Abstract
This first test of the role of REM (rapid eye movement) sleep in reversal spatial learning is also the first attempt to replicate a much cited pair of papers reporting that REM sleep deprivation impairs the consolidation of initial spatial learning in the Morris water maze. We hypothesized that REM sleep deprivation following training would impair both hippocampus-dependent spatial learning and learning a new target location within a familiar environment: reversal learning. A 6-d protocol was divided into the initial spatial learning phase (3.5 d) immediately followed by the reversal phase (2.5 d). During the 6 h following four or 12 training trials/day of initial or reversal learning phases, REM sleep was eliminated and non-REM sleep left intact using the multiple inverted flowerpot method. Contrary to our hypotheses, REM sleep deprivation during four or 12 trials/day of initial spatial or reversal learning did not affect training performance. However, some probe trial measures indicated REM sleep-deprivation–associated impairment in initial spatial learning with four trials/day and enhancement of subsequent reversal learning. In naive animals, REM sleep deprivation during normal initial spatial learning was followed by a lack of preference for the subsequent reversal platform location during the probe. Our findings contradict reports that REM sleep is essential for spatial learning in the Morris water maze and newly reveal that short periods of REM sleep deprivation do not impair concurrent reversal learning. Effects on subsequent reversal learning are consistent with the idea that REM sleep serves the consolidation of incompletely learned items.
While it has been widely shown that both total sleep deprivation and sleep fragmentation impair learning, there remains much debate over the role of REM sleep deprivation for learning (for reviews, see Smith 1995; Hobson and Pace-Schott 2002; Vertes 2004; Rauchs et al. 2005; Stickgold and Walker 2005; Vertes and Siegel 2005). Increases in REM sleep have been described following learning (e.g., Lucero 1970; Hennevin et al. 1971; Leconte and Hennevin 1971; Fishbein et al. 1974; Smith et al. 1980; Smith and Butler 1982; Smith and Lapp 1986; Portell-Cortes et al. 1989; Smith and Wong 1991; Bramham et al. 1994; Smith and Rose 1997; Mavanji and Datta 2003) that imply a functional relationship. Some studies suggest that REM sleep is tightly linked with specific types of learning such as spatial learning (e.g., Smith and Rose 1996, 1997; Youngblood et al. 1997; Smith et al. 1998; Beaulieu and Godbout 2000; Le Marec et al. 2001; Bjorness et al. 2005; Ruskin et al. 2006; Yang et al. 2008; Li et al. 2009; Wang et al. 2009), which is hippocampus-dependent.
The confounding effect of stress induced by the deprivation technique itself (Vertes and Eastman 2000) can be minimized with protocol manipulations (Van Hulzen and Coenen 1981; Suchecki et al. 1998; Suchecki et al. 2000; Suchecki and Tufik 2000; Suchecki et al. 2002; Machado et al. 2004, 2006). Therefore, observed performance deficits with short periods of REM sleep deprivation are likely due to REM sleep effects on learning. Furthermore, shorter periods of REM sleep deprivation (e.g., 4 or 6 h) should ameliorate the accumulation of side-effects such as stress and may be more representative of human sleep; rarely will a human go multiple days without REM sleep.
To date, four studies have utilized short periods of REM sleep deprivation after training to test the consolidation of spatial learning (Smith and Rose 1996, 1997; Smith et al. 1998; Bjorness et al. 2005), and all have found positive effects. The effects of REM sleep deprivation on the reversal of spatial learning, however, have not been studied.
Reversal of spatial learning is the learning of a new response (e.g., movement of the goal target to a new location) in a familiar environment with consistent environmental stimuli across both initial spatial and reversal learning. Hippocampal damage or an alteration in hippocampal activity disrupts reversal learning (Morris et al. 1986; Whishaw and Tomie 1997; for reviews, see Whishaw 1998; van der Meulen et al. 2003) and can have a greater effect on reversal learning than on initial spatial learning (Pouzet et al. 1999; Cirulli et al. 2000, 2004). Short periods of REM sleep deprivation have been shown to impair both concurrent and subsequent reversal of fear conditioning (extinction learning) (e.g., Silvestri 2005; Fu et al. 2007). Additionally, hippocampal firing patterns associated with the reversal of long-term potentiation (LTP, called synaptic depotentiation) which is thought to be important for reversal learning, are facilitated during REM sleep periods (Poe et al. 2000; Booth and Poe 2006). Such unique reversal-supporting hippocampal activity during REM sleep should render reversal learning even more susceptible to REM sleep deprivation than spatial learning.
Thus, we predicted that REM sleep deprivation would impair both concurrent spatial learning and concurrent spatial reversal learning and may also inhibit the subsequent reversal of spatial learning. Specifically, we hypothesized that short bouts of REM sleep deprivation concurrent to reversal learning leads to deficits in reversal performance. Further, we hypothesized that short bouts of REM sleep deprivation concurrent to initial spatial learning leads to performance deficits in the initial learning task and may cause performance deficits in subsequent reversal learning when REM sleep is no longer deprived. We are the first to describe the effects of 6 h of REM sleep deprivation on reversal of spatial learning in the Morris water maze.
Results
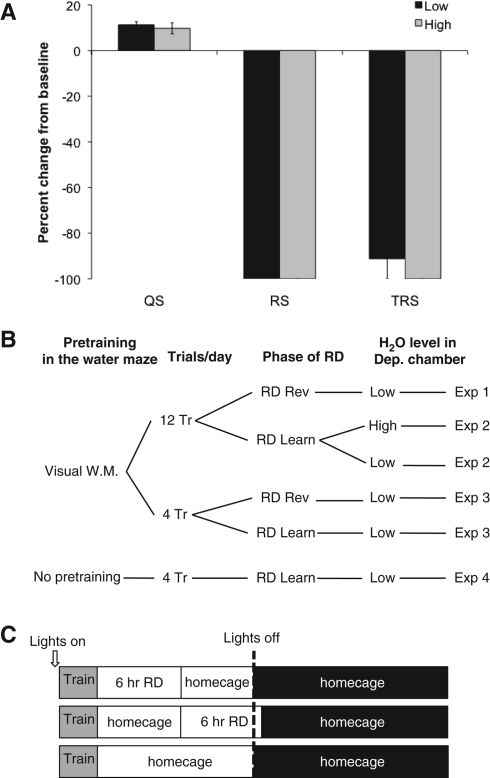
We verified that our inverted flowerpot technique resulted in 100% REM sleep deprivation, while preserving non-REM sleep (also called Slow Wave Sleep or Quiet Sleep) during a 6-h deprivation period (Fig. 1A).
Figure 1.
(A) Change in sleep state during the 6-h deprivation period from baseline for quiet sleep (QS), REM sleep (RS), and transitions to REM sleep (TRS). Within-group comparisons were made for deprivation with a low level of water (Low) and a high level of water (High) within the deprivation chamber. The 6-h deprivation period led to a complete loss of REM sleep (Low: P = 0.015; High: P = 0.023) and near complete loss of transitions to REM sleep with a low level of water in the deprivation chamber (Low: P = 0.042) but no effect on quiet sleep or waking (not shown) for either water level. (B) Description of experiments within the study. During the experiments, controls were always returned to their home cage. Experimental groups were deprived daily for 6 h following training during the phase indicated and otherwise were in their home cages. (W.M.) Water maze training, (Tr) training trials, (Dep) deprivation, (RD) REM sleep deprivation. (C) Daily protocol indicating timing of training and deprivation within each day. The first row indicates the daily protocol for rats deprived immediately following training. The second row indicates the daily protocol for rats deprived 6 h after training. The third row indicates the daily protocol for all controls and the REM sleep-deprived groups in the phases of the experiment when they were not being deprived.
Experiment 1—REM sleep deprivation effects on concurrent reversal learning with 12 trials/day
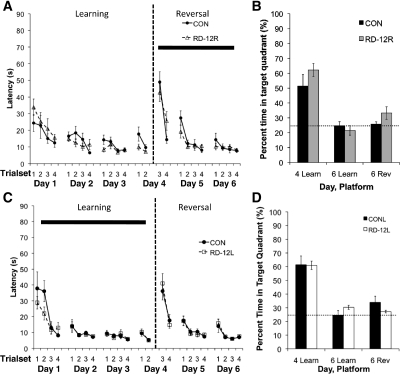
We tested the hypothesis that 6 h of REM sleep deprivation immediately following 12 trials of reversal learning each day would lead to poorer reversal learning performance compared to home cage controls (Fig. 1B). Rats were REM sleep-deprived only during the reversal learning phase, not during initial spatial learning. After passing initial visual screening on the visible platform version of the Morris water maze, rats were assigned to one of three groups (Fig. 1C). All groups were returned to their home cage after initial spatial learning sessions. During the reversal learning phase, controls were returned to their home cage after training (n = 8), and two REM sleep-deprived groups were either put in the REM sleep deprivation chamber immediately after training (RD-12R, n = 8) or with a 6-h delay after training was completed (RDdelay-12R, n = 8). Smith and Rose (1997) identified a REM sleep deprivation-sensitive window lasting 4 h immediately following 12 training trials but not later. Thus, we hypothesized that a REM sleep deprivation period delayed to begin 6 h after reversal learning would not lead to deficits in reversal learning. Our delayed deprivation group, therefore, served as a control for the possible stress effects that may be caused by the deprivation technique itself.
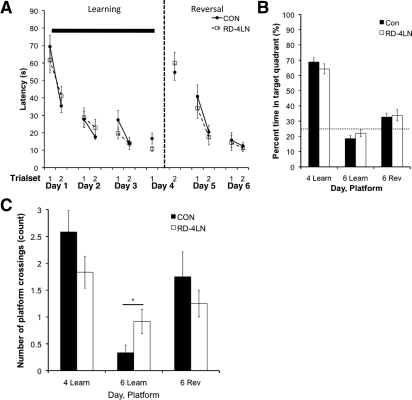
We found that all groups learned the reversal phase platform location, as measured by latency to the platform (Fig. 2A), path length to platform, and cumulative distance from the platform in the daily trials, and learned to near asymptotic levels on the first day of the reversal phase before the first REM sleep deprivation period began. On the day 6 probe trial, there were no group differences (P > 0.05). However, all rats showed no preference for the reversal platform location relative to the original platform location according to proximity measures and percent time in quadrant (Fig. 2B). This not adhering to the recently learned platform location could indicate impaired memory or adaptive learning, where the rat learns to search for the platform in alternative locations when not immediately found in the expected location. In either case, poor performance shown by the control group would create a floor effect that would prevent detection of possible impairments caused by REM sleep deprivation. In summary, the 6-h REM sleep deprivation condition during reversal training did not affect the quickly asymptotic reversal training or the relatively poor reversal probe trial performance.
Figure 2.
(A,B) Experiment 1 (CON [n = 8]; RD-12R [n = 8]): The effect of 6 h of REM sleep deprivation concurrent to reversal learning with 12 training trials per day. (C,D) Experiment 2 (CON [n = 7], RD-12L [n = 10]): The effect of 6 h of REM sleep deprivation with low-level water on initial learning and subsequent reversal learning with 12 training trials per day. A and C show latency to platform during the training trials. The solid bar indicates the phase of training when 6 h of RD each day occurred for the experimental groups (A, between days 4 and 5, and 5 and 6; C, between days 1 and 2, 2 and 3, and 3 and 4). The dashed line represents when the reversal phase begins. B and D show percent time spent in target quadrant during the probe trials. (Learn) learning phase platform location, (Rev) reversal phase platform location. Each trial set is the average performance of three consecutive trials. Data are shown as mean ± SEM.
Experiment 2—REM sleep deprivation effects on concurrent initial spatial learning and subsequent reversal learning with 12 trials/day
After initial visual screening on the visible platform version of the Morris water maze, rats were divided into one of two groups: home cage controls (n = 7) and those REM sleep-deprived for the first 6 h after training during the initial spatial learning phase (RD12-L, n = 10). All rats were trained for 12 trials per day and were tested on both concurrent initial spatial learning and on subsequent reversal learning performance, when the REM sleep-deprived group no longer had their REM sleep disturbed. We hypothesized that REM sleep deprivation would cause a performance deficit during the initial learning phase and might affect subsequent reversal learning.
However, we found that all rats learned across the initial 12 trials per day training period with no detectable REM sleep deprivation-associated performance deficits by any measure, either on the training trials or on the day 4 probe trial (P > 0.05) (Fig. 2C,D). During the reversal training trials on day 4, the rats previously deprived of REM sleep swam slightly faster than the home cage controls (0.25 meters/sec vs. 0.23 meters/sec, respectively, P < 0.025), but there were no group performance differences in any measure for reversal training. There were also no group differences in the day 6 reversal probe trials in the first 10 sec or across the entire probe trial (P > 0.05) (Fig. 2D), nor were there differences in the Gallagher measure that adjusts for differences in velocity. In this experiment, as in the last, both controls and previously REM sleep-deprived animals showed worse performance on the day 6 reversal probe trial as compared to their day 4 initial learning probe trial (P < 0.05). Neither group showed preference for the reversal platform location relative to the initial learning platform location during the initial 10 sec (not shown) or the full 60 sec (Fig. 2D) of the day 6 probe. Thus, there was no effect of REM sleep deprivation on initial spatial learning or on subsequent reversal learning with 12 training trials per day, although again, there could be a floor effect, as no group showed a preference for the reversal platform location on the probe trial.
To ensure that our results did not differ from the previously reported REM sleep deprivation-associated deficit (Smith and Rose 1997) due to differences in statistical approaches, we mimicked the analyses used in the Smith and Rose (1997) study. We compared the performance of the last four trials on day 1 with the first four trials on day 2 and performed pairwise comparisons. We found no group differences (P > 0.05). Thus, we were still unable to replicate the previously reported REM sleep deprivation-associated deficit.
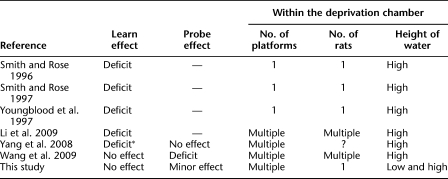
We next examined whether our unexpectedly weak results of Experiment 2 and lack of support for prior studies of initial learning deficits could be explained by our slightly different method of REM sleep deprivation. Though the deprivation chamber we used was similar to that used in experiments which showed REM sleep deprivation-associated spatial learning impairments in other tasks (Bjorness et al. 2005; Ravassard et al. 2009), we used a lower water level within our deprivation chamber than that used in previous Morris water maze/REM sleep deprivation studies (Table 1; Smith and Rose 1996, 1997; Youngblood et al. 1997; Yang et al. 2008; Li et al. 2009; Wang et al. 2009). Therefore, we retested the effects of REM sleep deprivation on 12 trials/day concurrent with spatial learning and subsequent reversal learning using a high level of water within the deprivation chamber. The high water-level deprivation technique also completely suppressed REM sleep during the deprivation period (Fig. 1A). And, as in our previous 12 trials/day experiments (Experiments 1 and 2), no differences in training or probe trial measures between the deprivation group and home cage controls were detected for either concurrent spatial learning (P > 0.05) or subsequent reversal learning (P > 0.05). As with the low water REM sleep-deprived group, the only group difference identified was that the high water, REM sleep-deprived group swam significantly faster than controls during the reversal phase, when their sleep was no longer disturbed (0.28 meters/sec vs. 0.25 meters/sec, respectively, P = 0.049).
Table 1.
Overview of the flowerpot deprivation chambers utilized in a subgroup of studies
The studies indicated are the key studies on the effects of REM sleep deprivation on performance in the Morris water maze using the inverted flowerpot technique. Our current study is also included. Asterisk (*) indicates mixed results; training was impaired although the probe trial showed no group differences.
Experiment 3—Effects of REM sleep deprivation on concurrent reversal or concurrent initial spatial and subsequent reversal learning with four trials/day
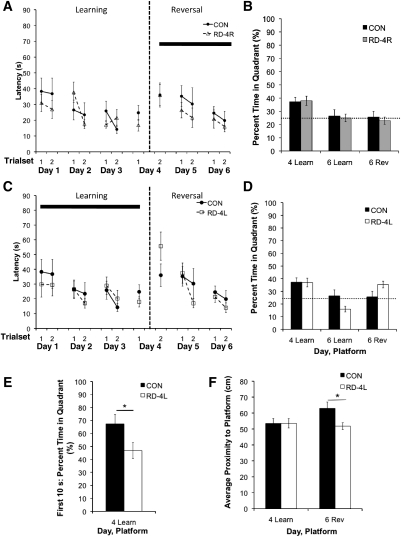
With 12 training trials/day, the animals may have sufficiently learned the task on day 1 (Experiment 2) prior to the first REM sleep manipulation. This determination was based on the similarities in the performance levels reached on trial set 4 (trials 10–12) of day 1 and on trial set 4 (cumulative trials 34–36) of day 3 (Fig. 2C) indicating near asymptotic performance on day 1. Therefore, after prescreening animals for visual acuity on the visible version of the Morris water maze, we tested the effects of 6 h of REM sleep deprivation immediately after training concurrent with reversal training (as in Experiment 1) and concurrent with initial learning (as in Experiment 2), but with only four training trials/day. There were three groups for this experiment: home cage controls (n = 9), those REM sleep-deprived for 6 h immediately following initial spatial learning (RD-4L, n = 8), and those REM sleep-deprived for 6 h immediately following reversal learning (RD-4R, n = 9). We chose four trials/day because in both Experiments 1 and 2, after four training trials on day 1, performance was at intermediate levels, not yet reaching the asymptotic levels achieved by the end of the learning phase.
We hypothesized that there would be a REM sleep deprivation-associated deficit during concurrent reversal learning when only four trials/day were administered. However, all rats learned the reversal task as measured by a general reduction in latency to platform for both groups during training trials. Although latency to platform, cumulative distance, and path length reduced significantly across training trials, without any between-group performance differences (P > 0.05) (Fig. 3A), neither group reached the 10-sec latency to platform mean that was achieved in the 12 trial/day experiments. Further, performance was poor in both the initial learning and reversal phases, with neither controls nor REM sleep-deprived animals showing a preference for the reversal phase platform location in the day 6 probe trial (Fig. 3B). REM sleep deprivation concurrent with reversal learning, therefore, had no deleterious effect on the already poor reversal performance and floored reversal probe trial with a reduced number of daily training trials.
Figure 3.
Experiment 3 [CON (n = 9); RD-4R (n = 9); RD-4L (n = 8)]: The effect of 6 h of REM sleep deprivation on initial spatial learning, reversal learning, and subsequent reversal learning across four training trials/day and probe trials. The solid bar indicates the phase of training with 6 h of RD each day for the experimental groups (A, between days 4 and 5, and 5 and 6; C, between days 1 and 2, 2 and 3, and 3 and 4). RD was applied during the reversal phase (A,B), and during the learning phase (C,D,E,F). The dashed line represents when the reversal phase begins. A and C show latency to platform during the training trials, and B and D show percent time in target quadrant during the probe trials. E shows percent time in target quadrant during the first 10 sec of the probe trial. F shows average proximity to platform location during the probe trial. (Learn) learning phase platform location, (Rev) reversal phase platform location. The previously REM sleep-deprived group performed better than controls during the day 6 probe trial for the reversal phase platform location. On the day 4 probe trial, the REM sleep-deprived group spent significantly less time in the target quadrant during the first 10 sec of the trial. Each trial set is the average performance of two consecutive trials. Data are shown as mean ± SEM. *P < 0.05.
To test the effect of REM sleep deprivation on initial spatial learning and subsequent reversal learning following fewer training trials, after visual prescreening, we administered 6 h of REM sleep deprivation immediately following spatial learning on days 1, 2, and 3, as in Experiment 2, but with only four training trials given per day instead of 12. We hypothesized that REM sleep deprivation would induce learning deficits during the initial learning phase and would interfere with subsequent reversal learning.
Again as in Experiment 2, both groups learned the platform locations equivalently during learning phase and subsequent reversal phase training trials (Fig. 3C), and on the day 4 probe trial, no group differences were identified when the entire 60-sec probe trial was analyzed (Fig. 3D). However, during the first 10 sec of the initial learning probe trial, the REM sleep-deprived group spent less time in the target quadrant than controls (P = 0.048) (Fig. 3E), indicating a deficit in initial spatial learning. This deficit in initial spatial learning was not robust across all measures, i.e., it did not appear as a group difference in the 60 sec proximity to platform (Fig. 3F) or the number of platform crossings (data not shown) in the initial day 4 learning probe. In contrast, these same animals performed better on the subsequent reversal platform location (day 6) probe trial, according to Gallagher's average proximity measure (60 sec), swimming closer to the reversal platform than home cage controls (P = 0.028) (Fig. 3F). These previously REM sleep-deprived animals also showed clear preference for the reversal platform location as opposed to the learning phase platform location (pairwise comparisons) on the day 6 probe trial for percent time in target quadrant (at 10 sec, data not shown, and at 60 sec, Fig. 3D, asterisk not shown) and number of platform crossings (data not shown, P < 0.05) whereas controls did not.
With only four trials per day, both groups did not reach the level of learning achieved with 12 trials per day in the initial and reversal learning trials (cf. Figs. 3C and 2C; note that latencies did not reach <10 sec, as seen during the 12 trials per day study in Experiment 2) or in the probe trial (Fig. 5, see below). However, since the group REM sleep-deprived during the learning phase actually performed better than controls on the reversal probes, the floor reached by controls did not impair the expression of group differences.
Experiment 4—Effects of REM sleep deprivation on initial spatial and subsequent reversal learning with four trials/day and without pre-exposure to the visible water maze
Most of the previous reports describing the effects of REM sleep deprivation on spatial learning in the Morris water maze did not initially screen the rats for visual acuity and swimming ability using the visible platform water maze, as we did in Experiments 1–3. Therefore, it was possible that our findings showing few REM sleep deprivation-associated changes may have been due to the already consolidated learning of the task procedures achieved during the visible platform version of the water maze, long before any REM sleep deprivation was instated. We hypothesized that learning of the task procedures during the visible platform maze may have altered the response to the hidden version of the Morris water maze, protecting against the effects of mild REM sleep deprivation. Thus, we expected that without such prior visible platform water maze exposure, we would reveal REM sleep deprivation-associated performance deficits during concurrent initial spatial learning as previously reported by other researchers.
We tested 12 rats per group on the Morris water maze with four training trials per day and REM sleep deprivation during initial spatial learning without prior visual acuity testing (naive). The REM sleep-deprived group tested was deprived of REM sleep for 6 h immediately following training. Again, contrary to our hypothesis and to previously published reports, both REM sleep-deprived (RD-4LN, n = 12) and home cage control (n = 12) groups learned both the initial platform location and the subsequent reversal phase platform location even when given only four trials/day. There were no group differences during the learning phase training (P > 0.05) (Fig. 4A) or on the learning phase probe trial on day 4 (Fig. 4B). Unlike the pre-exposed four training trials/day rats of Experiment 3, the level of learning achieved in this experiment was similar to that in the 12 training trials/day exposed rats of Experiment 2 as measured by latency to platform during training (Figs. 2C, 4A) and percent time in target quadrant in the probe trial (Figs. 2D, 4B). As was seen in the 12 training trials/day animals of Experiment 2, whether REM sleep-deprived with low or high water levels, these previously REM sleep-deprived rats swam faster than the home cage controls at the start of the reversal phase (day 4) (0.27 meters/sec vs. 0.24 meters/sec, respectively, P < 0.05). We found no group differences during reversal training or in the proximity measure of the day 6 reversal learning probe trial. However, during the reversal probe trial, the previously REM sleep-deprived animals swam across the learning phase platform location (first platform learned) about twice as often as the controls (P = 0.042) (Fig. 4C), indicating stronger persistence toward the original rather than reversed platform location. This was supported by pairwise comparisons showing that controls had a clear preference for the reversal phase platform in all measures (P < 0.05) (Fig. 4B,C, asterisks not shown), whereas previously REM sleep-deprived animals did not prefer the reversal phase platform location during the initial 10 sec (data not shown) or the full 60 sec of the probe trial as measured by percent time in target quadrant (Fig. 4B), nor did they distinguish locations by the number of platform crossings (Fig. 4C). Thus, contrary to our hypothesis, REM sleep deprivation in previously naive rats did not impair initial spatial learning or reversal training performance. However, prior REM sleep deprivation did have detrimental effects on subsequent reversal learning in the group comparison of platform crossings and in pairwise comparisons of percent time in target quadrant and platform crossings.
Figure 4.
Experiment 4 [CON (n = 12); RD-4LN (n = 12)]: The effect of 6 h of REM sleep deprivation on initial learning and subsequent reversal learning across four training trials per day in naive rats not previously exposed to the visual water maze. (A) Latency during the training trials. The solid bar indicates the phase of training when 6 h of RD each day occurred for the experimental groups (A, between days 1 and 2, 2 and 3, and 3 and 4). The dashed line represents when the reversal phase begins. (B) Percent time spent in target quadrant during the probe trials, and (C) number of platform crossings during the probe trials. During the day 6 probe trial, the previously REM sleep-deprived rats crossed the learning phase platform location significantly more times than controls. (Learn) learning phase platform location, (Rev) reversal phase platform location. Each trial set is the average of two consecutive trials. Data are shown as mean ± SEM. *P < 0.05.
Control performance across experiments
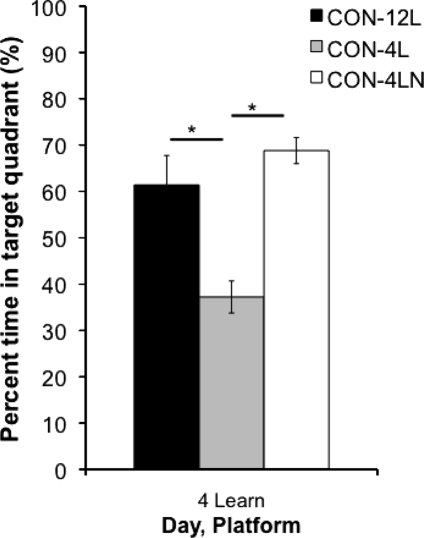
In order to assess the relative difficulty of four vs. 12 trials per day and learning the spatial maze after pre-exposure vs. no pre-exposure, we compared home cage controls from Experiments 2, 3, and 4 on percent time in the learning phase target quadrant on the day 4 probe trial. Controls that were trained with 12 training trials/day spent significantly more time in the target quadrant than controls with only four training trials/day (P = 0.003) (Fig. 5). Controls with four training trials/day that were not pretrained in the visible platform water maze spent significantly longer in the target quadrant than those four trials/day-rats who were pre-exposed to the visible water maze before spatial learning (P < 0.0001) (Fig. 5). There was no difference between the 12 training trials/day-controls (Experiment 2) and naive four training trials/day-controls (Experiment 4) in percent time in target quadrant. These results were replicated when we compared the groups that were REM sleep-deprived during the initial spatial learning phase across experiments (P < 0.0001, Experiment 2 vs. Experiment 3; P < 0.0001, Experiment 3 vs. Experiment 4; P > 0.01, Experiment 2 vs. Experiment 4). These comparisons indicate that both training load (trials per day) and pre-exposure to a swim task altered the level of learning achieved, though REM sleep deprivation itself had little effect.
Figure 5.
The percent time the control groups spent in the target quadrant on the day 4 probe trial. The controls from Experiment 2 (CON-12L) spent significantly more time in the learning phase target quadrant than the controls from Experiment 3 (CON-4L). The controls from Experiment 4 (CON-4LN) also spent significantly more time in the learning phase target quadrant than the controls from Experiment 3. (Learn) learning phase. Data are shown as mean ± SEM. *P < 0.05.
Summary
Overall it appears that, in contrast to our hypotheses, REM sleep deprivation and control groups performed similarly across training trials and most probe trial measures. When training intensity was reduced to four trials/day, some REM sleep deprivation-associated effects were found in either the initial or reversal probe trial measures depending on the level of control performance (Experiments 3 and 4) (Figs. 3D–F, 4B,C). Our findings did not coincide with previous reports indicating that short bouts (Smith and Rose 1996, 1997) of REM sleep deprivation following learning impaired latency performance during training in the Morris water maze.
Discussion
To date, this is the first study to determine the effects of short bouts of REM sleep deprivation on the reversal of spatial learning in the Morris water maze. Further, this is the first study to determine the effects of short bouts of REM sleep deprivation administered during initial spatial learning on subsequent reversal learning in the Morris water maze.
We used a comprehensive span of measurements on both training and probe trials, including the Gallagher measures, previously shown to be more robust and sensitive to group differences (Gallagher et al. 1993; discussed in Hodges 1996; Maei et al. 2009). Despite these sensitive metrics, in contrast to our hypotheses, we found that 6 h of REM sleep deprivation after reversal training each day, whether immediately following or delayed by 6 h, had no effect on reversal training or on reversal probe trial performance (Experiments 1 and 3). Further, we found no effect of previous REM sleep deprivation during the initial training on subsequent reversal training with12 trials/day (Experiment 2). With four trials/day, initial probe performance was impaired by REM sleep deprivation in one of three measures (percent time in target quadrant in first 10 sec), in accordance with our hypothesis, but subsequent reversal probe trial performance was enhanced compared to control performance in the proximity measure (Fig. 3F) and as shown in preference for the target quadrant and platform location, which controls lacked. This may suggest that the hippocampal spatial memory network of rats REM sleep-deprived during initial training was more free from the interference of a previously learned location than controls and thus better able to learn the reversal platform. However, in rats that were naive to the Morris water maze, initial probe performance after four trials/day training was good in both REM sleep-deprived and control groups. Under this condition, in agreement with our hypothesis, subsequent reversal learning was impaired by prior REM sleep deprivation as shown in the lack of preference for the target quadrant and target platform crossings and as shown in more original platform crossings relative to controls (Fig. 4B,C). This case may suggest that the hippocampal network was still occupied by the initial platform location mapping and not yet freed to learn a second location. Finally, in contrast to the literature, we found no effect of 6 h of REM sleep deprivation on initial spatial training or initial probe trial performance with either 12 trials/day in pre-exposed animals or four trials/day in naive animals (Experiments 2 and 4) according to any metric. Therefore, our study contradicts prior publications and only partially supported our hypotheses in the cases where performance was impaired by pre-exposure and/or made susceptible by a lower training intensity (fewer training trials/day).
In comparing performance across experiments (Fig. 5), we believe that prior exposure to the visible platform water maze may have taught rats that the platform could be moved around the tank and therefore located in other areas. During the probe trial, they did not persist searching in the one location for the platform. Performance was better during the probe trial in previously naive animals that were unaware that the platform could be located elsewhere (Experiments 3 and 4). In these two experiments, four training trials/day was enough to train rats to learn that the platform would be located in the one region. With 12 training trials/day in Experiments 1 and 2 (Fig. 2), there may have been ample reinforcement of a single platform location despite previous exposure to the visible water maze, such that the rats persisted in their search of that area during the probe trial. In support of this, during the reversal probe trial on day 6, when all groups had been exposed to the alternative, second hidden platform location, percent time in target quadrant was low for all groups as they searched in other locations for the missing platform.
Reversal learning concurrent to REM sleep deprivation
We are the first to test REM sleep deprivation effects on concurrent reversal spatial learning. The lack of effect following 6 h of REM sleep deprivation concurrent to reversal training with four or 12 training trials/day suggests that the reversal of spatial learning may be impervious to concurrent REM sleep deprivation, at least in rats that were not naive to a movable platform. Some issues surrounding this result, which ran counter to our hypothesis, are discussed below.
Initial spatial learning concurrent to REM sleep deprivation
We are not the first to show that REM sleep deprivation is not associated with concurrent performance deficits (Albert et al. 1970; Miller et al. 1971; Sloan 1972; Shiromani et al. 1979; Van Hulzen and Coenen 1979; Marti-Nicolovius et al. 1988; Gisquet-Verrier and Smith 1989; Yang et al. 2002). Even across the studies reporting REM sleep deprivation-associated deficits in performance, the results can be varying or contradictory (e.g., Youngblood et al. 1997; Yang et al. 2002; Ruskin et al. 2006). Though overall the number of trials in Experiment 2 was far more than in the previous Smith and Rose (1997) study, we performed statistical measures similar to the previous study, looking at the last four trials on day 1 compared to the first four trials on day 2. However, this did not alter our overall results, that REM sleep deprivation did not affect performance in the Morris water maze with 12 training trials/day.
Factors contributing to lack of concurrent REM sleep deprivation effects
Incomplete deprivation, overtraining, pretraining, and floor effects are factors that could contribute to the overall lack of effect of concurrent REM sleep deprivation on either initial spatial learning or reversal learning in the Morris water maze. Morris water maze protocol differences, including maze geometry and REM sleep deprivation technique differences, could also contribute to the lack of effect on concurrent learning. These factors are discussed below.
A lack of REM sleep deprivation-associated performance deficit could result from incomplete deprivation of REM sleep during the deprivation period (Horne and McGrath 1984). However, we showed a complete loss of REM sleep during the 6-h deprivation period (Fig. 1A). It is also unlikely that our mostly null effects result from using too short a REM sleep deprivation period, as prior research with spatial learning described performance deficits with only four hours of REM sleep deprivation (Smith and Rose 1996, 1997; Smith et al. 1998; Bjorness et al. 2005). It is possible that, regardless of our extended REM sleep deprivation period, our lack of REM sleep deprivation-associated deficit results from us not targeting the correct REM sleep critical window. We based our window on that previously described in the Smith and Rose (1997) study using 12 training trials/day, similar to our Experiments 1 and 2. While in Experiment 1, we tested a second, delayed window, we did not test for the effects of REM sleep deprivation between 13 and 24 h following training on our protocol.
Previously, it had been suggested that a lack of concurrent REM sleep deprivation-associated deficit could be due to the administration of overtraining, causing robust learning prior to the REM sleep deprivation period and no need for further REM sleep refinement (for review, see McGrath and Cohen 1978). Indeed, with sufficient training, learning in the Morris water maze becomes hippocampus-independent (Hoh et al. 1999) and would therefore be assumed to be REM sleep-independent as well. In our hands, animals show robust initial spatial training and initial probe trial performance and were not affected by concurrent REM sleep deprivation in those same initial learning metrics, except under the weakest learning conditions (four trials/day previously maze-exposed animals). However, in the four trials/day exposure condition (Experiments 3 and 4), learning is not yet asymptotic by the time of their first REM sleep deprivation exposure (Figs. 3B, 4A), yet the performance of the naive rat on initial learning is not affected by concurrent REM sleep deprivation in any measure. Our Experiment 4 average day 1 performance latencies were also poorer than those previously described (Smith and Rose 1996), which would suggest that our unexpectedly negative results in initial learning were not due to more learning in our study vs. that of others prior to the first REM sleep deprivation period. Therefore, sufficient training or hippocampal independence cannot fully explain our lack of effect of REM sleep deprivation on concurrent learning.
Pretraining on the visual water maze can also decrease the necessity for hippocampal processing of the spatial Morris water maze, as shown in a lesion study (Cain et al. 2006). It is possible that pretraining rendered the task sufficiently independent of the hippocampus to make it immune to REM sleep deprivation effects in the 12 trials/day experiments. Indeed, pretraining seemed to facilitate early day 1 training performance (cf. Experiment 3, Fig. 3C and Experiment 4, Fig. 4A) before any REM sleep deprivation was given. However, when animals received only four training trials/day, pretraining seemed to interfere with, rather than assist, performance in the day 4 probe trial (Fig. 5). As no REM sleep deprivation-associated deficits were observed during initial training in the previously naive rats (Experiment 4), prior training on the visible platform version of the water maze does not protect learning from the effects of REM sleep deprivation and cannot account for the differences between our results and those previously reported (Smith and Rose 1996, 1997).
The opposite possibility to too much training potentially protecting learning is that too few trials during reversal training could obscure REM sleep deprivation-associated deficits by a floor effect in reversal probe trial performance in Experiments 1–3. Although performance during reversal training trials themselves appears normal, both under concurrent REM sleep deprivation and prior REM sleep deprivation, a prior REM sleep deprivation-associated deficit was observed only when reversal probe trial performance showed no floor effect, i.e., controls performed well (Experiment 4).
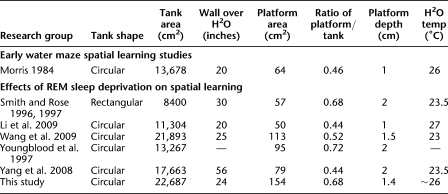
While other studies of short and long bouts of REM sleep deprivation described associated deficits in Morris water maze spatial learning (Smith and Rose 1996, 1997; Youngblood et al. 1997; Yang et al. 2008; Li et al. 2009; Wang et al. 2009), they differed on variables reported, and the findings sometimes conflicted. These inconsistencies across studies may be a result of variances within the protocols used, such as the period of REM sleep deprivation or the exact training procedure in the Morris water maze. Despite our attempts to match rat strain, the number of training trials used (Experiments 1–3), prior maze exposure (Experiment 4), and the level of water within the deprivation chamber (Experiment 2), we did not reproduce the previous findings using short bouts of REM sleep deprivation (Smith and Rose 1996, 1997). Differences between our results and previous reports is also likely not a result of variances in water temperature within the water maze (Woods et al. 1964; as described in Morris 1984), ratio of the platform size to the water maze size (see Vorhees and Williams 2006), or cue enrichment in the maze room, which can each affect learning; our parameters fall within those previously reported (Table 2).
Table 2.
Details on the Morris water maze tank utilized across a subgroup of studies
The studies included in this table are the original Morris 1984 paper and those focusing on the effects of REM sleep deprivation on the consolidation of spatial learning using the Morris water maze. For the platform measurements, unless indicated, the size provided was presumed to be the diameter of a circular platform surface.
One difference we did not address between our study and the two others with short periods of REM sleep deprivation following water maze training was the shape of the maze tank. Circular water mazes such as the one we employed are typically used when testing spatial learning via the Morris water maze. However, in the Smith and Rose (1996, 1997) studies, a rectangular tank was used. In addition to the typical distal or extramaze room cues provided, the corners of a rectangular tank could act as proximal or local cues (Jones et al. 2007). Mapping to local cues with geometric components can be sensitive to hippocampal damage (Pearce et al. 2004; Jones et al. 2007). While both proximal and distal cues together can form a spatial map (Collett et al. 1986; Biegler and Morris 1993; Gothard et al. 1996), reliance on proximal cues appears to be more difficult than using distal cues alone (Gothard et al. 1996; Parron et al. 2004). In our study, the circular tank would have reduced the chance of any overt geometric proximal cues, promoting distal cue-based learning on day 1 prior to the short REM sleep deprivation period. While we showed REM sleep deprivation had no strong effect on spatial learning based on mapping of distal cues, the previous reports of performance deficits in the rectangular water maze following short periods of REM sleep deprivation (Smith and Rose 1996, 1997) may have been describing the REM sleep deprivation effects on a more difficult spatial learning task relying on the challenging strategy of mapping local geometric cues.
Another protocol difference that could contribute to the inconsistent results between the previous studies (Smith and Rose 1996, 1997) and our study is that we used multiple inverted flowerpots in the REM sleep deprivation chamber (see Table 1) to reduce the stress associated with movement restriction (Van Hulzen and Coenen 1981). However, previous research on adrenalectomized rats who cannot mount the stress response still showed sleep deprivation-associated performance deficits, indicating that such deficits are likely not a result of the stress related to the deprivation technique itself (Ruskin et al. 2006). Further, both Li et al. (2009) and Wang et al. (2009) found REM sleep deprivation-associated deficits in spatial learning even with multiple platforms within the deprivation chambers. Therefore, the difference between our findings and those previously reported were likely not the result of variances in the deprivation chambers.
An additional protocol difference when considering our study in comparison to those of Smith and Rose (1996, 1997) is the source of the Sprague-Dawley rats. For our experiments, we used Sprague-Dawley rats acquired from Harlan, Indianapolis, IN. In contrast, those used in the Smith and Rose studies were bred in-house at Trent University. A more recent study by Fogel et al. (2009) indicated a different REM sleep-sensitive window compared to the in-house breed of Sprague-Dawleys previously used in Smith and Lapp (1986). It is possible that further differences exist between rodents sourced from different locations (external company vs. in-house breeding).
Reversal learning subsequent to REM sleep deprivation
Our results on reversal learning were not, on the whole, similar to the findings that REM sleep deprivation impairs concurrent or subsequent extinction learning (Silvestri 2005; Fu et al. 2007), suggesting that REM sleep deprivation has differential effects on reversal of spatial learning in the Morris water maze and extinction of conditioned learning.
With 12 trials/day, we found no differences during subsequent reversal training or in reversal probe trial measures (Fig. 2C,D). With four trials/day, again there was little effect of REM sleep deprivation during training, though we did find that prior REM sleep deprivation was associated with poorer initial learning (Fig. 3E) and stronger adherence to the subsequent reversal platform location in rats pretrained on the visual water maze (Fig. 3D,F) and with poorer reversal learning or greater persistence to the originally learned platform location after reversal training in naive rats (Fig. 4C). An enhancement of subsequent reversal learning with REM sleep deprivation could possibly be due to less proactive interference from poorer learning of the initial platform location as compared to controls. On the other hand, poorer reversal performance with REM sleep deprivation in animals that had only been exposed to one platform location may result from increased proactive interference from the as-yet-incompletely consolidated first learned platform location. Alternatively, the lower reversal performance could indicate better adaptive learning of the possibility that the platform could be located elsewhere. These results should be interpreted cautiously, as deficits were not observed in the training trial measures, nor in some of the probe trial measures tested.
Is REM sleep essential for learning?
Our results indicate that REM sleep is not essential for spatial learning in the Morris water maze. Though we found some differences in performance associated with REM sleep deprivation, we conclude that overall, REM sleep deprivation had little effect in Morris water maze performance in the rat model in our experiments.
To date, there has been much controversy on whether learning is facilitated by REM sleep (Fishbein 1971; Pearlman 1973; Leconte et al. 1974; Fishbein and Gutwein 1977; Gutwein and Fishbein 1980a,b; Smith and Butler 1982; Hars et al. 1985; Smith 1985, 1995; Smith and Lapp 1991; Smith and Wong 1991; Hennevin et al. 1995; Smith and Rose 1996, 1997; Youngblood et al. 1997; Smith et al. 1998; Poe et al. 2000; Le Marec et al. 2001; Bjorness et al. 2005; Silvestri 2005; Fu et al. 2007; Yang et al. 2008; Li et al. 2009; Wang et al. 2009) or is independent of REM sleep (Albert et al. 1970; Miller et al. 1971; Sloan 1972; McGrath and Cohen 1978; Shiromani et al. 1979; Van Hulzen and Coenen 1979; Horne and McGrath 1984; Smith 1985; Horne 1988; Yang et al. 2008). Only a few studies have focused on the effect of REM sleep deprivation on the consolidation of spatial learning (Smith and Rose 1996, 1997; Youngblood et al. 1997; Bjorness et al. 2005; Yang et al. 2008; Li et al. 2009; Wang et al. 2009). Short periods of REM sleep deprivation negatively affected performance in the Morris water maze and eight-box maze (Smith and Rose 1996, 1997). With longer periods of REM sleep deprivation, however, results were inconsistent and described by different performance measures.
Spatial learning in the Morris water maze is thought to be dependent on the cellular process of hippocampal long-term potentiation (LTP), which is disrupted by subsequent REM sleep deprivation (Ishikawa et al. 2006). REM sleep deprivation prior to hippocampal stimulation also impairs LTP induction and/or maintenance, as well as basal hippocampal excitability (Campbell et al. 2002; Davis et al. 2003; McDermott et al. 2003; Romcy-Pereira and Pavlides 2004; Marks and Wayner 2005). However, the Morris water maze can also be considered a threatening experience—posing the potential of drowning to the rat as incentive to find the platform. Though previously shown to be associated with the hippocampus, the added component of fear or heightened drive to find the platform may remove potential reliance on a sleep interaction with hippocampal learning. The majority of our results indicated that either our REM sleep deprivation protocol was not long enough to effect such plasticity changes or that, under the majority of our learning conditions, such REM sleep deprivation effect on hippocampal cellular mechanisms was not sufficient to meaningfully alter performance. A final alternative is that the neurocellular mechanisms necessary for a rat to perform our paradigm in the Morris water maze are not reliant on REM sleep. Under our protocol, in the Sprague-Dawley rat model, REM sleep was not essential for concurrent initial spatial or reversal learning.
It has been posited that REM sleep is integral for depotentiation in order to free up previously potentiated networks, allowing for subsequent learning, such as reversal learning (Crick and Mitchison 1983; Poe et al. 2000; Booth and Poe 2006). Some of our positive results (four trials/day reversal probe results in naive animals) supported this idea, showing that animals with normal REM sleep had more robust reversal learning as measured by the probe trial. However, six hours of REM sleep deprivation was not sufficient to result in reversal training trial performance deficits. Our negative probe trial test results were uninterpretable due to already low performance in all treatment groups. This area requires further research as does the effect of short bouts of REM sleep deprivation on depotentiation within the hippocampus.
A key issue with studying the effects of REM sleep deprivation on learning is to account for the effects of the deprivation protocol on the physiology of the subject. Other factors that can contribute to altered performance arising from REM sleep deprivation are decreased attention (Godoi et al. 2005), altered drive for making voluntary movements (Elomaa and Johansson 1986), decreased motivation for seeking food reward (Hanlon et al. 2005), but increased hunger (Kushida et al. 1989), and increased cold (Savourey and Bittel 1994) and pain sensitivity (Hakki Onen et al. 2001; Roehrs et al. 2006). Therefore, while an effect of REM sleep deprivation may be identified, it may not be an effect on the learning process itself but instead on the subject's physiological responses to various parameters and requirements of the task. Though short periods of REM sleep deprivation are less likely to be confounded with the side effects of longer REM sleep deprivation, most studies have not attempted to separate the effects of REM sleep deprivation physiology changes on performance vs. learning (for review, see Vertes and Eastman 2000). In our study, swim-speed increased slightly but significantly during subsequent reversal learning with prior REM sleep deprivation (Experiments 2 and 4), perhaps reflecting an altered physiological state during the REM sleep recovery period, such as recovery from heightened cold sensitivity. However, performance was much more greatly influenced by changing the number of trials per day and pre-exposure to a swim task (Fig. 5) than by REM sleep deprivation-associated effects.
Possibly there is no perfect task to test the effects of REM sleep deprivation on spatial learning. The variance in learning reported across studies for the effect of both long and short periods of REM sleep deprivation on performance in the Morris water maze may suggest that it is not a suitable tool to test sleep deprivation effects on spatial learning. Indeed, a pronounced effect of REM sleep deprivation would be unexpected since even hippocampal inactivation results in only mild deficits (Cimadevilla et al. 2005; Cimadevilla and Arias 2008). In light of such mild deficits in spatial learning with hippocampal inactivation, it is possible that the strong performance deficits observed in the rectangular maze with REM sleep deprivation are broader than the effects of REM sleep deprivation on spatial learning. Our results contribute to the ongoing debate of whether REM sleep deprivation impairs learning (McGrath and Cohen 1978; Smith 1985, 1995; Vertes and Eastman 2000; Hobson and Pace-Schott 2002; Vertes 2004; Rauchs et al. 2005; for example, Stickgold and Walker 2005; Vertes and Siegel 2005) by showing overall no effect of six hours of REM sleep deprivation on training trial performance, representing preserved working and short term memory.
Conclusion
We found that six hours of REM sleep deprivation had little detected effect on training trial performance in the Morris water maze, with some differences arising during the probe trials when training intensity was reduced from 12 to four trials/day. Overall, we found that varying the number of training trials and prior exposure to the swim task affected performance to a much greater extent than the 6 h of REM sleep deprivation protocol. Further research is required to determine whether REM sleep deprivation affects concurrent spatial, concurrent reversal, or subsequent reversal learning in other learning tasks.
Materials and Methods
Animals
All rats used in this study were Sprague-Dawley male rats (average weight: 390 g; Harlan, Indianapolis, IN). Animals were housed in a 12:12 light cycle at an average temperature of 23°C. Procedures were approved by the animal review board of the University Committee on Use and Care of Animals at the University of Michigan. Rats had ad libitum access to fresh drinking water and food at all times except while in the water maze. Each rat was weighed at the start of each experimental day, before testing, to monitor changes in percent body weight. Each rat was individually housed. Rats were allowed 5 d to acclimate to their housing environment. During these 5 d, rats were placed into individual REM sleep deprivation chambers for 45 min each on 2 sequential days (see REM sleep deprivation protocol for further description).
REM sleep deprivation protocol
The REM sleep deprivation tank (61 cm long × 47 cm wide × 50.8 cm tall) contained three inverted flowerpots (24 cm tall), forming 3 bases on which the rats could rest (Bjorness et al. 2005; Ravassard et al. 2009). Each base was 6 cm in diameter to maintain the necessary size ratio of rat weight-to-flowerpot base previously shown to induce REM sleep deprivation (Hicks et al. 1977; McGrath and Cohen 1978). The distance between the platforms was 9 cm to allow the rats to easily step between them. Drinking water and food were freely available in the deprivation chamber. A netted lid was placed over the REM sleep deprivation tanks, leaving enough space for the rats to rear up without reaching the lid. The netting enabled the experimenter to observe the rats remotely using an overhead camera projected to a neighboring room. Room temperature was kept constant at 23°C. A low level of water (2 cm in height) in the base of the deprivation tank was used for this study. This level of water allows the rats to relax their tails without touching the surface of the water, presenting no thermoregulation challenge. Rats were closely observed for signs of distress and to monitor their behavior. All REM sleep deprivation periods lasted for 6 h.
Visual water maze protocol
Rats were tested for visual and motor acuity using a visual platform in the water maze (Morris 1984) across two consecutive days to ensure that rats had the ability to perform the hidden platform version of the Morris water maze, both for the visual and motoric components. The water maze consisted of a circular tank (170 cm diameter), painted black and filled with clear water. The platform (14 cm diameter, 26.7 cm tall) was covered with a striped white and navy pattern and was 2 cm above the surface of the water. The water temperature was maintained at ∼ 26 ± 1°C. Surrounding the tank was a black curtain to remove all spatial cues, and the room was lit with overhead lighting. During testing, each rat was placed in an individual towel-lined cage with a microfilter lid. After 10 min acclimation to the room, each rat was placed, in turn, into the tank at one of four locations (North, South, East, or West) and allowed a maximum of 60 sec to find the platform. If the rat did not find the platform within the time limit, it was guided by hand to the platform location. Once on the platform, each rat remained there for an additional 20 sec. Each rat in the testing group completed its trial in turn, before the next trial was begun. At the start of each trial, the platform was moved to one of four different locations (Northeast, Northwest, Southeast, or Southwest). Each rat received a total of five trials per day for two consecutive days. The visual version of the water maze started either 5 d prior to the hidden version of the Morris water maze (Experiments 1–3), or the day after completion of the hidden version of the Morris water maze (Experiment 4). The rat's performance on the visual platform task indicated that they had both sufficient vision and the motor and mental competence to perform the task. For experiments 1–3, where rats were tested on the visible platform before the experiments, ∼25% of all tested rats were excluded due to poor performance.
Morris water maze protocol
The same water maze tank and pedestal as described in the visual platform protocol were used for the spatial learning version of the Morris water maze. However, the platform was covered with black material to match the color of the tank. Unlike the visual water maze, the platform for the standard Morris water maze was hidden 14 mm below the surface of the water. The room contained a number of spatial cues such as a large black curtain in one corner, a large picture on one side, a rack with hoses and mops on another side, and an additional four smaller but strongly contrasting images placed around the room.
At the start of lights-on on day 1, each rat was placed, in turn, onto the hidden platform for either 20 sec (Experiments 1–3) or 2 min (Experiment 4) to introduce the hidden platform. At the start of each trial, a rat was placed into the tank at one of four entry points, North, South, East, or West. The entry point for each trial was semirandomized across trials, but the order was constant across rats. No trial had the same entry point as the prior trial, but on any given trial number, all rats had the same entry point. Maximum trial length was set at 90 sec. If a rat did not find the platform within the allowed time, they were guided by hand to the hidden platform. After each trial, the rat remained on the hidden platform for an additional 20 sec. All rats were run in groups of six, and the whole group completed each trial before the next trial was begun.
In addition to daily training, spatial memory was tested using a probe trial at the start of day 4 and day 6 (see Fig. 1 for the protocol outline). For the two probe trials, the hidden platform was removed, and rats were placed into the water maze tank for 60 sec. At the end of the 60 sec, they were removed and returned to their water maze cages.
For the learning phase of this experiment on days 1–4, the hidden platform was located in the Northeast quadrant, 38 cm from the tank wall, equidistant from both the North and the East edge of the quadrant.
The reversal phase started after either six (Experiments 1 and 2) or two (Experiments 3 and 4) training trials on day 4, at which point the platform was moved to the opposite quadrant of the tank (Southwest quadrant). All room cues remained in their original positions, not changing between the learning phase and the reversal phase. The platform remained in this Southwest quadrant location for all training trials on both days 5 and 6, but not for the day 6 probe trial.
At the end of each day of training, the rats remained in their towel-lined cages for 10 min to dry and then were returned to either their home cage or to the REM sleep deprivation tanks. Home cage controls were tested in parallel with REM sleep deprivation experimental groups in each of our experiments (1–4).
Performance metrics
Many previous studies on the effects of REM sleep deprivation in the Morris water maze report performance as: latency to platform (Smith and Rose 1996, 1997; Li et al. 2009; Wang et al. 2009), path length (Li et al. 2009; Wang et al. 2009), number of target quadrant entries during training (Smith and Rose 1996), area under the curve for both latency and path length (Youngblood et al. 1997), or percent time spent in target quadrant during a probe trial (Wang et al. 2009). Though not previously used in the REM sleep deprivation and Morris water maze literature, Gallagher's cumulative distance and average proximity (Gallagher et al. 1993; Hodges 1996; Maei et al. 2009) are more sensitive and robust measures, which control for group differences in overall velocity. For convention, we depict latency for training trials and percent time in target quadrant for probe trials within the Figures (Figs. 2,3,4,5), rather than Gallagher's cumulative distance or average proximity measures. However, the Gallagher measures showed the same results in all cases except where noted (Fig. 5).
The dependent variables we measured during training trials were latency, path length, velocity, and Gallagher's cumulative distance from the platform (Gallagher et al. 1993). During probe trials, the dependent variables were Gallagher's average proximity error to the platform (Gallagher et al. 1993), number of platform crossings, percent time in target quadrant, path length, and velocity. All measures other than latency were acquired and processed using 4.1 EthoVision XT (Noldus Information Technology). Missing data points were interpolated off-line using an in-house program. Velocity for the missing data points was interpolated by the average of prior and post samples. Distance for the missing data points was determined by the duration of time and the relevant interpolated velocity. For the Gallagher and “in zone” measures (used to calculate percent time in quadrant and number of platform crossings), default EthoVision interpolation was retained for calculating the missing data points. When calculating the Gallagher measures (average proximity to the platform and cumulative distance from the platform), the time taken to swim directly between the initial start location and platform location for each individual rat was calculated and removed from the data set for each trial, and the data were down-sampled to 1 Hz. All other measures were sampled at 5 Hz.
Data were analyzed as trial sets (average performance across three consecutive trials for Experiments 1 and 2, and across two consecutive trials for Experiments 3 and 4) and in specific cases, as single trials. Retention was measured by comparing the last trial of a day with the first trial of the subsequent day. Retention was also calculated for the last trial set vs. the first trial set the subsequent day. Comparisons were not made for retention differences on days 4 and 6 due to potential interference resulting from the probe trial.
In addition to analyzing the entire length of the probe trial for the proximity, number of platform crossings, and percent time in target quadrant variables measured, the first 10 sec of the 60-sec probe trials were also analyzed to identify any initial performance differences in percent time in target quadrant and number of platform area crossings. Initial deficits may be masked by the entire trial length, e.g., due to lack of persistence in swimming in the target area once the platform was found to be missing (likely in rats previously exposed to a moving or missing platform).
Statistics
All analyses were done using SPSS (SPSS Inc.). In all cases, when sphericity could not be assumed during a Repeated Measures Analysis Of Variance (RMANOVA), the Huynh-Feldt correction was used. Statistical significance was set at P < 0.05.
RMANOVAs were used, and post-hoc analyses using a Tukey correction were administered when an effect was found. The learning phase was analyzed with all trial sets for 3 d (days 1, 2, and 3). To determine differences within each day, RMANOVAs were used across all trial sets on days 1, 2, and 3, and across the learning phase trial sets on day 4. The reversal phase was analyzed with all trial sets for 2 d (days 5 and 6). To determine differences within each day, RMANOVAs were used across all trial sets on days 5 and 6, and across the reversal phase trial sets on day 4. Additionally, we analyzed the first trial set alone and the single first trial on days 2, 3, and 5, using either independent t-tests or one-way ANOVA. Retention at the start of days 2, 3, and 5 was analyzed using a one-way ANOVA or independent t-tests on the difference between the last trial set (days 1, 2, and 4) and the first trial set on the subsequent day (days 2, 3, and 5, respectively). The retention analyses were also performed on the difference between the last single trial and the first single trial the subsequent day. Both the day 4 and day 6 probe trials were analyzed using one-way ANOVAs or independent t-tests to determine group differences. Pairwise t-tests were used where appropriate.
Sleep recording protocol
To determine the differences in sleep/waking characteristics with high level water vs. low level water REM sleep deprivation, four male Sprague-Dawley rats were tested for the effect of high vs. low levels of water in the deprivation chambers on the sleep cycle.
Four male Sprague-Dawley rats were implanted with four screw electroencephalogram (EEG) electrodes: two bilaterally over the frontal cortex and two bilaterally over the parietal cortex and two nuchal EMG. After 7 d of recovery from surgery, and habituation to the deprivation chambers, each rat was placed into one of two groups: low water level deprivation first, or high water level deprivation first. Each rat underwent deprivation with both water levels separated by prior baseline and post-recovery periods. Twenty-four hours prior to the 6-h deprivation period, baseline periods at the same circadian phase were recorded. Sleep/waking states were determined off-line using a within-lab designed sleep scoring program (Gross et al. 2009) based in MATLAB. A state was scored when its criteria were met in at least 50% of the 10-sec epoch. Active waking, quiet waking, quiet sleep (QS), REM sleep (RS), and transitions to RS (TRS) were scored. Data were compared as percent change from baseline.
Experiment 1—The effects of 6 h of REM sleep deprivation on concurrent reversal learning with 12 training trials per day
All rats in Experiment 1 were first tested using the visual platform maze and then trained on the hidden platform version of the Morris water maze using 12 training trials per day. The effect of 6 h of REM sleep deprivation on reversal learning was tested using three groups: (1) home cage controls (CON, n = 8); (2) delayed REM sleep deprivation with 6 h of REM sleep deprivation starting 6 h after the end of training on days 4, 5, and 6 (RDdelay-12R, n = 8); and (3) immediate REM sleep deprivation with 6 h of REM sleep deprivation starting immediately after the end of training on days 4, 5, and 6 (RD-12R, n = 8). For the latter part of the REM sleep deprivation protocol for RDdelay-12R, the lights were turned off to maintain their 12:12 light:dark cycle.
Experiment 2—The effects of six hours of REM sleep deprivation on concurrent initial spatial learning and on subsequent reversal learning with 12 training trials per day
Similar to Experiment 1, all rats in Experiment 2 were first tested using the visual platform maze and then trained on the hidden platform version of the Morris water maze using 12 training trials per day. The effect of 6 h of REM sleep deprivation on initial spatial learning was tested using two groups: home cage controls (CON, n = 7), and immediate REM sleep deprivation during the learning phase on days 1, 2, and 3 (RD-12L, n = 10). In addition, we tested the effect of 6 h of REM sleep deprivation using a high level of water within the REM sleep deprivation chamber on initial spatial learning. For this an additional group (High, n = 7) was tested compared to the home cage controls (CON, n = 7). Unlike the low level of water (∼22 cm below the base of the platforms [Bjorness et al. 2005; Ravassard et al. 2009]) within the deprivation chambers described in the REM sleep deprivation protocol, used in all our other experiments (1, 3, and 4), the high level of water used for the High group was at a height 1 cm below the base of the platforms. This high level of water is commonly used as described in a number of previous REM sleep deprivation and Morris water maze learning studies (Table 1; Smith and Rose 1996, 1997; Youngblood et al. 1997; Smith et al. 1998; Yang et al. 2008; Li et al. 2009; Wang et al. 2009).
Experiment 3—The effects of six hours of REM sleep deprivation on concurrent reversal learning, or on concurrent initial spatial learning and subsequent reversal learning with four training trials per day
Similar to Experiments 1 and 2, all rats were first tested on the visual form of the water maze prior to training on the hidden platform version of the Morris water maze. Similar to Experiment 1, rats were tested for the effect of REM sleep deprivation on reversal learning but with only four training trials per day. To test this, a group REM sleep-deprived for 6 h immediately following training during the reversal phase on days 4 and 5 (RD-4R, n = 9) were compared to home cage controls (CON, n = 9). Similar to Experiment 2, rats were tested for the effect of REM sleep deprivation on initial spatial learning and the effects on subsequent reversal learning when the rats were no longer sleep-disturbed. However, only four training trials per day and a low level of water within the deprivation chambers were used in this experiment. Rats were REM sleep-deprived for 6 h immediately following training during the learning phase on days 1, 2, and 3 (RD-4L, n = 8) and compared to the home cage controls.
Experiment 4—The effects of pre-exposure to the visible water maze prior to four training trials per day spatial learning
Rats were tested to determine the effect of REM sleep deprivation on spatial learning with only four training trials per day when not previously exposed to the visible platform version of the Morris water maze. Two groups were used for this experiment: home cage controls (CON, n = 12) and a group REM sleep-deprived for 6 h immediately following training during the learning phase on days 1, 2, and 3 (RD-4LN, n = 12). Unlike the previous experiments, the visible platform version of the Morris water maze was tested on the two days after the hidden platform version of the Morris water maze. All rats performed the visible version of the water maze following the hidden platform Morris water maze sufficiently, resulting in no exclusions from this experiment.
Acknowledgments
We thank Department of Anesthesiology members for their assistance: postdoctoral fellow Dr. Brooks Gross for programming; undergraduate research associate Michelle Kuznia and research technician Lori Saganek for assistance in data collection; and undergraduate research associates Jung-Ho Kim and Meghan Carroll for assistance in sleep scoring. This work was supported by MH60670 and the Department of Anesthesiology at the University of Michigan.
References
- Albert I, Cicala GA, Siegel J 1970. The behavioral effects of REM sleep deprivation in rats. Psychophysiology 6: 550–560 [DOI] [PubMed] [Google Scholar]
- Beaulieu I, Godbout R 2000. Spatial learning on the Morris Water Maze Test after a short-term paradoxical sleep deprivation in the rat. Brain Cogn 43: 27–31 [PubMed] [Google Scholar]
- Biegler R, Morris RG 1993. Landmark stability is a prerequisite for spatial but not discrimination learning. Nature 361: 631–633 [DOI] [PubMed] [Google Scholar]
- Bjorness TE, Riley BT, Tysor MK, Poe GR 2005. REM restriction persistently alters strategy used to solve a spatial task. Learn Mem 12: 352–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth V, Poe GR 2006. Input source and strength influences overall firing phase of model hippocampal CA1 pyramidal cells during theta: Relevance to REM sleep reactivation and memory consolidation. Hippocampus 16: 161–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Maho C, Laroche S 1994. Suppression of long-term potentiation induction during alert wakefulness but not during “enhanced” REM sleep after avoidance learning. Neuroscience 59: 501–509 [DOI] [PubMed] [Google Scholar]
- Cain DP, Boon F, Corcoran ME 2006. Thalamic and hippocampal mechanisms in spatial navigation: A dissociation between brain mechanisms for learning how versus learning where to navigate. Behav Brain Res 170: 241–256 [DOI] [PubMed] [Google Scholar]
- Campbell IG, Guinan MJ, Horowitz JM 2002. Sleep deprivation impairs long-term potentiation in rat hippocampal slices. J Neurophysiol 88: 1073–1076 [DOI] [PubMed] [Google Scholar]
- Cimadevilla JM, Arias JL 2008. Different vulnerability in female's spatial behavior after unilateral hippocampal inactivation. Neurosci Lett 439: 89–93 [DOI] [PubMed] [Google Scholar]
- Cimadevilla JM, Miranda R, Lopez L, Arias JL 2005. Partial unilateral inactivation of the dorsal hippocampus impairs spatial memory in the MWM. Brain Res Cogn Brain Res 25: 741–746 [DOI] [PubMed] [Google Scholar]
- Cirulli F, Berry A, Alleva E 2000. Intracerebroventricular administration of brain-derived neurotrophic factor in adult rats affects analgesia and spontaneous behavior but not memory retention in a Morris Water Maze task. Neurosci Lett 287: 207–210 [DOI] [PubMed] [Google Scholar]
- Cirulli F, Berry A, Chiarotti F, Alleva E 2004. Intrahippocampal administration of BDNF in adult rats affects short-term behavioral plasticity in the Morris water maze and performance in the elevated plus-maze. Hippocampus 14: 802–807 [DOI] [PubMed] [Google Scholar]
- Collett TS, Cartwright BA, Smith BA 1986. Landmark learning and visuo-spatial memories in gerbils. J Comp Physiol A 158: 835–851 [DOI] [PubMed] [Google Scholar]
- Crick F, Mitchison G 1983. The function of dream sleep. Nature 304: 111–114 [DOI] [PubMed] [Google Scholar]
- Davis CJ, Harding JW, Wright JW 2003. REM sleep deprivation-induced deficits in the latency-to-peak induction and maintenance of long-term potentiation within the CA1 region of the hippocampus. Brain Res 973: 293–297 [DOI] [PubMed] [Google Scholar]
- Elomaa E, Johansson GG 1986. Decision-making to initiate voluntary movements in the rat is altered during deprivation of rapid eye movement sleep. Neurosci Lett 63: 51–55 [DOI] [PubMed] [Google Scholar]
- Fishbein W 1971. Disruptive effects of rapid eye movement sleep deprivation on long-term memory. Physiol Behav 6: 279–282 [DOI] [PubMed] [Google Scholar]
- Fishbein W, Gutwein BM 1977. Paradoxical sleep and memory storage processes. Behav Biol 19: 425–464 [DOI] [PubMed] [Google Scholar]
- Fishbein W, Kastaniotis C, Chattman D 1974. Paradoxical sleep: Prolonged augmentation following learning. Brain Res 79: 61–75 [DOI] [PubMed] [Google Scholar]
- Fogel SM, Smith CT, Beninger RJ 2009. Evidence for 2-stage models of sleep and memory: Learning-dependent changes in spindles and theta in rats. Brain Res Bull 79: 445–451 [DOI] [PubMed] [Google Scholar]
- Fu J, Li P, Ouyang X, Gu C, Song Z, Gao J, Han L, Feng S, Tian S, Hu B 2007. Rapid eye movement sleep deprivation selectively impairs recall of fear extinction in hippocampus-independent tasks in rats. Neuroscience 144: 1186–1192 [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M 1993. Severity of spatial learning impairment in aging: Development of a learning index for performance in the Morris water maze. Behav Neurosci 107: 618–626 [DOI] [PubMed] [Google Scholar]
- Gisquet-Verrier P, Smith C 1989. Avoidance performance in rat enhanced by postlearning paradoxical sleep deprivation. Behav Neural Biol 52: 152–169 [DOI] [PubMed] [Google Scholar]
- Godoi FR, Oliveira MG, Tufik S 2005. Effects of paradoxical sleep deprivation on the performance of rats in a model of visual attention. Behav Brain Res 165: 138–145 [DOI] [PubMed] [Google Scholar]
- Gothard KM, Skaggs WE, Moore KM, McNaughton BL 1996. Binding of hippocampal CA1 neural activity to multiple reference frames in a landmark-based navigation task. J Neurosci 16: 823–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross BA, Walsh CM, Turakhia AA, Booth V, Mashour GA, Poe GR 2009. Open-source logic-based automated sleep scoring software using electrophysiological recordings in rats. J Neurosci Methods 184: 10–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutwein BM, Fishbein W 1980a. Paradoxical sleep and memory (I): Selective alterations following enriched and impoverished environmental rearing. Brain Res Bull 5: 9–12 [DOI] [PubMed] [Google Scholar]
- Gutwein BM, Fishbein W 1980b. Paradoxical sleep and memory (II): Sleep circadian rhythmicity following enriched and impoverished environmental rearing. Brain Res Bull 5: 105–109 [DOI] [PubMed] [Google Scholar]
- Hakki Onen S, Alloui A, Jourdan D, Eschalier A, Dubray C 2001. Effects of rapid eye movement (REM) sleep deprivation on pain sensitivity in the rat. Brain Res 900: 261–267 [DOI] [PubMed] [Google Scholar]
- Hanlon EC, Andrzejewski ME, Harder BK, Kelley AE, Benca RM 2005. The effect of REM sleep deprivation on motivation for food reward. Behav Brain Res 163: 58–69 [DOI] [PubMed] [Google Scholar]
- Hars B, Hennevin E, Pasques P 1985. Improvement of learning by cueing during postlearning paradoxical sleep. Behav Brain Res 18: 241–250 [DOI] [PubMed] [Google Scholar]
- Hennevin E, Leconte P, Bloch V 1971. [Effect of acquisition level on the increase of paradoxical sleep duration due to an avoidance conditioning in the rat]. C R Acad Sci Hebd Seances Acad Sci D 273: 2595–2598 [PubMed] [Google Scholar]
- Hennevin E, Hars B, Maho C, Bloch V 1995. Processing of learned information in paradoxical sleep: Relevance for memory. Behav Brain Res 69: 125–135 [DOI] [PubMed] [Google Scholar]
- Hicks RA, Okuda A, Thomsen D 1977. Depriving rats of REM sleep: The identification of a methodological problem. Am J Psychol 90: 95–102 [PubMed] [Google Scholar]
- Hobson JA, Pace-Schott EF 2002. The cognitive neuroscience of sleep: Neuronal systems, consciousness and learning. Nat Rev Neurosci 3: 679–693 [DOI] [PubMed] [Google Scholar]
- Hodges H 1996. Maze procedures: The radial-arm and water maze compared. Brain Res Cogn Brain Res 3: 167–181 [DOI] [PubMed] [Google Scholar]
- Hoh T, Beiko J, Boon F, Weiss S, Cain DP 1999. Complex behavioral strategy and reversal learning in the water maze without NMDA receptor-dependent long-term potentiation. J Neurosci 19: RC2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne JA 1988. Sleep loss and “divergent” thinking ability. Sleep 11: 528–536 [DOI] [PubMed] [Google Scholar]
- Horne JA, McGrath MJ 1984. The consolidation hypothesis for REM sleep function: Stress and other confounding factors—A review. Biol Psychol 18: 165–184 [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Kanayama Y, Matsumura H, Tsuchimochi H, Ishida Y, Nakamura S 2006. Selective rapid eye movement sleep deprivation impairs the maintenance of long-term potentiation in the rat hippocampus. Eur J Neurosci 24: 243–248 [DOI] [PubMed] [Google Scholar]
- Jones PM, Pearce JM, Davies VJ, Good MA, McGregor A 2007. Impaired processing of local geometric features during navigation in a water maze following hippocampal lesions in rats. Behav Neurosci 121: 1258–1271 [DOI] [PubMed] [Google Scholar]
- Kushida CA, Bergmann BM, Rechtschaffen A 1989. Sleep deprivation in the rat: IV. Paradoxical sleep deprivation. Sleep 12: 22–30 [DOI] [PubMed] [Google Scholar]
- Le Marec N, Beaulieu I, Godbout R 2001. Four hours of paradoxical sleep deprivation impairs alternation performance in a water maze in the rat. Brain Cogn 46: 195–197 [DOI] [PubMed] [Google Scholar]
- Leconte P, Hennevin E 1971. [Increase of the duration of paradoxical sleep due to learning in the rat]. C R Acad Sci Hebd Seances Acad Sci D 273: 86–88 [PubMed] [Google Scholar]
- Leconte P, Hennevin E, Bloch V 1974. Duration of paradoxical sleep necessary for the acquisition of conditioned avoidance in the rat. Physiol Behav 13: 675–681 [DOI] [PubMed] [Google Scholar]
- Li S, Tian Y, Ding Y, Jin X, Yan C, Shen X 2009. The effects of rapid eye movement sleep deprivation and recovery on spatial reference memory of young rats. Learn Behav 37: 246–253 [DOI] [PubMed] [Google Scholar]
- Lucero MA 1970. Lengthening of REM sleep duration consecutive to learning in the rat. Brain Res 20: 319–322 [DOI] [PubMed] [Google Scholar]
- Machado RB, Hipolide DC, Benedito-Silva AA, Tufik S 2004. Sleep deprivation induced by the modified multiple platform technique: Quantification of sleep loss and recovery. Brain Res 1004: 45–51 [DOI] [PubMed] [Google Scholar]
- Machado RB, Suchecki D, Tufik S 2006. Comparison of the sleep pattern throughout a protocol of chronic sleep restriction induced by two methods of paradoxical sleep deprivation. Brain Res Bull 70: 213–220 [DOI] [PubMed] [Google Scholar]
- Maei HR, Zaslavsky K, Teixeira CM, Frankland PW 2009. What is the most sensitive measure of Water Maze Probe Test performance? Front Integr Neurosci 3: 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks CA, Wayner MJ 2005. Effects of sleep disruption on rat dentate granule cell LTP in vivo. Brain Res Bull 66: 114–119 [DOI] [PubMed] [Google Scholar]
- Marti-Nicolovius M, Portell-Cortes I, Morgado-Bernal I 1988. Improvement of shuttle-box avoidance following post-training treatment in paradoxical sleep deprivation platforms in rats. Physiol Behav 43: 93–98 [DOI] [PubMed] [Google Scholar]
- Mavanji V, Datta S 2003. Activation of the phasic pontine-wave generator enhances improvement of learning performance: A mechanism for sleep-dependent plasticity. Eur J Neurosci 17: 359–370 [DOI] [PubMed] [Google Scholar]
- McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC 2003. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci 23: 9687–9695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath MJ, Cohen DB 1978. REM sleep facilitation of adaptive waking behavior: A review of the literature. Psychol Bull 85: 24–57 [PubMed] [Google Scholar]
- Miller L, Drew WG, Schwartz I 1971. Effect of REM sleep deprivation on retention of a one-trial passive avoidance response. Percept Mot Skills 33: 118. [DOI] [PubMed] [Google Scholar]
- Morris R 1984. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11: 47–60 [DOI] [PubMed] [Google Scholar]
- Morris RG, Hagan JJ, Rawlins JN 1986. Allocentric spatial learning by hippocampectomised rats: A further test of the “spatial mapping” and “working memory” theories of hippocampal function. Q J Exp Psychol B 38: 365–395 [PubMed] [Google Scholar]
- Parron C, Poucet B, Save E 2004. Entorhinal cortex lesions impair the use of distal but not proximal landmarks during place navigation in the rat. Behav Brain Res 154: 345–352 [DOI] [PubMed] [Google Scholar]
- Pearce JM, Good MA, Jones PM, McGregor A 2004. Transfer of spatial behavior between different environments: Implications for theories of spatial learning and for the role of the hippocampus in spatial learning. J Exp Psychol Anim Behav Process 30: 135–147 [DOI] [PubMed] [Google Scholar]
- Pearlman C 1973. REM sleep deprivation impairs latent extinction in rats. Physiol Behav 11: 233–237 [DOI] [PubMed] [Google Scholar]
- Poe GR, Nitz DA, McNaughton BL, Barnes CA 2000. Experience-dependent phase-reversal of hippocampal neuron firing during REM sleep. Brain Res 855: 176–180 [DOI] [PubMed] [Google Scholar]
- Portell-Cortes I, Marti-Nicolovius M, Segura-Torres P, Morgado-Bernal I 1989. Correlations between paradoxical sleep and shuttle-box conditioning in rats. Behav Neurosci 103: 984–990 [DOI] [PubMed] [Google Scholar]
- Pouzet B, Welzl H, Gubler MK, Broersen L, Veenman CL, Feldon J, Rawlins JN, Yee BK 1999. The effects of NMDA-induced retrohippocampal lesions on performance of four spatial memory tasks known to be sensitive to hippocampal damage in the rat. Eur J Neurosci 11: 123–140 [DOI] [PubMed] [Google Scholar]
- Rauchs G, Desgranges B, Foret J, Eustache F 2005. The relationships between memory systems and sleep stages. J Sleep Res 14: 123–140 [DOI] [PubMed] [Google Scholar]
- Ravassard P, Pachoud B, Comte JC, Mejia-Perez C, Scote-Blachon C, Gay N, Claustrat B, Touret M, Luppi PH, Salin PA 2009. Paradoxical (REM) sleep deprivation causes a large and rapidly reversible decrease in long-term potentiation, synaptic transmission, glutamate receptor protein levels, and ERK/MAPK activation in the dorsal hippocampus. Sleep 32: 227–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T 2006. Sleep loss and REM sleep loss are hyperalgesic. Sleep 29: 145–151 [DOI] [PubMed] [Google Scholar]
- Romcy-Pereira R, Pavlides C 2004. Distinct modulatory effects of sleep on the maintenance of hippocampal and medial prefrontal cortex LTP. Eur J Neurosci 20: 3453–3462 [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Dunn KE, Billiot I, Bazan NG, LaHoste GJ 2006. Eliminating the adrenal stress response does not affect sleep deprivation-induced acquisition deficits in the water maze. Life Sci 78: 2833–2838 [DOI] [PubMed] [Google Scholar]
- Savourey G, Bittel J 1994. Cold thermoregulatory changes induced by sleep deprivation in men. Eur J Appl Physiol Occup Physiol 69: 216–220 [DOI] [PubMed] [Google Scholar]
- Shiromani P, Gutwein BM, Fishbein W 1979. Development of learning and memory in mice after brief paradoxical sleep deprivation. Physiol Behav 22: 971–978 [DOI] [PubMed] [Google Scholar]
- Silvestri AJ 2005. REM sleep deprivation affects extinction of cued but not contextual fear conditioning. Physiol Behav 84: 343–349 [DOI] [PubMed] [Google Scholar]
- Sloan MA 1972. The effects of deprivation of rapid eye movement (REM) sleep on maze learning and aggression in the albino rat. J Psychiatr Res 9: 101–111 [DOI] [PubMed] [Google Scholar]
- Smith C 1985. Sleep states and learning: A review of the animal literature. Neurosci Biobehav Rev 9: 157–168 [DOI] [PubMed] [Google Scholar]
- Smith C 1995. Sleep states and memory processes. Behav Brain Res 69: 137–145 [DOI] [PubMed] [Google Scholar]
- Smith C, Butler S 1982. Paradoxical sleep at selective times following training is necessary for learning. Physiol Behav 29: 469–473 [DOI] [PubMed] [Google Scholar]
- Smith C, Lapp L 1986. Prolonged increases in both PS and number of REMS following a shuttle avoidance task. Physiol Behav 36: 1053–1057 [DOI] [PubMed] [Google Scholar]
- Smith C, Lapp L 1991. Increases in number of REMS and REM density in humans following an intensive learning period. Sleep 14: 325–330 [DOI] [PubMed] [Google Scholar]
- Smith C, Rose GM 1996. Evidence for a paradoxical sleep window for place learning in the Morris water maze. Physiol Behav 59: 93–97 [DOI] [PubMed] [Google Scholar]
- Smith C, Rose GM 1997. Posttraining paradoxical sleep in rats is increased after spatial learning in the Morris water maze. Behav Neurosci 111: 1197–1204 [DOI] [PubMed] [Google Scholar]
- Smith C, Wong PT 1991. Paradoxical sleep increases predict successful learning in a complex operant task. Behav Neurosci 105: 282–288 [DOI] [PubMed] [Google Scholar]
- Smith C, Young J, Young W 1980. Prolonged increases in paradoxical sleep during and after avoidance-task acquisition. Sleep 3: 67–81 [PubMed] [Google Scholar]
- Smith CT, Conway JM, Rose GM 1998. Brief paradoxical sleep deprivation impairs reference, but not working, memory in the radial arm maze task. Neurobiol Learn Mem 69: 211–217 [DOI] [PubMed] [Google Scholar]
- Stickgold R, Walker MP 2005. Sleep and memory: The ongoing debate. Sleep 28: 1225–1227 [DOI] [PubMed] [Google Scholar]
- Suchecki D, Tufik S 2000. Social stability attenuates the stress in the modified multiple platform method for paradoxical sleep deprivation in the rat. Physiol Behav 68: 309–316 [DOI] [PubMed] [Google Scholar]
- Suchecki D, Lobo LL, Hipolide DC, Tufik S 1998. Increased ACTH and corticosterone secretion induced by different methods of paradoxical sleep deprivation. J Sleep Res 7: 276–281 [DOI] [PubMed] [Google Scholar]
- Suchecki D, Duarte Palma B, Tufik S 2000. Sleep rebound in animals deprived of paradoxical sleep by the modified multiple platform method. Brain Res 875: 14–22 [DOI] [PubMed] [Google Scholar]
- Suchecki D, Tiba PA, Tufik S 2002. Hormonal and behavioral responses of paradoxical sleep-deprived rats to the elevated plus maze. J Neuroendocrinol 14: 549–554 [DOI] [PubMed] [Google Scholar]
- van der Meulen JA, Bilbija L, Joosten RN, de Bruin JP, Feenstra MG 2003. The NMDA-receptor antagonist MK-801 selectively disrupts reversal learning in rats. Neuroreport 14: 2225–2228 [DOI] [PubMed] [Google Scholar]
- Van Hulzen ZJ, Coenen AM 1979. Selective deprivation of paradoxical sleep and consolidation of shuttle-box avoidance. Physiol Behav 23: 821–826 [DOI] [PubMed] [Google Scholar]
- Van Hulzen ZJ, Coenen AM 1981. Paradoxical sleep deprivation and locomotor activity in rats. Physiol Behav 27: 741–744 [DOI] [PubMed] [Google Scholar]
- Vertes RP 2004. Memory consolidation in sleep: Dream or reality. Neuron 44: 135–148 [DOI] [PubMed] [Google Scholar]
- Vertes RP, Eastman KE 2000. The case against memory consolidation in REM sleep. Behav Brain Sci 23: 867–876 [DOI] [PubMed] [Google Scholar]
- Vertes RP, Siegel JM 2005. Time for the sleep community to take a critical look at the purported role of sleep in memory processing. Sleep 28: 1228–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT 2006. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1: 848–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GP, Huang LQ, Wu HJ, Zhang L, You ZD, Zhao ZX 2009. Calcineurin contributes to spatial memory impairment induced by rapid eye movement sleep deprivation. Neuroreport 20: 1172–1176 [DOI] [PubMed] [Google Scholar]
- Whishaw IQ 1998. Place learning in hippocampal rats and the path integration hypothesis. Neurosci Biobehav Rev 22: 209–220 [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie J 1997. Perseveration on place reversals in spatial swimming pool tasks: Further evidence for place learning in hippocampal rats. Hippocampus 7: 361–370 [DOI] [PubMed] [Google Scholar]
- Woods PJ, Davidson EH, Peters RJ Jr 1964. Instrumental escape conditioning in a water tank: Effects of variations in drive stimulus intensity and reinforcement magnitude. J Comp Physiol Psychol 57: 466–470 [DOI] [PubMed] [Google Scholar]
- Yang HW, Lin YW, Yen CD, Min MY 2002. Change in bi-directional plasticity at CA1 synapses in hippocampal slices taken from 6-hydroxydopamine-treated rats: The role of endogenous norepinephrine. Eur J Neurosci 16: 1117–1128 [DOI] [PubMed] [Google Scholar]
- Yang RH, Hu SJ, Wang Y, Zhang WB, Luo WJ, Chen JY 2008. Paradoxical sleep deprivation impairs spatial learning and affects membrane excitability and mitochondrial protein in the hippocampus. Brain Res 1230: 224–232 [DOI] [PubMed] [Google Scholar]
- Youngblood BD, Zhou J, Smagin GN, Ryan DH, Harris RB 1997. Sleep deprivation by the “flower pot” technique and spatial reference memory. Physiol Behav 61: 249–256 [DOI] [PubMed] [Google Scholar]