Abstract
Hearing loss encompasses both temporary and permanent deficits. If temporary threshold shift (TTS) and permanent threshold shift (PTS) share common pathological mechanisms, then agents that reduce PTS should also reduce TTS. Several antioxidant agents have reduced PTS in rodent models; however, reductions in TTS have been inconsistent. This study first determined whether dietary antioxidants (beta-carotene, and vitamins C and E) delivered in combination with magnesium (Mg) reliably increase plasma concentrations of the active agents. Then, additional manipulations tested the hypothesis that these nutrients reduce acute TTS insult in the first 24 hours following loud sound, as well as longer lasting changes in hearing measured up to 7 days post-noise. Saline or nutrients were administered to guinea pigs prior to and after noise exposure. Sound-evoked electrophysiological responses were measured before noise, with tests repeated 1-hour post-noise, as well as 1-, 3-, 5-, and 7-days post-noise. All subjects showed significant functional recovery; subjects treated with nutrients recovered more rapidly, and had better hearing outcomes at early post-noise times as well as the final test time. Thus, this combination of nutrients, which produced significant increases in plasma concentrations of vitamins C and E and Mg, effectively reduced hearing loss at multiple post-noise times. These data suggest free radical formation contributes to TTS as well as PTS insults, and suggest a potential opportunity to prevent TTS in human populations.
Keywords: Noise, temporary threshold shift (TTS), hearing, beta-carotene, vitamin C, vitamin E, magnesium
Introduction
Approximately 30 million American workers are exposed to hazardous levels of occupational noise, making noise-induced hearing loss (NIHL) one of the most common occupational diseases (1). Mechanical devices that attenuate sound coming into the ear remain the best choice for reducing occupation-related NIHL, and NIHL that occurs during other loud activities. However, it is increasingly clear that NIHL that occurs despite the use of hearing protectors could perhaps be reduced by decreasing oxidative stress events in the inner ear. Indeed, multiple agents that intervene in the oxidative stress pathway have been shown to reduce noise-induced permanent threshold shift (PTS) in animal subjects. The role of oxidative stress in NIHL and variety of antioxidant agents tested to date have been the subject of several recent reviews (see 2, 3, 4), and other agents continue to emerge as candidate therapeutics for reducing NIHL (5, 6).
Clinically significant reduction of human PTS is the ultimate clinical goal. Animal models clearly show reduction of PTS with antioxidant nutrient combinations (7), glutathione precursors such as D-methionine and N-acetylcysteine (NAC) (8–10), the glutathione peroxidase mimetic ebselen (11, 12), the mineral magnesium (Mg) (13–18), and other potential therapeutic agents such as calcium channel blockers, c-jun N-terminal kinase (JNK) antagonists, and caspase-inhibitors (for review, see 4). Prevention of temporary threshold shift (TTS) has also been considered a clinically appropriate target, and evidence that robust (≥40 dB) TTS has long-term consequences is emerging from current studies using the mouse as a model (19, 20). Given increasing emphasis on prevention of TTS, the potential prevention of TTS in human subjects treated with NAC was evaluated in two groups of students exposed to loud music (21, 22), as well as United States military recruits undergoing weapons training (see 9 for description of study design). No statistically reliable reductions in TTS were reported in these human studies. In contrast to the outcomes with NAC, treatment with oral Mg (122 mg Mg, delivered as Mg aspartate), reliably reduced TTS in human subjects exposed to 90 dB SL white noise for 10 min (23). Prevention of TTS in human subjects is clearly of significant interest. Upcoming studies will determine the potential for protection of the human inner ear using a proprietary ebselen formulation in a military population (for description of trial design, see 24) and using a nutrient combination in a population of students that use personal music players (for preliminary description of exposure model, see 25, 26, 27).
In animal models, the data on antioxidant protection against TTS is more variable than that for PTS. While NAC has effectively reduced PTS in noise-exposed guinea pigs (28, 29) and chinchillas (delivered in combination with salicylate, see 30), effects on TTS have been mixed. While some studies in animal models did show reductions of ~10 dB in TTS at early post-noise times (31, 32), the human studies described above and other animal studies have revealed minimal (29) or no (33) reduction in TTS with NAC treatment. D-methionine similarly reduced PTS, but not TTS, in chinchillas and mice (10, 34), although reductions in both PTS and TTS were reported in another study using guinea pigs (35). In contrast to NAC and D-methionine, ebselen has provided robust protection against both TTS (in guinea pigs) and PTS (in guinea pigs and rats) (12, 36–38). Mg, like ebselen, has reduced TTS (15, 39) as well as PTS (13–18), in guinea pig subjects, with level of protection varying both with dose and the timing of treatment onset relative to the time of noise insult (for review see 4).
In this study, we treated guinea pigs with doses of β-carotene, vitamin C, Trolox (a synthetic analogue of vitamin E), and magnesium (Mg) that have been shown to effectively reduce PTS with short treatment onset relative to the time of noise (treatment starting 1-hour pre-noise, see 7). The onset and duration of Mg dosing is an important parameter. Le Prell et al. (7) failed to detect benefits of Mg in reducing NIHL when Mg was delivered starting 1 hour pre-noise (2.85 mmol/kg MgSO4 s.c., 1-hr pre-noise plus once daily for 4 days post-noise; each dose equivalent to 343 mg/kg). Sendowski et al. (40) similarly failed to detect benefits with Mg in guinea pigs with treatement starting 1-hr post-noise (350 mg/kg MgSO4 s.c., 1-hr post-noise plus once daily for 3 days, plus 3.7g MgCl2/l water × 1 wk). Given that the benefits of Mg starting near the time of noise insult critically depend on the addition of vitamins to the therapy (and that the effects of vitamin therapy starting near the time of noise insult critically depend on Mg) (7), we did not include Mg only or vitamin only controls in this study. Rather than address dose-response and or synergy among agents, which clearly depends on timing of treatments relative to noise, the current investigation asked 2 questions of immediate translational value: 1) what are the plasma levels achieved using a dose that has been shown to reduce permanent NIHL, and 2) what effect does this treatment paradigm have on temporary noise-induced threshold shift, given that these TTS models have emerged as one potential paradigm for evaluating novel otoprotective drug agents in human subjects. The current data are of immediate translational value, as they provide a starting point for potential plasma levels to be targeted in human studies, and they provide confirmation that TTS models may have some utility for evaluation of nutrient combinations in human subjects.
We measured plasma concentrations of the active agents before and after dosing to estimate plasma concentrations that might be required to achieve similarly robust protection in future human studies. In a second group of guinea pigs treated using an identical dose paradigm, we tested the hypothesis that these nutrients reduce the acute noise insult occurring in the first 24 hours subsequent to loud sound, as well as hearing loss at later post-noise time points up to 7 days post-noise. The effects of this nutrient combination at early post-noise time points, during the period in which robust but reversible hearing loss is observed, have not been described previously. Data from other groups of noise-exposed guinea pigs provide little evidence for continued functional or morphological changes beyond post-noise day 7 (41), suggesting that hearing loss at this final test time provides a good estimate of PTS. As robust reductions in PTS using this nutrient combination have been well-demonstrated (7, 42), the primary aim of this study was to determine the potential for reduced TTS, with the achieved plasma levels measured as a guide for target therapeutic levels in human subjects.
In terms of clinical translation of animal data to humans, it is important to demonstrate efficacy against TTS, first in animal models, and then in human models. There are some cases of military noise exposure which result in PTS despite use of hearing protection, such as the M16 firearm training period previously used by the Israeli army (43, 44; personnel involved in those training exercises were randomized to Mg therapy or placebo control, and hearing loss >25 dB was reduced from a rate of 25% of control ears to 11% of Mg-treated ears). Access to such populations is difficult, and in general, it is not ethical to expose humans to experimental noise levels sufficient to induce PTS. Although some work environments eventually produce small levels of PTS, clinical trials to demonstrate protection of workers in such environments will take years to conduct. On the other hand, exposure to noise sufficient to produce TTS is common and controlled clinical trials can be performed to evaluate reductions in degree of TTS and/or more rapid recovery of normal hearing. Thus, it is critical to demonstrate that potential human treatments are effective in animal models of TTS in order to translate the efficacy into clinical trials. This study specifically evaluated a combination of β-carotene, vitamin C, Trolox® (a synthetic analogue of vitamin E), and Mg for potential translation to human TTS models.
Materials and Methods
Subject Group #1, Plasma Sampling and Analysis
To determine the plasma levels of the active agents, a pharmacokinetic study was conducted under a contract with MPI Research (Mattawan, MI). Ten male albino guineas pigs (CRL; HA) with a chronically implanted jugular vein cannula connected to a vascular access port were obtained from Charles River Laboratories (weight: 471–504g). The experimental protocol was reviewed and approved by MPI's IACUC. Of the eight animals included in the study, six were randomized to receive a nutrient treatment. Treatment included 2.1 mg/kg β-carotene delivered orally (p.o.) in vegetable oil (2.1 ml/kg volume), and vitamin C (71.4 mg/kg ascorbic acid), Trolox® (26 mg/kg; Trolox® is a water soluble analog of vitamin E), and Mg (2.85 mmol/kg magnesium sulfate) delivered subcutaneously (s.c.), as in a previous investigation (7). The vitamin C, Trolox®, and Mg were delivered with a single, 6 ml/kg injection. The other two subjects served as controls and were given an equivalent volume of saline (6 ml/kg, s.c.) and vegetable oil (2.1 ml/kg, p.o.). All treatments were delivered once daily for 5 days. The time at which the final day-5 dose was delivered was defined as time 0. Blood samples were taken via the jugular venous access port at −48 and −24 hrs [relative to the first (day 1) dose, for baseline measurements] and at 0.5, 1, 2, 4, 8, 12, 24 hrs after the last (day 5) dose.
Blood samples (approximately 0.5 mL) were collected from the jugular vein cannula into tubes containing lithium heparin and placed on ice. Following sample collection at each interval, 0.5 ml Sodium Heparin for Injection, USP, was administered through the jugular vein cannula to replace the volume of blood collected. The samples were centrifuged at 3 to 5°C at a speed of 1300 g for 10 minutes following completion of sample collection at each interval. The resulting plasma was separated and placed into a cryotube. A 30 μL aliquot of the plasma was removed and placed into a second cryotube. Then, 30 μL of a preservation buffer containing 10% meta-phosphoric acid and 2 mg/mL dithiothreitol (DTT) was added to the second cryotube and thoroughly mixed, to stabilize the ascorbic acid (vitamin C) in the plasma. Both aliquots were stored at approximately −70°C and protected from light.
The plasma levels of the actives were analyzed by KAR BioAnalytical (Kalamazoo, MI). β-carotene, retinol and vitamin C were analyzed using an HPLC System (Waters 2695) with UV detection. Standard curves were generated from spiked plasma samples. The linear ranges of detection were: β-carotene, 0.025 to 10.0 μg/mL; vitamin C, 1.67 to 83.3 μg/mL; retinol, 0.10 to 10.0 μg/mL. Trolox® was separated using an HPLC System (Waters 2695) and detected with a Micromass Quattro LC-MS/MS; linear detection range 0.42 to 16.7 μg/mL. Mg was analyzed with Atomic Absorption Spectrometry (Varian SpectraAA 20) with a linear range of 0.05 to 1.3 μg/mL.
Subject Group #2, Threshold Shift Post-Noise
Animals
A total of 31 pigmented male guinea pigs (250–300g; Elm Hill Breeding Labs, Chelmsford, MA) were used in the noise-exposure study. As in earlier studies (7, 41), male guinea pigs were selected based on sex differences in reactive oxygen species (ROS) detoxification (45), activity of glutathione S-transferase in the cochlea (46), and gender-based variation in susceptibility to NIHL (47). The experimental protocol was reviewed and approved by the University Committee for the Care and Use of Animals (UCUCA) at the University of Michigan, and all procedures conformed to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Surgical Procedure
All subjects underwent a single surgical intervention approximately 1-week after arrival at the University of Michigan (range = 5–14 days; mean = 8 +/− 3 days). The surgical procedure was closely modeled after those we have described previously (48, 49). Animals were anesthetized (29 mg/kg ketamine, 1.2 mg/kg xylazine, 0.6 mg/kg acepromazine), the bulla was exposed and gently opened using a post-auricular approach, and then a sterile ball electrode (0.25 mm diameter, constructed of teflon-coated platinum-iridium wire) was carefully placed on the round window membrane. A ground wire was inserted into the middle ear via the defect in the bulla, and carboxylate cement (Durelon, 3M/ESPE, St. Paul, MN) was used to seal the bulla defect and permanently fix both the cannula and the electrodes in place. The opposing ends of the electrodes, soldered to a two-pin connector (HSS-132-G2, Samtec Inc., New Albany, IN) prior to the onset of the surgical procedure, were fixed to the skull using methyl methacrylate cement (Jet Repair Acrylic, Lang Dental Manufacturing, Wheeling, IL). The post-auricular incision was then sutured and the incision cleaned. The indwelling electrode was used during subsequent sound-evoked electrophysiological testing. Subjects were allowed at least 1-week post-surgery recovery time prior to the first electrophysiological tests.
Electrophysiological Testing: Compound Action Potential (CAP)
After implanting the chronic electrode as described above, the sound-evoked whole-nerve compound action potential (CAP) was measured. Baseline CAP tests were conducted at least 1-week post-surgery (range = 7 – 18 days; mean = 11 +/− 2 days). Prior to CAP tests, the animals were anesthetized (29 mg/kg ketamine, 1.2 mg/kg xylazine, 0.6 mg/kg acepromazine), and placed on a warm heating pad to maintain body temperature. Acoustic stimuli were brief pure-tone stimuli (2, 4, 8, 16, 24, and 32 kHz) presented at levels ranging from 5 to 90-dB SPL in 5–10 dB increments (5, 10, 15, 20, 25, 30, 40, 50, 60, 70, 80, and 90 dB SPL). The 24 and 32 kHz test stimuli were included, given previous data suggesting protection mediated by this nutrient combination may be greatest in the basal regions of the cochlea (7). Acoustic signals were 5-msec in duration, with a 0.5-msec rise-fall time; signals were presented at a rate of 10/sec with 128 repetitions per frequency/level combination. Acoustic stimuli were generated using Tucker-Davis Technology (TDT; Alachua, FL) System III hardware and SigGenRP software. Signals were converted to analog, attenuated to set level (PA5), and presented using an EC1 transducer coupled to the animals' ear canal via vinyl tubing. Brainstem potentials were acquired and digitally filtered (300–3000 Hz) using BioSigRP (TDT).
CAP threshold was defined as the sound level that produced a 75-μV response (baseline-N1); threshold estimates were determined using linear interpolation. All CAP scoring was done by observers masked relative to subject treatment. Six animals were excluded from the study as a consequence of hearing loss measured during baseline (post-surgery) tests. Post-implant threshold sensitivity in those animals was elevated by 2–3 standard deviations relative to the mean. Subjects with normal CAP threshold sensitivity were randomly assigned to a treatment group, and the assigned treatment was initiated. One day later, subjects were exposed to noise (octave band noise, centered at 4 kHz, 110 dB SPL × 4 hours, see below). At 1 hour, 1 day, 3 days, 5 days, and 7 days post-noise exposure, the CAP tests were repeated.
Experimental groups
Implanted animals were assigned to receive control (n=9) or nutrient treatments (n=16) as described for the subjects in the initial plasma collection study. All test substances were purchased from Sigma-Aldrich (St. Louis, MO) (β-carotene, #C9750, CAS 7235-40-7; L-threoascorbic acid, #A5960, CAS 50-81-7; Trolox®, Fluka Chemika #56510, CAS 53188-07-1; magnesium sulfate, #M7506, CAS 7487-88-9). All treatments were initiated 1 day prior to noise exposure and continued daily at 24-hour intervals until day 5 post-noise, for a total of 6 days of treatment. The nutrient-treated animals received the entire daily dose during one daily treatment (QD) (n=8), or as two equal daily treatments (n=8), each providing 1/2 of the total daily dose of the active agents (BID). The BID treatments were separated by approximately 8 hours to possibly improve the treatment efficacy. However, manipulating the dose delivery paradigm did not influence the efficacy of the active agents on any measure of function (all p's>0.05) at any of the time points sampled, and all treated animals were therefore combined into a single treated group for the analyses in this report.
Noise exposure
Noise exposure was initiated 1-hour after the daily treatment (saline or nutrients) was delivered; a time at which plasma nutrient levels were elevated (see results). All subjects were exposed to octave-band noise (centered at 4 kHz, 110 dB SPL, 4 hours). This noise exposure was explicitly selected to induce robust TTS (i.e., 40–60 dB) and minimal PTS (i.e., less than 20 dB). Animals were exposed two at a time in separate cages in a ventilated sound exposure chamber fitted with speakers (Model 2450H, JBL, Salt Lake City, UT) driven by a noise generator (ME 60 graphic equalizer, Rane, Mukilteo, WA) and power amplifier (HCA-1000 high current power amplifier, Parasound Products, San Francisco, CA). Sound levels were calibrated (Type 2203 precision sound level meter, Type 4134 microphone, Brüel and Kjær Instruments, Norcross, GA) at multiple locations within the sound chamber to ensure uniformity of the stimulus, using a fast Fourier transform network analyzer with a linear scale. The stimulus intensity varied by a maximum of 3 dB across measured sites within the exposure chamber. During noise exposure, noise levels were monitored using a sound level meter, a pre-amplifier, and a condenser microphone positioned in the center of the chamber at the level of the animal's head.
Histological examinations
On day 7, immediately after the final CAP measurement, the animals were deeply anesthetized using an overdose of xylazine (1 ml delivered intra-cardiac), then decapitated. The cochleae were immediately removed and transferred into 4% paraformaldehyde in 0.1M phosphate-buffered saline (PBS, pH 7.4). Under a dissecting microscope, the bone nearest the apex and the round and oval windows were opened, followed by gentle local perfusion from the apex. The tissue was kept in fixative for 12 hours, then the bony capsule and the lateral wall tissues were removed, and the modiolar core was carefully removed from the temporal bone. Following permeabilization with Triton X-100 (0.3%, 30 min), the organ of Corti was stained for f-actin using rhodamine phalloidin (1%, 60–120 min) to outline hair cells and their stereocilia (50). After washing the tissues with PBS, the organ of Corti was dissected and surface preparations were mounted on glass slides. The tissues were observed under fluorescence microscopy, and the number of missing inner (IHC) and outer (OHC) hair cells were counted from the apex to the base in 0.19 mm segments (as described in 41). Counting was begun approximately 0.76–1.14 mm from the apex, thus omitting the initial irregular most-apical part of the cochlear spiral. Percentages of hair cell loss in each 0.19 mm length of tissue were plotted along the cochlear length.
Statistical analysis
Pharmacokinetic (PK) parameters were calculated by PharmOptima (Portage, MI) using the plasma-level test results and Excel (Microsoft) and PK solutions (Summit Research) software. These included maximum plasma concentration (Cmax), half life (t½), and area under the concentration time curve (AUC). All statistical comparisons of the CAP thresholds and hair cell counts were performed using SPSS for Windows (version 16.0). Threshold data were compared using repeated measure Analysis of Variance (ANOVA) tests. Repeated measures ANOVA require sphericity (equality of the variances) for the different levels of the repeated measures factors; Mauchly's Test of Sphericity was used to confirm sphericity. When the significance level of Mauchly's tests were less than 0.05, indicating that sphericity cannot be assumed, the Greenhouse-Geisser correction for sphericity was applied.
Results
Plasma Levels
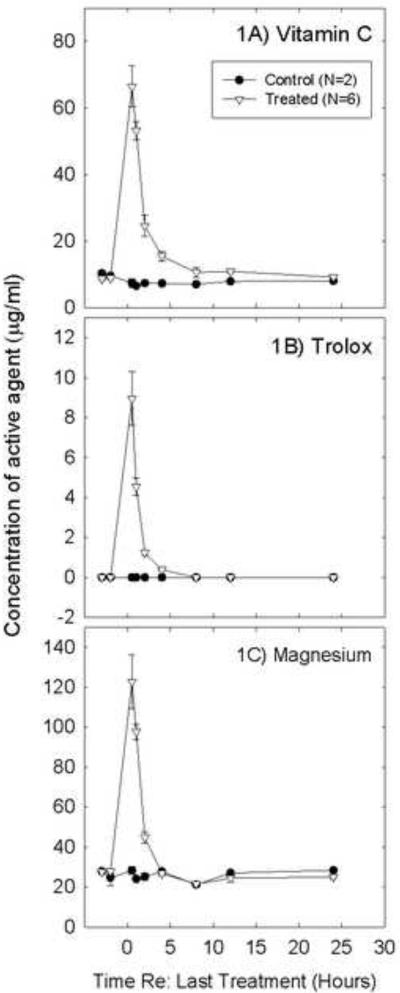
As shown in Figure 1, plasma levels for vitamin C (1A), Trolox® (1B), and Mg (1C) increased after daily injections. Time 0 was defined as the time at which the final day 5 dose was delivered, and samples collected after the final treatment showed robust increases in plasma level that were not evident in samples from control subjects. Mean baseline concentration of vitamin C (Figure 1A) was 8. 8 μg/mL which increased to a Cmax of 66.4 μg/mL at 0.5 hr. The plasma concentration returned to baseline by 24 hr with a t½ = 8.7 hr and an AUC(0–24hr) =160 μg•hr/mL. Since Trolox® is not a naturally occurring substance the baseline concentration was 0. Within 0.5 hr of dosing the Cmax of 8.9 μg/mL was reached (Figure 1B). Plasma concentrations returned rapidly to baseline by 8 hr. The calculated t½ = 0.59 hr and the AUC(0–8hr) = 10 μg•hr/mL. The mean pre-dose plasma concentration of Mg was 27.7 μg/mL (Figure 1C). After the last dose, the plasma concentration increased rapidly to a Cmax of 123 μg/mL at 0.5 hrs and fell rapidly to baseline by 4 hrs. The t½ = 1.32 hr and the AUC(0–4hr) = 112 μg•hr/mL. β-carotene was the only ingredient provided orally. At baseline, and at all post-dose time points in all animals, the plasma concentrations were below the level of quantification (0.025 μg/mL; data not plotted).
Figure 1.
Plasma concentrations of vitamin C (1A), Trolox (1B), and magnesium (Mg, 1C) rapidly increased after the final s.c. injection of the active agents. Two baseline samples (shown here prior to time 0) were collected via the jugular venous access port prior to the first injections; these samples were collected 48 and 24 hrs prior to the first dose. Time 0 was defined as the time of the fifth and final injection. Plasma increases were apparent at times extending from 2–8 hours post-injection. Samples were collected at 0.5, 1, 2, 4, 8, 12, and 24 hrs after the last (day 5) dose. In the control animals, which were maintained on a nutritionally complete diet, there was no evidence for robust changes across samples.
Since β-carotene can be converted into vitamin A (retinol), samples were also evaluated for the presence of retinol. Retinol was present at baseline (approximately 0.7 μg/mL), but there was no increase in retinol plasma concentrations after dosing. Taken together, β-carotene did not appear to reach the blood stream in significant quantities and/or was rapidly eliminated to maintain normal levels.
Baseline (Pre-Noise) CAP Threshold Sensitivity
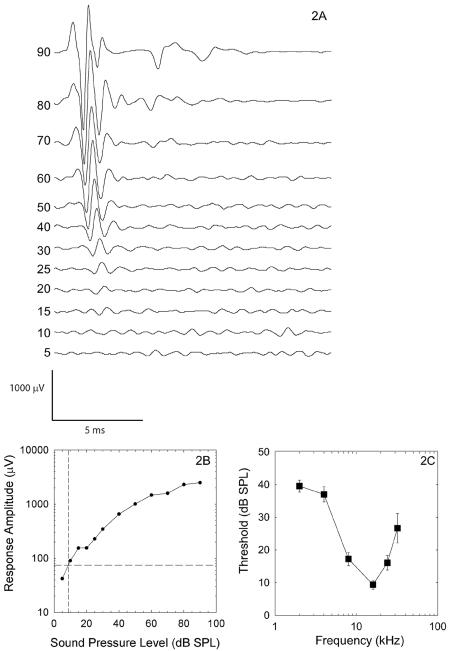
Typical pre-noise CAP waveforms are shown in Figure 2A. The linear interpolation procedure used to measure response thresholds is illustrated in Figure 2B, and average pre-noise thresholds are shown in Figure 2C. There were no systematic differences in pre-noise threshold sensitivity across groups (F=1.4; df=2,22; p=0.267) and there was no statistically reliable interaction for frequency × treatment (F=1.4; df=3,33.5; p=0.256). All subsequent analyses reported here are shift from baseline threshold, with larger shifts indicating greater hearing loss post-noise.
Figure 2.
Pre-noise compound action potential (CAP) waveforms evoked by 16 kHz tone pips are shown for tone pips ranging from 5 dB SPL to 90 dB SPL (2A). Baseline amplitude was measured at 0.09 msec. N1 is the first major negative peak and P1 is the first major positive peak subsequent to N1. Baseline to P1 amplitude was measured and plotted across levels as shown in Figure 2B, and CAP threshold was defined as the sound level that produced a 75-μV response using linear interpolation (see dashed lines). Pre-noise CAP thresholds (Figure 2C, N=25, Mean ± SEM) were consistent with other normative data on threshold sensitivity in guinea pigs (41).
Post-Noise CAP Data Sets
Some of the individual post-noise data sets contained no obvious sound-evoked response across test frequencies (2–32 kHz), even at the highest sound levels (90 dB SPL). The test sessions during which there was a lack of any sound-evoked response at any frequency/level combination are assumed to have been the result of electrode or other equipment issues if subjects had robust sound-evoked response during preceding and subsequent test sessions, and these data sets were excluded from analysis. Legends indicate the number of data sets used in each analysis. To assure that the overall outcome was not influenced by the missing data sets, we performed a secondary analysis limited to only those subjects with complete data sets at all test times. In that analysis, there were 7 control animals and 7 treated animals. Limiting the number of subjects did not change the direction of the effect, but did serve to reduce statistical power.
Noise-Induced Change in CAP Threshold: Threshold Protection
Maximum NIHL after exposure to an octave band noise centered at 4 kHz might be predicted to occur at approximately 6–8 kHz (i.e., the so-called `half-octave shift', see 51, 52–54). However, empirical data show robust hearing loss deficits extending from 8 kHz to at least 16 kHz after this noise exposure, with massive hair cell loss extending into the more basal regions of the cochlea (thus suggesting hearing loss at other, higher, frequencies not tested in those investigations) (7, 41). In fact, there is an extensive literature on extended high frequency loss with noise frequently reported to induce hearing loss at “unexpected” frequencies above the range predicted by the half-octave shift (55–58). Given empirical data suggesting differential effects of noise at lower frequencies (2 and 4 kHz) and higher frequencies (8–32 kHz), the initial statistical analyses to determine the reliability of treatment-based group differences were conducted separately for lower and higher frequencies.
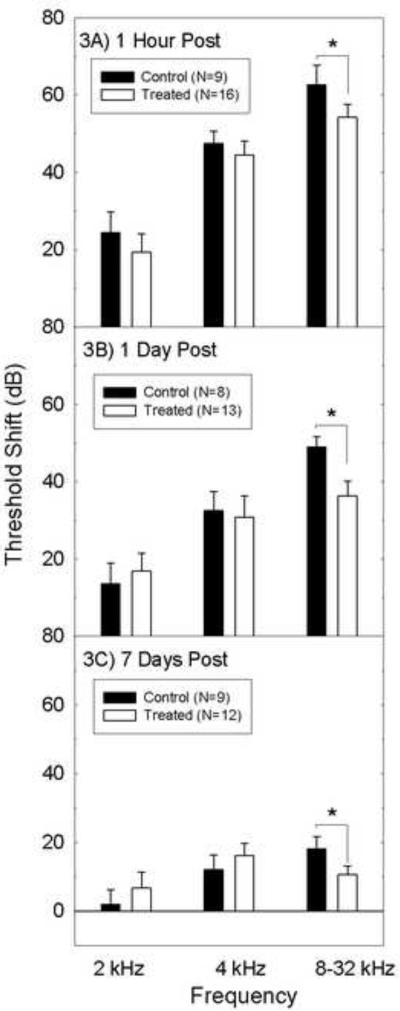
Initial threshold shift analyses evaluated the potential for group differences at 2 kHz and 4 kHz, where smaller threshold shifts are expected and thus the potential for protection against threshold shifts is reduced. There was no effect of treatment condition on post-noise threshold shift at 2 or 4 kHz. The higher frequencies are more likely to afford an opportunity to measure protection given that noise is more harmful at these higher frequencies. Threshold shift data were pooled for the other higher frequencies (8–32 kHz) where deficits are the most robust. In contrast to the 2 and 4 kHz outcomes, nutrient treatment significantly decreased noise-induced threshold shift at the pooled higher test frequencies (8–32 kHz; see Figures 3A, 3B, 3C). Treatment-induced differences were highly reliable (F=6.2, df=1,23, p=0.020). In treated animals, there was a 9-dB reduction in threshold shift measured 1-hour post-noise, a 13-dB reduction in threshold shift measured 1-day post-noise, and an 8-dB reduction in threshold shift was measured 7-days post-noise. There was a statistically significant main effect for time (F=100.9, df=4,92, p<0.001), with threshold shift decreasing with time post-noise. To determine whether the pooled differences reflected protection across frequencies, secondary analyses were conducted for individual test frequencies. When the data were broken down within individual frequencies, there were statistically reliable group differences at 8 kHz, 24 kHz, and 32 kHz (p's <0.05), with treated animals having smaller threshold shifts.
Figure 3.
Comparison of compound action potential (CAP) threshold shifts in guinea pigs treated with saline or the combination of nutrients at (3A) 1 hour, (3B) 1 day, and (3C) 7 days post noise insult. Threshold shifts at 2 and 4 kHz were smaller and more transient than those at the higher frequencies, and there was no reliable effect of treatment at 2 and 4 kHz (all p's >0.05). Nutrient treatment significantly reduced threshold shifts at all 3 time points relative to controls (* =p<0.05). All data are mean ± SEM.
Noise-Induced Change in CAP Threshold: Temporal Pattern of Change at Individual Frequencies
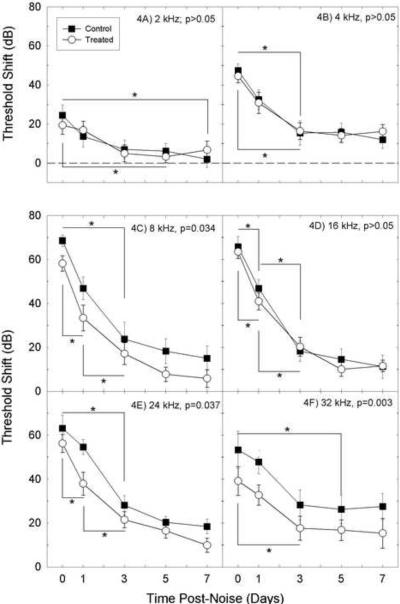
The time at which a statistically significant recovery occurred was defined as a time point at which we detected a statistically significant decrease in TTS compared to preceding within-group time points. The effects of nutrient treatment on rate of recovery were mixed; treated animals recovered more rapidly at most (2, 8, 24, and 32 kHz), but not all (4 and 16 kHz) frequencies (see Figure 4). Asterisks in Figure 4 indicate statistically reliable within-group pair-wise differences. For example, panel 4C shows that nutrient-treated subjects showed statistically reliable recovery of function at 8 kHz 1 day post-noise, and they showed additional statistically significant recovery of function between days 1 and 3 post-noise. In contrast, at 8 kHz, the control animals did not achieve statistically reliable recovery of function until 3 days post-noise. The most robust differences in the rate of recovery were detected at the frequencies where robust treatment effects were observed (i.e., 8, 24 and 32 kHz). At each of these three frequencies, statistically reliable improvement was detected at an earlier time in treated animals than in control animals. Taken together, the overall group differences in threshold shift were accompanied by differences in the timing of recovery across the first 7 days post noise.
Figure 4.
Temporal recovery of threshold shifts within saline and nutrient-treated groups of guinea pigs at 2 kHz (4A), 4 kHz (4B), 8 kHz (4C), 16 kHz (4D), 24 kHz (4E), and 32 kHz (4F). Statistical probability (p) values listed in each panel are the probability of obtaining a group difference test statistic (for amount of hearing loss in treated vs control conditions) that is at least as extreme as the one that was actually observed, assuming that the null hypothesis is true; i.e., the p-values provided indicate the probability that there is no reliable group difference at that frequency. At 2 kHz (4A), 4 kHz (4B), and 16 kHz, there was no effect of treatment on hearing outcomes (p's >0.05). At 8 kHz (4C), 24 kHz (4E), and 32 kHz (4F), robust protection was observed (p's <0.05). Asterisks and connecting lines are used to indicate times at which thresholds were reliably different within each treatment group (see asterisks). At 8 kHz (4C) and 24 kHz (4E), treated animals showed statistically reliable recovery between 1 hr and 1 day post noise as well as 1 day and 3 days post noise. Control animals showed statistically reliable recovery between 1 hour and 3 days post noise (see asterisks). Thus, statistically significant threshold recovery took longer in the control animals (3 days) than in the treated animals (1 day). Threshold shifts recovered with different time courses depending on frequency. At 32 kHz, treated animals showed statistically reliable recovery between 1 hr and 3 days post noise, whereas control animals did not show reliable recovery until 5 days post noise. All data are mean ± SEM.
CAP Input-Output Functions
We normalized post-noise CAP amplitude to pre-noise baseline amplitude to determine the percent reduction in CAP amplitude in treated and untreated groups at different post-noise times. CAP amplitude was significantly decreased post-noise for subjects in both treatment groups; antioxidant treatment did not reliably reduce the effects of noise on CAP amplitude (not shown).
Hair Cell Counts
We counted missing hair cells to determine if hair cell survival varied with treatment condition. Noise-induced cell death was limited to small lesions of row 1 OHCs approximately 10–12 mm from the apex (~4% missing cells) and row 3 OHCs in the first, most extreme apical section 1.14 mm from the apex (~25% missing); there was no evidence for group differences in hair cell survival (not shown).
Discussion
In establishing the efficacy of a substance or substances to protect against an insult like NIHL, it is critical to demonstrate both that the test subjects received reasonable exposure to the substance(s) and a relevant change in function. Meeting these criteria delineates the pathway for translation to human studies. In the present study, we have demonstrated that the nutrient combination produced large and reliable increases in plasma concentration of three of the active agents and that as a result of the exposure there was a significant reduction in noise-induced threshold shift as measured from 1-hour to 7-days post noise. The three agents provided by subcutaneous injections all produced significant increases in plasma concentrations.
Ascorbic acid (vitamin C) injections resulted in very rapid absorption and a large peak concentration 30 min post-injection, with relatively rapid elimination (return to baseline within 8 hours). Trolox® injections resulted in very rapid absorption and a large peak concentration 30 min post-injection, with relatively rapid elimination (return to baseline within 2 hours). Injection of Mg produced a rapid increase in plasma concentrations with a rapid clearance. Vitamin C, Trolox®, and Mg were thus biologically available at high levels during and after the noise exposure; changes in β-carotene status cannot b1e confirmed as β-carotene concentrations were below the level of quantification, both before and after the oral gavage of this agent. β-carotene may be an effective ROS scavenger at very low plasma concentrations (below the sensitivity of the current assay), it may work through a tissue depot effect, or it may be converted in vivo to an antioxidant species not measured in this study (i.e., not retinol). Furthermore, guinea pigs may metabolize β-carotene differently than other animals and humans (59, 60).
These data provide an initial quantification of nutrient plasma levels that reduced the effects of noise on the inner ear in this study, and in an earlier investigation that used the same dose paradigm (7). Because noise exposure started 1-hour post-injection in both the earlier study and the current study, and noise continued for 4–5 hours, the most relevant plasma levels for the purposes of protection during noise are those measured from 1–6 hours post-injection. Measurement of nutrient levels in the cochlear perilymph represents a significant challenge for this and any investigation, given potential CSF contamination with basal turn sampling strategies, and evidence that apical sample volumes are limited to no more than 1 μl per sample (61–64). However, the vitamins and minerals administered in this study are known to distribute well throughout the body. It is reasonable to assume that levels of the nutrients increased in the perilymph, although the exact concentrations and time course may be different from that seen in the plasma compartment. Further studies are required to determine the importance of β-carotene in this nutrient mixture. It will also be critical to confirm the effects of oral administration on functional protection. Preliminary data suggest oral treatments with these nutrients can be effective, showing dose-dependent protection (42, 65). Additional experiments directly comparing the efficacy of nutrient treatment with and without β-carotene in models of PTS and/or TTS would help clarify the importance of this constituent.
Noise-induced threshold shift was reduced by nutrient treatment; this effect was largely limited to the highest test frequencies, but was clearly observed both during initial acute noise-induced changes in hearing, and at later time points. Protection at the later time points is consistent with the reduction in PTS reported previously (7), where subjects were exposed to much higher noise levels. Protection at the earlier time points importantly extends the earlier findings, which only measured PTS. The lack of protection at 16 kHz was unexpected, given the robust group differences at 8, 24, and 32 kHz. Moreover, compelling protection was previously obtained at 16 kHz in guinea pigs exposed to a similar noise insult and treated with a similar combination of agents (7). One possible explanation is that subjects in the current cohort experienced increased vulnerability to noise at 16 kHz based on specific changes in resonance and/or cochlear vibration properties subsequent to surgical manipulation of the middle ear cavity and placement of an electrode on the round window membrane. Subjects in the earlier studies had not undergone surgical manipulation and implantation of a foreign body in the middle ear prior to noise insult.
The hair cell loss noted in this study was so small no significant difference between control and treated animals could be expected. The protection measured in this study was not a function of improved hair cell survival, as little hair cell loss was observed in cochleae from both the treated and the control group subjects. Minimal hair cell loss is, however, anticipated for noise insults that cause minimal PTS. Based on hair cell counts alone, we cannot rule out significant preservation of more subtle features such as hair cell shape, stereociliary stiffness, or conformation of the organ of Corti. To better understand mechanisms of protection, future investigations should include measurements of distortion product otoacoustic emission (DPOAE) amplitude. DPOAEs provide a sensitive and objective measure of OHC function (66–68), and OHCs are particularly sensitive to noise insult (69, 70). DPOAEs have demonstrated high sensitivity to noise in animal and human studies (for examples, see 71, 72, 73). DPOAE metrics have revealed functional hair cell protection with pre-noise Mg treatment (23), and transient evoked OAEs have similarly revealed protection of hair cell function with pre-noise vitamin C treatment (74). Based on these outcomes, and previous data showing improved hair cell survival after longer, more intense noise (7), functional protection of the OHCs by the nutrient treatment would not be surprising, but, remains to be confirmed.
In addition to the potential for subtle changes in hair cell structure and/or function (which might have been detected using DPOAE tests), it is also possible that neural swelling contributed to the measured TTS deficits. Temporary neural swelling and/or decreases in the number of synaptic contacts between the inner hair cells and the auditory nerve dendrites have been reported after noise insults that induce significant TTS in the absence of robust PTS (19, 20, 75, 76). We did not harvest cochlear tissues in a way that allowed us to directly measure neural swelling, or neural synaptic connections. However, the finding that CAP amplitude was reduced to a similar degree in treated and untreated subject groups suggests protection did not extend to the level of the auditory nerve synapse in this study. Another antioxidant agent, ebselen, has been shown to directly reduce neural excitotoxicity (38), confirming it is possible for antioxidant agents to prevent noise-induced neural swelling, and data using other dosing strategies suggest nutrient therapy could protect the nerve population with modification of the dosing paradigm. Importantly, both local (in the ear) and systemic treatments with vitamins E and vitamin C have enhanced auditory neural survival in guinea pigs treated with ototoxic drugs (77), and Mg supplements could also be expected to reduce neural swelling given an appropriate treatment paradigm. Mg modulates calcium channel permeability, influx of calcium into cochlear hair cells, and glutamate release (78, 79). Moreover, Mg can act as an antagonist at the NMDA class of glutamate receptors. Thus, the potential for synaptic and/or neural protection with nutrient therapy should not be excluded.
A final possibility with respect to site of action is protection of the lateral wall, as shown in mice fed a custom dietary supplement before and after noise insult (42, 65). PTS was reduced, and the density of the Type II fibrocyte population was reliably preserved, with some suggestion that the strial cell population was also protected against noise-induced cell loss. Noise-induced oxidative stress has been specifically shown in lateral wall tissues taken from guinea pig cochleae, and vitamin C supplements reduced free radical production in the lateral wall as well as NIHL (80). Thus, it would not be surprising if the nutrient combination had some benefits in preserving lateral wall cell structures in guinea pigs, as recently shown in mice. Taken together, the specific mechanism of protection associated with the functional protection obtained in this study is not known, and future investigations will be required to determine the extent to which more subtle outer hair cell protection, neural protection, protection of cells in the lateral wall, or other unknown morphological protection contributed. Functional protection measured in this study suggests the potential for protection of cells and/or structures not examined in the current investigation.
Guidance for Human Use and Human Trials
This report describes a treatment rationale which could readily be applied to human subjects, although translation of the dose paradigm from the current guinea pig doses into acceptable human doses requires some care. The mg/kg dosing for humans is likely to be lower than for guinea pigs, as it is well accepted that smaller mammals have greater energy requirements than larger ones, and correspondingly, that metabolic rates are greater in smaller animals than larger ones (81–85, for reviews, see 86). Indeed, species differences in drug metabolism are well known, even across rodents (87–93). Future human pharmacokinetic investigations will need to take into account the fast elimination of the active substances as revealed in the current guinea pig study, and the timing of the noise insult. Because noise exposure started 1 hour post injection in the earlier study and the current study, and continued for 4–5 hours, the most relevant plasma levels for the purposes of protection against NIHL are those measured from 1–6 hours post-injection. Agents used in this first plasma measurement study were injected, in the case of vitamin C, Trolox®, and Mg, and all showed rapid, robust increases in plasma level, followed by return to baseline within 2–8 hours. The following provides some guidance for each of the agents, based upon the plasma data generated and the United States Tolerable Upper Intake Levels (UL) as set by the Institute of Medicine (94, 95). Institute of Medicine guidelines provide important long-term safety data; data from multiple vitamin-based studies provide additional confidence that high-level supplements are reasonable for long-term use by human populations (for examples, see 96, 97).
Vitamin C (ascorbic acid)
Baseline plasma values were 0.88 ± 1.1 mg/dL (n=12, mean ± standard deviation of all pre-dose measurement), and the peak post-injection concentration was 6.6 ± 1.5 mg/dL with concentrations of 2.5 ± 0.78 mg/dL at 2 hours post-injection and 1.6 ± 0.36 mg/dL at 4 hours post-injection (n=6). Plasma levels in the range of 1.6–2.5 mg/dL are clearly achievable in human subjects. Across human studies, normal (unsupplemented) vitamin C levels range from 0.8 mg/dL (98) to 1.4 mg/dL (99–101). Vitamin C supplements of 400 mg/day or greater achieve plasma levels ≥ 2.0 mg/dL (102–104). In the Age-Related Eye Disease Study (AREDS), subjects treated with 500 mg/day vitamin C had a 25–30% increase in median plasma level (baseline = 1.1 mg/dL) after 1 year (96, 105), which suggests measured median plasma levels of ~1.4 mg/dL. In humans, peak vitamin C plasma levels increase with increasing vitamin C dose (106–108), although bioavailability does saturate between 200 and 400 mg/day given increased urinary excretion of vitamin C (106, 107). Time to achieve steady-state plateau concentration varies across subjects; single-subject examples presented in two earlier reports showed stable plateaus at 25–35 days of daily dosing (106, 107). The UL for vitamin C in adults is 2,000 mg/day (94,95) with many currently available supplements in the 400–500 mg range. Therefore, evaluation of a dose in the range of 500 mg to determine plasma levels would be reasonable starting point.
Vitamin E (α-tocopherol)
The dosing recommendation for therapeutically effective levels is more challenging, as vitamin E was delivered in the form of Trolox®. With respect to vitamin E, a 1:1 equivalence in oxygen-radical absorbing capacity (ORAC) has been reported for Trolox® and α-tocopherol (109), with a similar equivalence of Trolox® and α-tocopherol reported using another free radical measurement strategy (110). Depending on the specific test protocol, in vitro antioxidant efficiency can be either greater for Trolox® then α-tocopherol, or vice versa (for review see 111). Assuming a 1:1 equivalence, then the change from baseline (0 in the case of Trolox®) levels in plasma provide one possible target for human translation. The peak post-injection concentration measured here was 0.90 ±0.33 mg/dL with concentrations of 0.45 ± 0.11mg/dL at 2 hours post-injection and 0.12 ± 0.03 mg/dL at 4 hours post-injection (n=6).
Human oral dosing with vitamin E can readily achieve plasma level increases meeting or exceeding those reported here for Trolox®. Serum levels increased from 8.4 mg/dL to 20.7 mg/dL (12.3 mg/dL increase) in subjects taking 200 IU/day vitamin E for 8 weeks, and they increased from 8.9 mg/dL to 52.8 mg/dL (43.9 mg/dL increase) in those taking 2000 IU/day for 8 weeks (112). These increases far exceed those seen with Trolox®. Maximum levels appear to be reached approximately 4 weeks after onset of daily treatment (113). There is some reason for caution with respect to high level human dosing in patients with compromised health status; one meta-analysis of the dose-response relationship between vitamin E supplements and all-cause mortality revealed an increase in all-cause mortality and resulted in the author's recommendation to avoid vitamin E supplements ≥ 400 IU/day (114). That meta-analysis has been criticized based on multiple issues (115–120). Current US UL for healthy adults is 1000 mg/day, corresponding to 1500 IU/day. Taken together, a reasonable starting dose for human translation studies would be in the range of 200–400 IU (133–267 mg).
β-carotene
Dosing recommendations and suggestions for targeted levels are not possible based on this data, as β-carotene did not reach detectable levels in the guinea pig samples. We simply note here that human baseline β-carotene levels of 25–28 μg/dL have been reported; the lower limit of our assay was 2.5 μg/dL, indicating that guinea pigs have a basal level at least 10-fold lower than humans and assumedly metabolize/convert β-carotene differently than humans. A greater than 500% increase in median plasma levels after 1-year of daily vitamin use with a vitamin that includes 15 mg/day β-carotene has been reported (96, 105). In another group of subjects with a shorter (2-month) treatment duration, but a higher daily dose (20 mg), plasma β-carotene levels increased from 26.8 μg/dL to 267 μg/dL (121). Taken together, plasma β-carotene levels can be easily increased in human subjects, but, a target value for protection of the inner ear against noise insult is not currently known. Although there is no UL established, it should be noted that use of high-level β-carotene supplements (20–30 mg/day) is now contra-indicated for those with a significant history of tobacco use (for recent review, see 122). Specifically, an increase in the risk of lung cancer was reported for smokers who took high-level β-carotene supplements in the Alpha-Tocopherol, Beta-carotene Cancer Prevention (ATBC) trial in Finland (123, 124) as well as smokers and asbestos workers in the β-Carotene and Retinol Efficacy Trial (CARET) in the USA (124–126). We explicitly note here that while β-carotene is metabolized to vitamin A, use of β-carotene supplements is distinctly different from use of a preformed vitamin A supplement (such as retinol, or retinoic acid). Carotenoids are not toxic in animals or humans, and are nonteratogenic even at high doses in animals (127, for review, see 128). In contrast, high levels of pre-formed vitamin A have been reported to increase the risk of birth defects. In a large-scale study including 22,748 pregnant women who consumed greater than 10,000 IU/day pre-formed vitamin A during the first trimester of pregnancy, there was an increased risk of certain birth defects (129).
Magnesium
With respect to Mg, the plasma concentrations reached at 30 min and 1 hr post-injection in guinea pigs were higher than plasma concentrations previously reported in humans. Even in humans given a 50 mg/kg bolus of magnesium sulfate intravenously (IV) followed by 15 mg/kg/hr for 6 hrs, plasma concentrations were less than half the peak reached here (see 130). Nonetheless, the basal levels of Mg in humans and guinea pigs are similar (0.2–0.3 mg/dL), and, in humans Mg supplementation of as little as 122 mg/day (delivered as Mg aspartate for 10 days) reduced TTS with no apparent change in plasma concentration (106). In that study there was an increase in Mg concentration in mononuclear cells. There is little consensus on the optimal strategy for measuring the activity of Mg as plasma Mg is not sensitive to subtle change and may not reflect whole body Mg stores (131–133). The dose (343 mg/kg) and route (SC) of Mg provided here is not relevant to human translation. However, dietary supplementation including Mg (~5× normal chow) has recently been shown to reduce PTS in mice (42,65), and parenteral administration of Mg alone has been shown to reduce PTS in guinea pigs (16) [although as noted above this depends on both the dose and onset of treatment relative to the time of noise]. Taken together, this suggests that much lower doses than those used in this guinea pig study are able to prevent NIHL even with oral administration. Indeed, Mg supplementation at ≥ UL doses (350 mg/day) has a variety of health benefits in humans, including prevention of NIHL (43, 44, 134) and sudden sensorineural hearing loss (135, 136), as well as other general health benefits such as reduced blood pressure and improved serum lipid status (137). At levels exceeding UL, magnesium can act as a laxative. The available data indicates that doses of 150–350 mg/day should be beneficial in preventing TTS, do not exceed the UL, and should not cause adverse GI outcomes in most healthy adult populations.
One possible concern might be that administering combinations of vitamins may be more harmful than individual vitamin supplements. In the Age-Related Eye Disease study (AREDS), 3,640 participants (age 55–80) were treated with 1) a combination of antioxidants, including 500 mg vitamin C, 400 IU (267 mg) vitamin E, and 15 mg beta-carotene; 2) a combination of 80 mg zinc (as zinc oxide) and 2 mg copper (as cupric oxide); 3) a combination of antioxidants plus zinc and copper; or 4) placebo (96). In this long-term study (average follow-up: 6.3 years), the vitamin/zinc/copper combination reduced the progression of age-related macular degeneration, suggesting an important protective role for vitamins in at least one sensory disorder. There was no evidence of adverse side effects. In the United Kingdom (UK) Medical Research Council (MRC) Heart Protection Study, 20,536 participants (age 40–80), with coronary disease, other occlusive arterial disease, or diabetes were treated with 1) a combination of antioxidants, including 250 mg vitamin C, 600 mg vitamin E (896 IU), and 20 mg beta-carotene; or 2) placebo; as well as 3) the cholesterol-lowering drug simvastatin; or 4) placebo (97). In this 5-year study, there were no significant differences between the groups in any of the medical outcomes, leading the authors to conclude there was no clear evidence of either benefit or harm.
Summary
The current data provide critical new evidence documenting nutrient plasma levels achieved in animal subjects protected from TTS and PTS (7); these plasma levels serve as a guide to potentially protective levels to target in human studies. Additional animal studies measuring plasma concentrations with all agents administered orally and data from human pharmacokinetic studies with doses less than the UL will help establish appropriate doses for human clinical studies. While cochlear perilymph assays might be confirmatory, the required samples are technically challenging to collect, and it would not be feasible to replicate that sampling paradigm in human subjects. Comparisons between animal and human subjects are thus required to be limited to plasma levels.
Functionally, the current data extend our understanding of the times at which antioxidants may provide potential protection against noise insult, with the current data set showing functional protection at early (1 hour) post-noise times, as well as more rapid recovery in the first 1–3 days post noise. Protection was less robust than that previously reported after longer, louder sound insult (7) but was consistent with the level of protection recently reported by Tamir et al. (138). The use of antioxidants to reduce or prevent noise-induced hearing loss is of broad interest, with multiple groups soon to be initiating, or already conducting, clinical trials on prevention of NIHL (i.e., 9, 21, 22, 24, see also NCT00808470, NCT00552786, NCT00802425) as well as prevention of drug-induced hearing loss using antioxidant agents (139, 140, see also NCT00477607, NCT00578760, NCT01139281, NCT01131468). The ongoing translation of these agents from animal models to human trials provides a compelling rationale for continued investigations into the use of these and other antioxidant agents.
Acknowledgments
Portions of this research were presented at the 32nd Midwinter Meeting of the Association for Research in Otolaryngology (141). Support for this research was provided by the National Institutes of Health via NIH/NIDCD SBIR 1 R43 DC009106 awarded to OtoMedicine, Inc. Subcontracts were awarded to the University of Michigan (DFD), and the University of Florida (CGL). Additional support was provided by NIH/NIDCD P30 DC005188. The College of Public Health and Health Professions requires the following statement for all activities with direct support from industry; “Acceptance of support does not constitute endorsement of this sponsor by the College of Public Health and Health Professions, its departments, or members of the College.” The authors thank Glenn Green for helpful discussions on the use of these nutrient agents by human populations, and we thank Josef Miller for comments on earlier versions of this manuscript. We also thank Jennifer Benson, Susan DeRemer, Kärin Halsey, and Diane Prieskorn at the University of Michigan, and Michael Goodson, Laura Jacobsen and Jason Schmitt at the University of Florida, for technical assistance.
Sources of Support/Disclosure of Funding. Support for this research was provided by the National Institutes of Health via NIH/NIDCD SBIR 1 R43 DC009106 awarded to OtoMedicine, Inc. Subcontracts were awarded to the University of Michigan (DFD), and the University of Florida (CGL). Additional support was provided by NIH/NIDCD P30 DC005188.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Dr. Le Prell is a co-inventor on US Patent Application 20090155390, which is assigned to the University of Michigan. The nutrient treatment described in USPTO 20090155390 was licensed to OtoMedicine, Inc., at the time this study was conducted. Drs. Bennett and Boxer are founding members and were employees of OtoMedicine, Inc. Dr. Le Prell previously worked as a paid consultant to OtoMedicine, Inc. Dr. Le Prell has disclosed this relationship to the Conflict of Interest Board at the University of Florida.
References
- 1.NIOSH . Work related hearing loss. DHHS (NIOSH) Publication No. 2001-103. National Institute for Occupational Safety and Health; 2001. Contract No.: Document Number|. [Google Scholar]
- 2.Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27(1):1–19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- 3.Le Prell CG, Yamashita D, Minami S, Yamasoba T, Miller JM. Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hear Res. 2007;226:22–43. doi: 10.1016/j.heares.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Prell CG, Bao J. Prevention of noise-induced hearing loss: potential therapeutic agents. In: Le Prell CG, Henderson D, Fay RR, Popper AN, editors. Noise-Induced Hearing Loss: Scientific Advances, Springer Handbook of Auditory Research. Springer Science+Business Media, LLC; New York: 2011. [Google Scholar]
- 5.Bas E, Martinez-Soriano F, Lainez JM, Marco J. An experimental comparative study of dexamethasone, melatonin and tacrolimus in noise-induced hearing loss. Acta Otolaryngol. 2008 Dec 2;:1–5. doi: 10.1080/00016480802566279. [DOI] [PubMed] [Google Scholar]
- 6.Fetoni AR, Piacentini R, Fiorita A, Paludetti G, Troiani D. Water-soluble Coenzyme Q10 formulation (Q-ter) promotes outer hair cell survival in a guinea pig model of noise induced hearing loss (NIHL) Brain Res. 2009 Feb 27;1257:108–16. doi: 10.1016/j.brainres.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 7.Le Prell CG, Hughes LF, Miller JM. Free radical scavengers vitamins A, C, and E plus magnesium reduce noise trauma. Free Radic Biol Med. 2007;42:1454–63. doi: 10.1016/j.freeradbiomed.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell KCM, Meech RP, Klemens JJ, Gerberi MT, Dyrstad SSW, Larsen DL, et al. Prevention of noise- and drug-induced hearing loss with D-methionine. Hear Res. 2007;226:92–103. doi: 10.1016/j.heares.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Kopke RD, Jackson RL, Coleman JKM, Liu J, Bielefeld EC, Balough BJ. NAC for Noise: From the bench top to the clinic. Hear Res. 2007;226:114–25. doi: 10.1016/j.heares.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Kopke RD, Coleman JK, Liu J, Campbell KC, Riffenburgh RH. Candidate's thesis: enhancing intrinsic cochlear stress defenses to reduce noise-induced hearing loss. Laryngoscope. 2002 Sep;112(9):1515–32. doi: 10.1097/00005537-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Kil J, Pierce C, Tran H, Gu R, Lynch ED. Ebselen treatment reduces noise induced hearing loss via the mimicry and induction of glutathione peroxidase. Hear Res. 2007;226:44–51. doi: 10.1016/j.heares.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Lynch ED, Gu R, Pierce C, Kil J. Ebselen-mediated protection from single and repeated noise exposure in rat. Laryngoscope. 2004;114(2):333–7. doi: 10.1097/00005537-200402000-00029. [DOI] [PubMed] [Google Scholar]
- 13.Ising H, Handrock M, Gunther T, Fischer R, Dombrowski M. Increased noise trauma in guinea pigs through magnesium deficiency. Arch Otorhinolaryngol. 1982;236(2):139–46. doi: 10.1007/BF00454034. [DOI] [PubMed] [Google Scholar]
- 14.Joachims Z, Babisch W, Ising H, Gunther T, Handrock M. Dependence of noise-induced hearing loss upon perilymph magnesium concentration. J Acoust Soc Am. 1983 Jul;74(1):104–8. doi: 10.1121/1.389726. [DOI] [PubMed] [Google Scholar]
- 15.Scheibe F, Haupt H, Ising H. Preventive effect of magnesium supplement on noise-induced hearing loss in the guinea pig. Eur Arch Otorhinolaryngol. 2000;257(1):10–6. doi: 10.1007/pl00007505. [DOI] [PubMed] [Google Scholar]
- 16.Scheibe F, Haupt H, Ising H, Cherny L. Therapeutic effect of parenteral magnesium on noise-induced hearing loss in the guinea pig. Magnes Res. 2002 Mar;15(1–2):27–36. [PubMed] [Google Scholar]
- 17.Haupt H, Scheibe F, Mazurek B. Therapeutic efficacy of magnesium in acoustic trauma in the guinea pig. ORL J Otorhinolaryngol Relat Spec. 2003 May–Jun;65(3):134–9. doi: 10.1159/000072250. [DOI] [PubMed] [Google Scholar]
- 18.Abaamrane L, Raffin F, Gal M, Avan P, Sendowski I. Long-term administration of magnesium after acoustic trauma caused by gunshot noise in guinea pigs. Hear Res. 2009 Jan;247(2):137–45. doi: 10.1016/j.heares.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009 Nov 11;29(45):14077–85. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci. 2006;26(7):2115–23. doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kramer S, Dreisbach L, Lockwood J, Baldwin K, Kopke RD, Scranton S, et al. Efficacy of the antioxidant N-acetylcystein (NAC) in protecting ears exposed to loud music. J Am Acad Audiol. 2006;17:265–78. doi: 10.3766/jaaa.17.4.5. [DOI] [PubMed] [Google Scholar]
- 22.Toppila E, Starck J, Pyykko I, Miller JM. Protection against acute noise with antioxidants. Nordic Noise: An International Symposium on Noise and Health, in Nobel Forum; Karolinska Institutet: Stockholm, Sweden; 2002. [Google Scholar]
- 23.Attias J, Bresloff I, Haupt H, Scheibe F, Ising H. Preventing noise induced otoacoustic emission loss by increasing magnesium (Mg2+) intake in guinea-pigs. J Basic Clin Physiol Pharmacol. 2003;14(2):119–36. doi: 10.1515/jbcpp.2003.14.2.119. [DOI] [PubMed] [Google Scholar]
- 24.Lynch ED, Kil J. Development of ebselen, a glutathione peroxidase mimic, for the prevention and treatment of noise-induced hearing loss. Semin Hear. 2009;30:47–55. [Google Scholar]
- 25.Le Prell CG, Kujawa SG, Dell S, Hensley BN, Hall JWI, Campbell KCM, et al. Temporary threshold shifts and otoacoustic emission amplitude reductions subsequent to music player use by young adults. 36th Annual National Hearing Conservation Conference-Explore the World of Hearing Loss Prevention; NHCA Spectrum; 2011. in press. [Google Scholar]
- 26.Le Prell CG, Hall JWI, Sakowicz B, Campbell KCM, Kujawa SG, Antonelli PA, et al. Temporary threshold shift subsequent to music player use: comparison with hearing screenings in populations of adolescents and young adults. The National Hearing Conservation Association; Orlando, FL. February, 2010.2010. [Google Scholar]
- 27.Le Prell CG, Yang Q, Harris J, Schmitt J, Willis LM. Towards the development of a laboratory model of noise-induced hearing loss with real-world relevance for human subjects. Abs Assoc Res Otolaryngol. 2009;32:8. [Google Scholar]
- 28.Ohinata Y, Miller JM, Schacht J. Protection from noise-induced lipid peroxidation and hair cell loss in the cochlea. Brain Res. 2003 Mar 21;966(2):265–73. doi: 10.1016/s0006-8993(02)04205-1. [DOI] [PubMed] [Google Scholar]
- 29.Duan M, Qiu J, Laurell G, Olofsson A, Counter SA, Borg E. Dose and time-dependent protection of the antioxidant N-L-acetylcysteine against impulse noise trauma. Hear Res. 2004 Jun;192(1–2):1–9. doi: 10.1016/j.heares.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Kopke RD, Weisskopf PA, Boone JL, Jackson RL, Wester DC, Hoffer ME, et al. Reduction of noise-induced hearing loss using L-NAC and salicylate in the chinchilla. Hear Res. 2000 Nov;149(1–2):138–46. doi: 10.1016/s0378-5955(00)00176-3. [DOI] [PubMed] [Google Scholar]
- 31.Kopke R, Bielefeld E, Liu J, Zheng J, Jackson R, Henderson D, et al. Prevention of impulse noise-induced hearing loss with antioxidants. Acta Otolaryngol (Stockh) 2005 Mar;125(3):235–43. doi: 10.1080/00016480410023038. [DOI] [PubMed] [Google Scholar]
- 32.Bielefeld EC, Kopke RD, Jackson RL, Coleman JK, Liu J, Henderson D. Noise protection with N-acetyl-l-cysteine (NAC) using a variety of noise exposures, NAC doses, and routes of administration. Acta Otolaryngology. 2007 Sep;127(9):914–9. doi: 10.1080/00016480601110188. [DOI] [PubMed] [Google Scholar]
- 33.Fetoni AR, Ralli M, Sergi B, Parrilla C, Troiani D, Paludetti G. Protective effects of N-acetylcysteine on noise-induced hearing loss in guinea pigs. Acta Otorhinolaryngol Ital. 2009 Apr;29(2):70–5. [PMC free article] [PubMed] [Google Scholar]
- 34.Samson J, Wiktorek-Smagur A, Politanski P, Rajkowska E, Pawlaczyk-Luszczynska M, Dudarewicz A, et al. Noise-induced time-dependent changes in oxidative stress in the mouse cochlea and attenuation by D-methionine. Neuroscience. 2008 Mar 3;152(1):146–50. doi: 10.1016/j.neuroscience.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 35.Cheng PW, Liu SH, Young YH, Hsu CJ, Lin-Shiau SY. Protection from noise-induced temporary threshold shift by D-methionine is associated with preservation of ATPase activities. Ear Hear. 2008 Jan;29(1):65–75. doi: 10.1097/AUD.0b013e31815d635b. [DOI] [PubMed] [Google Scholar]
- 36.Lynch ED, Kil J. Compounds for the prevention and treatment of noise-induced hearing loss. Drug Discov Today. 2005 Oct 1;10(19):1291–8. doi: 10.1016/S1359-6446(05)03561-0. [DOI] [PubMed] [Google Scholar]
- 37.Pourbakht A, Yamasoba T. Ebselen attenuates cochlear damage caused by acoustic trauma. Hear Res. 2003;181(1–2):100–8. doi: 10.1016/s0378-5955(03)00178-3. [DOI] [PubMed] [Google Scholar]
- 38.Yamasoba T, Pourbakht A, Sakamoto T, Suzuki M. Ebselen prevents noise-induced excitotoxicity and temporary threshold shift. Neurosci Lett. 2005;380:234–8. doi: 10.1016/j.neulet.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 39.Haupt H, Scheibe F. Preventive magnesium supplement protects the inner ear against noise-induced impairment of blood flow and oxygenation in the guinea pig. Magnes Res. 2002 Mar;15(1–2):17–25. [PubMed] [Google Scholar]
- 40.Sendowski I, Raffin F, Braillon-Cros A. Therapeutic efficacy of magnesium after acoustic trauma caused by gunshot noise in guinea pigs. Acta Otolaryngol. 2006 Feb;126(2):122–9. doi: 10.1080/00016480500312547. [DOI] [PubMed] [Google Scholar]
- 41.Yamashita D, Jiang H, Schacht J, Miller JM. Delayed production of free radicals following noise exposure. Brain Res. 2004;1019:201–9. doi: 10.1016/j.brainres.2004.05.104. [DOI] [PubMed] [Google Scholar]
- 42.Le Prell CG, Ohlemiller KK, Gagnon PM, Bennett DC. Reduction in permanent noise-induced threshold deficits in mice fed a combination of dietary agents. Abs Assoc Res Otolaryngol. 2009;32:280. [Google Scholar]
- 43.Joachims Z, Netzer A, Ising H, Rebentisch E, Attias J, Weisz G, et al. Oral magnesium supplementation as prophylaxis for noise-induced hearing loss: results of a double blind field study. Schriftenr Ver Wasser Boden Lufthyg. 1993;88:503–16. [PubMed] [Google Scholar]
- 44.Attias J, Weisz G, Almog S, Shahar A, Wiener M, Joachims Z, et al. Oral magnesium intake reduces permanent hearing loss induced by noise exposure. Am J Otolaryngol. 1994 Jan–Feb;15(1):26–32. doi: 10.1016/0196-0709(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 45.Julicher RH, Sterrenberg L, Haenen GR, Bast A, Noordhoek J. Sex differences in the cellular defense system against free radicals from oxygen or drug metabolites in rat. Arch Toxicol. 1984;56:83–6. doi: 10.1007/BF00349076. [DOI] [PubMed] [Google Scholar]
- 46.El Barbary A, Altschuler RA, Schacht J. Glutathione S-transferases in the organ of Corti of the rat: Enzymatic activity, subunit composition, and immunohistochemical localization. Hear Res. 1993;71:80–90. doi: 10.1016/0378-5955(93)90023-t. [DOI] [PubMed] [Google Scholar]
- 47.McFadden SL, Henselman LW, Zheng XY. Sex differences in auditory sensitivity of chinchillas before and after exposure to impulse noise. Ear Hear. 1999 Apr;20(2):164–74. doi: 10.1097/00003446-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Le Prell CG, Halsey K, Hughes LF, Dolan DF, Bledsoe SC., Jr. Disruption of lateral olivocochlear neurons via a dopaminergic neurotoxin depresses sound-evoked auditory nerve activity. J Assoc Res Otolaryngol. 2005;6:48–62. doi: 10.1007/s10162-004-5009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Prell CG, Yagi M, Kawamoto K, Beyer LA, Atkin G, Raphael Y, et al. Chronic excitotoxicity in the guinea pig cochlea induces temporary functional deficits without disrupting otoacoustic emissions. J Acoust Soc Am. 2004;116:1044–56. doi: 10.1121/1.1772395. [DOI] [PubMed] [Google Scholar]
- 50.Raphael Y, Altschuler RA. Reorganization of cytoskeletal and junctional proteins during cochlear hair cell degeneration. Cell Motil Cytoskeleton. 1991;18(3):215–27. doi: 10.1002/cm.970180307. [DOI] [PubMed] [Google Scholar]
- 51.Cody AR, Johnstone BM. Acoustic trauma: Single neuron basis for the “half octave shift”. J Acoust Soc Am. 1981;70:707–11. doi: 10.1121/1.386906. [DOI] [PubMed] [Google Scholar]
- 52.Cody AR, Johnstone BM. Single auditory neuron response during acute acoustic trauma. Hear Res. 1980;3:3–16. doi: 10.1016/0378-5955(80)90004-0. [DOI] [PubMed] [Google Scholar]
- 53.Davis H, Morgan CT, Hawkins JE, Jr., Galambos R, Smith FW. Temporary deafness following exposure to loud tones and noise. Acta Otolaryngol Suppl (Stockh) 1950;88:1–57. [PubMed] [Google Scholar]
- 54.Mitchell C, Brummett RE, Vernon JA. Frequency effects of temporary N1 depression following acoustic overload. Arch Otolaryngol. 1977;103:117–23. doi: 10.1001/archotol.1977.00780200043001. [DOI] [PubMed] [Google Scholar]
- 55.Peng JH, Tao ZZ, Huang ZW. Risk of damage to hearing from personal listening devices in young adults. J Otolaryngol. 2007 Jun;36(3):181–5. [PubMed] [Google Scholar]
- 56.Schmuziger N, Patscheke J, Probst R. Hearing in nonprofessional pop/rock musicians. Ear Hear. 2006 Aug;27(4):321–30. doi: 10.1097/01.aud.0000224737.34907.5e. [DOI] [PubMed] [Google Scholar]
- 57.Balatsouras DG, Homsioglou E, Danielidis V. Extended high-frequency audiometry in patients with acoustic trauma. Clin Otolaryngol. 2005 Jun;30(3):249–54. doi: 10.1111/j.1365-2273.2005.00984.x. [DOI] [PubMed] [Google Scholar]
- 58.Kuronen P, Sorri MJ, Paakkonen R, Muhli A. Temporary threshold shift in military pilots measured using conventional and extended high-frequency audiometry after one flight. Int J Audiol. 2003 Jan;42(1):29–33. doi: 10.3109/14992020309056082. [DOI] [PubMed] [Google Scholar]
- 59.Wolf G. The enzymatic cleavage of beta-carotene: still controversial. Nutr Rev. 1995 May;53(5):134–7. doi: 10.1111/j.1753-4887.1995.tb01537.x. [DOI] [PubMed] [Google Scholar]
- 60.Worker NA. Studies on the in vitro conversion of beta-carotene into vitamin A in tissues from the rat, guinea-pig and sheep. Br J Nutr. 1959;13:400–18. doi: 10.1079/bjn19590054. [DOI] [PubMed] [Google Scholar]
- 61.Mynatt R, Hale SA, Gill RM, Plontke SK, Salt AN. Demonstration of a longitudinal concentration gradient along scala tympani by sequential sampling of perilymph from the cochlear apex. J Assoc Res Otolaryngol. 2006 Jun;7(2):182–93. doi: 10.1007/s10162-006-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Plontke SK, Biegner T, Kammerer B, Delabar U, Salt AN. Dexamethasone concentration gradients along scala tympani after application to the round window membrane. Otol Neurotol. 2008 Apr;29(3):401–6. doi: 10.1097/MAO.0b013e318161aaae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salt AN, Hale SA, Plonkte SK. Perilymph sampling from the cochlear apex: a reliable method to obtain higher purity perilymph samples from scala tympani. J Neurosci Methods. 2006 May 15;153(1):121–9. doi: 10.1016/j.jneumeth.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salt AN, Kellner C, Hale S. Contamination of perilymph sampled from the basal cochlear turn with cerebrospinal fluid. Hear Res. 2003 Aug;182(1–2):24–33. doi: 10.1016/s0378-5955(03)00137-0. [DOI] [PubMed] [Google Scholar]
- 65.Le Prell CG, Gagnon PM, Bennett DC, Ohlemiller KK. Antioxidant-enhanced diet reduces noise-induced damage to the inner ear and hearing loss. 2010 doi: 10.1016/j.trsl.2011.02.006. Manuscript submitted for publication. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kemp D. Otoacoustic emissions in perspective. In: Robinette M, Glattke T, editors. Otoacoustic emissions: clinical applications. Thieme; New York: 1997. pp. 1–21. [Google Scholar]
- 67.Kujawa SG, Glattke TJ, Fallon M, Bobbin RP. A nicotinic-like receptor mediates suppression of distortion product otoacoustic emissions by contralateral sound. Hear Res. 1994;74(1–2):122–34. doi: 10.1016/0378-5955(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 68.Hall JWI. Handbook of Otoacoustic Emissions. Singular Publishers; San Diego: 2000. [Google Scholar]
- 69.Dallos P. The active cochlea. J Neurosci. 1992;12(12):4575–85. doi: 10.1523/JNEUROSCI.12-12-04575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamernik RP, Qiu W. Correlations among evoked potential thresholds, distortion product otoacoustic emissions and hair cell loss following various noise exposures in the chinchilla. Hear Res. 2000 Dec;150(1–2):245–57. doi: 10.1016/s0378-5955(00)00204-5. [DOI] [PubMed] [Google Scholar]
- 71.Emmerich E, Richter F, Reinhold U, Linss V, Linss W. Effects of industrial noise exposure on distortion product otoacoustic emissions (DPOAEs) and hair cell loss of the cochlea--long term experiments in awake guinea pigs. Hear Res. 2000;148(1–2):9–17. doi: 10.1016/s0378-5955(00)00101-5. [DOI] [PubMed] [Google Scholar]
- 72.Fraenkel R, Freeman S, Sohmer H. Use of ABR threshold and OAEs in detection of noise induced hearing loss. J Basic Clin Physiol Pharmacol. 2003;14(2):95–118. doi: 10.1515/jbcpp.2003.14.2.95. [DOI] [PubMed] [Google Scholar]
- 73.Lapsley Miller JA, Marshall L, Heller LM. A longitudinal study of changes in evoked otoacoustic emissions and pure-tone thresholds as measured in a hearing conservation program. Int J Audiol. 2004 Jun;43(6):307–22. doi: 10.1080/14992020400050040. [DOI] [PubMed] [Google Scholar]
- 74.Derekoy FS, Koken T, Yilmaz D, Kahraman A, Altuntas A. Effects of ascorbic acid on oxidative system and transient evoked otoacoustic emissions in rabbits exposed to noise. Laryngoscope. 2004 Oct;114(10):1775–9. doi: 10.1097/00005537-200410000-00019. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002 Sep;3(3):248–68. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liberman MC, Dodds LW. Single-neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hear Res. 1984 Oct;16(1):55–74. doi: 10.1016/0378-5955(84)90025-x. [DOI] [PubMed] [Google Scholar]
- 77.Maruyama J, Miller JM, Ulfendahl M. Effects of antioxidants on auditory nerve function and survival in deafened guinea pigs. Neurobiol Dis. 2007;25(1):309–18. doi: 10.1016/j.nbd.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cevette MJ, Vormann J, Franz K. Magnesium and hearing. J Am Acad Audiol. 2003 May–Jun;14(4):202–12. [PubMed] [Google Scholar]
- 79.Gunther T, Ising H, Joachims Z. Biochemical mechanisms affecting susceptibility to noise-induced hearing loss. Am J Otol. 1989 Jan;10(1):36–41. [PubMed] [Google Scholar]
- 80.Heinrich UR, Fischer I, Brieger J, Rumelin A, Schmidtmann I, Li H, et al. Ascorbic acid reduces noise-induced nitric oxide production in the guinea pig ear. Laryngoscope. 2008 May;118(5):837–42. doi: 10.1097/MLG.0b013e31816381ae. [DOI] [PubMed] [Google Scholar]
- 81.White CR, Seymour RS. Sample size and mass range effects on the allometric exponent of basal metabolic rate. Comparative Biochemistry and Physiology Part A, Molecular and Integrative Physiology. 2005 Sep;142(1):74–8. doi: 10.1016/j.cbpa.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 82.Speakman JR. Body size, energy metabolism and lifespan. Journal of Experimental Biology. 2005 May;208(Pt 9):1717–30. doi: 10.1242/jeb.01556. [DOI] [PubMed] [Google Scholar]
- 83.Nagy KA. Field metabolic rate and body size. Journal of Experimental Biology. 2005 May;208(Pt 9):1621–5. doi: 10.1242/jeb.01553. [DOI] [PubMed] [Google Scholar]
- 84.White CR, Seymour RS. Allometric scaling of mammalian metabolism. Journal of Experimental Biology. 2005 May;208(Pt 9):1611–9. doi: 10.1242/jeb.01501. [DOI] [PubMed] [Google Scholar]
- 85.Singer D. Metabolic adaptation to hypoxia: cost and benefit of being small. Respiratory Physiology & Neurobiology. 2004 Aug 12;141(3):215–28. doi: 10.1016/j.resp.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 86.White CR, Seymour RS. Mammalian basal metabolic rate is proportional to body mass2/3. Proceedings of the National Academy of Sciences of the United States of America. 2003 Apr 1;100(7):4046–9. doi: 10.1073/pnas.0436428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gomez-Lechon MJ, Donato T, Ponsoda X, Castell JV. Human hepatic cell cultures: in vitro and in vivo drug metabolism. ATLA-Alternatives to Laboratory Animals. 2003 May–Jun;31(3):257–65. doi: 10.1177/026119290303100307. [DOI] [PubMed] [Google Scholar]
- 88.Lin JH, Chiba M, Balani SK, Chen IW, Kwei GY, Vastag KJ, et al. Species differences in the pharmacokinetics and metabolism of indinavir, a potent human immunodeficiency virus protease inhibitor. Drug Metabolism & Disposition. 1996 Oct;24(10):1111–20. [PubMed] [Google Scholar]
- 89.Lin JH. Species similarities and differences in pharmacokinetics. Drug Metabolism & Disposition. 1995 Oct;23(10):1008–21. [PubMed] [Google Scholar]
- 90.Chenery RJ, Ayrton A, Oldham HG, Standring P, Norman SJ, Seddon T, et al. Diazepam metabolism in cultured hepatocytes from rat, rabbit, dog, guinea pig, and man. Drug Metabolism & Disposition. 1987 May–Jun;15(3):312–7. [PubMed] [Google Scholar]
- 91.Al-Dabbagh SG, Smith RL. Species differences in oxidative drug metabolism: some basic considerations. Archives of Toxicology Supplement Archiv fur Toxikologie Supplement. 1984;7:219–31. doi: 10.1007/978-3-642-69132-4_31. [DOI] [PubMed] [Google Scholar]
- 92.Lorenz J, Glatt HR, Fleischmann R, Ferlinz R, Oesch F. Drug metabolism in man and its relationship to that in three rodent species: monooxygenase, epoxide hydrolase, and glutathione S-transferase activities in subcellular fractions of lung and liver. Biochemical Medicine. 1984 Aug;32(1):43–56. doi: 10.1016/0006-2944(84)90007-3. [DOI] [PubMed] [Google Scholar]
- 93.Litterst CL, Mimnaugh EG, Reagan RL, Gram TE. Comparison of in vitro drug metabolism by lung, liver, and kidney of several common laboratory species. Drug Metabolism & Disposition. 1975 Jul–Aug;3(4):259–65. [PubMed] [Google Scholar]
- 94.Institute of Medicine . Dietary Reference Intakes (DRIs): Tolerable Upper Intake Levels (UL), Vitamins. Food and Nutrition Board, Institute of Medicine, National Academies of Science; 2004. [Google Scholar]
- 95.Institute of Medicine . Dietary Reference Intakes (DRIs): Tolerable Upper Intake Levels (UL), Elements. Food and Nutrition Board, Institute of Medicine, National Academies of Science; 2004. [Google Scholar]
- 96.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol. 2001 Oct;119(10):1439–52. doi: 10.1001/archopht.119.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 98.Barbosa E, Faintuch J, Machado Moreira EA, Goncalves da Silva VR, Lopes Pereima MJ, Martins Fagundes RL, et al. Supplementation of vitamin E, vitamin C, and zinc attenuates oxidative stress in burned children: a randomized, double-blind, placebo-controlled pilot study. J Burn Care Res. 2009 Sep–Oct;30(5):859–66. doi: 10.1097/BCR.0b013e3181b487a8. [DOI] [PubMed] [Google Scholar]
- 99.Karajibani M, Hashemi M, Montazerifar F, Bolouri A, Dikshit M. The status of glutathione peroxidase, superoxide dismutase, vitamins A, C, E and malondialdehyde in patients with cardiovascular disease in Zahedan, Southeast Iran. J Nutr Sci Vitaminol (Tokyo) 2009 Aug;55(4):309–16. doi: 10.3177/jnsv.55.309. [DOI] [PubMed] [Google Scholar]
- 100.Pollard J, Wild CP, White KL, Greenwood DC, Cade JE, Kirk SF. Comparison of plasma biomarkers with dietary assessment methods for fruit and vegetable intake. Eur J Clin Nutr. 2003 Aug;57(8):988–98. doi: 10.1038/sj.ejcn.1601634. [DOI] [PubMed] [Google Scholar]
- 101.Aydin S, Aral I, Kilic N, Bakan I, Erman F. The level of antioxidant enzymes, plasma vitamins C and E in cement plant workers. Clin Chim Acta. 2004 Mar;341(1–2):193–8. doi: 10.1016/j.cccn.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 102.Pancorbo D, Vazquez C, Fletcher MA. Vitamin C-lipid metabolites: uptake and retention and effect on plasma C-reactive protein and oxidized LDL levels in healthy volunteers. Med Sci Monit. 2008 Nov;14(11):CR547–51. [PubMed] [Google Scholar]
- 103.Anderson D. Factors that contribute to biomarker responses in humans including a study in individuals taking Vitamin C supplementation. Mutat Res. 2001 Sep 1;480–481:337–47. doi: 10.1016/s0027-5107(01)00193-2. [DOI] [PubMed] [Google Scholar]
- 104.Washio K, Inagaki M, Tsuji M, Morio Y, Akiyama S, Gotoh H, et al. Oral vitamin C supplementation in hemodialysis patients and its effect on the plasma level of oxidized ascorbic acid and Cu/Zn superoxide dismutase, an oxidative stress marker. Nephron Clin Pract. 2008;109(2):c49–54. doi: 10.1159/000137628. [DOI] [PubMed] [Google Scholar]
- 105.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001 Oct;119(10):1417–36. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci USA. 2001;98(17):9842–6. doi: 10.1073/pnas.171318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci USA. 1996 Apr 16;93(8):3704–9. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Polidori MC, Mecocci P, Levine M, Frei B. Short-term and long-term vitamin C supplementation in humans dose-dependently increases the resistance of plasma to ex vivo lipid peroxidation. Arch Biochem Biophys. 2004;423(1):109–15. doi: 10.1016/j.abb.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 109.Cao G, Alessio HM, Cutler RG. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic Biol Med. 1993 Mar;14(3):303–11. doi: 10.1016/0891-5849(93)90027-r. [DOI] [PubMed] [Google Scholar]
- 110.Cano A, Acosta M, Arnao MB. A method to measure antioxidant activity in organic media: application to lipophilic vitamins. Redox Rep. 2000;5(6):365–70. doi: 10.1179/135100000101535933. [DOI] [PubMed] [Google Scholar]
- 111.Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007 Jul 1;43(1):4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Meagher EA, Barry OP, Lawson JA, Rokach J, FitzGerald GA. Effects of vitamin E on lipid peroxidation in healthy persons. JAMA. 2001 Mar 7;285(9):1178–82. doi: 10.1001/jama.285.9.1178. [DOI] [PubMed] [Google Scholar]
- 113.Kappus H, Diplock AT. Tolerance and safety of vitamin E: a toxicological position report. Free Radic Biol Med. 1992;13(1):55–74. doi: 10.1016/0891-5849(92)90166-e. [DOI] [PubMed] [Google Scholar]
- 114.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005 Jan 4;142(1):37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 115.DeZee KJ, Shimeall W, Douglas K, Jackson JL. High-dosage vitamin E supplementation and all-cause mortality. Ann Intern Med. 2005 Jul 19;143(2):153–4. doi: 10.7326/0003-4819-143-2-200507190-00024. author reply 6–8. [DOI] [PubMed] [Google Scholar]
- 116.Hemilä H. High-dosage vitamin E supplementation and all-cause mortality. Ann Intern Med. 2005 Jul 19;143(2):151–2. doi: 10.7326/0003-4819-143-2-200507190-00020. author reply 6–8. [DOI] [PubMed] [Google Scholar]
- 117.Krishnan K, Campbell S, Stone WL. High-dosage vitamin E supplementation and all-cause mortality. Ann Intern Med. 2005 Jul 19;143(2):151. doi: 10.7326/0003-4819-143-2-200507190-00019. author reply 6–8. [DOI] [PubMed] [Google Scholar]
- 118.Marras C, Lang AE, Oakes D, McDermott MP, Kieburtz K, Shoulson I, et al. High-dosage vitamin E supplementation and all-cause mortality. Ann Intern Med. 2005 Jul 19;143(2):152–3. doi: 10.7326/0003-4819-143-2-200507190-00022. author reply 6–8. [DOI] [PubMed] [Google Scholar]
- 119.Meydani SN, Lau J, Dallal GE, Meydani M. High-dosage vitamin E supplementation and all-cause mortality. Ann Intern Med. 2005 Jul 19;143(2):153. doi: 10.7326/0003-4819-143-2-200507190-00023. author reply 6–8. [DOI] [PubMed] [Google Scholar]
- 120.Possolo AM. High-dosage vitamin E supplementation and all-cause mortality. Ann Intern Med. 2005 Jul 19;143(2):154. doi: 10.7326/0003-4819-143-2-200507190-00025. author reply 6–8. [DOI] [PubMed] [Google Scholar]
- 121.Albanes D, Virtamo J, Rautalahti M, Haukka J, Palmgren J, Gref CG, et al. Serum beta-carotene before and after beta-carotene supplementation. Eur J Clin Nutr. 1992 Jan;46(1):15–24. [PubMed] [Google Scholar]
- 122.Druesne-Pecollo N, Latino-Martel P, Norat T, Barrandon E, Bertrais S, Galan P, et al. Beta-carotene supplementation and cancer risk: a systematic review and metaanalysis of randomized controlled trials. Int J Cancer. 2010 Jul 1;127(1):172–84. doi: 10.1002/ijc.25008. [DOI] [PubMed] [Google Scholar]
- 123.Albanes D, Heinonen OP, Huttunen JK, Taylor PR, Virtamo J, Edwards BK, et al. Effects of alpha-tocopherol and beta-carotene supplements on cancer incidence in the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study. Am J Clin Nutr. 1995 Dec;62(6 Suppl):1427S–30S. doi: 10.1093/ajcn/62.6.1427S. [DOI] [PubMed] [Google Scholar]
- 124.The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994 April;330(15):1029–35. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 125.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996 Nov 6;88(21):1550–9. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- 126.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996 May 2;334(18):1150–5. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 127.Miller RK, Hendrickx AG, Mills JL, Hummler H, Wiegand UW. Periconceptional vitamin A use: how much is teratogenic? Reprod Toxicol. 1998;12(1):75–88. doi: 10.1016/s0890-6238(97)00102-0. [DOI] [PubMed] [Google Scholar]
- 128.Dolk HM, Nau H, Hummler H, Barlow SM. Dietary vitamin A and teratogenic risk: European Teratology Society discussion paper. Eur J Obstet Gynecol Reprod Biol. 1999;83(1):31–6. doi: 10.1016/s0301-2115(98)00228-0. [DOI] [PubMed] [Google Scholar]
- 129.Rothman KJ, Moore LL, Singer MR, Nguyen US, Mannino S, Milunsky A. Teratogenicity of high vitamin A intake. N Engl J Med. 1995;333(21):1369–73. doi: 10.1056/NEJM199511233332101. [DOI] [PubMed] [Google Scholar]
- 130.Ko SH, Lim HR, Kim DC, Han YJ, Choe H, Song HS. Magnesium sulfate does not reduce postoperative analgesic requirements. Anesthesiology. 2001 Sep;95(3):640–6. doi: 10.1097/00000542-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 131.Feillet-Coudray C, Coudray C, Wolf FI, Henrotte JG, Rayssiguier Y, Mazur A. Magnesium metabolism in mice selected for high and low erythrocyte magnesium levels. Metabolism. 2004 May;53(5):660–5. doi: 10.1016/j.metabol.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 132.Thomas J, Millot JM, Sebille S, Delabroise AM, Thomas E, Manfait M, et al. Free and total magnesium in lymphocytes of migraine patients - effect of magnesium-rich mineral water intake. Clin Chim Acta. 2000 May;295(1–2):63–75. doi: 10.1016/s0009-8981(00)00186-8. [DOI] [PubMed] [Google Scholar]
- 133.Wary C, Brillault-Salvat C, Bloch G, Leroy-Willig A, Roumenov D, Grognet JM, et al. Effect of chronic magnesium supplementation on magnesium distribution in healthy volunteers evaluated by 31P-NMRS and ion selective electrodes. Br J Clin Pharmacol. 1999 Nov;48(5):655–62. doi: 10.1046/j.1365-2125.1999.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Attias J, Sapir S, Bresloff I, Reshef-Haran I, Ising H. Reduction in noise-induced temporary threshold shift in humans following oral magnesium intake. Clin Otolaryngol Allied Health Sci. 2004 Dec;29(6):635–41. doi: 10.1111/j.1365-2273.2004.00866.x. [DOI] [PubMed] [Google Scholar]
- 135.Gordin A, Goldenberg D, Golz A, Netzer A, Joachims HZ. Magnesium: a new therapy for idiopathic sudden sensorineural hearing loss. Otol Neurotol. 2002 Jul;23(4):447–51. doi: 10.1097/00129492-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 136.Nageris BI, Ulanovski D, Attias J. Magnesium treatment for sudden hearing loss. Ann Otol Rhinol Laryngol. 2004 Aug;113(8):672–5. doi: 10.1177/000348940411300814. [DOI] [PubMed] [Google Scholar]
- 137.Itoh K, Kawasaka T, Nakamura M. The effects of high oral magnesium supplementation on blood pressure, serum lipids and related variables in apparently healthy Japanese subjects. Br J Nutr. 1997 Nov;78(5):737–50. doi: 10.1079/bjn19970191. [DOI] [PubMed] [Google Scholar]
- 138.Tamir S, Adelman C, Weinberger JM, Sohmer H. Uniform comparison of several drugs which provide protection from noise induced hearing loss. Journal of Occupational Medicine and Toxicology. 2010;5:26–32. doi: 10.1186/1745-6673-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Campbell KCM, Nayar R, Borgonha S, Hughes L, Rehemtulla A, Ross BD, et al. Oral D-methionine (MRX-1024) significantly protects against cisplatin-induced hearing loss: A phase II study in humans. IX European Federation of Audiology Societies (EFAS) Congress; Tenerife, Spain. 2009. [Google Scholar]
- 140.Sha SH, Qiu J-H, Schacht J. Aspirin to prevent gentamicin-induced hearing loss. N Engl J Med. 2006;354(17):1856–7. doi: 10.1056/NEJMc053428. [DOI] [PubMed] [Google Scholar]
- 141.Le Prell CG, Schmitt J, Dolan DF, Boxer PA, Prieskorn DM, DeRemer S, et al. Prevention of temporary noise-induced threshold deficits using dietary agents. Abs Assoc Res Otolaryngol. 2009;32:280. [Google Scholar]