Abstract
Cyclosporin A (CsA) has been shown to be neuroprotective in mature animal models of traumatic brain injury (TBI), but its effects on immature animal models of TBI are unknown. In mature animal models, CsA inhibits the opening of the mitochondrial permeability transition pore (MPTP), thereby maintaining mitochondrial homeostasis following injury by inhibiting calcium influx and preserving mitochondrial membrane potential. The aim of the present study was to evaluate CsA's ability to preserve mitochondrial bioenergetic function following TBI (as measured by mitochondrial respiration and cerebral microdialysis), in two immature models (focal and diffuse), and in two different species (rat and piglet). Three groups were studied: injured+CsA, injured+saline vehicle, and uninjured shams. In addition, we evaluated CsA's effects on cerebral hemodynamics as measured by a novel thermal diffusion probe. The results demonstrate that post-injury administration of CsA ameliorates mitochondrial dysfunction, preserves cerebral blood flow (CBF), and limits neuropathology in immature animals 24 h post-TBI. Mitochondria were isolated 24 h after controlled cortical impact (CCI) in rats and rapid non-impact rotational injury (RNR) in piglets, and CsA ameliorated cerebral bioenergetic crisis with preservation of the respiratory control ratio (RCR) to sham levels. Results were more dramatic in RNR piglets than in CCI rats. In piglets, CsA also preserved lactate pyruvate ratios (LPR), as measured by cerebral microdialysis and CBF at sham levels 24 h after injury, in contrast to the significant alterations seen in injured piglets compared to shams (p<0.01). The administration of CsA to piglets following RNR promoted a 42% decrease in injured brain volume (p<0.01). We conclude that CsA exhibits significant neuroprotective activity in immature models of focal and diffuse TBI, and has exciting translational potential as a therapeutic agent for neuroprotection in children.
Key words: cerebral microdialysis, cyclosporin A, mitochondrial bioenergetics, neuroprotection, pediatric brain injury
Introduction
Every year more than 200,000 children in the United States suffer traumatic brain injuries (TBI; Hoyert et al., 2006). Although head injury is the leading cause of death and disability in children, there are only general clinical management guidelines, and no Class I evidence supporting any standard therapy (Adelson et al., 2003; Hoyert et al., 2006). Only a modest number of pediatric clinical trials for TBI have been conducted, and nearly all trials have failed to show any significant benefits of any specific therapy (Hutchison et al., 2008; Josan and Sgouros, 2006; Patrick et al., 2006; Prabhakaran et al., 2004; Turner, 2003). While much of the literature has led to changes in general critical care principles and some improvement in outcomes, neuroprotective interventions are limited by the heterogeneity of TBI, with its distinct pathophysiologies and mechanisms associated with contusions, hemorrhages, ischemia, and diffuse axonal injury, which contributes to the persistent failure of multiple, large head injury treatment trials in adults and children (Narayan et al., 2002; Saatman et al., 2008).
In children, developing effective treatments for TBI is complicated by the rapidly changing responses of the immature brain to each type of TBI during development from infancy through childhood (Armstead and Kurth, 1994; Durham and Duhaime 2007; Duhaime et al., 2000; Raghupathi and Margulies, 2002). Therefore, evaluation of head injury therapies for children must utilize immature animal models as a translational pathway to human trials in children.
A growing body of literature suggests that mitochondria play a key role in many pathological pathways in neurodegenerative disorders, focal/global ischemia, and trauma (Fiskum, 2000; Lin and Beal, 2006; Robertson et al., 2006; Saito et al., 2005). Mitochondrial respiratory stability and the bio-energetic production of adenosine-5′-triphosphate (ATP) is vital to overall brain function, and an adequate supply may be even more important for brain function in the developing and injured brain. Following pediatric TBI, mitochondrial dysfunction appears to be involved in excitotoxicity, oxidative stress, metabolic perturbations, and cell death (Bayir et al., 2003, 2006; Nakai et al., 2004; Robertson et al., 2006). Unfortunately, there are only a very small number of studies of immature models of TBI, and all are limited to small animals. Importantly, adult TBI data are difficult to extrapolate to pediatric models, because critical mitochondrial characteristics are very different between young and adult animals, including the number and density of complexes of the electron transfer chain, antioxidant enzyme activity and content, and lipid content (Bates et al., 1994; Del Maestro and McDonald, 1987). These unique features of the developing brain may make it more vulnerable and/or resistant to cell death cascades following TBI, thus requiring age-specific neuroprotective approaches (Polster et al., 2003; Robertson et al., 2009).
One potential mitochondrial-targeted neuroprotective agent with promise is cyclosporin A (CsA). Dozens of pre-clinical TBI and ischemia studies, primarily in adult rodent models, have demonstrated CsA's effectiveness and potential as a neuroprotective agent, using immunohistochemistry, isolated mitochondrial preparations, and behavioral tests (Alessandri et al., 2002; Ferrand-Drake et al., 2003; Folbergrova et al., 1997; Fukui et al., 2003; Hansson et al., 2003; Li et al., 2000; Signoretti et al., 2004; Suehiro et al., 2003; Sullivan et al., 2000b). In addition, in a TBI Phase I clinical trial in adults, CsA routinely increased mean arterial pressure (MAP), and satisfied a broad range of safety parameters (Mazzeo et al., 2008). Further advantages of CsA include: it is FDA-approved and off-patent, and therefore inexpensively manufactured by several companies, and it has well-described safety and dosing profiles. Currently there are 101 open clinical trials that use CsA in children for indications other than brain injury. CsA given in non-immunosuppressive dosages and without chronic administration, such as the dosages used for TBI, may lead to minimal adverse immunological concerns. To our knowledge, CsA has not been tested in immature models of TBI.
Informed by recent workshops of the TBI and stroke scientific communities that examined why agents with pre-clinical therapeutic efficacy have failed to translate into clinical success, we used two species (rodent and pig) and two different models of injury (focal and diffuse) to investigate the effectiveness of CsA after TBI in the immature brain (Fisher et al., 2009; Margulies et al., 2009). We hypothesize that CsA promotes neuro-metabolic preservation by stabilizing mitochondrial respiration and ATP production, increasing cerebral blood flow (CBF), and ultimately preventing secondary cascades of cellular injury and neuronal tissue loss following TBI in neonatal rats and piglets.
Methods
Controlled cortical impact injury in rats
The rat protocol was approved by the University of Maryland, Baltimore, Animal Care and Use Committee. All care and handling of rats were in compliance with the National Institutes of Health (NIH) guidelines. Immature (9–10 days old) female Sprague-Dawley rats were anesthetized in an acrylic glass chamber with 4% isoflurane. They were positioned in a nose-cone mask and anesthesia was maintained with 2% inhaled isoflurane. A midline scalp incision and left parietal craniotomy were performed. After a 30-min period of stable brain and rectal temperatures (37±0.5°C), TBI was performed using a controlled cortical impact (CCI) device, as previously described (Robertson et al., 2007). Injury was produced using a 3-mm metal impactor tip with a depth of penetration of 1.5 mm, a velocity of 5.5±0.3 m/sec, and a duration of deformation of 50 msec. Following injury, the bone flap was replaced, the craniotomy sealed, and the scalp incision closed with interrupted sutures. At the completion of surgery, isoflurane was discontinued and the rats emerged from anesthesia and were returned to their cages with their littermates and mother. Three groups were studied: sham, CCI, and CCI+CsA. Sham rats underwent identical surgeries, with the exclusion of the CCI. At 15 min after injury CCI+CsA rats were injected with CsA (20 mg/kg IP) in 100 μL saline vehicle. Sham and CCI rats received 100 μL IP injection of normal saline vehicle at the same time point. Dose selection in the immature rat was based on published rodent neuro-injury studies, Phase I and II clinical trials in human adults, and published dosing guidelines for CsA. Specifically, CsA administration was studied in an adult rodent model of focal TBI, and a U-shaped dose-response curve was observed (Alessandri et al., 2002; Mbye et al., 2009; Sullivan et al., 2000a), such that the most beneficial dose was 20 mg/kg/d (Sullivan et al., 2000a). Allometric scaling of this optimal dose (Reagan-Shaw et al., 2008) from the adult rat (Km of 6) to the immature rat (Km of 4) yields a dose of 30 mg/kg/d. More importantly, the only published post-trauma CsA treatment study in an immature model of neuro-injury demonstrated dramatic attenuation of hypoxic-ischemic brain injury at 20 mg/kg/d (Hwang et al., 2010). We weighed this data from an immature model more heavily than the scaled dose, and therefore we used 20 mg/kg/d for our study.
At 24 h after CCI, rat forebrains were quickly removed and placed on an acrylic brain matrix previously cooled in ice. The impacted region, as well as peri-trauma (non-impacted) lateral, hippocampal, and thalamic tissues inferior to the impact site, were rapidly dissected as a single ipsilateral tissue sample for mitochondrial isolation using razor blades placed vertically just in front of, behind, and horizontally below the area of injury on the matrix. A similar sample was removed for mitochondrial isolation from the contralateral hemisphere. Both samples were placed in ice-cold mitochondrial isolation buffer (10 mL). In order to obtain a sufficient quantity of rat mitochondria, tissue samples from three rats were pooled to create one mitochondrial preparation.
Rapid non-impact rotational injury in piglets
The piglet protocol was approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania. All care and handling of piglets were in compliance with NIH guidelines. In order to model pediatric TBI, we used our well-established porcine model of pediatric TBI (Raghupathi and Margulies, 2002). This purely inertial (non-impact) sagittal head rotation model creates diffuse axonal injury and subarachnoid hemorrhage. RNR was performed on infant piglets 3–5 days old. Similar to the rat, three groups were studied: sham, RNR, and RNR+CsA (n=3–5/group, a total of 38 piglets). Induction of anesthesia was instituted with isoflurane (4%) via snout mask. Monitors were placed to record oxygen saturation, heart rate, respiratory rate, and end-tidal CO2 (ETCO2; Surgivet v9240; Smiths Medical; Waukesha, WI). Buprenorphine (0.02 mg/kg IM) was administered for analgesia, and a rectal thermometer was placed to measure core body temperature. The animals were kept on a warming blanket to maintain a constant core temperature throughout the experiment (36.8°C and 38.5°C, normothermia for piglets). As soon as the desired depth of surgical anesthesia was attained, characterized by the absence of foot-withdrawal response to a mild toe pinch, a 3.5-mm cuffed endotracheal tube was inserted via direct laryngoscopy, and placement was confirmed by auscultation and ETCO2. Isoflurane (3%) was administered for maintenance of anesthesia, and the animals were allowed to breathe spontaneously. Oxygen saturation was maintained between 95% and 100% at all times with supplemental oxygen and ventilatory support as needed to maintain ETCO2 ∼55 mm Hg.
While maintained on isoflurane, the animal's head was secured to the inertial injury apparatus by a snout clamp (Eucker et al., 2008; Friess et al., 2009; Ibrahim et al., 2010). Isoflurane was withdrawn immediately prior to injury. Activation of the inertial loading device rapidly rotates the head in the sagittal plane through a 60- to 70-degree arc in 10–40 milliseconds. After injury, the animal's head is released from the clamp, and ventilatory support provided as needed for apnea and desaturation (Raghupathi and Margulies, 2002; Raghupathi et al., 2004). With return of airway-protective reflexes, eye opening, effective spontaneous ventilation, and movement, the pigs were extubated and monitored in a post-anesthesia recovery unit.
The RNR+CsA group received a total of 40 mg/kg of CsA divided into two intravenous doses: 20 mg/kg 5 min post-injury and 20 mg/kg 12 h post-injury, diluted in 10 mL of normal saline. The sham and RNR groups each received 10 mL of normal saline vehicle intravenously at the same time points. Because there are no published dose-response data for CsA in pigs following neuro-injury, we based our dose selection for the 3- to 5-day-old piglet on adult rodent neuro-injury CsA dose-response data (Sullivan et al., 2000a), published pediatric dosage guidelines for CsA (del Mar Fernandez De Gatta et al., 2002), and pharmacokinetic data for CsA in pigs from non-TBI studies (Lie et al., 2010). First, the optimal adult rodent dose of 20 mg/kg/d (Km of 6) would scale to 10 mg/kg/d in the 3- to 5-day-old piglet (Km of 12) using allometric scaling (Reagan-Shaw et al., 2008). Second, CsA is administered clinically in doses between 5 and 21 mg/kg/d (del Mar Fernandez De Gatta et al., 2002; Ghafari et al., 2007; Mazzeo et al., 2009) in humans (Km of 37), which scales to 13–65 mg/kg/d in the 3- to 5-day-old piglet (Km of 12; Reagan-Shaw et al., 2008). It should be noted that children have higher CsA systemic clearance than adults, requiring significantly higher doses and more frequent administration in a transplant cohort (del Mar Fernandez De Gatta et al., 2002). Lastly, even unscaled therapeutic rodent CsA doses (which are higher than scaled doses) failed to demonstrate therapeutic efficacy in a porcine model of myocardial ischemia-reperfusion (Lie et al., 2010), and the authors speculated that higher doses were required in porcine models, underscoring the challenge in scaling across species. In summary, these findings about the important influence of age and species led us to select a dose for the piglet that is higher than the scaled adult rat dose of 10 mg/kg/d. We chose 40 mg/kg/d, a dose still well within the range of published human dosage guidelines allometrically scaled to piglets.
The animals were returned to the animal care unit after the following criteria were met: vocalization (without squealing), steady ambulation, no aggression or avoidance behavior, no piloerection, and presence of proper feeding.
Tissue harvest was performed at 24 h. The animals were re-anesthetized using the same protocol as stated above. While under anesthesia, the apical portion of the cranium was resected and the pig was euthanized with a lethal intracardiac dose of sodium pentobarbital (150 mg/kg). The brain tissue regions of interest for mitochondrial sampling were rapidly dissected: left cortex, bilateral hippocampus, bilateral olfactory bulbs, and bilateral cerebellum.
Mitochondrial isolation
Regions of tissue were placed immediately in ice-cold MSH+isolation buffer (215 mM mannitol, 75 mM sucrose, 20 mM HEPES, 0.01% bovine serum albumin, and 1 mM EGTA, pH adjusted to 7.3; Robertson et al., 2007). Mitochondria were isolated as previously described (Starkov and Fiskum, 2003; Starkov et al., 2002, 2004), with the following modifications. Tissue was briefly minced with sharp scissors, the supernatant decanted, and the tissue re-suspended in 30 mL of fresh ice-cold buffer. The tissue was homogenized manually with six strokes of a chilled Dounce Homogenizer pestle A, followed by six stokes of pestle B (Kontes Glass Company, Vineland, NJ) The homogenized cell lysates were then poured into chilled 50-mL centrifuge tubes (Beckman Coulter, Brea, CA), and the volumes were brought to 45 mL and placed on ice. Whole cells and large organelles were removed from the homogenates by spinning at 5000g for 5 min in a Beckman Coulter Avanti JE centrifuge, using a fixed-angle JA 25.50 rotor kept at 4°C. The supernatants were reserved and placed on ice and the remaining pellets were discarded. The supernatants were then brought to a volume of 45 mL with fresh MSH+buffer and centrifuged at 12,000g for 10 min. The supernatants were discarded and the pellets re-suspended in 10 mL of MSH+buffer, and 20 μL of 10% digitonin (Sigma-Aldrich, St. Louis, MO) in DMSO was added. Following a 1-min incubation, the suspensions were centrifuged at 10,000g for 10 min. The supernatant was discarded and the mitochondrial pellet re-suspended in 10 mL MSH+EGTA. This suspension was centrifuged at 10,000g for 10 min, and the final pellet containing both synaptosomal and non-synaptosomal mitochondria was suspended in 400 μL MSH+EGTA and placed on ice. Mitochondrial concentration was estimated using the Bio-Rad DC Protein Assay Kit (Bio-Rad, Hercules, CA). Samples of isolated mitochondria were kept on ice for the mitochondrial respiration assay, and the remainder was frozen for further analysis.
Mitochondrial respiration
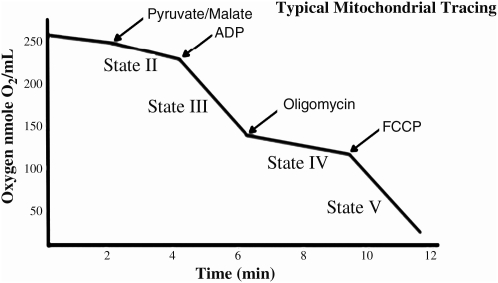
Mitochondrial oxygen consumption was measured using a Clark-type oxygen electrode (Hansatech Instruments, Norfolk, U.K.) in the presence of pyruvate and malate while at 37°C at a pH of 7.0 in KCL medium (125 μmol/L KCl, 2 μmol/L KH2PO4, 1 μmol/L MgCL2, and 20 μmol/L HEPES-KOH), in the absence of defatted bovine serum albumin (BSA). The chamber was supplemented with pyruvate (5 mM), malate (2.5 mM), 1 μmol/L EGTA, and 0.4 μmol/L ADP, in a total volume of 0.5 mL. State 3 respiration was initiated by the addition of mitochondria (0.5 mg/mL; Fig. 1), and state 4 respiration was induced by the addition of the ATP synthetase inhibitor oligomycin (2.5 μg/mL). State 4 respiration measured in the presence of oligomycin is not equivalent to the traditional state 4 respiration measured after all ADP has been converted to ATP. However, for our measurements, we wanted to eliminate the contribution of ATP cycling via hydrolysis by contaminating ATPases and resynthesis by mitochondrial ATP synthetase. Thus, the oligomycin-induced state 4 rate of respiration reflects mitochondrial proton cycling limited by passive proton leakiness of the inner membrane. Mitochondrial respiratory energy coupling was evaluated by determining the respiratory control ratio (RCR), calculated as the ratio of the rate of ADP-stimulated state 3 respiration to the state 4 rate in the presence of oligomycin. The mitochondrial respiration rates were calculated as nanomoles of oxygen/min/mg of protein.
FIG. 1.
Typical oxygen utilization trace taken from isolated mitochondria.
Piglet cerebral blood flow
Additional piglets (n=8 sham, n=9 RNR, n=10 RNR+CsA) were anesthetized at 24 h post-injury or sham operation as previously described, and a thermal diffusion probe (Bowman Perfusion Monitor; Hemedex, Cambridge MA) was inserted into the right frontal subcortical white matter via a burr hole to a depth of 10 mm and allowed to equilibrate. Thermal diffusion CBF monitoring is a validated, quantitative method of continuous measurement of CBF (Vajkoczy et al., 2000). All piglets were maintained on isoflurane at a constant 2.5%, with similar ETCO2, temperature, and arterial saturation. After equilibration, CBF readings were taken over a 30-min period and allowed to plateau.
Piglet cerebral microdialysis
Because elevated lactate:pyruvate ratios are neurochemical biomarkers of metabolic distress in the brain (Hillered et al., 2006), in a subset of these additional CBF piglets (n=4 sham, n=5 RNR, n=5 RNR+CsA), we obtained cerebral microdialysis measurements. At 24 h post-injury or sham operation, the piglets were anesthetized as described above, and a CMA 12 elite 4-mm microdialysis probe (CMA Microdialysis AB, North Chelmsford, MA) was placed via a burr hole into the left frontal subcortical white matter at a depth of 10 mm. After 30 min of equilibration, two cerebral microdialysis samples were collected over the next two 30 min intervals (flow rate 1 μL/min, using 0.9% NaCl as the dialysate). Immediately after collection, the samples were stored at −70°C. Levels of lactate and pyruvate were measured in the dialysate using a CMA 600 microdialysis analyzer, and lactate:pyruvate ratios were calculated from their respective values (lactate divided by pyruvate; Vespa et al., 2005).
Piglet pathology and histology
All piglets assigned to microdialysis assessment were sacrificed for histology and immunohistochemistry. The brains were fixed in situ by perfusion with a buffered solution of 10% formalin (3.5 L; Sigma-Aldrich; Friess et al., 2009). Formalin-fixed piglet brains were examined by a neuropathologist blinded to the animals' group assignments. Macroscopic examination documented focal pathology such as subdural and subarachnoid hemorrhages and surface contusions. In 3-mm-thick serial coronal sections, the cerebrum and brainstem were documented photographically. Following routine processing, the tissue was embedded in paraffin, and two 6-mm-thick sections were cut from every 3-mm-thick coronal block for microscopic evaluation. The sections were stained with hematoxylin and eosin (H&E), or with immunohistochemical markers for axonal injury (β-amyloid precursor protein, β-APP; dilution 1:5000; Chemicron 22C11; Millipore, Billerica, MA), and counterstained with Meyer's hematoxylin.
Every field in every slide was examined at a scanning power of 5–10× magnification. In addition, specific fields were examined at 20–40× magnification. Locations of axonal injury, subarachnoid and parenchymal hemorrhage, and cell death, were noted on digital photographs of the coronal sections. Assessment focused on the total volume of cellular injury and white matter damage. H&E-stained sections were examined to document hemorrhages, established infarcts (changes in staining intensity), and ischemic neurons (cell shrinkage and eosinophilia). Axonal injury was assessed from β-APP immunohistochemistry to identify disruption of axonal flow (Gentleman et al., 1995; Williams et al., 2001). Regions of ischemic damage in the grey matter were defined as focal tissue vacuolation due to localized edema, and neuronal ischemic cell changes, for which the damaged neurons have shrunken nuclei and eosinophilic (pink-stained) cytoplasm. The locations and extent of axonal and neuronal injury was documented on the digital photographs for each animal. From scaled digital photographs of each of the 3-mm-thick coronal slices, the brain periphery was traced and brain area determined (ImageJ software; NIH, Bethesda, MD), and then summed to determine the total slice area. The periphery of every region of axonal and neuronal damage was traced in each slice using the same procedure to determine the total injured slice area. Total and injured areas were then multiplied by section thickness to determine total and injured brain volumes. Injured brain volume (Vinj), as a percentage of total brain volume, was calculated by dividing the injured by the total brain volume.
Statistical analysis
Outcomes across regions and groups were compared using paired and unpaired Student's t-tests, as appropriate, with significance defined as p<0.05.
Results
RNR injury
Both the RNR and RNR+CsA groups had similar velocity and acceleration (Table 1). In addition, loss of consciousness following injury in piglets showed no significant difference in unconscious time between the two injured groups (RNR: 10±2.8 versus RNR+CsA: 7±3.2 min). Injured piglets (RNR and RNR+CsA) had significantly longer unconscious times, as measured by a return of pinch reflex, compared to sham piglets (Table 1).
Table 1.
Piglet Average Sagittal Angular Velocity, Acceleration, Latency of Pinch Response, and End-Tidal CO2a
| Group | Velocity (rads/sec) | Acceleration (rads/sec2) | Latency of pinch response (min) | End-tidal CO2 (mm Hg) |
|---|---|---|---|---|
| Sham | 2±1.1 | 55.9±6.1 | ||
| RNR | 151±2.2 | 54620±1703 | 10±2.8 | 54.6±6.1 |
| RNR+CsA | 151±2.3 | 51699±6108 | 7±3.2 | 58.3±6.6 |
Groups±SD; p<0.01.
There was no statistically significant difference between loading conditions or unconscious times between injured groups. There was no statistically significant difference between end-tidal CO2 measurements between all groups measured at 24 h.
RNR, rapid non-impact rotational injury; CsA, cyclosporin A; SD, standard deviation.
Mitochondrial bioenergetics
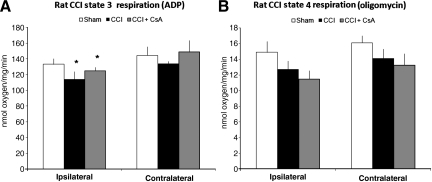
When measured 24 h post-injury, sham rat pups displayed no interhemispheric difference (ipsilateral versus contralateral) in state 3 respiration (ipsilateral=133±7, contralateral=144±11 nmol/min/mg), but significant differences were seen in injured rat pups (ipsilateral CCI=114±4.3, contralateral CCI=134±3, *p<0.01 nmol/min/mg; Fig. 2A). In the CCI+CsA group, the state 3 respiratory rate was significantly different between hemispheres (ipsilateral CCI+CsA=124±9.8, contralateral CCI+CsA=140±15 nmol/min/mg, *p<0.05; Fig. 2A). State 4 respiration rates did not differ significantly between hemispheres or with treatment (Fig. 2B).
FIG. 2.
State 3 respiration (adenosine diphosphate, ADP) and state 4 respiration (oligomycin) in rat pups at 24 hours after controlled cortical impact (CCI). (A) There was no hemispheric difference in sham rats (ipsilateral versus contralateral). State 3 respiration was significantly reduced at 24 h in the ipsilateral hemisphere compared to the contralateral hemisphere in the injury plus saline vehicle group (*p<0.01 for ipsilateral versus contralateral in CCI animals). In rats treated with cyclosporin A (CsA; CCI+CsA), state 3 respiration was not preserved and was significantly lower compared to the contralateral hemisphere (*p<0.05 for the ipsilateral versus the contralateral CCI+CsA). (B) State 4 respiration did not differ significantly between hemispheres or with treatment.
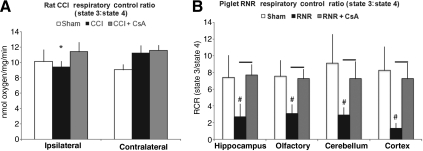
Sham rat pups did not have significant interhemispheric differences in RCR between hemispheres (ipsilateral=10.1±1.5, contralateral=9.1±0.7), but CCI rat pups showed a significant 20% reduction in RCR in ipsilateral hemispheres compared to contralateral hemispheres (ipsilateral=9.4±0.7, contralateral=11.2±1; *p<0.01, Fig. 3A). Administration of CsA (CCI+CsA) to rat pups preserved mitochondrial bioenergetics, and abolished the hemispheric RCR differences (Fig. 3A).
FIG. 3.
Respiratory control ratio (RCR; state 3:state 4) in rats (controlled cortical impact, CCI), and piglets (rapid non-impact rotational injury, RNR), 24 h after traumatic brain injury. (A) In rats, there was ∼ 20% reduction in RCR in the ipsilateral hemisphere of CCI rats (*p<0.01 ipsilateral versus contralateral in CCI animals). There were no hemispheric differences in RCR in uninjured (sham) or treated (CCI+CsA) rats (p=not significant for ipsilateral versus contralateral). (B) In piglets, RCRs were significantly lower, over 50%, in all four brain regions 24 h after RNR (#p<0.01 compared to shams). Post-injury administration of CsA preserved the RCR of isolated mitochondria from all four brain regions (bars=p<0.01 compared to RNR; CsA, cyclosporin A).
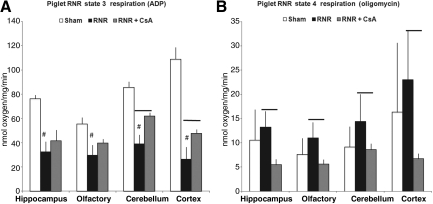
In piglets, state 3 respiration in injured animals treated with saline (RNR) displayed a significant reduction in all four brain regions at 24 h compared to sham piglets treated with saline (sham; Fig. 4A, #p<0.01). Post-injury administration of CsA (RNR+CsA) in piglets preserved state 3 respiration in the cerebellum and cortex compared to injured piglets (RNR; bars in Fig. 4A indicate p<0.05). State 4 respiration in piglets showed no significant differences in all four brain regions when comparing sham to RNR animals, similarly to rats. However, unlike the rat data, injured piglets treated with CsA had a significantly lower state 4 respiration in all four brain regions compared to RNR+saline animals (#p<0.05; Fig. 4B).
FIG. 4.
State 3 respiration (adenosine diphosphate, ADP) and state 4 respiration (oligomycin) in piglets 24 h after rapid non-impact rotational injury (RNR). (A) State 3 respiration in RNR piglets displayed a significant reduction in all four brain regions at 24 h (#p<0.01 compared to sham animals). RNR+CsA piglets had higher state 3 respiration, reaching significance in cerebellum and cortex (bars=p<0.01 compared to RNR animals). (B) State 4 respiration was significantly lower in all four brain regions in piglets treated with CsA (bars=p<0.01 compared to RNR animals; CsA, cyclosporin A).
RCR was significantly reduced, by over 50%, in all four brain regions following RNR compared to sham piglets (#p<0.01; Fig. 3B). Furthermore, piglets treated with CsA (RNR+CsA) maintained cellular homeostasis with significantly higher RCRs in all four brain regions compared to injured piglets treated with saline (bars in Fig. 3B=p<0.01). RCRs in RNR+CsA piglets were similar to sham levels.
Cerebral microdialysis
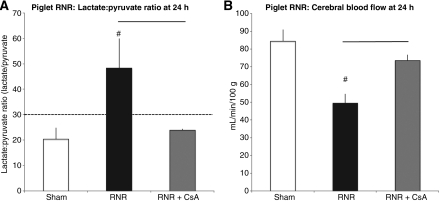
The lactate:pyruvate ratio (LPR) is a neurochemical biomarker of metabolic distress after brain injury. In several clinical studies LPRs greater than 30 at 24 h post-TBI have been associated with poor outcomes in humans (Bellander et al., 2004; Goodman et al., 1999; Hutchinson, 2005). LPRs measured in RNR piglets at 24 h showed a significant increase in LPRs compared to sham piglets (48.2±25.8 versus 20.4±7.6; #p<0.01, Fig. 5A). Piglets receiving CsA post-injury (RNR+CsA) maintained LPRs similar to those of sham piglets, and showed a significant reduction from RNR animals (RNR: 48.2±25.8 versus RNR+CsA: 23.8±0.8; bars in Fig. 5A=p<0.01).
FIG. 5.
Minimally-invasive non-terminal metrics: cerebral microdialysis, lactate:pyruvate ratios; cerebral blood flow, thermal diffusion. (A) Lactate:pyruvate ratios (LPRs) >30 as measured by cerebral microdialysis have been associated with cerebral metabolic crisis and poor outcomes in humans. Rapid non-impact rotational injury (RNR) causes a markedly significant increase in LPRs (#p<0.01 compared to sham animals). RNR+CsA piglets maintained LPRs similar to those of sham piglets, well below the levels associated with metabolic crisis and poor clinical outcomes (RNR: 48.2±25.8 versus RNR+CsA: 23.8±0.8; bar=p<0.01) (B) Cerebral blood flow (CBF) as measured by a thermal diffusion probe showed a markedly significant reduction in CBF, by over 50%, 24 h after RNR injury compared to sham piglets (49±5.2 versus 84±6.5 mL/min/100 g; #p<0.01). Post-injury CsA (RNR+CsA) maintained CBF at a significantly higher level following RNR injury (74±3.0 versus 49±5.2 mL/min/100 g; bar=p<0.01; CsA, cyclosporin A).
Cerebral blood flow
In the piglet, cerebral blood flow (CBF), as measured by thermal diffusion and reported in mL/min/100 g, was significantly reduced (over 50% reduction) at 24 h, in RNR piglets compared to sham piglets (49±5.2 versus 84±6.5 mL/min/100 g, #p<0.01; Fig. 5B) Piglets that were administered CsA post-injury (RNR+CsA) had significantly higher CBF compared to RNR piglets (74±3.0 versus 49±5.2 mL/min/100 g, bar=p<0.01; Fig. 5B), 94% of sham levels.
Histopathology
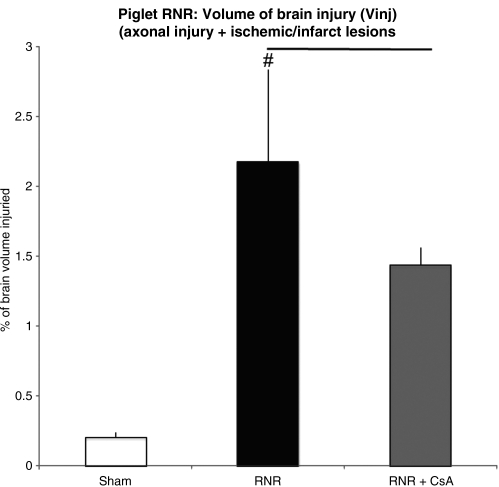
In the piglet, injured brain volume (Vinj) in RNR piglets was significantly larger compared to sham animals (2.18±0.65% versus 0.2±0.03%, #p<0.01; Fig. 6). Administration of CsA significantly reduced Vinj, by 42% (RNR+CsA 1.43±0.12, bar in Fig. 6=p<0.01).
FIG. 6.
Injured brain volume (Vinj) 24 hours after rapid non-impact rotational injury (RNR). Vinj in RNR piglets was significantly larger compared to sham animals (2.18±0.65% versus 0.2±0.03%; #p<0.01). Administration of cyclosporin A (CsA) resulted in a markedly significant reduction in Vinj, by 42%, compared to sham animals (RNR+CsA 1.43±0.12 versus 2.18±0.65%; bar=p<0.01). There was a 14% decrease in axonal injury with the administration of CsA following RNR in piglets, and an 83% decrease in infarct injury (data not shown).
Discussion
The present study is the first to demonstrate the potential efficacy of CsA for TBI in the developing brain, using two different mechanisms of TBI (CCI and RNR), and two different species (rat and piglet). CsA was first developed as a calcineurin inhibitor to inhibit T-cell activation for immunosuppressant therapy following solid organ transplant. However, recently CsA, a mitochondrial permeability transition pore inhibitor, has also shown benefits as a neuroprotectant in mature small animal models of focal and global ischemia, as well as focal TBI (Domanska-Janik et al., 2004; Li et al., 2000; Scheff and Sullivan, 1999; Shiga et al., 1992; Sullivan et al., 1999, 2000a, 2000b; Uchino et al., 1998, 2002; Yoshimoto and Siesjo, 1999). CsA's effects on the immature brain following TBI has not been studied; however, CsA has shown promise in limited immature rodent models of focal ischemia (Leger et al., 2010), and global hypoxia-ischemia (Hwang et al., 2010; Nakai et al., 2004; Setkowicz et al., 2004). Development of potential neuroprotectants has been complicated by the heterogeneity of TBI in children, which includes processes such as contusion, diffuse or focal axonal injury, hematomas, and subarachnoid hemorrhage (Adams et al., 1982, 1983; Moppett, 2007; Saatman et al., 2008), which can initiate ischemia, edema, inflammation, and brain herniation (Brain Trauma Foundation, 2007). Underlying these processes may be mitochondrial dysfunction, which leads to energy imbalance, ionic imbalance, release of cytochrome C, pro-apoptotic events, mitochondrial swelling, propagation of oxidative stress, and reduced brain ATP levels (Sullivan, 2005). Subsequent cellular and molecular responses progress over minutes, hours, and days, to mediate progressive cellular damage (Margulies et al., 2009; Marklund et al., 2006; Povlishock and Katz, 2005; Raghupathi et al., 2004; Schouten, 2007; Werner and Engelhard, 2007), and lead to decreased CBF and mitochondrial disturbances (Graham et al., 2000; Marklund et al., 2006; Povlishock and Katz, 2005; Smith et al., 2003; Thompson et al., 2005; Yi and Hazell, 2006). The first study to directly evaluate mitochondrial function after TBI in the developing brain was published by Robertson and associates in 2007, and showed evidence for very early (∼ 4 h) mitochondrial dysfunction following CCI (Robertson et al., 2007). Mitochondrial functional abnormalities included alterations in mitochondrial respiration, a strong trend toward reduced H2O2 production in the presence of rotenone, and reduced activity of the metabolic enzyme pyruvate dehydrogenase complex (PDHC). In addition, there was evidence for loss of mitochondrial cytochrome C content by 4 h after TBI. In a follow-up study by Casey and colleagues, investigators used proton magnetic resonance spectroscopy (1H MRS) to evaluate the metabolic profile of brain tissue at 4 h, 24 h, and 7 days in the same immature rat CCI model (Casey et al., 2008). In this study the authors concluded that metabolic alterations peaked at 24 h after TBI, with both persistent elevations in tissue lactate/creatine, and emerging reductions in the neuronal/mitochondrial marker N-acetyl-aspartate (NAA) at this time point. Mitochondrial dysfunction in immature rodent models following CCI injury begins early and is persistent for at least 24 h (Casey et al., 2008; Robertson et al., 2007). Our results are consistent with previously published data from a mature mouse model of TBI by Singh and colleagues, that showed significant cortical and hippocampal mitochondrial respiratory dysfunction at 24 h following TBI, that persisted up to 72 h (Singh et al., 2006). In fact, others have reported decreased mitochondrial function up to 14 days following TBI (Xiong et al., 1997). Given the heterogeneity of pediatric TBI, CsA's ability to preserve mitochondrial function in two different species and models of immature TBI greatly strengthens its potential for translation for use children suffering from TBI.
Effects of CsA on mitochondrial bioenergetics in immature animals
Guided by these studies, our data reveal significant mitochondrial dysfunction at 24 h after CCI and diffuse RNR in immature rodent and piglet models of TBI, and preservation of mitochondrial function with the post-injury administration of CsA. State 3 respiration is a measure of the electron transport chain's (ETC) ability to utilize oxygen and phosphorylate ADP, thus producing ATP for cellular homeostasis. Rat CCI injury induced a 20% reduction in state 3 respiration 24 h post-injury; piglets also displayed significantly reduced state 3 respiration in all four brain regions compared to sham animals, indicating a neuro-metabolic crisis and loss of ATP production. Piglets that received CsA had a preservation of ATP production, as measured by state 3 respiration. In diffuse injury in piglets, state 3 preservation was seen in all four brain regions, reaching significance in the cerebellum and cortex.
State 4 respiration represents the rate of proton leakage back across the inner mitochondrial matrix, maintaining (in healthy mitochondria) a proton gradient of the ETC, predominantly for synthesis of ATP (Nicholls and Budd, 2000). Therefore, if the inner mitochondrial matrix is damaged, there is an increase in oxygen utilization in isolated mitochondria, indicating an uncoupling of ETC and ATP production. Previous studies in mature rodent models of CCI TBI and ischemia/reperfusion have found state 4 respiration increases of ∼ 20–50% (Gilmer et al., 2009; Sciamanna et al., 1992; Singh et al., 2006) following brain injury. In the present study, rat pups displayed no statistical changes in state 4; however, piglet state 4 respiration increased ∼ 30% in all four brain regions 24 h after RNR, and piglets that received CsA had a significant decrease in state 4 respiration compared to injured-only animals (p<0.01), indicating preserved mitochondrial membrane potential and coupling of ETC to ATP production.
The mitochondrial respiratory control ratio (RCR; state 3:state 4) is a measure of how well the electron transport system is coupled to ATP synthesis (hydrolysis of ADP+Pi), an overall indicator of mitochondrial functional bioenergetics and integrity (Mbye et al., 2009). A high RCR (5–10) indicates fully-functioning organelles, while a low RCR (<5) indicates that neurons have mitochondrial bioenergetic dysfunction and difficulty maintaining cellular homeostasis, secondary to an inability to produce ATP efficiently. Insufficient production of ATP may lead to a cellular bioenergetic crisis and further neuronal injury. In rat pups, there was an approximately 20% reduction in RCR in ipsilateral regions of CCI compared to contralateral regions, which was not observed in animals treated with CsA, demonstrating stabilization of mitochondrial bioenergetics following CCI. Piglets with RNR TBI exhibit an even more dramatic RCR response to CsA administration (40 mg/kg) post-injury, with preservation of mitochondrial respiration to sham levels in four regions of the brain after a global reduction of RCR of approximately 50% across the same four brain regions in saline-treated piglets.
Our novel mitochondrial bioenergetic data in immature, developing brains supports results originally described in mature animals at 24 h, and is the first study to describe bioenergetic dysfunction in diffuse RNR injury in an immature large-animal model of TBI. Our study suggests that mitochondrial dysfunction in piglets is a combination of ETC component damage (state 3), and inner membrane damage (state 4). CsA administration post-injury improves the overall function of mitochondria and the ability to produce ATP in both rats and piglets, which may be critical to maintain cellular homeostasis in injured neurons.
CsA may have even greater potential for neuroprotection in children with TBI. CsA's mechanism of action primarily attenuates mitochondrial dysfunction by stabilizing the mitochondrial permeability transition pore (mPTP) by binding to cyclophilin D (CypD; Sullivan et al., 2000b). The mPTP is a nonselective, high-conductance channel with multiple macromolecular protein components that forms at sites where the inner and outer membranes fuse (Alano et al., 2002). Neurodegeneration and brain injury leads to the induction of the mPTP, which triggers a cascade of events. An increase in mitochondrial membrane permeability abolishes the mitochondrial membrane gradient (Δψ), resulting in the inability to produce ATP, and thus an energy deficiency in an injured cell. This may be even more important in immature animals, in which there is an increase in CypD compared to mature animals, making the developing brain particularly sensitive to CsA (Eliseev et al., 2007).
Effects of CsA on brain neurochemistry as measured by cerebral microdialysis
Cerebral microdialysis as a bedside monitor for alterations in neurochemistry following TBI is continuing to mature as a technique for improving outcomes following TBI (Hutchinson, 2005). Elevated lactate:pyruvate ratios (LPRs) may be a marker of the cerebral metabolic crisis and mitochondrial dysfunction that limit neuronal recovery following TBI. Increased brain lactate levels may indicate a shift from aerobic metabolism to anaerobic metabolism, and an LPR >25 in adult trials has been associated with worse outcomes (Goodman and Robertson, 2009; Goodman et al., 1999; Stahl et al., 2001). In piglets at 24 h post-RNR TBI, LPR ratios were significantly elevated compared to shams, with LPRs >30, RNR+CsA animals had LPRs maintained at sham levels. Our data show, for the first time, a correlation between mitochondrial dysfunction, as measured by isolated mitochondrial respiration, and metabolic crisis, as measured by cerebral microdialysis, in the developing brain following TBI. CsA not only preserves the mitochondrial bioenergetic state following TBI in the developing brain, but it also improves brain energy metabolism as measured by cerebral microdialysis, a relevant (minimally-invasive) bedside clinical monitoring technique.
Effects of CsA on cerebral blood flow
CsA-induced systemic hypertension is a frequently-cited side effect of CsA in patients receiving CsA as an immunosuppressive agent (Nishiyama et al., 2003). In phase I adult human TBI trials performed by Mazzeo and colleagues, CsA resulted in significantly elevated mean arterial pressure and higher resulting cerebral perfusion pressure compared to placebo, which continued post-CsA infusion for the entire 5-day monitoring period (Mazzeo et al., 2008). Our novel data in this large animal model for the first time show a real-time effect of CsA on CBF. At 24 h post-injury, piglets that ambulated, met criteria to return to the animal facility, and ate without difficulty, had a significant decrease in CBF, more than 50% from sham levels, which post-injury administration of CsA nearly completely abolished, returning CBF to sham levels. Clinically these findings may be extremely important, especially in repetitive injury. Children who may have mild to moderate injury, like our piglet model, and who clinically appear normal to caretakers and clinicians, may be at increased risk following a second insult, due to metabolic reserve (bioenergetic dysfunction) and metabolic delivery (cerebral blood flow) (Friess et al., 2009). CsA may have pleiotropic effects that reduce secondary brain injury by complementing mitochondrial-targeted effects that improve mitochondrial bioenergetics at the mPTP, with a significant vasoactive effect that leads to higher cerebral perfusion pressures (Mazzeo et al., 2008), maintenance of CBF, and improved cerebral metabolic delivery. Conversely, it is conceivable that a reduction in CBF after injury is a protective mechanism to match reperfusion with the observed reduction in cerebral bioenergetics. This minimally-invasive, non-terminal measurement of CBF shows promise as a clinical tool for the observation and guidance of therapeutic interventions in children with diffuse TBI.
Effects of CsA on piglet neuropathology
The administration of CsA to piglets following RNR promoted a 42% decrease in injured (total injury, including axonal injury and infarct) brain volume, composed of a 14% decrease in axonal injury volume, and an 83% decrease in tissue volume designated as infarct-injured. Pathologic sparing of the immature axon following rapid non-impact injury due to reductions in Ca2+-induced cytoskeletal injury, and preservation of bioenergetic function and ATP production, provides a milieu for neuronal healing and avoidance of secondary injury (Okonkwo and Povlishock, 1999; Okonkwo et al., 1999). The neuropathologic distribution of injury was consistent with bioenergetic dysfunction seen in the cortex, hippocampus, and olfactory lobes. However, despite significant bioenergetic dysfunction in the cerebellum, as measured by mitochondrial respiration, there was minimal pathologic injury.
Advantages of the model
With encouraging results from the small-animal rat model, we were able to further investigate the strengths of CsA in a large-animal model of diffuse injury that afforded a higher degree of fidelity when examining different brain regions, as well as when measuring cerebral microdialysis and CBF. In addition, this study provides proof of concept that our large-animal model of diffuse brain injury can provide the basis for a high-fidelity translational model that is able to investigate mitochondrial-targeted neuroprotective strategies from the molecular level to clinically-relevant FDA-approved bedside monitoring: cerebral microdialysis and thermal diffusion CBF. Our results suggest a higher degree of bioenergetic preservation in piglet RNR compared to rat CCI, illuminating the question of whether there was a difference due to the inciting pathology (focal versus diffuse), or species difference. We are currently investigating CCI in piglets and the benefits of CsA as a mitochondrial-targeted neuroprotectant.
Importantly, because there are no dose optimization data for neuro-injury in the pig, and we were informed that higher CsA doses were required for younger patients (del Mar Fernandez De Gatta et al., 2002), and in pigs compared to rats (Lie et al., 2010), we had to estimate a reasonable porcine dose. However, using 40 mg/kg/d of CsA in piglets and 20 mg/kg/d in immature rats may be not be equivalent doses across species, and may be responsible for our finding that immature rats with focal brain injury had decreased mitochondrial preservation compared to piglets with diffuse brain injury.
We propose that dose optimization studies are needed to elucidate optimal dosing for immature rats and pigs, and for focal and diffuse brain injuries. The optimal dose and pharmacodynamics will likely vary with age, species, and mitochondrial development.
Conclusions
CsA has a documented track record of efficacy in adult rodent focal neural injury models, and has an established safety profile in children. This study shows, for the first time, that CsA has exciting translational potential as a therapeutic agent for neuroprotection in children. In two immature models of TBI, and two different species, post-injury administration of CsA preserved mitochondrial bioenergetics and significantly limited neuropathology. Minimally-invasive, non-terminal metrics with potential for clinical translation into pediatric neurocritical care also demonstrated preservation of CBF and amelioration of elevated LPRs.
Acknowledgments
These studies were supported by NIH/NINDS grant R01NS039679 and the Endowed Chair of Anesthesiology and Critical Care Medicine at the Children's Hospital of Philadelphia. We are thankful for the technical and scientific assistance of Trupti Akella, Stuart Friess, Jill Ralston, and Gary Fiskum.
Author Disclosure Statement
No competing financial interests exist.
References
- Adams J.H. Graham D.I. Gennarelli T.A. Head injury in man and experimental animals: neuropathology. Acta Neurochir. Suppl. (Wien.) 1983;32:15–30. doi: 10.1007/978-3-7091-4147-2_2. [DOI] [PubMed] [Google Scholar]
- Adams J.H. Graham D.I. Murray L.S. Scott G. Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann. Neurol. 1982;12:557–563. doi: 10.1002/ana.410120610. [DOI] [PubMed] [Google Scholar]
- Adelson P.D. Bratton S.L. Carney N.A. Chesnut R.M. du Coudray H.E. Goldstein B. Kochanek P.M. Miller H.C. Partington M.D. Selden N.R. Warden C.R. Wright D.W. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 1: Introduction. Pediatr. Crit. Care Med. 2003;4:S2–S4. doi: 10.1097/01.CCM.0000066600.71233.01. [DOI] [PubMed] [Google Scholar]
- Alano C.C. Beutner G. Dirksen R.T. Gross R.A. Sheu S.S. Mitochondrial permeability transition and calcium dynamics in striatal neurons upon intense NMDA receptor activation. J. Neurochem. 2002;80:531–538. doi: 10.1046/j.0022-3042.2001.00738.x. [DOI] [PubMed] [Google Scholar]
- Alessandri B. Rice A.C. Levasseur J. DeFord M. Hamm R.J. Bullock M.R. Cyclosporin A improves brain tissue oxygen consumption and learning/memory performance after lateral fluid percussion injury in rats. J. Neurotrauma. 2002;19:829–841. doi: 10.1089/08977150260190429. [DOI] [PubMed] [Google Scholar]
- Armstead W. Kurth C. Different cerebral hemodynamic responses following fluid percussion brain injury in the newborn and juvenile pig. J. Neurotrauma. 1994;11:487–497. doi: 10.1089/neu.1994.11.487. [DOI] [PubMed] [Google Scholar]
- Bates T.E. Almeida A. Heales S.J. Clark J.B. Postnatal development of the complexes of the electron transport chain in isolated rat brain mitochondria. Dev. Neurosci. 1994;16:321–327. doi: 10.1159/000112126. [DOI] [PubMed] [Google Scholar]
- Bayir H. Kochanek P.M. Clark R.S. Traumatic brain injury in infants and children: mechanisms of secondary damage and treatment in the intensive care unit. Crit. Care Clin. 2003;19:529–549. doi: 10.1016/s0749-0704(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Bayir H. Kochanek P.M. Kagan V.E. Oxidative stress in immature brain after traumatic brain injury. Dev. Neurosci. 2006;28:420–431. doi: 10.1159/000094168. [DOI] [PubMed] [Google Scholar]
- Bellander B.-M. Cantais E. Enblad P. Hutchinson P. Nordstrom C.-H. Robertson C. Sahuquillo J. Smith M. Stocchetti N. Ungerstedt U. Unterberg A. Olsen N.V. Consensus meeting on microdialysis in neurointensive care. Intensive Care Med. 2004;30:2166–2169. doi: 10.1007/s00134-004-2461-8. [DOI] [PubMed] [Google Scholar]
- Brain Trauma Foundation. Guidelines for the management of severe head injury. J. Neurotrauma. 2007;24:S1–S106. doi: 10.1089/neu.2007.9999. [DOI] [PubMed] [Google Scholar]
- Casey P.A. McKenna M.C. Fiskum G. Saraswati M. Robertson C.L. Early and sustained alterations in cerebral metabolism after traumatic brain injury in immature rats. J. Neurotrauma. 2008;25:603–614. doi: 10.1089/neu.2007.0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Maestro R. McDonald W. Distribution of superoxide dismutase, glutathione peroxidase and catalase in developing rat brain. Mech. Ageing Dev. 1987;41:29–38. doi: 10.1016/0047-6374(87)90051-0. [DOI] [PubMed] [Google Scholar]
- Del Mar Fernandez De Gatta M. Santos-Buelga D. Dominguez-Gil A. Garcia M.J. Immunosuppressive therapy for paediatric transplant patients: pharmacokinetic considerations. Clin. Pharmacokinet. 2002;41:115–135. doi: 10.2165/00003088-200241020-00004. [DOI] [PubMed] [Google Scholar]
- Domanska-Janik K. Buzanska L. Dluzniewska J. Kozlowska H. Sarnowska A. Zablocka B. Neuroprotection by cyclosporin A following transient brain ischemia correlates with the inhibition of the early efflux of cytochrome C to cytoplasm. Brain Res. Mol. Brain Res. 2004;121:50–59. doi: 10.1016/j.molbrainres.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Duhaime A.C. Margulies S.S. Durham S.R. O'Rourke M.M. Golden J.A. Marwaha S. Raghupathi R. Maturation-dependent response of the piglet brain to scaled cortical impact. J. Neurosurg. 2000;93:455–462. doi: 10.3171/jns.2000.93.3.0455. [DOI] [PubMed] [Google Scholar]
- Durham S.R. Duhaime A.C. Maturation-dependent response of the immature brain to experimental subdural hematoma. J. Neurotrauma. 2007;24:5–14. doi: 10.1089/neu.2006.0054. [DOI] [PubMed] [Google Scholar]
- Eliseev R.A. Filippov G. Velos J. VanWinkle B. Goldman A. Rosier R.N. Gunter T.E. Role of cyclophilin D in the resistance of brain mitochondria to the permeability transition. Neurobiol. Aging. 2007;28:1532–1542. doi: 10.1016/j.neurobiolaging.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Eucker S. Friess S. Ralston J. Margulies S. Regional cerebral blood flow response following brain injury depends on direction of head motion. National Neurotrauma Society; Orlando, FL: 2008. [Google Scholar]
- Ferrand-Drake M. Zhu C. Gido G. Hansen A.J. Karlsson J.O. Bahr B.A. Zamzami N. Kroemer G. Chan P.H. Wieloch T. Blomgren K. Cyclosporin A prevents calpain activation despite increased intracellular calcium concentrations, as well as translocation of apoptosis-inducing factor, cytochrome c and caspase-3 activation in neurons exposed to transient hypoglycemia. J. Neurochem. 2003;85:1431–1442. doi: 10.1046/j.1471-4159.2003.01794.x. [DOI] [PubMed] [Google Scholar]
- Fisher M. Feuerstein G. Howells D.W. Hurn P.D. Kent T.A. Savitz S.I. Lo E.H. Update of the Stroke Therapy Academic Industry Roundtable Preclinical Recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskum G. Mitochondrial participation in ischemic and traumatic neural cell death. J. Neurotrauma. 2000;17:843–855. doi: 10.1089/neu.2000.17.843. [DOI] [PubMed] [Google Scholar]
- Folbergrova J. Li P.A. Uchino H. Smith M.L. Siesjo B.K. Changes in the bioenergetic state of rat hippocampus during 2.5 min of ischemia, and prevention of cell damage by cyclosporin A in hyperglycemic subjects. Exp. Brain Res. 1997;114:44–50. doi: 10.1007/pl00005622. [DOI] [PubMed] [Google Scholar]
- Friess S.H. Ichord R.N. Ralston J. Ryall K. Helfaer M.A. Smith C. Margulies S.S. Repeated traumatic brain injury affects composite cognitive function in piglets. J. Neurotrauma. 2009;26:1111–1121. doi: 10.1089/neu.2008.0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui S. Signoretti S. Dunbar J.G. Marmarou A. The effect of cyclosporin A on brain edema formation following experimental cortical contusion. Acta Neurochir. Suppl. 2003;86:301–303. doi: 10.1007/978-3-7091-0651-8_65. [DOI] [PubMed] [Google Scholar]
- Gentleman S.M. Roberts G.W. Gennarelli T.A. Maxwell W.L. Adams J.H. Kerr S. Graham D.I. Axonal injury: a universal consequence of fatal closed head injury? Acta Neuropathol. (Berl.) 1995;89:537–543. doi: 10.1007/BF00571509. [DOI] [PubMed] [Google Scholar]
- Ghafari A. Makhdoomi K. Ahmadpour P. Afshari A.T. Fallah M.M. Rad P.S. Low-dose versus high-dose cyclosporine induction protocols in renal transplantation. Transplant Proc. 2007;39:1219–1222. doi: 10.1016/j.transproceed.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Gilmer L.K. Roberts K.N. Joy K. Sullivan P.G. Scheff S.W. Early mitochondrial dysfunction after cortical contusion injury. J. Neurotrauma. 2009;26:1271–1280. doi: 10.1089/neu.2008.0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J.C. Robertson C.S. Microdialysis: is it ready for prime time? Curr. Opin. Crit. Care. 2009;15:110–117. doi: 10.1097/MCC.0b013e328325d142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J.C. Valadka A.B. Gopinath S.P. Uzura M. Robertson C.S. Extracellular lactate and glucose alterations in the brain after head injury measured by microdialysis. Crit. Care Med. 1999;27:1965–1973. doi: 10.1097/00003246-199909000-00041. [DOI] [PubMed] [Google Scholar]
- Graham D.I. McIntosh T.K. Maxwell W.L. Nicoll J.A. Recent advances in neurotrauma. J. Neuropathol. Exp. Neurol. 2000;59:641–651. doi: 10.1093/jnen/59.8.641. [DOI] [PubMed] [Google Scholar]
- Hansson M.J. Persson T. Friberg H. Keep M.F. Rees A. Wieloch T. Elmer E. Powerful cyclosporin inhibition of calcium-induced permeability transition in brain mitochondria. Brain Res. 2003;960:99–111. doi: 10.1016/s0006-8993(02)03798-8. [DOI] [PubMed] [Google Scholar]
- Hillered L. Persson L. Nilsson P. Ronne-Engstrom E. Enblad P. Continuous monitoring of cerebral metabolism in traumatic brain injury: a focus on cerebral microdialysis. Curr. Opin. Crit. Care. 2006;12:112–118. doi: 10.1097/01.ccx.0000216576.11439.df. [DOI] [PubMed] [Google Scholar]
- Hoyert D.L. Heron M.P. Murphy S.L. Kung H.C. Deaths: final data for 2003. Natl. Vital Stat. Rep. 2006;54:1–120. [PubMed] [Google Scholar]
- Hutchinson P.J. Microdialysis in traumatic brain injury—methodology and pathophysiology. Acta Neurochir. Suppl. 2005;95:441–445. doi: 10.1007/3-211-32318-x_91. [DOI] [PubMed] [Google Scholar]
- Hutchison J.S. Ward R.E. Lacroix J. Hebert P.C. Barnes M.A. Bohn D.J. Dirks P.B. Doucette S. Fergusson D. Gottesman R. Joffe A.R. Kirpalani H.M. Meyer P.G. Morris K.P. Moher D. Singh R.N. Skippen P.W. Hypothermia therapy after traumatic brain injury in children. N. Engl. J. Med. 2008;358:2447–2456. doi: 10.1056/NEJMoa0706930. [DOI] [PubMed] [Google Scholar]
- Hwang J.H. Lee J.H. Lee K.H. Bae E.J. Sung D.K. Chang Y.S. Park W.S.J. Cyclosporin a attentuates hypoxic-ischemic brain injury in newborn rats. Brain Res. 2010;1359:208–215. doi: 10.1016/j.brainres.2010.08.047. [DOI] [PubMed] [Google Scholar]
- Ibrahim N.G. Ralston J. Smith C. Margulies S.S. Physiological and pathological responses to head rotations in toddler piglets. J. Neurotrauma. 2010;27:1021–1035. doi: 10.1089/neu.2009.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josan V.A. Sgouros S. Early decompressive craniectomy may be effective in the treatment of refractory intracranial hypertension after traumatic brain injury. Childs Nerv. Syst. 2006;22:1268–1274. doi: 10.1007/s00381-006-0064-0. [DOI] [PubMed] [Google Scholar]
- Leger P.L. De Paulis D. Branco S. Bonnin P. Couture-Lepetit E. Baud O. Renolleau S. Ovize M. Gharib A. Charriaut-Marlangue C. Evaluation of cyclosporine A in a stroke model in the immature rat brain. Exp. Neurol. 2010 doi: 10.1016/j.expneurol.2010.06.009. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Lie R.H. Stoettrup N. Sloth E. Hasenkam J.M. Kroyer R. Nielsen T.T. Post-conditioning with cyclosporine A fails to reduce the infarct size in an in vivo porcine model. Acta Anaesthesiol. Scand. 2010;54:804–813. doi: 10.1111/j.1399-6576.2010.02241.x. [DOI] [PubMed] [Google Scholar]
- Lin M.T. Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Li P.A. Kristian T. He Q.P. Siesjo B.K. Cyclosporin A enhances survival, ameliorates brain damage, and prevents secondary mitochondrial dysfunction after a 30-minute period of transient cerebral ischemia. Exp. Neurol. 2000;165:153–163. doi: 10.1006/exnr.2000.7459. [DOI] [PubMed] [Google Scholar]
- Margulies S. Hicks R. Leaders, and Combination Therapies for Traumatic Brain Injury Workshop Leaders. Combination therapies for traumatic brain injury: Prospective considerations. J Neurotrauma. 2009;26:925–939. doi: 10.1089/neu.2008.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund N. Bakshi A. Castelbuono D.J. Conte V. McIntosh T.K. Evaluation of pharmacological treatment strategies in traumatic brain injury. Curr. Pharm. Des. 2006;12:1645–1680. doi: 10.2174/138161206776843340. [DOI] [PubMed] [Google Scholar]
- Mazzeo A.T. Alves O.L. Gilman C.B. Hayes R.L. Tolias C. Niki Kunene K. Ross Bullock M. Brain metabolic and hemodynamic effects of cyclosporin A after human severe traumatic brain injury: a microdialysis study. Acta Neurochir. (Wien.) 2008;150:1019–1031. doi: 10.1007/s00701-008-0021-7. discussion 1031. [DOI] [PubMed] [Google Scholar]
- Mazzeo A.T. Brophy G.M. Gilman C.B. Alves O.L. Robles J.R. Hayes R.L. Povlishock J.T. Bullock M.R. Safety and tolerability of cyclosporin A in severe traumatic brain injury patients: results from a prospective randomized trial. J. Neurotrauma. 2009;26:2195–2206. doi: 10.1089/neu.2009.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbye L.H. Singh I.N. Carrico K.M. Saatman K.E. Hall E.D. Comparative neuroprotective effects of cyclosporin A and NIM811, a nonimmunosuppressive cyclosporin A analog, following traumatic brain injury. J. Cereb. Blood Flow Metab. 2009;29:87–97. doi: 10.1038/jcbfm.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moppett I.K. Traumatic brain injury: assessment, resuscitation and early management. Br. J. Anaesth. 2007;99:18–31. doi: 10.1093/bja/aem128. [DOI] [PubMed] [Google Scholar]
- Nakai A. Shibazaki Y. Taniuchi Y. Miyake H. Oya A. Takeshita T. Role of mitochondrial permeability transition in fetal brain damage in rats. Pediatr. Neurol. 2004;30:247–253. doi: 10.1016/j.pediatrneurol.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Narayan R.K. Michel M.E. Ansell B. Baethmann A. Biegon A. Bracken M.B. Bullock M.R. Choi S.C. Clifton G.L. Contant C.F. Coplin W.M. Dietrich W.D. Ghajar J. Grady M.S. Grossman R.G. Hall E.D. Heetderks W. Hovda D.A. Jallo J. Katz R.L. Knoller N. Kochanek P.M. Maas A.I. Majde J. Marion D.W. Marmarou A. Marshall L.F. McIntosh T.K. Miller E. Mohberg N. Muizelaar J.P. Pitts L.H. Quinn P. Riesenfeld G. Robertson C.S. Strauss K.I. Teasdale G.M. Temkin N. Tuma R. Wade C. Walker M.D. Weinrich M. Whyte J. Wilberger J.E., Jr. Young A.B. Yurkewicz L. Clinical trials in head injury. J. Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D.G. Budd S.L. Mitochondria and neuronal survival. Physiol. Rev. 2000;80:315–360. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- Nishiyama A. Kobori H. Fukui T. Zhang G.X. Yao L. Rahman M. Hitomi H. Kiyomoto H. Shokoji T. Kimura S. Kohno M. Abe Y. Role of angiotensin II and reactive oxygen species in cyclosporine A-dependent hypertension. Hypertension. 2003;42:754–760. doi: 10.1161/01.HYP.0000085195.38870.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo D.O. Povlishock J.T. An intrathecal bolus of cyclosporin A before injury preserves mitochondrial integrity and attenuates axonal disruption in traumatic brain injury. J. Cereb. Blood Flow Metab. 1999;19:443–451. doi: 10.1097/00004647-199904000-00010. [DOI] [PubMed] [Google Scholar]
- Okonkwo D.O. Buki A. Siman R. Povlishock J.T. Cyclosporin A limits calcium-induced axonal damage following traumatic brain injury. Neuroreport. 1999;10:353–358. doi: 10.1097/00001756-199902050-00026. [DOI] [PubMed] [Google Scholar]
- Patrick P.D. Blackman J.A. Mabry J.L. Buck M.L. Gurka M.J. Conaway M.R. Dopamine agonist therapy in low-response children following traumatic brain injury. J. Child Neurol. 2006;21:879–885. doi: 10.1177/08830738060210100901. [DOI] [PubMed] [Google Scholar]
- Polster B.M. Robertson C.L. Bucci C.J. Suzuki M. Fiskum G. Postnatal brain development and neural cell differentiation modulate mitochondrial Bax and BH3 peptide-induced cytochrome c release. Cell Death Differ. 2003;10:365–370. doi: 10.1038/sj.cdd.4401158. [DOI] [PubMed] [Google Scholar]
- Povlishock J.T. Katz D.I. Update of neuropathology and neurological recovery after traumatic brain injury. J. Head Trauma Rehabil. 2005;20:76–94. doi: 10.1097/00001199-200501000-00008. [DOI] [PubMed] [Google Scholar]
- Prabhakaran P. Reddy A.T. Oakes W.J. King W.D. Winkler M.K. Givens T.G. A pilot trial comparing cerebral perfusion pressure-targeted therapy to intracranial pressure-targeted therapy in children with severe traumatic brain injury. J. Neurosurg. 2004;100:454–459. doi: 10.3171/ped.2004.100.5.0454. [DOI] [PubMed] [Google Scholar]
- Raghupathi R. Margulies S.S. Traumatic axonal injury after closed head injury in the neonatal pig. J. Neurotrauma. 2002;19:843–853. doi: 10.1089/08977150260190438. [DOI] [PubMed] [Google Scholar]
- Raghupathi R. Mehr M. Helfaer M. Margulies S. Traumatic axonal injury is exacerbated following repetitive close head injury in the neonatal pig. J. Neurotrauma. 2004;21:307–316. doi: 10.1089/089771504322972095. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S. Nihal M. Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Robertson C.L. Saraswati M. Fiskum G. Mitochondrial dysfunction early after traumatic brain injury in immature rats. J. Neurochem. 2007;101:1248–1257. doi: 10.1111/j.1471-4159.2007.04489.x. [DOI] [PubMed] [Google Scholar]
- Robertson C.L. Scafidi S. McKenna M.C. Fiskum G. Mitochondrial mechanisms of cell death and neuroprotection in pediatric ischemic and traumatic brain injury. Exp. Neurol. 2009;218:371–380. doi: 10.1016/j.expneurol.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson C.L. Soane L. Siegel Z.T. Fiskum G. The potential role of mitochondria in pediatric traumatic brain injury. Dev. Neurosci. 2006;28:432–446. doi: 10.1159/000094169. [DOI] [PubMed] [Google Scholar]
- Saatman K.E. Duhaime A.-C. Bullock R. Maas A.I.R. Valadka A. Manley G.T. Workshop Scientific Team and Advisory Panel Members. Classification of traumatic brain injury for targeted therapies. J. Neurotrauma. 2008;25:719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A. Maier C.M. Narasimhan P. Nishi T. Song Y.S. Yu F. Liu J. Lee Y.S. Nito C. Kamada H. Dodd R.L. Hsieh L.B. Hassid B. Kim E.E. Gonzalez M. Chan P.H. Oxidative stress and neuronal death/survival signaling in cerebral ischemia. Mol. Neurobiol. 2005;31:105–116. doi: 10.1385/MN:31:1-3:105. [DOI] [PubMed] [Google Scholar]
- Scheff S.W. Sullivan P.G. Cyclosporin A significantly ameliorates cortical damage following experimental traumatic brain injury in rodents. J. Neurotrauma. 1999;16:783–792. doi: 10.1089/neu.1999.16.783. [DOI] [PubMed] [Google Scholar]
- Schouten J.W. Neuroprotection in traumatic brain injury: a complex struggle against the biology of nature. Curr. Opin. Crit. Care. 2007;13:134–142. doi: 10.1097/MCC.0b013e3280895d5c. [DOI] [PubMed] [Google Scholar]
- Sciamanna M.A. Zinkel J. Fabi A.Y. Lee C.P. Ischemic injury to rat forebrain mitochondria and cellular calcium homeostasis. Biochim. Biophys. Acta. 1992;1134:223–232. doi: 10.1016/0167-4889(92)90180-j. [DOI] [PubMed] [Google Scholar]
- Setkowicz Z. Ciarach M. Guzik R. Janeczko K. Different effects of neuroprotectants FK-506 and cyclosporin A on susceptibility to pilocarpine-induced seizures in rats with brain injured at different developmental stages. Epilepsy Res. 2004;61:63–72. doi: 10.1016/j.eplepsyres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Shiga Y. Onodera H. Matsuo Y. Kogure K. Cyclosporin A protects against ischemia-reperfusion injury in the brain. Brain Res. 1992;595:145–148. doi: 10.1016/0006-8993(92)91465-q. [DOI] [PubMed] [Google Scholar]
- Signoretti S. Marmarou A. Tavazzi B. Dunbar J. Amorini A.M. Lazzarino G. Vagnozzi R. The protective effect of cyclosporin A upon N-acetylaspartate and mitochondrial dysfunction following experimental diffuse traumatic brain injury. J. Neurotrauma. 2004;21:1154–1167. doi: 10.1089/neu.2004.21.1154. [DOI] [PubMed] [Google Scholar]
- Singh I.N. Sullivan P.G. Deng Y. Mbye L.H. Hall E.D. Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J. Cereb. Blood Flow Metab. 2006;26:1407–1418. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- Smith D.H. Meaney D.F. Shull W.H. Diffuse axonal injury in head trauma. J. Head Trauma Rehabil. 2003;18:307–316. doi: 10.1097/00001199-200307000-00003. [DOI] [PubMed] [Google Scholar]
- Stahl N. Moellergard P. Hallstrom A. Ungerstedt U. Nordstrom C.H. Intracerebral microdialysis and bedside biochemical analysis in patients with fatal traumatic brain lesions. Acta Anaesthesiol. Scand. 2001;45:977–985. doi: 10.1034/j.1399-6576.2001.450810.x. [DOI] [PubMed] [Google Scholar]
- Starkov A.A. Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J. Neurochem. 2003;86:1101–1107. doi: 10.1046/j.1471-4159.2003.01908.x. [DOI] [PubMed] [Google Scholar]
- Starkov A.A. Chinopoulos C. Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium. 2004;36:257–264. doi: 10.1016/j.ceca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Starkov A.A. Polster B.M. Fiskum G. Regulation of hydrogen peroxide production by brain mitochondria by calcium and Bax. J. Neurochem. 2002;83:220–228. doi: 10.1046/j.1471-4159.2002.01153.x. [DOI] [PubMed] [Google Scholar]
- Suehiro E. Ueda Y. Wei E.P. Kontos H.A. Povlishock J.T. Posttraumatic hypothermia followed by slow rewarming protects the cerebral microcirculation. J. Neurotrauma. 2003;20:381–390. doi: 10.1089/089771503765172336. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Interventions with neuroprotective agents: novel targets and opportunities. Epilepsy Behav. 2005;7(Suppl. 3):S12–S17. doi: 10.1016/j.yebeh.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Rabchevsky A.G. Hicks R.R. Gibson T.R. Fletcher-Turner A. Scheff S.W. Dose-response curve and optimal dosing regimen of cyclosporin A after traumatic brain injury in rats. Neuroscience. 2000a;101:289–295. doi: 10.1016/s0306-4522(00)00380-8. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Thompson M. Scheff S.W. Continuous infusion of cyclosporin A postinjury significantly ameliorates cortical damage following traumatic brain injury. Exp. Neurol. 2000b;161:631–637. doi: 10.1006/exnr.1999.7282. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Thompson M.B. Scheff S.W. Cyclosporin A attenuates acute mitochondrial dysfunction following traumatic brain injury. Exp. Neurol. 1999;160:226–234. doi: 10.1006/exnr.1999.7197. [DOI] [PubMed] [Google Scholar]
- Thompson H.J. Lifshitz J. Marklund N. Grady M.S. Graham D.I. Hovda D.A. McIntosh T.K. Lateral fluid percussion brain injury: a 15-year review and evaluation. J. Neurotrauma. 2005;22:42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- Turner M.S. Early use of intrathecal baclofen in brain injury in pediatric patients. Acta Neurochir. Suppl. 2003;87:81–83. doi: 10.1007/978-3-7091-6081-7_17. [DOI] [PubMed] [Google Scholar]
- Uchino H. Elmer E. Uchino K. Li P.A. He Q.P. Smith M.L. Siesjo B.K. Amelioration by cyclosporin A of brain damage in transient forebrain ischemia in the rat. Brain Res. 1998;812:216–226. doi: 10.1016/s0006-8993(98)00902-0. [DOI] [PubMed] [Google Scholar]
- Uchino H. Minamikawa-Tachino R. Kristian T. Perkins G. Narazaki M. Siesjo B.K. Shibasaki F. Differential neuroprotection by cyclosporin A and FK506 following ischemia corresponds with differing abilities to inhibit calcineurin and the mitochondrial permeability transition. Neurobiol. Dis. 2002;10:219–233. doi: 10.1006/nbdi.2002.0514. [DOI] [PubMed] [Google Scholar]
- Vajkoczy P. Roth H. Horn P. Lucke T. Thome C. Hubner U. Martin G.T. Zappletal C. Klar E. Schilling L. Schmiedek P. Continuous monitoring of regional cerebral blood flow: experimental and clinical validation of a novel thermal diffusion microprobe. J. Neurosurg. 2000;93:265–274. doi: 10.3171/jns.2000.93.2.0265. [DOI] [PubMed] [Google Scholar]
- Vespa P. Bergsneider M. Hattori N. Wu H.-M. Huang S.-C. Martin N.A. Glenn T.C. McArthur D.L. Hovda D.A. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J. Cereb Blood Flow Metab. 2005;25:763–774. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner C. Engelhard K. Pathophysiology of traumatic brain injury. Br. J. Anaesth. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- Williams S. Raghupathi R. MacKinnon M.A. McIntosh T.K. Saatman K.E. Graham D.I. In situ DNA fragmentation occurs in white matter up to 12 months after head injury in man. Acta Neuropathol. (Berl.) 2001;102:581–590. doi: 10.1007/s004010100410. [DOI] [PubMed] [Google Scholar]
- Xiong Y. Gu Q. Peterson P.L. Muizelaar J.P. Lee C.P. Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J. Neurotrauma. 1997;14:23–34. doi: 10.1089/neu.1997.14.23. [DOI] [PubMed] [Google Scholar]
- Yi J.H. Hazell A.S. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem. Int. 2006;48:394–403. doi: 10.1016/j.neuint.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T. Siesjo B.K. Posttreatment with the immunosuppressant cyclosporin A in transient focal ischemia. Brain Res. 1999;839:283–291. doi: 10.1016/s0006-8993(99)01733-3. [DOI] [PubMed] [Google Scholar]