Abstract
Evidence suggests that focus of attention and cognitive load may each affect emotional processing and that individual differences in anxiety moderate such effects. We examined (a) fear-potentiated startle (FPS) under threat-focused (TF), low-load/alternative-set (LL/AS), and high-load/alternative-set (HL/AS) conditions and (b) the moderating effect of trait anxiety on FPS across these conditions. As predicted, redirecting attentional focus away from threat cues and increasing cognitive load reduced FPS. However, the moderating effects of anxiety were specific to the LL/AS condition. Whereas FPS was comparable for high-anxiety and low-anxiety subjects in the TF and HL/AS conditions, FPS was significantly greater for high-anxiety than for low-anxiety subjects in the LL/AS condition. These results suggest that affective processing requires attentional resources and that exaggerated threat processing in anxious individuals relates to direction of attention rather than emotional reactivity per se.
It has long been recognized that processing of emotional stimuli can occur in the absence of selective attention to these stimuli (Esteves & Öhman, 1993). Indeed, a large corpus of evidence demonstrates that emotional stimuli can elicit fast, involuntary autonomic responses (Globisch, Hamm, & Esteves, 1999) that take place outside conscious awareness (Esteves & Öhman, 1993; Maxwell & Davidson, 2004) and guide decision-making processes (Bechara, Damasio, & Damasio, 2000; Damasio, Tranel, & Damasio, 1991). However, neuroscience has also demonstrated that the subcortical neural systems responsible for establishing and maintaining emotional responses do not operate in isolation, but interact with higher-order brain regions (Curtin, Patrick, Lang, Cacioppo, & Birbaumer, 2001; Lang, 1995) and require some amount of cognitive resources (Pessoa, McKenna, Gutierrez, & Ungerleider, 2002). Indeed, researchers (Simpson, Snyder, Gusnard, & Raichle, 2001a, 2001b; Pessoa et al., 2002) have argued that attenuation of an emotional response during increasing levels of cognitive load provides a compelling illustration of this point.
The amygdala appears to play a critical role in the processing of emotional information (Davis & Whalen, 2001; LeDoux, 1995). In particular, there is compelling evidence that the amygdala is involved in the recognition of cues predicting threat, as well as the conditioning of stimulus-reinforcement contingencies (LeDoux, 1996). Moreover, research using positron emission tomography shows that higher levels of dispositional negative affect are associated with greater metabolic rate in the right amygdala (Abercrombie, Schaefer, & Larson, 1998). With regard to the automaticity of emotion, Whalen et al. (1998) reported that amygdala activation in response to emotional stimuli may occur without conscious awareness.
The fact that the amygdala is activated in the absence of conscious awareness does not negate the possibility that activation of the amygdala can be moderated by higher-order cognitive processes. In fact, research has demonstrated deactivation or suppression of the amygdala in tasks that involve higher-order cognitive processing (Drevets & Raichle, 1998). Research also suggests that amygdala-mediated emotional processes may be regulated by higher-order processes such as effortful evaluation and appraisal (Hariri, Mattay, Tessitore, Fera, & Weinberger, 2003), and may be eliminated when attention is directed to another task (Pessoa et al., 2002). Such findings highlight the importance of understanding the interaction between cognitive and affective processes.
Though evidence suggests that cognitive processes may interfere with or diminish emotion processing, the necessary and sufficient conditions for observing such effects are unclear. Two relevant considerations involve direction of attention and cognitive load. For example, Pessoa et al. (2002) employed an experimental manipulation that focused both spatial and object-based attention away from negative affective stimuli and observed significantly reduced amygdala responding. However, other studies that manipulated spatial (e.g., Vuilleumier, Armony, Driver, & Dolan, 2001) or object-based (Anderson & Phelps, 2001) attention failed to alter amygdala activation significantly. Such findings raise the possibility that task difficulty or cognitive load, as opposed to direction of attention alone, is the crucial variable that constrains processing of peripheral affective stimuli. Indeed, there is compelling evidence that experimental manipulations that tax cognitive resources effectively reduce amygdala activation (Pessoa, Padmala, & Morlan, in press). Thus, the first goal of this study was to clarify whether an experimental manipulation that directs attention away from the affective stimuli without taxing cognitive resources is sufficient to diminish processing of emotion or whether such effects depend on the extent to which a task diminishes cognitive resources.
Much of the research on the cognitive modulation of emotional responding has focused on amygdala activation as the primary dependent measure. Although this research has demonstrated cognitive or attentional modulation of amygdalar activation evoked by emotional or threatening cues, it has not demonstrated that such modulation actually leads to changes in affective response. Substantial evidence indicates that fear-potentiated startle (FPS) is a sensitive and specific index of fear responding (Lang, 1995). For example, the startle response in humans is potentiated during viewing of photographic images with negative valence (Vrana, Spence, & Lang, 1988), during negative emotional imagery (Cook, Davis, Hawk, Spence, & Gautier, 1992), and during the anticipation of electric shock (Curtin et al., 2001; Grillon, Ameli, & Foot, 1993). Moreover, in both animals and humans, FPS is reduced by anxiolytic drugs (Curtin et al., 2001; Curtin, Lang, Patrick, & Stritzke, 1998; Patrick, Berthot, & Moore, 1996) and enhanced by anxiogenics (Davis, Walker, & Lee, 1999). Finally, FPS during processing of threat cues is mediated by the amygdala (Davis et al., 1999). Given the evidence demonstrating cognitive or attentional moderation of amygdala activation and the established link between the amygdala and FPS, one might assume that cognitive or attentional load also moderates FPS. This study tested this assumption in an attempt to form a bridge between the literature on cognitive and attentional moderation of amygdala activation and other relevant research using FPS to more directly index affective responding.
The second goal of this study was to evaluate the role of individual differences in the predicted cognitive-affective interaction. Specifically, this study was designed to examine the effects of trait anxiety on the cognitive or attentional moderation of FPS. The magnitude of FPS appears to vary as a function of trait fearfulness (Cook et al., 1992; Cook, Hawk, Davis, & Stevenson, 1991), yet research has failed to find a similar effect of trait anxiety (Cook et al., 1991; Grillon et al., 1993; Nitschke, Larson, & Smoller, 2002). However, these studies have not investigated FPS response to threat cues as a function of cognitive task demands. Consequently, the absence of differences in FPS between high-anxiety and low-anxiety subjects may reflect the fact that all subjects allocate sufficient attention to the processing of threat when no additional demands are placed on attentional or cognitive resources. In contrast, some investigators have posited an association between trait anxiety and sustained vigilance for threat processing (Calvo & Eysenck, 2000). If individual differences in anxiety affect the maintenance or persistence of threat processing under cognitive or attentional constraints, these individual differences may be observed in the laboratory only if cognitive task demands are explicitly manipulated. Specifically, high-anxiety individuals may process threat cues longer than low-anxiety subjects as attention or cognitive demands increase. The second goal of this experiment was to evaluate this prediction.
We assessed subjects’ fear response, as indexed by FPS, in three conditions to examine the possible influence of trait anxiety and cognitive-attentional demands. In the threat-focus (TF) condition, subjects’ task explicitly required attention to the threat cues. In the low-load/alternative-set (LL/AS) condition, subjects’ task required focusing attention on threat-irrelevant information but placed minimal demands on working memory resources. In the high-load/alternative-set (HL/AS) condition, subjects’ task again required focusing attention on threat-irrelevant information, but also involved working memory so that a high cognitive load was imposed (Jonides, Schumacher, Smith, & Lauber, 1997).
With respect to the first goal of this experiment, if increased cognitive (working memory) load is necessary to modulate emotion responding, FPS would be reduced in the high-load (HL/AS) condition relative to the other two, low-load conditions (LL/AS and TF). However, if manipulating the direction of attention away from the threat stimulus is sufficient to modulate emotional responding, FPS would be reduced during the LL/AS condition as well. With respect to the second goal of this experiment, we predicted that trait anxiety would moderate the effects of load on emotional processing. Specifically, we hypothesized that individual differences in trait anxiety exert their influence only when demands are placed on attention or working memory resources, and therefore that the effects of trait anxiety on FPS would be observed in the HL/AS and LL/AS conditions, but not the TF condition (i.e., we predicted a Trait Anxiety × Condition interaction).
METHOD
Subjects were 39 right-handed undergraduates (17 female) between the ages of 17 and 21. They were divided into high- and low-anxiety groups using a median split on the Welsh Anxiety Scale (α = .86; median = 10; range = 0–33; Welsh, 1956).1 All procedures were compliant with guidelines for human subjects.
During the task, subjects viewed a series of letter cues, each presented for 500 ms, with a variable intertrial interval of 3 or 4 s. The letter cues were either upper- or lowercase and colored red or green. Subjects were instructed that in all three conditions, electric shocks would be administered on some trials following red letters (CUE+), but that no shocks would follow green letters (CUE−). In fact, electric shocks 200 ms in duration were administered to adjacent fingers on subjects’ left hands 1,750 s after cue onset on 20% of CUE+ trials in each condition, for a total of 30 shocks (10 per condition). Given the results from previous research demonstrating that the color of the letter cue for CUE+ trials has no effect on the intensity of FPS (see Curtin et al., 1998), the color connoting shock was not counterbalanced. Prior to the start of the experimental task, electrodes were attached to the subjects’ left hands, and subjects performed a shock sensitivity task in which they were instructed to use a scale from 0 to 100 to rate a number of shocks that increased linearly in severity. Shock intensity for the experimental task was calibrated to 75% of each subject’s shock tolerance threshold, which was determined as the midpoint between the ratings of 50 (uncomfortable) and 100 (maximum tolerable threshold) on the shock sensitivity task.2
For the TF condition, subjects were instructed to attend to the color of the letter cue on each trial and to press one of two buttons using their right hand to indicate the color. (Given that responses were made with the right hand in all conditions, the buttons indicating specific responses were not counterbalanced across conditions.) To ensure that subjects were sufficiently motivated to perform the task, we informed them that speed and accuracy would influence the number of shocks they received. This condition was designed to focus subjects on the feature of the letter cue (i.e., color) that connoted threat of shock.
For the LL/AS condition, subjects were instructed to attend to the case of the letter cue on each trial and to press one of the two buttons to indicate if the letter cue was upper- or lowercase. Thus, the color of the letter was not part of the feature set relevant to performing this simple task.3 For the HL/AS condition, subjects were instructed to attend to the identity (e.g., c, f, r) of each letter cue in the series and to press one of the two buttons to indicate whether or not the identity of the current letter matched the identity of the letter presented two trials back (i.e., a 2-back task). As in the LL/AS condition, the color of the letter was not part of the feature set relevant to performing the task. Moreover, other research with this 2-back task has confirmed that it places substantially increased demand on working memory and its neurobiological substrates relative to simpler identification tasks, such as those used in the LL/AS condition (Jonides et al., 1997). To ensure sufficient motivation in the LL/AS and HL/AS conditions, we informed subjects that speed and accuracy would influence their likelihood of receiving a reward (i.e., one of three prizes). In both these conditions, as in the TF condition, subjects were reminded that electric shocks would be administered on some trials following red letter cues, but that no shocks would follow green letters.
Letter cues were grouped into six task blocks of 50 trials. Subjects performed two consecutive blocks of each of the three tasks, and task order was counterbalanced across subjects.
Each of 48 startle-eliciting noise probes (50-ms, 102-dB white-noise bursts with nearly instantaneous rise time) was presented 1,750 ms after the onset of a cue. The noise probes were equally distributed across CUE+ and CUE− trials in all three task conditions, so that each subject experienced 16 startles (8 CUE+ and 8 CUE−) per condition. The time between startles in each condition averaged 27 s, with a minimum of 14 s and a maximum of 61 s. In addition, probes never occurred on the same trial as shock administration. Startle eyeblink electromyogram activity was sampled at 2000 Hz from electrodes under the right eye, band-pass filtered (30–500 Hz, 24 dB/octave roll off), smoothed (rectified, then low-pass filtered at 30 Hz, 24 dB/octave), and baseline corrected. Startle blink magnitude was scored as the peak response between 20 and 120 ms after probe onset. Fear response to threat cues was indexed by FPS, defined as the difference in blink-response magnitude to probes following CUE+ versus CUE− letters, in each of the three task conditions.
Following the completion of the experimental task, shock electrodes and measurement sensors were removed, and subjects completed our measure of anxiety, which was embedded within a battery of electronically administered questionnaires.
RESULTS
FPS
FPS was analyzed with a Trait Anxiety (low vs. high) × Condition (TF vs. LL/AS vs. HL/AS) multivariate repeated measures analysis of variance.4 A main effect of condition was observed, F(2, 36) = 11.61, p < .001, η2 = .39, indicating that FPS was linearly reduced from the TF to the LL/AS to the HL/AS condition. Orthogonal contrasts indicated that FPS was significantly lower in the LL/AS than in the TF condition, F(1, 37) = 9.65, p = .004, prep = .97, and was also significantly lower in the HL/AS than in the LL/AS condition, F(1, 37) = 8.21, p = .007, prep = .96. Furthermore, one-sample t tests revealed that FPS was significantly different from 0 for the TF (p < .001, prep > .999) and LL/AS (p < .001, prep = .999) conditions, but not the HL/AS condition (p = .073).
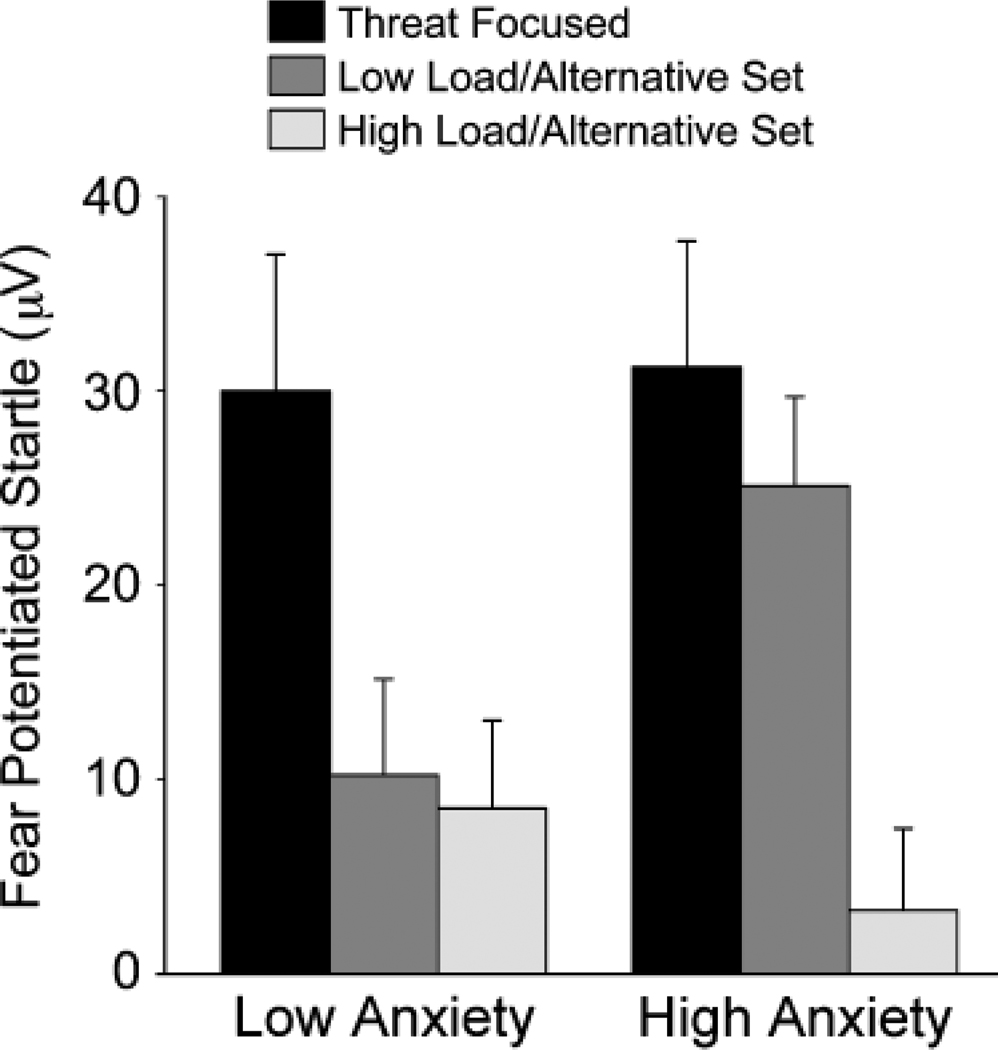
In addition, the main effect of condition was significantly moderated by trait anxiety, F(1, 36) = 3.45, p = .043, prep = .888, η2 = .16 (Fig. 1). Simple-effect tests revealed no significant effects of trait anxiety in the TF (p = .898) or HL/AS (p = .403) conditions. In contrast, a significant simple effect of trait anxiety was observed in the LL/AS condition (p =.034, prep = .9016), with high-anxiety subjects displaying greater FPS than low-anxiety subjects.
Fig. 1.
Fear-potentiated startle as a function of trait-anxiety group and task condition (threat-focused, low-load/alternative-set, and high-load/alternative-set).
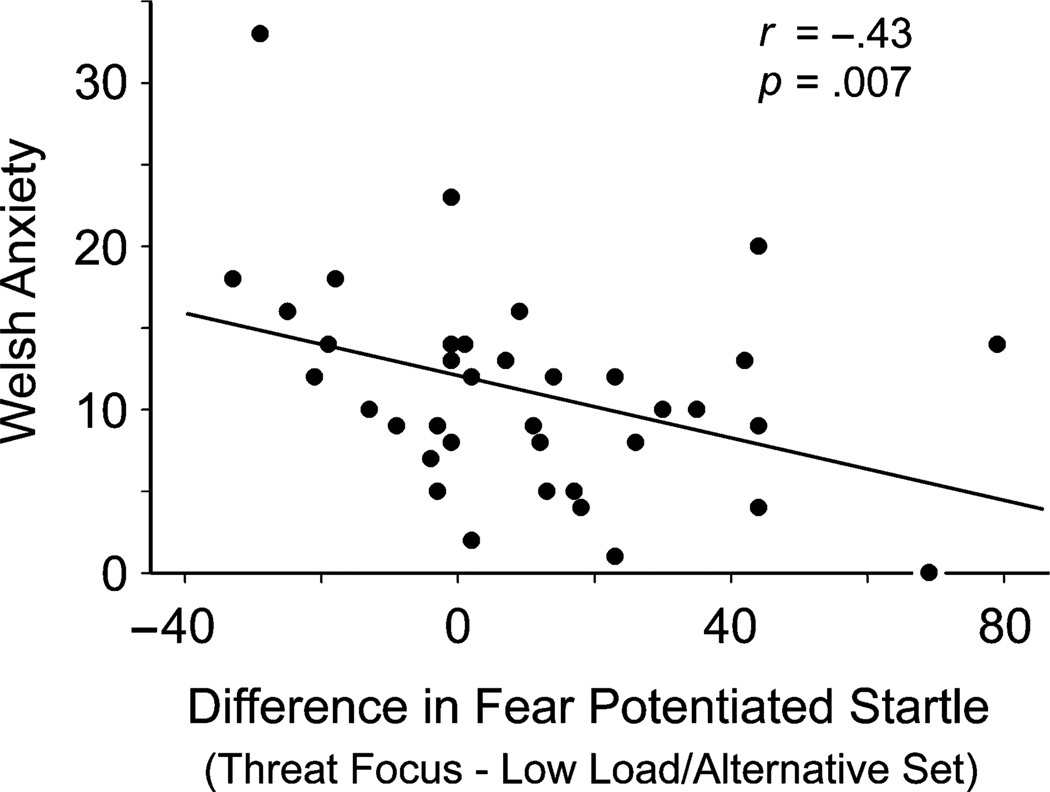
To examine further the impact of individual differences in trait anxiety on fear responding, we correlated raw Welsh anxiety scores with the difference in FPS between the TF and LL/AS condition. Welsh anxiety scores were negatively correlated (r = −.43, p = .007, prep = .96; Fig. 2) with the decrease in FPS from the TF to the LL/AS condition, indicating that as trait anxiety increased, subjects displayed less reduction in FPS when focused on task-relevant but threat-irrelevant features (i.e., case) of the letter cues. In contrast, no significant correlation was observed between Welsh anxiety scores and the decrease in FPS between the TF and HL/AS conditions (r = .09, p = .583).
Fig. 2.
Association between Welsh anxiety scores and the difference in fear-potentiated startle between the threat-focused and the low-load/alternative-set conditions.
Response Time and Accuracy
Response time and accuracy were analyzed separately with Trait Anxiety (low vs. high) × Condition (TF vs. LL/AS vs. HL/AS) × Cue Type (CUE+ vs. CUE−) multivariate repeated measures analyses of variance. For response time, a main effect of condition was observed, F(2, 37) = 126.49, p < .001, η2 = .87, indicating that response time increased from the TF (M = 531.9 ms, SD = 12.94) to the LL/AS (M = 613.2 ms, SD = 15.5) to the HL/AS (M = 843.27 ms, SD = 32.95) condition. A main effect of cue type was also observed, F(1, 38) = 12.22, p < .001, prep = .98, η2 = .24, indicating that subjects were quicker to respond to CUE+ trials (M = 654.8 ms, SD = 18.42) than to CUE− trials (M = 670.78 ms, SD = 18.28). No main effect of trait anxiety was observed, and trait anxiety was not involved in any significant interactions.
For accuracy, a main effect of condition was observed, F(2, 38) = 23.59, p < .001, η2 = .55; the number of errors was equivalent in the TF (M = 2.85, SD = 1.14) and LL/AS (M = 2.51, SD = 1.963) conditions, but increased in the HL/AS (M = 10.69, SD = 1.279) condition. A main effect of cue type was also observed, F(1, 39) = 7.7, p = .008, prep = .9557, η2 = .17, indicating that subjects made more errors in CUE− trials (M = 5.67, SD = 0.66) than in CUE+ trials (M = 5.01, SD = 0.63). As was the case for response time, trait anxiety did not have a main effect and was not involved in any significant interactions.
DISCUSSION
To our knowledge, this experiment provides the first direct demonstration that FPS to threat cues is moderated independently by working memory load and focus of attention. Specifically, the load manipulations used in this study significantly attenuated FPS in the LL/AS condition relative to the TF condition, and in the HL/AS condition relative to the LL/AS and TF conditions. Consistent with the results of previous studies, this finding suggests that amygdala-mediated affective processing is not completely automatic and requires attentional resources (Pessoa et al., 2002).
As reviewed earlier, Pessoa et al. (2002) found that a shift in attentional focus effectively reduced differential amygdala activation to emotional stimuli. Furthermore, research demonstrates that amygdala activation varies as a function of cognitive load (Pessoa et al., in press). Our study is consistent with these results and demonstrates that changes in attentional focus and cognitive load shown to influence activation of the amygdala are paralleled by changes in emotional (i.e., fear) responses. However, other studies in which attentional focus was manipulated without inducing substantial cognitive load have failed to demonstrate these reductions in amygdala activation (e.g., Vuilleumier et al., 2001). In contrast, our redirection of attentional focus was sufficient to reduce emotional responding without substantial cognitive load. Given the interaction between our attentional-focus manipulation and trait anxiety, it is possible that these discrepant findings reflect differences in the samples’ composition, particularly differences related to anxiety. Alternatively, it is possible that previous studies encouraged subjects to divide attention and maintain processing of the affective stimuli.
Past research provides little or no support for the intuitive connection between threat processing and trait anxiety (Cook et al., 1991; Grillon et al., 1993; Nitschke et al., 2002). To the extent that the conditions used in previous investigations of threat sensitivity in trait-anxious individuals resemble our TF condition, as appears to be the case, the failure of those studies to find significant associations is no longer surprising. The association between trait anxiety and FPS was found only in an experimental condition that both directed attention away from threat cues and did not exhaust cognitive capacity. Hence, this study has also served to clarify the conditions under which trait anxiety and threat processing are related and, thus, the nature of trait anxiety.
Several interpretations of our findings for trait anxiety are possible. High-anxiety individuals may have a deliberate attentional bias to process threat information and may thus persist in processing threat cues even when task demands require a redirection of attentional focus. To the extent that high-anxiety subjects in the LL/AS condition were more likely than low-anxiety subjects to divide attention between task-relevant stimulus features (letter case) and task-irrelevant threat information (letter color), they would have displayed more robust fear responses. However, the relatively high working memory load in the HL/AS condition would have interfered with this strategy of dividing attention and thus would have limited threat processing in high-anxiety as well as low-anxiety subjects.
Alternatively, high anxiety may increase threat reactivity, which, in effect, makes threat stimuli more salient. To the extent that threat stimuli are more salient for high-anxiety than for low-anxiety individuals, high-anxiety individuals would find such stimuli more difficult to ignore. Thus, threat stimuli might continue to influence emotion processing in high-anxiety individuals unless their cognitive capacity is essentially exhausted. This heightened-reactivity interpretation, however, is substantially undermined by the fact that high-anxiety subjects did not show exaggerated fear responses in the TF condition. Moreover, to the extent that threat cues are more salient for high-anxiety than for low-anxiety individuals, it follows that high-anxiety subjects would have displayed stronger fear responses than low-anxiety subjects even in the HL/AS condition, but they did not. Although proponents of a heightened-reactivity interpretation might suggest that the absence of group differences in the HL/AS condition reflects a floor effect, this interpretation is undermined by the fact that comparable variability in FPS was observed across all three conditions.
A final possibility relates to flexibility of attention, as opposed to attentional bias or threat reactivity per se. According to Gray and McNaughton (2000), high anxiety reflects the strength of a physiological system that monitors the environment for potentially relevant information (e.g., threat cues) when people are engaged in goal-directed behavior and facilitates a redirection of attention in response to such information. According to Newman and his colleagues, the calls for attention that initiate such reorienting are relatively automatic, but answering calls for processing relies on capacity-limited resources (Newman, MacCoon, Hiatt, Bertsch, & Buckholtz, in press; Patterson & Newman, 1993). Thus, the significant group difference observed in the LL/AS condition may reflect the fact that high-anxiety individuals are more strongly predisposed to reorient attention to potential threat cues than are low-anxiety individuals, and the absence of a group difference in the HL/AS condition may reflect the fact that high working memory load precluded answering the call for processing.
Given the emphasis that Gray and McNaughton (2000) placed on goal-directed behavior as a necessary condition for revealing anxiety-related differences in threat processing, it is noteworthy that we deliberately reinforced the focus of goal-directed behavior in the LL/AS and HL/AS conditions by informing subjects that the “amount of reward that you earn depends on the speed and accuracy of your responses.” Thus, their strategy in this condition may have been inherently different from their strategy in the TF condition. The fact that our alternative-set manipulation was combined with this instructional manipulation may, therefore, be important for fully understanding the significance of the alternative-set manipulation used in this study, as well as its interaction with trait anxiety.
In summary, this experiment advances understanding of cognitive-emotional interactions in three important ways. First, it clearly demonstrates that both redirection of attentional focus and working memory load can reduce fear response. Second, this experiment substantiates the claim that cognitive-emotional interactions are moderated by individual differences. Whereas redirection of attention was sufficient to curtail threat processing in low-anxiety individuals, it appears that high trait anxiety was associated with the persistence of threat processing unless working memory resources were exhausted. Finally, the fact that these results were obtained using FPS rather than amygdala activation as the primary index of fear responding strengthens confidence that the recent demonstrations that cognitive load moderates amygdala activation (e.g., Pessoa et al., 2002, in press) are directly relevant to emotional responding, rather than other functions of the amygdala. More generally, these findings clarify the circumstances under which the processing of emotion stimuli is privileged (Davis & Whalen, 2001).
Acknowledgments
Research described in this manuscript was supported by a grant from the National Institute of Mental Health (MH53041). We greatly appreciate Alex Shackman’s insightful contributions to the preparation of this manuscript. We also thank Josh Zeier, Samantha Glass, Kristina Hiatt, and Donal MacCoon for their feedback and suggestions regarding the manuscript.
Footnotes
Spielberger trait-anxiety scores were also available for a subset of subjects (n = 30). These scores were significantly and strongly correlated with Welsh anxiety scores (r = .74, p < .001). When analyses were conducted using Spielberger instead of Welsh anxiety scores, results were comparable to those reported here, though not significant, owing to the reduced number of subjects and associated loss of power.
Shock intensity levels were recorded on a 255 (8-bit) intensity scale, condensed to 25 possible levels of shock. Analyses of the shock intensity (75% tolerance threshold) across gender and anxiety groups revealed that there were no group differences, F(1, 39) < 1.
In the LL/AS condition, our intention was to simply direct attention away from the threat cues. However, we recognize that the task may also have entailed a low level of cognitive load; therefore, we refer to this as a low-load condition.
Preliminary analysis of the startle response (rather than FPS) revealed no significant effects for condition, indicating that the average startle magnitude was comparable across all three conditions. Initial analysis of FPS also revealed no significant effects for order of the tasks or for gender. Therefore, neither order nor gender was included as a factor in the analyses reported here.
REFERENCES
- Abercrombie HC, Schaefer SM, Larson CL. Metabolic rate in the right amygdala predicts negative affect in depressed patients. NeuroReport. 1998;9:3301–3307. doi: 10.1097/00001756-199810050-00028. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Calvo MG, Eysenck MW. Early vigilance and late avoidance of threat processing: Repressive coping versus low/ high anxiety. Cognition & Emotion. 2000;14:763–787. [Google Scholar]
- Cook EW, Davis TL, Hawk LW, Spence EL, Gautier CH. Fearfulness and startle potentiation during aversive visual stimuli. Psychophysiology. 1992;29:633–645. doi: 10.1111/j.1469-8986.1992.tb02038.x. [DOI] [PubMed] [Google Scholar]
- Cook EW, Hawk LW, Davis TL, Stevenson VE. Affective individual differences and startle reflex modulation. Journal of Abnormal Psychology. 1991;100:5–13. doi: 10.1037//0021-843x.100.1.5. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, Lang AR, Patrick CJ, Stritzke WGK. Alcohol and fear-potentiated startle: The role of competing cognitive demands in the stress-reducing effects of intoxication. Journal of Abnormal Psychology. 1998;107:547–565. doi: 10.1037//0021-843x.107.4.547. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, Patrick JP, Lang AL, Cacioppo JT, Birbaumer N. Alcohol affects emotion through cognition. Psychological Science. 2001;12:527–531. doi: 10.1111/1467-9280.00397. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio HC. Somatic markers and the guidance of behavior: Theory and preliminary testing. In: Levin HS, Eisenberg HM, Benton AL, editors. Frontal lobe function and dysfunction. New York: Oxford University Press; 1991. pp. 217–229. [Google Scholar]
- Davis M, Walker DL, Lee Y. Neurophysiology and neuropharmacology of startle and its affective modification. In: Dawson M, Schell A, Bohmelt M, editors. Startle modification: Implications for neuroscience, cognitive science, and clinical science. Cambridge, England: Cambridge University Press; 1999. pp. 95–113. [Google Scholar]
- Davis M, Whalen PJ. The amygdala: Vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cognition & Emotion. 1998;12:353–385. [Google Scholar]
- Esteves F, Öhman A. Masking the face: Recognition of emotion facial expressions as a function of parameters of backward-masking. Scandinavian Journal of Psychology. 1993;34:1–18. doi: 10.1111/j.1467-9450.1993.tb01096.x. [DOI] [PubMed] [Google Scholar]
- Globisch J, Hamm A, Esteves F. Fear appears fast: Temporal course of startle reflex potentiation in animal fearful subjects. Psychophysiology. 1999;36:66–75. doi: 10.1017/s0048577299970634. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety: An enquiry into the function of the septo-hippocampal system. Oxford, England: Oxford University Press; 2000. [Google Scholar]
- Grillon C, Ameli R, Foot M. Fear-potentiated startle: Relationship to the level of state/trait anxiety in healthy subjects. Biological Psychiatry. 1993;33:566–574. doi: 10.1016/0006-3223(93)90094-t. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Lauber EJ. Verbal working memory load affects regional brain activation as measured by PET. Journal of Cognitive Neuroscience. 1997;9:462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The emotion probe: Studies of motivation and attention. American Psychologist. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion: Clues from the brain. Annual Review of Psychology. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. The emotional brain: The mysterious underpinnings of emotional life. New York: Simon & Schuster; 1996. [Google Scholar]
- Maxwell JS, Davidson RJ. Unequally masked: Indexing differences in the perceptual salience of ‘unseen’ facial expressions. Cognition & Emotion. 2004;18:1009–1026. [Google Scholar]
- Newman JP, MacCoon DG, Hiatt KD, Bertsch J, Buckholtz J. Deficient integration of top-down and bottom-up influences on attention in psychopaths: Potential contribution of the septal-hippocampal system. In: Barch D, editor. Cognitive and affective neuroscience of psychopathology. New York: Oxford University Press; in press. [Google Scholar]
- Nitschke JB, Larson CL, Smoller MJ. Startle potentiation in aversive anticipation: Evidence for state but not trait effects. Psychophysiology. 2002;39:254–258. doi: 10.1017/S0048577202010156. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Berthot B, Moore JD. Diazepam blocks fear-potentiated startle in humans. Journal of Abnormal Psychology. 1996;105:89–96. doi: 10.1037//0021-843x.105.1.89. [DOI] [PubMed] [Google Scholar]
- Patterson CM, Newman JP. Reflectivity and learning from aversive events: Toward a psychological mechanism for the syndromes of disinhibition. Psychological Review. 1993;100:716–736. doi: 10.1037/0033-295x.100.4.716. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences, USA. 2002;98:683–687. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Padmala S, Morlan T. Fate of unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. Neuroimaging. doi: 10.1016/j.neuroimage.2005.05.048. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JR, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: I. During cognitive task performance. Proceedings of the National Academy of Sciences, USA. 2001a;98:683–687. doi: 10.1073/pnas.98.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JR, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proceedings of the National Academy of Sciences, USA. 2001b;98:688–693. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrana SR, Spence EL, Lang PJ. The startle probe response: A new measure of emotion? Journal of Abnormal Psychology. 1988;97:487–491. doi: 10.1037//0021-843x.97.4.487. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: An event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Welsh G. Factor dimensions A and R. In: Welsh GS, Dahlstrom WG, editors. Basic readings on the MMPI in psychology and medicine. Minneapolis: University of Minnesota Press; 1956. pp. 264–281. [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]