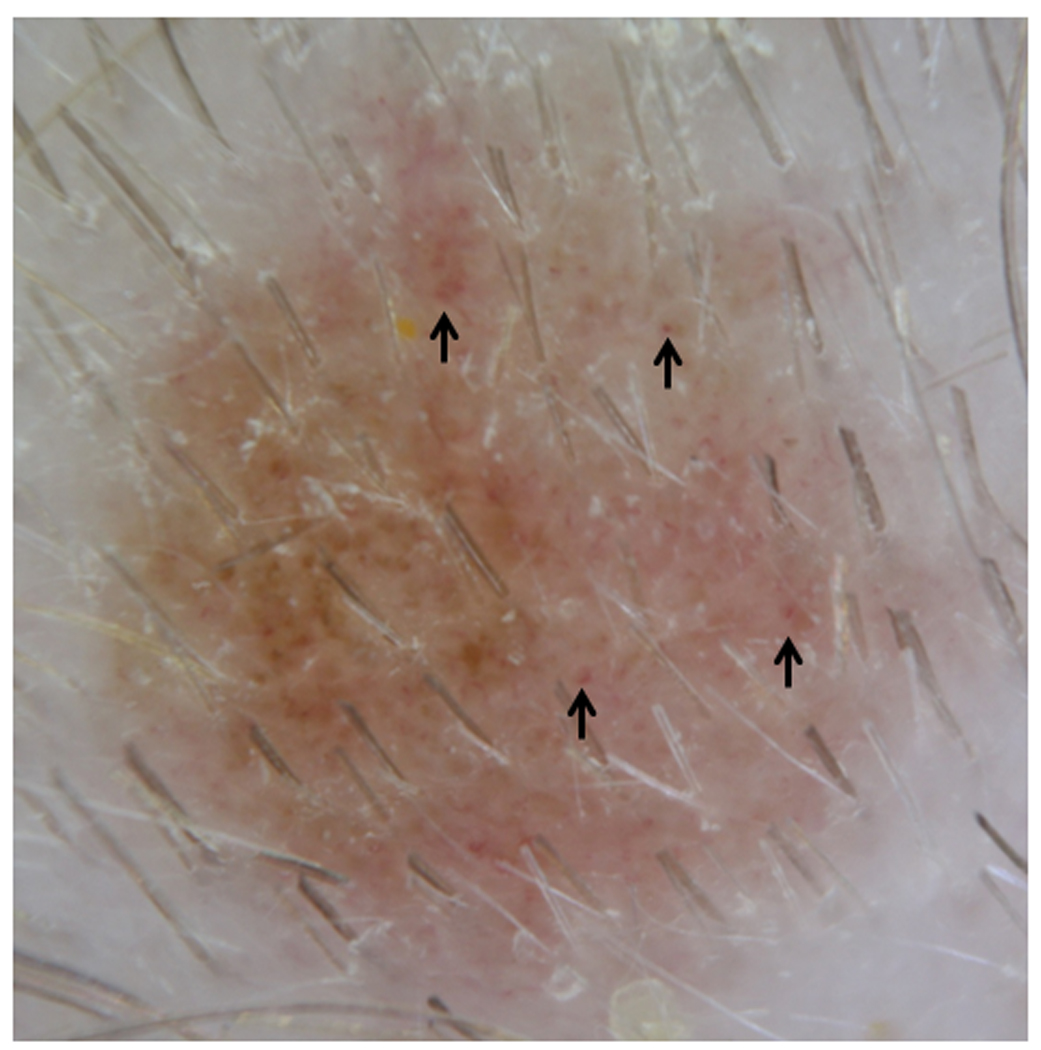
Dotted vascular pattern (arrows) seen on dermoscopy.
Discussion
Dermoscopic patterns of nevi vary by age and body location7,8. Not only do scalp nevi appear different clinically than cutaneous nevi in children, they often will have dysplastic features on histopathologic review,3,9,10 similar to nevi of “special sites” such as acral and genital skin. In addition, the number of scalp nevi is directly correlated with the number of body nevi; therefore, those with a large number of atypical body nevi will also have a larger number of atypical scalp nevi10,11. Prompted by concern about the large size, irregular borders, and colour variegation of scalp nevi, clinicians and parents may request excision of these nevi. Although melanoma of the scalp does occur in childhood12, frequently such excisions demonstrate nevi with mild to moderate atypia. The indiscriminate removal of scalp nevi may lead to heightened anxiety amongst patients and their parents, along with scarring, pain, and further procedures as new scalp nevi are found. Therefore, distinguishing benign scalp lesions from concerning lesions is prudent, and using tools, such as dermoscopy (able to increase diagnostic accuracy by 5–30%)13,14 may aid the practitioner with their clinical decision.
After examining the number of scalp nevi between male and female subjects, we found that scalp nevi had a striking male predominance, which is in agreement with past studies11,15. Males made significantly more scalp nevi (3.3 nevi per male) compared to females (1.3 nevi per female) (p-value=0.005). Interestingly, in a study on paediatric head and neck melanoma, most subjects with scalp/neck melanoma were also male12. The higher incidence of melanoma of the scalp in males could be attributed to males possessing more scalp nevi, as melanoma may develop from existing nevi.
In terms of anatomic location of scalp nevi, De Giorgi et al found that a large portion of their scalp nevi favoured the parietal region followed by the occipital region10. Our subjects’ scalp nevi also had an affinity for the parietal region (27%), but the occipital region (5%) was rarely represented. Of note, De Giorgio’s study also included adult subjects.
Clinical and dermoscopic features of benign eclipse and cockade scalp nevi in children have been described and are felt to be fairly common16. (Fig. 3 and Fig. 4) We agree as 24 % of our subjects’ nevi demonstrated these patterns. Past dermoscopic descriptions of eclipse nevi on the body illustrated a featureless, hypopigmented centre with a darker, reticulated network for an outer ring, which manifests clinically as stellate borders secondary to perifollicular hyper or hypopigmentation17. The central diminished pigmentation should not be confused with regression structures (white scar-like areas as seen in melanoma), which are asymmetrical compared to the well-organized and symmetrical central area of the eclipse nevus. Our dermoscopic observation is consistent with these features described, including notched borders due to perifollicular hypopigmentation. (Fig. 4) Histology of eclipse nevi of the body demonstrated no significant atypia17.
Another benign entity, the cockade nevus, appears with pigmented network centrally, structure-less inner ring, and peripheral, pigmented outer ring16,18. Our subjects with cockade nevi differed slightly on dermoscopy: the central pigmented portion had globules instead of a network, which may be due to the younger age of our subjects compared to those in the existing dermoscopic literature. (Fig. 4) Histologically, the cockade nevus from body sites demonstrates a central and peripheral junctional pattern17,19.
Signature solid pink and brown nevi, when found on body sites, were bland appearing with smooth borders20. These nevi may represent compound melanocytic or dermal nevi20. In comparison to nevi of the body, the higher concentration of hair follicles on the scalp, with resultant perifollicular hypopigmentation, imparts variation in colour and scalloped borders for scalp nevi. (Fig. 7) Perifollicular hypopigmentation was a common feature in our subjects’ scalp nevi (99%). Bolognia first noted that perifollicular hypopigmentation not only occurred within a pigmented lesion, creating a lightened halo around the hair shaft, but also on the edge of a lesion, creating a half moon, notched pattern. On histology, these nevi expressed fewer melanocytes, melanophages, and/or decrease in pigmented keratinocytes around hair follicles21.
Consistent with Gupta et al’s study, the number of scalp nevi increased with age11. Older subjects made significantly more scalp nevi than younger subjects—3.4 nevi per older subject (>=10 years) compared to 1.5 nevi per younger subject (<10 years) (p-value= p=0.0004).
The globular pattern is the most common dermoscopic pattern in melanocytic and congenital melanocytic nevi (CMN) found on the body in children15,22; this is also the most common CMN pattern found on the head and neck7. Among our subjects with solid-coloured scalp nevi, the most common pattern was globular, followed by complex (reticular-globular), reticular, homogenous, and finally fibrillar. Even after stratifying subjects by age (<10 years, mean of 5.6 years, and >= 10 years, mean of 13.9 years), we found that this same pattern prevailed and was consistent with Zalaudek et al’s findings15. However, a Framingham study of back nevi in 10–11 year old children found that the homogenous pattern (44%) was more prevalent than the globular pattern (37%), reticular pattern (13%), and complex (reticular-globular) pattern (5%)23. The difference between mean age and anatomic location between our subjects (8.8 years), the Framingham subjects (10.7 years), and Zalaudek’s subjects (13.3years) may account for and highlight these differences.
Haloed globules, target globules, and target networks have been used to describe pigmented lesions, especially congenital melanocytic nevi, in the past. The haloed globule appears as a dot surrounded by a hypopigmented ring, while the target globule has a hyperpigmented surrounding ring. A target network is a reticular network with dots, globules, and/or blood vessels within its space8. (Fig. 6) The presence of these ultrastrutures may highlight the congenital nature of some scalp nevi.
Dotted vessels (68%) were the main vascular pattern observed in our sample of scalp nevi. (Fig. 8) Dotted vessels observed in our patients were sparsely aligned in a well-organized fashion. They have been observed in other dermatologic lesions, as well, such as spitz nevi, dermal nevi, and psoriasis.
The overall patterns of signature scalp nevi in this study were organized and benign in appearance. In contrast to the dermoscopic pattern described of benign scalp lesions, dermoscopy of melanoma of the scalp demonstrates asymmetry with an atypical pigmented network, irregular streaks, and regression structures. The authors concluded that dermoscopic patterns of scalp melanoma resembled melanoma from other cutaneous sites (excluding face/acral sites)5.
Conclusion
Examination of the scalp is an important part of the skin examination. The predominant clinical patterns of signature scalp nevi in children include solid-coloured, eclipse, and cockade nevi. Fair-skinned children were more likely to have solid-pink nevi. Older subjects and males tended to harbour a larger percentage of scalp nevi compared to their younger, female counterparts.
Dermoscopy adds more information about features of scalp nevi than clinical examination alone. On dermoscopy, perifollicular hypopigmentation, with resultant clinically scalloped borders or variegation in pigmentation, is a hallmark feature of scalp nevi. The most common dermoscopic pattern of scalp nevi is the globular pattern (57%) followed by the complex/reticular-globular pattern (27%)—this was observed even after nevi were divided into different age groups. The number of younger and older subjects possessing each of these patterns was not significantly different. Dotted vessels are the predominant vascular pattern. Dermoscopy is a non-invasive tool in the evaluation of cutaneous melanocytic lesions in children--it provides detailed information, which may increase the accuracy of clinical diagnosis of benign and malignant skin lesions, thus decreasing the number of unnecessary excisions.
Based on the reassuring dermoscopy patterns of our subjects, we did not excise any scalp nevi in our paediatric patients in this study. One patient was scheduled for follow up of his scalp nevi six weeks after the initial visit—his nevi remained stable during that interval. Parents were given instructions to follow up if they thought their nevi were growing asymmetrically, bleeding spontaneously, ulcerating, and/or becoming tender/painful/itchy. In conclusion, we recommend that 1) clinicians consider using dermoscopy to help further characterize scalp nevi in children, and 2) if clinicians are uncertain about the atypia of a scalp nevus, consider referral to a paediatric dermatologist or pigmented lesion specialist for a second opinion.
Acknowledgments
Funding: Partially funded by Grant Number UL1RR024128 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research
Footnotes
Conflict of interest: none declared
References
- 1.Nachbar F, Stolz W, Merkle T, et al. The ABCD rule of dermatoscopy. High prospective value in the diagnosis of doubtful melanocytic skin lesions. J Am Acad Dermatol. 1994;30:551–559. doi: 10.1016/s0190-9622(94)70061-3. [DOI] [PubMed] [Google Scholar]
- 2.Rajpara SM, Botello AP, Townend J, et al. Systematic review of dermoscopy and digital dermoscopy/ artificial intelligence for the diagnosis of melanoma. Br J Dermatol. 2009;161:591–604. doi: 10.1111/j.1365-2133.2009.09093.x. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez M, Raimer SS, Sanchez RL. Dysplastic nevi of the scalp and forehead in children. Pediatr Dermatol. 2001;18:5–8. doi: 10.1046/j.1525-1470.2001.018001005.x. [DOI] [PubMed] [Google Scholar]
- 4.Strouse JJ, Fears TR, Tucker MA, et al. Pediatric melanoma: risk factor and survival analysis of the surveillance, epidemiology and end results database. J Clin Oncol. 2005;23:4735–4741. doi: 10.1200/JCO.2005.02.899. [DOI] [PubMed] [Google Scholar]
- 5.Zalaudek I, Leinweber B, Soyer HP, et al. Dermoscopic features of melanoma on the scalp. J Am Acad Dermatol. 2004;51:S88–S90. doi: 10.1016/j.jaad.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 6.Katz TM, Silapunt S, Goldberg LH, et al. Analysis of 197 female scalp tumors treated with Mohs micrographic surgery. J Am Acad Dermatol. 2005;52:291–294. doi: 10.1016/j.jaad.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Seidenari S, Pellacani G, Martella A, et al. Instrument-, age- and site-dependent variations of dermoscopic patterns of congenital melanocytic naevi: a multicentre study. Br J Dermatol. 2006;155:56–61. doi: 10.1111/j.1365-2133.2006.07182.x. [DOI] [PubMed] [Google Scholar]
- 8.Changchien L, Dusza SW, Agero AL, et al. Age- and site-specific variation in the dermoscopic patterns of congenital melanocytic nevi: an aid to accurate classification and assessment of melanocytic nevi. Arch Dermatol. 2007;143:1007–1014. doi: 10.1001/archderm.143.8.1007. [DOI] [PubMed] [Google Scholar]
- 9.Fabrizi G, Pagliarello C, Parente P, et al. Atypical nevi of the scalp in adolescents. J Cutan Pathol. 2007;34:365–369. doi: 10.1111/j.1600-0560.2006.00622.x. [DOI] [PubMed] [Google Scholar]
- 10.De Giorgi V, Sestini S, Grazzini M, et al. Prevalence and distribution of melanocytic naevi on the scalp: a prospective study. Br J Dermatol. 162:345–349. doi: 10.1111/j.1365-2133.2009.09486.x. [DOI] [PubMed] [Google Scholar]
- 11.Gupta M, Berk DR, Gray C, et al. Morphologic features and natural history of scalp nevi in children. Arch Dermatol. 146:506–511. doi: 10.1001/archdermatol.2010.88. [DOI] [PubMed] [Google Scholar]
- 12.Tcheung WJ, Marcello JE, Puri PK, et al. Evaluation of 39 cases of pediatric cutaneous head and neck melanoma. J Am Acad Dermatol. doi: 10.1016/j.jaad.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Bafounta ML, Beauchet A, Aegerter P, et al. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma? Results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch Dermatol. 2001;137:1343–1350. doi: 10.1001/archderm.137.10.1343. [DOI] [PubMed] [Google Scholar]
- 14.Kittler H, Pehamberger H, Wolff K, et al. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159–165. doi: 10.1016/s1470-2045(02)00679-4. [DOI] [PubMed] [Google Scholar]
- 15.Zalaudek I, Grinschgl S, Argenziano G, et al. Age-related prevalence of dermoscopy patterns in acquired melanocytic naevi. Br J Dermatol. 2006;154:299–304. doi: 10.1111/j.1365-2133.2005.06973.x. [DOI] [PubMed] [Google Scholar]
- 16.Kessides MC, Puttgen KB, Cohen BA. No biopsy needed for eclipse and cockade nevi found on the scalps of children. Arch Dermatol. 2009;145:1334–1336. doi: 10.1001/archdermatol.2009.282. [DOI] [PubMed] [Google Scholar]
- 17.Schaffer JV, Glusac EJ, Bolognia JL. The eclipse naevus: tan centre with stellate brown rim. Br J Dermatol. 2001;145:1023–1026. doi: 10.1046/j.1365-2133.2001.04538.x. [DOI] [PubMed] [Google Scholar]
- 18.Yazici AC, Ikizoglu G, Apa DD, et al. The eclipse naevus and cockade naevus: are they two of a kind? Clin Exp Dermatol. 2006;31:596–597. doi: 10.1111/j.1365-2230.2006.02141.x. [DOI] [PubMed] [Google Scholar]
- 19.Guzzo C, Johnson B, Honig P. Cockarde nevus: a case report and review of the literature. Pediatr Dermatol. 1988;5:250–253. doi: 10.1111/j.1525-1470.1988.tb00897.x. [DOI] [PubMed] [Google Scholar]
- 20.Suh KY, Bolognia JL. Signature nevi. J Am Acad Dermatol. 2009;60:508–514. doi: 10.1016/j.jaad.2008.10.056. [DOI] [PubMed] [Google Scholar]
- 21.Bolognia JL, Shapiro PE. Perifollicular hypopigmentation. A cause of variegate pigmentation and irregular border in melanocytic nevi. Arch Dermatol. 1992;128:514–517. doi: 10.1001/archderm.128.4.514. [DOI] [PubMed] [Google Scholar]
- 22.Westhafer J, Gildea J, Klepeiss S, et al. Age distribution of biopsied junctional nevi. J Am Acad Dermatol. 2007;56:825–827. doi: 10.1016/j.jaad.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 23.Scope A, Marghoob AA, Dusza SW, et al. Dermoscopic patterns of naevi in fifth grade children of the Framingham school system. Br J Dermatol. 2008;158:1041–1049. doi: 10.1111/j.1365-2133.2008.08510.x. [DOI] [PubMed] [Google Scholar]


