Abstract
Polycystic ovary syndrome (PCOS) is a common disorder characterized by hyperandrogenism and disordered gonadotropin secretion, often associated with insulin resistance. The syndrome, which modulates both hormonal and metabolic processes, is the most common endocrinopathy in reproductive-age women and increases a woman's risk of infertility, endometrial pathology, and cardiometabolic disease. As it is currently defined, PCOS most likely encompasses several distinct diseases with similar clinical phenotypes but different underlying pathophysiological processes. However, hyperandrogenism remains the syndrome's clinical hallmark. The clinical manifestations of PCOS often emerge during childhood or in the peripubertal years, suggesting that the syndrome is influenced by fetal programming and/or early postnatal events. However, given that the full clinical spectrum of PCOS does not typically appear until puberty, a “two-hit” hypothesis has been proposed: (1) a girl develops hyperandrogenism via one or more of many different potential mechanisms; (2) the preexisting hyperandrogenism subsequently disturbs the hypothalamic–pituitary–ovarian axis, resulting in ovulatory dysfunction and sustained hyperandrogenism. No consensus guidelines exist regarding the diagnosis and management of PCOS in the pediatric population; however, because the syndrome is a diagnosis of exclusion, the clinical evaluation of girls suspected of having PCOS is aimed at excluding other causes of androgen excess and menstrual dysfunction. For the syndrome's management, emphasis is placed on lifestyle and symptom-directed treatment.
Introduction
Polycystic ovary syndrome (PCOS) is a common endocrinopathy affecting an estimated 5–10% of reproductive-age women in the Unites States.1–4 Furthermore, the syndrome increases a woman's risk of infertility, dysfunctional uterine bleeding, endometrial carcinoma, depression, type 2 diabetes, hypertension, dyslipidemia, and metabolic syndrome, independent of obesity or insulin resistance.1–8 In the United States alone, it is also associated with an economic burden exceeding four billion dollars.9
PCOS is primarily characterized by: (1) Menstrual dysfunction (oligo- or amenorrhea), (2) cutaneous signs of hyperandrogenism (acne, hirsutism, or alopecia), (3) obesity, (4) disordered gonadotropin [luteinizing hormone (LH) and follicle-stimulating hormone (FSH)] secretion, and (5) polycystic ovaries by ultrasonography.10–14 However, the syndrome is also associated with defects in insulin action (insulin resistance) and/or insulin secretion (pancreatic β-cell dysfunction). Although the clinical and biochemical presentation of PCOS is heterogeneous, hyperandrogenemia is the most consistent biochemical abnormality, and thus is considered the hallmark of the syndrome.15 Furthermore, although various signs and symptoms of hyperandrogenism can manifest prepubertally, the onset of menstrual dysfunction in PCOS typically occurs peripubertally. The syndrome has also been associated with the childhood antecedents of reduced fetal growth, followed by excessive postnatal catch up and premature adrenarche/pubarche,16,17 suggesting a developmental aspect to its etiology. Moreover, being overweight or obese, a common problem in the pediatric and adult populations, amplifies the clinical severity of the syndrome and increases the risk of metabolic dysfunction.4
Definitions
The diagnosis of PCOS remains controversial and is based on various signs, symptoms, and/or laboratory findings that are not universally accepted. The four most common definitions of the syndrome are presented in Table 1. The 1990 National Institutes of Health (NIH) definition requires the simultaneous presence of hyperandrogenism (clinical and/or biochemical) and menstrual dysfunction in the absence of other causes,18 highlighting the importance of hyperandrogenism in the syndrome's etiology. In contrast, the 2003 Rotterdam [European Society for Human Reproduction and Embryology and American Society for Reproductive Medicine (ESHRE/ASRM)] definition requires only two of the following three criteria: (1) Hyperandrogenism (clinical and/or biochemical), (2) ovulatory dysfunction (oligo- or anovulation), and (3) ultrasonographic evidence of polycystic ovaries in the absence of other causes.19 Importantly, the Rotterdam criteria broadened the PCOS phenotype to include women with ovulatory dysfunction and polycystic ovaries but without hyperandrogenism, and eumenorrheic women with hyperandrogenism and polycystic ovaries (often called “ovulatory” PCOS).20 However, the 2006 Androgen Excess Society (AES) definition reemphasized the importance of hyperandrogenism in the etiology of PCOS, requiring: (1) The absence of other hyperandrogen-causing disorders, syndromes of severe insulin resistance, thyroid dysfunction, and hyperprolactinemia, (2) hyperandrogenism (clinical and/or biochemical), and (3) ovulatory dysfunction (oligo- or anovulation) or polycystic ovarian morphology.15 The 2009 Androgen Excess and Polycystic Ovary Syndrome Society's definition also emphasized the importance of hyperandrogenism in the syndrome's etiology, requiring: (1) Hyperandrogenism (clinical and/or biochemical), (2) ovarian dysfunction (oligo- or anovulation and/or polycystic ovaries), and (3) the exclusion of other androgen excess or related disorders.21
Table 1.
Commonly Used Definitions of Polycystic Ovary Disease
| Definition/year | Diagnostic criteriaa |
|---|---|
| NIH/1990 | Requires the simultaneous presence of: |
| 1. Hyperandrogenism (clinical and/or biochemical) | |
| 2. Ovarian dysfunction | |
| Rotterdam (ESHRE/ASRM)/2003 | Requires the presence of at least two criteria: |
| 1. Hyperandrogenism (clinical and/or biochemical) | |
| 2. Ovulatory dysfunction | |
| 3. Polycystic ovarian morphologyb | |
| AES/2006 | Requires the presence of hyperandrogenism (clinical and/or biochemical) and either: |
| 1. Ovulatory dysfunction | |
| 2. Polycystic ovarian morphologyb | |
| Androgen Excess and PCOS Society/2009 | Requires the simultaneous presence of: |
| 1. Hyperandrogenism (clinical and/or biochemical) | |
| 2. Ovarian dysfunction (ovulatory dysfunction and/or polycystic ovarian morphologyb) |
All of the diagnostic criteria for PCOS require the exclusion of other disorders such as nonclassical congenital adrenal hyperplasia, Cushing syndrome, hyperprolactinemia, hypothyroidism, acromegaly, premature ovarian failure, a virilizing adrenal or ovarian neoplasm, or a drug-related condition.
The ultrasound definition of polycystic ovarian morphology is the presence of ≥12 follciles with a 2- to 9-mm diameter on the ovary. An ovarian volume >10 mL is also suggestive. Only one ovary consistent with polycystic ovarian morphology is sufficient for the diagnosis.
Abbreviations: NIH, National Institutes of Health; ESHRE, European Society for Human Reproduction and Embryology; ASRM, American Society for Reproductive Medicine; AES, Androgen Excess Society.
However, diagnosing PCOS in adolescents using the above criteria poses several challenges. First, using menstrual irregularity to diagnose PCOS is difficult in adolescents, given that greater than 50% of menstrual cycles are anovulatory in the first 2 years after menarche.22,23 However, menstrual irregularity for more than 2 years after menarche is not considered physiological and is predictive of continued irregularity.24 Second, nonpathologic acne and mild hirsutism are common in the peripubertal years.25,26 Third, children develop physiologic insulin resistance during puberty.27–29 Fourth, limited normative data of androgen levels by body mass index (BMI) and pubertal stage exist.19 Fifth, ovarian size appears to be maximal in the perimenarchal period; ≈25% of adolescent girls have multifollicular ovaries, and polycystic-type ovaries can occur in up to 20–30% of reproductive-age women and 10% of healthy, regularly menstruating girls,30–32 making the differentiation of “normal” versus “abnormal” ovaries difficult for even experienced specialists.33 Moreover, a transvaginal ultrasound is often inappropriate for pediatric patients, particularly virginal girls, and the use of a transabdominal ultrasound yields limited resolution of ovarian morphology and has been shown to underestimate the presence of the syndrome.34
Thus, in an attempt to overcome these limitations, an alternative method for diagnosing the syndrome in adolescents has been advocated to avoid mislabeling an adolescent girl with transitional functional hyperandrogenism and menstrual irregularity as having PCOS.35 According to this proposal, four out of the following five criteria would be required for a PCOS diagnosis in adolescents: (1) Oligo- or amenorrhea 2 years after menarche, (2) clinical hyperandrogenism (hirsutism, acne, and/or alopecia), (3) biologic hyperandrogenism (an elevated testosterone concentration), (4) insulin resistance or hyperinsulinemia (acanthosis nigricans, abdominal obesity, and/or glucose intolerance), and (5) polycystic ovaries. However, the criteria used to diagnose PCOS in clinical studies are currently the same for all females, limiting the ability to study the syndrome's incidence and prevalence in the pediatric population.
Pathogenesis
PCOS is a complex multifactorial disorder influenced by the synergistic impact of environmental factors on a predisposed genetic background, which modulates both hormonal and metabolic processes.3,36–38 Moreover, several lines of evidence suggest a developmental origin of the syndrome.39,40 In particular, studies from nonhuman primates have shown that prenatal exposure to androgen excess in utero leads to the development of the human PCOS phenotype in adult monkeys,41–45 reinforcing the fetal origins of adult disease hypothesis (i.e., the Barker hypothesis).46,47
Androgen sources
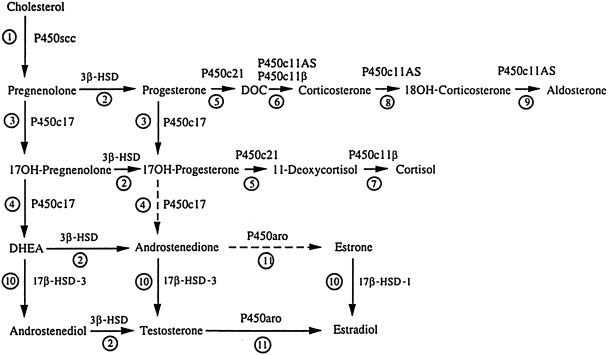
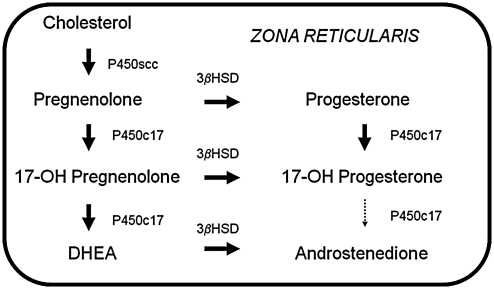
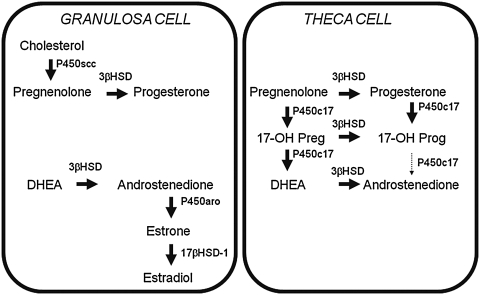
The production of all steroid hormones, including androgens, begins with cholesterol. It is then the tissue specificity of the various steroidogenic enzymes and the availability of their substrates/cofactors that determine the type of steroid produced by a particular gland.48 Although no gland expresses every steroidogenic enzyme, their interrelationships are demonstrated in the integrated pathway shown in Fig. 1. The major enzymes involved in adrenal and ovarian androgen production are shown in Figs. 2 and 3, respectively. In the past, the source of hyperandrogenemia in women with PCOS had been a topic of debate. However, the observation that hyperandrogenemia persists when ovarian steroidogenesis is suppressed with a long-acting gonadotropin-releasing hormone (GnRH) agonist49,50 and when adrenal steroidogenesis is suppressed with dexamethasone51,52 suggests that both glands play a role.
FIG. 1.
Integrated view of human steroidogenesis, showing adrenal and gonadal pathways. Reaction 1: P450scc converts cholesterol to pregnenolone. Reaction 2: 3β-hydroxysteroid dehydrogenase (3β-HSD) converts Δ5 steroids [pregnenolone, 17OH-pregnenolone, dehydroepiandrosterone (DHEA), androstenediol] to the corresponding Δ4 steroids (progesterone, androstenedione, testosterone). Reaction 3: P450c17 catalyzes the 17α-hydroxylation of pregnenolone and progesterone. Reaction 4: The 17,20-lyase activity of P450c17 converts 17OH-pregnenolone to DHEA; the conversion of 17OH-progesterone to androstenedione occurs in cattle and rodents, but human P450c17 cannot catalyze this reaction efficiently. Reaction 5: P450c21 catalyzes the 21-hydroxylation of progesterone and 17OH-progesterone. Reaction 6: Deoxycorticosterone (DOC) can be converted to corticosterone by either P450c11AS (in the adrenal zona glomerulosa) or P450c11β (in the adrenal zona fasciculata). Reaction 7: P450c11β converts 11-deoxycortisol to cortisol. Reactions 8 and 9: P450c11AS catalyzes 18 hydroxylase (reaction 8) and 18 methyl oxidase activities (reaction 9) to produce aldosterone in the adrenal zona glomerulosa. Reaction 10: Two isozymes of 17βHSD activate sex steroids: 17β-HSD1 produces estradiol and 17β-HSD3 produces androgens. In peripheral tissues 17β-HSD5 has similar activity to 17β-HSD3, and 17β-HSD2 and 4 catalyze the “reverse” reactions to inactivate sex steroids. Reaction 11: P450aro aromatizes C19 androgenic steroids to C18 estrogens.
FIG. 2.
Adrenal sex steroid synthesis. Sex steroid synthesis in the adrenal gland occurs in the zona reticularis in the cortex of the adrenal gland. Dehydroepiandrosterone (DHEA) and androstenedione are the principal androgen precursors produced in the adrenal gland. 3βHSD, 3β-hydroxysteroid dehydrogenase.
FIG. 3.
Ovarian sex steroid synthesis. Sex steroid synthesis in the ovary occurs in both the granulosa and theca cells. However, P450c17, the “qualitative” regulator of steroidogenesis, is only expressed in the theca cell. Androstenedione is the principal androgen precursor produced in the ovary. Isozymes of 17β-hydroxsteroid dehyrogenase (17βHSD) can convert androstenedione to testosterone; alternatively, aromatase (P450aro) can convert androstenedione to estrogens. 17-OH Preg, 17-OH pregnenolone; 17-OH Prog, 17-OH progesterone.
In ovarian tissue from women with PCOS, in vitro studies have demonstrated overexpression of steroidogenic enzymes (in particular, P450c17 and 3β-hydroxysteroid dehydrogenase) in theca cells.53 The majority of hyperandrogenic women with PCOS also have abnormal responses to GnRH agonists.50,54 In addition, adrenal hyperresponsiveness to adrenocorticotropic hormone (ACTH) occurs in ≈25% of women with PCOS, resulting in excess dehydroepiandrosterone (DHEA), DHEA-sulfate (DHEA-S), and androstenedione.55,56 Interestingly, the adrenal glands may be an even more important source of hyperandrogenism in nonobese subjects.57 Furthermore, although the ovaries and adrenal glands are the principal sources of excess androgen production in women with PCOS, enhanced 5α-reductase activity in the liver and peripheral tissues (e.g., adipose tissue) may also increase conversion of testosterone to the biologically more potent androgen, dihydrotestosterone (DHT).58
Androgen production
Whereas the steroidogenic enzyme P450scc is the “quantitative regulator” of steroidogenesis, determining the net “capacity” of a steroidogenic cell, the “qualitative regulator” of steroidogeneisis, the factor that determines whether a steroid precursor will become a mineralocorticoid, a glucocorticoid, or a sex steroid, is the microsomal enzyme P450c17. P450c17 is expressed in both the adrenal glands and gonads59 and sequentially catalyzes both 17α-hydroxylase activity and 17,20-lyase activity on sex steroid hormone precursors (see Fig. 1).60–62 In the absence of P450c17, a steroidogenic cell produces C21 17-deoxysteroids (e.g., progesterone in the ovarian granulosa cell or aldosterone in the adrenal glomerulosa cell). If only the 17α-hydroxylase activity of P450c17 is present (e.g., in the adrenal zona fasiculata), C21 17-hydroxysteroids (e.g., cortisol) are produced. If both the 17α-hydroxylase and 17,20-lyase activities of P450c17 are present (e.g., in ovarian theca cells, testicular Leydig cells, or adrenal zona reticularis), C19 precursors of sex steroids (e.g., DHEA) are produced. A detailed discussion of sex steroid production is beyond the scope of this article, but has recently been reviewed elsewhere.63,64
The ratio of P450c17's 17α-hydroxylase to 17,20-lyase activity determines the ratio of C21 to C19 steroids generated, varies in different cell types, and can be developmentally regulated (e.g., during human adrenarche). Specifically, regulation of P450c17's enzymatic activity is mediated posttranslationally by at least three factors: (1) The electron-donating protein P450 oxidoreductase (POR), (2) cytochrome b5, and (3) serine phosphorylation.65 Importantly, increased activity of P450c17 has been specifically implicated in the etiology of PCOS,66,67 and identifying the molecular factors regulating this enzyme is an area of active investigation.
Neuroendocrine abnormalities
The most common neuroendocrine aberration observed in women with PCOS is an alteration in their GnRH pulse frequency.68,69 As opposed to the cyclic variation seen with regular, ovulatory menstrual cycles, the GnRH pulse frequency in women with PCOS is ≈1 pulse/h.70 This rapid GnRH pulse frequency favors pituitary LH secretion over FSH secretion,71–73 resulting in elevated LH levels and LH:FSH ratios.74 The high LH concentrations then stimulate ovarian theca cells to produce androgens, whereas the “relative” FSH deficiency impairs aromatization of the androgens to estrogens in the granulosa cells, follicular development/maturation, and luteal progesterone release, leading to both sustained hyperandrogenism and ovulatory dysfunction.
Given that the observed GnRH pulse frequency of ≈1 pulse/h in women with PCOS is both comparable to the maximal GnRH pulse frequency that occurs during a normal, ovulatory menstrual cycle in the late follicular phase75 and similar to the GnRH pulse frequency that occurs in isolated hypothalamic GnRH neurons76 and hypogonadal women,77,78 the persistently rapid GnRH pulse frequency in PCOS is considered the result of impaired ovarian hormone feedback as opposed to an inherent acceleration of the GnRH pulse generator. Of the ovarian sex steroids, progesterone appears to be the primary modulator of GnRH pulse frequency,79 although estradiol also probably plays a permissive role by inducing the expression of progesterone receptors in the hypothalamus.80 Evidence to support the importance of progesterone in the regulation of the GnRH pulse generator stems from the observations that GnRH pulse frequency decreases during the endogenous luteal phase rise in progesterone during normal, ovulatory cycles,75 and exogenous progesterone slows GnRH pulse frequency in both ovulatory and postmenopausal women.78,81
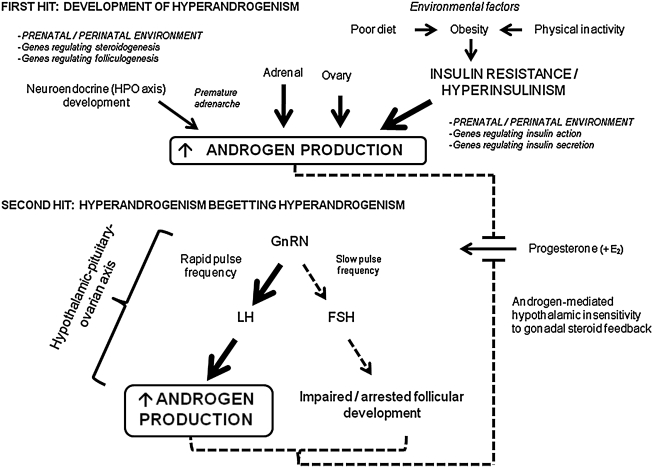
However, in women with PCOS, the importance of progesterone in the regulation of the GnRH pulse generator poses two potential issues. First, endogenous progesterone secretion is limited due to frequent anovulatory cycles. Second, the sensitivity of the hypothalamus to progesterone is impaired by androgens.82 This then creates a cycle whereby preexisting hyperandrogenism leads to further hyperandrogenism by impairing the sensitivity of the GnRH pulse generator to progesterone, leading to increased LH secretion from the pituitary, stimulating further ovarian androgen production (see Fig. 4).
FIG. 4.
The “two-hit” hypothesis of PCOS. The “two-hit” hypothesis of polycystic ovary syndrome (PCOS) suggests that two insults are required for the syndrome's full phenotypic expression. For the first “hit,” one or more of a number of different mechanisms, including: (1) Primary adrenal, ovarian, and/or neuroendocrine abnormalities; (2) insulin resistance and hyperinsulinemia; and/or (3) prenatal, immediate postnatal, and/or peripubertal androgen exposure, lead to increased androgen production. For the second “hit,” the preexisting hyperandrogenism reduces the sensitivity of the gonadotropin-releasing hormone (GnRH) pulse generator to progesterone-mediated slowing during pubertal maturation, thereby initiating a series of changes in the hypothalamic–pituitary–ovarian (HPO) axis that result in ovulatory dysfunction and sustained hyperandrogenism. Thus, a cycle is established whereby the presence of hyperandrogenism, the final common pathway for the development of PCOS, begets more hyperandrogenism. E2, Estradiol; LH, luteinizing hormone; FSH, follicle stimulating hormone; E2, estradiol. (Figure based on ref. 32.)
Genetics
A genetic predisposition for PCOS certainly exists,36 and the syndrome has been found to aggregate in families.83–87 However, despite a large number of genetic studies, no one single gene has been associated with the development of all the syndrome's phenotypes.88–90 Although a comprehensive review of the genetics of PCOS is beyond the scope of this review, to date, the most promising candidate gene associated with PCOS maps to a locus on chromosome 19p13.2 within an intron of the fibrillin-3 gene, which interestingly is located near the insulin receptor gene.91–94 Although the biological function of fibrillin-3 is unknown, fibrillins can bind transforming growth factor-β (TGF-β) and have been implicated in early follicle development and theca cell formation,95 presenting a potential link to the inherent ovarian dysfunction associated with the syndrome. Other potential genes associated with PCOS include those encoding 17β-hydroxsteroid dehydrogenase type 6, sex hormone-binding globulin (SHBG), the androgen receptor (AR), and aromatase.96–98 More comprehensive genome-wide association studies (GWAS) evaluating the genetic variation of women with a PCOS-like phenotype are currently ongoing; however, given its clinical and phenotypic diversity, the syndrome is most likely polygenic in nature.
Fetal programming
Interestingly, females exposed to high levels of androgens in the intrauterine environment, including women with virilizing congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency and congenital adrenal virilizing tumors, have an increased risk of PCOS in adolescence, despite the normalization of androgen levels after birth.99 Furthermore, prenatal exposure of female nonhuman primate fetuses to excess androgens in utero has been shown to disturb both the hypothalamic–pituitary–ovarian (HPO) and hypothalamic–pituitary–adrenal (HPA) endocrine axes and recapitulate the development of the human PCOS phenotype (hyperandrogenism, LH hypersecretion, oligo- or anovulation, and insulin resistance) as the monkeys age.41–45,100,101 The hyperandrogenic fetal environment in these monkeys specifically appears to upregulate P450c17's 17,20-lyase activity, leading to increased androgen production.44,102 In addition, intrauterine androgen exposure in these monkeys leads to the development of insulin resistance associated with visceral adiposity, impaired glucose metabolism, and dyslipidemia.101 The above observations in both humans and monkeys thus support a potential role of epigenetics and fetal programming in the syndrome's pathogenesis.
However, in the nonhuman primate studies in particular, pregnant dams were given very large doses of androgens and had androgen concentrations much higher than those typically observed in pregnant women with PCOS.103 Nevertheless, studies in hyperandrogenic pregnant women suggest that increased maternal androgens may be a source of in utero androgenicity103 and can adversely affect the intrauterine environment and retard fetal development.104,105 However, it is unlikely that maternal androgens in most pregnancies exceed the normal safeguards of high maternal circulating concentrations of SHBG and placental aromatase (which converts maternal androgens to estrogens), and cross the fetoplacental barrier in quantities sufficient to “androgenize” the fetus.106 Thus, the potential contribution of the fetal adrenal glands and ovaries to intrauterine “androgenization” must be considered. In fact, studies suggest that the fetal ovary is indeed capable of synthesizing androgens in utero,107 and clinical and biochemical manifestations of PCOS have been noted in young adults with nonclassic CAH.108 Furthermore, even if the fetal ovary does not produce enough androgen to cause prenatal virilization, it may nonetheless contribute to the “programming” of the HPO axis and may be genetically predisposed to hypersecrete androgen when the HPO axis is stimulated.4
Moreover, in other animal studies, exposure of fetuses to high levels of androgens in utero appear to mediate the postnatal development of obesity through increased food intake and decreased energy expenditure, and produce features of the metabolic syndrome.109 Interestingly, the dyslipidemia and hepatic steatosis found in the prenatally androgenized offspring appear to be regulated by prenatal androgenization-induced adiposity; in contrast, the hyperinsulinemia in the offspring appear to be regulated by prenatal androgenization directly.
Postnatal events
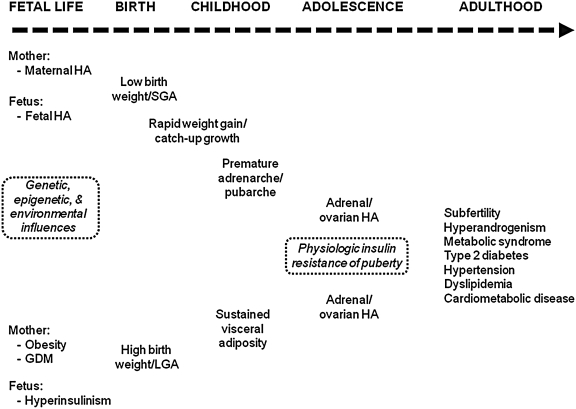
Despite the syndrome's genetic predisposition, the severity of PCOS and its phenotypic expression result from the impact of environmental influences on genetic and epigenetic factors (see Fig. 5).110
FIG. 5.
Proposed natural history of PCOS from fetal life to adulthood. The severity of polycystic ovary syndrome (PCOS) and the evolution of its phenotypic expression result from the impact of environmental influences (both pre- and postnatal) on genetic and epigenetic factors in utero. HA, Hyperandrogenism; GDM, gestational diabetes mellitus; SGA, small for gestational age; LGA, large for gestational age. (Figure based on ref. 204.)
First, premature adrenarche, a term used to describe an early increase in adrenal androgen production before 8 years in girls (and 9 years in boys), has been linked to the development of PCOS and metabolic syndrome during adolescence.17,33,111 The increased androgen production associated with adrenarche, which has been recently reviewed,112 typically leads to the development of pubic hair, or pubarche. Girls with premature adrenarche/pubarche also appear to have: (1) Adrenal hyperresponsiveness to ACTH, (2) elevated levels of insulin and insulin-like growth factor 1 (IGF-1), and (3) decreased levels of the binding proteins SHBP, thereby increasing free testosterone concentrations, and insulin-like growth factor binding protein 1 (IGFBP-1), thereby increasing free insulin and IGF-1 concentrations.113,114 Despite extensive evaluation, the cause of premature adrenarche currently remains unknown. One postulated reason for the condition is hypersecretion of a cortical adrenal stimulating hormone from the pituitary gland sharing amino acids 79–96 of proopiomelanocortin (POMC)115; however, in vitro studies have failed to confirm this hypothesis.116 Another theory is that the zona reticularis, the site of adrenal androgen production, develops prematurely.117 Early activation of P450c17's 17,20-lyase activity could also account for premature adrenal androgen secretion.118 Furthermore, corticotropin-releasing hormone (CRH) has been found to potentially affect adrenal androgen section, suggesting a role for this hormone in premature adrenarche as well.119,120
Second, rapid weight gain in small for gestational age (SGA) girls in the first few years of life and sustained adiposity in large for gestational age (LGA) girls during childhood accelerate the prepubertal appearance of PCOS, characterized by visceral obesity, insulin resistance, and premature adrenarche/pubarche.17,111,121,122 The final PCOS phenotype is then expressed during puberty following activation of the HPO axis. Interestingly, although a definitive biological mechanism has not been identified, women with a history of high birth weight are also more likely to have a polycystic ovarian morphology on ultrasound evaluation than women with low birth weight.121 Furthermore, hyperandrogenemia during childhood appears to alter normal pubertal development,123 increase the risk of postpubertal ovarian hyperandrogenism,123 and is a risk factor for metabolic syndrome independent of obesity.7 Hyperandrogenemia may also be involved with the development of central obesity and affect insulin, androgen, and glucocorticoid metabolism.124
Third, the normal physiologic insulin resistance that develops during puberty27–29 may aggravate the syndrome's symptoms and phenotypic expression. Specifically, a physiologic increase in insulin resistance and androgen levels occurs in response to growth hormone (GH) secretion, which peaks during adolescence125; this then leads to an increase in insulin and a decrease in SHBG concentrations, both of which may exacerbate the clinical manifestations of hyperandrogenism.27,126
Fourth, the “adipose tissue expandability hypothesis” may also account for the early origins of PCOS in some individuals.127 By suggesting that subcutaneous adipose tissue has a limited capacity to increase its mass safely, influenced by both environmental and genetic factors, this hypothesis accounts for the development of insulin resistance in states of obesity as well as the apparent paradox of insulin resistance in states of adipose tissue deficiency.128–131 According to this theory, an individual's “metabolic set-point” determines the caloric load that can be safely stored in their adipose tissues. Caloric loads exceeding this “set point” results in lipotoxicity, a condition associated with elevated free fatty acids (FFAs), hypertriglyceridemia, and an unfavorable adipocytokine profile, including low levels of adiponectin and high levels of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), and the potential for ectopic fat deposition (i.e., the deposition of fat in nonadipose tissues such as the liver, skeletal muscle, and pancreas), both of which could adversely affect insulin action. Thus, this theory suggests the concept of an “adiposity threshold” at which insulin resistance and other markers of lipotoxicity emerge. A caloric load in excess of a girl's ability to expand her subcutaneous adipose tissue in a metabolically safe manner (whether she be obese or of normal weight) could then potentially contribute to insulin resistance and hyperinsulinemic androgen excess.127
Effects of androgens
In addition to affecting insulin sensitivity, androgens can also influence adipocyte function.132 The AR is located in both the subcutaneous and visceral components of fat; however, its expression is higher in visceral preadipocytes than subcutaneous preadipocytes.133 Moreover, the observation that androgens can act in a sex-dimorphic manner in many tissues133 may account for the beneficial effects in fat mass distribution seen in testosterone-treated hypogonadal men but the visceral fat accumulation seen in hyperandrogenic PCOS women.124 Androgens also appear to regulate lipolysis in adipose tissue depots. Specifically, testosterone causes a dose-dependent AR-mediated decrease of catecholamine (β-adrenergic)-stimulated lipolysis in differentiated preadipocytes from abdominal subcutaneous fat depots but not from omental fat depots.134 This phenomenon has been observed in women with PCOS135 and may thus contribute to the development of upper-body obesity, an established risk factor for insulin resistance. However, the differential regulation of lipolysis between subcutaneous and omental fat does not explain the high prevalence of visceral adiposity in women with PCOS. Rather, androgen-mediated lipogenesis and lipid deposition may be the major factors involved. Specifically, androgens mediate lipoprotein lipase (LPL), the key enzyme for the hydrolysis of triglyercides into FFAs and glycerol and subsequent lipid storage in adipose tissue. In addition, androgens appear to stimulate lipogenesis in visceral adipose tissue by increasing the expression of several key lipogenic genes.136
Androgen excess is also associated with an atherogenic lipid profile in women.137,138 Specifically, testosterone lowers high-density lipoprotein cholesterol (HDL-C)139 and may contribute to increased circulating low-density lipoprotein cholesterol (LDL-C) concentrations.138 Beyond its metabolic effects, androgens may also act directly on the vasculature to promote endothelial dysfunction140,141 and accelerate atherosclerotic changes.142 Furthermore, although testosterone levels have been reported to be directly associated with the risk for hypertension in PCOS,143 the frequent prevalence of obesity in these women confounds this association.144 However, given that androgens stimulate the intrarenal renin–angiotensin–aldosterone system and modulate renal sodium homeostasis by increasing angiotensinogen and renin gene expression,145 augmenting proximal tubular transport,146 and upregulating expression of the α-subunit of the epithelial sodium channel (ENaC),147 they do have prohypertensive properties. Androgen excess may also play a role in the low-grade, chronic inflammation and oxidative stress associated with PCOS.148–150
The role of insulin
Hyperinsulinemia secondary to insulin resistance is common in PCOS and occurs independent of obesity or BMI.57,151,152 The degree of hyperinsulinemia also correlates with the syndrome's severity.153 Although it has been debated whether hyperandrogenism results from hyperinsulinemia, hyperinsulinemia results from hyperandrogenism, or they are each independent variables linked in a noncausal relationship, data showing that bilateral oophorectomy,154 or the administration of a long-acting GnRH agonist155,156 or an antiandrogenic compound,157 do not affect the hyperinsulinemia in women with PCOS suggest that hyperinsulinemia is the primary factor driving increased androgen production. If elevated levels of androgens were causing insulin resistance and hyperinsulinemia, the opposite effect would be expected. Conversely, androgen excess can cause insulin resistance. For example, women receiving testosterone158 and women with CAH159 have decreased insulin sensitivity. However, high levels of endogenous androgens do not cause insulin resistance in normal men; thus, the causal relationship between hyperandrogenemia and insulin resistance in women remains unclear.
Importantly, insulin has several direct and indirect effects in women with PCOS that potentiate the hyperandrogenic state. First, insulin may act alone to stimulate ovarian androgen secretion directly, and/or augment LH-stimulated androgen secretion.160–162 Second, insulin may act indirectly to: (1) Potentiate ACTH-mediated adrenal androgen production,163 (2) enhance the amplitude of GnRH-stimulated LH pulses,164,165 (3) decrease hepatic production of SHBG (thereby increasing free testosterone levels),166,167 and/or (4) decrease production of IGFBP-1.168,169 This latter effect would not only increase the availability of free insulin, but also the availability of free IGF-1, which can also stimulate androgen production.113,170 Furthermore, insulin may contribute to mid-antral follicular arrest,171 a characteristic feature of the polycystic ovary.
Mechanisms of insulin resistance
Most women with PCOS have decreased insulin sensitivity, independent of their degree of adiposity, body fat topography, and androgen levels.172 However, PCOS patients do not typically have structural abnormalities of their insulin receptors (IRs),173,174 decreased IR number,175,176 or altered insulin binding affinity.175,176 Therefore, a postreceptor mechanism causing insulin resistance is most likely responsible.
In particular, the potential role of serine phosphorylation of the IR as a cause of insulin resistance in women with PCOS has been widely studied. Mechanistically, serine phosphorylation of the IR's β-subunit (IRβ) inhibits IR tyrosine autophosphorylation without affecting insulin binding.177–180 Furthermore, serine phosphorylation of IRβ has been found to occur in many women with PCOS.176 Although the mechanism causing IRβ serine phosphorylation remains undefined, it appears to involve a serine/threonine kinase extrinsic to the receptor181; alternatively, it may involve an inhibitor of a serine/threonine phosphatase.176,182
Insulin resistance in PCOS patients without IRβ serine phosphorylation may be due to other postreceptor defects. For example, serine phosphorylation of insulin receptor substrate-1 (IRS-1) inhibits IRS-1-dependent signaling pathways183–185 and may contribute to the insulin resistance induced by FFAs186 and TNF-α,187 both of which can be elevated in PCOS.188–190 Furthermore, factors such as inflammatory cytokines (e.g., IL-1 and IL-6),191 glucosamine,192 and other proteins involved in the insulin signaling pathways, such as IRS-2193 and the β isoform of Akt (Akt2),194 may also play a role.
Tissue-selective insulin resistance
Importantly, not all tissues in women with PCOS are insulin resistant. Rather, the insulin resistance appears to be tissue selective. Specifically, resistance to the metabolic actions of insulin has been reported in the skeletal muscle, adipose tissue, and the liver172,195; however, sensitivity to the steroidogenic actions of insulin persists in the adrenal gland and ovary. In fact, insulin potentiates adrenal and ovarian androgen production in vitro.160–162 Hence the paradox: Whereas some tissues (muscle, fat, and liver) are insulin resistant in women with PCOS, others (the adrenal gland and ovary) are insulin sensitive.10,11,196
To explain this paradox, it has been suggested that insulin could act on the ovaries through either homodimeric IGF-1 receptors (IGF-1Rs) or heterodimeric receptors having one IR subunit and one IGF-1R subunit.196 Although the clinical observation that female patients with profound insulin resistance due to mutations in both IR alleles (i.e., female patients with leprechaunism) have severe hirsutism and elevated androgen levels197 suggests that the dominant action of insulin on the ovary in these individuals is mediated through a non-IR-specific mechanism, the finding that antibodies against IGF-1R do not inhibit insulin-stimulated sex steroid production in ovarian tissue from PCOS women suggest that other factors must be involved.171,198 Furthermore, data suggest that only insulin's action on glucose transport and metabolic pathways are affected in PCOS12,119; in fact, even in the ovary itself, the metabolic effects of insulin seem to be impaired whereas its ability to potentiate steroidogenesis is preserved.200–202
Thus, to date, the “paradox” remains unexplained, and the biological mechanisms underlying the apparent tissue-selective insulin resistance in PCOS remain unclear.
Hyperandrogenemia and insulin resistance: the serine phosphorylation hypothesis
Although P450c17's 17α-hydroxylase and 17,20-lyase activities are catalyzed on a single active site,60–62 they are differentially regulated. Specifically, serine phosphorylation of P450c17 dramatically increases the enzyme's latter (17,20-lyase) but not former (17α-hydroxylase) activity.118 Because serine phosphorylation of IRβ impairs insulin signaling179,180 and many women with PCOS have excess serine phosphorylation of IRβ,176 it has been postulated that a gain-of-function mutation in a hypothetical kinase (or in a regulator of a hypothetical kinase) might potentially serine phosphorylate both IRβ, causing insulin resistance, and P450c17, causing hyperandrogenemia.118,203 However, while the serine phosphorylation hypothesis provides a common biological mechanism for hyperandrogenemia and insulin resistance (two cardinal features of PCOS), it remains an unproven hypothesis until such time as the hypothetical kinase or its regulatory factors are identified and activating mutations are found.203
The “Two-Hit” Hypothesis of PCOS
Given that the full clinical spectrum of PCOS does not typically appear until pubertal maturation, a “two-hit” hypothesis has been proposed.4,32,204,205 For the first “hit,” one or more of a number of different mechanisms, including primary adrenal, ovarian, and/or neuroendocrine abnormalities, insulin resistance and hyperinsulinemia, and/or prenatal, immediate postnatal, and/or peripubertal androgen exposure, lead to increased androgen production. For the second “hit,” the preexisting hyperandrogenism reduces the sensitivity of the GnRH pulse generator to progesterone-mediated slowing during pubertal maturation, thereby initiating a series of changes in the HPO axis that result in ovulatory dysfunction and sustained hyperandrogenism (see Fig. 4). Thus, a cycle is established whereby the presence of hyperandrogenism, the final common pathway for the development of PCOS, begets more hyperandrogenism.
This “two-hit” hypothesis further reinforces the importance of diet and physical activity, and their effects on maintaining insulin sensitivity and appropriate body weight, on a woman's health. Although insulin resistance is common in PCOS, its presence is not invariable. But, as described above, insulin resistance and its resulting hyperinsulinemia can certainly promote androgen synthesis. Therefore, even in a genetically susceptible girl, the maintenance of insulin sensitivity may limit the syndrome's phenotypic expression. Alternatively, the presence of overweight/obesity can have additive adverse effects in PCOS206,207 and promote hyperandrogenism by diminishing insulin sensitivity (i.e., increasing insulin resistance) and/or upregulating peripheral 17β-hydroxysteroid dehydrogenase action.208 Furthermore, although neither necessary nor sufficient for the development of the syndrome, overweight/obesity amplifies the clinical severity of PCOS and increases the risk of metabolic dysfunction.4 This is particularly alarming given that an evaluation of the National Health and Nutrition Examination Survey (NHANES) data estimates that ≈30% of girls ages 6–19 in the United States are either overweight or at risk for becoming overweight.209 Thus, in approximately one third of U.S. adolescent girls, the presence of extra body fat may lead to PCOS-type symptoms in an otherwise asymptomatic girl, accelerate the syndrome's clinical manifestations, and/or aggravate the syndrome's clinical course. Furthermore, overweight and obese girls with PCOS are at increased risk for impaired glucose metabolism and have a greater than three-fold increased risk of developing type 2 diabetes later in life.210
Thus, in the natural history of PCOS, environmental influences (mainly diet and physical inactivity leading to obesity) may perpetuate not only the metabolic, but also the endocrine aberrations of the syndrome.
Clinical Evaluation
Given that PCOS is a diagnosis of exclusion, the clinical evaluation of the syndrome is aimed at excluding other causes of androgen excess and menstrual dysfunction, such as late-onset CAH, hyperprolactinemia, thyroid dysfunction, and premature ovarian failure. Furthermore, although only androgen levels (testosterone, free testosterone, and DHEA-S) are included in the diagnostic criteria for PCOS, reliable specialized assays, particularly for the measurement of sex steroid hormones in children, are inconsistently available.211 Moreover, the interpretation of the results must be made in the context of age-appropriate reference ranges. It is also important to remember that existing laboratory measurements do not permit the evaluation of hormonal bioactivity, explaining the poor correlation between circulating androgen levels and clinical symptoms.212 In addition, the importance of a complete medical history, including a detailed family history, information on menarche and the nature of a woman's menstrual cycles, and a history of any predisposing factors to PCOS (low birth weight with excessive catch-up growth or premature adrenarche/pubarche), and a thorough physical examination, specifically documenting any clinical signs of hyperandrogenism (hirsutism, acne, and/or alopecia) or insulin resistance (acanthosis nigricans), and an assessment of regional adiposity, cannot be overemphasized. The importance of the family history is exemplified by the observation that pubertal girls born to women with PCOS tend to have higher serum testosterone and lower SHBG concentrations compared to age- and BMI-matched controls.213 Moreover, determination of the waist-to-hip ratio (WHR), which is noninvasive and can easily be measured at each clinic visit, can be used as a surrogate marker for central fat accumulation, with a value greater than 0.8 suggestive of visceral adiposity.26
Although no consensus guidelines exist regarding the evaluation of suspected PCOS in the pediatric population, many practitioners measure the following analytes during the diagnostic evaluation: FSH, LH, prolactin, thyroid stimulating hormone (TSH), 17-hydroxyprogesterone (17-OHP), total and free testosterone, SHBG, a lipid panel, and a random blood glucose level. If the girl is overweight or has cutaneous signs of insulin resistance (acanthosis nigricans), fasting glucose and insulin levels are frequently obtained and a 2-h oral glucose tolerance test (OGTT) is performed.25,32,205 Although a pelvic ultrasound (transabdominal if the girl is virginal) may be performed in a girl with high testosterone levels or rapidly progressive hirsutism or virilization to evaluate for malignancy, routine ovarian imaging is not indicated for the diagnosis of PCOS in adolescents.214 If the evaluation suggests a potential adrenal tumor, a computed tomography (CT) scan or a magnetic resonance imaging (MRI) study should be performed.
Girls diagnosed with hyperandrogenism should then also be screened for other metabolic abnormalities (such as hypertension [using the appropriate age- and height-percentile reference values], dyslipidemia, and impaired glucose metabolism) given the approximately four-fold increased risk of metabolic syndrome in adolescents with PCOS independent of body weight.7,8
Treatment
The treatment of PCOS in adolescents is primarily focused on the symptomatic management of the reproductive, metabolic, and cosmetic manifestations of the syndrome. Given that most adolescent girls are not trying to conceive and unaware of the metabolic aberrations that can occur in PCOS, the dermatological manifestations and menstrual dysfunction (i.e., abnormal bleeding) associated with the syndrome are typically the most common concerns.
Lifestyle modifications
Certainly for overweight or obese girls with PCOS, a serious attempt at weight loss and increased physical activity should be first-line therapy.215 In nonobese girls with PCOS, weight management should be the goal. A weight loss of 5–10% has been shown to decrease testosterone concentrations, increase SHBG, normalize menses, and improve fertility in women with PCOS216–222; it can also attenuate insulin resistance and other metabolic aberrations.223 A low-calorie diet of ≈1,000–1,200 kcal/day typically reduces total body weight by ≈10% over 6 months.224 Moreover, a modest 500–1,000 kcal/day reduction in caloric intake typically results in 1–2 pounds of weight loss per week. The intake of sugar-sweetened beverages in particular is associated with weight gain225–228 and indices of insulin resistance in the adolescent population229 and thus should also be avoided. In addition to dietary modifications, regular physical activity is essential for weight loss and long-term weight management, and a minimum of 30 min of moderately intense exercise at least 3 days per week is recommended.230 Increased physical activity also decreases insulin resistance231–233 and has been associated with improved indices of insulin sensitivity in the pediatric population.229
Dermatological interventions
Hirsutism, the most common cutaneous sign of hyperandrogenism, appears to be progressive in women with PCOS. Therefore, the sooner it is treated, the better the outcome. Waxing, plucking, shaving, depilation, electrolysis, and laser hair removal techniques can all be used to remove current hair; however, pharmacological interventions are often needed to prevent new hair growth. Unfortunately for the affected adolescent, it may take up to 12 months to reverse the androgen-induced transformation of vellus to terminal hairs and see clinical improvement in hirsutism due to the prolonged growth cycle of hair.25 Eflornithine cream (Vaniqa®), an inhibitor of ornithine decarboxylase, is another option for the treatment of hirsutism, but it is expensive, not often covered by insurance carriers, and needs to be used continuously to yield its desired effect.234 For acne, topical treatment with salicylic acid, benzoyl peroxide, clindamycin/benzoyl peroxide preparations, tretinoin, and clindamycin/tretinoin combinations can be used. If topical therapies for acne are ineffective, oral isotretinoin can be used. However, given its teratogenicity, isotretinoin is typically only used in severe cases of acne and in combination with effective forms of contraception.
Combined hormonal agents
Combined hormonal oral contraceptive pills (OCPs) containing both estrogen and progestin are the most common form of therapy in adolescents with PCOS,235 improving hirsutism, acne, and menstrual irregularity. The estrogen component both suppresses LH secretion (and thus ovarian androgen production) and increases hepatic SHBG production (decreasing the amount of free testosterone); the progestin component protects the endometrium from unopposed estrogen.236 Combined OCPs also inhibit 5α-reductase in the skin, decreasing its exposure to DHT.237 Although no significant clinical differences with respect to androgenicity appear to exist among the progestins in currently available OCPs, the fourth-generation progestin drospirenone (Yasmin® [30 μg of ethinyl estradiol + 3 mg drospirenone] and Yaz® [20 μg of ethinyl estradiol + 3 mg drospirenone]) has been suggested as the ideal choice given that it is a derivative of spironolactone (equivalent to ≈25 mg of spironolactone) and thus has direct antiandrogenic activity. Both high-dose (30–35 μg of ethinyl estradiol) and low-dose (20 μg of ethinyl estradiol) OCPs appear comparable238; the preparation with the fewest side effects is preferable. OrthoEvra®, a transdermal contraceptive patch, is also a treatment option for girls with PCOS; however, it may be associated with an increased risk for venous thromboembolic events compared to OCPs.234 The NuvaRing®, a transvaginal contraceptive ring, is another option.234 Although combined hormonal agents have been shown to increase insulin resistance,239 this effect is not thought to outweigh their therapeutic benefits in PCOS. Furthermore, given that an imbalanced LH-to-FSH ratio is often the driving force for hyperandrogenism in lean girls with the syndrome, combined hormonal agents may be especially useful in this population.32
Antiandrogens
Antiandrogen medications either block androgen binding to the AR or inhibit 5α-reductase, limiting the conversion of testosterone to the more biologically potent androgen DHT. The most commonly used antiandrogen in the United States is spironolactone, which functions mainly as a competitive AR antagonist; however, it also inhibits 5α-reductase and decreases testosterone production.205,240 The recommended dosage is typically 100–200 mg/day in divided doses. The AR inhibitor flutamide is another antiandrogen that is commonly used in Europe.241 Although it appears to be well tolerated at the recommended dosage of 250–500 mg/day, the risks of hepatotoxicity and fetal abnormalities limit its use outside of clinical studies. Finasteride, a 5α-reductase inhibitor, is another antiandrogen that has demonstrated comparable efficacy with spironolactone and flutamide for the treatment of hirsutism at its recommended dosage of 5 mg/day242; however, it is rarely used clinically.
Insulin-sensitizing agents
Insulin-sensitizing agents are also frequently used in the management of PCOS.243,244 Of these agents, metformin is the most commonly prescribed, particularly in adolescents with impaired glucose tolerance, insulin resistance, and/or obesity.245,246 Metformin inhibits hepatic glucose production and increases peripheral tissue insulin sensitivity,247 and in women with PCOS, appears to improve insulin sensitivity, insulin and androgen levels, lipid parameters, and menstrual cyclicity.245,246,248 Moreover, it is effective in reducing the incidence of diabetes in those at high risk.249 Several studies evaluating the use of metformin in both obese and nonobese adolescents with PCOS (at dosages ranging from 750 to 2,250 mg/day) have been performed,250–257 and in general they all demonstrate the agent's efficacy. However, there is a lack of large, randomized controlled trials, and there are no prospective studies examining the long-term effects of metformin in the prevention or reduction of PCOS-associated metabolic complications. Although the observation that metformin's normalizing effects are reversed soon after therapy is discontinued258 is a concern, the favorable safety profile of metformin and its potential to benefit both the cardiometabolic as well as reproductive aspects of PCOS make it an attractive therapeutic agent.246 The thiazolidinediones (TZDs) (troglitazone, rosiglitazone, and pioglitazone) are another class of insulin-sensitizing agents that act as agonists for the nuclear peroxisome proliferator-activated receptor γ (PPARγ).259,260 Like metformin, they improve peripheral insulin sensitivity, androgen levels, and ovulatory function in women with PCOS.261–264 However, they have not been studied widely in the pediatric population. Moreover, given their potential side effects, they are unlikely to replace metformin as the insulin-sensitizing drugs of choice.
Other agents
Octreotide (Sandostatin®), an analog of somatostatin, has also been used in patients with PCOS.265–267 Mechanistically, somatostatin inhibits pancreatic insulin release268 in addition to decreasing pituitary GH secretion269 and blunting the LH response to GnRH.270 However, due to the parenteral nature by which the drug has to be given and its extensive side-effect profile, octreotide therapy is unlikely to play a major role in PCOS treatment.
Combination therapy
Given that no single pharmacological agent adequately addresses all of the symptoms associated with PCOS and each available agent has different mechanisms of action, combination regimens are common. In the United States, the combination of ethinyl estradiol/drospirenone-containing OCPs (Yasmin® or Yaz®) with metformin is often used, particularly in overweight girls.271 The combination of ethinyl estradiol/drospirenone, metformin, and flutamide has been studied in Europe and appears to have additive benefits on the syndrome's phenotype.272–274 However, as described above, flutamide is not widely used outside of clinical studies given its potential toxicities.
Conclusions
PCOS is a common endocrinopathy characterized by hyperandrogenism and disordered gonadotropin secretion, often associated with insulin resistance. The syndrome, which modulates both hormonal and metabolic processes, affects an estimated 5–10% of reproductive-age women in the United States and increases a woman's risk of infertility, endometrial pathology, and cardiometabolic disease. As it is currently defined, PCOS most likely includes a group of distinct diseases with similar clinical phenotypes but different underlying pathophysiological processes. However, hyperandrogenism remains the syndrome's clinical hallmark. The clinical manifestations of PCOS often emerge during childhood or in the peripubertal years, suggesting that the syndrome is influenced by fetal programming and/or early postnatal events. However, given that the full clinical spectrum of PCOS does not typically appear until pubertal maturation, a “two-hit” hypothesis has been proposed: (1) A girl develops hyperandrogenism through one or more of many different potential mechanisms; and (2) the preexisting hyperandrogenism, by whatever source, then disturbs the HPO axis, resulting in ovulatory dysfunction and sustained hyperandrogenism. No consensus guidelines exist regarding the diagnosis and management of PCOS in the pediatric population; however, because the syndrome is a diagnosis of exclusion, the clinical evaluation of suspected PCOS is aimed at excluding other causes of androgen excess and menstrual dysfunction. For the management of PCOS, the importance of lifestyle should not be overlooked, and a symptom-directed treatment strategy should be used.
Acknowledgments
The author would like to thank Professor Walter L. Miller for invaluable insight in the preparation of this manuscript. This work was supported by grant numbers KL2 RR024144 and UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the author and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at www.ncrr.nih.gov. Information on reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp/.
Author Disclosure Statement
The author has no conflicts of interest or financial interests to report.
References
- 1.Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R. Woods KS. Reyna R. Key TJ. Knochenhauer ES. Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 3.Ehrmann D. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 4.Franks S. Polycystic ovary syndrome in adolescents. Int J Obes (Lond) 2008;32:1035–1041. doi: 10.1038/ijo.2008.61. [DOI] [PubMed] [Google Scholar]
- 5.Carmina E. Chu MC. Longo RA. Rini GB. Lobo RA. Phenotypic variation in hyperandrogenic women influences the findings of abnormal metabolic and cardiovascular risk parameters. J Clin Endocrinol Metab. 2005;90:2545–2549. doi: 10.1210/jc.2004-2279. [DOI] [PubMed] [Google Scholar]
- 6.Ehrmann DA. Liljenquist DR. Dasza K. Azziz R. Legro RS. Ghazzi MN. Group PTS. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:48–53. doi: 10.1210/jc.2005-1329. [DOI] [PubMed] [Google Scholar]
- 7.Coviello A. Legro R. Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2006;91:492–497. doi: 10.1210/jc.2005-1666. [DOI] [PubMed] [Google Scholar]
- 8.Cussons AJ. Watts GF. Burke V. Shaw JE. Zimmet PZ. Stuckey BG. Cardiometabolic risk in polycystic ovary syndrome: A comparison of different approaches to defining the metabolic syndrome. Hum Reprod. 2008;23:2352–2358. doi: 10.1093/humrep/den263. [DOI] [PubMed] [Google Scholar]
- 9.Azziz R. Marin C. Hoq L. Badamgarav E. Song P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab. 2005;90:4650–4658. doi: 10.1210/jc.2005-0628. [DOI] [PubMed] [Google Scholar]
- 10.Dunaif A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 11.Dunaif A. Insulin action in the polycystic ovary syndrome. Endocrinol Metab Clin North Am. 1999;28:341–359. doi: 10.1016/s0889-8529(05)70073-6. [DOI] [PubMed] [Google Scholar]
- 12.Book C-B. Dunaif A. Selective insulin resistance in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1999;84:3110–3116. doi: 10.1210/jcem.84.9.6010. [DOI] [PubMed] [Google Scholar]
- 13.Venkatesan AM. Dunaif A. Corbould A. Insulin resistance in polycystic ovary syndrome: Progress and paradoxes. Recent Prog Horm Res. 2001;56:295–308. doi: 10.1210/rp.56.1.295. [DOI] [PubMed] [Google Scholar]
- 14.Diamanti-Kandarakis E. Xyrafis X. Boutzios G. Christakou C. Pancreatic beta-cells dysfunction in polycystic ovary syndrome. Panminerva Med. 2008;50:315–325. [PubMed] [Google Scholar]
- 15.Azziz R. Carmina E. Dewailly D. Diamanti-Kandarakis E. Escobar-Morreale HF. Futterweit W. Janssen OE. Legro RS. Norman RJ. Taylor AE. Witchel SF. Positions statement: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91:4237–4245. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 16.Franks S. Adult polycystic ovary syndrome begins in childhood. Best Pract Res Clin Endocrinol Metab. 2002;16:263–272. doi: 10.1053/beem.2002.0203. [DOI] [PubMed] [Google Scholar]
- 17.Ibanez L. Diaz R. Lopez-Bermejo A. Marcos MV. Clinical spectrum of premature pubarche: Links to metabolic syndrome and ovarian hyperandrogenism. Rev Endocr Metab Disord. 2009;10:63–76. doi: 10.1007/s11154-008-9096-y. [DOI] [PubMed] [Google Scholar]
- 18.Zawadski JK. Dunaif A. Diagnostic criteria for polycystic ovary syndrome: Towards a rational approach. In: Dunaif A, editor; Givens JR, editor; Haseltine F, editor. Polycystic Ovary Syndrome. Boston: Blackwell Scientific; 1992. pp. 377–384. [Google Scholar]
- 19.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Diamanti-Kandarakis E. Panidis D. Unravelling the phenotypic map of polycystic ovary syndrome (PCOS): A prospective study of 634 women with PCOS. Clin Endocrinol. 2007;67:735–742. doi: 10.1111/j.1365-2265.2007.02954.x. [DOI] [PubMed] [Google Scholar]
- 21.Azziz R. Carmina E. Dewailly D. Diamanti-Kandarakis E. Escobar-Morreale HF. Futterweit W. Janssen OE. Legro RS. Norman RJ. Taylor AE. Witchel SF. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: The complete task force report. Fertil Steril. 2009;91:456–488. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 22.Dewailly D. Catteau-Jonard S. Reyss AC. Leroy M. Pigny P. Oligoanovulation with polycystic ovaries but not overt hyperandrogenism. J Clin Endocrinol Metab. 2006;91:3922–3927. doi: 10.1210/jc.2006-1054. [DOI] [PubMed] [Google Scholar]
- 23.Diaz A. Laufer MR. Breech LL. Menstruation in girls and adolescents: Using the menstrual cycle as a vital sign. Pediatrics. 2006;118:2245–2250. doi: 10.1542/peds.2006-2481. [DOI] [PubMed] [Google Scholar]
- 24.Southam AL. Richart RM. The prognosis for adolescents with menstrual abnormalities. Am J Obstet Gynecol. 1966;94:637–645. doi: 10.1016/0002-9378(66)90398-x. [DOI] [PubMed] [Google Scholar]
- 25.Buggs C. Rosenfield RL. Polycystic ovary syndrome in adolescence. Endocrinol Metab Clin North Am. 2005;34:677–705. doi: 10.1016/j.ecl.2005.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodman NF. Bledsoe MB. Cobin RH. Futterweit W. Goldzieher JW. Petak SM. Smith KD. Steinberger E. American Association of Clinical Endocrinologists medical guidelines for the clinical practice for the diagnosis and treatment of hyperandrogenic disorders. Endocr Pract. 2001;7:120–134. [PubMed] [Google Scholar]
- 27.Hannon TS. Janosky J. Arslanian SA. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res. 2006;60:759–763. doi: 10.1203/01.pdr.0000246097.73031.27. [DOI] [PubMed] [Google Scholar]
- 28.Moran A. Jacobs DR., Jr. Steinberger J. Steffen LM. Pankow JS. Hong CP. Sinaiko AR. Changes in insulin resistance and cardiovascular risk during adolescence: Establishment of differential risk in males and females. Circulation. 2008;117:2361–2368. doi: 10.1161/CIRCULATIONAHA.107.704569. [DOI] [PubMed] [Google Scholar]
- 29.Moran A. Jacobs DR., Jr. Steinberger J. Hong CP. Prineas R. Luepker R. Sinaiko AR. Insulin resistance during puberty: Results from clamp studies in 357 children. Diabetes. 1999;48:2039–2044. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- 30.van Hooff MH. Voorhorst FJ. Kaptein MB. Hirasing RA. Koppenaal C. Schoemaker J. Polycystic ovaries in adolescents and the relationship with menstrual cycle patterns, luteinizing hormone, androgens, and insulin. Fertil Steril. 2000;74:49–58. doi: 10.1016/s0015-0282(00)00584-7. [DOI] [PubMed] [Google Scholar]
- 31.Azziz R. Controversy in clinical endocrinology: Diagnosis of polycystic ovarian syndrome: the Rotterdam criteria are premature. J Clin Endocrinol Metab. 2006;91:781–785. doi: 10.1210/jc.2005-2153. [DOI] [PubMed] [Google Scholar]
- 32.Blank SK. Helm KD. McCartney CR. Marshall JC. Polycystic ovary syndrome in adolescence. Ann NY Acad Sci. 2008;1135:76–84. doi: 10.1196/annals.1429.005. [DOI] [PubMed] [Google Scholar]
- 33.Rosenfield RL. Clinical review: Identifying children at risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:787–796. doi: 10.1210/jc.2006-2012. [DOI] [PubMed] [Google Scholar]
- 34.Biro FM. Emans SJ. Whither PCOS? The challenges of establishing hyperandrogenism in adolescent girls. J Adolesc Health. 2008;43:103–105. doi: 10.1016/j.jadohealth.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Sultan C. Paris F. Clinical expression of polycystic ovary syndrome in adolescent girls. Fertil Steril. 2006;86(Suppl 1):S6. doi: 10.1016/j.fertnstert.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Legro R. Driscoll D. Strauss JF., 3rd Fox J. Dunaif A. Evidence for a genetic basis for hyperandrogenism in polycystic ovary syndrome. Proc Natl Acad Sci USA. 1998;95:14956–1460. doi: 10.1073/pnas.95.25.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xita N. Georgiou I. Tsatsoulis A. The genetic basis of polycystic ovary syndrome. Eur J Endocrinol. 2002;147:717–725. doi: 10.1530/eje.0.1470717. [DOI] [PubMed] [Google Scholar]
- 38.Nardo LG. Patchava S. Laing I. Polycystic ovary syndrome: Pathophysiology, molecular aspects and clinical implications. Panminerva Med. 2008;50:267–278. [PubMed] [Google Scholar]
- 39.Abbott DH. Dumesic DA. Franks S. Developmental origin of polycystic ovary syndrome—a hypothesis. J Endocrinol. 2002;174:1–5. doi: 10.1677/joe.0.1740001. [DOI] [PubMed] [Google Scholar]
- 40.Franks S. McCarthy MI. Hardy K. Development of polycystic ovary syndrome: Involvement of genetic and environmental factors. Int J Androl. 2006;29:278–285. doi: 10.1111/j.1365-2605.2005.00623.x. [DOI] [PubMed] [Google Scholar]
- 41.Eisner JR. Dumesic DA. Kemnitz JW. Abbott DH. Timing of prenatal androgen excess determines differential impairment in insulin secretion and action in adult female rhesus monkeys. J Clin Endocrinol Metab. 2000;85:1206–1210. doi: 10.1210/jcem.85.3.6453. [DOI] [PubMed] [Google Scholar]
- 42.Eisner JR. Barnett MA. Dumesic DA. Abbott DH. Ovarian hyperandrogenism in adult female rhesus monkeys exposed to prenatal androgen excess. Fertil Steril. 2002;77:167–172. doi: 10.1016/s0015-0282(01)02947-8. [DOI] [PubMed] [Google Scholar]
- 43.Abbott DH. Barnett DK. Levine JE. Padmanabhan V. Dumesic DA. Jacoris S. Tarantal AF. Endocrine antecedents of polycystic ovary syndrome in fetal and infant prenatally androgenized female rhesus monkeys. Biol Reprod. 2008;79:154–163. doi: 10.1095/biolreprod.108.067702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abbott DH. Zhou R. Bird IM. Dumesic DA. Conley AJ. Fetal programming of adrenal androgen excess: lessons from a nonhuman primate model of polycystic ovary syndrome. Endocr Dev. 2008;13:145–158. doi: 10.1159/000134831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abbott DH. Tarantal AF. Dumesic DA. Fetal, infant, adolescent and adult phenotypes of polycystic ovary syndrome in prenatally androgenized female rhesus monkeys. Am J Primatol. 2009;71:1–9. doi: 10.1002/ajp.20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barker DJ. Clark PM. Fetal undernutrition and disease in later life. Rev Reprod. 1997;2:105–112. doi: 10.1530/ror.0.0020105. [DOI] [PubMed] [Google Scholar]
- 47.Barker DJ. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition. 1997;13:807–813. doi: 10.1016/s0899-9007(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 48.Miller WL. Molecular biology of steroid hormone synthesis. Endocr Rev. 1988;9:295–318. doi: 10.1210/edrv-9-3-295. [DOI] [PubMed] [Google Scholar]
- 49.Barnes RB. Rosenfield RL. Burstein S. Ehrmann DA. Pituitary-ovarian responses to nafarelin testing in the polycystic ovary syndrome. N Engl J Med. 1989;320:559–565. doi: 10.1056/NEJM198903023200904. [DOI] [PubMed] [Google Scholar]
- 50.Ehrmann DA. Rosenfield RL. Barnes RB. Brigell DF. Sheikh Z. Detection of functional ovarian hyperandrogenism in women with androgen excess. N Engl J Med. 1992;327:157–162. doi: 10.1056/NEJM199207163270304. [DOI] [PubMed] [Google Scholar]
- 51.Lachelin GC. Judd HL. Swanson SC. Hauck ME. Parker DC. Yen SS. Long term effects of nightly dexamethasone administration in patients with polycystic ovarian disease. J Clin Endocrinol Metab. 1982;55:768–773. doi: 10.1210/jcem-55-4-768. [DOI] [PubMed] [Google Scholar]
- 52.Rittmaster RS. Thompson DL. Effect of leuprolide and dexamethasone on hair growth and hormone levels in hirsute women: The relative importance of the ovary and the adrenal in the pathogenesis of hirsutism. J Clin Endocrinol Metab. 1990;70:1096–1102. doi: 10.1210/jcem-70-4-1096. [DOI] [PubMed] [Google Scholar]
- 53.Nelson VL. Legro RS. Strauss JF., 3rd McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 1999;13:946–957. doi: 10.1210/mend.13.6.0311. [DOI] [PubMed] [Google Scholar]
- 54.Ibanez L. Potau N. Zampolli M. Prat N. Gussinye M. Saenger P. Vicens-Calvet E. Carrascosa A. Source localization of androgen excess in adolescent girls. J Clin Endocrinol Metab. 1994;79:1778–1784. doi: 10.1210/jcem.79.6.7989484. [DOI] [PubMed] [Google Scholar]
- 55.Moran C. Reyna R. Boots LS. Azziz R. Adrenocortical hyperresponsiveness to corticotropin in polycystic ovary syndrome patients with adrenal androgen excess. Fertil Steril. 2004;81:126–131. doi: 10.1016/j.fertnstert.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 56.Kumar A. Woods KS. Bartolucci AA. Azziz R. Prevalence of adrenal androgen excess in patients with the polycystic ovary syndrome (PCOS) Clin Endocrinol (Oxf) 2005;62:644–649. doi: 10.1111/j.1365-2265.2005.02256.x. [DOI] [PubMed] [Google Scholar]
- 57.Silfen ME. Denburg MR. Manibo AM. Lobo RA. Jaffe R. Ferin M. Levine LS. Oberfield SE. Early endocrine, metabolic, and sonographic characteristics of polycystic ovary syndrome (PCOS): comparison between nonobese and obese adolescents. J Clin Endocrinol Metab. 2003;88:4682–4688. doi: 10.1210/jc.2003-030617. [DOI] [PubMed] [Google Scholar]
- 58.Fassnacht M. Schlenz N. Schneider SB. Wudy SA. Allolio B. Arlt W. Beyond adrenal and ovarian androgen generation: Increased peripheral 5α-reductase activity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2760–2766. doi: 10.1210/jc.2002-021875. [DOI] [PubMed] [Google Scholar]
- 59.Chung BC. Picado-Leonard J. Haniu M. Bienkowski M. Hall PF. Shively JE. Miller WL. Cytochrome P450c17 (steroid 17α-hydroxylase/17,20 lyase): Cloning of human adrenal and testis cDNAs indicates the same gene is expressed in both tissues. Proc Natl Acad Sci USA. 1987;84:407–411. doi: 10.1073/pnas.84.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakajin S. Shively JE. Yuan PM. Hall PF. Microsomal cytochrome P-450 from neonatal pig testis: Two enzymatic activities (17α-hydroxylase and c17,20-lyase) associated with one protein. Biochemistry. 1981;20:4037–4042. doi: 10.1021/bi00517a014. [DOI] [PubMed] [Google Scholar]
- 61.Zuber MX. Simpson ER. Waterman MR. Expression of bovine 17α-hydroxylase cytochrome P-450 cDNA in nonsteroidogenic (COS 1) cells. Science. 1986;234:1258–1261. doi: 10.1126/science.3535074. [DOI] [PubMed] [Google Scholar]
- 62.Auchus RJ. Miller WL. Molecular modeling of human P450c17 (17α-hydroxylase/17,20-lyase): Insights into reaction mechanisms and effects of mutations. Mol Endocrinol. 1999;13:1169–1182. doi: 10.1210/mend.13.7.0326. [DOI] [PubMed] [Google Scholar]
- 63.Miller WL. Steroidogenic enzymes. Endocr Dev. 2008;13:1–18. doi: 10.1159/000134751. [DOI] [PubMed] [Google Scholar]
- 64.Auchus RJ. Miller WL. The principles, pathways, and enzymes of human steroidogenesis. In: DeGroot LJ, editor; Jameson JL, editor. Endocrinology. 5th. Philadelphia: WB Saunders; 2005. pp. 2263–2285. [Google Scholar]
- 65.Miller WL. Auchus RJ. Geller DH. The regulation of 17,20 lyase activity. Steroids. 1997;62:133–142. doi: 10.1016/s0039-128x(96)00172-9. [DOI] [PubMed] [Google Scholar]
- 66.Wickenheisser JK. Quinn PG. Nelson VL. Legro RS. Stauss JF., 3rd McAllister JM. Differential activity of the cytochrome P450 17α-hydroxylase and steroidogenic acute regulatory protein gene promoters in normal and polycystic ovary syndrome theca cells. J Clin Endocrinol Metab. 2000;85:2304–2311. doi: 10.1210/jcem.85.6.6631. [DOI] [PubMed] [Google Scholar]
- 67.Jakimiuk AJ. Weitsman SR. Navab A. Magoffin DA. Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overexpressed in thecal and granulosa cells from polycystic ovaries. J Clin Endocrinol Metab. 2001;86:1318–1323. doi: 10.1210/jcem.86.3.7318. [DOI] [PubMed] [Google Scholar]
- 68.Blank SK. McCartney CR. Chhabra S. Helm KD. Eagleson CA. Chang RJ. Marshall JC. Modulation of gonadotropin-releasing hormone pulse generator sensitivity to progesterone inhibition in hyperandrogenic adolescent girls—implications for regulation of pubertal maturation. J Clin Endocrinol Metab. 2009;94:2360–2366. doi: 10.1210/jc.2008-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blank SK. McCartney CR. Helm KD. Marshall JC. Neuroendocrine effects of androgens in adult polycystic ovary syndrome and female puberty. Semin Reprod Med. 2007;25:352–359. doi: 10.1055/s-2007-984741. [DOI] [PubMed] [Google Scholar]
- 70.Waldstreicher J. Santoro NF. Hall JE. Filicori M. Crowley WF., Jr Hyperfunction of the hypothalamic-pituitary axis in women with polycystic ovarian disease: Indirect evidence for partial gonadotroph desensitization. J Clin Endocrinol Metab. 1988;66:165–172. doi: 10.1210/jcem-66-1-165. [DOI] [PubMed] [Google Scholar]
- 71.Gross KM. Matsumoto AM. Bremner WJ. Differential control of luteinizing hormone and follicle-stimulating hormone secretion by luteinizing hormone-releasing hormone pulse frequency in man. J Clin Endocrinol Metab. 1987;64:675–680. doi: 10.1210/jcem-64-4-675. [DOI] [PubMed] [Google Scholar]
- 72.Haisenleder DJ. Dalkin AC. Ortolano GA. Marshall JC. Shupnik MA. A pulsatile gonadotropin-releasing hormone stimulus is required to increase transcription of the gonadotropin subunit genes: evidence for differential regulation of transcription by pulse frequency in vivo. Endocrinology. 1991;128:509–517. doi: 10.1210/endo-128-1-509. [DOI] [PubMed] [Google Scholar]
- 73.Ciccone NA. Kaiser UB. The biology of gonadotroph regulation. Curr Opin Endocrinol Diabetes Obes. 2009;16:321–327. doi: 10.1097/MED.0b013e32832d88fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taylor AE. McCourt B. Martin KA. Anderson EJ. Adams JM. Schoenfeld D. Hall JE. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:2248–2256. doi: 10.1210/jcem.82.7.4105. [DOI] [PubMed] [Google Scholar]
- 75.Filicori M. Santoro N. Merriam GR. Crowley WF., Jr Characterization of the physiological pattern of episodic gonadotropin secretion throughout the human menstrual cycle. J Clin Endocrinol Metab. 1986;62:1136–1144. doi: 10.1210/jcem-62-6-1136. [DOI] [PubMed] [Google Scholar]
- 76.Rasmussen DD. Gambacciani M. Swartz W. Tueros VS. Yen SS. Pulsatile gonadotropin-releasing hormone release from the human mediobasal hypothalamus in vitro: opiate receptor-mediated suppression. Neuroendocrinology. 1989;49:150–156. doi: 10.1159/000125107. [DOI] [PubMed] [Google Scholar]
- 77.Rossmanith WG. Liu CH. Laughlin GA. Mortola JF. Suh BY. Yen SS. Relative changes in LH pulsatility during the menstrual cycle: Using data from hypogonadal women as a reference point. Clin Endocrinol (Oxf) 1990;32:647–660. doi: 10.1111/j.1365-2265.1990.tb00909.x. [DOI] [PubMed] [Google Scholar]
- 78.Gill S. Lavoie HB. Bo-Abbas Y. Hall JE. Negative feedback effects of gonadal steroids are preserved with aging in postmenopausal women. J Clin Endocrinol Metab. 2002;87:2297–2302. doi: 10.1210/jcem.87.5.8510. [DOI] [PubMed] [Google Scholar]
- 79.Nippoldt TB. Reame NE. Kelch RP. Marshall JC. The roles of estradiol and progesterone in decreasing luteinizing hormone pulse frequency in the luteal phase of the menstrual cycle. J Clin Endocrinol Metab. 1989;69:67–76. doi: 10.1210/jcem-69-1-67. [DOI] [PubMed] [Google Scholar]
- 80.Romano GJ. Krust A. Pfaff DW. Expression and estrogen regulation of progesterone receptor mRNA in neurons of the mediobasal hypothalamus: An in situ hybridization study. Mol Endocrinol. 1989;3:1295–1300. doi: 10.1210/mend-3-8-1295. [DOI] [PubMed] [Google Scholar]
- 81.Soules MR. Steiner RA. Clifton DK. Cohen NL. Aksel S. Bremner WJ. Progesterone modulation of pulsatile luteinizing hormone secretion in normal women. J Clin Endocrinol Metab. 1984;58:378–383. doi: 10.1210/jcem-58-2-378. [DOI] [PubMed] [Google Scholar]
- 82.Eagleson CA. Gingrich MB. Pastor CL. Arora TK. Burt CM. Evans WS. Marshall JC. Polycystic ovarian syndrome: Evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85:4047–4052. doi: 10.1210/jcem.85.11.6992. [DOI] [PubMed] [Google Scholar]
- 83.Goodarzi MO. Guo X. Yildiz BO. Stanczyk FZ. Azziz R. Correlation of adrenocorticotropin steroid levels between women with polycystic ovary syndrome and their sisters. Am J Obstet Gynecol. 2007;196:398. doi: 10.1016/j.ajog.2006.12.009. e1–e5. [DOI] [PubMed] [Google Scholar]
- 84.Baillargeon JP. Carpentier AC. Brothers of women with polycystic ovary syndrome are characterised by impaired glucose tolerance, reduced insulin sensitivity and related metabolic defects. Diabetologia. 2007;50:2424–2432. doi: 10.1007/s00125-007-0831-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Franks S. Webber LJ. Goh M. Valentine A. White DM. Conway GS. Wiltshire S. McCarthy MI. Ovarian morphology is a marker of heritable biochemical traits in sisters with polycystic ovaries. J Clin Endocrinol Metab. 2008;93:3396–3402. doi: 10.1210/jc.2008-0369. [DOI] [PubMed] [Google Scholar]
- 86.Sam S. Coviello AD. Sung YA. Legro RS. Dunaif A. Metabolic phenotype in the brothers of women with polycystic ovary syndrome. Diabetes Care. 2008;31:1237–1241. doi: 10.2337/dc07-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kent SC. Gnatuk CL. Kunselman AR. Demers LM. Lee PA. Legro RS. Hyperandrogenism and hyperinsulinism in children of women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2008;93:1662–1669. doi: 10.1210/jc.2007-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nam Menke M. Strauss JF., 3rd Genetics of polycystic ovarian syndrome. Clin Obstet Gynecol. 2007;50:188–204. doi: 10.1097/GRF.0b013e3180305f7c. [DOI] [PubMed] [Google Scholar]
- 89.Urbanek M. The genetics of the polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab. 2007;3:103–111. doi: 10.1038/ncpendmet0400. [DOI] [PubMed] [Google Scholar]
- 90.Unluturk U. Harmanci A. Kocaefe C. Yildiz BO. The genetic basis of the polycystic ovary syndrome: A literature review including discussion of PPAR-gamma. PPAR Res. 2007;2007:49109. doi: 10.1155/2007/49109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tucci S. Futterweit W. Concepcion ES. Greenberg DA. Villanueva R. Davies TF. Tomer Y. Evidence for association of polycystic ovary syndrome in caucasian women with a marker at the insulin receptor gene locus. J Clin Endocrinol Metab. 2001;86:446–449. doi: 10.1210/jcem.86.1.7274. [DOI] [PubMed] [Google Scholar]
- 92.Urbanek M. Woodroffe A. Ewens KG. Diamanti-Kandarakis E. Legro RS. Strauss JF., 3rd Dunaif A. Spielman RS. Candidate gene region for polycystic ovary syndrome on chromosome 19p13.2. J Clin Endocrinol Metab. 2005;90:6623–6629. doi: 10.1210/jc.2005-0622. [DOI] [PubMed] [Google Scholar]
- 93.Stewart DR. Dombroski BA. Urbanek M. Ankener W. Ewens KG. Wood JR. Legro RS. Strauss JF., 3rd Dunaif A. Spielman RS. Fine mapping of genetic susceptibility to polycystic ovary syndrome on chromosome 19p13.2 and tests for regulatory activity. J Clin Endocrinol Metab. 2006;91:4112–4117. doi: 10.1210/jc.2006-0951. [DOI] [PubMed] [Google Scholar]
- 94.Urbanek M. Sam S. Legro RS. Dunaif A. Identification of a polycystic ovary syndrome susceptibility variant in fibrillin-3 and association with a metabolic phenotype. J Clin Endocrinol Metab. 2007;92:4191–4198. doi: 10.1210/jc.2007-0761. [DOI] [PubMed] [Google Scholar]
- 95.Matzuk MM. Revelations of ovarian follicle biology from gene knockout mice. Mol Cell Endocrinol. 2000;163:61–66. doi: 10.1016/s0303-7207(99)00241-5. [DOI] [PubMed] [Google Scholar]
- 96.Xita N. Georgiou I. Lazaros L. Psofaki V. Kolios G. Tsatsoulis A. The role of sex hormone-binding globulin and androgen receptor gene variants in the development of polycystic ovary syndrome. Hum Reprod. 2008;23:693–698. doi: 10.1093/humrep/dem382. [DOI] [PubMed] [Google Scholar]
- 97.Xita N. Georgiou I. Lazaros L. Psofaki V. Kolios G. Tsatsoulis A. The synergistic effect of sex hormone-binding globulin and aromatase genes on polycystic ovary syndrome phenotype. Eur J Endocrinol. 2008;158:861–865. doi: 10.1530/EJE-07-0905. [DOI] [PubMed] [Google Scholar]
- 98.Jones MR. Mathur R. Cui J. Guo X. Azziz R. Goodarzi MO. Independent confirmation of association between metabolic phenotypes of polycystic ovary syndrome and variation in the type 6 17beta-hydroxysteroid dehydrogenase gene. J Clin Endocrinol Metab. 2009;94:5034–5038. doi: 10.1210/jc.2009-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xita N. Tsatsoulis A. Review: fetal programming of polycystic ovary syndrome by androgen excess: Evidence from experimental, clinical, and genetic association studies. J Clin Endocrinol Metab. 2006;91:1660–1666. doi: 10.1210/jc.2005-2757. [DOI] [PubMed] [Google Scholar]
- 100.Zhou R. Bird IM. Dumesic DA. Abbott DH. Adrenal hyperandrogenism is induced by fetal androgen excess in a rhesus monkey model of polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:6630–6637. doi: 10.1210/jc.2005-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abbott DH. Barnett DK. Bruns CM. Dumesic DA. Androgen excess fetal programming of female reproduction: A developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11:357–374. doi: 10.1093/humupd/dmi013. [DOI] [PubMed] [Google Scholar]
- 102.Abbott DH. Bird IM. Nonhuman primates as models for human adrenal androgen production: function and dysfunction. Rev Endocr Metab Disord. 2009;10:33–42. doi: 10.1007/s11154-008-9099-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sir-Petermann T. Maliqueo M. Angel B. Lara HE. Perez-Bravo F. Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17:2573–2579. doi: 10.1093/humrep/17.10.2573. [DOI] [PubMed] [Google Scholar]
- 104.Carlsen SM. Jacobsen G. Romundstad P. Maternal testosterone levels during pregnancy are associated with offspring size at birth. Eur J Endocrinol. 2006;155:365–370. doi: 10.1530/eje.1.02200. [DOI] [PubMed] [Google Scholar]
- 105.Sir-Petermann T. Hitchsfeld C. Maliqueo M. Codner E. Echiburu B. Gazitua R. Recabarren S. Cassorla F. Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum Reprod. 2005;20:2122–2126. doi: 10.1093/humrep/dei009. [DOI] [PubMed] [Google Scholar]
- 106.McClamrock HD. Adashi EY. Gestational hyperandrogenism. Fertil Steril. 1992;57:257–274. doi: 10.1016/s0015-0282(16)54828-6. [DOI] [PubMed] [Google Scholar]
- 107.Cole B. Hensinger K. Maciel GA. Chang RJ. Erickson GF. Human fetal ovary development involves the spatiotemporal expression of p450c17 protein. J Clin Endocrinol Metab. 2006;91:3654–3661. doi: 10.1210/jc.2006-0641. [DOI] [PubMed] [Google Scholar]
- 108.Barnes RB. Rosenfield RL. Ehrmann DA. Cara JF. Cuttler L. Levitsky LL. Rosenthal IM. Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: Evidence for perinatal masculinization of neuroendocrine function in women. J Clin Endocrinol Metab. 1994;79:1328–1333. doi: 10.1210/jcem.79.5.7962325. [DOI] [PubMed] [Google Scholar]
- 109.Demissie M. Lazic M. Foecking EM. Aird F. Dunaif A. Levine JE. Transient prenatal androgen exposure produces metabolic syndrome in adult female rats. Am J Physiol Endocrinol Metab. 2008;295:E262–E268. doi: 10.1152/ajpendo.90208.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Diamanti-Kandarakis E. Piperi C. Genetics of polycystic ovary syndrome: searching for the way out of the labyrinth. Hum Reprod Update. 2005;11:631–643. doi: 10.1093/humupd/dmi025. [DOI] [PubMed] [Google Scholar]
- 111.Ibanez L. Potau N. Francois I. de Zegher F. Precocious pubarche, hyperinsulinism, and ovarian hyperandrogenism in girls: relation to reduced fetal growth. J Clin Endocrinol Metab. 1998;83:3558–3562. doi: 10.1210/jcem.83.10.5205. [DOI] [PubMed] [Google Scholar]
- 112.Miller WL. Androgen synthesis in adrenarche. Rev Endocr Metab Disord. 2009;10:3–17. doi: 10.1007/s11154-008-9102-4. [DOI] [PubMed] [Google Scholar]
- 113.Ibanez L. Potau N. Zampolli M. Rique S. Saenger P. Carrascosa A. Hyperinsulinemia and decreased insulin-like growth factor-binding protein-1 are common features in prepubertal and pubertal girls with a history of premature pubarche. J Clin Endocrinol Metab. 1997;82:2283–2288. doi: 10.1210/jcem.82.7.4084. [DOI] [PubMed] [Google Scholar]
- 114.Ibanez L. Dimartino-Nardi J. Potau N. Saenger P. Premature adrenarche—normal variant or forerunner of adult disease? Endocr Rev. 2000;21:671–696. doi: 10.1210/edrv.21.6.0416. [DOI] [PubMed] [Google Scholar]
- 115.Parker LN. Lifrak ET. Odell WD. A 60,000 molecular weight human pituitary glycopeptide stimulates adrenal androgen secretion. Endocrinology. 1983;113:2092–2096. doi: 10.1210/endo-113-6-2092. [DOI] [PubMed] [Google Scholar]
- 116.Mellon SH. Shively JE. Miller WL. Human proopiomelanocortin-(79-96), a proposed androgen stimulatory hormone, does not affect steroidogenesis in cultured human fetal adrenal cells. J Clin Endocrinol Metab. 1991;72:19–22. doi: 10.1210/jcem-72-1-19. [DOI] [PubMed] [Google Scholar]
- 117.Dickerman Z. Grant DR. Faiman C. Winter JS. Intraadrenal steroid concentrations in man: Zonal differences and developmental changes. J Clin Endocrinol Metab. 1984;59:1031–1036. doi: 10.1210/jcem-59-6-1031. [DOI] [PubMed] [Google Scholar]
- 118.Zhang L-H. Rodriguez H. Ohno S. Miller WL. Serine phosphorylation of human P450c17 increases 17,20-lyase activity: Implications for adrenarche and the polycystic ovary syndrome. Proc Natl Acad Sci USA. 1995;92:10619–10623. doi: 10.1073/pnas.92.23.10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ibanez L. Potau N. Marcos MV. de Zegher F. Corticotropin-releasing hormone as adrenal androgen secretagogue. Pediatr Res. 1999;46:351–353. doi: 10.1203/00006450-199909000-00018. [DOI] [PubMed] [Google Scholar]
- 120.Ibanez L. Potau N. Marcos MV. de Zegher F. Corticotropin-releasing hormone: a potent androgen secretagogue in girls with hyperandrogenism after precocious pubarche. J Clin Endocrinol Metab. 1999;84:4602–4606. doi: 10.1210/jcem.84.12.6239. [DOI] [PubMed] [Google Scholar]
- 121.Ibanez L. Lopez-Bermejo A. Callejo J. Torres A. Cabre S. Dunger D. de Zegher F. Polycystic ovaries in nonobese adolescents and young women with ovarian androgen excess: relation to prenatal growth. J Clin Endocrinol Metab. 2008;93:196–199. doi: 10.1210/jc.2007-1800. [DOI] [PubMed] [Google Scholar]
- 122.Ibanez L. Valls C. Potau N. Marcos MV. de Zegher F. Polycystic ovary syndrome after precocious pubarche: Ontogeny of the low-birthweight effect. Clin Endocrinol (Oxf) 2001;55:667–672. doi: 10.1046/j.1365-2265.2001.01399.x. [DOI] [PubMed] [Google Scholar]
- 123.Ibanez L. Potau N. Virdis R. Zampolli M. Terzi C. Cussinye M. Carrascosa A. Vicens-Calvet E. Postpubertal outcome in girls diagnosed of premature pubarche during childhood: Increased frequency of functional ovarian hyperandrogenism. J Clin Endocrinol Metab. 1993;76:1599–1603. doi: 10.1210/jcem.76.6.8501168. [DOI] [PubMed] [Google Scholar]
- 124.Diamanti-Kandarakis E. Christakou C. Kandarakis H. Polycystic ovarian syndrome: the commonest cause of hyperandrogenemia in women as a risk factor for metabolic syndrome. Minerva Endocrinol. 2007;32:35–47. [PubMed] [Google Scholar]
- 125.Martha PM., Jr. Rogol AD. Veldhuis JD. Kerrigan JR. Goodman DW. Blizzard RM. Alterations in the pulsatile properties of circulating growth hormone concentrations during puberty in boys. J Clin Endocrinol Metab. 1989;69:563–570. doi: 10.1210/jcem-69-3-563. [DOI] [PubMed] [Google Scholar]
- 126.Caprio S. Plewe G. Diamond MP. Simonson DC. Boulware SD. Sherwin RS. Tamborlane WV. Increased insulin secretion in puberty: A compensatory response to reductions in insulin sensitivity. J Pediatr. 1989;114:963–967. doi: 10.1016/s0022-3476(89)80438-x. [DOI] [PubMed] [Google Scholar]
- 127.de Zegher F. Lopez-Bermejo A. Ibanez L. Adipose tissue expandability and the early origins of PCOS. Trends Endocrinol Metab. 2009;20:418–423. doi: 10.1016/j.tem.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 128.Virtue S. Vidal-Puig A. It's not how fat you are, it's what you do with it that counts. PLoS Biol. 2008;6:e237. doi: 10.1371/journal.pbio.0060237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Garg A. Adipose tissue dysfunction in obesity and lipodystrophy. Clin Cornerstone. 2006;8(Suppl 4):S7–S13. doi: 10.1016/s1098-3597(06)80039-6. [DOI] [PubMed] [Google Scholar]
- 130.Gray SL. Vidal-Puig AJ. Adipose tissue expandability in the maintenance of metabolic homeostasis. Nutr Rev. 2007;65:S7–S12. doi: 10.1111/j.1753-4887.2007.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 131.Virtue S. Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome—an allostatic perspective. Biochim Biophys Acta. 2010;1801:338–349. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 132.Christakou CD. Diamanti-Kandarakis E. Role of androgen excess on metabolic aberrations and cardiovascular risk in women with polycystic ovary syndrome. Womens Health (Lond Engl) 2008;4:583–594. doi: 10.2217/17455057.4.6.583. [DOI] [PubMed] [Google Scholar]
- 133.Mayes JS. Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev. 2004;5:197–216. doi: 10.1111/j.1467-789X.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 134.Anderson LA. McTernan PG. Harte AL. Barnett AH. Kumar S. The regulation of HSL and LPL expression by DHT and flutamide in human subcutaneous adipose tissue. Diabetes Obes Metab. 2002;4:209–213. doi: 10.1046/j.1463-1326.2002.00214.x. [DOI] [PubMed] [Google Scholar]
- 135.Arner P. Effects of testosterone on fat cell lipolysis. Species differences and possible role in polycystic ovarian syndrome. Biochimie. 2005;87:39–43. doi: 10.1016/j.biochi.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 136.McInnes KJ. Corbould A. Simpson ER. Jones ME. Regulation of adenosine 5′-monophosphate-activated protein kinase and lipogenesis by androgens contributes to visceral obesity in an estrogen-deficient state. Endocrinology. 2006;147:5907–5913. doi: 10.1210/en.2006-0879. [DOI] [PubMed] [Google Scholar]
- 137.Wu FC. von Eckardstein A. Androgens and coronary artery disease. Endocr Rev. 2003;24:183–217. doi: 10.1210/er.2001-0025. [DOI] [PubMed] [Google Scholar]
- 138.Diamanti-Kandarakis E. Papavassiliou AG. Kandarakis SA. Chrousos GP. Pathophysiology and types of dyslipidemia in PCOS. Trends Endocrinol Metab. 2007;18:280–285. doi: 10.1016/j.tem.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 139.Langer C. Gansz B. Goepfert C. Engel T. Uehara Y. von Dehn G. Jansen H. Assmann G. von Eckardstein A. Testosterone up-regulates scavenger receptor BI and stimulates cholesterol efflux from macrophages. Biochem Biophys Res Commun. 2002;296:1051–1057. doi: 10.1016/s0006-291x(02)02038-7. [DOI] [PubMed] [Google Scholar]
- 140.Paradisi G. Steinberg HO. Hempfling A. Cronin J. Hook G. Shepard MK. Baron AD. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation. 2001;103:1410–1415. doi: 10.1161/01.cir.103.10.1410. [DOI] [PubMed] [Google Scholar]
- 141.Kravariti M. Naka KK. Kalantaridou SN. Kazakos N. Katsouras CS. Makrigiannakis A. Paraskevaidis EA. Chrousos GP. Tsatsoulis A. Michalis LK. Predictors of endothelial dysfunction in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:5088–5095. doi: 10.1210/jc.2005-0151. [DOI] [PubMed] [Google Scholar]
- 142.Luque-Ramirez M. Mendieta-Azcona C. Alvarez-Blasco F. Escobar-Morreale HF. Androgen excess is associated with the increased carotid intima-media thickness observed in young women with polycystic ovary syndrome. Hum Reprod. 2007;22:3197–3203. doi: 10.1093/humrep/dem324. [DOI] [PubMed] [Google Scholar]
- 143.Chen MJ. Yang WS. Yang JH. Chen CL. Ho HN. Yang YS. Relationship between androgen levels and blood pressure in young women with polycystic ovary syndrome. Hypertension. 2007;49:1442–1447. doi: 10.1161/HYPERTENSIONAHA.106.083972. [DOI] [PubMed] [Google Scholar]
- 144.Luque-Ramirez M. Alvarez-Blasco F. Mendieta-Azcona C. Botella-Carretero JI. Escobar-Morreale HF. Obesity is the major determinant of the abnormalities in blood pressure found in young women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:2141–2148. doi: 10.1210/jc.2007-0190. [DOI] [PubMed] [Google Scholar]
- 145.Chen YF. Naftilan AJ. Oparil S. Androgen-dependent angiotensinogen and renin messenger RNA expression in hypertensive rats. Hypertension. 1992;19:456–463. doi: 10.1161/01.hyp.19.5.456. [DOI] [PubMed] [Google Scholar]
- 146.Quan A. Chakravarty S. Chen JK. Chen JC. Loleh S. Saini N. Harris RC. Capdevila J. Quigley R. Androgens augment proximal tubule transport. Am J Physiol Renal Physiol. 2004;287:F452–F459. doi: 10.1152/ajprenal.00188.2003. [DOI] [PubMed] [Google Scholar]
- 147.Quinkler M. Bujalska IJ. Kaur K. Onyimba CU. Buhner S. Allolio B. Hughes SV. Hewison M. Stewart PM. Androgen receptor-mediated regulation of the alpha-subunit of the epithelial sodium channel in human kidney. Hypertension. 2005;46:787–798. doi: 10.1161/01.HYP.0000184362.61744.c1. [DOI] [PubMed] [Google Scholar]
- 148.Diamanti-Kandarakis E. Paterakis T. Kandarakis HA. Indices of low-grade inflammation in polycystic ovary syndrome. Ann NY Acad Sci. 2006;1092:175–186. doi: 10.1196/annals.1365.015. [DOI] [PubMed] [Google Scholar]
- 149.Diamanti-Kandarakis E. Alexandraki K. Piperi C. Protogerou A. Katsikis I. Paterakis T. Lekakis J. Panidis D. Inflammatory and endothelial markers in women with polycystic ovary syndrome. Eur J Clin Invest. 2006;36:691–697. doi: 10.1111/j.1365-2362.2006.01712.x. [DOI] [PubMed] [Google Scholar]
- 150.Fenkci V. Fenkci S. Yilmazer M. Serteser M. Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil Steril. 2003;80:123–127. doi: 10.1016/s0015-0282(03)00571-5. [DOI] [PubMed] [Google Scholar]
- 151.Chang RJ. Nakamura RM. Judd HL. Kaplan SA. Insulin resistance in nonobese patients with polycystic ovarian disease. J Clin Endocrinol Metab. 1983;57:356–359. doi: 10.1210/jcem-57-2-356. [DOI] [PubMed] [Google Scholar]
- 152.Jialal I. Naiker P. Reddi K. Moodley J. Joubert SM. Evidence for insulin resistance in nonobese patients with polycystic ovarian disease. J Clin Endocrinol Metab. 1987;64:1066–1069. doi: 10.1210/jcem-64-5-1066. [DOI] [PubMed] [Google Scholar]
- 153.Burghen GA. Givens JR. Kitabchi AE. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab. 1980;50:113–116. doi: 10.1210/jcem-50-1-113. [DOI] [PubMed] [Google Scholar]
- 154.Nagamani M. Van Dinh T. Kelver ME. Hyperinsulinemia in hyperthecosis of the ovaries. Am J Obstet Gynecol. 1986;154:384–349. doi: 10.1016/0002-9378(86)90676-9. [DOI] [PubMed] [Google Scholar]
- 155.Geffner ME. Kaplan SA. Bersch N. Golde DW. Landaw EM. Chang RJ. Persistence of insulin resistance in polycystic ovarian disease after inhibition of ovarian steroid secretion. Fertil Steril. 1986;45:327–333. [PubMed] [Google Scholar]
- 156.Dunaif A. Green G. Futterweit W. Dobrjansky A. Suppression of hyperandrogenism does not improve peripheral or hepatic insulin resistance in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1990;70:699–704. doi: 10.1210/jcem-70-3-699. [DOI] [PubMed] [Google Scholar]
- 157.Diamanti-Kandarakis E. Mitrakou A. Hennes MM. Platanissiotis D. Kaklas N. Spina J. Georgiadou E. Hoffmann RG. Kissebah AH. Raptis S. Insulin sensitivity and antiandrogenic therapy in women with polycystic ovary syndrome. Metabolism. 1995;44:525–531. doi: 10.1016/0026-0495(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 158.Diamond MP. Grainger D. Diamond MC. Sherwin RS. Defronzo RA. Effects of methyltestosterone and insulin secretion and sensitivity in women. J Clin Endocrinol Metab. 1998;83:4420–4425. doi: 10.1210/jcem.83.12.5333. [DOI] [PubMed] [Google Scholar]
- 159.Speiser PW. Serrat J. New MI. Gertner JM. Insulin insensitivity in adrenal hyperplasia due to nonclassical steroid 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1992;75:1421–1424. doi: 10.1210/jcem.75.6.1464643. [DOI] [PubMed] [Google Scholar]
- 160.Barbieri RL. Makris A. Ryan KJ. Insulin stimulates androgen accumulation in incubations of human ovarian stroma and theca. Obstet Gynecol. 1984;64:73S–80S. doi: 10.1097/00006250-198409001-00019. [DOI] [PubMed] [Google Scholar]
- 161.Cara JF. Rosenfield RL. Insulin-like growth factor I and insulin potentiate luteinizing hormone-induced androgen synthesis by rat ovarian thecal-interstitial cells. Endocrinology. 1988;123:733–739. doi: 10.1210/endo-123-2-733. [DOI] [PubMed] [Google Scholar]
- 162.Hernandez ER. Resnick CE. Holtzclaw WD. Payne DW. Adashi EY. Insulin as a regulator of androgen biosynthesis by cultured rat ovarian cells: cellular mechanism(s) underlying physiological and pharmacological hormonal actions. Endocrinology. 1988;122:2034–2043. doi: 10.1210/endo-122-5-2034. [DOI] [PubMed] [Google Scholar]
- 163.Moghetti P. Castello R. Nigri C. Tosi F. Spiazzi GG. Brun E. Balducci R. Toscano V. Muggeo M. Insulin infusion amplifies 17 alpha-hydroxycorticosteroid intermediates response to adrenocorticotropin in hyperandrogenic women: apparent relative impairment of 17,20-lyase activity. J Clin Endocrinol Metab. 1996;81:881–886. doi: 10.1210/jcem.81.3.8772544. [DOI] [PubMed] [Google Scholar]
- 164.Adashi EY. Hsueh AJ. Yen SS. Insulin enhancement of luteinizing hormone and follicle-stimulating hormone release by cultured pituitary cells. Endocrinology. 1981;108:1441–1449. doi: 10.1210/endo-108-4-1441. [DOI] [PubMed] [Google Scholar]
- 165.Soldani R. Cagnacci A. Yen SS. Insulin, insulin-like growth factor I (IGF-I) and IGF-II enhance basal and gonadotrophin-releasing hormone-stimulated luteinizing hormone release from rat anterior pituitary cells in vitro. Eur J Endocrinol. 1994;131:641–645. doi: 10.1530/eje.0.1310641. [DOI] [PubMed] [Google Scholar]
- 166.Dunkel L. Sorva R. Voutilainen R. Low levels of sex hormone-binding globulin in obese children. J Pediatr. 1985;107:95–97. doi: 10.1016/s0022-3476(85)80623-5. [DOI] [PubMed] [Google Scholar]
- 167.Nestler JE. Powers LP. Matt DW. Steingold KA. Plymate SR. Rittmaster RS. Clore JN. Blackard WG. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1991;72:83–89. doi: 10.1210/jcem-72-1-83. [DOI] [PubMed] [Google Scholar]
- 168.Suikkari AM. Koivisto VA. Rutanen EM. Yki-Jarvinen H. Karonen SL. Seppala M. Insulin regulates the serum levels of low molecular weight insulin-like growth factor-binding protein. J Clin Endocrinol Metab. 1988;66:266–272. doi: 10.1210/jcem-66-2-266. [DOI] [PubMed] [Google Scholar]
- 169.Lee PD. Conover CA. Powell DR. Regulation and function of insulin-like growth factor-binding protein-1. Proc Soc Exp Biol Med. 1993;204:4–29. doi: 10.3181/00379727-204-43630. [DOI] [PubMed] [Google Scholar]
- 170.LeRoith D. McGuinness M. Shemer J. Stannard B. Lanau F. Faria TN. Kato H. Werner H. Adamo M. Robers CTJ. Insulin-like growth factors. Biol Signals. 1992;1:173–181. doi: 10.1159/000109323. [DOI] [PubMed] [Google Scholar]
- 171.Franks S. Gilling-Smith C. Watson H. Willis D. Insulin action in the normal and polycystic ovary. Endocrinol Metab Clin North Am. 1999;28:361–378. doi: 10.1016/s0889-8529(05)70074-8. [DOI] [PubMed] [Google Scholar]
- 172.Dunaif A. Segal KR. Shelley DR. Green G. Dobrjansky A. Licholai T. Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes. 1992;41:1257–1266. doi: 10.2337/diab.41.10.1257. [DOI] [PubMed] [Google Scholar]
- 173.Conway GS. Avey C. Rumsby G. The tyrosine kinase domain of the insulin receptor gene is normal in women with hyperinsulinaemia and polycystic ovary syndrome. Hum Reprod. 1994;9:1681–1683. doi: 10.1093/oxfordjournals.humrep.a138773. [DOI] [PubMed] [Google Scholar]
- 174.Talbot JA. Bicknell EJ. Rajkhowa M. Krook A. O'Rahilly S. Clayton RN. Molecular scanning of the insulin receptor gene in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1996;81:1979–1983. doi: 10.1210/jcem.81.5.8626868. [DOI] [PubMed] [Google Scholar]
- 175.Ciaraldi TP. el-Roeiy A. Madar Z. Reichart D. Olefsky JM. Yen SS. Cellular mechanisms of insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab. 1992;75:577–583. doi: 10.1210/jcem.75.2.1322430. [DOI] [PubMed] [Google Scholar]
- 176.Dunaif A. Xia J. Book C-B. Schenker E. Tang Z. Excessive insulin receptor serine phosphorylation in cultured fibroblasts and in skeletal muscle. A potential mechanism for insulin resistance in the polycystic ovary syndrome. J Clin Invest. 1995;96:801–810. doi: 10.1172/JCI118126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bollage GE. Roth RA. Beaudoin J. Mochly-Rosen D. Doshland DEJ. Protein kinase C directly phosphorylates the insulin receptor in vitro and reduces its protein-tyrosine kinase activity. Proc Natl Acad Sci USA. 1986;83:5822–5824. doi: 10.1073/pnas.83.16.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Stadtmauer L. Rosen OM. Increasing the cAMP content of IM-9 cells alters the phosphorylation state and protein kinase activity of the insulin receptor. J Biol Chem. 1986;261:3402–3407. [PubMed] [Google Scholar]
- 179.Takayama S. White MF. Kahn CR. Phorbol ester-induced serine phosphorylation of the insulin receptor decreases its tyrosine kinase activity. J Biol Chem. 1988;263:3440–3447. [PubMed] [Google Scholar]
- 180.Chin JE. Dickens M. Tavare JM. Roth RA. Overexpression of protein kinse C isoenzymes α, βI, γ, and ε in cells overexpressing the insulin receptor. Effects on receptor phosphorylation and signaling. J Biol Chem. 1993;268:6338–6347. [PubMed] [Google Scholar]
- 181.Li M. Youngren JF. Dunaif A. Goldfine ID. Maddux BA. Zhang BB. Evans JL. Decreased insulin receptor (IR) autophosphorylation in fibroblasts from patients with PCOS: Effects of serine kinase inhibitors and IR activators. J Clin Endocrinol Metab. 2002;87:4088–4093. doi: 10.1210/jc.2002-020363. [DOI] [PubMed] [Google Scholar]
- 182.Guo H. Damuni Z. Autophosphorylation-activated protein kinase phosphorylates and inactivates protein phosphatase 2A. Proc Natl Acad Sci USA. 1993;90:2500–2504. doi: 10.1073/pnas.90.6.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Paz K. Hemi R. LeRoith D. Karasik A. Elhanany E. Kanety H. Zick Y. A molecular basis for insulin resistance. J Biol Chem. 1997;272:29911–9918. doi: 10.1074/jbc.272.47.29911. [DOI] [PubMed] [Google Scholar]
- 184.Pirola L. Johnston AM. Obberghen EV. Modulation of insulin action. Diabetologia. 2004;47:170–184. doi: 10.1007/s00125-003-1313-3. [DOI] [PubMed] [Google Scholar]
- 185.Liberman Z. Eldar-Finkelman H. Serine 332 phosphorylation of insulin receptor substrate-1 by glycogen synthase kinase-3 attenuates insulin signaling. J Biol Chem. 2005;280:4422–4428. doi: 10.1074/jbc.M410610200. [DOI] [PubMed] [Google Scholar]
- 186.Dresner A. Laurent D. Marcucci M. Griffin ME. Dufour S. Cline GW. Slezak LA. Andersen DK. Hundal RS. Rothman DL. Petersen KF. Shulman GI. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hotamisligil GS. Peraldi P. Budavari A. Ellis R. White MF. Speigelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 188.Holte J. Bergh T. Berne C. Lithell H. Serum lipoprotein lipid profile in women with the polycystic ovary syndrome: Relation to anthropometric, endocrine and metabolic variables. Clin Endocrinol. 1994;41:463–471. doi: 10.1111/j.1365-2265.1994.tb02577.x. [DOI] [PubMed] [Google Scholar]
- 189.Robinson S. Henderson AD. Gelding SV. Kiddy D. Niththyananthan R. Bush A. Richmond W. Johnston DG. Franks S. Dyslipidaemia is associated with insulin resistance in women with polycystic ovaries. Clin Endocrinol. 1996;44:277–284. doi: 10.1046/j.1365-2265.1996.674495.x. [DOI] [PubMed] [Google Scholar]
- 190.Naz RK. Thurston D. Santoro N. Circulating tumor necrosis factor (TNF)-α in normally cycling women and patients with premature ovarian failure and polycystic ovaries. Am J Reprod Immunol. 1995;34:170–175. doi: 10.1111/j.1600-0897.1995.tb00934.x. [DOI] [PubMed] [Google Scholar]
- 191.Spranger J. Kroke A. Mohlig M. Hoffmann K. Bergmann MM. Ristow M. Boeing H. Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: Results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 192.Ciaraldi TP. Carter L. Nikoulina S. Mudaliar S. McClain DA. Henry RR. Glucosamine regulation of glucose metabolism in cultured human skeletal muscle cells: divergent effects on glucose transport/phosphorylation and glycogen synthase in non-diabetic and type 2 diabetic subjects. Endocrinology. 1999;140:3971–3980. doi: 10.1210/endo.140.9.6974. [DOI] [PubMed] [Google Scholar]
- 193.Previs SF. Withers DJ. Ren JM. White MF. Shulman GI. Contrasting effects of IRS-1 versus IRS-2 gene disruption on carbohydrate and lipid metabolism. J Biol Chem. 2000;275:38990–38994. doi: 10.1074/jbc.M006490200. [DOI] [PubMed] [Google Scholar]
- 194.Cho H. Mu J. Kim JK. Thorvaldsen JL. Chu Q. Crenshaw EB., 3rd Kaestner KH. Bartolomei MS. Shulman GI. Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 195.Dunaif A. Segal KR. Futterweit W. Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165–1174. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 196.Poretsky L. On the paradox of insulin-induced hyperandrogenism in insulin-resistant states. Endocr Rev. 1991;12:3–13. doi: 10.1210/edrv-12-1-3. [DOI] [PubMed] [Google Scholar]
- 197.Taylor SI. Molecular mechanisms of insulin resistance. Lessons learned from patients with mutations in the insulin-receptor gene. Diabetes. 1992;41:1473–1490. doi: 10.2337/diab.41.11.1473. [DOI] [PubMed] [Google Scholar]
- 198.Willis D. Franks S. Insulin action in human granulosa cells from normal and polycystic ovaries is mediated by the insulin receptor and not the type-I insulin-like growth factor receptor. J Clin Endocrinol Metab. 1995;80:3788–3790. doi: 10.1210/jcem.80.12.8530637. [DOI] [PubMed] [Google Scholar]
- 199.Corbould A. Zhao H. Mirzoeva S. Aird F. Dunaif A. Enhanced mitogenic signaling in skeletal muscle of women with polycystic ovary syndrome. Diabetes. 2006;55:751–759. doi: 10.2337/diabetes.55.03.06.db05-0453. [DOI] [PubMed] [Google Scholar]
- 200.Lin Y. Fridstrom M. Hillensjo T. Insulin stimulation of lactate accumulation in isolated human granulosa-luteal cells: A comparison between normal and polycystic ovaries. Hum Reprod. 1997;12:2469–2472. doi: 10.1093/humrep/12.11.2469. [DOI] [PubMed] [Google Scholar]
- 201.Fedorcsak P. Storeng R. Dale PO. Tanbo T. Abyholm T. Impaired insulin action on granulosa-lutein cells in women with polycystic ovary syndrome and insulin resistance. Gynecol Endocrinol. 2000;14:327–336. doi: 10.3109/09513590009167701. [DOI] [PubMed] [Google Scholar]
- 202.Rice S. Christoforidis N. Gadd C. Nikolaou D. Seyani L. Donaldson A. Margara R. Hardy K. Franks S. Impaired insulin-dependent glucose metabolism in granulosa-lutein cells from anovulatory women with polycystic ovaries. Hum Reprod. 2005;20:373–381. doi: 10.1093/humrep/deh609. [DOI] [PubMed] [Google Scholar]
- 203.Bremer AA. Miller WL. The serine phosphorylation hypothesis of polycystic ovary syndrome: A unifying mechanism for hyperandrogenemia and insulin resistance. Fertil Steril. 2008;89:1039–1048. doi: 10.1016/j.fertnstert.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 204.Diamanti-Kandarakis E. Christakou C. Palioura E. Kandaraki E. Livadas S. Does polycystic ovary syndrome start in childhood? Pediatr Endocrinol Rev. 2008;5:904–911. [PubMed] [Google Scholar]
- 205.O'Brien RF. Emans SJ. Polycystic ovary syndrome in adolescents. J Pediatr Adolesc Gynecol. 2008;21:119–128. doi: 10.1016/j.jpag.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 206.Dunaif A. Mandeli J. Fluhr H. Dobrjansky A. The impact of obesity and chronic hyperinsulinemia on gonadotropin release and gonadal steroid secretion in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1988;66:131–139. doi: 10.1210/jcem-66-1-131. [DOI] [PubMed] [Google Scholar]
- 207.Ciampelli M. Fulghesu AM. Cucinelli F. Pavone V. Ronsisvalle E. Guido M. Caruso A. Lanzone A. Impact of insulin and body mass index on metabolic and endocrine variables in polycystic ovary syndrome. Metabolism. 1999;48:167–172. doi: 10.1016/s0026-0495(99)90028-8. [DOI] [PubMed] [Google Scholar]
- 208.Moran C. Renteria JL. Moran S. Herrera J. Gonzalez S. Bermudez JA. Obesity differentially affects serum levels of androstenedione and testosterone in polycystic ovary syndrome. Fertil Steril. 2008;90:2310–2317. doi: 10.1016/j.fertnstert.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 209.Hedley AA. Ogden CL. Johnson CL. Carroll MD. Curtin LR. Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 210.Palmert MR. Gordon CM. Kartashov AI. Legro RS. Emans SJ. Dunaif A. Screening for abnormal glucose tolerance in adolescents with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:1017–1023. doi: 10.1210/jcem.87.3.8305. [DOI] [PubMed] [Google Scholar]
- 211.Albrecht L. Styne D. Laboratory testing of gonadal steroids in children. Pediatr Endocrinol Rev. 2007;5(Suppl 1):599–607. [PubMed] [Google Scholar]
- 212.Nisenblat V. Norman RJ. Androgens and polycystic ovary syndrome. Curr Opin Endocrinol Diabetes Obes. 2009;16:224–231. doi: 10.1097/MED.0b013e32832afd4d. [DOI] [PubMed] [Google Scholar]
- 213.Sir-Petermann T. Maliqueo M. Codner E. Echiburu B. Crisosto N. Perez V. Perez-Bravo F. Cassorla F. Early metabolic derangements in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:4637–4642. doi: 10.1210/jc.2007-1036. [DOI] [PubMed] [Google Scholar]
- 214.Chang RJ. Coffler MS. Polycystic ovary syndrome: early detection in the adolescent. Clin Obstet Gynecol. 2007;50:178–187. doi: 10.1097/GRF.0b013e31802f50fc. [DOI] [PubMed] [Google Scholar]
- 215.Giallauria F. Palomba S. Vigorito C. Tafuri MG. Colao A. Lombardi G. Orio F. Androgens in polycystic ovary syndrome: The role of exercise and diet. Semin Reprod Med. 2009;27:306–315. doi: 10.1055/s-0029-1225258. [DOI] [PubMed] [Google Scholar]
- 216.Kiddy DS. Hamilton-Fairley D. Bush A. Short F. Anyaoku V. Reed MJ. Franks S. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 1992;36:105–111. doi: 10.1111/j.1365-2265.1992.tb02909.x. [DOI] [PubMed] [Google Scholar]
- 217.Clark AM. Ledger W. Galletly C. Tomlinson L. Blaney F. Wang X. Norman RJ. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum Reprod. 1995;10:2705–2712. doi: 10.1093/oxfordjournals.humrep.a135772. [DOI] [PubMed] [Google Scholar]
- 218.Holte J. Bergh T. Berne C. Wide L. Lithell H. Restored insulin sensitivity but persistently increased early insulin secretion after weight loss in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1995;80:2586–2593. doi: 10.1210/jcem.80.9.7673399. [DOI] [PubMed] [Google Scholar]
- 219.Clark AM. Thornley B. Tomlinson L. Galletley C. Norman RJ. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod. 1998;13:1502–1505. doi: 10.1093/humrep/13.6.1502. [DOI] [PubMed] [Google Scholar]
- 220.Huber-Buchholz MM. Carey DG. Norman RJ. Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome: Role of insulin sensitivity and luteinizing hormone. J Clin Endocrinol Metab. 1999;84:1470–1474. doi: 10.1210/jcem.84.4.5596. [DOI] [PubMed] [Google Scholar]
- 221.Crosignani PG. Colombo M. Vegetti W. Somigliana E. Gessati A. Ragni G. Overweight and obese anovulatory patients with polycystic ovaries: Parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Hum Reprod. 2003;18:1928–1932. doi: 10.1093/humrep/deg367. [DOI] [PubMed] [Google Scholar]
- 222.Moran LJ. Noakes M. Clifton PM. Tomlinson L. Galletly C. Norman RJ. Dietary composition in restoring reproductive and metabolic physiology in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:812–819. doi: 10.1210/jc.2002-020815. [DOI] [PubMed] [Google Scholar]
- 223.Homburg R. Lambalk CB. Polycystic ovary syndrome in adolescence—a therapeutic conundrum. Hum Reprod. 2004;19:1039–1042. doi: 10.1093/humrep/deh207. [DOI] [PubMed] [Google Scholar]
- 224.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 225.Ludwig DS. Peterson KE. Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: A prospective, observational analysis. Lancet. 2001;357:505–508. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 226.Bray GA. Nielsen SJ. Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 227.Mrdjenovic G. Levitsky DA. Nutritional and energetic consequences of sweetened drink consumption in 6- to 13-year-old children. J Pediatr. 2003;142:604–610. doi: 10.1067/mpd.2003.200. [DOI] [PubMed] [Google Scholar]
- 228.Johnson L. Mander AP. Jones LR. Emmett PM. Jebb SA. Is sugar-sweetened beverage consumption associated with increased fatness in children? Nutrition. 2007;23:557–563. doi: 10.1016/j.nut.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 229.Bremer AA. Auinger P. Byrd RS. Relationship between insulin resistance-associated metabolic parameters and anthropometric measurements with sugar-sweetened beverage intake and physical activity levels in US adolescents: Findings from the 1999–2004 National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2009;163:328–335. doi: 10.1001/archpediatrics.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Pate RR. Pratt M. Blair SN. Haskell WL. Macera CA. Bouchard C. Buchner D. Ettinger W. Heath GW. King AC. Kriska A. Leon AS; Marcus BH. Morris J. Paffenbarger RS Jr. Patrick K. Pollock ML. Rippe JM. Sallis J. Wilmore JH. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 231.Venables MC. Jeukendrup AE. Endurance training and obesity: Effect on substrate metabolism and insulin sensitivity. Med Sci Sports Exerc. 2008;40:495–502. doi: 10.1249/MSS.0b013e31815f256f. [DOI] [PubMed] [Google Scholar]
- 232.Ren JM. Semenkovich CF. Gulve EA. Gao J. Holloszy JO. Exercise induces rapid increases in GLUT4 expression, glucose transport capacity, and insulin-stimulated glycogen storage in muscle. J Biol Chem. 1994;269:14396–14401. [PubMed] [Google Scholar]
- 233.Perseghin G. Price TB. Petersen KF. Roden M. Cline GW. Gerow K. Rothman DL. Shulman GI. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med. 1996;335:1357–1362. doi: 10.1056/NEJM199610313351804. [DOI] [PubMed] [Google Scholar]
- 234.Berlan ED. Emans SJ. Managing polycystic ovary syndrome in adolescent patients. J Pediatr Adolesc Gynecol. 2009;22:137–140. doi: 10.1016/j.jpag.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 235.Guttmann-Bauman I. Approach to adolescent polycystic ovary syndrome (PCOS) in the pediatric endocrine community in the U.S.A. J Pediatr Endocrinol Metab. 2005;18:499–506. doi: 10.1515/jpem.2005.18.5.499. [DOI] [PubMed] [Google Scholar]
- 236.Korytkowski MT. Mokan M. Horwitz MJ. Berga SL. Metabolic effects of oral contraceptives in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1995;80:3327–3334. doi: 10.1210/jcem.80.11.7593446. [DOI] [PubMed] [Google Scholar]
- 237.Hillard PJ. Oral contraceptives and the management of hyperandrogenism-polycystic ovary syndrome in adolescents. Endocrinol Metab Clin North Am. 2005;34:707–723. doi: 10.1016/j.ecl.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 238.Thorneycroft IH. Stanczyk FZ. Bradshaw KD. Ballagh SA. Nichols M. Weber ME. Effect of low-dose oral contraceptives on Hillard PJ. Oral contraceptives and the management of hyperandrogenism-polycystic ovary syndrome in adolescents androgenic markers and acne. Contraception. 1999;60:255–262. doi: 10.1016/s0010-7824(99)00093-1. [DOI] [PubMed] [Google Scholar]
- 239.Mastorakos G. Koliopoulos C. Deligeoroglou E. Diamanti-Kandarakis E. Creatsas G. Effects of two forms of combined oral contraceptives on carbohydrate metabolism in adolescents with polycystic ovary syndrome. Fertil Steril. 2006;85:420–427. doi: 10.1016/j.fertnstert.2005.07.1306. [DOI] [PubMed] [Google Scholar]
- 240.Pfeifer SM. Kives S. Polycystic ovary syndrome in the adolescent. Obstet Gynecol Clin North Am. 2009;36:129–152. doi: 10.1016/j.ogc.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 241.Ibanez L. Potau N. Marcos MV. de Zegher F. Treatment of hirsutism, hyperandrogenism, oligomenorrhea, dyslipidemia, and hyperinsulinism in nonobese, adolescent girls: Effect of flutamide. J Clin Endocrinol Metab. 2000;85:3251–3255. doi: 10.1210/jcem.85.9.6814. [DOI] [PubMed] [Google Scholar]
- 242.Moghetti P. Tosi F. Tosti A. Negri C. Misciali C. Perrone F. Caputo M. Muggeo M. Castello R. Comparison of spironolactone, flutamide, and finasteride efficacy in the treatment of hirsutism: A randomized, double blind, placebo-controlled trial. J Clin Endocrinol Metab. 2000;85:89–94. doi: 10.1210/jcem.85.1.6245. [DOI] [PubMed] [Google Scholar]
- 243.Practice Committee of American Society for Reproductive Medicine. Use of insulin-sensitizing agents in the treatment of polycystic ovary syndrome. Fertil Steril. 2008;90:S69–S73. doi: 10.1016/j.fertnstert.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 244.Katsiki N. Georgiadou E. Hatzitolios AI. The role of insulin-sensitizing agents in the treatment of polycystic ovary syndrome. Drugs. 2009;69:1417–1431. doi: 10.2165/00003495-200969110-00001. [DOI] [PubMed] [Google Scholar]
- 245.Palomba S. Falbo A. Zullo F. Orio F., Jr Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: a comprehensive review. Endocr Rev. 2009;30:1–50. doi: 10.1210/er.2008-0030. [DOI] [PubMed] [Google Scholar]
- 246.Diamanti-Kandarakis E. Christakou CD. Kandaraki E. Economou FN. Metformin: An old medication of new fashion: Evolving new molecular mechanisms and clinical implications in polycystic ovary syndrome. Eur J Endocrinol. 2010;162:193–212. doi: 10.1530/EJE-09-0733. [DOI] [PubMed] [Google Scholar]
- 247.Inzucchi SE. Maggs DG. Spollett GR. Page SL. Rife FS. Walton V. Shulman GI. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med. 1998;338:867–872. doi: 10.1056/NEJM199803263381303. [DOI] [PubMed] [Google Scholar]
- 248.La Marca A. Artensio AC. Stabile G. Volpe A. Metformin treatment of PCOS during adolescence and the reproductive period. Eur J Obstet Gynecol Reprod Biol. 2005;121:3–7. doi: 10.1016/j.ejogrb.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 249.Knowler WC. Barrett-Connor E. Fowler SE. Hamman RF. Lachin JM. Walker EA. Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ibanez L. Ferrer A. Ong K. Amin R. Dunger D. de Zegher F. Insulin sensitization early after menarche prevents progression from precocious pubarche to polycystic ovary syndrome. J Pediatr. 2004;144:23–29. doi: 10.1016/j.jpeds.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 251.Arslanian SA. Lewy V. Danadian K. Saad R. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: Amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. J Clin Endocrinol Metab. 2002;87:1555–1559. doi: 10.1210/jcem.87.4.8398. [DOI] [PubMed] [Google Scholar]
- 252.Glueck CJ. Wang P. Fontaine R. Tracy T. Sieve-Smith L. Metformin to restore normal menses in oligo-amenorrheic teenage girls with polycystic ovary syndrome (PCOS) J Adolesc Health. 2001;29:160–169. doi: 10.1016/s1054-139x(01)00202-6. [DOI] [PubMed] [Google Scholar]
- 253.Ibanez L. Valls C. Ferrer A. Marcos MV. Rodriguez-Hierro F. de Zegher F. Sensitization to insulin induces ovulation in nonobese adolescents with anovulatory hyperandrogenism. J Clin Endocrinol Metab. 2001;86:3595–3598. doi: 10.1210/jcem.86.8.7756. [DOI] [PubMed] [Google Scholar]
- 254.Freemark M. Bursey D. The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes. Pediatrics. 2001;107:E55. doi: 10.1542/peds.107.4.e55. [DOI] [PubMed] [Google Scholar]
- 255.Kay JP. Alemzadeh R. Langley G. D'Angelo L. Smith P. Holshouser S. Beneficial effects of metformin in normoglycemic morbidly obese adolescents. Metabolism. 2001;50:1457–1461. doi: 10.1053/meta.2001.28078. [DOI] [PubMed] [Google Scholar]
- 256.Bridger T. MacDonald S. Baltzer F. Rodd C. Randomized placebo-controlled trial of metformin for adolescents with polycystic ovary syndrome. Arch Pediatr Adolesc Med. 2006;160:241–246. doi: 10.1001/archpedi.160.3.241. [DOI] [PubMed] [Google Scholar]
- 257.De Leo V. Musacchio MC. Morgante G. Piomboni P. Petraglia F. Metformin treatment is effective in obese teenage girls with PCOS. Hum Reprod. 2006;21:2252–2256. doi: 10.1093/humrep/del185. [DOI] [PubMed] [Google Scholar]
- 258.Ibanez L. Valls C. Marcos MV. Ong K. Dunger DB. De Zegher F. Insulin sensitization for girls with precocious pubarche and with risk for polycystic ovary syndrome: Effects of prepubertal initiation and postpubertal discontinuation of metformin treatment. J Clin Endocrinol Metab. 2004;89:4331–4337. doi: 10.1210/jc.2004-0463. [DOI] [PubMed] [Google Scholar]
- 259.Spiegelman BM. PPAR-gamma: Adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507–514. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- 260.Kersten S. Desvergne B. Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 261.Hasegawa I. Murakawa H. Suzuki M. Yamamoto Y. Kurabayashi T. Tanaka K. Effect of troglitazone on endocrine and ovulatory performance in women with insulin resistance-related polycystic ovary syndrome. Fertil Steril. 1999;71:323–327. doi: 10.1016/s0015-0282(98)00454-3. [DOI] [PubMed] [Google Scholar]
- 262.Azziz R. Ehrmann D. Legro RS. Whitcomb RW. Hanely R. Fereshetian AG. O'Keefe M. Ghzaai MN. Group. PTS. Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: A multicenter, double blind, placebo-controlled trial. J Clin Endocrinol Metab. 2001;86:1626–1632. doi: 10.1210/jcem.86.4.7375. [DOI] [PubMed] [Google Scholar]
- 263.Azziz R. Ehrmann D. Legro R. Fereshetian A. O'Keefe M. Ghazzi MN PCOS/Troglitazone Study Group. Troglitazone decreases adrenal androgen levels in women with polycystic ovary syndrome. Fertil Steril. 2003;79:932–937. doi: 10.1016/s0015-0282(02)04914-2. [DOI] [PubMed] [Google Scholar]
- 264.Ortega-Gonzalez C. Luna S. Hernandez L. Crespo G. Aguayo P. Arteaga-Troncoso G. Parra A. Responses of serum androgen and insulin resistance to metformin and pioglitazone in obese, insulin-resistant women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:1360–1365. doi: 10.1210/jc.2004-1965. [DOI] [PubMed] [Google Scholar]
- 265.Prelevic GM. Wurzburger MI. Balint-Peric L. Hardiman P. Okolo S. Maletic D. Ginsburg J. Effects of the somatostatin analogue, octreotide, in polycystic ovary syndrome. Metabolism. 1992;41:76–79. doi: 10.1016/0026-0495(92)90036-a. [DOI] [PubMed] [Google Scholar]
- 266.Prelevic GM. Wurzburger MI. Balint-Peric L. Nesic JS. Inhibitory effect of sandostatin on secretion of luteinising hormone and ovarian steroids in polycystic ovary syndrome. Lancet. 1990;336:900–903. doi: 10.1016/0140-6736(90)92270-r. [DOI] [PubMed] [Google Scholar]
- 267.Fulghesu AM. Lanzone A. Andreani CL. Pierro E. Caruso A. Mancuso S. Effectiveness of a somatostatin analogue in lowering luteinizing hormone and insulin-stimulated secretion in hyperinsulinemic women with polycystic ovary disease. Fertil Steril. 1995;64:703–708. doi: 10.1016/s0015-0282(16)57842-x. [DOI] [PubMed] [Google Scholar]
- 268.Hsu WH. Xiang HD. Rajan AS. Kunze DL. Boyd AE., 3rd Somatostatin inhibits insulin secretion by a G-protein-mediated decrease in Ca2+ entry through voltage-dependent Ca2+ channels in the beta cell. J Biol Chem. 1991;266:837–843. [PubMed] [Google Scholar]
- 269.Brazeau P. Vale W. Burgus R. Ling N. Butcher M. Rivier J. Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- 270.Chiodera P. Volpi R. d'Amato L. Fatone M. Cigarini C. Fava A. Caiazza A. Rossi G. Coiro V. Inhibition by somatostatin of LH-RH-induced LH release in normal menstruating women. Gynecol Obstet Invest. 1986;22:17–21. doi: 10.1159/000298884. [DOI] [PubMed] [Google Scholar]
- 271.Hoeger K. Davidson K. Kochman L. Cherry T. Kopin L. Guzick DS. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. J Clin Endocrinol Metab. 2008;93:4299–4306. doi: 10.1210/jc.2008-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ibanez L. de Zegher F. Ethinylestradiol-drospirenone, flutamide-metformin, or both for adolescents and women with hyperinsulinemic hyperandrogenism: Opposite effects on adipocytokines and body adiposity. J Clin Endocrinol Metab. 2004;89:1592–1597. doi: 10.1210/jc.2003-031281. [DOI] [PubMed] [Google Scholar]
- 273.Ibanez L. Valls C. Cabre S. De Zegher F. Flutamide-metformin plus ethinylestradiol-drospirenone for lipolysis and antiatherogenesis in young women with ovarian hyperandrogenism: The key role of early, low-dose flutamide. J Clin Endocrinol Metab. 2004;89:4716–4720. doi: 10.1210/jc.2004-0047. [DOI] [PubMed] [Google Scholar]
- 274.Ibanez L. de Zegher F. Flutamide-metformin plus ethinylestradiol-drospirenone for lipolysis and antiatherogenesis in young women with ovarian hyperandrogenism: the key role of metformin at the start and after more than one year of therapy. J Clin Endocrinol Metab. 2005;90:39–43. doi: 10.1210/jc.2004-1405. [DOI] [PubMed] [Google Scholar]