Abstract
RATIONALE
Heat shock proteins (Hsps) are known to enhance cell survival under various stress conditions. In the heart, the small Hsp20 has emerged as a key mediator of protection against apoptosis, remodeling and ischemia/reperfusion injury. Moreover, Hsp20 has been implicated in modulation of cardiac contractility ex vivo.
OBJECTIVE
To determine the in vivo role of Hsp20 in the heart and the mechanisms underlying its regulatory effects in Ca-cycling.
METHODS and RESULTS
Hsp20 overexpression resulted in significant enhancement of cardiac function in intact animals, coupled with augmented Ca-cycling and sarcoplasmic reticulum (SR) Ca-load in isolated cardiomyocytes. This was associated with specific increases in phosphorylation of phospholamban (PLN) at both Ser16 and Thr17, relieving its inhibition of the apparent Ca-affinity of SERCA2a. Accordingly, the inotropic effects of Hsp20 were abrogated in cardiomyocytes expressing non-phosphorylatable PLN (S16A/T17A). Interestingly, the activity of type 1 protein phosphatase (PP1), a known regulator of PLN signaling, was significantly reduced by Hsp20-overexpression, suggesting that the Hsp20 stimulatory effects are partially mediated through the PP1/PLN axis. This hypothesis was supported by cell-fractionation, co-immunoprecipitation and co-immunolocalization studies, which revealed an association between Hsp20, PP1 and PLN. Furthermore, recombinant protein studies confirmed a physical interaction between AA 73–160 in Hsp20 and AA 163–330 in PP1.
CONCLUSIONS
Hsp20 is a novel regulator of SR Ca-cycling by targeting the PP1/PLN axis. These findings, coupled with the well-recognized cardioprotective role of Hsp20, suggest a dual benefit of targeting Hsp20 in heart disease.
Keywords: Hsp20, contractility, phosphatase, phospholamban, SR
Introduction
The 17-kDa chaperone protein Hsp20 belongs to a family of at least 10 different small Hsps, which transiently increase to enhance cell survival in stress conditions.1 Hsp20 is expressed in multiple tissues but it is most abundant in muscle.1 Of note, the levels of Hsp20 are increased in animal or human hearts under ischemic conditions,2 exercise training,3 rapid right ventricular pacing,4 pharmacological treatment by doxorubicin5 and chronic β-adrenergic stimulation.6 Currently, the functional significance of Hsp20 has been extensively studied in smooth and cardiac muscles.1, 7 In the heart, Hsp20 protects against ischemia/reperfusion-induced injury,8 and β-agonist-mediated remodeling6 as well as apoptosis.6, 9 Thus, the increased Hsp20 expression in failing hearts may constitute an important compensatory mechanism. Interestingly, acute increases of Hsp20 levels or activity in isolated cardiomyocytes are also associated with enhanced contractile parameters, suggesting an additional role of Hsp20 function in the heart.10, 11 However, the mechanisms and sub-cellular pathways underlying the Hsp20-mediated effects on contractility have not been identified.
Cardiac contractility and Ca-cycling are regulated by a fine equilibrium of protein phosphorylation, which is enacted by the balance of protein kinase and phosphatase activities.12 Reversible Ser/Thr protein phosphorylation represents the cellular basis for integration of key signaling pathways and the heart’s responses to increased demands during sympathetic stimulation.12 The majority of Ser/Thr phosphatase activity is attributed to type 1 protein phosphatase PP1, and type 2 phosphatases.13–16 Among these phosphatases, PP1 is of particular importance since it has been implicated as an important negative regulator of cardiac function.13, 17, 18 In fact, increases in its levels and activity have been suggested to play a major role in the aberrant SR Ca-cycling and pathogenesis of heart failure.12, 19 Accordingly, overexpression of the PP1 catalytic subunit (PP1c) in the mouse heart, at levels similar to those observed in human failing hearts, strongly suppressed contractility and induced heart failure.18 Current evidence indicates that substrate specificity for this versatile enzyme is achieved through its auxiliary proteins, which target it to sub-cellular compartments.12 In cardiac muscle, PP1 is present in the sarcoplasmic reticulum and it is the main phosphatase that dephosphorylates PLN, impacting SERCA2a activity and cardiac performance.12, 14, 19 Furthermore, PP1 activity is under regulation by two endogenous proteins, inhibitor-1 and inhibitor-2.12, 20 Genetic manipulations of either inhibitor molecule have supported their important role in regulation of PP1, and the critical involvement of this enzyme in the pathogenesis of heart failure.18, 20–23 However, it is not currently clear whether there are any additional endogenous protein partners of PP1 that may modify its activity.
Interestingly, the present study identified the small chaperone Hsp20 as a novel regulator of PP1 activity in the heart. Regulation appears to involve a direct physical interaction of Hsp20 with PP1 and inhibition of its enzymatic activity. This is associated with specific increases in PLN phosphorylation, SR Ca-transport and Ca-load, leading to augmented function. The present findings, coupled with previous observations on the cardioprotective function of Hsp20 in vivo, suggest that Hsp20 may have a dual beneficial role in the heart, increasing both cell survival and SR Ca-cycling.
Methods
An expanded Methods section is available in the Online Data Supplement at http://circres.ahajournals.org, and includes detailed information regarding the generation of Hsp20 transgenic mice, in vivo catheterization, ex vivo Langendorff perfusion, mouse left ventricular myocyte isolation and measurements of mechanics, Ca-kinetics and electrophysiology, quantitative immunoblotting, SR Ca-uptake, adult rat and mouse ventricular myocyte isolation and adenovirus-mediated gene transfer, isolation of cardiac microsomes enriched in SR membranes, PP1 activity assay, co-immunoprecipitations, immunofluorescence studies, generation of recombinant proteins, blot-overlay assay and statistical analyses.
Results
Overexpression of Hsp20 Enhances Global Cardiac Contractile Function
Previous ex vivo studies suggested an effect of Hsp20 on cardiac contractility.10, 11,24 To delineate the functional effects of Hsp20 in vivo, left ventricular (LV) contractile indices of Hsp20 transgenic mice were measured, using cardiac catheterization. As shown in Table 1, the systolic function, determined by the maximal rate of pressure rise (+dP/dt), was significantly higher in Hsp20 hearts, compared with wild types. The arterial blood pressure (BP) and maximal pressure (Pmax) were not different between the two groups. Diastolic parameters were also increased in Hsp20 hearts, as evidenced by restoration of the maximal rate of decline of left ventricular systolic pressure (−dP/dt) (Table 1). In addition, the minimum pressure (Pmin, a surrogate of ventricular diastolic suction) and relaxation constant (Tau, a measure of active relaxation) were decreased in Hsp20 hearts. Consistently, Langendorff perfused Hsp20 TG hearts with a fixed pressure of 70cm H2O showed increased contractile parameters, compared with WT hearts (Online Figure I). Taken together, these findings indicate that increased Hsp20 expression in the adult heart enhances cardiac performance.
Table 1.
Baseline Functional Parameters in WT and Hsp20 TG Hearts
| HR (beats/min) |
BP (mmHg) |
Pmax (mmHg) |
Pmin (mmHg) |
+dP/dt (mmHg/s) |
−dP/dt (mmHg/s) |
Tau (Weiss) |
|
|---|---|---|---|---|---|---|---|
| WT (n=8) | 350±11 | 110/78 | 99±2 | 7.9±1.7 | 7215±135 | 6167±275 | 12.6±0.8 |
| Hsp20 TG (n=5) | 368±14 | 110/75 | 104±9.2* | 5.7±1.1* | 9335±216* | 8321±179* | 8.8±0.6* |
HR: heart rate; BP: blood pressure; Pmax: maximum pressure; Pmin: minimum pressure; +dP/dt: rate of contraction; −dP/dt: rate of relaxation; and Tau: relaxation constant.
(P<0.05 vs. corresponding wild-type group).
Hsp20 Enhances Contractility and SR Ca-Cycling in Cardiomyocytes
To determine the mechanisms associated with the enhanced cardiac contractility by Hsp20, we evaluated the mechanical parameters and Ca-transients in isolated cardiomyocytes, which represent a load-independent system. Overexpression of Hsp20 resulted in significant increases in fractional shortening (FS%), rates of contraction (+dL/dt) and rates of relaxation (−dL/dt), compared to wild types (P<0.05, Figure 1A–D). Accordingly, the peak of the Ca-transient was significantly increased and the Ca-decay rate (T50) was abbreviated in TG myocytes, relative to controls (P<0.01, Figure 1E–G). Furthermore, the caffeine-induced Ca-transient peak was elevated compared with WT myocytes (P<0.01, Figure 1H–I), indicating a higher SR Ca content. However, the caffeine-induced 50% time constant of Ca-transient decay (T50), which mainly reflects the Ca extrusion by sodium/calcium exchanger (NCX), was not different between TG and WT myocytes (Figure 1J). Collectively, these data indicate that overexpression of Hsp20 increases cardiomyocyte Ca-cycling and contractile performance.
Figure 1. Mechanics and Ca transients of isolated wild type and Hsp20 TG cardiomyocytes.
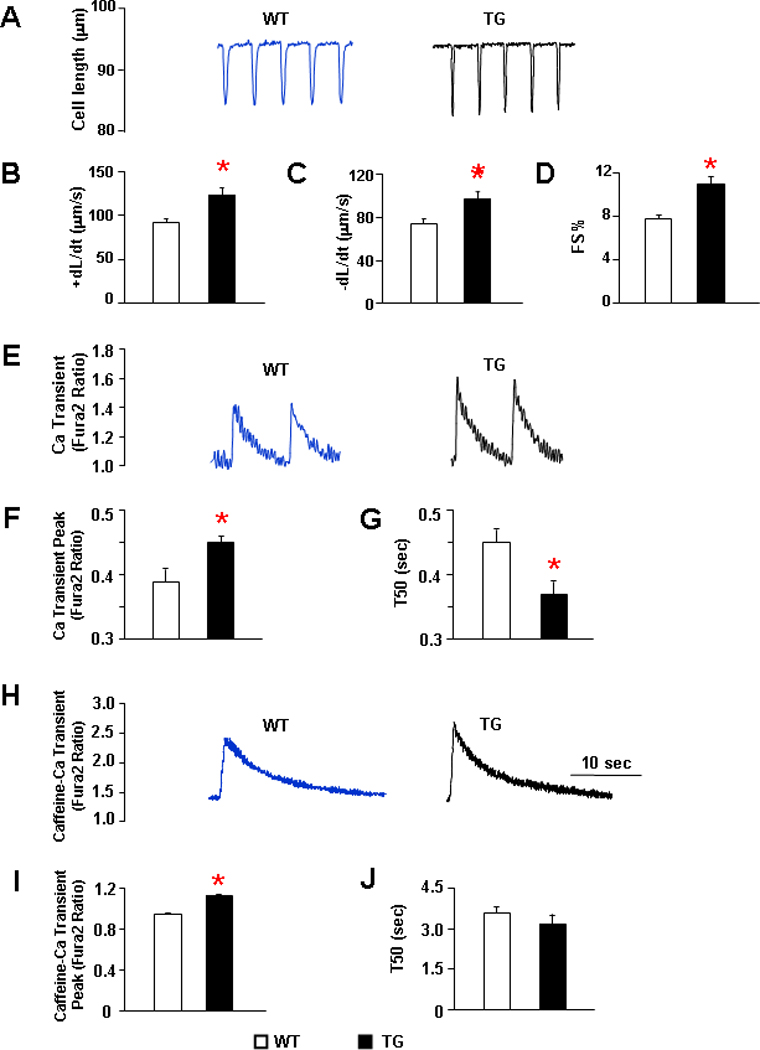
Isolated cardiomyocytes from 12–16 weeks old mice were suspended in 1.8mM Ca-Tyrode solution and field-stimulated at 0.5Hz. (A) Representative cell shortening traces of WT and TG cells. (B) Maximum rates of contraction (+dL/dt). (C) Maximum rates of relaxation (−dL/dt). (D) Fractional shortening (FS%) and (E) Representative tracings of Ca transients in WT and TG cardiomyocytes. (F) Ca transient peak. (G) Time to 50% of decay of Ca transient in WT and Hsp20 TG myocytes. (H) Representative tracings of caffeine induced Ca transient. (I) Caffeine-induced Ca transient amplitude and (J) Time to 50% decay of caffeine-induced Ca transient peak. n = 36–42 cells from 5 hearts for each group. Values = mean ± SEM. *: P<0.01 vs. WT.
Hsp20 Does Not Alter the Levels of Major Ca-Handling Proteins
Given the central role of SR Ca-cycling proteins in cardiac contractility,25 it was important to examine whether overexpression of Hsp20 poses any effects on their expression levels. Quantitative immunoblotting analysis did not reveal any significant changes in total levels of SERCA2a, phospholamban, ryanodine receptor 2 (RyR2), calsequestrin, NCX and L-type Ca channel (LTCC) (Figure 2A–B). The activities of the LTCC and NCX were further investigated using whole-cell voltage clamps. Neither Ca-currents in response to a series of depolarizing steps from a holding potential of −50 mV (Figure 2C) nor the average Ca current-voltage relationship (Figure 2D) showed differences between TG and WT ventricular cells. Furthermore, the Ni2+-sensitive INCX current (Figure 2E) and the INCX density (Figure 2F), which was calculated by normalizing total current to cell membrane capacitance, were similar between Hsp20-TG and WT myocytes. Together, these data indicate that the enhanced cardiac function by Hsp20 is not associated with alterations in SR Ca-handling proteins, or the activities of LTCC and NCX.
Figure 2. SR Ca regulatory proteins in Hsp20 TG hearts.
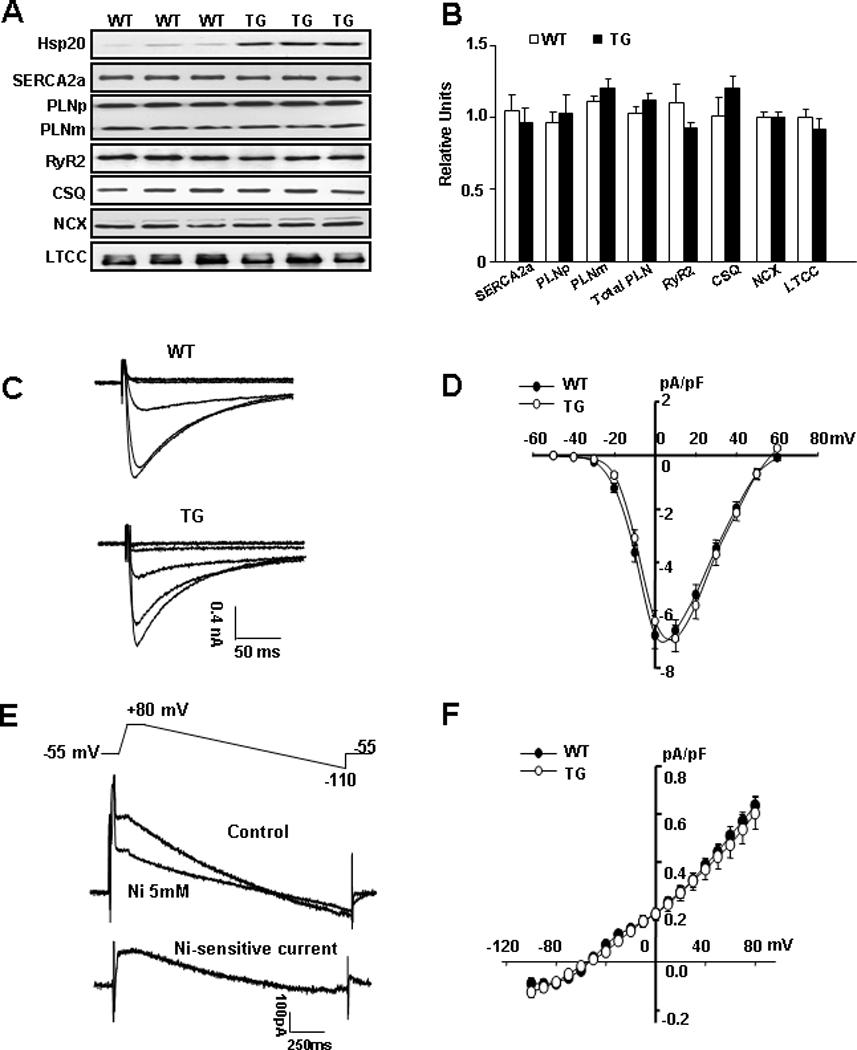
(A) Representative blots of SR Ca-cycling proteins in WT and Hsp20 TG hearts. (B) Quantitative results revealed that SERCA2a, phospholamban pentamer (PLNp), phospholamban monomer (PLNm), total PLN, ryanodine receptor (RYR2), calsequestrin (CSQ), sodium/calcium exchanger (NCX) and L-type Ca channel (LTCC) levels were not altered. (n=6 for each protein). (C) Typical L-type Ca currents in WT and TG cardiomyocytes elicited by a series of depolarizing steps from a holding potential of −50 mV. (D) Average peak current density-voltage relationships of L-type Ca currents recorded in WT (n=15) and TG (n=12) cardiomyocytes. (E) Ramp voltage protocol (top panel) is used to elicit membrane currents (middle panel) in absence and presence of 5mM NiCl2, and the subtraction of the 2 traces gives the Ni2+-sensitive NCX current (bottom panel). (F) Average current density-voltage relationships of NCX currents recorded in WT (n=10) and TG (n=11) ventricular myocytes.
Hsp20 Increases Phosphorylation of PLN and Enhances SR Ca-Transport
Since there were no differences in the expression levels of SR Ca-cycling proteins or the activities of LTCC and NCX, we further investigated the phosphorylation status of key Ca-cycling proteins in TG and WT hearts, which constitutes an important regulatory mechanism governing cardiac contractility.25 Interestingly, phosphorylation of PLN at Ser16 and Thr17 in TG hearts was significantly increased, compared to WTs (Figure 3A–B, P<0.01). However, the phosphorylation of RyR2 at Ser2809 or Ser2815 was not altered (Figure 3A–B). Furthermore, there was no increase in the phosphorylation of myofibrillar proteins, troponin I (TnI) or myosin-binding protein-C (MyBP-C) (Figure 3A–B). These results suggest that Hsp20 shows apparent specificity for PLN phosphorylation.
Figure 3. Overexpression of Hsp20 facilitates SR Ca-cycling by phosphorylation of PLN.
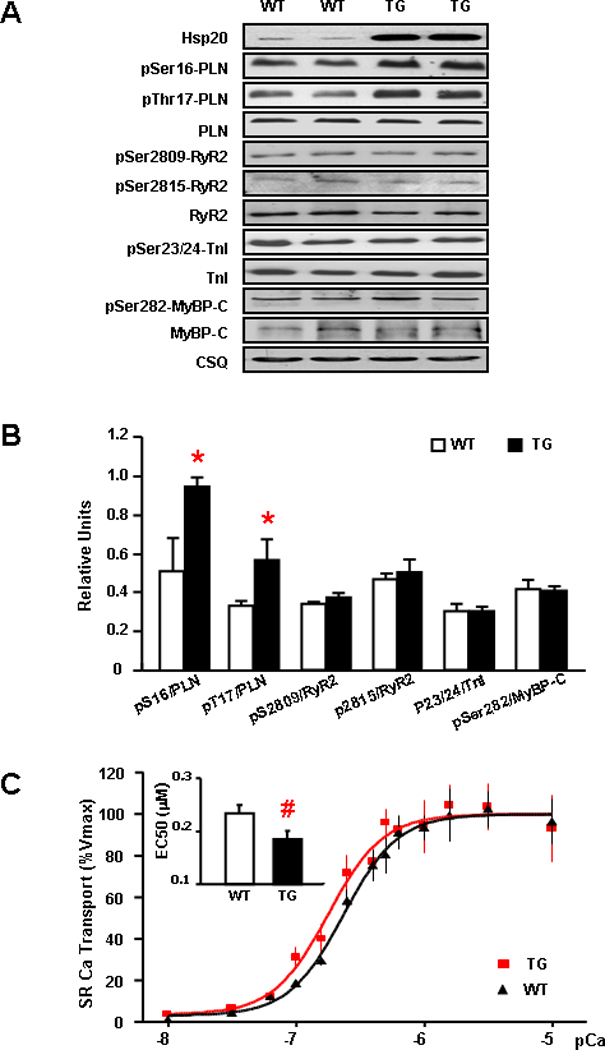
(A) Representative blots of phosphorylation and total levels of PLN, RyR2, TnI and MyBP-C in WT and Hsp20 TG hearts. (B) Quantitative results of phosphorylated Ca regulatory proteins. Immunoblots revealed that pSer16-PLN/PLN and pThr17-PLN/PLN were significantly increased in TGs compared with wild types, but the phosphorylation levels of Ser2809-RYR2, Ser2815-RyR2, Ser23/24-TnI and Ser282-MyBP-C were not different between WTs and TGs. n=6 for each protein. Values = mean ± SEM. *: P<0.01. (C) Initial rates of oxalate-supported SR Ca-uptake in hearts from WT (▲) and Hsp20 TG (■) mice. There was no difference in the maximal SR Ca- uptake rates, but the EC50 value significantly decreased (inset) in TG hearts, compared to WTs. n= 5 for each group; experiments were performed in duplicates. Values = mean ± SEM, #: P < 0.05.
Since the degree of PLN phosphorylation profoundly affects the activation state of SERCA2a and contractility,26 we next assessed the initial rates of ATP dependent, oxalate-facilitated SR Ca-uptake in cardiac homogenates over a wide range of Ca-concentrations, similar to those present in the cardiomyocyte during relaxation and contraction. The incubation conditions in cardiac homogenates, which restrict Ca-uptake to SR vesicles, have been defined previously.27 Our results revealed that Hsp20 overexpression significantly enhanced the affinity of SERCA2a for Ca (EC50, Figure 3C inset). Furthermore, no differences in the maximal velocity of SR Ca-uptake were noted between the two groups (Figure 3C). These findings indicate that overexpression of Hsp20 in the heart alleviates the PLN inhibition on SERCA2a by enhancing the phosphorylation status of PLN.
Further support for the apparent specificity of Hsp20 for PLN was provided by studies in a transgenic mouse model, which expresses non-phosphorylatable PLN (S16A, T17A double-mutant; DM) in the null background27 without any alterations in endogenous Hsp20 levels (Online Figure II). Isolated adult cardiomyocytes from DM and WT mice were infected with Ad.GFP or Ad.Hsp20 for 24 hours prior to assessment of their contractile parameters. As expected, overexpression of Hsp20 in WT cardiomyocytes significantly increased FS%, +dL/dt, and −dL/dt, which were accompanied by enhanced Ca-kinetics, compared with Ad.GFP controls (Figure 4A–D). However, there were no significant stimulatory effects of Hsp20 in DM-PLN cardiomyocytes (Figure 4A–D). Furthermore, infection of adult mouse cardiomyocytes from the PLN-KO model with Ad.GFP or Ad.Hsp20 showed no differences in either contractile parameters or Ca-transients between these two groups (Online Figure III). Collectively, these data further support the role of PLN phosphorylation in the inotropic effects of Hsp20 in the heart.
Figure 4. The inotropic effects of Hsp20 are abrogated in the presence of non-phosphorylatable PLN.
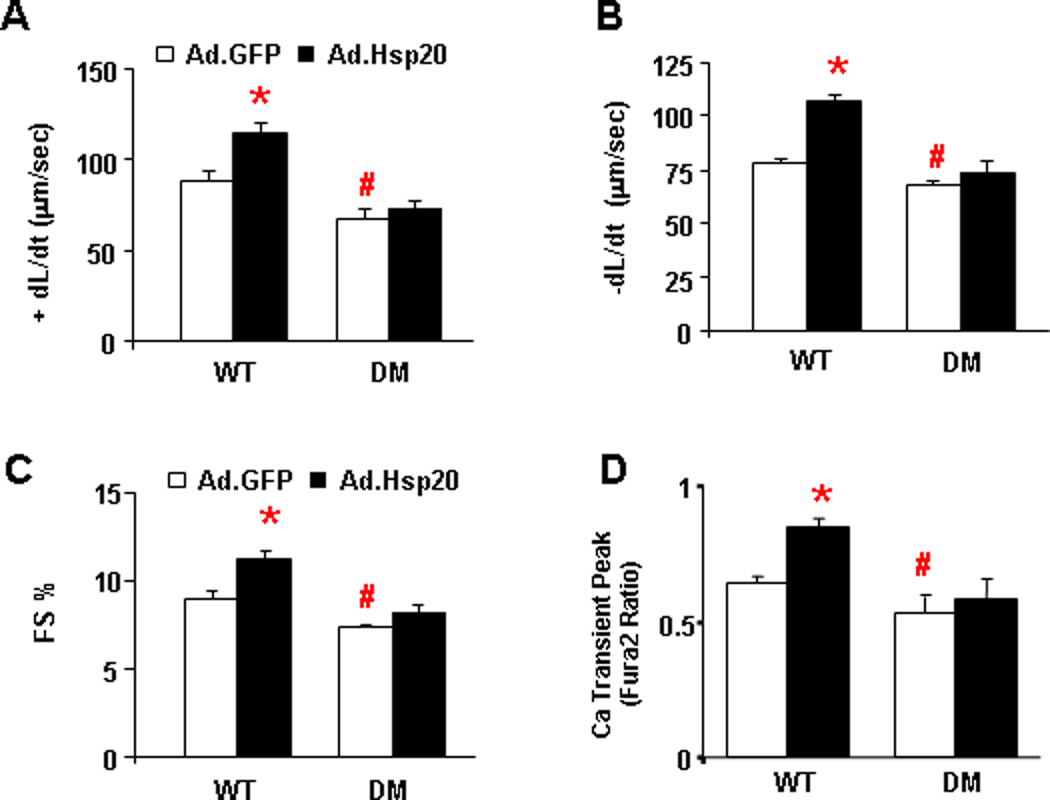
(A) Maximum rates of contraction (+dL/dt), (B) maximum rates of relaxation (−dL/dt), (C) fractional shortening (FS%) and (D) twitch Ca amplitude in cultured WT and PLN double-mutant (DM: S16A/T17A) cardiomyocytes upon Ad.GFP and Ad.Hsp20 infection. n=20 cells for WT and n=22 cells for DM. Values = mean ± SEM. *:P<0.05. WT-Ad.Hsp20 vs. WT- Ad.GFP; #: P<0.05. DM-Ad.GFP vs. WT-Ad.GFP.
Overexpression of Hsp20 Regulates PP1 Activity
The phosphorylation status of PLN is largely determined by the balance between protein kinases and phosphatases, including PP1 and PP2A. To investigate whether Hsp20 may tilt this fine-tuned balance resulting in PLN hyper-phosphorylation, we determined the protein phosphatase activities in Hsp20 TG and WT hearts. Our results showed that PP1 activity was significantly decreased in Hsp20-hearts, compared with WTs (Figure 5A). However, there was no difference in PP2A activity between Hsp20 and wild-type hearts (Figure 5A). To further verify the effects of Hsp20 on PP1 activity, adult rat cardiomyocytes were infected with either Ad.Hsp20 or Ad.GFP. Consistent with our in vivo findings, acute Hsp20 overexpression was associated with decreases in PP1 activity (Figure 5B). Taken together, these results suggest that Hsp20 may specifically regulate PP1 activity.
Figure 5. Hsp20 decreases PP1 activity.
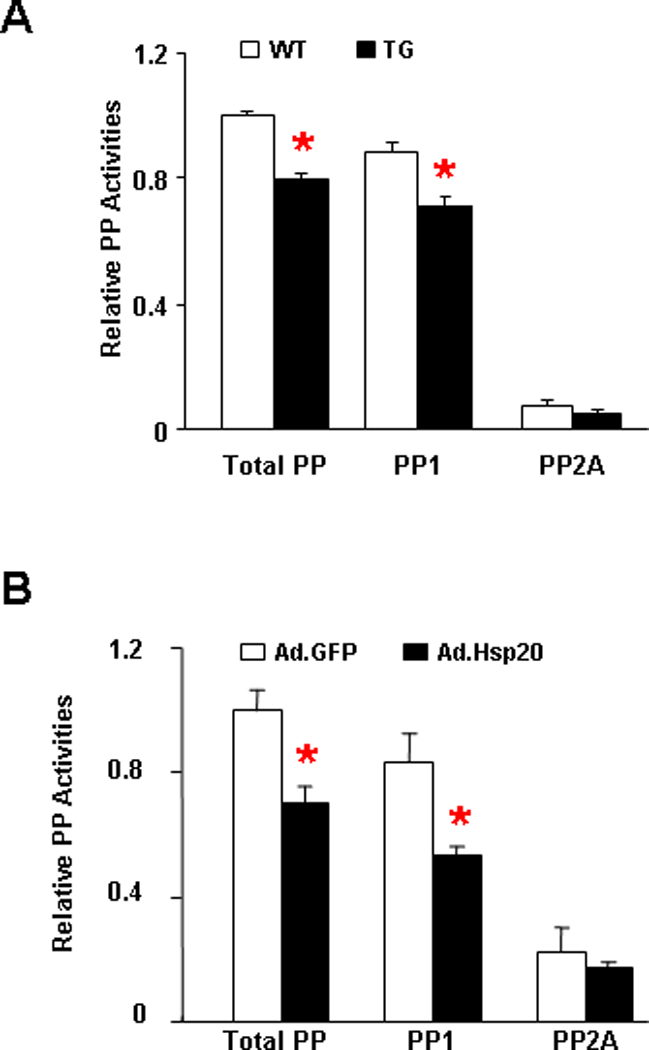
(A) Total, PP1 and PP2A activity were measured in the homogenate from the LV myocardium of wild type and Hsp20 transgenic hearts, which were normalized to total phosphatase activity in WT (n=9). Values=mean ± SEM, *:P <0.01 vs. wild type. (B) Total, PP1 and PP2A activity in adenoviral GFP and Hsp20 infected adult rat cardiomyocytes, normalized to total phosphatase activity in Ad.GFP group (n=6). Values= mean ± SEM. *: P<0.01 vs. Ad.GFP, #: P<0.05 vs. Ad.Hsp20.
Hsp20 Interacts with PP1 in Cardiomyocytes
Early studies indicated that the substrate specificity of PP1 is dictated by its sub-cellular localization, and this enzyme appears to be the main SR phosphatase responsible for dephosphorylating PLN.28 To determine whether the regulatory effect of Hsp20 on PP1 is due to its sub-cellular localization in SR, microsomal preparations enriched in SR membranes and their respective cytosolic fractions were isolated from TG and WT hearts (Figure 6A). Both PLN and PP1 were abundantly present in the SR enriched membrane fraction. Hsp20 was mainly present in the cytosolic fraction and overexpression increased its abundance in both cytosolic and microsomal fractions of TG hearts.
Figure 6. Interaction of Hsp20 with PP1.
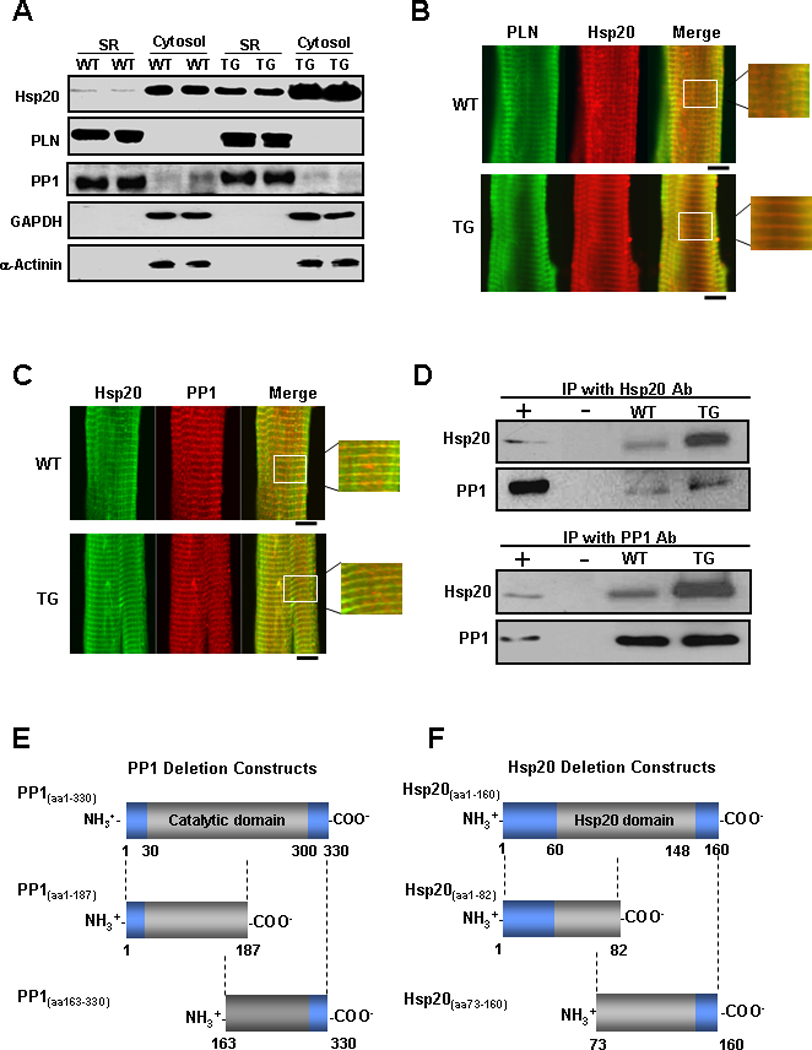
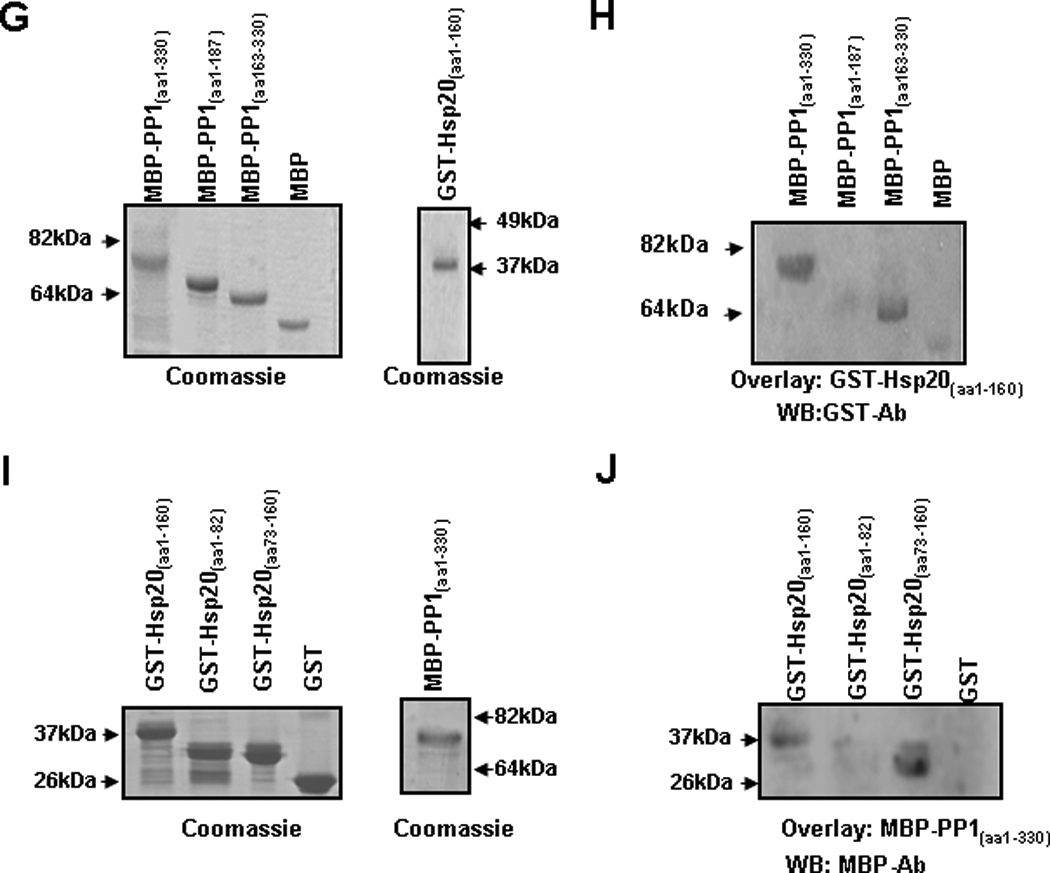
(A) Representative blots of Hsp20, PLN, PP1, GAPDH and α-actinin in microsomes enriched in SR membranes or in cytosolic fractions from WT and Hsp20 TG hearts (n=6). The yield of cardiac SR and cytosolic proteins averaged 1.5mg/heart and 12mg/ heart, respectively. The amount of total protein on each gel lane was the same (20 µg for each fraction), which diluted the cytosolic fraction by 10× compared to the SR fraction. (B) WT and TG cardiomyocytes were immunostained for Hsp20 (red) in combination with PLN (green). (C) WT and TG cardiomyocytes were immunostained for Hsp20 (green) in combination with PP1 (red). Scale bar, 10 µm. (D) Co-immunoprecipitation was performed using anti-Hsp20 or anti-PP1 antibody and cardiac homogenates (200µg total protein) of wild type and Hsp20 TG mice. The precipitates were analyzed by immunoblotting with anti-Hsp20 or anti-PP1 antibodies, as indicated. Preimmunoprecipitated WT heart homogenate was used as positive control (+), and immunoprecipitate with anti-IgG PLUS agarose was used as negative control (−). IP: immunoprecipitation. (E) Diagrammatic representation of the full length and the two deletion constructs of PP1 and (F) the full length and the two deletion constructs of Hsp20. Predicted protein domains are shown in grey. (G) SDS-gel stained with Coomassie blue showing the purified MBP-PP1 full-length or deletion proteins. (H) Blot overlay assays with anti-GST antibody (WB : GST-Ab) were performed to determine the protein region of PP1 required for its association with GST-Hsp20(aa1–160). This narrowed down the PP1 binding region to a C-terminal fragment including amino acids 163–330. (I) SDS-gel stained with Coomassie blue showing the purified GST-Hsp20 full-length or deletion proteins. (J) Blot overlay assays with anti-MBP antibody (WB: MBP-Ab) determined that the protein region of Hsp20 responsible for its binding with MBP-PP1(aa1–330) includes amino acids 73–160. WB: Western blot.
Considering that Hsp20 was present in the SR membrane fraction, we hypothesized that this protein may co-localize with PLN and PP1. Therefore, its sub-cellular localization was assessed using co-immunostaining with PLN and PP1 antibodies in WT and TG cardiomyocytes. PLN staining revealed a striated pattern, corresponding to the SR membrane system (Figure 6B). Interestingly, immunofluorescence staining of Hsp20 also produced a partially striated pattern, overlapping with PLN (Figure 6B). Furthermore, double staining with PP1 and Hsp20 antibodies revealed colocalization of these two proteins in a similar pattern (Figure 6C). These results suggest that Hsp20 co-localizes with PP1 and PLN in the heart.
The potential association between Hsp20 and PP1 was further confirmed by reciprocal co-immunoprecipitations, using antibodies against Hsp20 and PP1 in cardiac homogenates. PP1 was detected by Western blot analysis of anti-Hsp20 immunoprecipitates (Figure 6D, upper panel). Accordingly, the reverse immunoprecipitation indicated that Hsp20 was associated with PP1 (Figure 6D, lower panel).
To determine whether the observed association between Hsp20 and PP1 is direct and to narrow down the regions on these two proteins responsible for their physical interaction, various recombinant PP1 and Hsp20 constructs were generated. Recombinant full length (PP1(aa1–330)) and two deletion constructs of PP1 (PP1(aa1–187) and PP1(aa163–330)) were generated in fusion with the maltose-binding protein (MBP) (Figure 6E & G). In addition, an Hsp20 construct containing residues 1–160 (Hsp20(aa1–160)) and two deletion constructs (Hsp20(aa1–82) and Hsp20(aa73–160)) were expressed as glutathione-S-transferase (GST)-fusion proteins in Escherichia coli (Figure 6F & I). Using blot overlay assays we evaluated binding of GST-Hsp20(aa1–160) (~40 kDa) to the full length and the two truncated MBP-PP1 proteins. Full length MBP-PP1(aa1–330) and MBP-PP1(aa163–330) showed strong binding to GST-Hsp20(aa1–160), therefore mapping the region of PP1 required for its association with Hsp20 to residues 163–330 (Figure 6H). Similarly, on reciprocal blot overlay experiments, we evaluated binding of MBP-PP1(aa1–330) (~80kDa) to the full length GST-Hsp20(aa1–160) and the two Hsp20 deletion constructs GST-Hsp20(aa1–82) and GST-Hsp20(aa73–160). We found that GST-Hsp20(aa1–160) and GST-Hsp20(aa73–160) interacted with MBP-PP1(aa1–330) (Figure 6J). Taken together, these data suggest that Hsp20 and PP1 directly interact with each other and that the region involved in their binding is within their C-terminal domains.
Discussion
This study presents the first evidence on inhibition of PP1 by the small Hsp20. PP1 is a major Ser/Thr protein phosphatase in the cardiomyocytes, where its activity is regulated by the endogenous inhibitor-1 and inhibitor-2 proteins.12, 13, 18, 20–23, 29 Herein we identified an additional control switch for PP1 enzymatic activity, provided by the cardioprotective protein Hsp20. Regulation of PP1 by Hsp20 involves a physical direct interaction between these two proteins, leading to amplified SR Ca-cycling and augmented cardiac inotropy (Figure 7).
Figure 7. Proposed Mechanism of Hsp20 Regulating Cardiac Contractility.
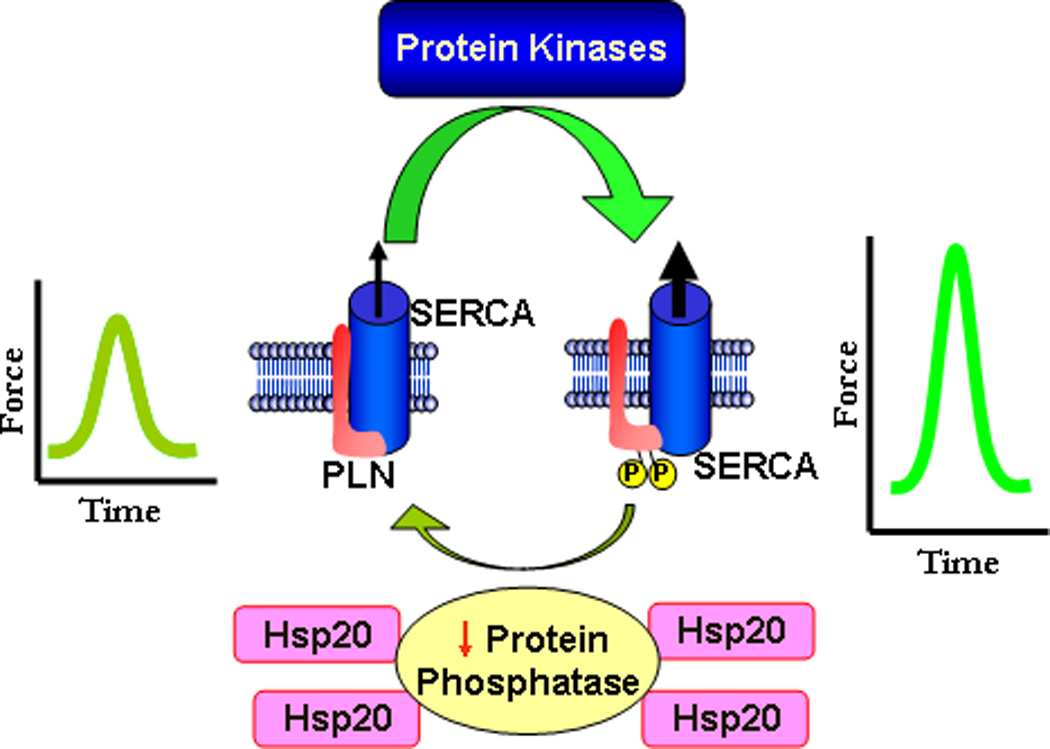
The intricate balance between protein kinase and phosphatase activities regulates the phosphorylation of phospholamban in cardiomyocytes. Dephosphorylated PLN poses an inhibitory effect on the activity of SERCA. Dephosphorylation of PLN is mainly mediated by PP1. PKA- and CaMKII-dependent phosphorylation of PLN at Ser16 and Thr17, respectively, relieves PLN inhibition of SERCA and allows for increased Ca-pumping into the SR. Through inhibition of PP1 activity, overexpression of Hsp20 shifts the balance of kinase and PP1 activities, and favors enhanced phosphorylation of PLN and increased cardiac performance.
Previous studies have shown that inhibition of PP1 activity in genetic models through overexpression/activation of inhibitor-1 or inhibitor-2 results in a hypercontractile cardiac phenotype.22, 29, 30 These findings are compatible with the idea that suppression of PP1 activity increases the phosphorylation status of PLN with subsequent disinhibition of SERCA2a, enhanced SR Ca-uptake and SR Ca-load. The apparent specificity of Hsp20 toward PLN was further confirmed in acutely infected cardiomyocytes from: a) PLN double-mutant mice, which abrogated the stimulatory effects of Hsp20 on contractile and Ca-kinetic parameters in the absence of the Ser16 and Thr17 phosphorylation sites (Figure 4A–D); and b) PLN-KO mice, which prevented the Hsp20 stimulatory effects in the absence of PLN (Online Figure III). Although these studies in isolated cardiomyocytes represent an ex vivo system with limited functional analysis, they offer an advantage over cross models, which may develop compensatory mechanisms contributing to the observed phenotype. This unique preference of Hsp20 for PLN may be important from a therapeutic point of view, since increased RyR phosphorylation may potentially lead to diastolic leakiness and arrhythmogenic activity in failing hearts.31
It should be also noted that additional modulators of SERCA activity exist, such as the membrane-associated anti-apoptotic protein Bcl-232 which has been shown to underlie one of the Hsp20 mechanisms, preventing cardiac ischemia/reperfusion injury.8 However, Hsp20 did not alter the expression level of Bcl-2 and coimmunoprecipitation studies excluded a direct association between these two proteins8. Thus, the Hsp20 regulatory effects on cardiac contractility do not appear to be mediated by the Bcl-2/SERCA pathway. Interestingly, Hsp20 appeared to coimmunoprecipitate not only with PP1, as indicated above, but also inhibitor-1 (Online Figure IV), suggesting the presence of a multi-protein regulatory complex. However, it is not currently known whether Hsp20 associates with inhibitor-1 directly or indirectly via PP1.
The mechanisms underlying the apparent specificity of Hsp20 for hyperphosphorylation of PLN may involve the location of PP1 within the proper SERCA/PLN functional domain of the SR. It has been recognized that PP1 associates with specific subunits or regulatory proteins, which target it to different subcellular locations/compartments, modulating its activity toward unique substrates.12 In the heart, it is suggested that PP1 associates with SR through its GM subunit, which contains a hydrophobic SR anchoring region.33 This would allow for PP1 compartmentalization and amplification of the Hsp20 effects on phospholamban phosphorylation through the PP1/PLN axis. Interestingly, Hsp20 is also present in the SR enriched fraction and there appears to be an association between PP1 and Hsp20. Indeed, recombinant protein assays confirmed a physical interaction between Hsp20 and PP1. Thus, Hsp20, PP1 and PLN may comprise a supra-molecular complex, facilitating the targeting of PP1 towards the SR-associated PLN.
Recently, two distinct peptide motifs, which are responsible for the interaction of regulatory proteins with PP1 have been documented: (1) [R/K]-x(0,1)-V-x-F, a well characterized and conventional consensus motif, which binds to the C-terminal region of PP1c; and (2) F-x-x-[R/K]-x-[R/K], which was initially identified in Bcl-xL, Bcl-w and Bad proteins, but its binding site on PP1c is still unknown.34, 35 By sequence screening, we found that Hsp20 does not contain the [R/K]-x(0,1)-V-x-F motif. However, the region between residues 117 and 122 (FHRRYR), which locates within a predicted C-terminal protein-protein interaction domain, possesses criteria for a putative F-x-x-[R/K]-x-[R/K] motif binding to PP1 (Online Figure V). Indeed, our GST-pull down assay confirmed that the C-terminal region of Hsp20 (aa73–160) binds to PP1c (Figure 6I & J).
A previous study showed that incubation of transiently permeabilized myocytes with phospho-Hsp20 peptide analogues, which contain the N-terminal 13 amino acids of Hsp20, increased contractility, associated with an abbreviated Ca transient decay.11 However, the non-phosphorylated peptide with similar length had no effects on contractility.11 The apparent discrepancy between the effects of non-phosphorylated peptide analogues and our results may be due to differences in peptide length, techniques used, experimental conditions and models. Consistent with our current findings, another chaperone protein, Hsp70, has been identified as a SR Ca-regulatory protein.36 Deletion of Hsp70 resulted in a delayed decline of Ca-transients, decreased SR Ca-content, and decreased rates of contraction and relaxation, which were related to decreases in SERCA2a expression.36 However, we did not observe any alterations in SERCA2a expression levels by either acute or chronic overexpression of Hsp20. Collectively, these findings underscore the need for further studies on the interactions between chaperones and SR Ca-regulatory proteins.
In heart failure, reduced output evokes an increase in catecholamines and other neuroendocrine factors, which negatively impact function by downregulation of β-adrenergic receptors and decreased PKA activity, resulting in dephosphorylation of key phosphoproteins.12 Overexpression of Hsp20 may hold benefits in improving ventricular performance of failing hearts, through targeting the PP1-PLN signaling axis. This is especially important in light of the elevated PP1 enzyme activity in SR.19 However, it should be pointed out that disturbed Ca-handling is not the only cause of heart failure. Loss of cardiomyocytes in the infarcted heart, increased load work on the surviving myocardium by the scar tissue, limited perfusion distal to coronary stenosis, suboptimal preload due to stiffness of the hypertrophic ventricle, and matrix remodeling are but a few additional factors that can contribute to global cardiac dysfunction.37 Interestingly, Hsp20 also possesses cardioprotective effects by preserving the viability of injured myocardium.2, 5, 6, 8, 9, 38 Thus, our findings lead to the suggestion that Hsp20 may hold a double benefit in the failing myocardium by a combination of contractile-dependent and cardioprotection-dependent effects.
Taken together, this is the first study that defines a role for Hsp20 in enhancing cardiac contractility and calcium handling. In addition to its recognized cardioprotective effects against myocardial injuries, our results strongly support the hypothesis that Hsp20 may represent a “refined” target, augmenting contractility and providing cardiac protection in heart failure.
Novelty and Significance.
What is Known?
Heat Shock Proteins (Hsps) are important mediators of cell survival under stress conditions
Hsp20 is a small heat shock protein that protects the heart against ischemic injury, β-agonist remodeling and apoptosis
Acute expression of Hsp20 in cardiomyocytes stimulates contractility but the underlying in vivo mechanisms are not currently known
What New Information Does This Article Contribute?
Hsp20 enhances cardiac function accompanied by increased SR Ca-cycling
The enhanced contractility is associated with specific increase in phospholamban (PLN) phosphorylation by Hsp20
The stimulatory effects of Hsp20 are ascribed to inhibition of protein phosphatase 1 (PP1) activity by its direct physical interaction, indicating that Hsp20 represents a novel regulator of PP1
Hsp20 is constitutively expressed in multiple tissues but it is most abundant in muscle. Cardiac overexpression of Hsp20 is associated with protection against ischemia/reperfusion-induced injury, β-agonist-mediated remodeling and apoptosis. Here we identified Hsp20 as a novel regulator of PP1 activity in the heart. Regulation appears to involve a direct physical interaction of Hsp20 with PP1 and inhibition of its enzymatic activity. The reduced PP1 activity by Hsp20 results in specific increases of PLN phosphorylation, SR Ca-transport and Ca-load, which lead to augmented function. These findings have major implications in heart failure, where depressed SR Ca-handling is attributed partially to increased PP1 activity. Hsp20 may target the PP1-PLN signaling axis and improve ventricular performance as well as increase cell survival, holding a dual benefit in heart disease.
Supplementary Material
Acknowledgements
We appreciate the kind gifts of the antibody to RyR2-S2815p by Dr. Andrew R. Marks (Columbia University) and to PLM C2 by Dr. Joseph Y. Cheung (Thomas Jefferson University).
Sources of Funding
This study was supported by NIH grants HL-26057 and HL-64018 (to E.G.K), HL 087861 and HL 087861-3S (to G.C.F), NIH training grant HL007382 (to T.J.P) and the European Community's Seventh Framework Programme FP7/2007–2013 under grant agreement No. HEALTH-F2-2009-241526, EUTrigTreat.
Non-Standard Abbreviations and Acronyms
- Ad
adenovirus
- Ca
calcium
- GST
glutathione-S-transferase
- Hsp20
heat shock protein 20
- LTCC
L-type Ca channel
- MBP
maltose-binding protein
- MyBP-C
myosin-binding protein-C
- NCX
sodium-calcium exchanger
- PLN
phospholamban
- PP1
protein phosphatase 1
- PP1c
catalytic protein phosphatase 1
- RYR2
ryanodine receptor 2
- Ser
serine
- SR
sarcoplasmic reticulum
- TG
transgenic
- TnI
troponin I
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Dr. E.G. Kranias is a scientific founder of Nanocor.
References
- 1.Dreiza CM, Komalavilas P, Furnish EJ, Flynn CR, Sheller MR, Smoke CC, Lopes LB, Brophy CM. The small heat shock protein, HSPB6, in muscle function and disease. Cell Stress Chaperones. 2010;15:1–11. doi: 10.1007/s12192-009-0127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qian J, Ren X, Wang X, Zhang P, Jones WK, Molkentin JD, Fan GC, Kranias EG. Blockade of Hsp20 phosphorylation exacerbates cardiac ischemia/reperfusion injury by suppressed autophagy and increased cell death. Circ Res. 2009;105:1223–1231. doi: 10.1161/CIRCRESAHA.109.200378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boluyt M, Brevick J, Rogers D, Randall M, Scalia A, Li Z. Changes in the rat heart proteome induced by exercise training: Increased abundance of heat shock protein hsp20. Proteomics. 2006;6:3154–3169. doi: 10.1002/pmic.200401356. [DOI] [PubMed] [Google Scholar]
- 4.Dohke T, Wada A, Isono T, Fujii M, Yamamoto T, Tsutamoto T, Horie M. Proteomic analysis reveals significant alternations of cardiac small heat shock protein expression in congestive heart failure. J Card Fail. 2006;12:77–84. doi: 10.1016/j.cardfail.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Fan G, Zhou X, Wang X, Song G, Qian J, Nicolaou P, Chen G, Ren X, Kranias E. Heat shock protein 20 interacting with phosphorylated Akt reduces doxorubicin-triggered oxidative stress and cardiotoxicity. Circ Res. 2008;103:1270–1279. doi: 10.1161/CIRCRESAHA.108.182832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan G, Yuan Q, Song G, Wang Y, Chen G, Qian J, Zhou X, Lee Y, Ashraf M, Kranias E. Small heat-shock protein Hsp20 attenuates beta-agonist-mediated cardiac remodeling through apoptosis signal-regulating kinase 1. Circ Res. 2006;99:1233–1242. doi: 10.1161/01.RES.0000251074.19348.af. [DOI] [PubMed] [Google Scholar]
- 7.Fan G, Chu G, Kranias E. Hsp20 and its cardioprotection. Trends Cardiovasc Med. 2005;15:138–141. doi: 10.1016/j.tcm.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Fan G, Ren X, Qian J, Yuan Q, Nicolaou P, Wang Y, Jones W, Chu G, Kranias E. Novel cardioprotective role of a small heat-shock protein, Hsp20, against ischemia/reperfusion injury. Circulation. 2005;111:1792–1799. doi: 10.1161/01.CIR.0000160851.41872.C6. [DOI] [PubMed] [Google Scholar]
- 9.Fan G, Chu G, Mitton B, Song Q, Yuan Q, Kranias E. Small heat-shock protein Hsp20 phosphorylation inhibits beta-agonist-induced cardiac apoptosis. Circ Res. 2004;94:1474–1482. doi: 10.1161/01.RES.0000129179.66631.00. [DOI] [PubMed] [Google Scholar]
- 10.Chu G, Egnaczyk G, Zhao W, Jo S, Fan G, Maggio J, Xiao R, Kranias E. Phosphoproteome analysis of cardiomyocytes subjected to beta-adrenergic stimulation: identification and characterization of a cardiac heat shock protein p20. Circ Res. 2004;94:184–193. doi: 10.1161/01.RES.0000107198.90218.21. [DOI] [PubMed] [Google Scholar]
- 11.Pipkin W, Johnson J, Creazzo T, Burch J, Komalavilas P, Brophy C. Localization, macromolecular associations, and function of the small heat shock-related protein HSP20 in rat heart. Circulation. 2003;107:469–476. doi: 10.1161/01.cir.0000044386.27444.5a. [DOI] [PubMed] [Google Scholar]
- 12.Nicolaou P, Hajjar R, Kranias E. Role of protein phosphatase-1 inhibitor-1 in cardiac physiology and pathophysiology. J Mol Cell Cardiol. 2009;47:365–371. doi: 10.1016/j.yjmcc.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta R, Mishra S, Rastogi S, Imai M, Habib O, Sabbah H. Cardiac SR-coupled PP1 activity and expression are increased and inhibitor 1 protein expression is decreased in failing hearts. Am J Physiol Heart Circ Physiol. 2003;285:H2373–H2381. doi: 10.1152/ajpheart.00442.2003. [DOI] [PubMed] [Google Scholar]
- 14.Depaoli-Roach A, Park I, Cerovsky V, Csortos C, Durbin S, Kuntz M, Sitikov A, Tang P, Verin A, Zolnierowicz S. Serine/threonine protein phosphatases in the control of cell function. Adv Enzyme Regul. 1994;34:199–224. doi: 10.1016/0065-2571(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 15.Kranias E, Steenaart N, Di Salvo J. Purification and characterization of phospholamban phosphatase from cardiac muscle. J Biol Chem. 1988;263:15681–15687. [PubMed] [Google Scholar]
- 16.Herzig S, Neumann J. Effects of serine/threonine protein phosphatases on ion channels in excitable membranes. Physiol Rev. 2000;80:173–210. doi: 10.1152/physrev.2000.80.1.173. [DOI] [PubMed] [Google Scholar]
- 17.El-Armouche A, Pamminger T, Ditz D, Zolk O, Eschenhagen T. Decreased protein and phosphorylation level of the protein phosphatase inhibitor-1 in failing human hearts. Cardiovasc Res. 2004;61:87–93. doi: 10.1016/j.cardiores.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Carr A, Schmidt A, Suzuki Y, del Monte F, Sato Y, Lanner C, Breeden K, Jing S, Allen P, Greengard P, Yatani A, Hoit B, Grupp I, Hajjar R, DePaoli-Roach A, Kranias E. Type 1 phosphatase, a negative regulator of cardiac function. Mol Cell Biol. 2002;22:4124–4135. doi: 10.1128/MCB.22.12.4124-4135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neumann J. Altered phosphatase activity in heart failure, influence on Ca2+ movement. Basic Res Cardiol. 2002;97:I91–I95. doi: 10.1007/s003950200036. [DOI] [PubMed] [Google Scholar]
- 20.Grote-Wessels S, Baba HA, Boknik P, El-Armouche A, Fabritz L, Gillmann HJ, Kucerova D, Matus M, Muller FU, Neumann J, Schmitz M, Stumpel F, Theilmeier G, Wohlschlaeger J, Schmitz W, Kirchhefer U. Inhibition of protein phosphatase 1 by inhibitor-2 exacerbates progression of cardiac failure in a model with pressure overload. Cardiovasc Res. 2008;79:464–471. doi: 10.1093/cvr/cvn113. [DOI] [PubMed] [Google Scholar]
- 21.Nicolaou P, Rodriguez P, Ren X, Zhou X, Qian J, Sadayappan S, Mitton B, Pathak A, Robbins J, Hajjar R, Jones K, Kranias E. Inducible expression of active protein phosphatase-1 inhibitor-1 enhances basal cardiac function and protects against ischemia/reperfusion injury. Circ Res. 2009;104:1012–1020. doi: 10.1161/CIRCRESAHA.108.189811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pathak A, del Monte F, Zhao W, Schultz J, Lorenz J, Bodi I, Weiser D, Hahn H, Carr A, Syed F, Mavila N, Jha L, Qian J, Marreez Y, Chen G, McGraw D, Heist E, Guerrero J, DePaoli-Roach A, Hajjar R, Kranias E. Enhancement of cardiac function and suppression of heart failure progression by inhibition of protein phosphatase 1. Circ Res. 2005;96:756–766. doi: 10.1161/01.RES.0000161256.85833.fa. [DOI] [PubMed] [Google Scholar]
- 23.Wittkopper K, Fabritz L, Neef S, Ort KR, Grefe C, Unsold B, Kirchhof P, Maier LS, Hasenfuss G, Dobrev D, Eschenhagen T, El-Armouche A. Constitutively active phosphatase inhibitor-1 improves cardiac contractility in young mice but is deleterious after catecholaminergic stress and with aging. J Clin Invest. 2010;120:617–626. doi: 10.1172/JCI40545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Islamovic E, Duncan A, Bers DM, Gerthoffer WT, Mestril R. Importance of small heat shock protein 20 (hsp20) C-terminal extension in cardioprotection. J Mol Cell Cardiol. 2007;42:862–869. doi: 10.1016/j.yjmcc.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Bers D. Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 26.MacLennan D, Kranias E. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 27.Brittsan A, Ginsburg K, Chu G, Yatani A, Wolska B, Schmidt A, Asahi M, MacLennan D, Bers D, Kranias E. Chronic SR Ca2+-ATPase inhibition causes adaptive changes in cellular Ca2+ transport. Circ Res. 2003;92:769–776. doi: 10.1161/01.RES.0000066661.49920.59. [DOI] [PubMed] [Google Scholar]
- 28.MacDougall LK, Jones LR, Cohen P. Identification of the major protein phosphatases in mammalian cardiac muscle which dephosphorylate phospholamban. Eur. J. Biochem. 1991;296:725–734. doi: 10.1111/j.1432-1033.1991.tb15871.x. [DOI] [PubMed] [Google Scholar]
- 29.Kirchhefer U, Baba H, Bokník P, Breeden K, Mavila N, Brüchert N, Justus I, Matus M, Schmitz W, Depaoli-Roach A, Neumann J. Enhanced cardiac function in mice overexpressing protein phosphatase Inhibitor-2. Cardiovasc Res. 2005;68:98–108. doi: 10.1016/j.cardiores.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Braz J, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball T, Lorenz J, Nairn A, Liggett S, Bodi I, Wang S, Schwartz A, Lakatta E, DePaoli-Roach A, Robbins J, Hewett T, Bibb J, Westfall M, Kranias E, Molkentin J. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10:248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 31.Eisner D, Kashimura T, O'Neill S, Venetucci L, Trafford A. What role does modulation of the ryanodine receptor play in cardiac inotropy and arrhythmogenesis? J Mol Cell Cardiol. 2009;46:474–481. doi: 10.1016/j.yjmcc.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Dremina ES, Sharov VS, Kumar K, Zaidi A, Michaelis EK, Schoneich C. Anti-apoptotic protein Bcl-2 interacts with and destabilizes the sarcoplasmic/endoplasmic reticulum Ca2t-ATPase (SERCA) Biochem. J. 2004;383:361–370. doi: 10.1042/BJ20040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berrebi-Bertrand I, Souchet M, Camelin J, Laville M, Calmels T, Bril A. Biophysical interaction between phospholamban and protein phosphatase 1 regulatory subunit GM. FEBS Lett. 1998;439:224–230. doi: 10.1016/s0014-5793(98)01364-7. [DOI] [PubMed] [Google Scholar]
- 34.Garcia A, Caylab X, Guergnona J, Dessaugea F, Hospitala V, Rebolloc MP, Fleischerd A, Rebollo A. Serine/threonine protein phosphatases PP1 and PP2A are key players in apoptosis. Biochimie. 2003;85:721–726. doi: 10.1016/j.biochi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Meiselbach H, Sticht H, Enz R. Structural analysis of the protein phosphatase 1 docking motif: Molecular description of binding specificities identifies interacting proteins. Chem. Biol. 2006;13:49–59. doi: 10.1016/j.chembiol.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Kim Y, Suarez J, Hu Y, McDonough P, Boer C, Dix D, Dillmann W. Deletion of the inducible 70-kDa heat shock protein genes in mice impairs cardiac contractile function and calcium handling associated with hypertrophy. Circulation. 2006;113:2589–2597. doi: 10.1161/CIRCULATIONAHA.105.598409. [DOI] [PubMed] [Google Scholar]
- 37.Sipido K, Vangheluwe P. Targeting sarcoplasmic reticulum Ca2+ uptake to improve heart failure: hit or miss. Circ Res. 2010;6:230–233. doi: 10.1161/CIRCRESAHA.109.210740. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Zingarelli B, O'Connor M, Zhang P, Adeyemo A, Kranias E, Wang Y, Fan G. Overexpression of Hsp20 prevents endotoxin-induced myocardial dysfunction and apoptosis via inhibition of NF-kappaB activation. J Mol Cell Cardiol. 2009;47:382–390. doi: 10.1016/j.yjmcc.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


