Abstract
Nicotine functions as a negative feature in a Pavlovian discriminated goal-tracking task. Whether withholding of responding to the conditional stimulus (CS) reflects nicotine functioning as a conditioned inhibitor is unknown. Accordingly, the present research sought to determine whether nicotine trained as a negative feature passed the retardation-of-acquisition and summation tests, thus characterizing it as a pharmacological (interoceptive) conditioned inhibitor. In the retardation test, rats received either nicotine (0.4 mg/kg) or chlordiazepoxide (5 mg/kg) negative feature training in which the drug state signaled when a 15-sec light CS would not be paired with sucrose; light was paired with sucrose on intermixed saline sessions. Following acquisition of the discrimination, both groups received nicotine CS training in which sucrose was intermittently available on nicotine but not intermixed saline sessions. Acquisition of conditioned responding to the nicotine CS was slower in the nicotine negative feature group than in the chlordiazepoxide negative feature group. In the summation test, rats were assigned to either the nicotine negative feature group or a pseudoconditioning control. In this control, the light CS was paired with sucrose on half the nicotine and half the saline sessions. Both groups also received excitatory training in which a white noise CS was paired with sucrose. The summation test consisted of presenting the white noise in conjunction with nicotine. Conditioned responding evoked by the white noise was decreased in the negative feature but not the pseudoconditioning group. Combined, the results provide the first evidence that an interoceptive stimulus (nicotine) can become a conditioned inhibitor.
Learning involving the interoceptive stimuli generated by abused drugs is thought to contribute to the development and persistence of addictions, as well as relapse following abstinence (Bevins and Murray 2011). Over the years, a variety of tasks has been developed to more closely study drug stimuli and learning. For instance, administration of nicotine, considered to be the primary addictive constituent of tobacco products and the drug of primary interest in this report, can serve as a discriminative stimulus for rats in an operant two-lever discrimination task indicating which lever press (right or left) will be reinforced (for reviews, see Smith and Stolerman 2009 and Wooters et al. 2009). More recently, a discriminated goal-tracking task has been developed to study how nicotine (and other drugs) can modulate conditioned responding to a discrete conditioned stimulus (CS) in rats. Goal-tracking in this case refers to a rat's tendency to approach a place where an appetitive stimulus (reward) has occurred in the past (e.g., a liquid sucrose receptacle) (Boakes 1977; Farwell and Ayres 1979). This task differs in several ways from the operant two-lever tasks (cf. Bevins 2009). Most notable is that there is no experimenter-imposed schedule of reinforcement; that is, the reinforcer (sucrose) is delivered independent of the rat's behavior.
As a feature positive modulator, nicotine in this discriminated goal-tracking task occasions when illumination of a light CS will be paired with access to a liquid sucrose unconditioned stimulus (US); placebo (saline) indicates non-reinforced presentations of light. Notably, this interoceptive drug modulator shares processes in common with discrete exteroceptive feature positive modulators (cf. Holland 1995). For example, non-reinforced presentation of the drug feature (i.e., extinction) does not eliminate its modulation of a goal-tracking response to the CS (Palmatier and Bevins 2007). Further, a positive nicotine feature can transfer its modulatory control to a separate stimulus such as a white noise CS that was trained separately with the unrelated chlordiazepoxide drug state as the feature (Palmatier and Bevins 2008).
Far less is known about the behavioral processes involved with nicotine as a negative feature. To date, there has been only one published demonstration that nicotine can signal that a discrete light CS will not be paired with the sucrose US in this discriminated goal-tracking task (Bevins et al. 2006). In that experiment, the light CS evoked a goal-tracking conditioned response (CR) on saline sessions; on nicotine sessions, rats withheld the goal-tracking response to the light CS. Although others have reported examples of response inhibition with negative drug features in several different tasks (e.g., Jaeger and Mucha 1990; Troisi and Akins 2004), no study has examined whether a negative drug feature will pass the two standard tests of conditioned inhibition: (1) the retardation-of-acquisition test, in which prior inhibitory training slows subsequent excitatory conditioning, and (2) the summation test, in which prior inhibitory training has an additive effect with responding to an excitatory stimulus, thus reducing overall responding (Rescorla 1969).
To our knowledge, the closest attempt was by Skinner et al. (1995) using a discriminated taste aversion task in rats with morphine as the negative feature. As a negative feature, morphine indicated when a vinegar solution was safe to drink (i.e., the emetic lithium chloride [LiCl] US was withheld). In a summation test, they found that when the negative morphine feature was present, rats increased consumption of a saccharin solution that had been separately paired with LiCl. Although the negative morphine feature passed the summation test, Skinner and colleagues could not determine whether it could pass the retardation-of-acquisition test because there is no obvious way to move morphine from being an interoceptive drug feature to serving as a taste CS that can be paired with an emetic US.
This limitation is not present with the discriminated goal-tracking task. Nicotine readily serves as an interoceptive contextual CS for intermittent access to sucrose (e.g., Besheer et al. 2004; Reichel et al. 2010). Accordingly, the two experiments reported here examined whether nicotine trained as a negative feature would pass the retardation and summation tests of conditioned inhibition. If it does, then this will be the first demonstration of a drug stimulus acquiring such properties. Such a demonstration would not only help dissociate between associative learning models that predict that a negative feature should pass these tests of conditioned inhibition (e.g., Rescorla and Wagner 1972; Miller and Matzel 1988) from those that do not (e.g., Mackintosh 1975), but it could have important implications for treatment approaches to addiction and other psychopathologies that involve interoceptive stimuli (cf. Pavlov 1927; Bevins and Murray 2011).
Results
Experiment 1: Retardation-of-acquisition test
For the retardation-of-acquisition test, two groups of rats were trained with a negative drug feature. One group had nicotine (0.4 mg/kg) as the negative feature (NIC-NF); the other group had chlordiazepoxide (CDP-NF). This latter group served as a control that received equal training with a negative drug feature, but chlordiazepoxide (5 mg/kg) does not share stimulus properties with nicotine as demonstrated by a lack of pharmacological substitution when nicotine is trained as a negative feature (cf. Bevins et al. 2006), thus avoiding pharmacological generalization of this training to the retardation-of-acquisition phase. During discrimination training, the drug feature indicated when each of the eight 15-sec light CS presentations was not followed by the sucrose US; no drug (saline) signaled when each of the light CS presentations was followed by 4-sec access to sucrose. All training sessions were 20 min. Exposure to drug was controlled by home cage administration of the nontraining drug. Following acquisition of the discrimination, both groups received excitatory conditioning in which nicotine served as the interoceptive contextual CS, and there were no light presentations. In this phase, sucrose was intermittently available eight separate times on nicotine sessions but not on intermixed saline. Under these training conditions, past research has shown that rats readily acquire this discrimination, as evidenced by a differential increase in goal-tracking before any sucrose deliveries on nicotine sessions (e.g., Besheer et al. 2004; Wilkinson et al. 2006). If feature negative training with nicotine imbued it with conditioned inhibitory properties, then subsequent acquisition of a conditioned response to the nicotine CS should be impaired relative to the control that had chlordiazepoxide trained as the negative feature.
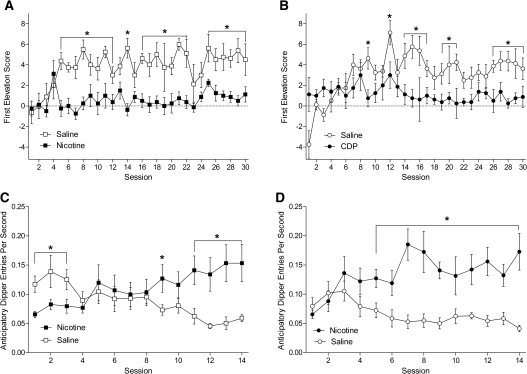
The discrimination was acquired regardless of which drug served as the negative feature (Fig. 1A,B). For group NIC-NF, there was a main effect of Drug and Session, F's ≥ 5.06, P's < 0.001, and a Drug × Session interaction, F(29,230) = 3.06, P < 0.001. Responding evoked by the first light presentation was higher on saline than nicotine for sessions 4–5, 9, 11–14, 16–22, and 25–30 (Fig. 1A). For group CDP-NF, there was a main effect of Drug and Session, F's ≥ 2.81, P's ≤ 0.005, and a Drug × Session interaction, F(29,203) = 1.96, P = 0.004. There was higher responding on saline than CDP for sessions 12, 15–16, 18–22, and 26–30 (Fig. 1B).
Figure 1.
Panel A shows acquisition of the nicotine negative feature discrimination for the NIC-NF group. Panel B shows acquisition of the chlordiazepoxide negative feature discrimination for the CDP-NF group. Panels C and D show the excitatory nicotine CS discrimination for the NIC-NF and CDP-NF groups, respectively. All results shown as ±SEM. (*) Significant difference between saline and drug sessions.
Feature negative training with nicotine slowed later acquisition of discriminated responding controlled by the nicotine CS (Fig. 1C,D). For group NIC-NF, there was a main effect of Drug, F(1,7) = 5.97, P = 0.045, and a Drug × Session interaction, F(13,91) = 6.36, P < 0.001. Dipper entry rate on saline sessions was higher than on nicotine for sessions 1–3. This flipped later in training with higher responding on nicotine than saline for sessions 9 and 11–14 (Fig. 1C). In contrast, the CDP-NF group displayed a reliable discrimination on earlier sessions. There was a main effect of Drug, F(1,7) = 28.23, P < 0.001, and a Drug × Session interaction, F(13,91) = 4.86, P < 0.001. Responding on nicotine was higher than saline for sessions 5–14 (Fig. 1D).
Experiment 2: Summation test
Experiment 2 sought to determine if a negative nicotine feature would pass the summation test of conditioned inhibition. In this experiment, one group of rats was trained with nicotine (0.4 mg/kg) as the negative drug feature (NIC-NF). A second group of rats, pseudoconditioning control (NIC-Pseudo), was given equal exposure to all stimuli (nicotine, light CS, sucrose US), but nicotine was presented such that it did not reliably indicate the presence or absence of the sucrose US after the light CS presentations. Following this phase, all rats received excitatory training in which a 15-sec white noise CS was paired with sucrose (eight presentations per session) until conditioned responding stabilized. The subsequent summation test presented the nicotine stimulus with the separately trained white noise CS. To assess response disruption by a shift in drug state (see later), there was a subsequent test with 1 mg/kg naloxone. To pass the summation test, nicotine trained as a negative feature should selectively reduce conditioned responding evoked by the white noise CS.
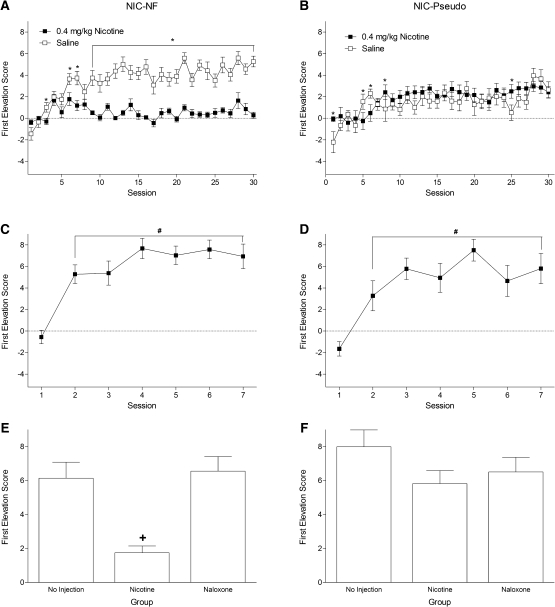
Rats in group NIC-NF acquired the discrimination (Fig. 2A). There was increased goal-tracking during the first light CS presentation on saline relative to nicotine sessions as training continued. There were main effects of Drug and Session, F's ≥ 7.99, P's ≤ 0.001, and a significant Drug × Session interaction, F(29,435) = 5.81, P < 0.001. Responding was higher on saline than nicotine for sessions 3, 6, 7, and 9–30. As expected, by the end of the initial training phase for group NIC-Pseudo, the increase in dipper entry time was moderate and comparable, whether it was a nicotine or saline session (Fig. 2B). There were main effects of Drug and Session, F's ≥ 5.28, P's ≤ 0.023, and a significant Drug × Session interaction, F(29,406) = 1.65, P = 0.02. Responding was different between nicotine and saline for sessions 1, 5, 6, 8, and 25.
Figure 2.
Panel A shows acquisition of the nicotine negative feature discrimination for the NIC-NF group. Panel B shows pseudoconditioning in the non-differential control group (NIC-Pseudo). Panels C and D show the excitatory conditioning with the white noise CS for the NIC-NF and NIC-Pseudo groups, respectively—no drugs were given during this training phase. Panels E and F show the results of the 4-min test sessions for the NIC-NF and NIC-Pseudo groups, respectively. All results shown as ±SEM. (*) Significant difference between saline and nicotine states, (#) significant difference from session 1, (+) significant difference from the no-pretreatment test.
In the white noise conditioning phase, rats in group NIC-NF (Fig. 2C) and NIC-Pseudo (Fig. 2D) acquired a robust goal-tracking response evoked by the first white noise CS presentation of each training session. For both groups there were main effects of Session, F's ≥ 8.91, P's < 0.001. Sessions 2–7 had higher conditioned responding than session 1 in both groups.
Test results for group NIC-NF are shown in Figure 2E. The one-way ANOVA was significant, F(2,30) = 20.81, P < 0.001. Post-hoc tests indicated that nicotine weakened responding evoked by the white noise CS relative to no pretreatment or pretreatment with naloxone. Test results for group NIC-Pseudo are shown in Figure 2F. The one-way ANOVA was not significant, F(2,28) = 1.69, P = 0.203, indicating that white noise evoked responding remained stable regardless of test condition.
Discussion
In the Pavlovian conditioning field, there has been a long history of studying learning processes involving interoceptive stimuli as CSs and modulators (e.g., Pavlov 1927; Bykov 1957; Cook et al. 1960; Doty 1961; Davidson 1993; Bevins 2009). Despite this history, until now, there does not appear to be a clear demonstration that an interoceptive Pavlovian stimulus functioned as a conditioned inhibitor. The two experiments in this report fill this important gap by demonstrating that the interoceptive stimulus effects of nicotine trained as a negative feature passed the retardation-of-acquisition and summation tests of conditioned inhibition (Rescorla 1969). As detailed by Rescorla and others, both tests are necessary in order to eliminate an alternative attentional explanation of any one particular test. Slowed acquisition of excitatory conditioning to the nicotine CS in the retardation-of-acquisition test may reflect decreased attention to the putative inhibitor (i.e., nicotine) imbued by the training. Conversely, an attentional explanation for disruption of responding in the summation test is that increased attention to the putative inhibitor (i.e., nicotine) occurs at the expense of the excitor (i.e., white noise). Nicotine trained as a negative feature for a light CS was slow to acquire discriminative control of an excitatory CR on its own, and it was able to disrupt conditioned responding to a separately trained white noise CS. It seems more likely that nicotine in this case is functioning as a conditioned inhibitor and not simultaneously increasing and decreasing attention.
The experiments in this report also included key control groups and manipulations to help winnow through alternative explanations for the findings. In the retardation-of-acquisition study (Experiment 1), we had a control group that had chlordiazepoxide, a ligand pharmacologically unrelated to nicotine, trained as a negative feature. Differential control of the goal-tracking CR by nicotine in this group (CDP-NF) was quick and comparable to previously published reports using similar parameters (Besheer et al. 2004; Wilkinson et al. 2006). Thus, simply having a learning history with a drug state as a negative feature was not sufficient to slow acquisition of a nicotine-evoked CR. Further, group CDP-NF received equal exposure to nicotine in the home cage (see Methods). This appears to challenge any explanation based on a CS pre-exposure effect (Ayres et al. 1992) or alteration of neurobiological processes related to simply receiving repeated exposure to this psychoactive drug (Besheer and Bevins 2004). Also, note that the NIC-NF group received equal exposure to chlordiazepoxide in the home cage; this further negates any account invoking differences in drug exposure.
In the summation study (Experiment 2), we had a control group (NIC-Pseudo) that received equal exposure to nicotine, saline, chambers, light CS, and sucrose US, yet nicotine did not reliably occasion whether or not the light CS would be paired with sucrose—the chamber was the best predictor of sucrose (i.e., 50%). The fact that nicotine did not disrupt responding evoked by the white noise CS in this group eliminates an account based on state-dependent recall (Overton 1964). This finding is consistent with other research from our lab showing that shifting to or away from a nicotine training state does not disrupt a goal-tracking CR (Bevins et al. 2007; Murray et al. 2011). We also had a within-subject test of this state-dependent recall account using naloxone as the alternate state. We included this test in case the feature negative training received by group NIC-FN encouraged greater sensitivity to state changes than the training history of group NIC-Pseudo. Naloxone is an opioid antagonist, whereas nicotine is a nicotinic acetylcholine agonist (Takemori and Portoghese 1984; Besheer and Bevins 2004). Further, naloxone at the 1 mg/kg dose used in this study serves as a stimulus in the two-lever drug discrimination task (Smurthwaite et al. 1992), indicating that it is a perceptible stimulus (i.e., change in state from no drug). Conditioned responding evoked by the white noise CS was not affected by pretreatment with naloxone, further diminishing any account based on altered drug state affecting recall.
There are many theoretical conceptualizations of the potential underlying associative structure of what is learned in a feature negative task that produces a conditioned inhibitor (Swartzentruber 1995; Savastano et al. 1999; Bevins et al. 2006). For instance, the negative nicotine feature may be directly affecting the US representation (e.g., Rescorla 1979; Bouton and Nelson 1994), dampening expression of the excitatory CS-US association (e.g., Ross and Holland 1981; Holland 1992) or activating an inhibitory association between the CS and US that parallels the excitatory CS-US association (e.g., Bouton and Nelson 1994). The present research was not designed to select among these alternatives. As such, it will be of much interest in the future to design experiments that begin to differentiate between these different possibilities. Different associative structures may suggest different ways in which to provide competing learning histories that may help increase the rate of quitting or decrease the high rate of relapse in chronic tobacco users. Take as a general example legislative action that has made many public places and business establishments (e.g., bars, restaurants) smoke-free. If a smoker decides to have a cigarette while out to dinner with family and friends, then they must leave the pleasure of dinner conversation and the meal. Thus, the nicotine state has the opportunity in this situation to occasion the absence of pleasurable events within the context of a restaurant (i.e., negative feature). Note that these smoke-free laws not only have the health benefit of decreasing secondhand smoke exposure of the restaurant staff and patrons (Farrelly et al. 2005), but the policy may be providing a learning history that could influence smoking rate and perhaps cessation. Albeit speculative, if this is the case, then finding ways to further increase a smoker's experience with nicotine as a negative feature may decrease consumption of cigarettes and eventually facilitate quit attempts.
Materials and Methods
Experiment 1: Retardation-of-acquisition test
Subjects
Male Sprague-Dawley rats (n = 16) were obtained from Harlan (Indianapolis, IN). Rats were housed individually in clear plastic cages [48.3 × 26.7 × 20.3 cm (l × w × h)] lined with wood shavings. Water was freely available in the home cage. Free-feeding body weights (297 ± 6 g) were maintained at 85% by post-session feedings (Harlan Teklad Rodent Diet). All sessions were conducted during the light portion of a 12-h light:dark cycle. Protocols were approved by the University of Nebraska-Lincoln Animal Care and Use Committee and followed the Guide for the Care and Use of Laboratory Animals (National Research Council 1996).
Apparatus
Experiments were conducted in eight conditioning chambers (ENV-008CT; Med Associates, Inc.) measuring 30.5 × 24.1 × 21.0 cm (l × w × h), each enclosed in a light- and sound-attenuating cubicle (ENV-018) fitted with an exhaust fan. The ceilings and front and back walls of the chambers were clear polycarbonate; the sidewalls were aluminum. All stimulus elements were attached to the sidewalls. On one sidewall, there was a recessed dipper receptacle [5.2 × 5.2 × 3.8 cm (l × w × d)]. A dipper arm raised a 0.1-mL cup into the receptacle. Head entries into the dipper were detected by an infrared beam 1.2 cm into the receptacle and 3 cm from the floor. Positioned on the sidewall containing the receptacle were two white stimulus lights (2.54 cm diameter; 28 V, 100 mA), 14.6 cm above the metal rod floor and 3.5 cm from either the front or back wall. Illumination of both lights served as the light CS. A computer with Med Associates, Inc. interface and software (Med-PC for Windows, version IV) controlled stimulus events and recorded dipper entries.
Drugs
(–)-Nicotine hydrogen tartrate and chlordiazepoxide hydrochloride (all from Sigma) were mixed in 0.9% saline solution (w/v). Nicotine was adjusted to a pH of 7.0 ± 0.2 using a dilute NaOH solution. All drugs were injected at 1 mL/kg. Nicotine injections were subcutaneous (SC); chlordiazepoxide injections were intraperitoneal (IP). Nicotine doses are reported in the base form; other drug doses are given as the salt form.
Dipper training
Rats were trained to access sucrose in the receptacle in three 50-min sessions. Across the sessions, the probability of receiving 4-sec access to 26% sucrose (w/v) decreased from about four deliveries per min to one delivery every 2 min. The session began with the rat's first dipper entry. By the end of the three days, all rats displayed robust dipper-entry behavior.
Negative feature training
Eight of the rats (group NIC-NF) received an injection of either saline or 0.4 mg/kg nicotine SC 5 min before placement in the chamber for daily 20-min training sessions. On saline sessions, eight presentations of a 15-sec light CS were each followed by 4-sec access to sucrose. On nicotine sessions, sucrose was withheld after each light presentation. Four different programs of each type were created such that the time before onset of the first CS ranged from 90 to 180 sec (mean 135 sec), and the time between the CS offset and the next CS presentation ranged from 79 to 169 sec (mean 124 sec). Session types were intermixed in a unique order for each rat with the restriction that no more than two saline or two nicotine sessions occurred in a row. Negative feature training continued for 30 saline and 30 nicotine sessions. The other eight rats (group CDP-NF) received an IP injection of either saline or 5 mg/kg chlordiazepoxide (CDP) 15 min before placement in the chamber and start of the sessions. Saline and CDP sessions were conducted in the same manner as group NIC-NF, except CDP was administered instead of nicotine. To control for drug exposure and route of administration between groups, rats in group NIC-NF received intermixed IP injections of saline and CDP in the colony at least 6 h after the end of a daily training session. Group CDP-NF received intermixed SC injections of saline and nicotine. Order of the control injections was such that half of the sessions with sucrose were followed with drug and half were followed with saline in a unique order for each rat, again with the restriction that no more than two drug or two saline injections occurred in a row.
Retardation-of-acquisition (nicotine CS training)
Following negative feature training, all rats began excitatory conditioning with nicotine as the CS. On a given day, a rat was administered either 0.4 mg/kg nicotine or saline 5 min before placement in the chamber and the start of the session (∼20 min). On nicotine sessions, rats had 4-sec access to sucrose on eight separate occasions. Four separate Med-PC programs were used to vary time of sucrose delivery; average time between deliveries was 141 sec (range 90–210 sec) with average time of the initial delivery being 120 sec (range 90–150 sec). Sucrose was not available on saline sessions. The light was not presented in this phase. As in the previous phase, session types were intermixed such that no more than two nicotine or two saline sessions occurred in a row. This phase continued for 14 nicotine and 14 saline sessions.
Dependent measures and data analyses
Because chlordiazepoxide served as one of the negative drug features, we used the increase in head-entry time for the first light CS presentation of a session as the dependent measure assessing whether the discrimination was acquired (see Palmatier and Bevins 2008 for more discussion of how response form varies with drug and CS type). This measure was calculated by subtracting the time spent breaking the beam in the dipper receptacle during the 15 sec before the CS onset (pre-CS period) from the time spent in the dipper receptacle during the 15-sec light CS presentation. A positive value indicates more time in the dipper receptacle during the CS (i.e., a goal-tracking CR). We only use the first CS presentation to avoid any influence of the reinforcer on this index of conditioning. Comparable to all our previously published work on nicotine as a CS, for the retardation-of-acquisition phase, the dependent measure was the number of dipper entries per second before the first sucrose delivery on nicotine sessions or equivalent interval on saline sessions. Again, only dipper entries before sucrose delivery were used to avoid any influence of US delivery on the measure of conditioning. A rate measure was necessary given that the programmed time to first sucrose delivery varied across sessions. For each group, acquisition with these dependent measures was examined using two-way analyses of variance (ANOVAs) with Session and Drug State as within-subject factors. A significant ANOVA was followed with protected Fisher's Least Significant Difference (LSD) tests comparing drug to saline sessions. Statistical significance was declared at P < 0.05 for all tests.
Experiment 2: Summation test
Subjects and materials
Rats (n = 32; 301 ± 8 g) were handled and maintained as described in Experiment 1. A speaker was mounted in the back upper corner of the sidewall opposite the lights and dipper receptacle. This speaker was used to deliver a white noise CS (74 dB). Nicotine preparation and administration was as previously described. As a control condition, the test phase of Experiment 2 also used naloxone hydrochloride (Sigma). Naloxone was mixed in 0.9% saline solution and injected SC at 1 mL/kg. All other details were similar to Experiment 1 unless noted.
Negative feature training
Sixteen of the experimentally naive rats received nicotine negative feature training exactly as described in Experiment 1 (group NIC-NF). A separate set of 16 rats served as a pseudoconditioning control (NIC-Pseudo). Rats in this group received an injection of either saline or nicotine 5 min before chamber placement for the 20-min session. On half of the saline and half of the nicotine sessions, eight presentations of a 15-sec light CS were each followed by 4-sec access to sucrose. On the other half of the saline and nicotine sessions, sucrose was withheld after each light presentation. For each rat, session types were intermixed so that no more than two saline or two nicotine sessions occurred successively. In addition, no more than two positive (sucrose) or two negative sessions occurred in a row. Training continued for 30 saline and 30 nicotine sessions. During the training, one rat in group NIC-Pseudo was removed for illness reducing this group to 15 rats.
White noise CS+ training
During each session of this phase, there were eight 15-sec white noise presentations, each followed by 4-sec access to sucrose. There were no injections given during this phase, and the timing of the white noise CS within and across session varied as described for the light CS in the negative feature training. Excitatory conditioning continued for seven sessions.
Summation test
Before summation testing, rats received training with earlier session types to ensure that responding was stable before testing. For the NIC-NF group, this training included three cycles in which each cycle contained one session with white noise paired with sucrose (no injection), one saline session in which the light CS was reinforced, and two nicotine sessions in which the light CS was not followed by sucrose. Each rat had a randomly assigned order for each cycle. Two nicotine sessions were given to balance the drug/no-drug exposure and the sucrose/no sucrose presentations. For the NIC-Pseudo group, there were three cycles in which each cycle contained a reinforced white noise session (no injection) and sessions both reinforced and nonreinforced following nicotine and saline injections. With responding stable and comparable to early phases, rats were moved to summation testing. There were two test sessions conducted on consecutive days. Both tests were 4 min, with one white noise presentation 120 sec into the session; sucrose was withheld. Baseline responding evoked by the white noise was assessed in an injection-free test. For the other test (summation), 0.4 mg/kg nicotine was administered SC 5 min before placement in the chamber and start of the test session. Half the rats had nicotine administered before the first test; the remaining half had no injection before the first test. Because order of testing did not affect performance, the data were pooled for overall analyses.
Novel drug test
To ensure responding was still stable under earlier training conditions, rats had one cycle of intermixed session types as described previously. We then conducted a 4-min test session with a single white noise presentation. All rats received 1 mg/kg naloxone SC 10 min before the session. Naloxone at this dose has perceptible stimulus properties (e.g., Smurthwaite et al. 1992). Thus, this test provided a within-subject evaluation of whether response inhibition in the summation test reflected an acquired inhibitory property of nicotine or rather was a nonspecific response disruption because the internal state differed from the white noise no-drug training state in group NIC-NF. This test was conducted last, given that it would have been unnecessary if the white noise-evoked CR was not selectively reduced in group NIC-NF.
Dependent measures and data analyses
The dependent measure was the increase in head-entry duration during the CS presentation described in Experiment 1. Acquisition of excitatory conditioning was evaluated using 1- and 2-way repeated measures ANOVAs. For testing, each group was analyzed by repeated measures ANOVAs. Significant results, P < 0.05, were followed by protected Fisher's post-hoc analyses.
Acknowledgments
We thank Jessica D. Barr and Jonathan D. Fullner for their assistance with the studies. We thank Anushka Fernando for her thoughtful comments on an earlier version of this report. The research and R.A.B. were supported by NIH grant DA018114. J.E.M. was supported by NIH F31-DA025399 and MRC 9536855 (latter awarded to B.J. Everitt), and C.L. was partially supported by UNL Undergraduate Creative Activities and Research Experiences while conducting the research. MED-PC programs used in the present article are available upon request.
References
- Ayres JJB, Philbin D, Cassidy S, Bellino L, Redlinger E 1992. Some parameters of latent inhibition. Learn Motiv 23: 269–287 [Google Scholar]
- Besheer J, Bevins RA 2004. Acetylcholine: II. Nicotinic receptors. In From messenger to molecules: Memories are made of these (ed. Riedel G, Platt B), pp. 113–124 Landes Bioscience, Austin, TX [Google Scholar]
- Besheer J, Palmatier MI, Metschke DM, Bevins RA 2004. Nicotine as a signal for the presence or absence of sucrose reward: A Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology 172: 108–117 [DOI] [PubMed] [Google Scholar]
- Bevins RA 2009. Altering the motivational function of nicotine through conditioning processes. In The motivational impact of nicotine and its role in tobacco use: The 55th Nebraska symposium on motivation (ed. Bevins RA, Caggiula AR), pp. 111–129 Springer, New York: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Murray JE 2011. Internal stimuli generated by abused substances: Role of Pavlovian conditioning and its implications for drug addiction. In Associative learning and conditioning: Human and non-human applications (ed. Schachtman T, Reilly S), pp. 270–289 Oxford University Press, New York [Google Scholar]
- Bevins RA, Wilkinson JL, Palmatier MI, Siebert HL, Wiltgen SM 2006. Characterization of nicotine's ability to serve as a negative feature in a Pavlovian appetitive conditioning task in rats. Psychopharmacology 184: 470–481 [DOI] [PubMed] [Google Scholar]
- Bevins RA, Penrod RD, Reichel CM 2007. Nicotine does not produce state-dependent effects on learning in a Pavlovian appetitive goal tracking task with rats. Behav Brain Res 177: 134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boakes RA 1977. Performance on learning to associate a stimulus with positive reinforcement. In operant-Pavlovian interactions (ed. Davis H, Hurwitz HMB), pp. 67–97 Erlbaum, Hillsdale, NJ [Google Scholar]
- Bouton ME, Nelson JB 1994. Context-specificity of target versus feature inhibition in a feature negative discrimination. J Exp Psychol Anim Behav Process 20: 51–65 [PubMed] [Google Scholar]
- Bykov KM 1957. The cerebral cortex and the internal organs. Chemical Publishing Co., New York [Google Scholar]
- Cook L, Davidson A, Davis DJ, Kelleher RT 1960. Epinephrine, norepinepherine, and acetylcholine as conditioned stimuli for avoidance behavior. Science 131: 990–991 [DOI] [PubMed] [Google Scholar]
- Davidson TL 1993. The nature and function of interoceptive signals to feed: Toward integration of physiological and learning perspectives. Psychol Rev 100: 640–657 [DOI] [PubMed] [Google Scholar]
- Doty RW 1961. Conditioned reflexes formed and evoked by brain stimulation. In Electrical stimulation of the brain: An interdisciplinary survey of neurobehavioral integrative systems (ed. Sheer DE), pp. 397–412 University of Texas Press, Austin, TX [Google Scholar]
- Farrelly MC, Nonnemaker JM, Chou R, Hyland A, Peterson KK, Bauer UE 2005. Changes in hospitality workers' exposure to secondhand smoke following the implementation of New York's smoke-free law. Tob Control 14: 236–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwell BJ, Ayres JJB 1979. Stimulus-reinforcer and response-reinforcer relations in the control of conditioned appetitive headpoking (“goal-tracking”) in rats. Learn Motiv 10: 295–312 [Google Scholar]
- Holland PC 1992. Occasion setting in Pavlovian conditioning. In The psychology of learning and motivation (ed. Medin DL), Vol. 28, pp. 69–125 Academic Press, New York [Google Scholar]
- Holland PC 1995. Transfer of occasion setting across stimulus and response in operant feature positive discriminations. Learn Motiv 26: 239–263 [Google Scholar]
- Jaeger TV, Mucha RF 1990. A taste aversion model of drug discrimination learning: Training drug and condition influence rate of learning, sensitivity, and drug specificity. Psychopharmacology 100: 145–150 [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ 1975. A theory of attention: Variations in the associability of stimuli with reinforcement. Psychol Rev 82: 276–298 [Google Scholar]
- Miller RR, Matzel LD 1988. The comparator hypothesis: A response rule for the expression of associations. In The psychology of learning and motivation (ed. Bower GH), Vol. 22, pp. 51–92 Academic Press, San Diego [Google Scholar]
- Murray JE, Walker AW, Polewan RJ, Bevins RA 2011. An examination of NMDA receptor contribution to conditioned responding evoked by the conditional stimulus effects of nicotine. Psychopharmacology 213: 131–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington [Google Scholar]
- Overton DA 1964. State-dependent or “dissociated” learning produced with pentobarbital. J Comp Physiol Psychol 57: 3–12 [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Bevins RA 2007. Facilitation by drug states does not depend on acquired excitatory strength. Behav Brain Res 176: 292–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Bevins RA 2008. Occasion setting by drug states: Functional equivalence following similar training history. Behav Brain Res 195: 260–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP 1927. Conditioned reflexes. Oxford University Press, London [Google Scholar]
- Reichel CM, Murray JE, Barr JD, Bevins RA 2010. Extinction with varenicline and nornicotine, but not ABT-418, weakens conditioned responding evoked by the interoceptive stimulus effects of nicotine. Neuropharmacology 58: 1237–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA 1969. Pavlovian conditioned inhibition. Psychol Bull 72: 77–94 [Google Scholar]
- Rescorla RA 1979. Conditioned inhibition and extinction. In Mechanisms of learning and motivation (ed. Dickinson A, Boakes RA), pp. 83–110 Lawrence Erlbaum Associates, Hillsdale, NJ [Google Scholar]
- Rescorla RA, Wagner AR 1972. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In Classical conditioning II: Current research and theory (ed. Black AH, Prokasy WF), pp. 64–99 Meredith Corp, New York [Google Scholar]
- Ross RT, Holland PC 1981. Conditioning of simultaneous and serial feature positive discriminations. Anim Learn Behav 9: 292–303 [Google Scholar]
- Savastano HI, Cole RP, Barnet RC, Miller RR 1999. Reconsidering conditioned inhibition. Learn Motiv 30: 101–127 [Google Scholar]
- Skinner DM, Martin GM, Howe RD, Pridgar A, van der Kooy D 1995. Drug discrimination learning using a taste aversion paradigm: An assessment of the role of safety cues. Learn Motiv 26: 343–369 [Google Scholar]
- Smith JW, Stolerman IP 2009. Recognizing nicotine: The neurobiological basis of nicotine discrimination. In Nicotine pharmacology: Handbook of experimental psychology (ed. Henningfield JE, et al. ), Vol. 192, pp. 295–333 Springer-Verlag, Berlin, Heidelberg: [DOI] [PubMed] [Google Scholar]
- Smurthwaite S, Kautz M, Geter B, Riley A 1992. Naloxone as a stimulus in drug discrimination learning: Generalization to other opiate antagonists. Pharmacol Biochem Behav 41: 43–47 [DOI] [PubMed] [Google Scholar]
- Swartzentruber D 1995. Modulatory mechanisms in Pavlovian conditioning. Anim Learn Behav 23: 123–143 [Google Scholar]
- Takemori AE, Portoghese PS 1984. Comparative antagonism by naltrexone and naloxone of mu, kappa, and delta agonists. Eur J Pharmacol 104: 101–104 [DOI] [PubMed] [Google Scholar]
- Troisi JR, Akins C 2004. The discriminative stimulus effects of cocaine in a Pavlovian sexual approach paradigm in male Japanese quail. Exp Clin Psychopharmacol 12: 237–242 [DOI] [PubMed] [Google Scholar]
- Wilkinson JL, Murray JE, Li C, Wiltgen SM, Penrod RD, Berg SA, Bevins RA 2006. Interoceptive Pavlovian conditioning with nicotine as the conditional stimulus varies as function of number of conditioning trials and unpaired sucrose deliveries. Behav Pharmacol 17: 161–172 [DOI] [PubMed] [Google Scholar]
- Wooters TE, Bevins RA, Bardo MT 2009. Neuropharmacology of the interoceptive stimulus properties of nicotine. Curr Drug Abuse Rev 2: 243–255 [DOI] [PMC free article] [PubMed] [Google Scholar]