Abstract
The purpose of this study was to determine if there was a connection between adherence to antiretroviral therapy (ART) and use of risk reduction behaviors (RRB) in HIV-infected women who were prescribed antiretroviral therapy. The sample consisted of 193 predominately African American women with an average age of 44 who had been on ARV for approximately 9 years and had low annual incomes. All women were participating in a behavioral clinical trial focused on these dual outcomes. Using a risk index developed for this study, we examined the relationship of a composite of risk behaviors to electronically measured and self-reported adherence over the approximately 13-month study period. Women were categorized based on levels of adherence and risky behaviors, and we sought to determine if these classifications were associated with clinical outcomes of HIV viral load and CD4 counts. High levels of adherence were correlated with low risk behaviors (abstinence, consistent use of condoms, etc.). Those classified as high adherence and low-risk behavior (HALR) as well as those classified as high adherence and high-risk behavior (HAHR) had lower mean viral loads and higher CD4 counts than those in the other categories. Women in the low adherence and high-risk category (LAHR) had detectable viral loads and the lowest CD4 counts and are at higher risk for transmitting HIV to partners and unborn children. Our findings underscore the importance of addressing adherence to both ART and RRB in HIV clinical settings to improve clinical outcomes and reduce HIV transmission.
Introduction
Adherence to antiretroviral therapy (ART) is effective in reducing viral load and prolonging life of HIV-infected persons. Depending on the medications included in the regimen, adherence requirements needed to maintain the desired undectable level of viral load, raise CD4 cell counts, and prevent opportunistic infections range from 54 to 100% for non-nucleoside reverse transcriptase inhibitors (NNRTI) and 95 to 100% for unboosted protease inhibitors.1,2 Consistent use of ART also has implications for HIV transmission.3 More recently the importance of low viral loads for HIV prevention has been described.4,5 As viral load decreases, the potential for HIV transmission also decreases. Because there is no “magic number” or cut point for viral load at which transmission is considered impossible, use of risk reduction behaviors is still important for prevention of HIV transmission. Therefore HIV infected women are expected to be highly adherent and consistently practice risk reduction behaviors to prevent transmission as well as protect themselves from sexually transmitted infections or drug resistant strains of HIV.
There is evidence to suggest that those who are adherent are more likely to practice safer sex.6–9 Diamond et al.7 reported that self-reported adherence (≥95%) and viral load suppression were associated with fewer episodes of unprotected anal or vaginal sex in 874 HIV-infected men and women. In 2002, Wilson and colleagues6 found that HIV infected women who self-reported less than 95% adherence had twice the risk for inconsistent condom use when compared to women who consistently take their medications. In a later study, Wilson et al.9 followed sexual risk behaviors of 724 women before and after initiating ART. They found that the women had fewer partners, but were at higher risk for unprotected sex compared to their pre-ART behaviors. When Remien et al.8 examined characteristics of subgroups of HIV-infected men and women who had high and low levels of self-reported adherence and sexual risk in a large sample of 2849 adults, there were no consistent factors associated with poor adherence and use of risky behaviors. However, being female and recent substance use had strong association with risky sexual behavior. All of the above studies were limited by use of self-reported adherence and cross-sectional methods, except that Wilson et al.9 studied women over a 3-month period.
One possible explanation for the combined personal risk taking behaviors of sexual risk behaviors with medication nonadherence could be the existence of a “risk taking” personality as Wilson et al.6 has suggested. Women who for whatever reason, such as lack of education, lack of impulse control, low sense of personal responsibility, denial of HIV disease, or other reasons, take more risks with their health and lives. VanZile-Tamsen and colleagues10 studied behavioral risk taking and personality in a sample of 1004 community dwelling women. They identified two highly correlated factors of risk taking: sexual and substance use and found that sensation seeking personality trait was significantly correlated with both but was stronger for substance use. Negative affect was also significantly correlated with both but to a lesser degree. Others have documented sensation seeking as associated with HIV risk behavior in gay men11 and to some extent in women,12,13 however, we could find no studies of sensation seeking in HIV-infected women. O'Cleirigh and colleagues14 examined the trait of conscientiousness (n=119 HIV-infected persons, 13% women) and found that it predicted CD4 counts and viral load levels over a 12-month period. Those with high levels of conscientiousness had higher CD4 counts and lower viral load levels at the end of the study period compared to those with low levels. They also noted that adherence, active coping, depression, and perceived stress were associated with conscientiousness, but did not mediate the relationship between it and CD4 or viral load changes. The primary ways women are infected with HIV are through sexual transmission, or substance use, or often risky sex while under the influence of substances. So an element of sensation seeking might be present in this population to some degree. Conscientiousness, in terms of health and self-care behaviors, could promote adherence and use of safer sex behaviors.
Gender-related factors might also play a role. Low-income HIV-positive women may depend on men or others for economic support and women are, to some degree, dependent on men for using condoms. Women are often the caregivers and in this role, often might take unknown risks by putting caring for themselves behind caring for others. Compared to HIV-negative women and HIV-positive men, HIV-positive women also are more depressed. Depression can diminish adherence to ART and use of risk reduction behaviors in women,6,15 and as noted above is associated with use of substances, and lower levels of conscientiousness. Fear of stigma resulting from disclosure of HIV status, may impact medication taking behavior and insistence on condom use. These variables may play an important role in differentiating those women who do well from those who do not.
In this study, we sought to determine if there was a connection between adherence to medication and use of risk reduction behaviors (RRB) in HIV-infected women prescribed ART. Specifically, we were interested in the association between adherence (measured using self-report and electronic drug monitoring) to ART and risk taking (measured with a risk index). We also wanted to know if those categorized according to adherence and risk levels showed differences in clinical outcomes of viral load (log) and CD4 counts, and if so, did these effects change over time.
Methods
Data for this project were from The KHARMA (Keeping Healthy and Active with Risk Reduction and Medication Adherence) Project, a randomized controlled behavioral clinical trial that tested the efficacy of a nurse-led motivational group intervention to promote adherence to ART and RRB in HIV-infected women. Motivational interviewing techniques were used by nurses to deliver content to the motivational group (MI), which was compared to an attention equivalent health promotion program (HPP) control group led by nurses and a health educator. Data were collected between January 2005 and January 2008. The study was approved by the Emory University Institutional Review Board and research committees at the recruitment sites, when required.
Recruitment
Recruitment for KHARMA took place at 5 HIV clinical sites in a large southeastern metropolitan city. The primary recruitment site was an infectious disease clinic at a large public hospital system that serves over 4000 HIV infected men, women, and children. The other sites were an HIV/AIDS nonprofit service organization, a hospital-based infectious disease clinic, a health department HIV clinic, and a private practice that provided care for HIV-infected persons. Women were recruited for the KHARMA Project through providers, nurse educators, case managers, and self-referral at the sites. Potential participants were asked to have their providers sign a referral form that stated they were prescribed antiretroviral medications. Eligibility criteria consisted of: (1) HIV infected; (2) female by birth; (3) prescribed antiretroviral therapy; (4) 18 years of age or older; (5) English speaking; (6) mentally stable as determined by a screening assessment; (7) willing to participate by completing computerized assessments, use electronic drug monitoring (EDM) caps, be randomly assigned and participate in group sessions. Interested women signed an informed consent form and completed a screening interview.
Study procedures
Three hundred ninety-one women were referred to the KHARMA Project; 229 were eligible and 207 women were enrolled into the study. Participants who met the eligibility criteria received a MEMS 6 TrackCap® (Aardex Ltd, Zug, Switzerland) for electronic drug monitoring and began using the MEMS 6 Track Cap immediately upon enrollment for a minimum of 2 weeks to gather baseline adherence information. When a group of 16–18 newly enrolled women accrued, they were scheduled for a baseline assessment. Following the baseline, each woman was randomly assigned to either the intervention (MI) group or the control (HPP) group. Groups started within 1–2 weeks after baseline and met for 8 weekly sessions. Follow-up assessments occurred at 2 weeks (immediate), 3 months, 6 months, and 9 months after the final group session. Assessments were conducted using audio computerized assessment self-interview (ACASI) technology. Participants also returned for monthly downloads of the EDM caps and the completion of a short in-person questionnaire on MEMS 6 Track Cap use. Participants received $25 for each of the 5 assessments and transportation tokens and snacks for EDM cap downloads. A more complete description of the project is found in Holstad et al.16,17
Measures
Adherence was measured using both electronic monitoring caps and self-report. One medication from the regimen was electronically monitored using the MEMS cap, and all staff followed an algorithm to determine which medication would receive the cap. In general, in a boosted PI regimen, that medication was the primary protease inhibitor; in an NNRTI based regimen, it was the NNRTI. A sticker was placed on the monitored medication bottle and the participant was instructed to write times on the sticker when the medication was pocketed, or if the cap was opened by mistake. At each download, information on this sticker was reviewed and an EDM questionnaire was completed. The information collected on the questionnaire included changes in medication regimen, problems with the cap, if someone else had administered the medication, and if the medication had been stopped. EDM data were adjusted by setting certain days as “nonmonitored” on which the following events occurred: cap malfunction, lost cap, medication stopped by health care provider, someone else administered the medication (e.g., hospital, group home), participant incarcerated, pocketing pills, reported exclusive use of pill box, and excessive openings (a form of cap malfunction defined as more than twice the dose plus one). The EDM data were captured over time in phases consistent with the assessment periods. The MEMS Track caps were used by participants during the entire study period (approximately 13 months) including at least 2 weeks prior to the baseline to obtain an estimate of baseline adherence. For this analysis, we used the “Percentage of Doses Taken” from the MEMS report. The possible range was 0–100% of prescribed doses taken over the assessment period. To categorize participants by adherence rates, 90% or more was considered high adherence and less than 90% was considered low adherence. We assigned this cut point conservatively, based on the wide range of adherence needed per type of medication, given that our participants were on NNRTI and boosted PI-based regimens.
Self-reported adherence was measured by the Antiretroviral General Adherence Scale (AGAS). This is a five-item unidimensional measure that assesses the ease and ability of participants to take ART according to a health care provider's recommendations in the previous 30 days.16,18 The possible scores for this instrument range from 0 to 30, and higher scores reflect better adherence. Cronbach α for this sample was 0.85 at baseline.
CD4 counts and viral loads are indicators of the immune status and HIV viral activity and are clinical indicators of adherence. Both are expected to improve with ART; however, the intended effect of ART, to prevent viral replication, is more directly assessed by the viral load. We extracted laboratory results from participants' medical records during the time they were enrolled in the study. We used the viral load criteria of 400 copies per milliliter or less and corresponding log 2.6 or less as undectable. Study sites used viral load tests with varying levels of detection over the study time period and this level was the most inclusive. Dates that the laboratory tests were drawn were grouped according to proximity with the study assessment periods. For example, CD4 and viral loads drawn as close as possible and prior to or on the day of the baseline assessment were classified as the baseline results. The median was approximately 27 days prior to the baseline. Participants did not always have both tests performed on the same date and therefore may have only had one test result available during an assessment period. Since these tests are typically ordered every 3–4 months once a patient has stabilized, participants had fewer lab results available that corresponded to study time points by the end of the follow-up period.
Risk behaviors were measured using a risk index we developed for this study. The index was composed of 11 items taken from two instruments: our modified version of the Centers for Disease Control Sexual Behavior Questions19 and a Substance Use Questionnaire. The modified version of the Centers for Disease Control Sexual Behavior Questions contained 58 items about current sexual activity, sexual activity with a main partner and a casual partner, male and female partners. Examples of behaviors addressed are vaginal, oral, and anal sex; use of alcohol, drugs before sex; use of protection (such as male condom, female condom, and dental dam) during sex. Items require a “yes/no” for use of behavior and scaled responses for frequency of a behavior. The Substance Use Questionnaire contained 35 items regarding use of all types of substances such as alcohol, marijuana, cocaine, including injection drug use. It was an adaptation of items from two instruments, the HIVNET Risk Assessment developed with National Institutes of Health (NIH) funding by the Statistical Center for HIV/AIDS Research and Prevention (ACHARP) for the HIV Prevention Trials Network (HPTN)20 and the Elicitation of Compliance and Adherence Behaviors Survey (ECAB).21 Items on this questionnaire also required a “yes/no” for whether a certain drug or behavior was practiced and scaled response for the frequency.
The risk index was calculated based on scores from 11 risky behavior items from the questionnaires described above. With the exception of sexual activity during menstrual period, the items were selected because they are commonly cited as outcomes in research studies related to risky behaviors. We chose items related to the main partner because fewer women in the sample reported casual partners and women might be more likely to relax risk practices with a primary partner. Sexual activity during menses was included because of the additional high risk related to contact with blood and body fluids. We then recoded the responses to the 11 items for ease of scoring and standardization. To recode, we used a 3-point scale, where 0=no risk, 1=moderate risk, and 2=high risk. This scale was chosen based on the work of Susser and colleagues22,23 on the Vaginal Episode Equivalent Risk Index (VEE) and the recommendations of Crosby and colleagues24 with respect to categorizing condom users. The items included: sexual activity in the last 3 months (0=no; 1=yes), number of partners (0=none, 1=1, 2=3 or more), HIV status of partners (0=HIV positive, 2=HIV negative or unknown), vaginal sex in past 3 months (0=no, 1=yes), anal sex in past 3 months (0=no, 2=yes), frequency of sex during menstrual period (0=never or no longer has periods, 1=almost never to sometimes, 2=monthly), frequency of protection/condom use in past 3 months (0=always, 1=half the time to less than half the time, 2=never), decided not to have sex at some time in past 3 months because no protection was available (0=yes; 1=no), in past 3 months frequency of getting high or drinking before sex (0=never, 1=occasionally, 2=often, all the time), in past 3 months did substance use make it difficult to practice safer sex (0=never, 1=occasionally, 2=often, all the time), in the past 3 months using a needle to inject drugs (0=no, 2=yes). The risk score was a summary of the scores on these items and possible scores ranged from 0 to 18 with higher scores indicating riskier behaviors. To classify risk we assigned a summary score of ≤2 on the Risk Index as low risk and a summary score of 3 or more on the risk index as high risk.
In order to determine if participants' clinical outcomes differed according to levels of adherence and risk, we cross-classified participants by adherence levels and risk levels, using the risk index scores above and the 90% or more cut point described above as high adherence. The categories chosen were similar to those reported by Remien et al.8 The four resulting group categories were: (1) high adherence, low risk (HALR, n=51), (2) high adherence, high risk (HAHR, n=37), (3) low adherence, low risk (LALR, n=40), and (4) low adherence, high risk (LAHR, n=56).
Analysis
Descriptive statistics were computed for all variables and the distributions of continuous variables were screened for normality using skewness and kurtosis values and q-q plots. Since the data for these analyses were from a randomized controlled behavioral clinical trial, we examined relevant variables with respect to identification of any baseline differences in the two treatment groups using independent samples t tests, Mann-Whitney U tests, and χ2 tests based on the nature and distributional properties of the variable. To examine the association between adherence (measured using self-report and electronic drug monitoring) to ART and risk taking (measured with the risk index) we used Spearman's ρ because of the non-normality of the risk index.
We also wanted to know if those categorized according to adherence and risk levels showed differences in clinical outcomes of viral load (log) and CD4 counts, and if so, did these effects change over time. To address this question, we used a generalized estimating equations (GEE) approach to assess the effects of adherence/risk category and time controlling for intervention group, number of sessions attended, the number of years the participant had been HIV-positive and participant's age. We used a similar approach to conduct preliminary analyses assessing the effects of intervention group and time on the Risk Index and classification as high or low risk with respect to risk behaviors. A GEE approach was chosen because of the correlated data structure and to maximize the number of observations included in the analyses. For hypothesis testing, a significance level of 0.05 was used.
Results
Of the 207 women who enrolled in KHARMA, we have at least one follow-up assessment (including at least four weeks of EDM data) on 193 (93%) participants and these were included in the analyses. The characteristics of these 193 participants are displayed in Table 1. They ranged in age from 18 to 68 with a mean of 44 years. Most of the women were African American, unmarried, unemployed, and had very low income. About half of the participants reported being sexually active at the time of the baseline assessment. Based on the results of χ2 and t tests, the groups did differ significantly at baseline on three variables. Those in the HPP group had been HIV positive somewhat longer (M=10.4 years) than those in the MI group (M=8.6 years). A greater percentage of those in the MI group reported having children (89% versus 78%) with the median number of children the same in both groups. The mean CD4 count was higher in the HPP group (463.8) compared to the MI group (337.0). Mean adherence for the percentage of prescribed doses taken from the EDM data and self report from the AGAS data did not differ significantly between groups (Table 1).
Table 1.
Baseline Characteristics of KHARMA Participants (n=193)
| Variable | MI group (n=97) | HPP group (n=96) | Total (n=193) |
|---|---|---|---|
| Age | |||
| Mean (SD) | 43.5 (9.2) | 44.1 (9.0) | 43.8 (9.1) |
| Number of years HIV-positive | |||
| Mean (SD)a | 8.6 (6.0) | 10.4 (6.5) | 9.5 (6.3) |
| Number of years on ARVs | |||
| Mean (SD) | 5.7 (4.9) | 6.8 (5.0) | 6.2 (5.0) |
| Ethnicity, n (%) | |||
| African American | 88 (90.7) | 91 (94.8) | 179 (92.7) |
| White | 6 (6.2) | 1 (1.0) | 7 (3.6) |
| Other | 3 (3.1) | 4 (4.2) | 7 (3.6) |
| Education, n(%) | |||
| <High school | 17 (17.5) | 21 (21.9) | 38 (19.7) |
| High school | 54 (55.7) | 49 (51.0) | 103 (53.4) |
| >High school | 26 (26.8) | 26 (27.1) | 52 (26.9) |
| Employment, n(%) | |||
| Employed | 16 (16.5) | 14 (14.6) | 30 (15.5) |
| Marital status, n (%) | |||
| Married | 5 (5.2) | 13 (13.5) | 18 (9.4) |
| Committed relationship | 21 (21.9) | 13 (13.5) | 34 (17.7) |
| Never married | 26 (27.1) | 27 (28.1) | 53 (27.6) |
| Separated/divorced/widowed | 44 (45.8) | 43 (44.8) | 87 (45.3) |
| Children, n(%) | |||
| Yesb | 86 (88.7) | 75 (78.1) | 161 (83.4) |
| Median, range | 3, 1-7 | 3, 1-9 | 3, 1-9 |
| Annual Income, n (%) | |||
| ≤$10,000 | 64 (69.6) | 62 (66.0) | 126 (67.7) |
| >$10,000 | 28 (30.4) | 32 (34.0) | 60 (32.3) |
| Sexual identity, n (%) | |||
| Heterosexual | 78 (80.4) | 72 (75.0) | 150 (77.7) |
| Gay, homosexual | 2 (2.1) | 3 (3.1) | 5 (2.6) |
| Bisexual | 5 (5.2) | 3 (3.1) | 8 (4.1) |
| None of above/unsure | 12 (12.4) | 18 (18.8) | 30 (15.5) |
| Sexually active (past 3 months), n (%) | |||
| Yes | 50 (51.5) | 52 (54.2) | 102 (51.5) |
| CD 4 count | |||
| Mean (SD),cn | 337.0 (267.4), 69 | 463.8 (388.2), 75 | 403.0 (340.6), 144 |
| Viral load (log10) | |||
| Mean (SD), n | 2.6 (1.0), 76 | 2.5 (1.0), 77 | 2.5 (1.0), 153 |
| Percent of prescribed doses taken | |||
| Mean (SD), n | 72.9 (35.8), 94 | 76.4 (32.6), 96 | 74.6 (34.2), 190 |
| AGAS (self-report adherence) | |||
| Mean (SD) | 20.5 (3.2) | 20.3 (3.1) | 20.4 (3.2) |
| Risk index score | |||
| Mean (SD) | 2.6 (2.8) | 3.0 (3.1) | 2.8 (2.9) |
t(188)=2.09, p=0.038.
χ2(1)=3.87, p=0.049.
t(131.9)=2.29, p=0.023, unequal variances.
KHARMA, Keeping Healthy and Active with Risk Reduction and Medication Adherence Project; AGAS, Antiretroviral General Adherence Scale; SD, standard deviation; ARVs, antiretrovirals.
Risk index scores and adherence scores by group and for the total sample for all assessment periods are found in Table 2. Using the high adherence cut point of 90% or more and the summary risk score cut point of 0 to 3 as low risk, 47.8% were categorized as highly adherent and 50.3% were classified as having low risk behaviors at baseline.
Table 2.
Electronic Drug Monitoring, Antiretroviral General Adherence Scale, and Risk Index Means and Standard Deviations by Group and Time
| |
|
|
Time |
|||
|---|---|---|---|---|---|---|
| Group | Baseline | F1 | F2 | F3 | F4 | |
| EDM | ||||||
| HPP | Mean (SD) | 76.6 (31.2) | 68.6 (31.6) | 60.4 (33.9) | 59.7 (35.8) | 56.9 (36.4) |
| n | 96 | 89 | 85 | 81 | 82 | |
| MI | Mean (SD) | 71.4 (35.1) | 71.6 (32.7) | 64.0 (36.2) | 61.0 (38.0) | 58.1 (38.4) |
| n | 88 | 79 | 75 | 73 | 67 | |
| Total | Mean (SD) | 74.1 (33.2) | 70.0 (32.1) | 62.1 (34.9) | 60.3 (36.8) | 57.4 (37.2) |
| N | 184 | 168 | 160 | 154 | 149 | |
| AGAS | ||||||
| HPP | Mean (SD) | 20.3 (3.1) | 20.8 (3.1) | 20.8 (3.5) | 20.7 (3.7) | 20.5 (3.9) |
| n | 96 | 94 | 91 | 90 | 95 | |
| MI | Mean (SD) | 20.5 (3.2) | 20.3 (3.4) | 20.1 (4.1) | 20.5 (3.4) | 20.6 (4.0) |
| n | 97 | 95 | 92 | 86 | 89 | |
| Total | Mean (SD) | 20.4 (3.2) | 20.5 (3.3) | 20.4 (3.8) | 20.6 (3.6) | 20.5 (3.9) |
| N | 193 | 189 | 183 | 176 | 184 | |
| Risk index | ||||||
| HPP | Mean (SD) | 3.0 (3.1) | 2.7 (2.8) | 2.5 (3.0) | 2.4 (3.4) | 2.6 (3.1) |
| Min-Max | 0–11 | 0–10 | 0–12 | 0–15 | 0–9 | |
| n | 96 | 94 | 91 | 90 | 95 | |
| MI | Mean (SD) | 2.6 (2.8) | 2.3 (2.8) | 2.6 (3.0) | 2.6 (2.8) | 2.7 (3.0) |
| Min-Max | 0–14 | 0–9 | 0–9 | 0–9 | 0–12 | |
| n | 97 | 95 | 92 | 88 | 88 | |
| Total | Mean (SD) | 2.8 (2.9) | 2.5 (2.8) | 2.6 (3.0) | 2.5 (3.1) | 2.7 (3.0) |
| Min-Max | 0–14 | 0–10 | 0–12 | 0–15 | 0–12 | |
| N | 193 | 189 | 183 | 178 | 183 | |
EDM, electronic drug monitoring; HPP, health promotion program; MI, motivational group; SD, standard deviation.
One of the primary foci of the MI intervention group was to reduce risky behaviors, and based on our final results there were differences at some time points between groups in some behaviors (practicing abstinence, always using protection) in those who attended at least seven group sessions that could confound the results. However, when these behaviors were included with other behaviors in the risk index, without the effect of attendance, there was no significant difference in mean Risk Index scores at baseline or any of the follow-up periods between the MI and HPP groups. Therefore, for the analyses, the MI and HPP groups were combined.
To determine if an association existed between adherence and risky behaviors, we evaluated the Spearman correlations assessing the association between adherence as measured by the percent of doses taken from the EDM data and the AGAS and the risk index scores. We found a significant negative correlation between EDM data and the risk index scores for baseline (rs=−0.207, p=0.005), and consistent negative correlations between AGAS scores and participant risk index scores at each time point (rs=−0.141 to −0.265, p<0.05). Lower adherence rates were associated with higher scores on the risk index, meaning that participants who were less adherent were also more likely to practice risky behaviors.
Prior to conducting the analysis focused on comparison of clinical outcomes by risk group classification, we examined the group, time, and group*time effects on risk behavior and adherence classifications controlling for the number of years a participant had been HIV positive and their intervention attendance. For risk behavior classification, we did not find statistically significant effects for group, time, or group*time. The attendance covariate was statistically significant (p=0.015) with those having higher attendance having a lower odds of being in the high behavior risk group compared to those with lower attendance. In addition, age was found to be a significant covariate (p<0.001) with older age being associated with a decreased odds of being in the high risk behavior group. For medication adherence (≥90%), there was no group or group*time effect. However, there was an overall effect for time with the proportion achieving 90% or greater adherence being significantly (p<0.05) lower at follow-up 2, 3, and 4 compared to baseline.
Finally, we used a multinomial model to examine group, time, and, group*time effects on the four level adherence/risk classification variable controlling for years HIV positive and intervention attendance. The group, time, and group*time effects were not found to be statistically significant in this model. Similar to the risk behavior group analysis, age (p<0.001) and attendance (p=0.038) were both statistically significant predictors with those having higher attendance and age being more likely to be in the HALR compared to the other three groups. Since these analyses did not reveal any intervention related effects, we elected to analyze the effect of overall adherence/risk group classification on clinical outcomes including intervention group as a covariate along with attendance, years HIV positive, and age at baseline.
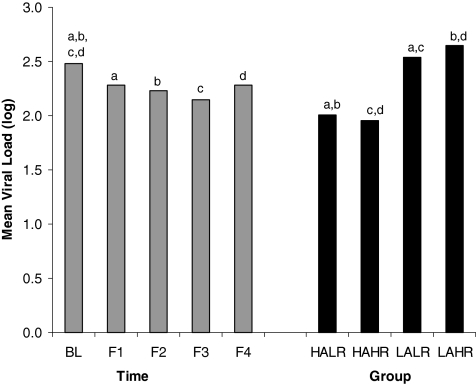
As noted previously, to determine whether overall adherence/risk classification (4 groups) was associated with the clinical outcomes of viral load (log) and CD4 counts, we used a GEE approach. For each outcome, the model included an effect for classification, time, and classification*time with intervention group, number of sessions attended, the number of years the participant had been HIV-positive and participant's age included as covariates. For viral load (log), the results of this analysis indicated a statistically significant effect for time (Wald χ2(4)=13.98, p=0.007) and classification (Wald χ2(3)=50.42, p<0.001) and a marginally significant effect for the time*classification effect (Wald χ2(12)=19.56, p=0.076). In this model, the only covariate that was statistically significant was age at baseline (p=0.018) with higher age being associated with lower viral load. For the time effect, pairwise comparisons indicated that adjusted mean viral load (log) at each follow-up was significantly lower than viral load at baseline (p=0.010, p=0.004, p<0.001, and p=0.038 for baseline compared to follow-up 1, 2, 3, and 4, respectively). However, the adjusted means for the follow-up periods did not differ significantly. For the adherence/risk classification effect, pairwise comparisons indicated that the HALR had a significantly lower mean viral load (log) compared to the LALR group (p<0.001) and the LAHR group (p<0.001). The HAHR group also had a significantly lower mean viral load (log) compared to the LALR (p<0.001) and LAHR (p<0.001) groups. The adjusted means for the time and group main effects are displayed in Fig. 1.
FIG. 1.
Adjusted mean viral load (log) by time period of assessment and by adherence/risk group classification. HALR, high adherence, low risk (n=51); HAHR, high adherence, high risk (n=37); LALR, low adherence, low risk (n=40); LAHR, low adherence, high risk (n=56). Bars with the same letters on top differ significantly with each other at p<0.05 for the time comparisons and p<0.001 for the group comparisons. Covariates included intervention group, intervention attendance, age at baseline, and number of years HIV-positive.
Similar results were noted for CD4 count with significant overall effects for time (Wald χ2(4)=13.33, p=.010) and group classification (Wald χ2(3)=10.52, p=0.015) and no significant effect for the group classification by time interaction. The only statistically significant covariate in this model was number of years positive (Wald χ2(1)=4.85, p=0.028) with years positive showing a positive relationship with CD4 count. For the overall effect of time, pairwise comparisons indicated that CD4 count was significantly higher at follow-up 2 (M=448.8, standard error [SE]=26.4, p=0.003) and follow-up 3 (M=466.4, SE=26.6, p=0.001) than at baseline (M=395.5, SE=24.5). Although higher, mean CD4 count at follow-up 1 (432.5, SE=30.6, p=0.055) and at follow-up 4 (438.7, SE=26.9, p=0.083) did not differ significantly from the mean at baseline. The remaining pairwise comparisons were not statistically significant. For the overall group effect, pairwise comparisons indicated that the HALR group (M=478.7, SE=42.7) had a significantly (p=0.019) higher mean CD4 count compared to the LALR group (M=389.8, SE=25.9). The mean for the HALR group was marginally (p=0.078) higher than the mean for the LAHR group (M=393.8, SE=29.0). Interestingly, the mean for the HAHR group (M=483.3, SE=32.5) was significantly higher than the mean for both the LALR (p=0.009) and LAHR groups (p=0.013). All reported means are adjusted for the previously mentioned covariates.
Discussion
The purpose of this study was to determine if there was a relationship between ART medication adherence and risky sexual behaviors, and if women had differences in clinical outcomes of CD4 counts and viral loads based on their adherence-risk category. Using a risk index developed for this study, we combined several risky behaviors into one measure that provided a more comprehensive assessment of risk than that of examining discreet behaviors separately.
We found a consistently significant negative correlation between adherence to ART and the use of risky behaviors over time. High levels of self-reported adherence were associated with low-risk behaviors (e.g., abstinence, consistent use of condoms/protection, avoidance of alcohol or drugs before sex, and avoidance of sex during menses). Few authors have examined this relationship, and none that we could find, examined it longitudinally. Our findings are comparable to those of other researchers who examined discreet behaviors. For example, Wilson et al.4 found a similar association between ART adherence and condom use, and Diamond et al.5 found an association between adherence and unprotected anal or vaginal sex.
When we classified the sample into four categories based on levels of adherence and risk, we found that, controlling for age, viral load in all groups decreased (improved) significantly over time, there was a significant difference in viral load by adherence/risk classification, and there was a trend toward a classification group*time effect. Those women in each of the HALR and HAHR categories had significantly lower mean viral loads (the desired clinical effect) than those in the LALR and LAHR categories. Those classified as LAHR, which represents low adherence and high risk, had the highest mean viral loads and were above the level of detection. With the exception of no significant classification*time effect, findings were similar for CD4 counts as well. Controlling for years HIV positive, those in the HALR and HAHR categories had significantly higher mean CD4 counts than the other groups and the LAHR category had the lowest mean CD4 counts. This seems to indicate that although adherence and risk behavior are correlated, clinical outcomes are driven by adherence.
Of note is that those who have lower adherence and higher risk behavior are at high risk for transmitting HIV, due to their higher viral loads. Compared to the other three categories, in this study, the LAHR group contained the largest number of participants. Our participants were HIV infected an average of over 9 years and perhaps they developed adherence and risk “fatigue” over time. It may also be possible that characteristics such as depression, sensation seeking, and low levels of conscientiousness were predominate in this group. These women are not only at risk for progression of their HIV disease, but also development of drug resistant strains due to poor adherence. They are also at risk of transmitting HIV, including drug-resistant strains, to others such as partners and unborn children. The greater risk of HIV transmission increases the public health significance of their behaviors.
An important next step is to identify characteristics of women who do not routinely take their medications or practice safer sex behaviors. Remien and colleagues8 attempted this in a cross sectional study of both men and women, however they found no consistent factors. Female gender (our population) and substance use were associated with sexual risk; and psychological distress, stress, self-efficacy, attitudes about treatment, and social support were associated with ART adherence.
Limitations
The primary limitation is that this study was not originally powered for this type of subset analysis and future studies will require adequate sample size. Another possible limitation is that about half women in this study were exposed to an MI based intervention focused on promoting adherence to ART and reducing risky behaviors. When dichotomized based on the 90% cut point, adherence did not differ between intervention and control groups. Some risk behaviors did differ based on intervention group and attendance, although when risk behaviors were combined into the composite index, there were no significant differences between intervention or control groups. We controlled for intervention group and attendance in the analysis, however the possibility of intervention group as a confounder could exist. This study is also limited by the potential bias associated with self-report of sexual behaviors due to memory and social desirability. In addition, laboratory data were extracted from medical records, results closest to the assessment time points were used. Labs are typically drawn every 3–4 months and with time, fewer results were available at the end of the study. The sample for this study comprised predominately African American women, which is consistent with the demographics of HIV/AIDS in women in the southeast, but the findings cannot be generalized to other groups. These limitations are offset by the objective adherence data from electronic monitoring and use of ACASI to enhance confidential self-reporting of adherence and sexual behaviors.
Clinical implications
In 2009 the Centers for Disease Control and Prevention4 issued a document on the effects of ART on HIV transmission. They affirmed that a low viral load in the setting of ART substantially reduces but doesn't eliminate the risk of sexual transmission. Thus, HIV-infected women on ART still should practice safer sex behaviors. Our findings underscore the importance of combining ART adherence counseling with safer sex/risk reduction discussions. More research is needed to identify characteristics of women who do not adhere to both and to develop evidenced based interventions effective for both behaviors.
Acknowledgments
This research was funded by a grant from the National Institutes of Nursing Research R01 NR008094.
We wish to thank the women who participated in this project and the providers and staff of the clinics from which we recruited and conducted the study. We also acknowledge the work of the KHARMA Project staff, including Bridget Jones, Carol Corkran, Sally Carpentier, Versey McLendon, Lisa Weaver, Kate Yeager, Ilya Teplinskiy, and Samaha Norris.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43:939–941. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- 2.Paterson D. Swindells S. Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 3.Lalani T., MBBS Hicks C., MD Does antiretroviral therapy prevent HIV transmission to sexual partners. Curr Infect Dis Rep. 2008;10:140–145. doi: 10.1007/s11908-008-0025-8. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Effect of Antiretroviral Theraphy on Risk of Sexual Transmission of HIV Infection and Superinfection. 2009. www.cdc.gov/hiv/topics/treatment/resources/factsheets/pdf/art.pdf. [Jun 14;2010 ]. www.cdc.gov/hiv/topics/treatment/resources/factsheets/pdf/art.pdf
- 5.Donnell D. Baeten JD. Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: A prospective cohort analysis. Lancet. 2010;375:2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson TE. Barron Y. Cohen M, et al. Adherence to antiretroviral therapy and its association with sexual behavior in a national sample of women with human immunodeficiency virus. Clin Infect Dis. 2002;34:529–534. doi: 10.1086/338397. [DOI] [PubMed] [Google Scholar]
- 7.Diamond C. Kemper C. McCutchan A, et al. Use of and adherence to antiretroviral therapy is associated with decreased sexual risk behavior in HIV clinic patients. J Acquir Immune Defic Syndr. 2005;39:211–218. [PubMed] [Google Scholar]
- 8.Remien RH. Exner TM. Morin SF, et al. Medication adherence and sexual risk behavior among HIV-infected adults: Implications for transmission of resistant virus. AIDS Behav. 2007;11:663–675. doi: 10.1007/s10461-006-9201-8. [DOI] [PubMed] [Google Scholar]
- 9.Wilson TE. Gore ME. Greenblatt R, et al. Changes in sexual behavior among HIV-infected women after initiation of HAART. Am J Public Health. 2004;94:1141. doi: 10.2105/ajph.94.7.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.VanZile-Tamsen C. Testa M. Harlow LL. Livingston JA. A measurement model of women's behavioral risk taking. Heatlh Psychol. 2006;25:249–254. doi: 10.1037/0278-6133.25.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalichman SC. Heckman T. Kelly JA. Sensation seeking as an explanation for the association between substance use and HIV-related risky sexual behavior. Arch Sex Behav. 1996;25:141–154. doi: 10.1007/BF02437933. [DOI] [PubMed] [Google Scholar]
- 12.Spitalnick JS. DiClemente RJ. Wingood GM, et al. Brief report: Sexual sensation seeking and its relationship to risky sexual behaviour among African-American adolescent females. J Adolesc. 2007;30:165–173. doi: 10.1016/j.adolescence.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones R. Relationships of sexual imposition, dyadic trust, and sensation seeking with sexual risk behavior in young urban women. Res Nurs Health. 2004;27:185–197. doi: 10.1002/nur.20016. [DOI] [PubMed] [Google Scholar]
- 14.O'Cleirigh C. Ironson G. Weiss A. Costa PT., Jr Conscientiousness predicts disease progression (CD4 number and viral load) in people living with HIV. Heatlh Psychol. 2007;26:473–480. doi: 10.1037/0278-6133.26.4.473. [DOI] [PubMed] [Google Scholar]
- 15.Remien RH. Exner T. Kertzner RM, et al. Depressive symptomatology among HIV-positive women in the era of HAART: A stress and coping model. Am J Commun Psychol. 2006;38:275–285. doi: 10.1007/s10464-006-9083-y. [DOI] [PubMed] [Google Scholar]
- 16.Holstad MM. DiIorio C. Magowe MK. Motivating HIV positive women to adhere to antiretroviral therapy and risk reduction behavior: The KHARMA Project. Online J Issues Nurs. 2006;11:5. [PubMed] [Google Scholar]
- 17.Holstad MM. DiIorio C. Kelley ME. Resnicow K. Sharma S. Group Motivational Interviewing to promote adherence to antiretroviral medications and risk reduction behaviors in HIV infected women. AIDS Behav. doi: 10.1007/s10461-010-9865-y. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holstad MM. Foster V. DiIorio C. McCarty F. Teplinskiy I. An examination of the psychometric properties of the Antiretroviral General Adherence Scale (AGAS) in two samples of HIV-infected individuals. J Assoc Nurses AIDS Care. 2010;21:162–172. doi: 10.1016/j.jana.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Core measures for HIV/STD risk behavior and prevention: Questionnaire-based measurement for surveys and other data systems. Sexual Behavior Questions Version 5.00. 2001. http://chipts.cch.ucla.edu/assessment/IB/List_ScalesCDC%20sexual%20Behavior%20Questions%20(CSBQ).htm. [Jun 14;2010 ]. http://chipts.cch.ucla.edu/assessment/IB/List_ScalesCDC%20sexual%20Behavior%20Questions%20(CSBQ).htm
- 20.Metzger DS. Koblin B. Turner C, et al. Randomized controlled trial of audio computer-assisted self-interviewing: Utility and acceptability in longitudinal studies. HIVNET Vaccine Preparedness Study Protocol Team. Am J Epidemiol. 2000;152:99–106. doi: 10.1093/aje/152.2.99. [DOI] [PubMed] [Google Scholar]
- 21.Williams M. Bowen A. Ross M. Freeman R. Elwood W. Perceived compliance with AZT dosing among a sample of African-American drug users. Int J STD AIDS. 2000;11:57–63. doi: 10.1258/0956462001914797. [DOI] [PubMed] [Google Scholar]
- 22.Susser E. Desvarieux M. Wittkowski KM. Reporting sexual risk behavior for HIV: A practical risk index and a method for improving risk indices. Am J Public Health. 1998;88:671–674. doi: 10.2105/ajph.88.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Susser E. Valencia E. Berkman A, et al. Human immunodeficiency virus sexual risk reduction in homeless men with mental illness. Arch Gen Psychiatry. 1998;55:266–272. doi: 10.1001/archpsyc.55.3.266. [DOI] [PubMed] [Google Scholar]
- 24.Crosby RA. Yarber WL. Sanders SA. Graham CA. Condom use as a dependent variable: A brief commentary about classification of inconsistent users. AIDS Behav. 2004;8:99–103. doi: 10.1023/b:aibe.0000017529.20932.73. [DOI] [PubMed] [Google Scholar]