Abstract
Several growth factors are effective for salvaging myocardium and limiting infarct size in experimental studies with small animals. Their benefit in large animals and feasibility in clinical practice remains to be elucidated. We investigated the cardioprotective effect of midkine (MK) in swine subjected to ischemia/reperfusion (I/R). I/R was created by left anterior descending coronary artery occlusion for 45 min using a percutaneous over-the-wire balloon catheter. MK protein was injected as a bolus through the catheter at the initiation of reperfusion [MK-treated (MKT) group]. Saline was injected in controls (CONT). Infarct size/area at risk (24 h after I/R) in MKT was almost five times smaller than in CONT. Echocardiography in MKT revealed a significantly higher percent wall thickening of the interventricular septum, a higher left ventricular (LV) fractional shortening, and a lower E/e′ (ratio of transmitral to annular flow) compared with CONT. LV catheterization in MKT showed a lower LV end-diastolic pressure, and a higher dP/dtmax compared with CONT. Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end-labeling-positive myocytes and CD45-positive cell infiltration in the peri-infarct area were significantly less in MKT than in CONT. Here, we demonstrate that a single intracoronary injection of MK protein in swine hearts at the onset of reperfusion dramatically reduces infarct size and ameliorates systolic/diastolic LV function. This beneficial effect is associated with a reduction of apoptotic and inflammatory reactions. MK application during percutaneous coronary intervention may become a promising adjunctive therapy in acute coronary syndromes.
Keywords: midkine, ischemia/reperfusion injury, intracoronary injection, acute coronary syndrome
Introduction
Current treatment of acute myocardial infarction is focused on limiting the duration of the ischemic period by disrupting the occlusion in the coronary arteries and reestablishment of coronary flow. However, post ischemic reperfusion may induce myocardial dysfunction due to microcirculatory flow disturbances, aggravation of oxidative stress, calcium overload, excessive inflammation, and activation of apoptosis (Moens et al., 2005). Despite numerous strategies aiming to prevent ischemia/reperfusion (I/R) induced myocardial damage, no effective agent is available in clinical practice. Several growth factors have been shown to protect against I/R injury in small animals like rodents, but their way into clinical practice is still far away by insufficient effects or unacceptable side effects in large animals or humans. Recently, we have reported that midkine (MK) has a protective role against reperfusion injury, and that it can diminish myocardial infarct size in both acute and chronic ischemic mouse models (Horiba et al., 2006; Takenaka et al., 2009). However, its cardioprotective effect in swine is unknown.
Midkine is a heparin-binding growth factor with 45% sequence homology with pleiotrophin (also called heparin-binding growth-associated molecule, HB-GAM). It is structurally unrelated to other heparin-binding growth factors such as fibroblast growth factors. MK gene expression is high in malignant tumors in various tissues and is expected to become a new tumor marker (Muramatsu, 2002). MK is involved in various anabolic activities, such as neural growth (Michikawa et al., 1993), migration of inflammatory leukocytes, and preservation and repair of injured tissues (Horiba et al., 2006). MK has anti-apoptotic activity in embryonic neural cells (Yokota et al., 1998; Kadomatsu and Muramatsu, 2004) and in retinal photoreceptor cells (Unoki et al., 1994). In the murine heart, we have shown that this effect occurs not only in acute ischemia but also in chronic myocardial infarction (Takenaka et al., 2009). This study aims to elucidate whether MK may be attractive for clinical use. We assessed the acute effect of MK in swine hearts subjected to I/R.
Materials and Methods
Ischemia and reperfusion
All animal experiments were performed in accordance with the regulations adopted by the National Institute of Health and approved by the Animal Care and Use Committee of Nagoya University. In male castrated swine weighing 37–42 kg, anesthesia was induced by ketamine (10 mg/kg, i.m.) and thiamylal sodium (0.3 mg/kg, i.v.), and maintained by inhalation of sevoflurane (1%). Oxygen saturation, aortic blood pressure, central body temperature, and a three-lead (I, II, III) electrocardiogram (ECG) were monitored continuously throughout the procedure. An over-the-wire balloon was placed in the left anterior descending (LAD) coronary artery distal to the bifurcation of the first diagonal branch and inflated. Total coronary occlusion and patency of the first diagonal branch was confirmed by injection of contrast agent under fluoroscopy and was further confirmed by ST segment elevation on the ECG. Because the swine is susceptible to ischemia-induced ventricular fibrillation (VF), we intravenously infused 100 mg of lidocaine 5 min before balloon inflation. Five minutes before the end of the 45-min period of ischemia, the guide wire was removed and human derived MK 5 μg/kg dissolved in 10 ml saline [MK-treated (MKT) group, n = 9] or 10 ml saline alone [control (CONT) group, n = 12] were injected at 1 ml/min through the guide-wire lumen of the balloon catheter. The total infusion time was 10 min and the infusion lasted during the first 5 min of reperfusion. The dose of MK protein employed in the present study (5 μg/kg) was comparable to that used in our previous experiments in a mouse model of I/R (Horiba et al., 2006), where direct injection of 20 μl human recombinant MK protein (10 μg/ml) into the left ventricular (LV) free wall – corresponding to 8–9 μg/kg of body weight – was shown to reduce infarct size significantly 24 h after I/R. Human recombinant MK protein was generated and purified as described previously (Horiba et al., 2000).
Infarct size determination
Twenty-four hours after reperfusion, swine were anesthetized again as above. After assessment of left ventriculographical (LVG) and echocardiographical parameters, KCl was injected through an 8-Fr catheter inserted in the ascending aorta to arrest the heart in diastole. The heart was rapidly harvested and the LAD was occluded surgically at the site where the balloon distal marker was positioned. The hearts from three CONT and three MKT swine were then perfused with 20 ml of 1% Evans blue from the ascending aorta, and each was dissected into four slices. The slices were further stained with 1% 2,3,5-triphenyltetrazolium chloride (TTC) to demarcate the unperfused area (area at risk, AAR) and the TTC-positive viable tissue. The stained slices were photographed to evaluate the AAR and infarct size as described previously (Horiba et al., 2006).
Serum troponin T levels were measured as an indicator of cardiac cytolysis in seven CONT and seven MKT swine before balloon inflation (“PRE”) and at 2 and 24 h of reperfusion.
Measurement of systolic and diastolic function
Left ventricular catheterization, LVG, and echocardiography were performed to evaluate LV systolic and diastolic function before balloon inflation (“PRE”) and at 30 min (“POST”) and 24 h of reperfusion. Ejection fraction (EF) and hypokinetic areas were measured by LVG (60° right anterior oblique projection). To estimate the LV local hypokinetic area, the percentage of abnormal contracting segments (%ACS) was measured as the percent ratio of hypokinetic chords among the total chords in the centerline method of regional wall motion analysis (Sweet et al., 1975). The number of hypokinetic chords was automatically counted with computer software (QCA-CMS, Goodman, Japan). LV end-diastolic pressure (LVEDP), the maximum first derivative of pressure over time (dP/dtmax) and the minimum first derivative of pressure over time (dP/dtmin) were calculated from the LV tracings by computer software (OP-310G, Nihon Kohden, Japan).
In transthoracic echocardiography (SONOS 5500, Philips), LV fractional shortening (%FS), and the percentage thickening of the interventricular septum (%IVS) and the LV posterior wall (%PW) were measured as indices of LV systolic function, and the ratio of transmitral and annular flow (E/e′) was measured as an index of LV diastolic function (Nagueh et al., 1998; Kasner et al., 2007).
Detection of apoptosis and inflammation
In five CONT and five MKT hearts excised at 24 h after reperfusion, apoptotic myocardial cells were identified by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end-labeling (TUNEL) staining with the use of an in situ apoptosis detection kit (Apoptag, Millipore). In those hearts, immunostaining of inflammatory cells was also performed by labeling the LV tissue sections with anti-mouse CD45 (leukocyte common antigen) antibody (Laboratory Vision Corporation, Fremont, CA, USA). Exposure to secondary antibody conjugated with goat anti-rat IgG (Jackson Laboratory) was followed by incubation with biotinyl-tyramide and streptavidin–horseradish peroxidase.
Organ deposition of exogenous MK
In 3 swine (in addition to the 21 for the I/R experiments), MK protein labeled with rhodamine was injected into the LAD through the over-the-wire balloon catheter at the initiation of reperfusion (total infusion time was 10 min from 5 min before the end of 45-min ischemia period). The labeling was performed using EZ-Label™ rhodamine Protein Labeling Kit (PIERCE Biotechnology). Briefly, after pre-dialysis with borate buffer (pH 8.5), 100 μg MK was mixed with 8 μl rhodamine solution and then incubated for 1 h at room temperature. Rhodamine-labeled MK solution was then dialyzed with phosphate-buffered saline (PBS) to remove excess fluorescent dye. The hearts were excised 24 h after I/R, and the LV tissue sections were prepared to visualize the tissue distribution of rhodamine-labeled MK protein under laser microscopy.
Statistical analysis
Data were expressed as mean ± SEM. Statistical comparisons were either performed by ANOVA, or the paired Student’s t-test, or Fischer’s exact test when appropriate. Details are described in the legends to the Figures. Differences were considered significant when p < 0.05.
Results
Protocol and mortality rate
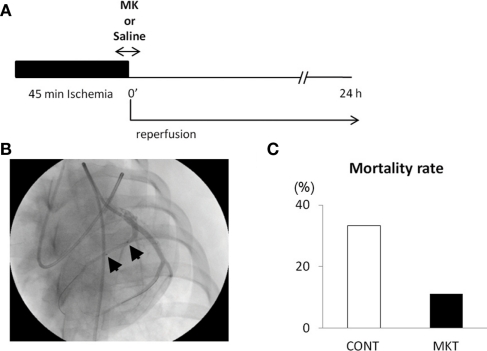
Figure 1A shows the protocol of I/R insult in swine hearts by balloon inflation for 45 min, and Figure 1B shows an angiographical view of the balloon location. There was not a single episode of VF during the ischemic period, but it should be emphasized that this was in the prophylactic presence of lidocaine. VF occurred in 11/12 CONT and 8/9 MKT swine during 2 h of reperfusion. All episodes of VF were successfully defibrillated by transthoracic DC shocks. Figure 1C shows the mortality rate. Although all swine survived at 2 h of reperfusion, 4/12 (33.3%) of CONT died within 24 h after reperfusion, whereas 1/9 (11.1%) of MKT died during the same period (p = 0.34, Fischer’s exact).
Figure 1.
Ischemia/reperfusion (I/R) experiments. (A) Protocol of I/R insult. The left anterior descending (LAD) coronary artery distal to the bifurcation to the first diagonal branch was occluded by inflation of an over-the-wire balloon to create myocardial ischemia for 45 min followed by reperfusion for 24 h. Midkine protein (MK 5 μg/kg) or saline (CONT) was injected into the coronary artery for 10 min through the guide-wire lumen, beginning 5 min before the end of ischemia period. (B) Position of the occlusion balloon. An X-ray image was taken during LAD angiography. Two arrows indicate proximal and distal ends of the balloon positioned at the site of occlusion. (C) The mortality rate of animals during the 24-h reperfusion. Four of 12 (33.3%) CONT, whereas 1/9 (11.1%) MK-treated swines died (p = 0.34, Fischer’s exact test).
Reduction of infarct size
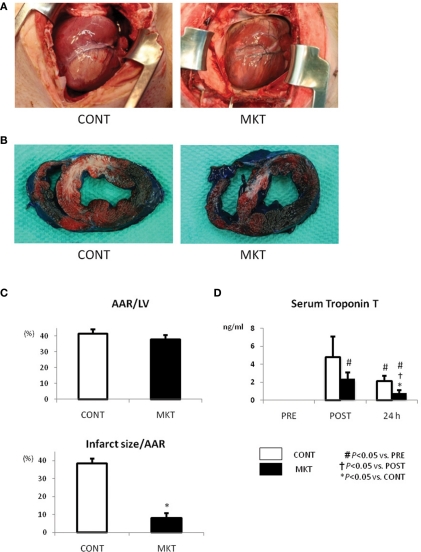
Figure 2A shows the appearance of the ventricular area distal from the site of coronary occlusion just before extirpation of the hearts. As seen at the surface, a uniform mass of whitish tissue was seen at the coronary occlusion area including the LAD area in the CONT (Figure 2A, left), whereas smaller mottled whitish lesions were seen in the same area of MKT (Figure 2A, right). To assess infarct dimensions, we stained the hearts with Evans blue and TTC. Figure 2B shows representative examples of TTC and Evans blue double staining in infarcted hearts. TTC stains surviving tissue red, leaving infarcted areas white. Although the areas at risk for occlusion were similar between the two groups (Figure 2C, top panel), MK treatment reduced infarct size by 79% (Figure 2C, bottom panel).
Figure 2.
Infarct size and serum troponin T of swine subjected to I/R insult. (A) External view of the hearts prior to extirpation (24 h after I/R insult). Macroscopic infarct area (whitish lesion) downstream the coronary occlusion in the MK-treated (MKT) heart was much smaller than that in CONT heart. (B) Representative pictures of ventricular tissue sections from CONT and MKT swine at 24 h after I/R insult. Tissue stained blue by Evans blue represents non-ischemic area; tissue stained red by TTC indicates viable area. Tissue not stained by either Evans blue or TTC appears pale to white and represents infarct myocardium. (C) Infarct size was measured as the percentage of LV area of that of total ischemic area at risk (AAR). Values are mean ± SEM of AAR/LV and infarct size/AAR (CONT n = 3, MKT n = 3; *p < 0.05 vs. CONT, ANOVA). (D) Serum troponin T levels before (“PRE”), 2 and 24 h after I/R insult. Values are mean ± SEM (CONT, n = 7; MKT, n = 7). The differences between “PRE,” “POST,” and “24 h” within the CONT and MKT groups were tested by the paired Student’s t-test in such a way that each animal served as its own control (#p < 0.05 vs. PRE, †p < 0.05 vs. POST). The difference between the CONT and MKT groups at any moment was tested by ANOVA (*p < 0.05 vs. CONT).
Serum troponin T, a marker of myocardial cell damage, was elevated at 2 and 24 h after I/R insult. The elevation at 24 h in MKT was significantly less than in CONT (Figure 2D).
Preservation of systolic and diastolic LV function
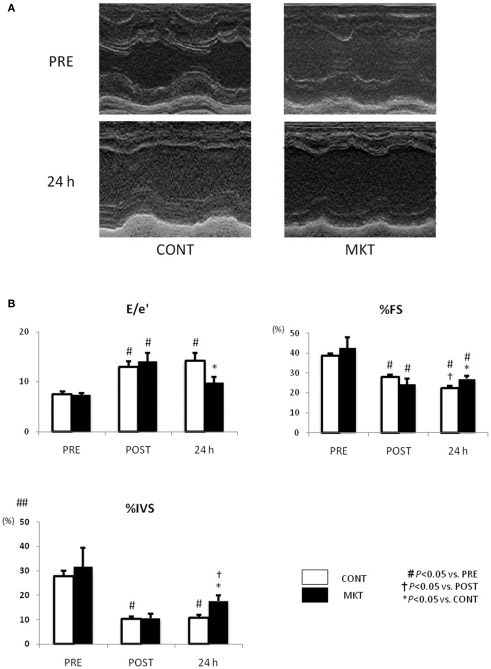
We investigated LV systolic and diastolic function by echocardiography (Figure 3). At baseline (“PRE”) and at 30 min of reperfusion (“POST”), there were no significant differences between the CONT and MKT in any assessed parameter. However, at 24 h of reperfusion (“24 h”), lower E/e′, higher percentages of fractional shortening (%FS), and higher %IVS were observed in the MKT compared with the CONT. Thus, MKT swine showed partial recovery of E/e′ and %IVS, whereas CONT swine showed no recovery in these parameters. Percentage thickening of posterior wall (%PW) was unaffected by the I/R insult both in CONT and MKT swine (data not shown).
Figure 3.
Echocardiographic assessment of LV function before and after I/R insult. (A) Representative M-mode LV images of a CONT and a MKT swine 24 h after I/R. (B) Parameters representing diastolic and systolic LV function. Values are mean ± SEM of the ratio of transmitral to annular flow (E/e′), percentage LV fractional shortening (%FS), and percentage thickening of interventricular septum (%IVS) measured before (“PRE”), 30 min after (“POST”), and 24 h after I/R (CONT, n = 8; MKT, n = 8). The differences between “PRE,” “POST,” and “24 h” within the CONT and MKT groups were tested by the paired Student’s t-test in such a way that each animal served as its own control (#p < 0.05 vs. PRE, †p < 0.05 vs. POST). The difference between the CONT and MKT groups at any moment was tested by ANOVA (*p < 0.05 vs. CONT).
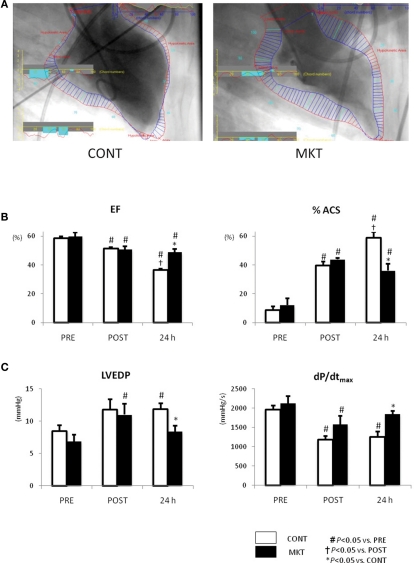
Figure 4 shows the results obtained in LVG and LV catheterization. In both CONT and MKT swine, EF decreased and %ACS increased significantly at 30 min of reperfusion (“POST”). At 24 h reperfusion, CONT swine showed a further decrease in EF and a further increase of %ACS, whereas MKT swine did not show such a deterioration (Figure 4B). In the CONT group LVEDP was significantly higher at 24 h reperfusion compared with baseline (Figure 4C). This was not the case in the MKT group, although there had been a significant difference between “POST” and “PRE” in the MKT group, but not the CONT group (Figure 4C). Also, at 24 h reperfusion LVEDP was significantly lower in the MKT group compared with the CONT group (ANOVA). The dP/dtmax was significantly lower at “POST” compared with “PRE” in both groups (Figure 4C). However, at 24 h reperfusion this still was the case (compared with “PRE”) in the CONT group, but no longer in the MKT group. At 24 h of reperfusion dP/dtmax was significantly higher in the MKT group compared with the CONT group (Figure 4C, ANOVA). In the CONT and the MKT groups, the dP/dtmin at “POST” decreased similarly from baseline (“POST”), and the change was reversed at 24 h of reperfusion (data not shown).
Figure 4.
Left ventricular function assessed by catheterization and LVG before and after I/R. (A) Representative images of LVG (60° right anterior oblique projection) at 24 h after I/R. Normal kinetic and hypokinetic chords (blue and red bars, respectively) were drawn along the centerline methods, and percentage abnormality segments (%ACS) was counted automatically using a computer software (QCA-CMS, Goodman, Japan). Hypokinetic area (a zone of red bars) in the MKT swine is appreciably less than that in the CONT swine. (B) Ejection fraction (EF) and %ACS measured in LVG before (“PRE”), 30 min after I/R (“POST”), and 24 h after I/R. Values are mean ± SEM (CONT, n = 7; MKT, n = 8). (C) LV end-diastolic pressure (LVEDP) and maximum first derivative of LV pressure (dP/dtmax) measured before (PRE), 30 min after I/R (“POST”), and 24 h after I/R. Values are mean ± SEM (CONT, n = 8; MKT, n = 8). The differences between “PRE,” “POST,” and “24 h” within the CONT and MKT groups were tested by the paired Student’s t-test in such a way that each animal served as its own control (#p < 0.05 vs. PRE, †p < 0.05 vs. POST). The difference between the CONT and MKT groups at any moment was tested by ANOVA (*p < 0.05 vs. CONT).
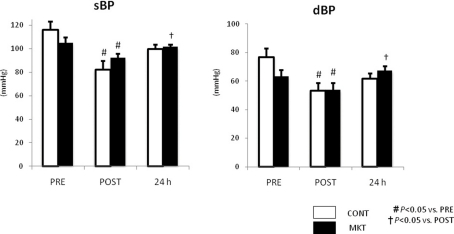
Figure 5 summarizes systolic and diastolic blood pressure (sBP, dBP) at baseline (“PRE”), and at 30 min (“POST”), and 24 h of reperfusion. There were no significant difference in these parameters between CONT and MKT swine at any moment. In both CONT and MKT swine, sBP and dBP at “POST” decreased significantly from “PRE.” At 24 h of reperfusion, sBP and dBP in MKT increased significantly compared with “POST.” However, both in the CONT and the MKT groups the sBP and dBP values were not significantly different at 24 h of reperfusion and at baseline (“PRE”). We emphasize that each animal in fact served as its own control.
Figure 5.
Blood pressure (BP) response to I/R. Systolic BP (sBP) and diastolic BP (dBP) were measured before (“PRE”), 30 min after I/R (“POST”), and 24 h after I/R. Values are mean ± SEM (CONT, n = 8; MKT, n = 8). The differences between “PRE,” “POST,” and “24 h” within the CONT and MKT groups were tested by the paired Student’s t-test in such a way that each animal served as its own control (#p < 0.05 vs. PRE, †p < 0.05 vs. POST). The difference between the CONT and MKT groups at any moment was tested by ANOVA.
Table 1 summarizes ECG parameters measured at baseline (“PRE”), 30 min (“POST”), and 24 h of reperfusion. In both CONT and MKT swine, I/R caused no significant changes in PQ interval, QRS duration, and QT interval. RR interval at 24 h was decreased similarly in CONT and MKT. There were no significant differences in these parameters between CONT and MKT at any moment of the ECG recording. The ratio of heart weight/body weight measured after 24 h of reperfusion was comparable in CONT and MKT.
Table 1.
Electrocardiogram parameters of control and MK-treated swine.
| Condition | Group | HW/BW | RR (ms) | PQ (ms) | QRS (ms) | QT (ms) | QTc (ms) |
|---|---|---|---|---|---|---|---|
| PRE | CONT | n.a. | 617 ± 41 | 97 ± 3 | 37 ± 1 | 325 ± 25 | 412 ± 19 |
| MKT | n.a. | 649 ± 28 | 97 ± 2 | 39 ± 1 | 337 ± 17 | 417 ± 12 | |
| POST | CONT | n.a. | 653 ± 30 | 102 ± 3 | 40 ± 2 | 338 ± 17 | 417 ± 11 |
| MKT | n.a. | 580 ± 31 | 98 ± 4 | 38 ± 2 | 303 ± 15 | 398 ± 9 | |
| 24 h | CONT | 0.45 ± 0.02 | 542 ± 27# | 96 ± 2 | 39 ± 1 | 278 ± 13 | 378 ± 8 |
| MKT | 0.43 ± 0.01 | 560 ± 20# | 98 ± 2 | 40 ± 1 | 293 ± 10 | 391 ± 7 |
Values are mean ± SEM. ECG data were obtained before I/R (PRE), 30 min of reperfusion (POST), and 24 h of reperfusion (24 h). CONT, control swine (n =8); MKT, midkine-treated swine (n =8); QTc = QT/(RR/1000)1/2. Body weight (BW) measured at PRE was 40 ±1 kg for CONT, and 39 ± 1 kg for MKT (ns). Heart weight (HW) measured at 24 h was 178 ± 3 g for CONT, and 167 ± 3 g for MKT (ns). The differences between “CONT,” “POST,” and “24 h” within the CONT and MKT groups were tested by the paired Student’s t-test in such a way that each animal served as its own control (#p < 0.05 vs. PRE). The difference between the CONT and MKT groups at any moment was tested by ANOVA.
Apoptotic response and infiltration of inflammatory cells
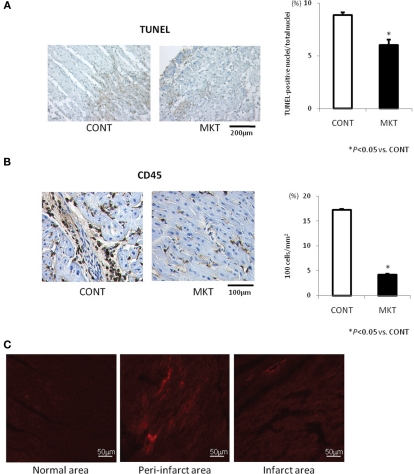
Apoptotic and inflammatory responses of swine hearts were assessed 24 h after I/R insult by TUNEL staining and immunolabeling of CD45 in the peri-infarct area of LV tissue. MK treatment significantly decreased the number of TUNEL-positive cells compared with CONT (Figure 6A). MK treatment also caused a significant reduction of CD45-positive cells compared with CONT (Figure 6B).
Figure 6.
Apoptotic and inflammatory reaction to I/R, and myocardial deposition of exogenous MK protein. (A) TUNEL staining and quantification of apoptosis in the LV peri-infarct area of CONT and MKT swine. Left, representative TUNEL-stained sections from a CONT and a MKT swine at 24 h after I/R. Right, apoptotic cell populations were estimated by TUNEL-positive nuclei/total nuclei (%). Values are mean ± SEM (CONT, n = 5; MKT, n = 5; five fields of each specimen; *p < 0.05 vs. CONT, ANOVA). (B) CD45 staining and quantification of inflammatory cell infiltration. Left, representative CD45-stained LV sections from a CONT and a MKT swine at 24 h after I/R. Right, inflammatory cell infiltration was estimated by CD45-positive cell counts per unit area (102 cells/mm2). Values are mean ± SEM (CONT, n = 5; MKT, n = 5; five fields of each specimen; *p < 0.05 vs. CONT, ANOVA). (C) Deposition of exogenous MK protein labeled with rhodamine in the peri-infarct area. Images were taken from LV tissue sections in a swine 24 h after I/R. Fluorescence signals of rhodamine are recognized predominantly in the peri-infarct area (center) but not in the normal area (left) and infarct area (right).
Exogenous MK deposition in the infarct area
In three swine, we injected rhodamine-labeled MK protein into the LAD at the onset of reperfusion in order to evaluate the local distribution of exogenous MK. Representative results are shown in Figure 6C. The deposition of MK-rhodamine was clearly recognized in the peri-infarct area; it was absent in the normal and infarct areas. Similar results were obtained in two other swine hearts. This observation is consistent with our previous report on the localization of endogenous MK protein in mice subjected to I/R (Horiba et al., 2006).
Discussion
The present study demonstrates that MK plays a crucial role in cardioprotection in response to I/R. We used a swine model of 45 min myocardial ischemia by LAD occlusion with subsequent reperfusion to investigate the effect of acute MK administration on infarct size and myocardial dysfunction. Our study indicates that favorable effects can be produced with a single, intracoronary administration of MK, delivered just before a 24-h period of reperfusion. In our experimental setting, no significant hemodynamic perturbation was noted by such MK treatment.
Infarct size reduction and functional aspects
Current evidence suggests that prevention of infarct expansion during the vulnerable very early period after coronary occlusion may abort the whole process of remodeling (Kelley et al., 1999). At present, the therapeutic strategy consists of acute unloading of the left ventricle, within the first 24 h of myocardial damage (Jugdutt, 1993; Sutton and Sharpe, 2000). Many successful strategies have been developed in animal studies to reduce reperfusion damage and reperfusion-induced apoptosis. However, most of these approaches have limited relevance in a clinical setting and the results in clinical trials have been disappointing (Baran et al., 2001; Simons et al., 2002; Henry et al., 2003). Using this swine model of I/R, we have demonstrated that a single intracoronary injection of MK protein at the onset of reperfusion leads to a reduction in infarct size, a more normal ventricular geometry and function and an anti-apoptotic reaction. Mortality rate of MKT swine tended to be less than that of CONT swine, but the difference did not reach statistical significance. The lack of significance could be due to the small experimental group size and the short follow-up period (24 h).
A localized intracoronary delivery strategy rather than global myocardial infusion is appealing from the point of safety. This relates to the ease of administration, the avoidance of open chest surgery, and first of all, an only 10 min delay to recanalization as adjunctive therapy with percutaneous coronary intervention (PCI). In this study, MK was administered through the guide-wire lumen of the balloon catheter, starting 5 min before reperfusion. This mimics the clinical situation of a patient with 100% occlusion subjected to PCI as an adjunctive therapy in the same surgical session. An over-the-wire balloon was used because it permits injection of MK through the guide-wire lumen of the catheter. As a result, MK can be administered selectively into the ischemic lesion without a wash-out effect by coronary flow.
Mechanistic aspects
In a previous report, we compared the apoptotic response in association with ischemic myocardial injury in MK knock-out mice (Mdk−/−) and wild type mice subjected to I/R (Horiba et al., 2006). The infarct size and apoptosis in the peri-infarct area, which was quantified by TUNEL staining, were significantly worse in Mdk−/− mice than in wild type mice. In mouse cultured cardiomyocytes subjected to hypoxia/reoxygenation, application of exogenous MK prevented apoptosis through upregulation of Bcl-2 and phosphorylation of ERK-1/2 (Horiba et al., 2006). In another study we assessed the long-term effects of MK treatment in a mouse model of myocardial infarction. We demonstrated that the ratio of Bax to Bcl-2 and the expression of p-Bad (p-bcl-xl-bcl-associated death promoter) in LV myocardium 28 days after the creation of infarction were significantly decreased and increased, respectively in the mice treated with exogenous MK (Takenaka et al., 2009). These observations suggest that MK plays a protective role against myocardial injury by prevention of apoptosis. In the present study, we could demonstrate the anti-apoptotic effect of MK by TUNEL staining in a large mammalian species. The benefit of anti-apoptotic action of MK may lead to a reduction of secondary necrosis, which activates inflammation.
Side effects, limitations, and future aspects
In this study, MK treatment did not cause any undesirable effect on the hemodynamic parameters throughout the whole period of 45 min ischemia followed by 24 h reperfusion. ECG parameters associated with conduction and repolarization were also unchanged. It suggests that MK has enough safety margin from the cardiovascular point of view when we consider future clinical use. To confirm the localization of exogenously injected MK, we used MK protein labeled with rhodamine. Intracoronary injected MK-rhodamine was restricted to the peri-infarct area at 24 h after injection. The MK-rhodamine was not found in the normal area. The mechanism is probably the specific expression of a MK receptor in the injured myocardium (Takenaka et al., 2009). This aspect is pivotal, because it may pave the way to intravenous medication in a clinical setting.
There was a certain animal to animal variation of hemodynamic parameters at baseline (before I/R insult). Both sBP and dBP in CONT swine at baseline tended to be higher than those in MKT swine (Figure 5). MKT baseline values of %IVS thickening in echocardiography had a considerable SEM (Figure 3B). We, therefore, cannot rule out possible influence of inadequate randomization on the beneficial effects of MK against myocardial injury. This may be a limitation of the present study. However, the statistical significance of our data is not at debate, because both for the echocardiographic data and the hemodynamic data each animal served as its own control from baseline (“PRE”) till 24 h after reperfusion.
We evaluated a practical treatment approach, aiming at preventing cardiac damage, during the acute phase of myocardial infarction. The proposed strategy consists of acute MK administration targeted at the infarct area and this report is the first that shows cardioprotective effects in a large animal model of myocardial infarction and reperfusion.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Sadatoshi Sakuma for critical advice and helpful discussion. We thank M. Hojo and K. Miwa for technical assistance. This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology Japan (to M. Horiba).
References
- Baran K. W., Nguyen M., McKendall G. R., Lambrew C. T., Dykstra G., Palmeri S. T., Gibbons R. J., Borzak S., Sobel B. E., Gourlay S. G., Rundle A. C., Gibson C. M., Barron H. V. (2001). Double-blind, randomized trial of an anti-CD18 antibody in conjunction with recombinant tissue plasminogen activator for acute myocardial infarction – limitation of myocardial infarction following thrombolysis in acute myocardial infarction (LIMIT AMI) study. Circulation 104, 2778–2783 10.1161/hc4801.100236 [DOI] [PubMed] [Google Scholar]
- Henry T. D., Annex B. H., McKendall G. R., Azrin M. A., Lopez J. J., Giordano F. J., Shah P. K., Willerson J. T., Benza R. L., Berman D. S., Gibson C. M., Bajamonde A., Rundle A. C., Fine J., McCluskey E. R. (2003). The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation 107, 1359–1365 10.1161/01.CIR.0000065222.34933.FC [DOI] [PubMed] [Google Scholar]
- Horiba M., Kadomatsu K., Nakamura E., Muramatsu H., Ikematsu S., Sakuma S., Hayashi K., Yuzawa Y., Matsuo S., Kuzuya M., Kaname T., Hirai M., Saito H., Muramatsu T. (2000). Neointima formation in a restenosis model is suppressed in midkine-deficient mice. J. Clin. Invest. 105, 489–495 10.1172/JCI7208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiba M., Kadomatsu K., Yasui K., Lee J. K., Takenaka H., Sumida A., Kamiya K., Chen S., Sakuma S., Muramatsu T., Kodama I. (2006). Midkine plays a protective role against cardiac ischemia/ reperfusion injury through a reduction of apoptotic reaction. Circulation 114, 1713–1720 10.1161/CIRCULATIONAHA.106.632273 [DOI] [PubMed] [Google Scholar]
- Jugdutt B. I. (1993). Prevention of ventricular remodeling post myocardial-infarction – timing and duration of therapy. Can. J. Cardiol. 9, 103–114 [PubMed] [Google Scholar]
- Kadomatsu K., Muramatsu T. (2004). Midkine and pleiotrophin in neural development and cancer. Cancer Lett. 204, 127–143 10.1016/S0304-3835(03)00450-6 [DOI] [PubMed] [Google Scholar]
- Kasner M., Westermann D., Steendijk P., Gaub R., Wilkenshoff U., Weitmann K., Hoffmann W., Poller W., Schultheiss H. P., Pauschinger M., Tschope C. (2007). Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction – a comparative Doppler-conductance catheterization study. Circulation 116, 637–647 10.1161/CIRCULATIONAHA.106.661983 [DOI] [PubMed] [Google Scholar]
- Kelley S. T., Malekan R., Gorman J. H., Jackson B. M., Gorman R. C., Suzuki Y., Plappert T., Bogen D. K., Sutton M. G. S., Edmunds L. H. (1999). Restraining infarct expansion preserves left ventricular geometry and function after acute anteroapical infarction. Circulation 99, 135–142 [DOI] [PubMed] [Google Scholar]
- Michikawa M., Xu R. Y., Muramatsu H., Muramatsu T., Kim S. U. (1993). Midkine is a mediator of retinoic acid-induced neuronal differentiation of embryonal carcinoma-cells. Biochem. Biophys. Res. Commun. 192, 1312–1318 10.1006/bbrc.1993.1559 [DOI] [PubMed] [Google Scholar]
- Moens A. L., Claeys M. J., Timmermans J. P., Vrints C. J. (2005). Myocardial ischemia/reperfusion-injury, a clinical view on a complex pathophysiological process. Int. J. Cardiol. 100, 179–190 10.1016/j.ijcard.2004.04.013 [DOI] [PubMed] [Google Scholar]
- Muramatsu T. (2002). Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J. Biochem. 132, 359–371 [DOI] [PubMed] [Google Scholar]
- Nagueh S. F., Mikati I., Kopelen H. A., Middleton K. J., Quinones M. A., Zoghbi W. A. (1998). Doppler estimation of left ventricular filling pressure in sinus tachycardia – a new application of tissue Doppler imaging. Circulation 98, 1644–1650 [DOI] [PubMed] [Google Scholar]
- Simons M., Annex B. H., Laham R. J., Kleiman N., Henry T., Dauerman H., Udelson J. E., Gervino E. V., Pike M., Whitehouse M. J., Moon T., Chronos N. A. (2002). Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2 – double-blind, randomized, controlled clinical trial. Circulation 105, 788–793 10.1161/hc0802.104407 [DOI] [PubMed] [Google Scholar]
- Sutton M. G. S., Sharpe N. (2000). Left ventricular remodeling after myocardial infarction – pathophysiology and therapy. Circulation 101, 2981–2988 [DOI] [PubMed] [Google Scholar]
- Sweet R. L., Moraski R. E., Russell R. O., Rackley C. E. (1975). Relationship between echocardiography, cardiac-output, and abnormally contracting segments in patients with ischemic heart-disease. Circulation 52, 634–641 [DOI] [PubMed] [Google Scholar]
- Takenaka H., Horiba M., Ishiguro H., Sumida A., Hojo M., Usui A., Akita T., Sakuma S., Ueda Y., Kodama I., Kadomatsu K. (2009). Midkine prevents ventricular remodeling and improves long-term survival after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 296, H462–H469 10.1152/ajpheart.00733.2008 [DOI] [PubMed] [Google Scholar]
- Unoki K., Ohba N., Arimura H., Muramatsu H., Muramatsu T. (1994). Rescue of photoreceptors from the damaging effects of constant light by midkine, a retinoic acid-responsive gene product. Invest. Ophthalmol. Vis. Sci. 35, 4063–4068 [PubMed] [Google Scholar]
- Yokota C., Takahashi S., Eisaki A., Asashima M., Akhter S., Muramatsu T., Kadomatsu K. (1998). Midkine counteracts the activin signal in mesoderm induction and promotes neural formation. J. Biochem. 123, 339–346 [DOI] [PubMed] [Google Scholar]