Abstract
An increasing number of antiretroviral agents (ARVs) are approved for use, but their use during pregnancy in the United States has not been completely described. We used data from the Pediatric HIV/AIDS Cohort Study (PHACS) Surveillance Monitoring for ART Toxicities (SMARTT) study, a United States-based prospective cohort study of HIV-exposed but uninfected children, to assess temporal trends and maternal characteristics associated with the use of ARVs during pregnancy. The proportion of children exposed in utero to ARVs was calculated over time. A multivariable logistic regression model was used to estimate associations of maternal characteristics with use of highly active antiretroviral therapy (HAART) during pregnancy. We studied 1768 HIV-exposed but uninfected children born between 1995 and 2009 and enrolled in SMARTT. Prenatal HAART exposure increased from 19% in 1997 to 88% in 2009. Of children born in 2009, 99% had prenatal exposure to NRTIs (including zidovudine, 73%; lamivudine, 72%; tenofovir, 39%; and emtricitabine, 37%). Exposure to protease inhibitors increased from 15% in 1997 to 86% in 2009, while exposure to non-nucleoside reverse transcriptase inhibitors (NNRTIs) declined from 33% in 2003 to 11% in 2009. Higher maternal HIV RNA viral load (VL) concentration, lower maternal CD4 count, and earlier timing of the first maternal CD4 or VL measurement during pregnancy were associated with increased odds of HAART exposure. Prenatal HAART exposure has increased but is not universal. As ARV use during pregnancy continues to evolve, follow-up of children is needed to assess long-term effects of ARV exposures.
Introduction
Several thousand infants are born in the United States each year to HIV-infected women.1 Due to successful prevention programs, the vast majority of these infants are not infected with HIV. U.S. guidelines recommend combination antiretroviral (ARV) regimens during pregnancy, both for treatment of maternal HIV infection and for prevention of mother-to-child transmission of HIV, regardless of maternal plasma HIV RNA concentration (viral load).2 The increased availability of ARVs and knowledge about their use has resulted in dramatic reduction in the risk of transmission of HIV to the fetus and infant.
A number of new ARVs have been approved for HIV treatment and prophylaxis in recent years. As new ARVs become available, the number of potential combinations of individual ARVs rapidly grows. Selection of an appropriate ARV regimen may depend on a number of factors, including maternal health status, previous use of ARVs, resistance testing, individual clinical practice, changes in guidelines, and cost of specific agents. While several first-line combinations may dominate clinical practice, the number of alternative regimens in current use continues to increase.
The potential therefore exists for wide variation in clinical practice, but no studies have described recent trends in actual clinical practice in the United States. Earlier studies have described trends in use of ARVs in high-resource countries.3–6 Several other studies have presented data on recent in utero ARV exposure in U.S. cohorts, but these studies offer exposure information only on select ARVs or regimens7,8 or do not report temporal trends in exposure.9
We used data from the Surveillance Monitoring for ART Toxicities (SMARTT) study to describe changes in ARV use during pregnancy in the United States and Puerto Rico, and to identify key predictors of maternal use of combination ARV regimens during pregnancy.
Methods
The source population for this study was the SMARTT study, which is an observational cohort study conducted at 22 sites in the United States and Puerto Rico designed to study the effects of in utero and early infant ARV exposure on outcomes and toxicities in HIV-uninfected children born to HIV-infected mothers. Enrollment in SMARTT began in March, 2007.
SMARTT consists of two cohorts. The Static cohort enrolls HIV-uninfected children born to HIV-infected mothers from the early neonatal period until 12 years of age who either were previously enrolled in an approved prior study (the largest of which are the cohort studies PACTG 219C, the Women and Infants Transmission Study, and IMPAACT P1025, all of which have been described elsewhere6,8,9) or who have perinatal and early infant ARV and pregnancy complication data available in their medical chart. Participants were enrolled into the Dynamic cohort either through enrollment of the mother during pregnancy (>22 weeks gestation) or of the infant at birth. Mothers or caregivers of the children in the Static cohort were enrolled if willing; the mother's enrollment was required for the Dynamic cohort. The SMARTT protocol was approved by human subject research review boards at each of the participating sites and by the Harvard School of Public Health. Written informed consent was obtained from the participant or from the parent or legal guardian for participating children by staff at the local sites.
All children enrolled in SMARTT with data regarding maternal use of ARVs during pregnancy were included in the analysis. At SMARTT enrollment, the child's medical and clinical histories, including ARV use during early infancy, and the mother's pregnancy history, including the first and last HIV viral load and CD4 measurement during pregnancy, were abstracted from clinical records. In utero and early childhood ARV exposure data, including start and stop dates from the beginning of pregnancy, were collected from the approved prior studies or was abstracted from clinical records, regardless of whether the ARVs were intended for prevention of mother-to-child transmission or for the mother's own health.
Information on race/ethnicity was provided by each child's parent or guardian. Information on alcohol, tobacco, and illicit substance use during pregnancy was reported by the child's mother if she was enrolled on the study, and meconium was collected and analyzed for children enrolled in the Dynamic cohort to verify this self-reported use.10
In utero ARV exposure was categorized as: no ARVs, zidovudine (ZDV) only, two nucleoside reverse transcriptase inhibitors (NRTIs), three or more NRTIs, HAART-equivalent combination ARVs (cARVs), and other ARV regimens. For the purposes of this analysis, HAART-equivalent cARV use was defined as maternal use of at least three drugs from at least two drug classes, whether use was for treatment or prevention of HIV transmission. In defining HAART-equivalent cARVs, ritonavir (RTV) used as a boosting agent did not count towards the total number of drugs. Because the mother's regimen may have changed during pregnancy, a single maternal regimen was chosen as the most intense regimen among all regimens received for at least three days. Regimen intensity was assigned to a hierarchy (least to most intensive): no ARVs, ZDV only, two NRTIs, other ARV regimens, three or more NRTIs, and HAART-equivalent cARVs. We use the term combination ARVs (cARVs) to refer to the two most intense types of regimens (regimens consisting of three or more NRTIs or HAART-equivalent cARVs). As a sensitivity analysis, we also considered the regimen used for the longest duration rather than the most intensive regimen, and determined the number of children with a change in assigned regimen.
We also considered infant prophylaxis based on exposure to ARVs during the first 2 months of life for each infant enrolled in SMARTT. The median and interquartile range of the duration of infant ARV prophylaxis were calculated for the most common ARV drugs and classes.
Temporal trends in in utero ARV exposure, ARV classes, and the most intense ARV regimen were evaluated by calculating the proportion of children exposed in a calendar year. ARVs initiated less than 3 days prior to birth were not considered in the analysis. The in utero regimen with the longest duration was also identified for each child, and the number and proportion of children exposed to each regimen was reported for each birth cohort for any regimen comprising more than 5% of the total.
Predictors of cARV and HAART-equivalent cARV use were estimated using univariate and multivariable logistic regression models. To account for the temporal availability and use of cARVs, children were classified into three birth cohorts. The cohort of children born in 1995–2002 had disparate and variable patterns of ARV exposure as new drugs became available and ARV in pregnancy guidelines were established. Therefore, this era was excluded from the modeling. The remaining time period was subdivided into two cohorts: 2003–2006 and 2007–2009. Variables considered as potential predictors of cARV use during pregnancy included birth cohort; the child's race, ethnicity, and gender; the trimester of the first maternal viral load or CD4 count during pregnancy; the first maternal viral load and CD4 measurements during pregnancy; and indicators for maternal tobacco, alcohol, marijuana, and other substance use during pregnancy. Children whose mothers did not have a viral load or CD4 count during pregnancy were retained in the models using indicator variables for these categories. Children with other missing covariates were excluded from the modeling.
Univariate logistic regression models were fit for each covariate and p values were calculated using a Wald χ2 test. Exact logistic regression was used when all participants in the same level of a covariate had the same outcome. Variables with a p value less than 0.20 from the univariate models were included in a multivariate logistic regression model, and variables with adjusted p values greater than 0.10 were removed. Exact logistic regression was performed using logXact (version 8.0; Cytel Systems, Marlboro, MA); all other calculations were performed using SAS (version 9.1; SAS Institute, Cary, NC).
Results
Size and characteristics of the study population
Of 1975 children enrolled in the Static and Dynamic cohorts of SMARTT as of December 1, 2009, 1787 had any in utero ARV exposure data available, and 1768 had such data available with dates of initiation and discontinuation of ARVs. Therefore, the study population comprised 1768 children, of whom 905 (51%) were male, 1117 (63%) were black/African American, and 587 (33%) were Hispanic/Latino (Table 1). The children were born in 1995–2009, but most were born in more recent years (median year of birth was 2006).
Table 1.
Characteristics of the SMARTT Study Population of HIV-Exposed but Uninfected Infants by Year of Birth (n=1768)
| |
|
Year of birth |
|||
|---|---|---|---|---|---|
| Characteristic | Total (n=1768) | 1995–1997 (n=67) | 1998–2002 (n=399) | 2003–2006 (n=487) | 2007–2009 (n=815) |
| Gender | |||||
| Male | 905 (51%) | 31 (46%) | 211 (53%) | 253 (52%) | 410 (50%) |
| Female | 863 (49%) | 36 (54%) | 188 (47%) | 234 (48%) | 405 (50%) |
| Race | |||||
| Black or African American | 1117 (63%) | 44 (66%) | 262 (66%) | 281 (58%) | 530 (65%) |
| White | 496 (28%) | 19 (28%) | 93 (23%) | 158 (32%) | 226 (28%) |
| Other/more than one race/unknown | 155 (9%) | 4 (6%) | 44 (11%) | 48 (10%) | 59 (7%) |
| Ethnicity | |||||
| Hispanic or Latino | 587 (33%) | 15 (22%) | 126 (32%) | 193 (40%) | 253 (31%) |
| Not Hispanic or Latino | 1168 (66%) | 52 (78%) | 272 (68%) | 290 (60%) | 554 (68%) |
| More than one ethnicity/unknown | 13 (1%) | 0 (0%) | 1 (0%) | 4 (1%) | 8 (1%) |
| First maternal viral load during pregnancy (copies/mL) | |||||
| None | 137 (8%) | 43 (64%) | 52 (13%) | 28 (6%) | 14 (2%) |
| <1000 | 673 (38%) | 5 (7%) | 125 (31%) | 197 (40%) | 346 (42%) |
| 1000–9999 | 444 (25%) | 6 (9%) | 105 (26%) | 128 (26%) | 205 (25%) |
| 10,000–100,000 | 391 (22%) | 11 (16%) | 80 (20%) | 108 (22%) | 192 (24%) |
| ≥100,000 | 98 (6%) | 0 (0%) | 26 (7%) | 23 (5%) | 49 (6%) |
| Unknown | 25 (1%) | 2 (3%) | 11 (3%) | 3 (1%) | 9 (1%) |
| Last maternal viral load during pregnancy (copies/mL) | |||||
| None | 137 (8%) | 43 (64%) | 52 (13%) | 28 (6%) | 14 (2%) |
| <1000 | 1301 (74%) | 8 (12%) | 245 (61%) | 380 (78%) | 668 (82%) |
| 1000–9999 | 176 (10%) | 4 (6%) | 46 (12%) | 51 (10%) | 75 (9%) |
| 10,000–100,000 | 88 (5%) | 7 (10%) | 34 (9%) | 17 (3%) | 30 (4%) |
| ≥100,000 | 10 (1%) | 0 (0%) | 2 (1%) | 4 (1%) | 4 (0%) |
| Unknown | 56 (3%) | 5 (7%) | 20 (5%) | 7 (1%) | 24 (3%) |
| First maternal CD4 count during pregnancy (cells/mm3) | |||||
| None | 226 (13%) | 6 (9%) | 48 (12%) | 61 (13%) | 111 (14%) |
| 0–199 | 397 (22%) | 16 (24%) | 78 (20%) | 103 (21%) | 200 (25%) |
| 200–349 | 404 (23%) | 17 (25%) | 90 (23%) | 101 (21%) | 196 (24%) |
| 350–499 | 614 (35%) | 16 (24%) | 127 (32%) | 187 (38%) | 284 (35%) |
| ≥500 | 102 (6%) | 10 (15%) | 45 (11%) | 32 (7%) | 15 (2%) |
| Unknown | 25 (1%) | 2 (3%) | 11 (3%) | 3 (1%) | 9 (1%) |
| Trimester of first maternal viral load or CD4 measurement during pregnancy | |||||
| No measurement | 82 (5%) | 9 (13%) | 38 (10%) | 24 (5%) | 11 (1%) |
| Trimester 1 | 912 (52%) | 18 (27%) | 162 (41%) | 298 (61%) | 434 (53%) |
| Trimester 2 | 609 (34%) | 27 (40%) | 145 (36%) | 126 (26%) | 311 (38%) |
| Trimester 3 | 138 (8%) | 11 (16%) | 41 (10%) | 36 (7%) | 50 (6%) |
| Unknown | 27 (2%) | 2 (3%) | 13 (3%) | 3 (1%) | 9 (1%) |
| Maternal substance use during pregnancy | |||||
| Tobacco | 296 (17%) | 8 (12%) | 69 (17%) | 84 (17%) | 135 (17%) |
| Alcohol | 130 (7%) | 1 (1%) | 26 (7%) | 26 (5%) | 77 (9%) |
| Marijuana | 99 (6%) | 1 (1%) | 18 (5%) | 30 (6%) | 50 (6%) |
| Other substances | 156 (9%) | 4 (6%) | 27 (7%) | 45 (9%) | 80 (10%) |
| Unknown | 159 (9%) | 13 (19%) | 65 (16%) | 48 (10%) | 33 (4%) |
SMARTT, Surveillance Monitoring for ART Toxicities.
Maternal use of ARVs during pregnancy
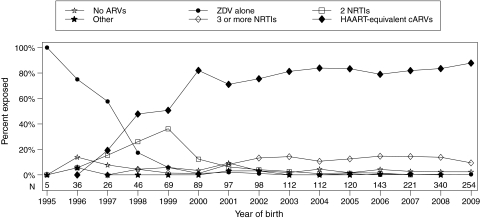
Almost all children (n=1704, 96.4%) had in utero ARV exposure. Of these 1704 children, most (70%) were exposed in utero to only one ARV regimen, with 23% exposed to two regimens. HAART-equivalent cARVs was the most common in utero regimen (77%). Maternal use of HAART-equivalent cARVs in this cohort began in 1997 with 19% reporting use and became the most common regimen from 1998 onwards (Fig. 1). Over 79% of infants were exposed to HAART-equivalent cARVs from 2003 to the present. By 2009, 88% were exposed to HAART-equivalent cARVs and 97% were exposed to cARVs.
FIG. 1.
Proportion of children exposed to in utero antiretroviral (ARV) regimens by year of birth. Because the mother's regimen may have changed during pregnancy, a single maternal regimen was chosen as the most intense regimen among all regimens received at least 3 days. Regimen intensity was assigned to a hierarchy (least to most intensive): no ARVs, ZDV only, two NRTIs, other ARV regimens, three or more NRTIs, and HAART-equivalent combination ARVs (regimens consisting of three or more NRTIs or three ARVs from two or more classes). ZDV, zidovudine; NRTI, nucleoside reverse transcriptase inhibitor; HAART, highly active antiretroviral therapy.
In the sensitivity analysis based on the regimen of longest duration instead of the most intensive, the assigned regimen changed for 76 children (4.3%). HAART-equivalent cARVs demonstrated the greatest change, falling from 77% exposed (using the most-intensive regimen) to 74% (using the longest regimen).
After HAART, the next most common regimens were those regimens consisting of three or more NRTIs (11%). Between 2002 and 2009, these regimens were consistently the second most common regimens each year, with between 9% and 15% exposed. The most common was the regimen of ZDV, lamivudine, and abacavir (ZDV+3TC+ABC), with 13% of the population exposed from 2003 to 2009. The remainder of the population was almost equally split between children exposed to ZDV alone (3.8%), two NRTIs (4.1%), and no ARVs (3.6%). The proportion of children without in utero ARV exposure peaked in 1996 (14%) and declined to 2.4% in 2009. Few children (0.6%) were exposed to other regimens.
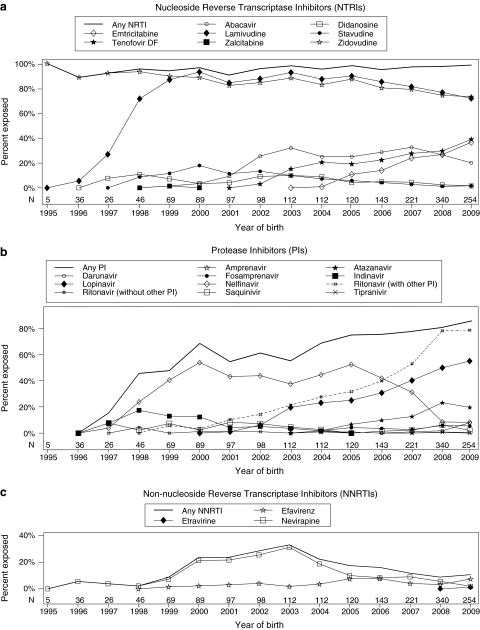
When individual ARV use was examined, the NRTIs as a group were the most common drug class used in the cohort (97% exposed), with ZDV and 3TC the most commonly used NRTIs (Fig. 2A). However, both ZDV and 3TC exposure declined between 2003 and 2009; ZDV exposure fell from 88% in 2003 to 73% in 2009, and 3TC exposure fell from 93% in 2003 to 72% in 2009. While ZDV and 3TC use declined, use of tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC) increased since their first appearance (TDF in 2002 and FTC in 2004). By 2009, these were the most common NRTIs after ZDV and 3TC. Other NRTIs used include abacavir (ABC), stavudine (d4T), didanosine (ddI), and zalcitabine (ddC), although exposure to all but ABC have been limited in recent years (less than 2% exposed to d4T and ddI in 2009 and no ddC use since 1999).
FIG. 2.
Proportion of children exposed to in utero antiretrovirals (ARVs) by year of birth. Denominator includes patients not exposed to antiretroviral therapy (ART).
After NRTIs, the most common class of drugs observed were protease inhibitors (PIs). Seventy percent were exposed in utero to a PI, reaching 86% exposure in 2009 (Fig. 2B). The most common PI since 2007 was lopinavir coformulated with ritonavir (LPV/r). In 2009, LPV/r exposure was more than double that of the next most common PI, atazanavir (ATV) (55–20%, respectively). Other PIs used include nelfinavir, the most common PI from 1998 to 2006, and indinavir, the second most common PI from 1998 to 2000. Use of amprenavir, fosamprenavir, saquinavir, tipranivir, and therapeutic dose RTV was also reported (Fig. 2B). RTV boosting was common, especially in later years. Seventy-nine percent of children in 2009 were exposed to a RTV-boosted PI regimen.
Non-nucleoside reverse transcriptase inhibitors (NNRTIs) were used less often than NRTIs or PIs, and use has declined in recent years. Overall, 15% were exposed to an NNRTI, but exposure to NNRTIs peaked in 2003 (33%) and fell to 11% by 2009 (Fig. 2C). Nevirapine (NVP) was the most common NNRTI overall, but was overtaken by efavirenz (EFV) in 2009 (7.5%). Etravirine exposure was only observed in 2009 (1.2% in 2009).
Use of fusion, integrase, and other entry inhibitors was limited (1.3% overall). Raltegravir was the most common of these agents in 2009 (5.1% in 2009, 1.0% overall). Enfuviritide exposure was 0.8% in 2009. No maraviroc use was reported. Since 2003, the most common in utero exposure was to regimens consisting of two NRTIs and one PI, followed by regimens consisting of three NRTIs (Table 2). Since 1998, 65–70% of the children in each birth cohort were exposed to one of the four most common regimens for that cohort. The remaining were exposed to a large number of combination regimens. From 2007 to 2009, the 293 children not exposed to the four most common regimens were exposed to 90 different regimens. For most drugs and classes, the proportion of children exposed in later trimesters was at least as high as those exposed in earlier trimesters. The exception was EFV, used in 3.4% of children in the first trimester compared to 1.2% in the second and 0.9% in the third (data not shown). Children exposed to EFV in the first trimester were exposed for a median (interquartile range, IQR) of 48 days (34–76 days).
Table 2.
Most Common In Utero ARV Regimens by Year of Birth
|
1995–1997 (n=67) |
1998–2002 (n=399) |
2003–2006 (n=487) |
2007–2009 (n=815) |
||||
|---|---|---|---|---|---|---|---|
| Regimen | n (%) | Regimen | n (%) | Regimen | n (%) | Regimen | n (%) |
| Showing constituent ARVs, ordered by frequency | |||||||
| ZDV alone | 50 (75%) | ZDV+3TC+NFV | 116 (29%) | ZDV+3TC+NFV | 158 (32%) | ZDV+3TC+LPV/r | 258 (32%) |
| ZDV+3TC | 6 (9.0%) | ZDV+3TC | 65 (16%) | ZDV+3TC+ABC | 64 (13%) | ZDV+3TC+ABC | 103 (13%) |
| ZDV+3TC+NVP | 43 (11%) | ZDV+3TC+LPV/r | 45 (9.2%) | ZDV+3TC+NFV | 77 (9.5%) | ||
| ZDV alone | 29 (7.3%) | ZDV+3TC+NVP | 45 (9.2%) | TDF+FTC+ATV/r | 67 (8.2%) | ||
| 3 other regimensa | 4 (6.0%) | 42 other regimensa | 125 (31%) | 81 other regimensa | 160 (33%) | 90 other regimensa | 293 (36%) |
| No in utero ARV exposure | 7 (10%) | No in utero ARV exposure | 21 (5.3%) | No in utero ARV exposure | 15 (3.1%) | No in utero ARV exposure | 17 (2.1%) |
| Summarized by constituent classes | |||||||
| No in utero ARV exposure | 7 (10%) | 21 (5.3%) | 15 (3.1%) | 17 (2.1%) | |||
| 1 NRTI | 50 (75%) | 29 (7.3%) | 2 (0.4%) | 3 (0.4%) | |||
| 2 NRTI | 6 (9.0%) | 69 (17.3%) | 14 (2.9%) | 4 (0.5%) | |||
| 3 NRTI | 0 | 23 (5.8%) | 72 (15%) | 105 (13%) | |||
| 2 NRTI + 1 NNRTI | 0 | 51 (13%) | 55 (11%) | 36 (4%) | |||
| 2 NRTI + 1 PI | 2 (3.0%) | 180 (45%) | 266 (55%) | 577 (71%) | |||
| Other HAART-equivalent cARV | 1 (1.5%) | 16 (4%) | 54 (11%) | 65 (8%) | |||
| Other regimen | 1 (1.5%) | 10 (2.5%) | 9 (1.8%) | 8 (1%) | |||
Only regimens that represent no in utero ARV exposure or regimens with more than 5% of children exposed are shown.
ZDV, zidovudine; 3TC, lamivudine; NFV, nelfinavir; NVP, nevaripine; ABC, abacavir; LPV/r, lopinavir/ritonavir; TDF, tenofovir disproxil fumarate; FTC, emtricitabine; ARV, antiretroviral; HAART, highly active antiretroviral therapy.
Characteristics associated with cARV and HAART-equivalent cARV exposure are shown in Table 3. A multivariate model was not constructed to model cARV exposure because only a small number of children (n=46) were unexposed to cARVs in utero. The first maternal CD4 and viral load were both significantly associated with both overall cARV and HAART-equivalent cARV exposure. Women without CD4 and viral load measurements during pregnancy were least likely to have received cARV or HAART-equivalent cARVs. Women with CD4 counts above 350 cells/mm3 had significantly lower odds of HAART-equivalent cARV use than those with less than 200 cells/mm3 (adjusted odds ratio [aOR]=0.33 [95% confidence interval {CI}: 0.13, 0.85] and aOR=0.24 [95% CI: 0.10, 0.61], respectively, for 350–500 and >500 versus <200 cells/mm3). Similarly, women with first maternal viral load during pregnancy above 10,000 copies per milliliter had significantly higher odds of HAART-equivalent cARV use (aOR=2.36 (95% CI: 1.39, 4.01) and aOR=13.4 (95% CI: 1.75, 102), respectively, for 10,000 to <100,000 and >100,000 versus <1000 copies per milliliter, respectively). The trimester of the first CD4 or viral load measurement during pregnancy was also associated with cARV and HAART-equivalent cARV use; earlier trimesters of the first available measurement were associated with higher odds of exposure.
Table 3.
Predictors of cARV and HAART-Equivalent cARV Exposure: Analysis Restricted to Children Born After 2002
| |
|
Exposed in utero to cARV |
Exposed in utero to HAART-equivalent cARV |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Total (n=1302) | n (%) | Crude OR | Crude p value | n (%) | Crude OR | Crude p value | Adjusted OR (n=1271) | Adjusted p value |
| Year of birth | |||||||||
| 2003–2006 | 487 | 462 (94.9%) | 1.00 (reference) | 0.02 | 398 (81.7%) | 1.00 (reference) | 0.10 | ||
| 2007–2009 | 815 | 794 (97.4%) | 2.05 (1.13–3.70) | 694 (85.2%) | 1.28 (0.95–1.73) | ||||
| Gender | |||||||||
| Male | 663 | 644 (97.1%) | 1.00 (reference) | 0.19 | 566 (85.4%) | 1.00 (reference) | 0.13 | ||
| Female | 639 | 612 (95.8%) | 0.67 (0.37–1.22) | 526 (82.3%) | 0.80 (0.59–1.07) | ||||
| Race/ethnicity (4 missing) | |||||||||
| Black/African American | 840 | 807 (96.1%) | 1.00 (reference) | 0.52 | 691 (82.3%) | 1.00 (reference) | 0.07 | ||
| Hispanic/Latino | 395 | 383 (97.0%) | 1.31 (0.67–2.56) | 345 (87.3%) | 1.49 (1.05–2.10) | ||||
| White/other | 63 | 62 (98.4%) | 2.53 (0.34–18.8) | 54 (85.7%) | 1.29 (0.63–2.68) | ||||
| First maternal HIV viral load during pregnancy (copies/mL) (20 missing) | |||||||||
| <1000 | 180 | 163 (90.6%) | 1.00 (reference) | <0.001 | 127 (70.6%) | 1.00 (reference) | <0.001 | 1.00 (reference) | <0.001 |
| 1000–9999 | 252 | 246 (97.6%) | 2.19 (1.20–4.02) | 182 (72.2%) | 1.09 (0.71–1.66) | 0.92 (0.59–1.44) | |||
| 10,000–99,999 | 244 | 241 (98.8%) | 5.54 (2.52–13.5) | 214 (87.7%) | 2.98 (1.81–4.90) | 2.36 (1.39–4.01) | |||
| ≥100,000 | 57 | 57 (100%) | 18.8 (3.29–inf) | 56 (98.2%) | 23.4 (3.15–173) | 13.4 (1.75–102) | |||
| No measurement | 35 | 23 (65.7%) | 0.16 (0.09–0.29) | 21 (60.0%) | 0.63 (0.30–1.32) | 0.78 (0.35–1.73) | |||
| First viral load after ARV initiation | 514 | 509 (99.0%) | 3.41 (1.97–5.91) | 481 (93.6%) | 6.08 (3.78–9.80) | 7.80 (4.71–12.9) | |||
| First maternal CD4 count during pregnancy (cells/mm3) (20 missing) | |||||||||
| 0–199 | 91 | 87 (95.6%) | 1.00 (reference) | <0.001 | 85 (93.4%) | 1.00 (reference) | <0.001 | 1.00 (reference) | <0.001 |
| 200–349 | 215 | 211 (98.1%) | 2.43 (0.59–9.92) | 181 (84.2%) | 0.38 (0.15–0.93) | 0.42 (0.17–1.08) | |||
| 350–500 | 183 | 177 (96.7%) | 1.36 (0.37–4.93) | 142 (77.6%) | 0.24 (0.10–0.60) | 0.33 (0.13–0.85) | |||
| ≥501 | 244 | 232 (95.1%) | 0.89 (0.28–2.83) | 171 (70.1%) | 0.17 (0.07–0.40) | 0.24 (0.10–0.61) | |||
| No measurement | 35 | 23 (65.7%) | 0.09 (0.03–0.30) | 21 (60.0%) | 0.11 (0.04–0.31) | 0.01 (0.00–0.13) | |||
| First CD4 count after ARV initiation | 514 | 509 (99.0%) | 4.68 (1.23–17.8) | 481 (93.6%) | 1.03 (0.42–2.53) | 0.14 (0.02–1.20) | |||
| Time of first maternal viral load/CD4 during pregnancy (12 missing) | |||||||||
| Trimester 3 | 86 | 75 (87.2%) | 1.00 (reference) | <0.001 | 62 (72.1%) | 1.00 (reference) | <0.001 | 1.00 (reference) | <0.001 |
| Trimester 2 | 437 | 431 (98.6%) | 10.5 (3.78–29.4) | 369 (84.4%) | 2.10 (1.23–3.60) | 2.64 (1.43–4.85) | |||
| Trimester 1 | 732 | 716 (97.8%) | 6.57 (2.94–14.7) | 631 (86.2%) | 2.42 (1.44–4.05) | 3.10 (1.72–5.59) | |||
| No measurement | 35 | 23 (65.7%) | 0.28 (0.11–0.72) | 21 (60.0%) | 0.58 (0.25–1.32) | 0.26 (0.11–0.65) | |||
| Substance use during pregnancy (81 missing) | |||||||||
| Tobacco | 219 | 209 (95.4%) | 0.65 (0.31–1.34) | 0.24 | 186 (84.9%) | 1.09 (0.72–1.63) | 0.69 | ||
| Alcohol | 103 | 99 (96.1%) | 0.82 (0.29–2.36) | 0.72 | 83 (80.6%) | 0.77 (0.46–1.29) | 0.32 | ||
| Marijuana | 80 | 76 (95.0%) | 0.62 (0.21–1.78) | 0.37 | 68 (85.0%) | 1.08 (0.57–2.04) | 0.81 | ||
| Other substance usea | 125 | 121 (96.8%) | 1.03 (0.36–2.93) | 0.96 | 108 (86.4%) | 1.23 (0.72–2.11) | 0.45 | ||
Other substance use includes pain medications (n=50), antidepressants (n=31), cocaine/crack (n=31), methadone (n=12), benzodiazepines/tranquilizers (n=5), barbiturates/sedatives (n=4), methamphetamines (n=2), ecstasy/MDMA (n=2), heroin (n=4), opium (n=1), PCP (n=2), LSD (n=1), other/unknown (n=4). No stimulant, inhalant, ketamine, or lysergic acid diethylamide use was reported.
Race/ethnicity and maternal substance use during pregnancy were not significantly associated with maternal cARV or HAART exposure in either the crude or adjusted models.
Data regarding receipt of ARVs during the first 2 months of life were available on 1422 children. Nine (0.6%) had no reported receipt of ARVs during this period. The remaining 1413 (99.4%) received ZDV (median duration [IQR]: 43 days [43, 45]); 1265 (89%) received no other ARVs, while 148 (10%) received ZDV in combination with at least one other ARV (6.9% NVP, 3.4% 3TC). Among the 98 infants who received NVP, 63 received only a single dose. Sixty-four (4.5%) children received ZDV and another ARV that was not NVP. Forty-eight children (3.3%) received 3TC (median duration [IQR]: 36 days [25, 43]), and 27 children (1.9%) received another ARV.
Discussion
This study summarizes trends in in utero ARV exposure and infant prophylaxis among U.S. children over the last 15 years. We observed a temporal trend in changes in ARV exposure over time, with HAART-equivalent cARVs becoming the most common regimens in 2000. Despite the general trend, use of other non-HAART regimens remained above 10% for all years since 2001 except for 2009.
Because we describe the change in in utero ARV exposure over time, we are able to relate the variation in clinical practice over our study period with changes in the U.S. perinatal guidelines.2 We found that many of the observed changes in clinical practice reflect changes in these guidelines. Among PIs, we observed a transition from NFV-based therapy to LPV/r based therapy. LPV/r became the most common protease inhibitor in 2007 after it was one of two recommended protease inhibitors with NFV in the October 2006 guidelines.11 The transition from NFV to LPV/r was possibly hastened by a temporary safety alert that recommended that pregnant women starting ARVs should not be offered NFV-containing regimens due to the presence of ethyl methanesulfonate, a teratogenic process-related impurity.12 We observed a decline in NNRTI use from 33% in 2003 to 11% in 2009, primarily due to a decrease in NVP use. This decline is likely due to concerns about severe hepatoxicity and rash for women with CD4 counts greater than 250 cells/mm3 as well as concerns about the risk of NVP resistance.2
We observed limited EFV use in our population even though it is classified as a FDA Pregnancy Category D drug with the risk of teratogenicity. Its use is contraindicated during the first trimester of pregnancy.2 EFV was the only ARV in this study with a greater use in the first trimester than in the second or third trimester, consistent with the practice that clinicians replace EFV-containing regimens with other regimens after pregnancy is identified. Further analyses are planned to investigate the relationship between in utero exposure to EFV and other ARVs with congenital anomalies in the SMARTT cohort.
ZDV and 3TC were the most common NRTIs used each year, consistent with the U.S. guidelines.2 However, we observed a steady increase in the number of children exposed to TDF and FTC with a corresponding decline in ZDV and 3TC exposure since 2005. The combination of TDF+FTC represents a first-line NRTI backbone in non-pregnant adults because of its efficacy, tolerability, and simplicity of dosing, and because both TDF and FTC are FDA Pregnancy Category B drugs, while ZDV and 3TC are both Category C.13,14 Despite these potential advantages, current U.S. guidelines recommend the use of TDF+FTC in pregnant women only after careful consideration of the alternatives2 due to decreased fetal growth and reduction in bone porosity in animal studies15,16 as well as bone-demineralization in HIV-infected children on chronic TDF-based therapy.17 Given the increasing proportion of children exposed in utero to TDF in recent years, further study is needed to clarify the risks and benefits of this exposure.
Our observed changes in ARV use in the U.S. are similar to those observed in other high-resource countries. Analysis of data through 2008 from an Italian cohort also revealed a change from NFV to LPV/r as the most common PI, increasing TDF+FTC use, and a 40% decline in NVP use.3 A small Danish study reported that among thirteen pregnant women, none received HAART-equivalent cARV without a PI in the first half of 2008, compared to 6 of 34 (18%) in 2007, suggesting a decline in NVP use.4
Although use of cARVs during pregnancy increased during the study period, use of such regimens was not universal, even during later years. Those women least likely to have used such regimens were those without CD4 or viral load measurements during pregnancy, possibly because they were not identified as HIV positive or were not in prenatal care. Women with higher viral loads and lower CD4 counts were more likely to use cARVs. This is expected since these are two of the characteristics that determine whether ARVs should be used for treatment or prophylaxis during pregnancy. Current U.S. guidelines recommend that cARVs containing at least three drugs should be offered to women during pregnancy, although a regimen of ZDV alone, while controversial, might be appropriate for women with viral load less than 1000 copies per milliliter.2
Almost all of the children (>99%) in our population received ARVs during the first 2 months of life and 85% received at least 6 weeks of prophylaxis. This near-universal receipt of ARV prophylaxis is in accordance with the U.S. guidelines and contrasts with the findings of the European Collaborative Study that reported that 40% of their study population born between 2004 and 2007 in Western Europe received no such prophylaxis in spite of European guidelines.18
In addition to evaluating individual ARV drugs, we also explored the proportion of children exposed to ARV regimens and found that while 62% of children born since 2007 were exposed to the four most common regimens during pregnancy, the remaining 38% were exposed to 91 different regimens. This regimen diversity complicates the conduct of observational studies of the effects of in utero ARV exposure as such studies often do not have the power to examine the effects of less common regimens.
Our study has certain limitations. Although the clinics participating in our study are from a number of U.S. states and Puerto Rico, they are likely to be located at urban research institutions and the prescribing patterns at these clinics may differ from others in the United States. In addition, older children in our study must have been uninfected when the study began in 2007. Therefore, selection bias likely understates the proportion of pregnant women who used less effective ARV regimens before 2007.
In conclusion, use of cARVs, including HAART-equivalent cARVs, among women in the U.S. is increasing, but not universal. A large number of regimens are used during pregnancy and the frequency of in utero exposure has changed over time—two trends that will likely continue as new agents are introduced. Therefore, long-term follow-up of already exposed infants and children as well as continued study of prospective cohorts of newborns is essential to identify any long-term effects of in utero and postnatal ARV exposure.
Acknowledgments
We thank the children and families for their participation in the PHACS protocol “Surveillance Monitoring for ART Toxicities” (SMARTT), and the individuals and institutions involved in the conduct of PHACS SMARTT. The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute of Allergy and Infectious Diseases, the National Institute on Drug Abuse, the National Institute of Mental Health, National Institute of Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, National Institute of Neurological Disorders and Stroke, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard University School of Public Health (U01 HD052102-04) (Principal Investigator: George R. Seage III; Project Director: Julie Alperen) and the Tulane University School of Medicine (U01 HD052104-01) (Principal Investigator: Russell Van Dyke; Co-Principal Investigator: Kenneth Rich; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (PI: Mercy Swatson). The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or U.S. Department of Health and Human Services.
The following institutions, clinical site investigators and staff participated in conducting PHACS SMARTT in 2009, in alphabetical order: Baylor College of Medicine: William Shearer, Norma Cooper, Lynette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Emma Stuard, Anna Cintron; Children's Diagnostic & Treatment Center: Ana Puga, Dia Cooley, Doyle Patton; Children's Hospital of Philadelphia: Richard Rutstein, Carol Vincent, Nancy Silverman; Children's Memorial Hospital: Ram Yogev, Kathleen Malee, Scott Hunter, Eric Cagwin; Jacobi Medical Center: Andrew Wiznia, Marlene Burey, Molly Nozyce; New York University School of Medicine: William Borkowsky, Sandra Deygoo, Helen Rozelman; St. Jude Children's Research Hospital: Katherine Knapp, Kim Allison, Patricia Garvie; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Lourdes Angeli-Nieves, Vivian Olivera; SUNY Downstate Medical Center: Hermann Mendez, Ava Dennie, Susan Bewley; SUNY Stony Brook: Sharon Nachman, Margaret Oliver, Helen Rozelman; Tulane University Health Sciences Center: Russell Van Dyke, Karen Craig, Patricia Sirois; University of Alabama, Birmingham: Marilyn Crain, Newana Beatty, Dan Marullo; University of California, San Diego: Stephen Spector, Jean Manning, Sharon Nichols; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Emily Barr, Robin McEvoy; University of Florida/Jacksonville: Mobeen Rathore, Kathleen Thoma, Ann Usitalo; University of Illinois, Chicago: Kenneth Rich, Delmyra Turpin, Renee Smith; University of Maryland, Baltimore: Douglas Watson, LaToya Stubbs, Rose Belanger; University of Medicine and Dentistry of New Jersey: Arry Dieudonne, Linda Bettica, Susan Adubato; University of Miami: Gwendolyn Scott, Erika Lopez, Elizabeth Willen; University of Southern California: Toinette Frederick, Mariam Davtyan, Maribel Mejia; University of Puerto Rico Medical Center: Zoe Rodriguez, Ibet Heyer, Nydia Scalley Trifilio.
The author contributions are as follows: R.G.: design of this analysis, data analysis and interpretation, statistical analysis, drafting the manuscript. P.L.W.: design of parent study, design of this analysis, data analysis and interpretation, manuscript revisions. J.S.R.: data analysis and interpretation, manuscript revisions. G.R.S.: design of parent study, data analysis and interpretation, manuscript revisions. M.C. and R.Y.: manuscript revisions. R.H.: design of parent study, design of this analysis, data analysis and interpretation, manuscript revisions. K.R.: design of parent study, manuscript revisions.
Data presented previously at the 17th Conference on Retroviruses and Opportunistic Infections (February 16–19, 2010, San Francisco, California).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Whitmore S. Zhang X. Taylor A. Estimated number of births to HIV-positive women in the United States, 2006 [Abstract 924]. 16th Conference on Retroviruses and Opportunistic Infections; Montreal. Feb 8–11;2009 . [Google Scholar]
- 2.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. May 24, 2010. http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf. [Apr 13;2011 ]. pp. 1–117.http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf
- 3.Baroncelli S. Tamburrini E. Ravizza M, et al. Antiretroviral treatment in pregnancy: A six-year perspective on recent trends in prescription patterns, viral load suppression, and pregnancy outcomes. AIDS Patient Care STDs. 2009;23:513–520. doi: 10.1089/apc.2008.0263. [DOI] [PubMed] [Google Scholar]
- 4.von Linstow ML. Rosenfeldt V. Lebech AM, et al. Prevention of mother-to-child transmission of HIV in Denmark, 1994–2008. HIV Med. 2010;11:448–456. doi: 10.1111/j.1468-1293.2009.00811.x. [DOI] [PubMed] [Google Scholar]
- 5.Townsend CL. Cortina-Borja M. Peckham CS. Tookey PA. Trends in management and outcome of pregnancies in HIV-infected women in the UK and Ireland, 1990–2006. BJOG. 2008;115:1078–1086. doi: 10.1111/j.1471-0528.2008.01706.x. [DOI] [PubMed] [Google Scholar]
- 6.Cooper ER. Charurat M. Mofenson L, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1–infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29:484–494. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 7.Brogly SB. Ylitalo N. Mofenson LM, et al. In utero nucleoside reverse transcriptase inhibitor exposure and signs of possible mitochondrial dysfunction in HIV-uninfected children. AIDS. 2007;21:929–938. doi: 10.1097/QAD.0b013e3280d5a786. [DOI] [PubMed] [Google Scholar]
- 8.Patel K. Shapiro DE. Brogly SB, et al. Prenatal protease inhibitor use and risk of preterm birth among HIV-infected women initiating antiretroviral drugs during pregnancy. J Infect Dis. 2010;201:1035–1044. doi: 10.1086/651232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brogly SB. Abzug MJ. Watts DH, et al. Birth defects among children born to human immunodeficiency virus-infected women: Pediatric AIDS clinical trials protocols 219 and 219C. Pediatr Infect Dis J. 2010;29:721–727. doi: 10.1097/INF.0b013e3181e74a2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tassiopoulos K. Read JS. Brogly S, et al. Substance use in HIV-Infected women during pregnancy: Self-report versus meconium analysis. AIDS Behav. 2010;14:1269–1278. doi: 10.1007/s10461-010-9705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. Oct 12, 2006. http://aidsinfo.nih.gov/ContentFiles/PerinatalGL000616.pdf. [Apr 13;2011 ]. p. 43.http://aidsinfo.nih.gov/ContentFiles/PerinatalGL000616.pdf
- 12.[Healthcare Professional Letter] New York, NY: Pfizer Inc.; Sep 10, 2007. [Apr 13;2011 ]. VIRACEPT® (nelfinavir mesylate) 250 mg, 625 mg tablets, powder for oral suspension. [Google Scholar]
- 13.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1–infected adults and adolescents. Department of Health and Human Services. Dec 1, 2009. www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL001419.pdf. [Apr 13;2011 ]. pp. 1–161.www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL001419.pdf
- 14.Foster C. Lyall H. Olmscheid B. Pearce G. Zhang S. Gibb DM. Tenofovir disoproxil fumarate in pregnancy and prevention of mother-to-child transmission of HIV-1: Is it time to move on from zidovudine? HIV Med. 2009;10:397–406. doi: 10.1111/j.1468-1293.2009.00709.x. [DOI] [PubMed] [Google Scholar]
- 15.Tarantal AF. Marthas ML. Shaw JP. Cundy K. Bischofberger N. Administration of 9-[2-(R)-(phosphonomethoxy)propyl]adenine (PMPA) to gravid and infant rhesus macaques (Macaca mulatta): Safety and efficacy studies. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:323–333. doi: 10.1097/00042560-199904010-00001. [DOI] [PubMed] [Google Scholar]
- 16.Tarantal AF. Castillo A. Ekert JE. Bischofberger N. Martin RB. Fetal and maternal outcome after administration of tenofovir to gravid rhesus monkeys (Macaca mulatta) J Acquir Immune Defic Syndr. 2002;29:207–220. doi: 10.1097/00042560-200203010-00001. [DOI] [PubMed] [Google Scholar]
- 17.Gafni RI. Hazra R. Reynolds JC, et al. Tenofovir disoproxil fumarate and an optimized background regimen of antiretroviral agents as salvage therapy: Impact on bone mineral density in HIV-infected children. Pediatrics. 2006;118:e711–718. doi: 10.1542/peds.2005-2525. [DOI] [PubMed] [Google Scholar]
- 18.England K. Thorne C European Collaborative Study. Use of neonatal antiretroviral prophylaxis for prevention of mother-to-child transmission of HIV is decreasing in Western Europe. Clin Infect Dis. 2009;48:1797–1800. doi: 10.1086/599230. [DOI] [PMC free article] [PubMed] [Google Scholar]