Abstract
Thymic stromal lymphopoietin (TSLP) is an essential cytokine for the initiation and development of allergic inflammation. Here, we have investigated the role of TSLP in the breakdown of immune tolerance and generation of inducible regulatory T cells (iTregs). Our results demonstrated that TSLP diverted airway tolerance against ovalbumin to Th2 sensitization and inhibited the generation of OVA-specific iTregs. TSLP exerted a direct inhibitory effect on both human and mouse iTreg development in vitro. Low doses of TSLP were capable of inhibiting iTreg induction without significantly promoting Th2 development, indicating that these two functions of TSLP are separable. Moreover, the TSLP-mediated inhibition of iTreg generation was only partially dependent on IL-4 and Stat6, and was effective when TSLP was present for the first 24 h of T cell activation. These results define a novel role for TSLP in regulating the balance of airway tolerance and allergic inflammation.
Introduction
The immune response in the respiratory system maintains a tightly controlled balance between immunity against invading pathogens and the regulatory mechanisms of airway tolerance towards harmless environmental antigens. Disruption of this balance can lead to persistent infections or allergic diseases, including asthma and allergic rhinitis. Asthma is a chronic disease of dysregulated T helper type-2 (Th2) immunity in the airways, which may be caused by a combination of environmental and genetic factors (1).
Thymic stromal lymphopoietin (TSLP) was identified as a key initiator and Th2-driven force of allergic inflammation in asthma and atopic dermatitis (2). TSLP is highly expressed in acute and chronic atopic dermatitis lesions, and airways of allergic asthma patients (3–6). Over-expression of TSLP in lung leads to eosinophilic airway inflammation and hyperactivity (7), while in skin results in skin inflammation characteristic of atopic dermatitis (8, 9). TSLP receptor-deficient (Tslpr−/−) mice are protected from developing allergic airway inflammation and skin inflammation (7, 10, 11).
TSLP strongly activated peripheral blood derived DCs in vitro to up-regulate MHC II and co-stimulatory molecules, and to secrete chemokines that attract eosinophils, neutrophils, and Th2 cells, but not Th1/Th17-polarizing cytokines IL-12 and IL-23 (3). TSLP activated DCs also expressed high levels of OX40L, which interacts with OX40 expressed on T cells to drive Th2 differentiation (12). In addition to DCs, TSLP was shown to be able to directly act on naïve CD4+ T cells to promote Th2 differentiation and/or IL-4 secretion in vivo and in vitro (11, 13, 14).
Recent studies also suggest that TSLP acting through DCs promotes the differentiation and development of regulatory T (Treg) cells. In humans, thymic CD11c+ DCs (15) or plasmacytoid DCs (16) activated by TSLP induced proliferation and differentiation of CD4+CD8−CD25− thymic T cells into CD4+CD25+FOXP3+ Tregs. In mouse embryonic day 17 fetal thymus organ culture, addition of TSLP also promoted expression of Foxp3 while blocking TSLP receptor resulted in reduced Foxp3 expression (17). TSLP may also play a role in inducible Treg (iTreg) differentiation since bone marrow-derived DCs from NOD mice (18) or human monocytes-derived DCs (19) when treated with TSLP were able to increase iTreg differentiation.
Induction of antigen-specific regulatory T cells is an important mechanism to maintain mucosal tolerance against harmless antigens (20, 21). In this study, we demonstrated that TSLP acts directly on CD4+ T cells to inhibit the generation of both human and mouse iTregs in vitro. More importantly, the presence of TSLP during the first 24 hr of T cell activation was sufficient to inhibit Treg development from naïve T cell. TSLP is efficient in suppressing iTreg generation, even in the absence of endogenous IL-4 and Stat6. Since significantly increased serum TSLP was reported in children with atopic dermatitis (22), inhibition of iTreg differentiation may be an important component of the function of TSLP to suppress airway tolerance and initiate allergen sensitization leading to the development of allergic airway inflammation.
Materials and Methods
Animals
Wild-type BALB/c mice, DO11.10 TCR transgenic mice, Foxp3eGFP knockin mice, and Il4−/− mice were purchased from Harlan Laboratories and Jackson Laboratories, respectively. Tslpr−/− and Stat6−/− mice were described previously (7, 23). All animals were housed at pathogen-free condition and all studies were approved by the Indiana University School of Medicine Animal Care and Use Committee.
OVA sensitization, airway challenge, and airway hyperresponsiveness
Groups of 6–10 weeks old mice were sensitized on days 1, 2, and 3 by intranasal instillation of 100 μg OVA (Worthington Biochemicals), or 100 μg OVA with 500 ng TSLP in 40 μl PBS. As controls, mice received PBS or TSLP. Mice were challenged intranasally with 50 μg OVA in PBS for 3 consecutive days from day 14. Airway hyperresponsiveness (24) in these mice was tested 24 hours after the last challenge. Enhanced pause (Penh) in response to increasing doses of aerosolized methacholine (Sigma-Aldrich) in PBS was analyzed using unrestrained whole body plethysmography (Buxco Electronics).
Bronchoalveolar lavage (BAL), tissue fixation, and staining
After the mice were euthanized, lungs were lavaged three times with 1 ml each warm PBS via a tracheal polyethylene catheter. Total cells in BAL fluid were counted with a hemocytometer, while differential cell counts were performed using cytospin (Shandon) cell preparations stained with modified Wright-Giemsa stain.
After lavage, lungs were excised from thorax cavity. The lungs were inflated with 4% neutral buffered formaldehyde (Fisher Biotech) and fixed overnight at room temperature. Tissues were then embedded in paraffin, sectioned and stained with hematoxylin & eosin, or Periodic acid-Schiff (PAS) stain.
qRT-PCR
RNA from lungs or cell cultures was extracted using TRIzol (Invitrogen) according to the manufacture’s protocol, and reverse transcribed to cDNA using the high capacity cDNA reverse transcription kit (Applied Biosystems). qRT-PCR was performed using TaqMan chemistry on a 7500 Fast Real-Time PCR System (Applied Biosystems). TaqMan primer and probe mixtures were purchased from Applied Biosystems. GAPDH was used as endogenous control reference gene to normalize sample variation and relative expression was calculated by the change-in-threshold (−ΔΔCT) method.
Naïve T cell isolation, culture, and in vitro Treg generation
Naïve mouse CD4 +CD62L+ T cells were isolated from spleens and lymph nodes, and naïve human CD4+ cells were isolated from PBMC using MACS isolation kit (Miltenyi Biotec). RPMI 1640 medium was supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate, 10 mM HEPES buffer, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μM 2-mercaptoethanol. Effects of TSLP on iTreg differentiation in vitro was studied using either a standard protocol (25) or a protocol without exogenous TGF-β1 modified from Sauer et al. (2008) and Walker et al. (2005)(26, 27). Briefly, in the standard protocol, naïve T cells were cultured for 5 days in the presence of 10 μg/ml of plate-bound anti-CD3 (17A2, Biolegend), 2 μg/ml of soluble anti-CD28 mAb (37.51, Biolegend), and 3 ng/ml TGF-β1 (PeproTech). For the protocol without exogenous TGF-β1, naïve T cells were activated with 5 μg/ml of plate-bound anti-CD3 and 1 μg/ml anti-CD28 without TGF-β for 24 hours. On the second day, cells were removed from activation stimuli, washed, and cultured for 9 more days. For selected treatments, recombinant TSLP (R&D systems) was added to culture as indicated.
Adoptive transfer experiments
Naïve CD4+ CD62L+ T cells were isolated from DO11.10 mice, and adoptively transferred into wild type or TSLPR deficient recipient mice via tail vein injection at a 3 – 4 × 106 T cells in 200 μl PBS. 24 h after cell transfer, mice were treated intranasally with 100 μg OVA or 100 μg OVA plus 500 ng TSLP for 3 or 5 days.
To examine the effect of TSLP on antigen-specific Treg generation in an antigen- driven asthma model, groups of wild type Balb/c mice were sensitized to OVA by intraperitoneal injection of 50 μg OVA emulsified in 1.3 mg aluminum hydroxide on days 0 and 7. On day 13, 3 × 106 purified wild type or TSLPR deficient CD4+ naïve T cells were i.v. injected into these sensitized mice. Starting from day 14, the mice were challenged with 25 μg OVA for 5 days.
To track OVA-specific iTreg generation, lung draining mediastinal lymph nodes were harvested from the recipient mice. Cells were stained with CD4 and KJ1-26 antibodies followed by intracellular staining for Foxp3 (Biolegend).
Flow cytometry, antibodies, and reagents
Following monoclonal antibodies were purchased from Biolegend unless stated otherwise: CD4 (clone GK1.5), DO11.10 TCR (clone KJ1-26), Foxp3 (clone 150D), IL-4 (clone 11B11), IFN-γ (clone XMG1.2). Rat IgG1 (clone RTK2071). Rat IgG2a (clone RTK2758), and rat IgG2b (RTK4530) were included as isotype controls. All surface and Foxp3 staining was carried out according to manufacturer’s protocol. To detect intracellular cytokine production, cells were restimulated with 50 ng/ml phorbol 12-myristate 13-acetate and 500 ng/ml ionomycin for 6 hours. Monensin (Biolegend) was added to the culture for the last 4 hour incubation. Cells were then stained for IL-4 and IFN-γ production as described (24).
Results
TSLP breaks immune tolerance while promoting Th2 sensitization in vivo
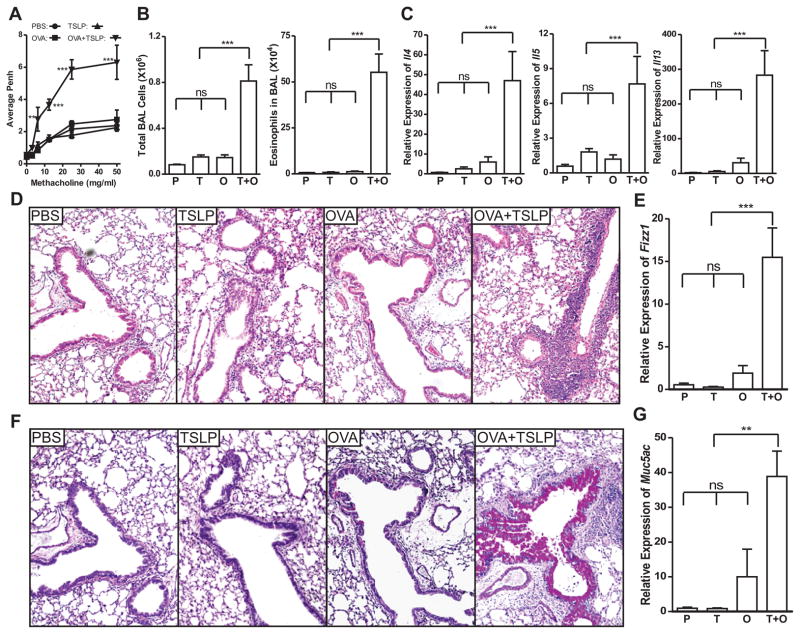
Previous studies showed that TSLP is strongly associated with respiratory and dermal allergic inflammatory diseases (7, 9–11, 28, 29). However, repeated intranasal (i.n.) instillation of TSLP did not induce airway inflammation, but rather predisposed airway mucosa towards the development of aberrant responses against innocuous environmental antigens (30). To determine whether TSLP can break airway tolerance towards harmless antigens like OVA, we examined the effect of TSLP at primary immunization when a tolerance-vs-immunity decision was made in the airway mucosa. BALB/c mice were subjected to i.n. TSLP, administered in the presence or absence of OVA, for three consecutive days. Control groups received OVA or PBS. After 10 days, these mice were challenged three times to examine the airway response against OVA. OVA plus TSLP-conditioned mice developed strong airway hyperresponsiveness (Fig. 1A) and airway eosinophilia (Fig. 1B), while OVA conditioned mice were tolerant to OVA challenge. Neither TSLP- nor PBS conditioned mice displayed pulmonary inflammation. In addition, lungs from OVA + TSLP-conditioned mice showed a Th2 biased cytokine environment with high Il4, Il5, and Il13 expression in the lungs (Fig. 1C). Histopathology analysis demonstrated that only OVA + TSLP-conditioned mice developed allergic airway inflammation (Fig. 1D), which was confirmed by significantly increased expression of Fizz1 (Fig. 1E), a gene associated with pulmonary inflammation. On PAS stained slides, the magenta-colored mucus-producing cells were rarely seen in PBS-, TSLP- and OVA-conditioned mice but were abundant in OVA + TSLP-conditioned mice, indicating goblet cell hyperplasia/metaplasia and increased mucus production (Fig. 1F). Consistent with the PAS staining, expression of mucus gene Muc5ac in the lungs was significantly elevated in OVA + TSLP conditioned mice (Fig. 1G). Therefore, intranasal exposure to OVA renders wild type BALB/c mice tolerant to OVA challenge, while the presence of TSLP during primary immune response is sufficient to divert this tolerance to Th2 sensitization, leading to strong allergic airway inflammation with all the cardinal features of asthma following OVA challenge.
Figure 1. TSLP diverts airway tolerance to Th2 sensitization against innocuous antigen OVA.
BALB/c mice were conditioned i.n. with PBS, TSLP, OVA, or OVA + TSLP for 3 days. Ten days later, the animals were challenged i.n. with OVA for three times. A, Airway responsiveness to increasing dose of methacholine was analyzed by unrestrained whole body plethysmography and is presented as average enhanced pause (Penh) over a three minute period. B, Total cell and eosinophil count in BAL fluid. C, Quantitative PCR analysis of the expression of Th2 cytokines Il-4, Il-5 and Il-13 in the lungs, presented relative to the expression in PBS conditioned and OVA challenged mice. D, Lung tissue sections stained with H&E showing peribronchiolar inflammatory infiltration in OVA + TSLP conditioned mice after challenge. E, Quantitative PCR analysis of the expression of Fizz1 gene in the lungs, presented relative to the expression in PBS conditioned and OVA challenged mice. F, Lung tissue sections stained with PAS showing goblet cell hyperplasia/metaplasia in OVA + TSLP conditioned mice after challenge. G, Quantitative PCR analysis of the expression of mucus gene Muc5ac, presented relative to PBS conditioned and OVA challenged mice. P: PBS-conditioned mice; T: TSLP-conditioned mice; O: OVA-conditioned mice; T + O: OVA plus TSLP conditioned mice. Data shown represent mean ± SEM (n = 4) from one of two independent experiments. **: p < 0.01; ***: P < 0.001; ns: not significant by analysis of variance with Bonferroni’s post-hoc tests.
TSLP inhibits antigen-specific iTreg development in vivo
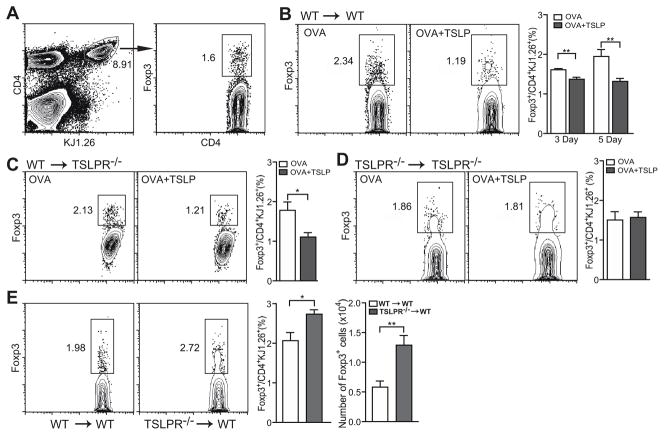
It is well established that TSLP is capable of promoting Th2 differentiation (2). To determine whether elevated TSLP can suppress the development of airway mucosal tolerance, we examined the capacity of TSLP to inhibit antigen-specific iTreg generation in an in vivo system. BALB/c wild type or TSLPR-deficient mice were transferred with naïve CD4+CD62L+ DO11.10 TCR transgenic T cells i.v. and 24 h later were treated intranasally with OVA in the presence or absence of TSLP daily for three or five days. Expression of Foxp3 in donor OVA-specific KJ1.26+ T cells from lung draining mediastinal lymph nodes was analyzed using flow cytometry (Fig. 2A). Mice that received OVA plus TSLP treatment had significantly lower percentages of OVA-specific iTregs in their MdLNs, compared to OVA only treated mice (Fig. 2B). While antigen-specific iTregs continued to accumulate in the lung draining lymph nodes of mice from Day 3 to Day 5, such increase of OVA-specific iTregs was completely prevented by TSLP (Fig. 2B). These results indicate that TSLP can antagonize antigen induced airway tolerance through suppressing antigen-specific iTreg generation. More strikingly, TSLP was equally efficient in suppressing OVA-specific iTreg accumulation in mediastinal lymph nodes of TSLPR-deficient recipient mice (Fig. 2C). The fact that only donor DO11.10 T cells were able to respond to TSLP suggests that TSLP directly acts on CD4+ T cell to suppress their conversion to iTregs, although we cannot rule out the possibility that TSLP can also affect other cell types such as DCs to indirectly suppress iTreg development in vivo.
Figure 2. Effects of TSLP on antigen-specific iTreg generation in vivo.
Naïve CD4+CD62L+ T cells were isolated from DO11.10 TCR transgenic mice. Approximate 3–4 × 106 purified T cells were i.v. transferred into BALB/c or TSLPR-deficient mice, which were then treated i.n. with OVA or OVA + TSLP for 3 or 5 days. A, Gating strategies to examine OVA-specific iTreg development in vivo. B, Antigen-specific iTreg induction in wild type BALB/c recipient mice. C, Antigen-specific iTreg induction TSLPR-deficient recipient mice. Expression of Foxp3 was stained with an intracellular staining kit from Biolegend. Percentage of Foxp3+ iTreg cells in the donor cells, as marked by KJ1-26+, were analyzed by FACS. Representative dot plots and graphs from three independent experiments were shown. Data represent mean ± SEM (n = 4). *: p < 0.05; **: p < 0.01, two-tailed t-test.
It has been reported that LPS induced OX40L expression on dendritic cells and B cells that resulted in a synergistic activity between TLR4 and OX40 signals, leading to production of IL-4, IFN-γ, and IL-6, which blocked Treg development (31). To rule out the possibility that contaminants in the recombinant TSLP played a similar role, we adoptively transferred TSLPR deficient DO11.10 T cells into TSLPR deficient recipient mice. Similar amount of OVA-specific iTregs were generated in the lung draining lymph nodes after i.n. OVA treatment regardless of TSLP (Fig. 2D). These results indicate that the iTreg suppressive effect seen in Fig. 2B and 2C are TSLP-dependent.
To further confirm the role of TSLP in inhibiting antigen-specific iTreg differentiation, we examined iTreg generation in a murine asthma model. Wild type or TSLPR deficient DO11.10 T cells were adoptively transferred into groups of OVA-sensitized Balb/c mice 24 hr before intranasal challenge. After 5 day OVA challenge, both percentage and number of Foxp3+ iTregs differentiated from TSLPR deficient DO11.10 T cells were significantly higher than those differentiated from wild type DO11.10 T cells (Fig. 2E), indicating that endogenous TSLP was sufficient to inhibit antigen-specific iTreg generation in airway mucosa.
TSLP inhibits the generation of iTreg cells in vitro
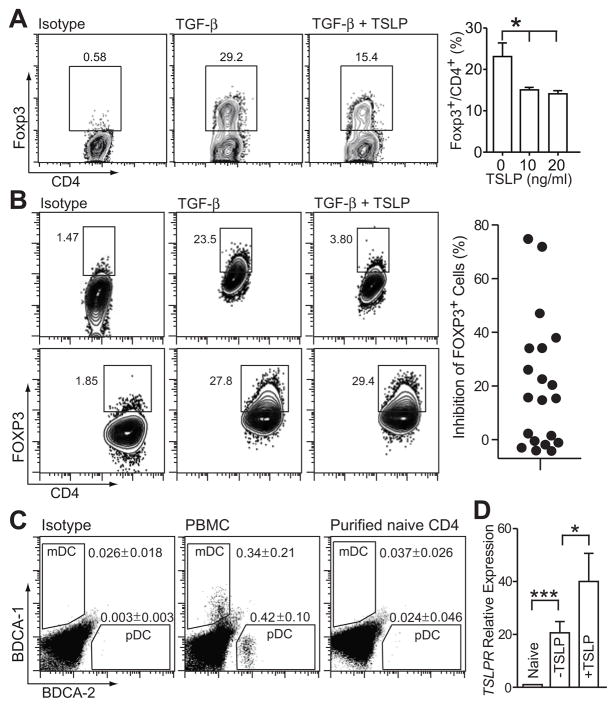
To further assess the ability of TSLP to directly act on CD4+ T cells, we analyzed the effect of TSLP on mouse and human iTreg differentiation in vitro. Naïve CD4+CD62L+ T cells were isolated from mouse spleens and lymph nodes, and stimulated with plate-bound anti-CD3, soluble anti-CD28, and TGF-β. After culture for 5 days, approximately 30% of murine CD4+ T cells differentiated to iTregs as indicated by intracellular staining for Foxp3. In the presence of 10 ng/ml TSLP, the CD4 +Foxp3+ T cell population was significantly reduced (Fig. 3A).
Figure 3. Effects of TSLP on inducible regulatory T cell generation in vitro.
A, TSLP suppressed murine iTreg differentiation in the presence of TGF-β1. Naïve CD4+CD62L+ T cells were isolated from spleens and lymph nodes, cultured with 10 μg/ml plate-bound anti-CD3 and 2 μg/ml anti-CD28 in the presence of 3 ng/ml TGF-β1 for 5 days. TSLP was used at 10 ng/ml. Data represent mean ± SEM (n = 3). B, TSLP suppressed human iTreg differentiation in the presence of TGF-β1. Naïve CD4+ T cells were isolated from PBMC and cultured in the same condition as (A). Human T cells showed considerable variability in responding to TSLP. A responder is shown on top, a non-responder on the bottom. The percentage inhibition of iTreg differentiation by TSLP from all 20 donor samples is indicated in the graph on the right. C, Purity of naïve CD4+ T cells isolated from human PBMC. Samples of PBMC and purified T cells from the same donor were stained with Blood Dendritic Cell Enumeration Kit (Miltenyi Biotec) and analyzed by flow cytometry. Data represent mean ± SEM (n = 6). D, Quantitative PCR analysis of the expression of human TSLP receptor TSLPR in activated CD4+ T cells, presented relative to naïve cells. Including of TSLP in culture (+TSLP) further enhanced TSLPR expression. Naïve CD4+ T cells were purified from PBMC and cultured in the same condition as (A). Data represent mean ± SEM (n = 6). *: p < 0.05; **: p < 0.01 One way analysis of variance.
TSLP exhibited inhibitory effects on human iTreg generation in vitro from naïve CD4+ T cells isolated from some donors but not all subjects (Fig. 3B). Naïve CD4+ T cells obtained from anonymous donors showed a wide range of susceptibility to TSLP-mediated iTreg differentiation. TSLP had no effects on some donors while inhibiting over 70% iTreg differentiation in others (Fig. 3B). We are currently investigating whether the response of human CD4+ T cells to TSLP is related to predisposition to allergic diseases. Since it is reported that human TSLP mainly exerts its effect through DCs and had little effect on CD4+ T cells (32), we examined DC contamination in our purified human naïve CD4+ T cells by Blood Dendritic Cell Enumeration Kit (Miltenyi Biotec). Compared to PBMC which contained 0.34% (± 0.21, n = 6) myeloid DCs and 0.42% (± 0.1) plasmacytoid DCs, our purified naïve human CD4+ T cells contained only 0.037% (± 0.026, n = 6) mDCs and 0.024% (± 0.046) pDCs, not significantly different from isotype stained PBMC (Fig. 3C). These results suggest that TSLP is acting directly on human T cells, and not indirectly through DC. Consistent with other reported results (32–34), TSLPR expression was increased following T cell activation and was further enhanced when 10 ng/ml TSLP was included in the culture (Fig. 3D).
Potency of TSLP in inhibiting iTreg generation in vitro
Similar to other cytokines antagonizing iTreg induction (35), TSLP was more potent in suppressing iTreg differentiation when TGF-β levels were low. To eliminate the interference from TGF-β, we adapted an iTreg differentiation protocol without adding TGF-β based upon two published studies (26, 27). Purified CD4 +CD62L+ T cells were activated with plate bound anti-CD3 and soluble anti-CD28 for 24 hrs. Following activation, cells were removed from TCR stimulation, washed and cultured for 9 more days. Comparing to the standard protocol in which TCR stimulation was present during the entire 5-day culture, the 24 hour activation more closely mimics the in vivo scenario, since it has been reported that interaction of DCs with naive antigen-specific CD4+ T cells occurred within 18–24 hr after immunization followed by resumed movement and dispersion of activated CD4+ T cells, in both tolerizing and priming conditions (36, 37).
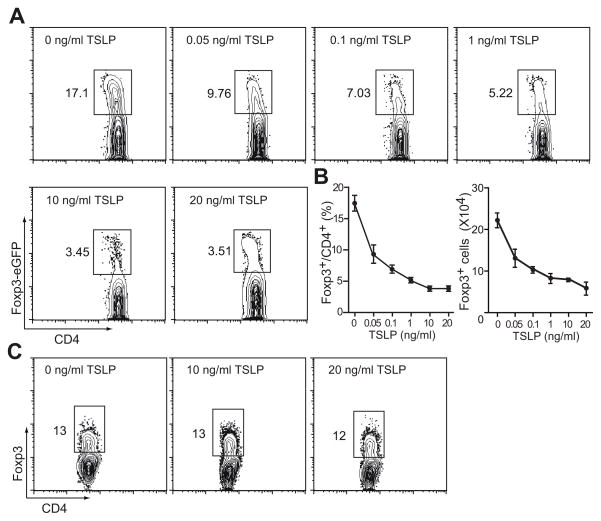
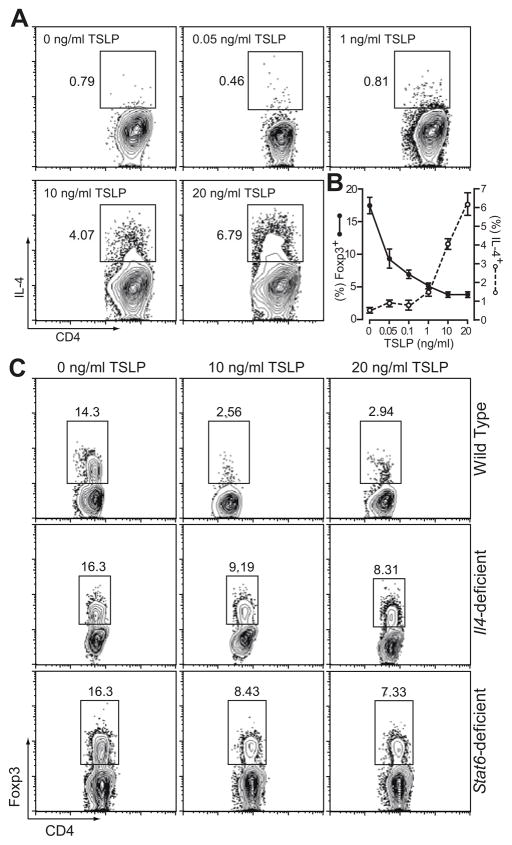
Using Foxp3eGFP knockin mice as the source of naïve CD4+ T cells, we found that after 10 day culture 17.1% cells differentiated into iTregs as identified by eGFP expression. Inclusion of TSLP in the culture inhibited the induction of iTreg in a dose dependent manner with 3.45% eGFP positive cells at 10 ng/ml TSLP (Fig. 4A,B). In this culture condition without adding TGF-β, iTreg differentiation is very sensitive to TSLP since as low as 0.05 ng/ml TSLP was enough to result in a 50% reduction in Foxp3eGFP+ cells. The number of Foxp3eGFP+ generated in these cultures was also reduced in a dose dependent manner (Fig. 4B). Such inhibitory effect is TSLP-specific, as naïve CD4+ T cells isolated from TSLPR-deficient mice showed normal iTreg induction even in the presence of 20 ng/ml TSLP (Fig. 4C).
Figure 4. Potency of TSLP in inhibiting iTreg generation.
A, TSLP suppressed murine iTreg differentiation in a dose dependent manner. Naïve CD4+ T cells isolated from spleens and lymph nodes of Foxp3eGFP mice were cultured with 5 μg/ml plate-bound anti-CD3 and 1 μg/ml anti-CD28 for 24 hours, removed from stimuli and cultured for another 9 days for Treg generation. TSLP was included in the culture at concentrations as indicated throughout 10 days of culture. Data represent mean ± SEM (n = 3) from one of three independent experiments. B, Both percentage and number of Foxp3eGFP+ cells were reduced in response to increasing dose of TSLP. C, Specificity of TSLP in suppressing iTreg differentiation. Naïve CD4+ T cells were isolated from TSLPR deficient mice and cultured in the conditions as described in (A). No significant inhibition of iTreg differentiation was seen even when 20 ng/ml TSLP was included in the culture.
TSLP can inhibit iTreg induction independent of IL-4 and Stat6
Studies have shown that transgenic expression of TSLP in skin and lung induced strong Th2 inflammation. In vitro experiments demonstrated that TSLP-treated DCs promoted naïve T cells to differentiate into Th2 lineage and produce Th2 cytokines, including IL-4, IL-5 and IL-13 (3, 12). TSLP could also directly exert its effect on CD4+ T cells to enhance their IL-4 and IL-13 secretion without the help of DCs in vivo and in vitro (11, 13). Indeed, in our culture system, TSLP induced IL-4+ Th2 cell differentiation in a dose dependent manner (Fig. 5A). T helper differentiation cytokines IL-4, IL-6, IL-12 and IFN-γ have been demonstrated to suppress the generation of Foxp3-expressing inducible regulatory T cells (35, 38). However, no significant increase of IL-4-producing cells was observed when 1 ng/ml TSLP was included in the culture (Fig. 5A). Thus, TSLP appeared to be more efficient in inhibiting iTreg generation than in promoting Th2 polarization. We superimposed the TSLP dose response curves in inhibiting iTreg generation and in promoting Th2 differentiation (Fig. 5B). As low as 0.05 ng/ml TSLP was sufficient to induce a 50% reduction in Foxp3+ iTreg population while greater than 1 ng/ml TSLP was needed to induce significant IL-4+ Th2 population in the same culture.
Figure 5. TSLP exerts its iTreg inhibiting effect independent of IL-4 and its signaling pathway.
A, TSLP promoted Th2 polarization in a dose dependent manner. Naïve CD4+ T cells were isolated from Foxp3eGFP mice and cultured as described in Figure 3C. After the culture, cells were restimulated and stained for intracellular IL-4 production. iTreg generation (eGFP expression) and Th2 differentiation (IL-4 expression) of the cultured cells were examined by flow cytometry. B, Comparison of dose response curves of TSLP in inhibiting iTreg generation and in promoting Th2 differentiation. Data represent mean ± SEM (n = 3) from one of three independent experiments. C, TSLP suppressed iTreg induction from IL-4- and Stat6-deficient naïve CD4+ T cells with reduced efficiency. Naïve CD4+ T cells were isolated from wild type, Il4−/− and Stat6−/− mice, and cultured as described in Figure 4A.
To further analyze whether TSLP was able to inhibit iTreg induction independent of its Th2 promoting effects, we cultured naïve CD4+ T cells isolated from Il4- or Stat6-deficient mice with or without TSLP. The Foxp3+ iTreg population in wild type CD4+ T cell culture decreased from 14.3% to below 3% in the presence of 10 to 20 ng/ml TSLP. In both Il4−/− and Stat6−/− CD4+ T cell cultures, iTreg population decreased from 16% to approximately 8% in the presence of TSLP (Fig. 5C). Thus, TSLP was still capable of inhibiting Treg generation in the absence of IL-4 or Stat6, though the efficiency was decreased. Taken together, these data indicate that TSLP is able to suppress iTreg induction even at low levels without inducing significant Th2 differentiation. With increasing concentrations, TSLP can further dampen iTreg generation through promoting Th2 polarization.
Presence of TSLP during T cell activation is sufficient to inhibit iTreg generation
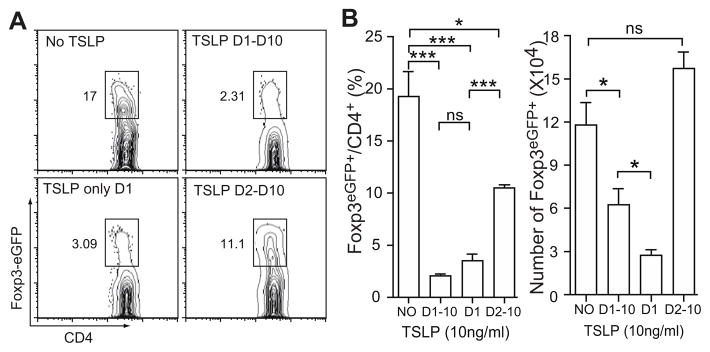
Studies indicated that T cell activation in vivo is likely to occur within 24 hr after antigen administration (36, 37). We next investigated whether TSLP is required throughout the 10-day culture period or only during the 24 hr T cell activation phase. We added TSLP to culture at various time points during and/or after activation and performed FACS analysis after 10 days of culture for Foxp3 expression. As shown in Figure 6A, the presence of TSLP during TCR stimulation on Day 1 was sufficient to suppress Foxp3 expression in those T cells. The inhibitory effect was as efficient as the presence of TSLP throughout the 10-day culture. Adding TSLP after TCR activation from Day 2 to Day 10 resulted in a much weaker suppression on iTreg generation (Fig. 6B). It is known that TSLP promotes both human and mouse CD4+ T cell proliferation and survival (14, 32, 33, 39). We found that cultures with TSLP throughout the 10-day culture and from Day 2 to Day 10 generated significantly more Foxp3eGFP+ cells than cultures with TSLP only on Day 1 (Fig. 6B). These data suggest that TSLP present after CD4+ T cell activation promotes T cell survival and/or proliferation without discriminating regulatory T cells or effector T cells. Therefore, it is critical for TSLP to be present at the time of T cell activation to inhibit iTreg generation.
Figure 6. Presence of TSLP during 24 hr TCR stimulation is critical to inhibit iTreg development.
A, Representative dot plots showing TSLP-mediated inhibition of iTreg differentiation when TSLP was included at different culture stage. B, Percentage and absolute Foxp3eGFP+ cell number in the culture. Naïve CD4+ T cells isolated from Foxp3eGFP mice were culture in a condition similar to that described in Figure 3C except that 10 ng/ml TSLP was included in the culture as indicated on the plots. D1–D10: TSLP was included through 10 day culture; D1: TSLP was included only on day 1 during TCR activation. D2–D10: TSLP was added after TCR activation for the rest of 9 days of culture. Data represent mean ± SEM (n = 3) from one of two independent experiments. *: p < 0.05; ***: p < 0.001; ns: not significant. One-way analysis of variance with Bonferroni’s post-hoc test.
Discussion
Development of tolerance against environmental allergens protects individuals from developing pathological responses and failures in the mechanisms of tolerance lead to the sensitization to allergens and to inflammatory reactions such as allergic asthma. The induction of adaptive Foxp3+ iTreg cells is essential to establish mucosal tolerance by the oral and respiratory routes (20, 21). We report here a novel function of TSLP on inhibiting iTreg induction. It is well established that TSLP promotes Th2 differentiation (2). The fact that low concentration of TSLP significantly inhibits iTreg induction without promoting Th2 development presents a new mechanism for TSLP to regulate the balance of airway tolerance and allergic inflammation.
It is well established that TSLP is a proallergic factor to promote Th2 differentiation by activating DCs (2). Recently, TSLP was also shown to positively affect Treg generation indirectly through its action on dendritic cells (15–19). Such seemingly contradictory results are most likely stem from tissue-specificity of TSLP action. For example, TSLP-activated DCs in thymus (15–17) and intestine (19) positively select Tregs. We report here that TSLP directly suppresses both human and mouse iTreg induction, which is separable from its ability to promote Th2 differentiation (Fig. 5). Our findings are of clinical significance since it has been shown that allergic asthma patients exhibit decreased frequency and diminished suppressive activity of pulmonary Tregs (40). Elevated TSLP in the bronchoalveolar lavage fluid from allergic asthmatic subjects inhibited IL-10 production and suppressive activity of pulmonary Tregs isolated from healthy controls (5). As we showed here that human TSLP inhibited iTreg differentiation (Fig. 3B), the increased pulmonary TSLP in allergic asthmatics could contribute to diminished pulmonary Tregs in these patients.
Recent animal studies suggest that TSLP might be the underlying factor to drive the “atopic march”, the progression from atopic dermatitis to asthma (41, 42). The keratinocyte derived systemic TSLP in these mice, but not the skin lesions, was shown to be responsible for augmenting allergic airway inflammation after the mice were sensitized with OVA + Alum and intranasally challenged with OVA (41, 42). Such augmentation might be attributed to the ability of TSLP to promote Th2 response. However, it was unclear how elevated TSLP at the time of sensitization still aggravated allergic airway inflammation and AHR in mice challenged 50 days later when TSLP levels were returned to normal (42). Our finding that TSLP even in low amounts suppressed iTreg induction during sensitization offers a possible explanation. Along with inducing Th2 priming, OVA + Alum sensitization also induced antigen-specific iTregs (20, 43). The antigen-specific iTregs accumulated in the inflamed lung after OVA challenge and helped to control the severity of inflammation (20). Elevated serum TSLP ranging from 50 pg/ml to 1.5 ng/ml was detected in the mice with severe atopic dermatitis (41, 42). While TSLP might not be necessary for promoting Th2 priming since these mice were sensitized with the strong Th2 polarizing adjuvant Alum, elevated serum TSLP at sensitization would suppress the induction of antigen-specific iTregs. When the animals were challenged, there would be fewer OVA-specific iTregs accumulated in the lung, resulting in severer airway inflammation.
Where TSLP would act to inhibit iTreg generation during natural allergen exposure is still unclear. TSLP is an epithelia-derived cytokine and is upregulated in skin and lung of atopic dermatitis and asthma patients (3–5). The ability of TSLP exposure within the first 24 h to profoundly inhibit subsequent Treg development suggests it may function at sites of priming. TSLP might function in lymph nodes, where Tslp mRNA has been detected (44). TSLP may also function systemically, following local production and release into the circulation. Epidermal keratinocytes in mice with severe AD-like skin inflammation expressed high TSLP leading to increased serum concentration of the cytokine (41, 42). More importantly, recent data showed that serum TSLP levels in children with AD were significantly higher than normal controls (22). The increased systemic TSLP, even at a low level that is unable to promote Th2 differentiation, could inhibit the induction of antigen-specific iTreg, altering mucosal tolerance against harmless antigens, and rendering these individuals more susceptible to the development of Th2 dominated immune responses upon subsequent allergen exposure.
Abbreviations used
- AD
atopic dermatitis
- AHR
airway hyperresponsiveness
- BAL
bronchoalveolar lavage
- DC
dendritic cell
- iTreg
inducible regulatory T cells
- OVA
ovalbumin
- PBMC
peripheral blood mononuclear cells
- TSLP
thymic stromal lymphopoietin
Footnotes
This work was supported by grants R21 AI072617, R01 AI085046 and Showalter Trust Fund to BZ, and U19 AI070448 to MHK.
References
- 1.Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat Immunol. 2002;3:715–720. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- 2.Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt Rde W, Omori M, Zhou B, Ziegler SF. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 3.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, de Waal-Malefyt Rd R, Bazan F, Kastelein RA, Liu YJ. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 4.Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, Corrigan C. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen KD, Vanichsarn C, Nadeau KC. TSLP directly impairs pulmonary Treg function: association with aberrant tolerogenic immunity in asthmatic airway. Allergy Asthma Clin Immunol. 2010;6:4. doi: 10.1186/1710-1492-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ying S, O’Connor B, Ratoff J, Meng Q, Fang C, Cousins D, Zhang G, Gu S, Gao Z, Shamji B, Edwards MJ, Lee TH, Corrigan CJ. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181:2790–2798. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- 7.Zhou B, Comeau MR, Smedt TD, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 8.Li M, Messaddeq N, Teletin M, Pasquali JL, Metzger D, Chambon P. Retinoid X receptor ablation in adult mouse keratinocytes generates an atopic dermatitis triggered by thymic stromal lymphopoietin. Proc Natl Acad Sci U S A. 2005;102:14795–14800. doi: 10.1073/pnas.0507385102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, Comeau MR, Campbell DJ, Ziegler SF. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He R, Oyoshi MK, Garibyan L, Kumar L, Ziegler SF, Geha RS. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc Natl Acad Sci U S A. 2008;105:11875–11880. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007;178:1396–1404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- 14.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe N, Wang YH, Lee HK, Ito T, Wang YH, Cao W, Liu YJ. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 16.Hanabuchi S, Ito T, Park WR, Watanabe N, Shaw JL, Roman E, Arima K, Wang YH, Voo KS, Cao W, Liu YJ. Thymic stromal lymphopoietin-activated plasmacytoid dendritic cells induce the generation of FOXP3+ regulatory T cells in human thymus. J Immunol. 2010;184:2999–3007. doi: 10.4049/jimmunol.0804106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Q, Su H, Knudsen G, Helms W, Su L. Delayed functional maturation of natural regulatory T cells in the medulla of postnatal thymus: role of TSLP. BMC Immunol. 2006;7:6. doi: 10.1186/1471-2172-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Besin G, Gaudreau S, Menard M, Guindi C, Dupuis G, Amrani A. Thymic stromal lymphopoietin and thymic stromal lymphopoietin-conditioned dendritic cells induce regulatory T-cell differentiation and protection of NOD mice against diabetes. Diabetes. 2008;57:2107–2117. doi: 10.2337/db08-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iliev ID, Spadoni I, Mileti E, Matteoli G, Sonzogni A, Sampietro GM, Foschi D, Caprioli F, Viale G, Rescigno M. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut. 2009;58:1481–1489. doi: 10.1136/gut.2008.175166. [DOI] [PubMed] [Google Scholar]
- 20.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Lee EB, Kim KW, Hong JY, Jee HM, Sohn MH, Kim KE. Increased serum thymic stromal lymphopoietin in children with atopic dermatitis. Pediatr Allergy Immunol. 2010;21:e457–460. doi: 10.1111/j.1399-3038.2009.00919.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 24.Zhou B, Headley MB, Aye T, Tocker J, Comeau MR, Ziegler SF. Reversal of Thymic Stromal Lymphopoietin-Induced Airway Inflammation through Inhibition of Th2 Responses. J Immunol. 2008;181:6557–6562. doi: 10.4049/jimmunol.181.9.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fantini MC, Dominitzki S, Rizzo A, Neurath MF, Becker C. In vitro generation of CD4+ CD25+ regulatory cells from murine naive T cells. Nat Protoc. 2007;2:1789–1794. doi: 10.1038/nprot.2007.258. [DOI] [PubMed] [Google Scholar]
- 26.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker MR, Carson BD, Nepom GT, Ziegler SF, Buckner JH. De novo generation of antigen-specific CD4+CD25+ regulatory T cells from human CD4+CD25− cells. Proc Natl Acad Sci U S A. 2005;102:4103–4108. doi: 10.1073/pnas.0407691102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci U S A. 2006;103:11736–11741. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demehri S, Liu Z, Lee J, Lin MH, Crosby SD, Roberts CJ, Grigsby PW, Miner JH, Farr AG, Kopan R. Notch-deficient skin induces a lethal systemic B-lymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity. PLoS Biol. 2008;6:e123. doi: 10.1371/journal.pbio.0060123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Headley MB, Zhou B, Shih WX, Aye T, Comeau MR, Ziegler SF. TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses. J Immunol. 2009;182:1641–1647. doi: 10.4049/jimmunol.182.3.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan W, So T, Croft M. Antagonism of airway tolerance by endotoxin/lipopolysaccharide through promoting OX40L and suppressing antigen-specific Foxp3+ T regulatory cells. J Immunol. 2008;181:8650–8659. doi: 10.4049/jimmunol.181.12.8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu N, Wang YH, Arima K, Hanabuchi S, Liu YJ. TSLP and IL-7 use two different mechanisms to regulate human CD4+ T cell homeostasis. J Exp Med. 2009;206:2111–2119. doi: 10.1084/jem.20090153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rochman I, Watanabe N, Arima K, Liu YJ, Leonard WJ. Cutting Edge: Direct Action of Thymic Stromal Lymphopoietin on Activated Human CD4+ T Cells. J Immunol. 2007;178:6720–6724. doi: 10.4049/jimmunol.178.11.6720. [DOI] [PubMed] [Google Scholar]
- 34.Rochman Y, Kashyap M, Robinson GW, Sakamoto K, Gomez-Rodriguez J, Wagner KU, Leonard WJ. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc Natl Acad Sci U S A. 2010;107:19455–19460. doi: 10.1073/pnas.1008271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2007;104:18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shakhar G, Lindquist RL, Skokos D, Dudziak D, Huang JH, Nussenzweig MC, Dustin ML. Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat Immunol. 2005;6:707–714. doi: 10.1038/ni1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 38.O’Malley JT, Sehra S, Thieu VT, Yu Q, Chang HC, Stritesky GL, Nguyen ET, Mathur AN, Levy DE, Kaplan MH. Signal transducer and activator of transcription 4 limits the development of adaptive regulatory T cells. Immunology. 2009;127:587–595. doi: 10.1111/j.1365-2567.2008.03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Shami A, Spolski R, Kelly J, Fry T, Schwartzberg PL, Pandey A, Mackall CL, Leonard WJ. A role for thymic stromal lymphopoietin in CD4(+) T cell development. J Exp Med. 2004;200:159–168. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartl D, Koller B, Mehlhorn AT, Reinhardt D, Nicolai T, Schendel DJ, Griese M, Krauss-Etschmann S. Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J Allergy Clin Immunol. 2007;119:1258–1266. doi: 10.1016/j.jaci.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 41.Demehri S, Morimoto M, Holtzman MJ, Kopan R. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067. doi: 10.1371/journal.pbio.1000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Z, Hener P, Frossard N, Kato S, Metzger D, Li M, Chambon P. Thymic stromal lymphopoietin overproduced by keratinocytes in mouse skin aggravates experimental asthma. Proc Natl Acad Sci U S A. 2009;106:1536–1541. doi: 10.1073/pnas.0812668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sims JE, Williams DE, Morrissey PJ, Garka K, Foxworthe D, Price V, Friend SL, Farr A, Bedell MA, Jenkins NA, Copeland NG, Grabstein K, Paxton RJ. Molecular cloning and biological characterization of a novel murine lymphoid growth factor. J Exp Med. 2000;192:671–680. doi: 10.1084/jem.192.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]