Abstract
Background
Carotid artery intima-media thickness (IMT) is greater in adults with elevated metabolic risk profiles. However, the influence of body mass index (BMI) or waist circumference (WC) on the relationship between IMT and metabolic risk is unclear.
Methods
Adults from the Bogalusa Heart Study were classified as normal weight, overweight, or obese and into WC categories (men, low <94 cm, moderate 94–101.9 cm, high ≥102 cm; women, low <80 cm, moderate 80–87.9 cm, high ≥88 cm). Elevated metabolic risk was defined by cardiovascular risk factor clustering (≥2 abnormal risk factors or insulin resistance (upper quartile of homeostasis model of insulin resistance). Carotid ultrasound measurements were obtained and mean IMT was calculated. General linear models compared IMT between elevated versus normal metabolic risk groups, adjusting for sex, age, race/ethnicity, and either BMI or WC category.
Results
Adults were 24–43 years of age (n = 991) and 41% had elevated metabolic risk (42% male, 28% African American, 38% obese). IMT (mm) was greater in adults with elevated metabolic risk (0.83 ± 0.007) versus normal risk (0.80 ± 0.006) whether adjusted by BMI or WC (both P < 0.0005). IMT was greater in adults with elevated compared to normal metabolic risk within normal-weight (0.84 ± 0.016 vs. 0.79 ± 0.008; P = 0.002), and obese adults (0.86 ± 0.009 vs. 0.80 ± 0.01; P = 0.03), but not significantly different between risk groups in overweight adults. Similar results were found when stratified by WC category.
Conclusion
Adults with elevated metabolic risk have greater IMT than those with normal risk in normal-weight, overweight, low WC, and high WC, but not significant for overweight or moderate WC categories.
Introduction
Measurement of intima media thickness (IMT) is assessed by a noninvasive ultrasound imaging technique that can measure the extent of generalized atherosclerosis detected in the arterial wall. IMT reflects cardiovascular disease development in asymptomatic, healthy individuals. IMT is positively associated with the number of abnormal cardiovascular disease risk factors,1 presence of metabolic syndrome,2–5 insulin resistance,6,7 diabetes,8–10 and occurrence of myocardial infarction and stroke.11–13 Thus, IMT is an important early screening tool to assess subclinical manifestation of cardiovascular and metabolic diseases.
Despite the known relationship between IMT and cardiovascular disease and diabetes, the influence of anthropometric markers of obesity, such as body mass index (BMI) and waist circumference (WC), is not well understood. Higher IMT has been shown to be associated with both elevated BMI and WC,6,14–18 although this relationship is not always significant.7,19–21 Possible reasons for this inconsistency may be due to the influences of existing chronic disease, insulin resistance, or cardiometabolic risk on both IMT and the surrogate measures of adiposity. Due to its possible influence, BMI is often controlled for in studies that examine the relationship of cardiometabolic risk on IMT,22–25 but few studies explicitly explore the relationship of IMT and metabolic risk on BMI. Some evidence suggests that obese individuals with insulin resistance have greater IMT compared to their obese, normal metabolic risk counterparts26; however, whether this relationship is consistent across the other BMI groups has not yet been investigated. Furthermore, when participants were matched for BMI and waist-to-hip circumference, there were no differences in IMT between those with normal versus impaired glucose tolerance,27 possibly suggesting some interactions of BMI and WC on the relationships between IMT and metabolic risk. Thus, the purpose of this study is to examine IMT between elevated and normal metabolic profiles within BMI (normal weight, overweight, and obese) and WC (low, moderate, and high) groups.
Methods
Study population
The Bogalusa Heart Study is a long-term community-based epidemiologic study of the early natural history of cardiovascular disease in children and young adults from the semirural, biracial (65% white, 35% black) community of Bogalusa, Louisiana.28 The present study sample includes adults measured in the 2001–2002 survey (n = 1,144) who had IMT measurements. Participants were excluded from the analysis if they were underweight (BMI ≤ 18.5) (n = 12), nonfasted (n = 14), pregnant (n = 7), had missing variables (n = 18), or had values greater than ± 3 standard deviations (SD) from the mean for IMT, BMI, WC, systolic blood pressure (SBP), diastolic blood pressure (DBP), triglycerides, high-density lipoprotein cholesterol (HDL-C), insulin, and glucose (n = 102). The final sample for this particular analysis included 991 (87% of total sample) adults (24–43 years of age; mean 36.2 ± 4.4). Institutional review board (IRB) approval was given from both Tulane University for the data collection, and from Pennington Biomedical Research Center for data analysis.
General examination
Duplicate measurements of height and weight were obtained to the nearest 0.1 cm and 0.1 kg, respectively, and averaged for analysis. BMI was calculated as weight in kilograms divided by the height in meters squared (kg/m2). WC was measured with a flexible tape midway between the lowest rib and the superior border of the iliac crest. SBP and 5th phase DBP were measured from the right arm with a mercury sphygmomanometer and averaged from six readings performed by two randomly assigned nurses.
Laboratory analyses
All participants were requested to fast for a minimum of 12 h for venipuncture. Blood sample assays were performed in the Core Lipid Laboratory in New Orleans, Louisiana. Blood lipids were measured by a VP instrument (Abbott Laboratories, North Chicago, IL) using enzymatic procedures29,30 under quality controls by the Centers for Disease Control and Prevention (Atlanta, GA). Serum triglyceride concentrations were measured with enzymatic procedures, and serum HDL-C was measured using a heparin–calcium precipitation in combination with an agar–agarose gel electrophoresis.31 Plasma glucose was measured using an enzymatic procedure, whereas insulin was measured with a commercial radioimmunoassay kit (Phadebas, Pharmacia Diagnostics, Piscataway, NJ).
Carotid ultrasonography
Carotid ultrasound measurements were performed with a Toshiba Ultrasound instrument (Power Vision Toshiba SSH-380 ultrasound system, Toshiba American Medical Systems, Carrollton, TX), using a 7.5-MHz linear array transducer recording images at the common carotid (CC), carotid bulb (CB) (bifurcation), and internal carotid (IC) arteries. Images were obtained bilaterally according to previously developed protocols for the Atherosclerosis Risk in Communities study.32 Images were recorded on super VHS videotapes and read by certified readers from the Vascular Ultrasound Research Laboratory in Wake Forest Medical Center, North Carolina, using semiautomatic ultrasound imaging. The maximum carotid IMT readings of left and right far walls were averaged for each segment; if bilateral images could not be obtained, one side was used in lieu of the average. Mean carotid IMT was calculated as the average carotid IMT from available measures for CC, CB, and IC segments. Due to some missing data for individual carotid segments, the total n differed between the groups for mean IMT (n = 991), CC (n = 991), CB (n = 949), and IC (n = 974).
Data treatment
Participants were classified as normal weight (BMI, 18.5–24.9 kg/m2), overweight (BMI, 25–29.9 kg/m2), or obese (BMI, ≥30 kg/m2), and were also placed into sex-specific low, moderate, or high WC categories (men, low <94 cm, moderate 94–101.9 cm, high ≥102 cm; women, low <80 cm, moderate 80–87.9 cm, high ≥88 cm).33
“Elevated” metabolic risk was defined by meeting the criteria for either cardiovascular risk factor clustering and/or insulin resistance. Cardiovascular risk factor clustering was defined by having two or more abnormal cardiometabolic risk factors: (1) triglycerides ≥150 mg/dL, or on drug treatment; (2) HDL-C <40 mg/dL for men; <50 mg/dL for women, or on drug treatment; (3) blood pressure ≥130/85 mmHg, or on drug treatment; and (4) fasting glucose ≥100 mg/dL, or on drug treatment.34 Insulin resistance was estimated by the homeostasis model of insulin resistance (HOMA-IR), which is calculated by [(fasting glucose * fasting insulin)/22.5].35 Elevated metabolic risk from insulin resistance was estimated as the top quartile of HOMA-IR (≥3.2) or on insulin or glucose drug treatment. Similar definitions combining insulin resistance and clustering of cardiovascular risk factors have been used in other studies to identify metabolic risk profiles.36–39
Statistical analysis
Differences between elevated and normal metabolic risk profiles were analyzed with t-tests for normally distributed cardiovascular disease risk factors, and nonnormally distributed variables (triglycerides, insulin, HOMA-IR) were analyzed with Wilcoxon–Mann–Whitney tests. General linear models were used to compare mean IMT as well as each individual carotid segment (CC, CB, IC) among those classified as elevated versus normal metabolic risk adjusting for sex, age, and race/ethnicity and either BMI group or WC group. Additional analyses were stratified by BMI and WC categories. BMI and WC were also analyzed continuously in the models to compare consistency of results.
Results
Descriptive characteristics of the Bogalusa Heart Study sample (n = 991) are presented in Table 1. The mean (±SD) age of the sample was 36.2 ± 4.4 years and mean BMI was 29.0 ± 6.4 kg/m2. A total of 38% of the sample was obese, 42% were male, and 28% were African American. Significant differences were present between normal and elevated metabolic risk groups for BMI, WC, triglycerides, SBP, DBP, HDL-C, glucose, insulin, HOMA-IR, and IMT (all P < 0.0001).
Table 1.
Descriptive Characteristics of the Bogalusa Heart Study Sample (n = 991)
| Total (n = 991) | Normal risk (n = 585, 59%) | Elevated risk (n = 406, 41%) | P valuea | |
|---|---|---|---|---|
| Male (n, %) | 417 (42) | 223 (38) | 194 (48) | 0.002 |
| White (n, %) | 717 (72) | 438 (75) | 279 (69) | 0.03 |
| Age (years) | 36.2 ± 4.4 | 36.1 ± 4.3 | 36.4 ± 4.6 | 0.32 |
| BMI (kg/m2) | 29.0 ± 6.5 | 26.4 ± 5.0 | 32.7 ± 6.6 | <0.0001 |
| Normal weight, n (%) | 309 (31) | 263 (45) | 46 (11) | |
| Overweight, n (%) | 306 (31) | 204 (35) | 102 (25) | <0.0001b |
| Obese, n (%) | 376 (38) | 118 (20) | 258 (64) | |
| Waist circumference (cm) | 92.5 ± 16.3 | 85.5 ± 12.7 | 102.6 ± 15.5 | <0.0001 |
| Low, n (%) | 402 (41) | 333 (57) | 69 (17) | |
| Moderate, n (%) | 192 (19) | 124 (21) | 68 (17) | <0.0001b |
| High, n (%) | 397 (40) | 128 (22) | 269 (66) | |
| Triglycerides (mg/dL)c | 102 (73–146) | 85 (64–113) | 147 (102–204) | <0.0001 |
| Systolic blood pressure (mmHg) | 115.5 ± 12.3 | 111.7 ± 10.5 | 121.1 ± 12.5 | <0.0001 |
| Diastolic blood pressure (mmHg) | 78.2 ± 9.0 | 75.3 ± 7.9 | 82.4 ± 8.9 | <0.0001 |
| HDL-C (mg/dL) | 47.3 ± 12.0 | 50.9 ± 11.8 | 42.2 ± 10.2 | <0.0001 |
| Glucose (mg/dL) | 83.3 ± 10.9 | 79.9 ± 7.8 | 88.2 ± 12.8 | <0.0001 |
| Insulin (μU/mL)c | 10 (7–15) | 8 (6.0–11.0) | 17 (11–22) | <0.0001 |
| HOMA-IRc | 2.0 (1.3–3.2) | 1.5 (1.1–2.1) | 3.6 (2.2–5.0) | <0.0001 |
| IMT (mm) | 0.80 ± 0.13 | 0.78 ± 0.12 | 0.83 ± 0.14 | <0.0001 |
P value represents the comparison of the unadjusted t-tests between normal and elevated risk.
P value represents the chi-squared comparison between normal and elevated risk.
Due to nonnormal distribution, nonparametric Wilcoxon rank sum tests were used to compare values between normal and elevated risk.
BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; IMT, intima media thickness.
Effect of metabolic risk by individual carotid artery segments and mean IMT
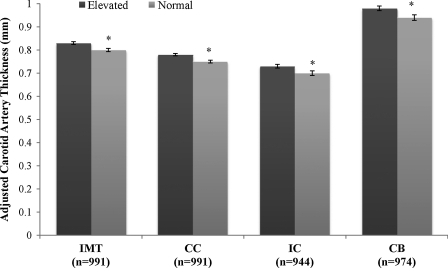
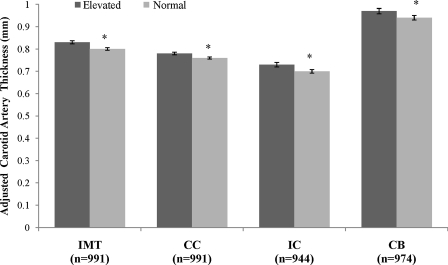
Overall, adults with elevated metabolic risk had higher mean IMT values than adults with normal metabolic risk, whether adjusting for BMI category (0.83 ± 0.007 versus 0.80 ± 0.006; P = 0.0001) (Fig. 1) or WC group (0.83 ± 0.007 versus 0.80 ± 0.006; P = 0.0004) (Fig. 2). Significant effects of race/ethnicity and sex were also found for both WC- and BMI-adjusted models whereby men had higher IMT values than women (P < 0.0001) and African-American adults had higher IMT then white adults (P = 0.02). The statistical significance of these results did not change when BMI or WC were included as a continuous rather than a categorical covariate.
FIG. 1.
Individual carotid segment thickness [mean ± standard error (SE)] between metabolic risk profiles adjusted for body mass index (BMI) group. Adjusted for age, sex, race, and BMI group (normal weight, overweight, and obese). (*) Significant difference between elevated and normal metabolic risk profile (P < 0.05). IMT, mean intima media thickness; CC, common carotid segment; IC, internal carotid segment; CB, carotid bulb segment.
FIG. 2.
Individual carotid segment thickness [mean ± standard error (SE)] between metabolic risk profiles adjusted for waist circumference (WC) group. Adjusted for age, sex, race, and WC group (sex-specific low, moderate, and high). (*) Significant difference between elevated and normal metabolic risk profile (P < 0.05). IMT, Mean intima media thickness; CC, common carotid segment; IC, internal carotid segment; CB, carotid bulb segment.
These results were also consistent for the individual carotid artery segments. In the analysis adjusted by BMI group, adults with elevated metabolic risk had a higher CC (mean difference, 0.02 ± 0.008, P = 0.003), IC (mean difference, 0.03 ± 0.013, P = 0.01), and CB (mean difference, 0.04 ± 0.02, P = 0.005) than adults in the normal metabolic risk group (Fig. 1). When adjusted by WC group, adults with elevated metabolic risk had a higher CC (mean difference, 0.02 ± 0.008, P = 0.01), IC (mean difference, 0.03 ± 0.01, P = 0.006), and CB (mean difference, 0.04 ± 0.02, P = 0.02) than adults with normal metabolic risk (Fig. 2). Statistical significance did not change whether BMI or WC was analyzed as continuous versus categorical variables, except in the case of WC-adjusted CC. When waist circumference was added as a continuous variable, differences between elevated and normal metabolic risk groups were no longer significant for CC (P = 0.15).
Effect of metabolic risk by BMI and WC groups
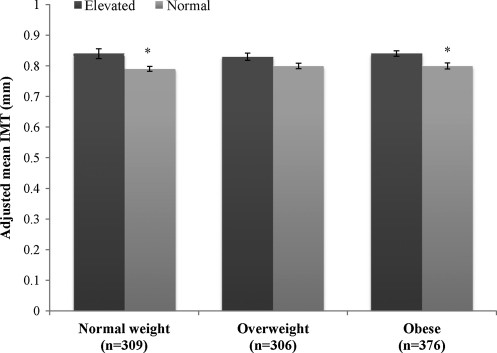
The prevalence of elevated metabolic risk was 41% overall, and 11%, 25%, and 64% in normal-weight, overweight, and obese groups, respectively, and 17%, 17%, and 66% in low, moderate, and high WC groups, respectively (Table 1). When regression analyses were stratified by BMI category, adjusted IMT was greater in those with elevated metabolic risk compared to normal metabolic risk within normal-weight (0.84 ± 0.016 vs. 0.79 ± 0.008; P = 0.002) and obese adults (0.86 ± 0.009 vs. 0.80 ± 0.01; P = 0.03), but not in the overweight adults (0.83 ± 0.012 vs. 0.80 ± 0.009; P = 0.06). Figure 3 presents the analysis stratified by BMI groups.
FIG. 3.
Mean intima media thickness (IMT) [mean ± standard error (SE)] between metabolic risk profiles within body mass index (BMI) groups. Adjusted for age, sex, and race. (*) Significant difference between elevated and normal metabolic risk profile (P < 0.05).
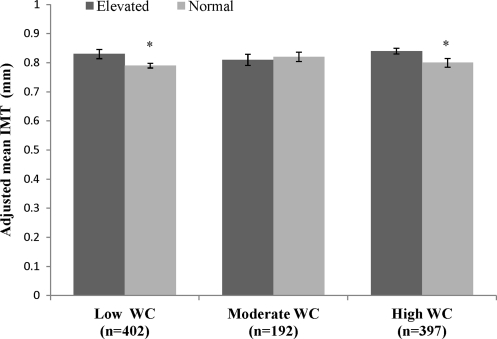
When regression analyses were stratified by WC category, adjusted IMT was greater in those with elevated metabolic risk compared to normal metabolic risk in the low WC (0.83 ± 0.013 vs. 0.79 ± 0.007; P = 0.003), and high WC group (0.84 ± 0.009 vs. 0.80 ± 0.012; P = 0.003), but not in the moderate WC group (0.81 ± 0.012 vs. 0.82 ± 0.015; P = 0.79). Figure 4 presents the analysis stratified by WC groups.
FIG. 4.
Mean intima media thickness (IMT) [mean ±standard error (SE)] between metabolic risk profiles within waist circumference (WC) groups. Adjusted for age, sex, and race. (*) Significant difference between elevated and normal metabolic risk profile (P < 0.05).
Discussion
In the current study, young adults had IMT values that varied by metabolic risk profile, independently of BMI or WC. Adults classified with elevated metabolic risk, defined as having either cardiovascular risk factor clustering or insulin resistance, showed greater IMT than their normal risk counterparts. These results were consistent across mean IMT and the individual carotid segments (CC, IC, and CB), as well as within BMI and WC groups.
Others have also found IMT to differ by metabolic risk status employing a definition of either insulin resistance7 or cardiometabolic risk factor clustering,1,4 although few have tested whether this relationship was independent of BMI or WC.7 Freedman et al. have suggested that cardiovascular risk factors have a stronger association with IMT than BMI.40 Metabolic risk assessment, regardless of BMI, can also predict the development of type 2 diabetes, cardiovascular disease, and death.37,41 Kawamoto et al. have shown that IMT is greater in normal-weight adults with metabolic syndrome versus those without metabolic syndrome in a clinical sample of Japanese elderly.42 In addition, Stefan et al. found greater IMT in obese adults with insulin resistance versus without in a German sample.26 The current study provides additional evidence that metabolic risk is significantly associated with subclinical atherosclerosis in a large biracial sample of men and women.
Specific differences in IMT were found between metabolic risk groups in normal-weight and obese adults classified by BMI and in low WC or high WC adults. There were no significant differences in elevated versus normal metabolic risk for IMT in either the overweight or moderate WC groups. However the differences between the normal versus elevated metabolic risk in the overweight group approached significance (P = 0.06). Possible reasons for nonsignificance in the moderate WC groups are uncertain, but could be due to the lower number of adults classified into this group or the lower amount of variation due to an upper and lower bound category. In addition, there were significant effects of sex within the moderate WC group. When we stratified the analysis by sex (data not shown), we found that women had significant differences in IMT between normal and elevated metabolic risk, whereas men did not. Thus, possible gender/sex influences may also explain the lack of statistical difference within the whole group.
The physiological mechanisms for the relationships between IMT and metabolic risk may relate to insulin levels acting on the arterial wall, resulting in cellular remodeling, or through insulin's association with clustering of multiple cardiovascular disease risk factors.43 Stefan et al. found that ectopic liver fat was the most important predictor of a person's metabolic status, whether they were normal weight, overweight, or obese. However, these findings need to be confirmed in larger population-based studies.26 Possible influences of metabolic status on IMT may relate to lifestyle behaviors, such as dietary intake or physical activity, and future studies are needed to help explain greater IMT in individuals with elevated metabolic risk, regardless of obesity status.
We used WC and BMI as surrogate measures for obesity; however, the Bogalusa Heart Study also has triceps and subscapular skinfold measures that estimate subcutaneous adiposity. Using the sum of the two skinfolds, IMT was significantly greater in elevated metabolic risk versus normal metabolic risk groups within each tertile of skinfolds (data not shown). These results are consistent with our results for BMI and WC, also suggesting that metabolic risk is related to IMT, independent of adiposity.
A marked strength of this study is the use of the Bogalusa Heart Study data, which includes a large cohort of young adults with IMT measures. We were able to employ measures from three carotid segments for a computed IMT mean, which has been shown to provide a better prediction for cardiovascular-related events.13 IMT is known to be influenced by aging,20,44 and using this measure in asymptomatic young, healthy asymptomatic adults is indicative of early stages of atherosclerosis. IMT measurement in young adults may reflect the local arterial shear stress and pressure environment influenced by other lipid and metabolic risk factors, as opposed to actual atherosclerotic lesions.20 Furthermore, previous evidence from the Bogalusa Heart Study has shown both sex and racial/ethnic influences of the relationship of BMI to IMT.24 Although we do not have the statistical power to specifically analyze racial/ethnic effects due to smaller number of African-American adults overall, we were able to use the full sample of African-American and white men and women and control for demographic characteristics in our analysis. Finally, because we have performed only cross-sectional analysis, the direction or causality of the observed relationships could not be determined.
In conclusion, IMT is associated with metabolic risk profiles, independent of BMI or WC. These results imply that assessment of metabolic risk, regardless of a person's BMI or WC, can provide valuable information concerning atherosclerosis status, even in young adults. Research is needed to identify the casual links between specific metabolic risk indicators and IMT for given levels of adiposity, and to determine the effects of interventions designed to decrease metabolic risk and their effects on IMT across classes of BMI.
Acknowledgments
This study was supported by grants HD-061437 and HD-062783 from the National Institute of Child Health and Human Development, 0855082E from American Heart Association, and AG-16592 from the National Institute on Aging.
Author Disclosure Statement
Dr. Katzmarzyk is partially funded by the Louisiana Public Facilities Authority Endowed Chair in Nutrition. Dr. Bouchard is partially funded by the John Barton Sr. Chair in Genetics and Nutrition. S.M.C., S.T.B., SRS, W.C., J.H.X., and G.S.B. have no competing financial interests to report.
References
- 1.Urbina EM. Srinivasan SR. Tang R. Bond MG. Kieltyka L. Berenson GS. Impact of multiple coronary risk factors on the intima-media thickness of different segments of carotid artery in healthy young adults (The Bogalusa Heart Study) Am J Cardiol. 2002;90:953–958. doi: 10.1016/s0002-9149(02)02660-7. [DOI] [PubMed] [Google Scholar]
- 2.Empana JP. Zureik M. Gariepy J. Courbon D. Dartigues JF. Ritchie K. Tzourio C. Alperovitch A. Ducimetiere P. The metabolic syndrome and the carotid artery structure in noninstitutionalized elderly subjects: The three-city study. Stroke. 2007;38:893–899. doi: 10.1161/01.STR.0000257983.62530.75. [DOI] [PubMed] [Google Scholar]
- 3.McNeill AM. Rosamond WD. Girman CJ. Heiss G. Golden SH. Duncan BB. East HE. Ballantyne C. Prevalence of coronary heart disease and carotid arterial thickening in patients with the metabolic syndrome (The ARIC Study) Am J Cardiol. 2004;94:1249–1254. doi: 10.1016/j.amjcard.2004.07.107. [DOI] [PubMed] [Google Scholar]
- 4.Tzou WS. Douglas PS. Srinivasan SR. Bond MG. Tan R. Chen W. Berenson GS. Stein JH. Increased subclinical atherosclerosis in young adults with metabolic syndrome: the Bogalusa Heart Study. J Am Coll Cardiol. 2005;46:457–463. doi: 10.1016/j.jacc.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 5.Zanchetti A. Prevalence and incidence of the metabolic syndrome in the European Lacidipine Study on Atherosclerosis (ELSA) and its relation with carotid intima-media thickness. J Hypertens. 2007;25:2463–2470. doi: 10.1097/HJH.0b013e3282f063d5. [DOI] [PubMed] [Google Scholar]
- 6.Ciccone M. Maiorano A. De Pergola G. Minenna A. Giorgino R. Rizzon P. Microcirculatory damage of common carotid artery wall in obese and non obese subjects. Clin Hemorheol Microcirc. 1999;21:365–374. [PubMed] [Google Scholar]
- 7.De Pergola G. Ciccone M. Pannacciulli N. Modugno M. Sciaraffia M. Minenna A. Rizzon P. Giorgino R. Lower insulin sensitivity as an independent risk factor for carotid wall thickening in normotensive, non-diabetic, non-smoking normal weight and obese premenopausal women. Int J Obes Relat Metab Disord. 2000;24:825–829. doi: 10.1038/sj.ijo.0801239. [DOI] [PubMed] [Google Scholar]
- 8.Bonora E. Tessari R. Micciolo R. Zenere M. Targher G. Padovani R. Falezza G. Muggeo M. Intimal-medial thickness of the carotid artery in nondiabetic and NIDDM patients. Relationship with insulin resistance. Diabetes Care. 1997;20:627–631. doi: 10.2337/diacare.20.4.627. [DOI] [PubMed] [Google Scholar]
- 9.Folsom AR. Eckfeldt JH. Weitzman S. Ma J. Chambless LE. Barnes RW. Cram KB. Hutchinson RG. Relation of carotid artery wall thickness to diabetes mellitus, fasting glucose and insulin, body size, and physical activity. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke. 1994;25:66–73. doi: 10.1161/01.str.25.1.66. [DOI] [PubMed] [Google Scholar]
- 10.Wagenknecht LE. D'Agostino RB., Jr. Haffner SM. Savage PJ. Rewers M. Impaired glucose tolerance, type 2 diabetes, and carotid wall thickness: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 1998;21:1812–1818. doi: 10.2337/diacare.21.11.1812. [DOI] [PubMed] [Google Scholar]
- 11.Bots ML. Hoes AW. Koudstaal PJ. Hofman A. Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: The Rotterdam Study. Circulation. 1997;96:1432–1437. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 12.Chambless LE. Heiss G. Folsom AR. Rosamond W. Szklo M. Sharrett AR. Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol. 1997;146:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 13.O'Leary DH. Polak JF. Kronmal RA. Manolio TA. Burke GL. Wolfson SK., Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 14.Czernichow S. Bertrais S. Oppert JM. Galan P. Blacher J. Ducimetiere P. Hercberg S. Zureik M. Body composition and fat repartition in relation to structure and function of large arteries in middle-aged adults (the SU.VI.MAX study) Int J Obes (Lond) 2005;29:826–832. doi: 10.1038/sj.ijo.0802986. [DOI] [PubMed] [Google Scholar]
- 15.De Michele M. Panico S. Iannuzzi A. Celentano E. Ciardullo AV. Galasso R. Sacchetti L. Zarrilli F. Bond MG. Rubba P. Association of obesity and central fat distribution with carotid artery wall thickening in middle-aged women. Stroke. 2002;33:2923–2928. doi: 10.1161/01.str.0000038989.90931.be. [DOI] [PubMed] [Google Scholar]
- 16.Hassinen M. Lakka TA. Komulainen P. Haapala I. Nissinen A. Rauramaa R. Association of waist and hip circumference with 12-year progression of carotid intima-media thickness in elderly women. Int J Obes (Lond) 2007;31:1406–1411. doi: 10.1038/sj.ijo.0803613. [DOI] [PubMed] [Google Scholar]
- 17.Lakka TA. Lakka HM. Salonen R. Kaplan GA. Salonen JT. Abdominal obesity is associated with accelerated progression of carotid atherosclerosis in men. Atherosclerosis. 2001;154:497–504. doi: 10.1016/s0021-9150(00)00514-1. [DOI] [PubMed] [Google Scholar]
- 18.Krishnan P. Balamurugan A. Urbina E. Srinivasan SR. Bond G. Tang R. Berenson GS. Cardiovascular risk profile of asymptomatic healthy young adults with increased carotid artery intima-media thickness: the Bogalusa Heart Study. J La State Med Soc. 2003;155:165–169. [PubMed] [Google Scholar]
- 19.Ebrahim S. Papacosta O. Whincup P. Wannamethee G. Walker M. Nicolaides AN. Dhanjil S. Griffin M. Belcaro G. Rumley A. Lowe GDO. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke. 1999;30:841–850. doi: 10.1161/01.str.30.4.841. [DOI] [PubMed] [Google Scholar]
- 20.Mizia-Stec K. Gasior Z. Zahorska-Markiewicz B. Holecki M. Haberka M. Mizia M. Gomulka S. Zak-Golab A. Goscinska A. The indexes of arterial structure and function in women with simple obesity: A preliminary study. Heart Vessels. 2008;23:224–229. doi: 10.1007/s00380-007-1030-9. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki M. Shinozaki K. Kanazawa A. Hara Y. Hattori Y. Tsushima M. Harano Y. Insulin resistance as an independent risk factor for carotid wall thickening. Hypertension. 1996;28:593–598. doi: 10.1161/01.hyp.28.4.593. [DOI] [PubMed] [Google Scholar]
- 22.Frontini MG. Srinivasan SR. Xu JH. Tang R. Bond MG. Berenson G. Utility of non-high-density lipoprotein cholesterol versus other lipoprotein measures in detecting subclinical atherosclerosis in young adults (The Bogalusa Heart Study) Am J Cardiol. 2007;100:64–68. doi: 10.1016/j.amjcard.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 23.Kieltyka L. Urbina EM. Tang R. Bond MG. Srinivasan SR. Berenson GS. Framingham risk score is related to carotid artery intima-media thickness in both white and black young adults: The Bogalusa Heart Study. Atherosclerosis. 2003;170:125–130. doi: 10.1016/s0021-9150(03)00244-2. [DOI] [PubMed] [Google Scholar]
- 24.Li S. Chen W. Srinivasan SR. Tang R. Bond MG. Berenson GS. Race (black-white) and gender divergences in the relationship of childhood cardiovascular risk factors to carotid artery intima-media thickness in adulthood: The Bogalusa Heart Study. Atherosclerosis. 2007;194:421–425. doi: 10.1016/j.atherosclerosis.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 25.Ruan L. Chen W. Srinivasan SR. Sun M. Wang H. Toprak A. Verenson G. Correlates of common carotid artery lumen diameter in black and white younger adults: The Bogalusa Heart Study. Stroke. 2009;40:702–707. doi: 10.1161/STROKEAHA.108.531608. [DOI] [PubMed] [Google Scholar]
- 26.Stefan N. Kantartzis K. Machann J. Schick F. Thamer C. Rittig K. Balletshofer B. Machicao F. Fristsche A. Haring H-U. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 27.Snehalatha C. Vijay V. Suresh Mohan R. Satyavani K. Sivasankari S. Megha T. Radhika S. Ramachandran A. Lack of association of insulin resistance and carotid intimal medial thickness in non-diabetic Asian Indian subjects. Diabetes Metab Res Rev. 2001;17:444–447. doi: 10.1002/dmrr.226. [DOI] [PubMed] [Google Scholar]
- 28.The Bogalusa Heart Study 20th Anniversary Symposium. Am J Med Sci. 1995;310:S1–S138. doi: 10.1097/00000441-199512000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Allain CC. Poon LS. Chan CS. Richmond W. Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 30.Bucolo G. David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19:476–482. [PubMed] [Google Scholar]
- 31.Srinivasan SR. Webber LS. Whitaker CF. Berenson GS. Quantification of lipoprotein cholesterol in serum from children with different lipoprotein profiles: Heparin-calcium precipitation and ultracentrifugation compared. Clin Chem. 1983;29:481–485. [PubMed] [Google Scholar]
- 32.Bond MG. Barnes RW. Riley WA. Wilmoth SK. Chambless LE. Howard G. Owens SB. High resolution B-mode ultrasound scanning methods in the Atherosclerosis Risk in Communities study (ARIC) J Neuroimaging. 1991;1:68–73. [PubMed] [Google Scholar]
- 33.Lean ME. Han TS. Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ. 1995;311:158–161. doi: 10.1136/bmj.311.6998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grundy SM. Cleeman JI. Daniels SR. Donato KA. Eckel RH. Franklin BA. Gordon DJ. Krauss RM. Savage PJ. Smith SC. Spertus JA. Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 35.Matthews DR. Hosker JP. Rudenski AS. Naylor BA. Treacher DF. Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 36.Karelis AD. Brochu M. Rabasa-Lhoret R. Can we identify metabolically healthy but obese individuals (MHO)? Diabetes Metab. 2004;30:569–572. doi: 10.1016/s1262-3636(07)70156-8. [DOI] [PubMed] [Google Scholar]
- 37.Meigs JB. Wilson PW. Fox CS. Vasan RS. Nathan DM. Sullivan LM. D'Agostino RB. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 38.Shin MJ. Hyun YJ. Kim OY. Kim JY. Jang Y. Lee JH. Weight loss effect on inflammation and LDL oxidation in metabolically healthy but obese (MHO) individuals: low inflammation and LDL oxidation in MHO women. Int J Obes (Lond) 2006;30:1529–1534. doi: 10.1038/sj.ijo.0803304. [DOI] [PubMed] [Google Scholar]
- 39.Wildman RP. Muntner P. Reynolds K. McGinn AP. Rajpathak S. Wylie-Rosett J. Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 40.Freedman DS. Patel DA. Srinivasan SR. Chen W. Tang R. Bond MG. Berenson GS. The contribution of childhood obesity to adult carotid intima-media thickness: The Bogalusa Heart Study. Int J Obes (Lond) 2008;32:749–756. doi: 10.1038/sj.ijo.0803798. [DOI] [PubMed] [Google Scholar]
- 41.Arnlov J. Ingelsson E. Sundstrom J. Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation. 2010;121:230–236. doi: 10.1161/CIRCULATIONAHA.109.887521. [DOI] [PubMed] [Google Scholar]
- 42.Kawamoto R. Ohtsuka N. Ninomiya D. Nakamura S. Carotid atherosclerosis in normal-weight metabolic syndrome. Intern Med. 2007;46:1771–1777. doi: 10.2169/internalmedicine.46.0261. [DOI] [PubMed] [Google Scholar]
- 43.Laakso M. Insulin resistance and coronary heart disease. Curr Opin Lipidol. 1996;7:217–226. doi: 10.1097/00041433-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Stevens J. Juhaeri Cai J. Evans GW. Impact of body mass index on changes in common carotid artery wall thickness. Obes Res. 2002;10:1000–1007. doi: 10.1038/oby.2002.136. [DOI] [PubMed] [Google Scholar]