Abstract
Background
Fibroblast growth factor 23 (FGF-23), a phosphaturic peptide hormone secreted by the osteoblasts, is an important regulator of phosphorus and vitamin D metabolism. In chronic kidney disease, FGF-23 levels rise with declining kidney function. Increasing FGF-23 levels are associated with increasing risk of mortality in dialysis patients. Two assays for FGF-23 have been reported. One assay detects only full-length/intact FGF-23. In contrast, the carboxy-terminal assay recognizes both intact and carboxy-terminal FGF-23.
Aim/Methods
The aim of this study was to evaluate both assays for FGF-23. Test samples were analyzed with both the intact and carboxy-terminal FGF-23 enzyme-linked immunosorbent assay (ELISA) kits according to manufacturers' instructions.
Results
Carboxy-terminal FGF-23 showed very good precision with coefficients of variation (CV) ranging from 4% to 10.5%, whereas the CVs for intact FGF-23 were not very good (6–37.5%). The carboxy-terminal assay was linear, stable in plasma samples, and was not affected by common interferents. Also, the carboxy-terminal FGF-23 assay appeared to correlate better with worsening of kidney function as assessed by plasma creatinine and calculated estimated glomerular filtration rate (eGFR).
Conclusion
Thus, the carboxy-terminal FGF-23 assay is robust and can be used in prospective trials to validate its utility as a biomarker of adverse outcomes in patients with renal disease.
Introduction
Fibroblast growth factor 23 (FGF-23), a phosphaturic peptide hormone secreted by the osteoblasts, is an important regulator of phosphorus and vitamin D metabolism.1–3 FGF-23 was recently shown to be involved in the development of several hypophosphatemic diseases, including X-linked hypophosphatemic rickets/osteomalacia (XLH) and tumor-induced rickets/osteomalacia (TIO).1–5 FGF-23 is processed between Arg179 and Ser180, and only full-length FGF-23 was shown to cause hypophosphatemia, due to impaired renal tubular phosphate absorption.6
End-stage renal disease is a common complication of long-term diabetes and subjects with the metabolic syndrome. In chronic kidney disease, phosphate levels rise with declining glomerular filtration rate (GFR) and phosphate accumulates. Also, FGF-23 levels increase and promote increased renal phosphate excretion.1–5 FGF-23 inhibits 1-α-hydrolase expression, leading to decreased hydroxylation of 25-hydroxyvitamin D [25(OH)D] to 1,25-dihydroxyvitamin D [1,25 (OH)2D]. FGF-23 null mice exhibit increased cardiovascular calcification, which is associated with hyperphosphatemia and excess 1,25 (OH)2D levels, and they die prematurely.7 In long-term hemodialysis patients, high levels of carboxy-terminal FGF-23 were significantly associated with the 2-year mortality rate, with a significantly increased hazard ratio (HR) of 2.5.8 Also, Gutierrez et al.9 demonstrated in a prospective cohort of 10,044 patients with chronic kidney disease that median carboxy-terminal FGF-23 levels were significantly higher compared to controls. Furthermore, multivariate analyses showed that increasing FGF-23 levels were associated with a monotonically increasing risk of death when examined on a continuous scale [odds ratio (OR) 1.8 (1.4–2.4), P < 0.01].9 Also, the carboxy-terminal FGF-23 assay has been shown to independently predict progression of chronic kidney disease.10 However, neither group reported on a thorough validation and choice of the carboxy-terminal assay.
Two assays for FGF-23 have been reported. One assay detects only full-length (intact) FGF-23. In contrast, the carboxy-terminal assay recognizes both full-length and processed carboxy-terminal fragments of FGF-23.11–13 However, discrepant results concerning circulatory levels of FGF-23 have been reported using these two assays. Because FGF-23 appears to be an indicator of mortality in patients with renal disease and chronic kidney disease patients exhibit features of the metabolic syndrome,14 it is important that a thorough evaluation of the assays be done to determine the validity and utility of these assay in long-term clinical trials. This was the aim of this study.
Materials and Methods
Assays
The intact FGF-23 enzyme-linked immunosorbent assay (ELISA) and carboxy-terminal FGF-23 ELISA were evaluated (Immutopics, Inc., San Clemente, CA). The intact FGF-23 assay is a sandwich ELISA and consists of a 96-well plate coated with goat anti-human FGF-23 (carboxy-terminal portion) to which sample is added, and a solution of goat anti-human FGF-23 (amino-terminal portion) conjugated to horseradish peroxidase (HRP) for detection. Values are expressed in pg/mL.
Carboxy-terminal FGF-23 ELISA is a sandwich assay, consisting of a 96-well plate coated with streptavidin, biotinylated goat anti-human FGF-23 (carboxy-terminal portion) antibody, and goat anti-human FGF-23 (carboxy-terminal portion) conjugated to HRP for colorimetric detection of bound carboxy-terminal FGF-23. Values are expressed in relative units (RU)/mL, and 1 RU/mL roughly equates to 2 pg/mL according to the manufacturer.
Each ELISA was performed manually according to instructions provided with the kit. Absorbances were measured with a BioTek Synergy HT Multi-Mode Microplate Reader. All samples and standards were assayed in duplicate.
Precision studies
Interassay and intra-assay precision studies were performed according to the Clinical and Laboratory Standards Institute (CLSI) EP5-A2 protocol.
Interassay precision
Plasma samples with low, medium, and high concentrations of FGF-23, both intact and carboxy-terminal, were aliquoted in 1-mL aliquots and stored at −20°C. These samples were then assayed on 20 separate occasions over the next several days to determine interassay precision.
Intra-assay precision
Plasma samples with different concentrations of FGF-23, both intact and carboxy-terminal, were run in multiples of 20 to determine intra-assay precision.
Linearity and dilution
Samples with high concentrations of caboxy-terminal and intact FGF-23 were diluted with the zero standard (serum matrix) and linearity of each of the assays was determined. Recovery was calculated by dividing the result obtained by the expected value.
Human serum and plasma
Although plasma is the sample of choice according to the manufacturer, we also tested levels of FGF-23 in serum and plasma obtained from the same subject at the same time (n = 20).
Interference studies
A pool of different plasma samples (n = 5) was aliquoted. Aliquots were then individually spiked with different concentrations of free hemoglobin, bilirubin, and triglycerides (TGs), substances known to frequently interfere with similar assays. Hemoglobin was added at 100, 40, and 20 g/dL, values far higher than what would be expected in either normal plasma or hemolysed plasma. Bilirubin was added at 450, 125, and 62.5 mg/L (770, 214, and 107 umoL/L; 45, 12.5, and 6.25 mg/dL). These values are higher than what would be expected even in a jaundiced patient with high levels of bilirubin. TGs (Intralipid) were added at 1500, 750, and 375 mg/dL, levels that would be expected from a dyslipidemic patient or from highly lipemic plasma samples. Each spiked plasma sample was assayed in duplicate; results were compared with those from an aliquot of native plasma.
Cross-reactivity studies
We used recombinant human intact FGF-23 protein (GenWay, San Diego, CA) for cross-reactivity tests. To determine cross reactivity of the intact protein in the carboxy-terminal assay, we spiked plasma samples with different concentrations of intact FGF-23 and assayed them with the carboxy-terminal FGF-23 ELISA.
Evaluation of intact and carboxy-terminal FGF-23 in patients with renal impairment compared to healthy controls
All procedures were in accord with the Helsinki Declaration of 1975. Following waiver of Institutional Review Board (IRB) consent, plasma samples were obtained from the UCDMC Clinical Pathology laboratories that came in for evaluation of creatinine levels. Carboxy-terminal and intact FGF assays were performed according to manufacturers instructions. Also, carboxy-terminal FGF-23 levels were assayed in 100 healthy controls with normal creatinine and eGFR >60. The latter had given informed consent. All creatinine values were performed on the Beckman autoanalyzer, and estimated glomerular filtration rate (eGFR) was calculated using the modified Modification of Diet in Renal Disease (MDRD) equation in the Clinical laboratory at University of California, Davis.
Statistics
Intra-assay and interassay precision were evaluated by determining means, standard deviation, and coefficient of variation. Differences between serum and plasma and interference studies were evaluated by paired t-tests. Linear regression analysis was used to correlate the assays with creatinine and eGFR calculated using the MDRD equation.
Results
Precision studies
For the precision studies, plasma samples with different concentrations of FGF-23 were studied. Intra-assay and interassay precision results are provided in Table 1a and b, respectively. For the intra-assay precision, the carboxy-terminal FGF-23 showed very good precision whereas the intra-assay CVs for intact FGF-23 were not very good at the lower range (Tables 1a,b). With regard to interassay precision, again carboxy-terminal FGF-23 demonstrated better precision whereas the intact assay had a very poor interassay precision.
Table 1a.
Precision: Intra-assay CV
| Intact FGF-23 (pg/mL) | N | Mean ± SD | %CV |
|---|---|---|---|
| 20 | 18.9 ± 3.4 | 18.1 | |
| 20 | 27.2 ± 3.6 | 13.3 | |
| 20 | 374 ± 22.5 | 6.0 | |
| C-Terminal FGF-23 (RU/mL) | |||
| 20 | 69.4 ± 2.8 | 4.0 | |
| 20 | 359.9 ± 25.7 | 7.1 | |
| 20 | 594.8 ± 36.8 | 6.2 | |
Samples with low, medium, and high levels of intact and C-terminal FGF-23 were run 20 times. Data are provided as mean ± SD and % CV.
Table 1b.
Precision: Interassay CV
| N | Mean ± SD | %CV | |
|---|---|---|---|
| Intact FGF-23 (pg/mL) | |||
| 20 | 16.1 ± 4.9 | 30.4 | |
| 20 | 24.7 ± 9.1 | 36.8 | |
| 20 | 86.2 ± 12.1 | 14.0 | |
| 20 | 255.9 ± 101.5 | 39.7 | |
| C-Terminal FGF-23 (RU/mL) | |||
| 20 | 54.4 ± 5.4 | 9.9 | |
| 20 | 333.5 ± 26.8 | 8.0 | |
| 20 | 756.3 ± 77.8 | 10.3 | |
| 20 | 13780 ± 1212 | 8.8 | |
Samples with low, medium, and high levels of intact and C-terminal FGF-23 were run in duplicate for 20 days. Data are provided as mean ± SD and % CV.
Linearity and dilution
Samples with high concentrations of carboxy-terminal and intact FGF-23 were diluted to determine linearity. Again, compared to the carboxy-terminal assay, the intact assay did not perform well on dilutions of 1:4 or more; the carboxy-terminal assay performed well up to a 1:16 dilution (Table 2).
Table 2.
Linearity and Dilution
| Expected Concentration | Observed Concentration | %Recovery | |
|---|---|---|---|
| Intact FGF-23 (pg/mL) | |||
| Sample 1 | 69 | 69 | 100 |
| 35 | 40 | 114 | |
| 18 | 21 | 116 | |
| 9 | 12 | 133 | |
| 5 | 10 | 200 | |
| Sample 2 | 96 | 96 | 100 |
| 48 | 49 | 102 | |
| 24 | 37 | 154 | |
| 12 | 19 | 158 | |
| 6 | 10 | 167 | |
| C-terminal FGF-23 (RLU/mL) | |||
| Sample 1 | 97.2 | 97.2 | 100 |
| 48.4 | 48.48 | 93 | |
| 24.2 | 24.4 | 101 | |
| 12.2 | 12.0 | 98 | |
| 6.0 | 6.6 | 110 | |
| 3.0 | 2.0 | 67 | |
| Sample 2 | 182.4 | 182.4 | 100 |
| 90.2 | 90.8 | 100 | |
| 55.6 | 55.4 | 98 | |
| 37.8 | 37.2 | 98 | |
| 19.0 | 19.2 | 101 | |
| 9.4 | 8.8 | 94 | |
Samples were diluted using zero standard (serum matrix) and linearity of the intact and C-terminal FGF-23 assays was determined.
Human serum and plasma
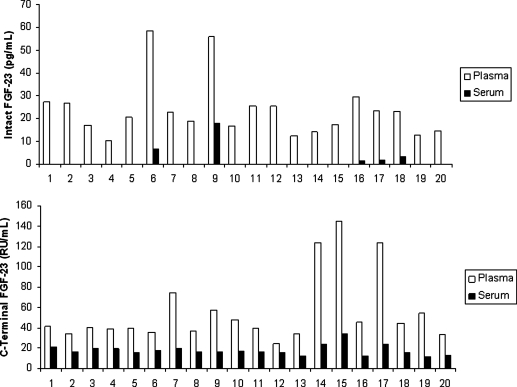
Although plasma is the sample of choice according to the manufacturer, we also tested levels of FGF-23 in serum and plasma obtained from the same subject at the same time (n = 20). As is evident in Fig. 1, whereas intact FGF-23 was detected in all of the plasma samples, it was detected in only 5 of the 20 serum samples (25%). With the carboxy-terminal FGF-23 assay, there were significant differences between plasma and serum values (P < 0.001) (Fig. 1).
FIG. 1.
Intact and carboxy-terminal fibroblast growth factor-23 (FGF-23) levels in plasma versus serum. Plasma and serum from the same individuals (n = 20) were evaluated for intact (upper panel) and carboxy-terminal (lower panel) FGF-23 as described in Materials and Methods. Some values for serum for the intact FGF-23 were undetectable. C-terminal, carboxy-terminal.
Interference studies
The results of the interference studies are provided in Fig. 2. Very high bilirubin and TGs resulted in a significant reduction of FGF-23 in the intact assay. However, there was no significant reduction in carboxy-terminal FGF-23, even at these high concentrations.
FIG. 2.
Interference studies. A pool of plasma samples (n = 5) was aliquoted. Aliquots were then individually spiked with different concentrations of free hemoglobin (Hb), bilirubin (Bil), and triglycerides (TG), as described in Materials and Methods. Each spiked plasma sample was assayed in duplicate by the two methods and results were compared with those from an aliquot of native plasma. (*) P < 0.05 compared to native plasma; n = 5 experiments. FGF, fibroblast growth factor; C-terminal, carboxy-terminal.
Cross-reactivity
Because the carboxy-terminal assay detects full-length and carboxy-terminal FGF-23, addition of intact FGF-23 protein at various concentrations was detected in the carboxy-terminal assay. However, using standards of carboxy-terminal FGF-23 in the intact assay failed to show any cross-reactivity (data not shown).
Patients' comparison studies
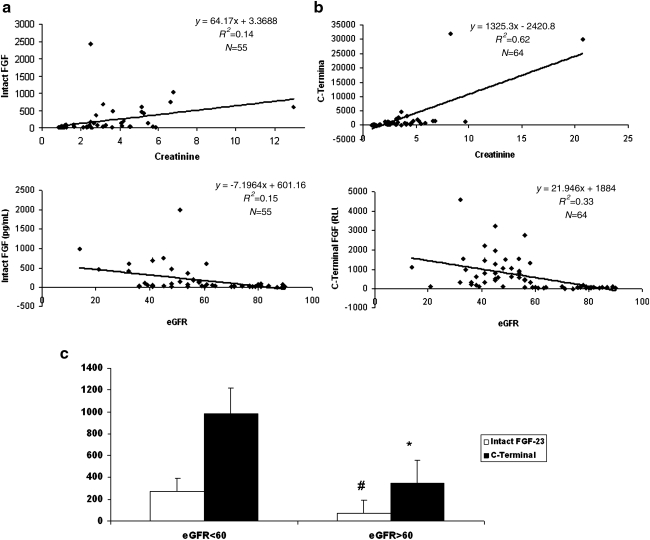
Correlation of the intact and carboxy-terminal FGF-23 assays with creatinine and eGFR levels is provided. As expected, for both assays there was a positive correlation with creatinine levels and a negative correlation with eGFR (Fig.3a,b). Furthermore, the carboxy-terminal FGF-23 assay appeared to correlate better with worsening of kidney function, as assessed by plasma creatinine and calculated eGFR. The reference range of the carboxy-terminal FGF-23 in healthy volunteers was determined to be 21–424 RU/mL (mean ± SD, 129 ± 33 RU/mL; 10th percentile, 76 RU/mL; 90th percentile, 201 RU/mL). Also, there was a significant increase in intact (P < 0.05) and carboxy-terminal FGF-23 in subjects with eGFR <60 (Fig. 3c, P < 0.001).
FIG. 3.
Correlation of intact fibroblast growth factor (FGF) (a) and carboxy-terminal FGF (b) with creatinine and estimated glomerular filtration rate (eGFR). Plasma samples were obtained from the UCDMC Clinical Pathology laboratories that came in for evaluation of creatinine levels. Carboxy-terminal and intact FGF assays were performed according to manufacturers instructions. (c) Values for intact and carboxy-terminal FGF-23 as a function of eGFR: Divided into eGFR >60 and <60, respectively. (#) P < 0.05 and (*) P < 0.001 compared to eGFR <60. C-terminal, Carboxy-terminal.
Discussion
Serum phosphate levels are maintained in a narrow range, and hyperphosphatemia leads to ectopic calcification and functional impairment of several organs. FGF-23 appears to be a key adaptive factor preventing early hyperphosphatemia in progressive chronic kidney disease.1–5 Furthermore, in these patients, FGF-23 is a strong predictor of mortality.9,10 FGF-23 null mice exhibit increased cardiovascular calcification.7
It will be of considerable interest to look at FGF-23 levels and their prognostic value because patients with metabolic syndrome have evidence of incipient renal disease manifesting as microalbuminuria. Commercially available assays for both intact FGF-23 and the splice product carboxy-terminal FGF-23 are now available.11–13 In this report, we evaluated both the intact and carboxy-terminal FGF-23 assays and demonstrate the superiority of the carboxy-terminal FGF-23 in terms of interassay and intra-assay precision and interference, and in stratifying patients according to kidney function. Our interassay and intra-assay precision studies demonstrate that the carboxy-terminal assay is clearly superior, especially with regard to interassay precision, in which the intact assay was highly variable (CV up to 37.5%).
Also, dilutional studies demonstrate that the carboxy-terminal assay was linear and demonstrated good linearity at the low end compared to the intact FGF-23 assay. Furthermore, although the manufacturer recommends the use of EDTA plasma, we examined levels of intact and carboxy-terminal FGF-23 in serum and plasma and report that for both intact and carboxy-terminal FGF-23 assays, plasma appears to be the sample of choice.
Because common interferents such as bilirubin and triglycerides could affect results, we tested these common interferents and free hemoglobin interference in the two assays. Very high bilirubin and TGs resulted in a significant reduction of FGF-23 in the intact assay, but there was no significant reduction in carboxy-terminal FGF-23, even at these high concentrations of interferents.
For both assays, there was a positive correlation with creatinine levels and a negative correlation with eGFR. Furthermore, the carboxy-terminal FGF-23 assay appeared to correlate better with worsening of kidney function as assessed by plasma creatinine and calculated eGFR. Also, there was a significant increase in carboxy-terminal FGF-23 in subjects with eGFR <60.
Previous studies have examined the utility of assays for FGF-23 in renal disease; however, there has not been a comprehensive evaluation of both assays head-to-head in a comprehensive manner. Fassbender et al.12 did not report on any precision studies and, like our report, reported that plasma was a superior sample than serum for the measurement of FGF-23. Heijboer et al.13 evaluated two intact assays, including an assay from Kainos and the carboxy-terminal assay and also reported on poor linearity and interassay precision of the intact assay. They did not report on any interferents or recovery.
FGF-23 appears to be a novel marker in the workup of chronic kidney disease, tumor-induced osteomalacia, and rare genetic causes of rickets. Thus, this assay requires standardization and needs to be available in clinical laboratories at academic centers. In this paper, we have demonstrated that for prospective trials to examine the utility of FGF-23 as a biomarker of adverse outcomes in end-stage renal disease patients, it is important that the carboxy-terminal FGF-23 assay be used because it has better precision, stability in plasma, is linear, and correlates well with loss of kidney function when compared to the intact FGF-23 assay. In future studies, we will examine the relationship of carboxy-terminal FGF-23 levels with mortality and progression of renal disease in patients with chronic kidney disease.
Acknowledgments
This work was supported by the K24 AT00596 grant from National Institutes of Health, Bethesda, MD, and the Institute of Kidney Lifesciences Technology, Ontario, Canada. We would also like to thank Manpreet Kaur for her editorial assistance.
Author Disclosure Statement
No competing financial interests exist for any of the authors.
References
- 1.Fukagawa M. Nii-Kono T. Kazama JJ. Role of fibroblast growth factor-23 in health and in chronic kidney disease. Curr Opin Nephrol Hypertens. 2005;14:325–329. doi: 10.1097/01.mnh.0000172717.49476.80. [DOI] [PubMed] [Google Scholar]
- 2.Ketteler M. Biggar PH. As nature did not predict dialysis—what we can learn from FGF-23 in end stage renal disease? Nephrol Dial Transplant. 2009;24:2618–2620. doi: 10.1093/ndt/gfp323. [DOI] [PubMed] [Google Scholar]
- 3.Yamashita T. Structural and biochemical properties of fibroblast growth factor 23. Ther Apher Dial. 2005;9:313–318. doi: 10.1111/j.1744-9987.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 4.Shimada T. Urakawa I. Yamazaki Y. Hasegawa H. Hino R. Yoneya T. Takeuchi Y. Fujita T. Fukumoto S. Yamashita T. FGF-23 trransgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun. 2004;314:409–414. doi: 10.1016/j.bbrc.2003.12.102. [DOI] [PubMed] [Google Scholar]
- 5.Larsson T. Nisbeth U. Ljunggren O. Jüppner H. Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 6.Shimada T. Muto T. Urakawa I. Yoneya T. Yamazaki Y. Okawa K. Takeuchi Y. Fujita T. Fukumoto S. Yamashita T. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology. 2002;143:3179–3182. doi: 10.1210/endo.143.8.8795. [DOI] [PubMed] [Google Scholar]
- 7.Stubbs JR. Liu S. Tang W. Zhou J. Wang Y. Yao X. Quarles LD. Role of hyperphosphatemia and vitamin D in vascular calcification and mortality in FGF23 null mice. J Am Soc Nephrol. 2007;18:2116–2124. doi: 10.1681/ASN.2006121385. [DOI] [PubMed] [Google Scholar]
- 8.Jean G. Terrat J. Vanel T. Hurot JM. Lorriaux C. Mayor B. Chazot C. High levels of serum fibroblast growth factor (FGF-23) are associated with increased mortality in long-term haemodialysis patients. Nephrol Dial Transplant. 2009;24:2792–2796. doi: 10.1093/ndt/gfp191. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez OM. Mannstadt M. Isakova T. Rauh-Hain JA. Tamez H. Shah A. Smtih K. Lee H. Thadhani R. Juppner H. Wolf M. FGF-23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fliser D. Kollerits B. Neyer U. Ankerst DP. Lhotta K. Lingenhel A. Ritz E. Kronenberg F MMKD Study Group. Kuen E. Konig P. Kraatz G. Mann JF. Muller GA. Kohler H. Riegler P. FGF-23 predicts progression of chronic kidney disease: The Mild to Moderate Kidney Disease (MMKD) study. J Am Soc Nephrol. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 11.Ito N. Fukumoto S. Takeuchi Y. Yasuda T. Hasegawa Y. Takemoto F. Tajima T. Dobashi K. Yamazaki Y. Yamashita T. Fujita T. Comparison of 2 assays for FGF-23. J Bone Miner Metab. 2005;23:435–440. doi: 10.1007/s00774-005-0625-4. [DOI] [PubMed] [Google Scholar]
- 12.Fassbender WJ. Brandenburg V. Schmitz S. Sandig D. Simon SA. Windolf J. Stumpf UC. Evaluation of Human FGF-23 C-terminal and intact ELISA in ESRD patients. Clin Lab. 2009;55:144–152. [PubMed] [Google Scholar]
- 13.Heijboer AC. Levitus M. Vervloet MG. Lips P. ter Wee PM. Dijstelbloem HM. Blankenstein MA. Determination of FGF-23. Ann Clin Biochem. 2009;46:338–340. doi: 10.1258/acb.2009.009066. [DOI] [PubMed] [Google Scholar]
- 14.Ting SM. Nair H. Ching I. Taheri S. Dasgupta I. Overweight, obesity and chronic kidney disease. Nephron Clin Pract. 2009;112:c121–127. doi: 10.1159/000214206. [DOI] [PubMed] [Google Scholar]