Abstract
Background
The aim of this study was to examine the distribution of alanine aminotrasferase (ALT) and its association with metabolic syndrome variables and their clustering in apparently healthy children.
Methods
A cross-sectional study of 1,524 preadolescents (age, 4–11 years, 62% white, 51% male) and 1,060 adolescents (age, 12–18 years, 58% white, 51% male) enrolled in the Bogalusa Heart Study was performed.
Results
ALT levels showed a significant race (whites > blacks) difference in preadolescents and a gender (males > females) difference in adolescents. Both preadolescents and adolescents in the age, race, and gender-specific top versus bottom quartiles of ALT had significant increases in the prevalence of adverse levels (>75th percentile specific for age, race, and gender) of body mass index (BMI), systolic blood pressure, total cholesterol to high-density lipoprotein cholesterol (HDL-C) ratio (adolescents only), insulin resistance index (HOMA-IR), and clustering of all four of these metabolic syndrome variables. In multivariate analyses, BMI was the major independent predictor of ALT in both preadolescents and adolescents; other independent predictors were total cholesterol to HDL-C ratio, HOMA-IR, white race in preadolescents and male gender in adolescents. With respect to the ability of ALT to identify children with clustering of the metabolic syndrome variables, area under the receiver operating characteristic curve analysis (c-statistics) adjusted for age, race, and gender yielded a value of 0.67 for preadolescents and 0.82 for adolescents.
Conclusion
An elevation in serum ALT within the reference range relate adversely to all of the major components of metabolic syndrome and their clustering in children and, thus, may be useful as a biomarker of the presence of metabolic syndrome and related risk in pediatric population, especially adolescents.
Introduction
Elevated levels of serum alanine aminotransferase (ALT) is considered an early noninvasive biomarker of nonalcoholic fatty liver disease (NAFLD), recognized as the most common liver disease in the pediatrics population.1–4 The parallel relationship of ALT with obesity and insulin resistance in NAFLD implicates this liver function enzyme as a biomarker of hepatic expression of adverse metabolic profile related to the metabolic syndrome and related coronary heart disease and type 2 diabetes.3–11
Although the above-mentioned associations have been studied extensively in adults and limited number of children, data on the distribution of ALT and its relationship to metabolic syndrome variables and their clustering are scant in an apparently healthy community-based pediatric population. Furthermore, information is lacking on the ability of ALT to identify clustering of adverse levels of metabolic syndrome components in children. The present study examined these aspects in children as part of the Bogalusa Heart Study, a biracial (black–white) community-based investigation of the early natural history of cardiovascular disease.12
Methods
Study population
Individuals (n = 3,352) aged 4–22 years, residing in the biracial (65% white, 35% black) community of Bogalusa, Louisiana, were examined in 1987–1988 as part of a long-term cohort follow-up study. Of these, 1,524 preadolescents (age, 4–11 years, 62% white, 51% male) and 1,060 adolescents (age, 12–18 years, 58% white, 51% male), who were fasting and had data on ALT along with other risk factor variables, formed the study sample. Individuals with ALT values above the normal limit of 55 IU/L were excluded. This study was approved by the Institutional Review Board of the Tulane University Health Sciences Center. All participants gave their informed consent.
General examination
Standardized protocols were used by trained observers in all examinations. Subjects were instructed to fast for 12 h before the screening, with compliance ascertained by an interview on the day of examination. Anthropometric and blood pressure measurements were made in replicate, and mean values were used in all analyses. Height and weight were measured to calculate body mass index (BMI = weight in kilograms divided by the square of the height in meters). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) measurements were obtained on the right arm of the subjects in a relaxed, sitting position.
Laboratory analyses
Cholesterol levels in the serum were assayed using enzymatic procedures on the Hitachi 902 Automatic Analyzer (Roche Diagnostics, Indianapolis, IN). Serum lipoprotein cholesterol levels were analyzed by a combination of heparin–calcium precipitation and agar–agarose gel electrophoresis procedures.13 The laboratory was monitored for precision and accuracy of lipid measurements by the Lipid Standardization and Surveillance Program of the Centers for Disease Control and Prevention (Atlanta, GA). A commercial radioimmunoassay kit was used for measuring plasma immunoreactive insulin levels (Phadebas, Pharmacia Diagnostics, Piscataway, NJ). Glucose and ALT levels were measured as part of a multiple chemistry profile (SMA20) by enzymatic procedures with the multichannel Olympus Au-5000 analyzer (Olympus, Lake Success, NY). Insulin resistance status was assessed as homeostasis model assessment of insulin resistance (HOMA-IR) according to the formula described previously14: [insulin (μU/mL) × glucose (mmol/L)/22.5].
Statistical methods
All statistical analyses were performed with SAS version 9.1 (SAS institute, Cary, NC). The criterion metabolic syndrome variables considered in the analyses were: (1) BMI, (2) SBP, (3) total cholesterol to high-density lipoprotein cholesterol (HDL-C) ratio, and (4) HOMA-IR. Because there are no acceptable standard age-, race-, and gender-specific cutoff points for the metabolic syndrome variables available for use in pediatric population,15,16 adverse levels were defined as values above the age-, race-, and gender-specific 75th percentiles, as previously reported.17,18 Clustering was defined as coexistence of adverse levels of all four criterion risk variables.
General linear models (GLM) were used to examine race and gender differences in risk factor variables. All P values were two-tailed and adjusted for covariates where appropriate. Wherever race–gender interaction was present, separate models were used by race or gender. Values of HOMA-IR and ALT were log transformed in the analyses to improve normality. Univariate analysis was used to obtain the percentile distribution of ALT by race and gender. Multivariate regression analysis was used to assess the independent relation between metabolic syndrome variables and ALT in preadolescents and adolescents. The prevalence of adverse levels of individual metabolic syndrome variables and their clustering was compared between bottom versus top quartiles of ALT in preadolescents and adolescents with the use of chi-squared analysis.
The receiver-operating characteristic (ROC) curve value (c-statistics) of ALT was used as a measure of its ability to identify individuals with clustering of adverse levels of all of the criterion metabolic syndrome components. Logistic regression model was used to calculate area under the ROC curve, where the c-statistic is the nonparametric estimate of the area under the curve (AUC).19 An AUC value of 0.5 indicates that the screening test is no better than chance and a value of 1.0 indicates perfect classification.
Results
Mean levels of age and metabolic syndrome variables in preadolescents and adolescents are shown in Table 1 by race and gender. In preadolescents, significant race (whites > blacks) and gender (females > males) differences were noted for total cholesterol-to-HDL-C ratio; there was a gender difference (females > males) for HOMA-IR. In adolescents, gender difference was observed for SBP (males > females) and HOMA-IR (females > males); there was a race difference (whites > blacks) for total cholesterol to HDL-C ratio.
Table 1.
Preadolescent and Adolescent Levels of Metabolic Syndrome Variables by Race and Gender: The Bogalusa Heart Study
| |
Male |
Female |
Comparison (P valuea) |
|||
|---|---|---|---|---|---|---|
| Variable (mean ± SD) | White | Black | White | Black | Gender | Race |
| Preadolescents (4–11years) | ||||||
| N | 480 | 297 | 458 | 286 | ||
| Age (years) | 8.4 ± 1.7 | 8.4 ± 1.9 | 8.4 ± 1.8 | 8.5 ± 1.8 | N.S. | N.S. |
| BMI (kg/m2) | 17.2 ± 2.8 | 17.5 ± 3.7 | 17.7 ± 3.6 | 17.5 ± 3.6 | N.S. | N.S. |
| SBP (mmHg) | 95.2 ± 7.9 | 95.9 ± 9.0 | 95.9 ± 8.4 | 95.0 ± 9.5 | N.S. | N.S. |
| TC/HDL-C | 3.3 ± 0.8 | 3.0 ± 0.7 | 3.5 ± 0.8 | 3.2 ± 0.7 | < 0.0001 | < 0.0001 |
| HOMA-IR | 1.5 ± 2.2 | 1.6 ± 1.4 | 1.8 ± 1.9 | 1.9 ± 2.0 | 0.001 | N.S. |
| Adolescents (12–18 years) | ||||||
| N | 314 | 235 | 302 | 209 | ||
| Age (years) | 14.5 ± 2.0 | 14.8 ± 2.1 | 14.3 ± 1.9 | 14.7 ± 2.1 | N.S. | N.S. |
| BMI (kg/m2) | 21.7 ± 4.5 | 21.7 ± 4.6 | 22.0 ± 5.0 | 21.9 ± 4.9 | N.S. | N.S. |
| SBP (mmHg) | 106.8 ± 9.7 | 109.8 ± 10.6 | 105.3 ± 8.7 | 107.5 ± 9.9 | 0.001 | N.S. |
| TC/HDL-C | 3.4 ± 0.7 | 3.1 ± 0.6 | 3.3 ± 0.8 | 3.1 ± 0.7 | N.S. | < 0.01 |
| HOMA-IR | 2.3 ± 1.5 | 2.4 ± 1.7 | 2.8 ± 1.6 | 3.0 ± 3.3 | < 0.0001 | N.S. |
Adjusted for covariates as appropriate.
SD, standard deviation; BMI, body mass index; SBP, systolic blood pressure; TC/HDL-C, total cholesterol to high-density lipoprotein cholesterol ratio; HOMA-IR, insulin resistance index, homeostasis model assessment of insulin resistance; N.S., not significant.
Mean levels and quartiles of ALT in preadolescents and adolescents by race and gender are given in Table 2. Whites versus blacks in preadolescents and females versus males in adolescents had significantly higher ALT levels.
Table 2.
Mean Levels and Percentile Distribution of Serum Alanine Aminotrasferase by Race and Gender in Preadolescents and Adolescents: The Bogalusa Heart Study
| |
|
Selected percentiles |
||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD (IU/L) | 5th | 10th | 25th | 50th | 75th | 90th | 95th | |
| Preadolescents (4–11 years)a | ||||||||
| White male | 16.8 ± 5.9 | 10.0 | 11.0 | 13.0 | 15.0 | 19.0 | 24.0 | 27.5 |
| Black male | 15.9 ± 5.5 | 9.0 | 10.0 | 13.0 | 15.0 | 18.0 | 22.0 | 27.0 |
| White female | 17.0 ± 5.7 | 10.0 | 11.0 | 13.0 | 16.0 | 19.0 | 24.0 | 29.0 |
| Black female | 15.3 ± 5.5 | 8.0 | 10.0 | 12.0 | 14.0 | 18.0 | 22.0 | 26.0 |
| Total | 16.4 ± 5.7 | 9.0 | 10.0 | 13.0 | 15.0 | 19.0 | 23.0 | 27.0 |
| Adolescents (12–18 years)b | ||||||||
| White male | 19.2 ± 7.7 | 8.0 | 11.0 | 14.0 | 18.0 | 24.0 | 29.0 | 33.0 |
| Black male | 19.3 ± 8.6 | 9.0 | 11.0 | 13.0 | 17.0 | 23.0 | 31.0 | 35.0 |
| White female | 16.1 ± 7.3 | 6.0 | 9.0 | 12.0 | 15.0 | 19.0 | 25.0 | 29.0 |
| Black female | 14.6 ± 7.3 | 3.0 | 7.0 | 10.0 | 13.0 | 18.0 | 23.0 | 30.0 |
| Total | 17.4 ± 7.9 | 7.0 | 9.0 | 12.0 | 16.0 | 22.0 | 28.0 | 32.0 |
Race (white > black) difference: P = < 0.0001.
Gender (male > female) difference: P = < 0.0001.
SD, standard deviation.
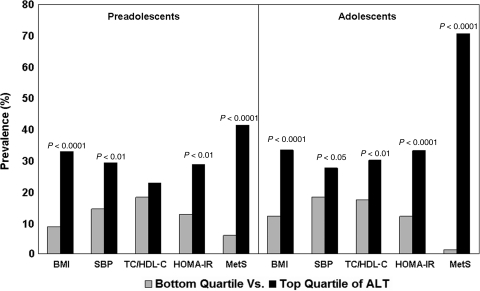
As shown in Fig. 1, both preadolescents and adolescents in the age-, race-, and gender-specific top versus bottom quartiles of ALT had significant increases in the prevalence of adverse levels (>75th percentile specific for age, race, and gender) of BMI, SBP, total cholesterol-to-HDL-C ratio (adolescents only), HOMA-IR, and their clustering. Predictor variables of ALT levels in preadolescents and adolescents are listed in Table 3. BMI was the major independent predictor of ALT levels in both preadolescents and adolescents; other independent predictors were white race in preadolescents and total cholesterol-to-HDL-C ratio, HOMA-IR, and male gender in adolescents.
FIG. 1.
Prevalence of adverse levels of metabolic syndrome variables and their clustering by alanine aminotransferase (ALT) levels in preadolescents and adolescents. BMI, body mass index; SBP, systolic blood pressure; TC/HDLC, total cholesterol-to-HDL cholesterol ratio; HOMA-IR, insulin resistance index, homeostasis model assessment of insulin resistance; MetS, metabolic syndrome. The Bogalusa Heart Study.
Table 3.
Independent Predictors of Alanine Aminotrasferase Levels in Preadolescents and Adolescents: The Bogalusa Heart Study
| Independent variable | Preadolescents (4–11years) (β) | Adolescents (12–18) (β) |
|---|---|---|
| Age | −0.033 | 0.015 |
| Whites > blacks | 0.089a | −0.027 |
| Males > females | −0.043 | 0.236a |
| BMI | 0.285a | 0.200a |
| SBP | 0.023 | 0.061 |
| TC/HDL-C | −0.001 | 0.088b |
| HOMA-IR | -0.037 | 0.066b |
All values are regression coefficient β.
P < 0.0001.
P = 0.01.
The bold values highlight the significant values in the table.
BMI, body mass index; SBP, systolic blood pressure; TC/HDL-C, total cholesterol to high-density lipoprotein cholesterol ratio; HOMA-IR, insulin resistance index, homeostasis model assessment of insulin resistance.
The AUC values (c-statistics) to determine the ability of ALT to identify preadolescents and adolescents with clustering of adverse levels of all four metabolic syndrome variables were 0.67 and 0.82, respectively. These values, by being greater than 0.50, highlight the efficiency of the test as a screening tool, especially in adolescents.
Discussion
The present community-based study provides normative values for serum ALT, an early noninvasive biomarker of liver dysfunction and NAFLD, among preadolescents and adolescents by race and gender and demonstrates that elevations in ALT, even within the normal range associated adversely with metabolic syndrome variable and their clustering. In addition, the current study also shows the usefulness of ALT in identifying the clustering of criterion metabolic syndrome variables in children, especially adolescents. These observations in an apparently healthy cohort, free of selection bias, are noteworthy in that they underscore the utility of ALT as a biomarker in the cardiovascular and type 2 diabetes risk assessment in childhood.
The observed white–black (preadolescents) and male–female (adolescents) differences in ALT levels are in agreement with earlier studies.6,20,21 Of interest, the observed race–gender differences were independent of metabolic syndrome risk variables, known correlates of ALT.10 The male versus female excess in ALT among adolescents may be mediated by androgens.22,23 The reasons for the observed higher ALT in whites compared to blacks only among preadolescents is not clear on the basis of the available data. Moreover, the race–gender differences in metabolic syndrome variables noted in this study are consistent with those previously reported.24 These race–gender differences in insulin, lipids, and blood pressure during preadolescent and adolescent periods have been found to be influenced by a complex interplay among various gonadal and adrenal steroid hormones, growth hormones, and growth factors.25–27
The adverse influence of elevations in ALT (or related pediatric NAFLD) on the pathophysiologically interrelated components of metabolic syndrome have been reported previously.7–10 In the present study, higher prevalence of adverse levels of metabolic syndrome variables such as BMI, SBP, total cholesterol-to-HDL-C ratio, and insulin resistance index were noted at higher levels of ALT in preadolescents and adolescents. In agreement with previous findings,7,10 BMI was the strongest independent predictor of ALT levels in both preadolescents and adolescents. On the other hand, the insulin resistance index and total cholesterol to HDL-C ratio were also related to ALT levels, independent of obesity measure in adolescents, but not in preadolescents. The observed difference in predictive variables of ALT between preadolescents and adolescents may be due to the fact that adverse changes in fat mass and distribution as well as insulin sensitivity and lipoprotein profile occur during puberty.22,28–31 Furthermore, childhood obesity is known to be the primary antecedent factor in the development of insulin resistance and related metabolic syndrome.12,32
The adverse relationship of ALT to risk variables of metabolic syndrome may be the consequence of a link between excess adiposity and hepatic insulin resistance mediated by increased hepatic free fatty acid flux from adipocytes, leading to increased hepatic lipogenensis and triglyceride-rich lipoprotein secretion.7,33 In addition, excess adiposity and free fatty acids (and by inference NAFL) enhance the expression of proinflamatory adipocytokines, including tumor necrosis factor-α and decreases the expression of insulin-sensitizing and antiinflammatory adiponectin, resulting in increase in insulin resistance.34–36
The concept of NAFLD as a hepatic component of metabolic syndrome is now widely being recognized.7,37,38 On the basis of this and other findings discussed above, ALT as a biomarker has the potential to help identify individual at risk. In the present study, the area (c-value) under the ROC curve values of 0.67 and 0.82 for preadolescents and adolescents, respectively, support the value of ALT as a screening test, especially in adolescents. However, because no such comparable data are available, further studies in other pediatric populations are needed to validate the current findings. This study has certain limitations in that it lacks direct assessment of body fat mass and distribution, liver fat content, and in vivo insulin action used in clinical and etiological studies. Instead, we used well-established measures that are appropriate for population studies. Furthermore, this observational cross-sectional study cannot address the issue of causality and underlying mechanisms governing the observed associations.
Conclusions
In summary, an elevation in serum ALT, even within the reference range, relates strongly to metabolic syndrome components, especially obesity, and their clustering in children. In view of the upward secular trend for being obese during childhood,39,40 ALT, as an established biomarker of ectopic fat accumulation in the liver, may help improve the risk assessment of metabolic syndrome and related disorders in the pediatric population, especially adolescents.
Acknowledgments
This study was supported by National Institute of Health Grants AG-16592 from the National Institute of Aging and HL-38844 from the National Heart, Lung, and Blood Institute.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Clark JM. Brancati FL. Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 2.Clark JM. Diehl AM. Nonalcoholic fatty liver disease: An underrecognized cause of cryptogenic cirrhosis. JAMA. 2003;289:3000–3004. doi: 10.1001/jama.289.22.3000. [DOI] [PubMed] [Google Scholar]
- 3.Lavine JE. Schwimmer JB. NAFLD in the pediatric population. Clin Liver Dis. 2004;8:549–558. doi: 10.1016/j.cld.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Wieckowska Feldstein AE. Non alcoholic fatty liver disease in the pediatric population: A review. Curr Opin Pediatr. 2005;17:636–641. doi: 10.1097/01.mop.0000172816.79637.c5. [DOI] [PubMed] [Google Scholar]
- 5.Strauss RS. Barlow SE. Dietz WH. Prevalence of abnormal serum aminotransferase values in overweight and obese adolescents. J Pediatr. 2000;136:727–733. [PubMed] [Google Scholar]
- 6.Schwimmer JB. Deutsch R. Rauch JB. Behling C. Newbury R. Lavine JE. Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatr. 2003;143:500–505. doi: 10.1067/S0022-3476(03)00325-1. [DOI] [PubMed] [Google Scholar]
- 7.Kotronen A. Yki-Jarvinen H. Fatty liver: A novel component of the metabolic syndrome. Aeterioscler Thromb Vasc Biol. 2008;28:27–38. doi: 10.1161/ATVBAHA.107.147538. [DOI] [PubMed] [Google Scholar]
- 8.Schindhelm RK. Dekker JM. Nijpels G. Bouter LM. Stehouwer CD. Heine RJ. Diamant M. Alanine aminotransferase predicts coronary heart disease events: A 10-year follow-up of the Hoorn Study. Atherosclerosis. 2007;191:391–396. doi: 10.1016/j.atherosclerosis.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Marchesini G. Brizi M. Bianchi G. Tomassetti S. Bugianesi E. Lenzi M. McCullough AJ. Natale S. Forlani G. Melchionda N. Nonalcoholic fatty liver disease: A feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 10.Hanley AJ. Williams K. Festa A. Wagenknecht LE. D'Agostino RB., Jr Haffner SM. Liver Markers and development of the metabolic syndrome: The Insulin Resistance Atherosclerosis Study. Diabetes. 2005;54:3140–3147. doi: 10.2337/diabetes.54.11.3140. [DOI] [PubMed] [Google Scholar]
- 11.Saviano MC. Brunetti F. Rubino A. Franzese A. Vajro P. Argenziano A. Puzziello A. Iannucci MP. Liver involvement in obese children. Ultrasonography and liver enzyme levels at diagnosis and during follow-up in an Italian population. Dig Dis Sci. 1997;42:1428–1432. doi: 10.1023/a:1018850223495. [DOI] [PubMed] [Google Scholar]
- 12.The Bogalusa Heart Study 20th anniversary symposium. Am J Med Sci. 1995;(suppl 1):S1–138. doi: 10.1097/00000441-199512000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Srinivasan SR. Berenson GS. Serum lipoproteins in children and methods for study. In: Lewis LA, editor. Handbook of Electrophoresis. Boca Raton, FL: CRC; 1983. pp. 185–204. , [Google Scholar]
- 14.Matthews DR. Hosker JP. Rudenski AS. Naylor BA. Treacher DF. Turner RC. Homeostasis model assessment: insulin resistance and cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 15.Ford ES. Li C. Defining the metabolic syndrome in children and adolescents: Will the real definition please stand up? J Pediatr. 2008;152:165–170. doi: 10.1016/j.jpeds.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 16.Steinberger J. Daniels SR. Eckel RH. Hayman L. Lustig RH. McCrindle B. Mietus-Snyder ML. Progress and challenges in metabolic syndrome in children and adolescents: A scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009;119:628–647. doi: 10.1161/CIRCULATIONAHA.108.191394. [DOI] [PubMed] [Google Scholar]
- 17.Srinivasan SR. Myers L. Berenson GS. Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (Syndrome X) in young adults: The Bogalusa Heart Study. Diabetes. 2002;51:204–209. doi: 10.2337/diabetes.51.1.204. [DOI] [PubMed] [Google Scholar]
- 18.Chen W. Srinivasan SR. Li S. Xu JH. Berenson GS. Metabolic syndrome variables at low levels in childhood are beneficially associated with adulthood cardiovascular risk. The Bogalusa Heart Study. Diabetes Care. 2005;28:138–144. doi: 10.2337/diacare.28.1.126. [DOI] [PubMed] [Google Scholar]
- 19.Hosmer DW. Lemeshow S. Applied Logistic Regression. 2nd. New York: John Wiley and Sons; 2000. [Google Scholar]
- 20.Schwimmer JB. McGreal N. Deutsch R. Finegold MJ. Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115:561–565. doi: 10.1542/peds.2004-1832. [DOI] [PubMed] [Google Scholar]
- 21.Rashid M. Roberts EA. Nonalcoholic steatohepatitis in children. J Pediatr Gastroenterol Nutr. 2000;30:48–53. doi: 10.1097/00005176-200001000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Deutsch MI. Mueller WH. Malina RM. Adrogeny in fat patterning is associated with obesity in adolescents and young adults. Am J Hum Biol. 1985;12:275–286. doi: 10.1080/03014468500007781. [DOI] [PubMed] [Google Scholar]
- 23.Schwimmer JB. Khorram O. Chiu V. Schwimmer WB. Abnormal aminotransferase activity in women with polycystic ovary syndrome. Fertil Steril. 2005;83:494–497. doi: 10.1016/j.fertnstert.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 24.Chen W. Bao W. Begum S. Elkasabany A. Srinivasan SR. Berenson GS. Age-related patterns of the clustering of cardiovascular risk variables of syndrome X from childhood to young adulthood in a population made up of black and white subjects: The Bogalusa Heart Study. Diabetes. 2000;49:1042–1048. doi: 10.2337/diabetes.49.6.1042. [DOI] [PubMed] [Google Scholar]
- 25.Smith CP. Dunger DB. Williams AJK. Taylor AM. Perry LA. Gale EA. Preece MA. Savage MO. Relationship between insulin, insulin-like growth factor 1, and dehydroepiandrosterone sulfate concentrations during children, puberty, and adult life. J Clin Endocrinol Metab. 1989;68:932–937. doi: 10.1210/jcem-68-5-932. [DOI] [PubMed] [Google Scholar]
- 26.Srinivasan SR. Freedman DS. Sundaram GS. Webber LS. Berenson GS. Racial (black-white) comparisons of the relationship of levels of endogenous sex hormones to serum lipoproteins during male adolescence: The Bogalusa Heart Study. Circulation. 1986;74:1226–1234. doi: 10.1161/01.cir.74.6.1226. [DOI] [PubMed] [Google Scholar]
- 27.Jiang X. Srinivasan SR. Dalferes ER., Jr Berenson GS. Plasma insulin-like growth factor-1 distribution and its relation to blood pressure in adolescents: The Bogalusa Heart Study. Am J Hypertens. 1997;10:714–719. doi: 10.1016/s0895-7061(97)00065-4. [DOI] [PubMed] [Google Scholar]
- 28.Frisancho AR. Flegel PN. Advanced maturation associated with centripetal fat pattern. Hum Biol. 1982;54:717–727. [PubMed] [Google Scholar]
- 29.Berenson GS. Srinivasan SR. Cresanta JL. Foster TA. Webber LS. Dynamic changes of serum lipoproteins in children during adolescence and sexual maturation. Am J Epidemiol. 1981;113:157–170. doi: 10.1093/oxfordjournals.aje.a113080. [DOI] [PubMed] [Google Scholar]
- 30.Amiel SA. Sherwin RS. Simonson DC. Lauritano AA. Tamborlane WV. Impaired insulin action in puberty. N Engl J Med. 1986;315:215–219. doi: 10.1056/NEJM198607243150402. [DOI] [PubMed] [Google Scholar]
- 31.Caprio S. Plewe G. Diamond MP. Simonson DC. Boulware SD. Sherwin RS. Tamborlane WV. Increased insulin secretion in puberty: A compensatory response to reductions in insulin sensitivity. J Pediatr. 1989;114:963–967. doi: 10.1016/s0022-3476(89)80438-x. [DOI] [PubMed] [Google Scholar]
- 32.Sinaiko A. Donahue RP. Jacobs DR. Prineas RJ. Relation of weight and rate of increase in weight during childhood and adolescence to body size, blood pressure, fasting insulin, and lipids in young adults. The Minneapolis Children's Blood Pressure Study. Circulation. 1999;99:1471–1476. doi: 10.1161/01.cir.99.11.1471. [DOI] [PubMed] [Google Scholar]
- 33.Bloomgarden ZT. Non alcoholic fatty liver disease and malignancy as complications of insulin resistance. Metabol Syndr Rel Disord. 2005;3:316–327. doi: 10.1089/met.2005.3.316. [DOI] [PubMed] [Google Scholar]
- 34.Weisberg SP. McCann D. Desai M. Rosenbaum M. Leibel RL. Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuzawa Y. Funahashi T. Kihara S. Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 36.Kern PA. Di Gregorio GB. Lu T. Rassouli N. Ranganathan G. Adiponectin expression from human adipose tissue-relation to obesity, insulin resistance, and tumor necrosis factor-α expression. Diabetes. 2003;52:1779–1785. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 37.Angulo P. Non-alcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 38.Angelico F. Del Ben M. Conti R. Francioso S. Feole K. Maccioni D. Antonini TM. Alessandri C. Non-alcoholic fatty liver syndrome: a hepatic consequence of common metabolic diseases. J Gastroenterol Hepatol. 2003;18:588–594. doi: 10.1046/j.1440-1746.2003.02958.x. [DOI] [PubMed] [Google Scholar]
- 39.Freedman DS. Srinivasan SR. Valdez RA. Williamson DF. Berenson GS. Secular increase in relative weight and adiposity among children over two decades: The Bogalusa Heart Study. Pediatrics. 1997;99:420–426. doi: 10.1542/peds.99.3.420. [DOI] [PubMed] [Google Scholar]
- 40.Ogden CL. Flegal KM. Carroll MD. Johnson CL. Prevalence and trends in overweight among U.S. children and adolescents 1999–2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]