Abstract
Here we attempted to clarify the role of dopamine signaling in reward seeking. In Experiment 1, we assessed the effects of the dopamine D1/D2 receptor antagonist flupenthixol (0.5 mg/kg i.p.) on Pavlovian incentive motivation and found that flupenthixol blocked the ability of a conditioned stimulus to enhance both goal approach and instrumental performance (Pavlovian-to-instrumental transfer). In Experiment 2 we assessed the effects of flupenthixol on reward palatability during post-training noncontingent re-exposure to the sucrose reward in either a control 3-h or novel 23-h food-deprived state. Flupenthixol, although effective in blocking the Pavlovian goal approach, was without effect on palatability or the increase in reward palatability induced by the upshift in motivational state. This noncontingent re-exposure provided an opportunity for instrumental incentive learning, the process by which rats encode the value of a reward for use in updating reward-seeking actions. Flupenthixol administered prior to the instrumental incentive learning opportunity did not affect the increase in subsequent off-drug reward-seeking actions induced by that experience. These data suggest that although dopamine signaling is necessary for Pavlovian incentive motivation, it is not necessary for changes in reward experience, or for the instrumental incentive learning process that translates this experience into the incentive value used to drive reward-seeking actions, and provide further evidence that Pavlovian and instrumental incentive learning processes are dissociable.
Dopamine signaling has long been implicated in reward processing, but its precise contribution remains a subject of intense debate. Early theories proposed that it was involved primarily in mediating the pleasurable effects of reward consumption (Wise and Rompre 1989; Koob and Le Moal 2001; Wise 2004). This model fell out of favor when it was noted that manipulation of dopamine transmission failed to produce the predicted effects on measures of reward palatability (Heath 1972; Portenoy et al. 1986; Berridge and Aldridge 2008), and attention shifted to the study of dopamine's role in reward learning.
The performance of actions that result in access to a reward is modulated by an incentive learning process, which can take two forms: Pavlovian and instrumental (Rescorla and Solomon 1967; Bindra 1974; Dickinson and Balleine 1994). Pavlovian incentive learning mediates the ability of a stimulus previously paired with a reward to acquire incentive motivational properties and thereby enhance reward approach behaviors, as well as invigorate the performance of actions instrumental to gaining rewards (Pavlovian-to-instrumental transfer) (Rescorla and Solomon 1967; Bindra 1974; Lovibond 1983; Berridge 1996). Instrumental incentive learning is the process by which a new reward value is encoded as a result of a change in reward experience during consumption. This instrumental incentive value is then used to control subsequent pursuit of that particular reward (Dickinson and Balleine 1994; Balleine 2005).
There is substantial evidence for dopaminergic involvement in the effects of Pavlovian incentive motivation on appetitive behavior. Dopamine receptor activation enhances Pavlovian incentive motivation as measured by conditioned approach behavior and enhanced Pavlovian-to-instrumental transfer (Wyvell and Berridge 2000; Pecina et al. 2003). Conversely, acute administration of dopamine antagonists attenuates these responses (Dickinson et al. 2000; Lopez and Ettenberg 2002; Danna and Elmer 2010). Robinson and Berridge (1993) highlighted the dissociability of neural processes underlying this phenomenon from those mediating the palatability responses elicited by reward consumption, which they refer to as “wanting” and “liking,” respectively.
Recently, we demonstrated using a heterogeneous chain of instrumental actions that the neural bases of instrumental incentive learning are similarly separable from those underlying the expression of palatability (Wassum et al. 2009, 2011). In these papers, we speculated that shifts in reward-specific, goal-directed performance brought about by changes in consumptive experience (instrumental incentive learning) reflect the “desire” for the specific reward. This specific instrumental desire and its associated neural processes may be separable from the Pavlovian processes, which allow cues or contexts to invigorate instrumental performance for reward generally, or “wanting” in the lexicon of Robinson and Berridge. However, as both Pavlovian and instrumental incentive processes can control the performance of goal-directed actions in similar ways, e.g., enhance responding, it is not unreasonable to consider that these processes may share mechanisms in common and, therefore, that dopamine signaling regulates both Pavlovian incentive motivation and instrumental incentive learning.
There is, indeed, evidence in support of such a scenario; dopamine receptor antagonism has been shown to produce a within session decrement in un-cued instrumental performance that has been attributed to interference with instrumental incentive processes (Wise et al. 1978; Willner et al. 1988; Beninger and Miller 1998). More recently, it has been suggested that dopamine neuron activity and dopamine receptor activation is involved in the reward value evaluation process (Tobler et al. 2005; Costa et al. 2007; Canu et al. 2010; D'Aquila 2010). However, contrary to this notion, acute dopamine receptor antagonism over repeated incentive learning opportunities has been reported not to affect the impact of such learning on subsequent reward-seeking actions (Dickinson et al. 2000). Similarly, both chronic enhancement of dopamine activity, and chronic dopamine depletion failed to affect the sensitivity of lever pressing behavior to decrements in reward value (Yin et al. 2006; Lex and Hauber 2009). However, these latter studies do not rule out a role for dopamine in instrumental incentive learning for two reasons: (1) Chronic manipulations of the dopamine system may result in the development of compensatory mechanisms for control of instrumental incentive learning, and (2) recent data suggest that neurochemical manipulations that do not affect the ability of natural decrements in reward value to control goal-directed actions can be effective in blocking the influence of an increase in reward value on goal-directed action performance (Wassum et al. 2011).
Here we attempted to clarify this issue further by evaluating the susceptibility of incentive learning to blockade with the dopamine D1/D2 receptor antagonist, flupenthixol, during a single opportunity for reward revaluation in an instrumental incentive learning task. Further, given some discrepancy in the literature regarding the role of dopamine in mediating palatability reactions (Leeb et al. 1991; Parker and Leeb 1994; Pecina et al. 1997; Higgs and Cooper 2000; Genn et al. 2003; Pecina et al. 2003; D'Aquila 2010), we also incorporated evaluation of flupenthixol's effect on reward palatability (or “liking”) into the experimental design, in addition to a measure of the Pavlovian goal approach (or “wanting”).
Rats were trained under 3 h of food deprivation to earn sucrose on a heterogeneous seeking–taking chain of actions previously shown to establish a reward-seeking action specifically sensitive to incentive value changes induced by instrumental incentive learning and free from the general influence of Pavlovian cues or motivational state (Balleine et al. 1995; Corbit and Balleine 2003; Wassum et al. 2011). After training, rats were given two separate re-exposure opportunities, one in which they were noncontingently re-exposed to the sucrose in the control 3-h deprived state and one in which they were re-exposed to the sucrose in a novel elevated hunger state. These re-exposure instrumental incentive learning opportunities were conducted either under vehicle or flupenthixol, at a dose and interval determined to be effective in blocking both conditioned approach and Pavlovian-to-instrumental transfer in a preliminary experiment (Experiment 1), and followed the next day by an off drug nonrewarded test of the effects of the instrumental incentive learning opportunity on reward seeking. During the re-exposure session we evaluated the effects of acute administration of flupenthixol on a lickometer measure of palatability. In this session we also simultaneously assessed the effects of acute flupenthixol on goal-approach behavior. The off-drug nonrewarded test of reward seeking allowed for a measure of the effects of flupenthixol on the instrumental incentive learning used to guide reward-seeking actions.
Results
Experiment 1: Dopamine receptor blockade attenuates conditioned approach and Pavlovian-to-instrumental transfer
Experiment 1 was conducted in three phases. In phase 1, Pavlovian training was used to pair an auditory cue (the CS+) with delivery of a sucrose solution reward and an alternate auditory cue (the CS−) with no reward. For all rats the Pavlovian training produced a robust conditioned approach behavior such that on the last training day rats entered the sucrose delivery magazine during the CS+ probe period (during the CS+ but prior to reward delivery; 13.01 entries/min, SEM = 1.34) significantly more than the pre-CS+ period (9.02 entries/min, SEM = 0.99; t(15) = 4.20, P = 0.0008). Phase 2 involved single lever instrumental training for the same sucrose reward. All rats acquired this behavior and on the last day of training pressed the lever at an average rate of 15.27 presses/min. In Phase 3 rats were given a general Pavlovian-to-instrumental transfer test whereby, following a short extinction period, both the CS+ and CS− were presented pseudo-randomly with the levers available, but not rewarded. This Pavlovian-to-instrumental transfer test was conducted after an injection of either saline vehicle or flupenthixol (0.5 mg/kg i.p.) to block dopamine D1/D2 receptors, counterbalanced for order.
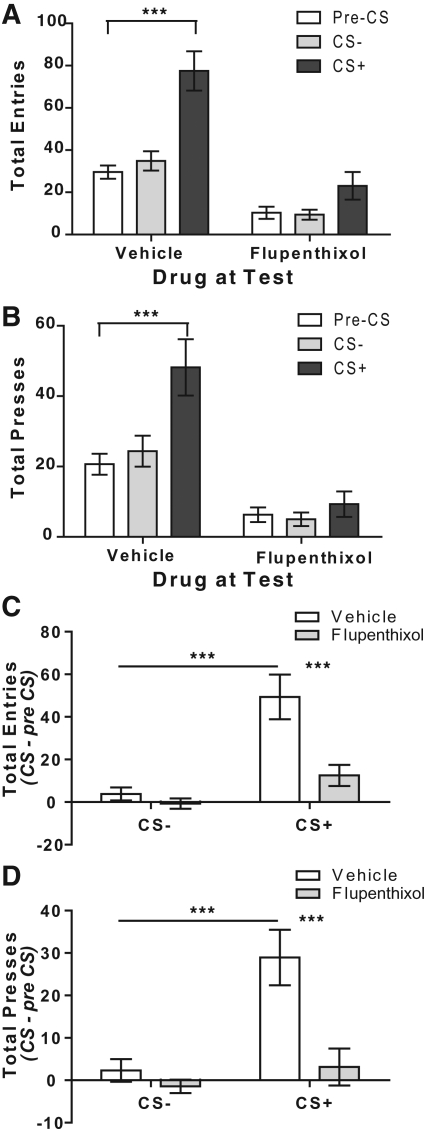
Figure 1 presents data for the total number of magazine entries/conditioned goal approach (A) and lever presses (B) during each period of the Pavlovian-to-instrumental transfer test. As is clear from this figure, when treated with vehicle, rats showed an elevation in both conditioned goal approach and lever presses during the CS+, but not CS− period relative to the pre-CS period. Administration of flupenthixol prior to testing produced an overall reduction in magazine entries and lever presses across test and also blocked the elevation in these measures during presentation of the CS+. Statistical analysis of these data reveals a significant main effect of both drug (total entries: F(1,14) = 38.12, P < 0.0001; total presses: F(1,14) = 20.95, P < 0.0001) and period (total entries: F(2,13) = 12.75, P = 0.001; total presses: F(2,13) = 6.52, P = 0.01), as well as a significant interaction between these factors (total entries: F(2,13) = 7.15, P = 0.008; total presses: F(2,13) = 8.41, P = 0.005). Bonferroni post hoc analyses controlling for multiple comparisons clarify these interactions to show that both conditioned goal approach and lever pressing activity were significantly elevated in the CS+ period relative to the pre-CS period in the vehicle- (P < 0.0001), but not flupenthixol-treated conditions (P > 0.05). Although there was clearly an effect of flupenthixol on overall activity in these measures, this is not likely to account for the drug's effect to suppress the ability of the CS+ to promote goal approach and Pavlovian-to-instrumental transfer as there was no significant correlation between press rate during the pre-CS period and the elevation in responding during the CS+ period under control vehicle-treated conditions (R2 = 0.18). The data were therefore analyzed further using elevation above pre-CS period responding as the dependent variable.
Figure 1.
Flupenthixol blocks conditioned approach and Pavlovian-to-instrumental transfer. (A) The effects of acute administration of flupenthixol (0.5 mg/kg i.p) on the total number of conditioned goal-approach responses in the periods prior to the CS deliveries (pre-CS), during presentation of an auditory cue previously paired with a sucrose reward (CS+, 4X) and during presentation of an unpaired auditory cue (CS−, 4X). (B) The effects of acute administration of flupenthixol during the same test as in A on the total number of lever presses in the pre-CS period, during presentation of the CS+ and during presentation of the CS−. (C) The total number of magazine entries during each pre-CS period was subtracted from its subsequent CS period (either CS− or CS+) to compare the effects of acute administration of flupenthixol on conditioned goal-approach behavior measured by an increase in magazine entries during presentation of an auditory cue previously paired with a sucrose reward (CS+) to that during presentation of an unpaired auditory cue (CS−). (D) The total number of lever presses during each pre-CS period was subtracted from its subsequent CS period (either CS− or CS+) to compare the effects of acute administration of flupenthixol during the same test as in B on Pavlovian-to-instrumental transfer as measured by the increase in the lever pressing activity during the CS+ relative to the CS− period. ***P < 0.001. N = 16.
As is clear from Figure 1C, when rats received vehicle injections (open bars) prior to test there was a robust increase in magazine entries/conditioned approach during the CS+ period relative to the pre-CS period, but no change in responding during the CS− period. Importantly, there was also a clear Pavlovian-to-instrumental transfer effect marked by elevated lever pressing activity during the CS+ period relative to the pre-CS period, which again was not apparent for the CS− period (Fig. 1D, open bars). This effect of the CS+ on both conditioned approach and Pavlovian-to-instrumental transfer was blocked by administration of flupenthixol; there was no elevation in either magazine approach or lever pressing during the CS+ period (Fig. 1C,D, shaded bars). Statistical analysis of the data presented in Figure 1C revealed a main effect of the CS period (F(1,30) = 18.34, P = 0.0002) and drug (F(1,30) = 15.43, P = 0.0005) on conditioned approach, as well as a significant interaction between these factors (F(1,30) = 9.44, P = 0.005). Similarly, statistical analysis of the data presented in Figure 1D also revealed a main effect of the CS period (F(1,30) = 9.96, P = 0.004) and drug (F(1,30) = 19.49, P = 0.0001) on lever pressing, as well as a significant interaction between these factors (F(1,30) = 10.86, P = 0.003). Bonferroni post hoc analyses clarify these interactions to show that, for both magazine entries and lever pressing, the difference between pre- vs. post-CS measure was significantly greater for the CS+ relative to the CS− in vehicle (P < 0.001 in both cases), but not in the flupenthixol condition (P > 0.05). Moreover, separate post hoc analyses indicate that within the CS+ period both entry and lever pressing rate were significantly lower under flupenthixol relative to vehicle (P < 0.001 in both cases). These data confirm that blockade of dopamine receptors attenuates the conditioned approach and Pavlovian-to-instrumental transfer, both measures of Pavlovian incentive motivation, incentive salience or, so-called, “wanting.”
Experiment 2: Dopamine receptor blockade has no effect on reward palatability or instrumental incentive learning
Experiment 2 was conducted in three phases involving initial training (3-h food deprived), an opportunity for instrumental incentive learning (noncontingent re-exposure to the reward in a novel 23-h food-deprived state) prior to which separate groups of rats received an injection of either flupenthixol or vehicle, and an off-drug nonrewarded test of the effects of the instrumental incentive learning opportunity on reward-seeking actions. Within each drug treatment group all rats also received a control condition in which they were re-exposed to the sucrose in the control 3-h food-deprived state under the same vehicle or flupenthixol treatment and then tested as above off drug for comparison. The order of the control 3-h and novel 23-h re-exposure and test was counterbalanced across the drug groups. All of the rats acquired and maintained lever-pressing performance, and, in the final session of training, performed the seeking lever response at a rate of 6.66 (SEM 1.45) and 6.94 (1.16) presses/min in the vehicle, and flupenthixol groups, respectively.
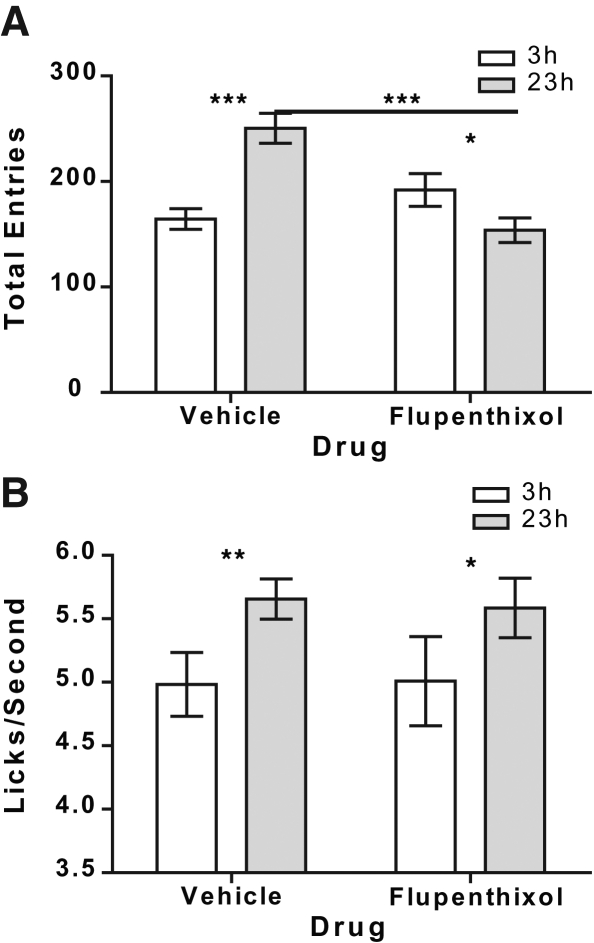
Figure 2 shows the direct effects of flupenthixol on both magazine approach behavior (Fig. 2A) and sucrose licking frequency during the re-exposure phases (Fig. 2B). As is clear from this figure, flupenthixol blocked the enhancement of the magazine approach behavior induced by an increase in motivational state (increased food deprivation). Based on the results of Experiment 1 this effect might be predicted given that such a goal approach was likely signaled by the contextual or sucrose pump cues. Interestingly, flupenthixol did not affect the apparent increase in palatability, reflected by consummatory licking reactions elicited by the sucrose, as a result of the deprivation state increase.
Figure 2.
Flupenthixol blocks an increase in goal approach induced by a motivational state shift, but does not affect reward palatability. (A) The effects of acute administration of flupenthixol (0.5 mg/kg i.p) on magazine entries during noncontingent re-exposure to the sucrose reward in either the control 3-h or novel 23-h elevated food deprivation state. (B) The effects of acute administration of flupenthixol during the same test on a reward “liking”-related lick frequency measure (the y-axis is truncated at 3.5 licks/sec based on our observation of this frequency as the floor licking rate). *P < 0.05, **P < 0.01, ***P < 0.001. N = 16, per drug group.
Statistical analysis of the data from Figure 2A found a main effect of both deprivation state (F(1,30) = 4.45, P = 0.04) and drug (F(1,30) = 5.71, P = 0.02) on magazine entries, as well as a significant interaction between these factors (F(1,30) = 29.98, P < 0.0001). Bonferroni post hoc analysis, correcting for multiple comparisons, clarified this interaction to show that there was a significant elevation in magazine entries during the noncontingent re-exposure session in the vehicle-treated group (P < 0.001), but not in the flupenthixol-treated group. In fact, this analysis found that, in rats tested in the 23-h food-deprived state, flupenthixol induced the opposite effect to controls; a decrease in magazine entry rate (P < 0.05), likely reflecting longer magazine entry bouts. Separate post hoc analysis further supported the argument that flupenthixol blocked the effect of increased motivational state on magazine entries in the 23-h food-deprived condition; rats treated with flupenthixol showed significantly lower magazine entry rates than those treated with vehicle (P < 0.001). As in Experiment 1 these data suggest that blockade of the D1 and D2 dopamine receptors attenuates Pavlovian incentive processes as measured by goal-approach behaviors.
However, as mentioned earlier, this same treatment neither affected the palatability responses elicited by the sucrose in the control 3-h food-deprived state, nor did it block the increase in palatability responses induced by increased food deprivation. Statistical analysis of the data of Figure 2B reveals only a main effect of deprivation (F(1,30) = 18.18, P = 0.0002) on the palatability-related lick frequency measure, with neither a main effect of drug (F(1,30) = 0.004, P = 0.95), nor an interaction between these factors (F(1,30) = 0.11, P = 0.75). Indeed, post hoc analysis confirmed that the elevation in lick frequency induced by the increase in food deprivation was significant in both vehicle- (P < 0.01) and flupenthixol- (P < 0.05) treated groups. For further confirmation we applied a Bayesian analysis (Gallistel 2009; Rouder et al. 2009) to evaluate the null effect of flupenthixol on palatability-related sucrose lick frequency. This analysis confirms the lack of effect of flupenthixol, showing the null hypothesis, that flupenthixol did not affect licking frequency, to be 3.91 and 3.84 times more likely than the alternate hypothesis in the 3-h and 23-h food-deprived conditions, respectively. These data suggest that, whereas the dopamine receptor antagonist was effective in blocking an increase in goal-approach behaviors (those thought to reflect “wanting”) induced by a motivational state change, at the very same time it did not affect the concomitant increase in consummatory licking responses (thought to reflect reward “liking”).
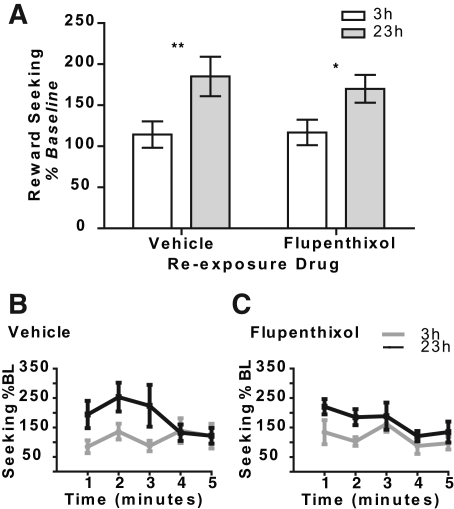
The data from the off-drug, nonrewarded test of instrumental incentive learning, conducted the day after the on-drug noncontingent re-exposure session are presented in Figure 3. An increase in seeking actions after exposure to the sucrose when in the novel heightened (23-h) food-deprived state, relative to after noncontingent exposure to the sucrose in the control 3-h deprived state, would suggest that the incentive value of the sucrose was increased as a consequence of that experience, and that this information was used to direct subsequent reward-seeking actions (Balleine 1992; Balleine et al. 1995). A clear instrumental incentive learning effect on reward-seeking actions was indeed observed in vehicle-treated rats (Fig. 3A, left). Interestingly, this instrumental incentive learning effect was also clearly apparent in flupenthixol-treated rats (Fig. 3A, right).
Figure 3.
Flupenthixol does not affect instrumental incentive learning. The test of the effects of instrumental incentive learning on reward seeking was conducted off drug and lever press performance was unrewarded. (A) The effects of flupenthixol (0.5 mg/kg i.p) during the previous day's noncontingent re-exposure session on reward-seeking actions (normalized to baseline pretest response rates) in both the control 3-h and novel elevated 23-h food-deprived state. (B) For the vehicle-treated rats only, reward-seeking response rate plotted as a percentage of baseline (BL) for the test of incentive learning separated into five 1-min time bins. (C) For the flupenthixol-treated rats only, reward-seeking response rate, plotted as a percentage of BL, for the test of incentive learning separated into five 1-min time bins. *P < 0.05, **P < 0.01. N = 16, per drug group.
Statistical analysis of the data from Figure 3A found only a main effect of deprivation state (F(1,30) = 17.94, P = 0.0002) on nonrewarded seeking actions, with no main effect of drug at re-exposure (F(1,30) = 0.08, P = 0.77), nor an interaction between these factors (F(1,30) = 0.36, P = 0.55). Post hoc analysis confirmed that the effect of deprivation on value-driven reward-seeking actions was significant in both the vehicle- (P < 0.01) and flupenthixol-treated rats (P < 0.05). As with the palatability data, Bayesian analysis confirmed the lack of effect of flupenthixol, showing the null hypothesis, that flupenthixol did not affect reward-seeking actions, to be 3.89 and 3.38 times more likely than the alternate hypothesis in the 3- and 23-h food-deprived conditions, respectively. Importantly also, the lack of an effect of flupenthixol on reward-seeking actions was consistent throughout the duration of the nonrewarded test. Analysis of the data presented in Figure 3B,C found a main effect of deprivation (F(1,30) = 18.44, P < 0.0001) and time (F(4,27) = 2.81, P = 0.04), with no effect of drug (F(1,30) = 0.07, P = 0.79), nor an interaction between any of these factors (deprivation × time: F(4,27) = 1.37, P = 0.27; deprivation × drug: F(1,30) = 0.40, P = 0.53; time × drug: F(4,27) = 1.02, P = 0.41; deprivation × time × drug F(4,27) = 0.51, P = 0.73). These data confirm, therefore, that blockade of dopamine D1/D2 receptors does not alone affect instrumental incentive learning to decrease reward-seeking actions, nor does it block the increase in value used to increase reward-seeking actions brought about by a positive instrumental incentive learning opportunity.
Discussion
Using acute administration of the dopamine D1/D2 receptor antagonist, flupenthixol, we provide data that help clarify the role of dopamine signaling in discrete aspects of reward processing. The data of Experiment 1 confirm that dopamine D1/D2 receptor activation is necessary for general Pavlovian incentive motivation, commonly referred to as reward “wanting,” measured both by conditioned goal approach and Pavlovian-to-instrumental transfer (Fig. 1). The data of Experiment 2 add to a body of literature suggesting that dopamine D1/D2 receptor activation is not necessary for expression of reward palatability, thought to reflect reward “liking,” or indeed for the increase in palatability resulting from increased food deprivation (Fig. 2). Most importantly, these data indicate a further distinction between the role of dopamine signaling in Pavlovian incentive motivation vs. instrumental incentive learning processes by demonstrating that dopamine receptor blockade during reward revaluation had no effect on subsequent shifts in instrumental performance, taken to reflect current incentive value or the desire for the reward (Fig. 3).
Our finding that acute dopamine receptor antagonism attenuates conditioned approach responses accords with findings of others suggesting that dopamine receptor activation is necessary for a conditioned stimulus to elicit approach behaviors (Lopez and Ettenberg 2002; Danna and Elmer 2010) and that enhancement in dopamine activity will enhance such a conditioned approach (Pecina et al. 2003). Interestingly, it has recently been shown that systemic flupenthixol given during Pavlovian learning sessions attenuated the expression of a goal-tracking conditioned response, similar to the result we show here on goal approach (Flagel et al. 2011). However, when tested off drug after 7 d of Pavlovian training these flupenthixol-treated rats showed a goal-tracking response (Flagel et al. 2011). These results suggest that dopamine receptor activation is not necessary for Pavlovian incentive learning, but rather only necessary for the Pavlovian incentive motivation that allows a reward-paired cue to invigorate goal approach (Flagel et al. 2011). Thus, although the current data do not address the role of dopamine in Pavlovian incentive learning, they are consistent with reports of a role for dopamine in Pavlovian incentive motivation.
Importantly, our finding that dopamine receptor blockade also attenuates the ability of a conditioned stimulus to invigorate instrumental responding is a direct replication of the data reported by Dickinson et al. (2000) showing that flupenthixol blocks such general Pavlovian-to-instrumental transfer, and is consistent with other reports showing a role for dopamine signaling in the influence of Pavlovian cues on instrumental actions (Wyvell and Berridge 2000). Use of dopamine antagonists to probe dopamine's role in this process is inevitably subject to limitations given the effect of these drugs to reduce general activity. Although dopamine receptor blockade did reduce overall response rate, we found no correlation between pre-CS lever press activity and the facilitation of responding during presentation of the CS+, suggesting that this was not a major factor. Additionally, in Experiment 2 we show that flupenthixol alone did not attenuate goal-approach behaviors in the control 3-h deprived state, but rather only blocked the increase in goal approach brought about by an increase in motivational state, suggesting that flupenthixol's effects on locomotor activity were not sufficient to block all appetitive behavior. Indeed, previous data have indicated that manipulations, such as reward devaluation, which can reduce overall activity and response rate, leave intact Pavlovian-to-instrumental transfer effects (Holland 2004). Considerable neurochemical and electrophysiological data suggest that presentation of conditioned stimuli can both activate mesolimbic dopamine neurons (Waelti et al. 2001) and result in dopamine release in the nucleus accumbens (Cheng et al. 2003; Day et al. 2007). Indeed, it is likely that the effects we report of systemic flupenthixol-induced attenuation of Pavlovian incentive response invigoration are mediated by nucleus accumbens dopamine receptors as manipulation of dopamine signaling specifically in this region has been shown to produce similar results (Wyvell and Berridge 2000; Lex and Hauber 2008, 2009).
Our current results extend from those of Dickinson et al. (2000) to provide data that are also consistent with contentions in the literature that the hedonic experience of reward consumption, or “liking,” is mediated by a dopamine-independent process (Pecina et al. 2003; Berridge and Aldridge 2008). By assessing licking frequency during multiple, short interval, small quantity sucrose reward deliveries we circumvented potential sensorimotor confounds (Pecina et al. 1997) in one previous study (Leeb et al. 1991) that may have accounted for the reported positive effect of a dopamine antagonist to decrease taste reactivity. There are, however, further data to suggest that dopamine receptor antagonism can reduce sucrose intake and alter microstructural licking patterns, interpreted as evidence that dopamine receptor activation is necessary for hedonic processing of an oral tastant (Geary and Smith 1985). Similarly, more recent data have shown that a D2 receptor antagonist can decrease licking activity for both a sucrose (D'Aquila 2010) and sodium chloride solution (Canu et al. 2010). Although these data may seem to contradict our current results, in those studies reward delivery was contingent upon licking behavior, and, as such, any dopamine-related effects could be interpreted as acting on motivational processes, such as those associated with conditioned approach responses or Pavlovian incentive motivation (e.g., as in Experiment 1 in this series). Our licking measure was used to specifically address the palatability of discrete noncontingent sucrose reward events and is less subject to motivational influences. Indeed, not only did dopamine receptor blockade fail to influence basal licking frequency, it also failed to attenuate an increase in this palatability response induced by increased food deprivation. Further, this dose of flupenthixol was at the very same time effective in blocking the increase in goal approach induced by an enhanced motivational state and, in Experiment 1, was effective in blocking conditioned goal approach and Pavlovian-to-instrumental transfer. It should be pointed out, however, that our study employed a relatively short flupenthixol injection-to-test interval, such that dopamine receptor blockade may not have been maximal. Thus, we cannot rule out the possibility that palatability-related lick frequency does depend in part on dopamine receptor activation and that this behavior is simply less sensitive to flupenthixol than a conditioned goal approach. Nonetheless, these data fit well with the theory, advanced by Berridge and Robinson, that how much a reward is “liked” upon consumption is dissociable from the Pavlovian incentive processes that control how much it is wanted, with the former being a dopamine-independent process and the latter being a dopamine-dependent process (Robinson and Berridge 1993; Berridge and Robinson 1998). Moreover, these data also fit well with those of Robinson et al. (2005) suggesting that dopamine in not necessary for “liking” or learning about rewards, but rather only for Pavlovian incentive motivation (Robinson et al. 2005).
Our current data also align with previous reports suggesting that instrumental incentive processes are also dopamine-independent and therefore dissociable from Pavlovian reward “wanting” processes. Chronic enhancement or depletion of dopamine activity has been shown not to affect the sensitivity of lever pressing actions to degradations in reward value (Yin et al. 2006; Lex and Hauber 2009). These chronic manipulations may, however, have allowed for compensatory mechanisms for instrumental incentive learning. Moreover, the lack of role for dopamine in such negative instrumental incentive learning does not rule out dopaminergic function in positive incentive learning (Wassum et al. 2011). Our current data overcame these obstacles to show that acute dopamine receptor antagonism is without effect on positive instrumental incentive learning. Interestingly, a conflicting report has shown that chronic enhancement of dopamine activity results in positive bias in the updating of tastant values to suggest that dopamine is involved in incentive learning processes (Costa et al. 2007). However, this study used a forced choice task, which could have resulted in multiple processes controlling the lick output measure, including general arousal, consummatory reactivity, or an instrumental incentive process. Indeed, elevated dopamine levels have been found to increase sucrose consumption (Perona et al. 2008). In our current study we used a paradigm designed to specifically address instrumental incentive learning processes and their effects on reward-seeking actions that is free from the confounds of general arousal, excitatory Pavlovian cues, and consumption (Balleine et al. 1995; Corbit and Balleine 2003; Wassum et al. 2009, 2011). This dissection of the actual instrumental incentive learning process allowed for evidence supporting the lack of a dopaminergic function in instrumental incentive learning.
The current data complement those of Dickinson et al. (2000), who used a dual outcome paradigm to demonstrate that acute dopamine receptor antagonism did not affect instrumental incentive processes to alter subsequent reward-seeking actions. Similar to our findings, that report noted that flupenthixol did not impact the effects of an upshift in motivational state on subsequent choice activity (Dickinson et al. 2000). However, whereas Dickinson and colleagues employed multiple rounds of revaluation and flupenthixol administration, we found that dopamine receptor antagonism during a single, instrumental incentive learning opportunity was without effect on subsequent reward seeking, ruling out the possibility that the negative data of Dickinson et al. (2000) resulted from multiple instrumental incentive learning opportunities overcoming the effects of dopamine receptor antagonism. Further, the concern raised by the investigators that the lack of an effect of dopamine receptor antagonism may have resulted from reward generalization in their dual outcome paradigm is assuaged by our data showing flupenthixol to be without effect in a single outcome paradigm. Our study also adds further weight to the original interpretation laid out by Dickinson et al. (2000) that dopamine signaling is unnecessary for instrumental incentive processes by employing a heterogeneous seeking–taking chain paradigm that specifically targets instrumental incentive learning processes free from general motivational and Pavlovian influences. This paradigm has previously been useful in uncovering neuromodulators, such opioid peptides in the basolateral amygdala, necessary specifically for instrumental incentive learning (Wassum et al. 2009, 2011). Last, unlike the study by Dickinson et al. (2000), the design of our experiment allowed for measurement of palatability, goal-approach and instrumental incentive learning in one behavioral task, revealing dopamine to be necessary for Pavlovian incentive motivation, but neither palatability, nor instrumental incentive learning.
There is, however, ample research implicating dopamine in effort-based instrumental decision making (Walton et al. 2006; Salamone et al. 2007) and response cost processing (Gan et al. 2009; Day et al. 2010; Ostlund et al. 2011), suggesting that the role of dopamine is not solely Pavlovian in nature and can contribute to changes in action values in these cases. Combined, these data suggest that Pavlovian and instrumental incentive processes are dissociable and support theories suggesting that they do not involve a common representation of the reward (Dickinson and Balleine 1994; Corbit et al. 2001; Corbit and Balleine 2003). It is important to note that cues that have been paired with a reward can have both a response invigorating effect and can bias response selection. The general form of Pavlovian-to-instrumental transfer used herein is thought to model the response-invigorating effects of reward-associated cues, which is a process distinct from reward-specific Pavlovian-to-instrumental transfer used to bias response selection (Corbit and Balleine 2005). Indeed, the general form of Pavlovian-to-instrumental transfer is tightly regulated by a general motivational state, and is thought not to require a specific representation of the reward (Dickinson and Balleine 1990; Balleine 1994).
In summary, Pavlovian incentive processes were found to alter goal-directed behavior through an effect on endogenous dopamine signaling, whereas instrumental incentive processes were found to affect goal-directed reward seeking through a dopamine-independent system, that we have previously shown to involve μ opioid receptors in the basolateral amygdala (Wassum et al. 2009). Further, both of these processes were dissociable from reward palatability. The current findings highlight both the behavioral and neurochemical complexity of these incentive processes.
Materials and Methods
Experiment 1
Subjects
Male Long Evans rats weighing between 240 and 280 g (N = 16, Charles River Laboratories, Wilmington, MA) were group housed and handled prior to training. Rats were maintained on a food-deprived schedule whereby they received 10–12 g of their maintenance diet daily in order to maintain ∼85% free-feeding body weight. Rats had free access to tap water in the home cage and were fed ∼3–4 h after each day's training session. All procedures were conducted in accordance with the NIH Guide for the Care and use of Laboratory Animals and approved by the UCLA Institutional Animal Care and Use Committee.
Apparatus and training
Training and testing took place during the light phase of the 12:12 h light:dark cycle in 8 Med Associates (East Fairfield, VT) operant chambers described previously (Corbit and Balleine 2003). Each session started with the illumination of the house light and insertion of the levers where appropriate and ended with the retraction of the levers and turning off of the house light. Rats received only one training session per day.
Magazine training
Rats received 1 d of magazine training in which they were exposed to noncontingent sucrose deliveries (0.1 mL of 20% sucrose per delivery; 20 outcomes over 30 min) in the operant chamber with the levers retracted, in order to learn where to receive the sucrose reward.
Pavlovian training
Following magazine training rats received 8 d of Pavlovian training in which one of two auditory cues (tone or white noise) was paired with noncontingent delivery of the sucrose solution outcome to provide a conditioned stimulus (CS+). The auditory cue that served as the CS+ was counterbalanced across rats. Each CS+ was presented for 2 min, during which the sucrose solution was presented on a random time (RT)-30s schedule. The CS+ was presented six times per session with an intertrial interval between 2 and 4 min (mean = 3 min).
Instrumental training
Rats then received 8 total days of instrumental training in which the lever to the right of the magazine was rewarded with delivery of sucrose solution. Each session lasted until 20 outcomes had been earned, or 30 min elapsed. Rats received 1 d each of continuous, RI-15s, RI-30s schedules of reinforcement, followed by 5 total days of instrumental training on the final RI-60s schedule.
Pavlovian reminder and CS− habituation
After instrumental training, rats were then given a single Pavlovian reminder session and habituation to the unpaired conditioned stimulus (CS−), which was also an auditory cue (either tone or white noise) that was the opposite cue to that which served at the CS+ for each rat. During this session rats were given four 2-min presentations of the CS+ during which sucrose was delivered on a RI-30s schedule, followed by four 2-min CS− presentations during which no reward was delivered. These CSs were delivered with an intertrial interval of 2–4 min.
Pavlovian-to-instrumental transfer test
On the day prior to each Pavlovian-to-instrumental transfer test rats were given a single 30-min instrumental extinction session in which their lever pressing activity was not rewarded with sucrose solution to establish a low level of responding. The day following this extinction session rats were injected with either vehicle (saline 1 mL/kg i.p.) or flupenthixol (0.5 mg/mL/kg i.p.) 15 min prior to the test. At test, rats were placed in the operant chamber and the lever was inserted in the box throughout the session, but lever presses were not rewarded. Responding was again extinguished for 5 min to establish a low rate of baseline performance, after which each CS was presented four times over the next 40 min following the order noise-tone-tone-noise-tone-noise-noise-tone. Each CS lasted 2 min with a 3-min fixed intertrial interval.
After the first Pavlovian-to-instrumental transfer test rats were then given 2 d of instrumental retraining on the final RI-60s schedule and 1 d of Pavlovian training, with the CS+ only and another 30-min instrumental extinction session. For the second Pavlovian-to-instrumental transfer test rats were given the opposite drug treatment to that which they received prior to the first test, 15 min prior to the test. The second test was otherwise conducted exactly as the first. The order of saline/drug administration was counterbalanced across animals.
Drug administration
The dopamine D1/D2 receptor antagonist flupenthixol (0.5 mg/kg, 1 mL/kg, Sigma) was dissolved in sterile saline and injected i.p. 15 min prior to the Pavlovian-to-instrumental transfer test. The same volume of sterile saline was injected i.p. as the vehicle control. This dose and interval was selected based on previous evidence suggesting it to be effective in blocking Pavlovian-to-instrumental transfer (Dickinson et al. 2000).
Data analysis
Data from the Pavlovian-to-instrumental transfer tests are shown in Figure 1. For Figure 1A,B the number of magazine entries (A) and lever presses (B) were totaled across the four 2-min pre-CS, CS+, and CS− periods and analyzed with two-way repeated measures ANOVA with variables drug (vehicle or flupenthixol) and CS period (pre-CS, CS−, or CS+) followed by Bonferroni post hoc analysis correcting for multiple comparisons (GraphPad Prismand SPSS). To specifically assess the effect of flupenthixol on the elevation in responding during the CS period relative to the pre-CS period the magazine entry/lever pressing rate during each 2-min pre-CS period was subtracted from the subsequent 2-min CS period (either CS+ or CS−) and averaged across the session. These data were similarly analyzed with two-way repeated measures ANOVAs followed with Bonferroni post hoc analysis to compare the effects of drug treatment within each CS period and then separately to compare the effects of CS within each drug group. For all hypothesis tests the α level for significance was set to P < 0.05.
In several cases a manipulation, such as drug treatment, was critically found to have no effect on lever press actions. In these select cases, we computed Bayes Factors for use in supporting the null hypothesis (Gallistel 2009; Rouder et al. 2009) using a freely available Bayes Factor calculator (http://pcl.missouri.edu/bayesfactor) (Rouder et al. 2009). This analysis has been argued to provide an appropriate method for expressing a preference for the null hypothesis (Gallistel 2009; Rouder et al. 2009).
Experiment 2
Subjects
Male Long Evans rats weighing between 240 and 280 g (N = 32) were group housed and handled prior to training. Rats were food-deprived for 3 h each day prior to training and had free access to tap water in the home cage. Rats were fed ∼3–4 h after each day's training session.
Apparatus and training
Training and testing took place during the light phase of the 12:12 h light:dark cycle in 8 Med Associates operant chambers described previously.
Magazine training
Rats received 2 d of magazine training in which they were exposed to noncontingent sucrose deliveries (0.1 mL of 20% sucrose per delivery; 20 outcomes over 30 min) in the operant chamber with the levers retracted, in order to learn where to receive the sucrose reward.
Single-action instrumental training
Rats were then given 4 d of single-action training on the lever to the right of the magazine (taking lever) with the sucrose delivered on a continuous reinforcement schedule. Each session lasted until 20 outcomes had been earned, or 30 min elapsed.
Training on the seeking–taking chain
On day 6 of instrumental training the seeking lever (i.e., the lever to the left of the magazine) was introduced into the chamber. Initially, only the seeking lever was present. One response on the left, seeking lever resulted in the presentation of the right, taking lever a response on which resulted in delivery of a single 0.1 mL sucrose solution and retraction of the taking lever. This session continued until 20 outcomes were earned, or 30 min elapsed. The seeking lever was continuously rewarded with the presentation of the taking lever for four daily sessions, then the reinforcement schedule was increased to random ratio (RR)-2 for four sessions then finally to RR-4 until stable lever pressing was obtained (approximately four to six daily sessions). The taking lever was always continuously rewarded and retracted after sucrose delivery.
Test of incentive learning
After the last training session half of the rats in each of the two drug treatment groups were shifted to a 23-h food-deprived state and half were maintained 3-h deprived before receiving either vehicle (N = 16; saline 1 mL/kg i.p.) or flupenthixol (N = 16; 0.5 mg/kg i.p.) 15 min prior to being placed in the operant chamber with the levers retracted and given 30 noncontingent sucrose presentations over 40 min. Lickometer measures were collected during this phase of the experiment. The next day, in the same food-deprived state as during the re-exposure, but devoid of drug, all the rats were tested for their responding on the chain under nonrewarded conditions for 5 min. This nonrewarded test was conducted just as in training with rats responding on the seeking lever on a RR-4 schedule to receive the second taking lever, which once pressed was retracted, but no reward was delivered. Rats were then given 1 d without training in which they were food-deprived for 3 h/d and then retrained for 3 d in the 3-h food-deprived state. For the second half of the test, rats previously 23-h food-restricted remained 3-h restricted and those previously tested 3-h deprived were shifted to the 23-h deprived state. Rats were then given the same drug treatment as in the first test (either vehicle or flupenthixol) 15 min prior to being placed in the operant chamber with the levers retracted and given 30 noncontingent sucrose presentations over 40 min. Lickometer measures were again collected during this phase of the experiment. The next day, in the same food-deprived state as during this second re-exposure, but devoid of drug, all the rats were tested for their responding on the chain under nonrewarded conditions for 5 min. In all cases data from the second series of tests showed a pattern of results that was not significantly different from those data in the first series and they were therefore collapsed together.
Palatability analysis
During instrumental training and testing the sucrose solution was delivered into the magazine through stainless steel tubes into an electrically isolated Plexiglas well. A lickometer circuit, connecting the grid floor of the boxes and the stainless steel tubes, with the circuit closed by the rat's tongue, allowed recording of lick frequencies and lick pattern. This circuit passed a current of no more than 60 nA through the rat each time its tongue made contact with the solution which was amplified and fed through an interface to a PC programmed to record the time of each lick to the nearest 1 msec. Based on previous reports (Davis and Smith 1992; Kaplan et al. 1995; Baird et al. 2006; Thornton-Jones et al. 2007; Wassum et al. 2009) we used licking frequency as a measure of sucrose palatability. This measure of licking microstructure during consumption provides a similar analysis of palatability changes as those assessing taste reactivity following oral infusions (Davis and Perez 1993).
Drug administration
Flupenthixol (0.5 mg/kg, 1 mL/kg) was dissolved in sterile saline and injected i.p. 15 min prior to the revaluation re-exposure session only. No drug was given during the test of lever pressing on the chain. This dose was selected based on previous evidence from Experiment 1 of its efficacy in blocking Pavlovian-to-instrumental transfer.
Data analysis
During the re-exposure session we collected data on both magazine entries and lickometer measures. Both entry rate and lick frequency data during this re-exposure session (Fig. 2) were analyzed with two-way ANOVAs with within-subjects variable deprivation state (either control 3-h deprived or 23-h deprived) and between-subjects variable drug treatment (vehicle or flupenthixol). Bonferroni post hoc analyses correcting for multiple comparisons were then used to compare the effects of deprivation state within each drug treatment group and then separately to compare the effects of drug treatment at each deprivation state. For all hypothesis tests the α level for significance was set to P < 0.05.
For the lever pressing data collected during the off-drug test of incentive learning on chain performance (Fig. 3) all data were normalized to baseline levels of responding, with the baseline being the average of the rate of performance during the last two training sessions prior to the test. A two-way ANOVA was then used to analyze these data with within-subjects variable deprivation state and between-subjects variable drug treatment at re-exposure. Again Bonferroni post hoc analyses correcting for multiple comparisons were then used to compare the effects of deprivation state within each drug treatment group and then separately to compare the effects of drug treatment at each deprivation state. Bayesian analysis was used for supporting the null hypothesis as previously described. Data were also analyzed with a three-way ANOVA with within-subjects variables deprivation state and time (separating the nonrewarded test into five 1-min bins) and between subjects variable drug treatment at re-exposure. For all hypothesis tests the α level for significance was set to P < 0.05.
Acknowledgments
This research was supported by grant nos. DA09359 and DA05010 from NIDA (to N.T.M.), DA029035 (to S.B.O.), MH56446 (to B.W.B.), and DA023774 as well as a Hatos scholarship (to K.M.W.).
References
- Baird JP, Rios C, Gray NE, Walsh CE, Fischer SG, Pecora AL 2006. Effects of melanin-concentrating hormone on licking microstructure and brief-access taste responses. Am J Physiol Regul Integr Comp Physiol 291: R1265–R1274 [DOI] [PubMed] [Google Scholar]
- Balleine B 1992. Instrumental performance following a shift in primary motivation depends on incentive learning. J Exp Psychol Anim Behav Process 18: 236–250 [PubMed] [Google Scholar]
- Balleine B 1994. Asymmetrical interactions between thirst and hunger in Pavlovian-instrumental transfer. Q J Exp Psychol B 47: 211–231 [PubMed] [Google Scholar]
- Balleine BW 2005. Neural bases of food seeking: Affect, arousal and reward in corticostriatolimbic circuits. Physiol Behav 86: 717–730 [DOI] [PubMed] [Google Scholar]
- Balleine BW, Garner C, Gonzalez F, Dickinson A 1995. Motivational control of heterogeneous instrumental chains. J Exp Psychol Anim Behav Process 21: 203–217 [Google Scholar]
- Beninger RJ, Miller R 1998. Dopamine D1-like receptors and reward-related incentive learning. Neurosci Biobehav Rev 22: 335–345 [DOI] [PubMed] [Google Scholar]
- Berridge KC 1996. Food reward: Brain substrates of wanting and liking. Neurosci Biobehav Rev 20: 1–25 [DOI] [PubMed] [Google Scholar]
- Berridge KC, Aldridge JW 2008. Decision utility, the brain, and pursuit of hedonic goals. Soc Cogn 26: 621–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE 1998. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 28: 309–369 [DOI] [PubMed] [Google Scholar]
- Bindra D 1974. A motivational view of learning, performance, and behavior modification. Psychol Rev 81: 199–213 [DOI] [PubMed] [Google Scholar]
- Canu ME, Carta D, Murgia E, Serra G, D'Aquila PS 2010. Dopamine on D2-like receptors is involved in reward evaluation in water-deprived rats licking for NaCl and water. Pharmacol Biochem Behav 96: 194–197 [DOI] [PubMed] [Google Scholar]
- Cheng JJ, de Bruin JP, Feenstra MG 2003. Dopamine efflux in nucleus accumbens shell and core in response to appetitive classical conditioning. Eur J Neurosci 18: 1306–1314 [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW 2003. Instrumental and Pavlovian incentive processes have dissociable effects on components of a heterogeneous instrumental chain. J Exp Psychol Anim Behav Process 29: 99–106 [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW 2005. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of Pavlovian-instrumental transfer. J Neurosci 25: 962–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW 2001. The role of the nucleus accumbens in instrumental conditioning: Evidence of a functional dissociation between accumbens core and shell. J Neurosci 21: 3251–3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa RM, Gutierrez R, de Araujo IE, Coelho MR, Kloth AD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA 2007. Dopamine levels modulate the updating of tastant values. Genes Brain Behav 6: 314–320 [DOI] [PubMed] [Google Scholar]
- D'Aquila PS 2010. Dopamine on D2-like receptors “reboosts” dopamine D1-like receptor-mediated behavioural activation in rats licking for sucrose. Neuropharmacology 58: 1085–1096 [DOI] [PubMed] [Google Scholar]
- Danna CL, Elmer GI 2010. Disruption of conditioned reward association by typical and atypical antipsychotics. Pharmacol Biochem Behav 96: 40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JD, Perez MC 1993. Food deprivation- and palatability-induced microstructural changes in ingestive behavior. Am J Physiol 264: R97–R103 [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP 1992. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci 106: 217–228 [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM 2007. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci 10: 1020–1028 [DOI] [PubMed] [Google Scholar]
- Day JJ, Jones JL, Wightman RM, Carelli RM 2010. Phasic nucleus accumbens dopamine release encodes effort- and delay-related costs. Biol Psychiatry 68: 306–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Balleine B 1990. Motivational control of instrumental performance following a shift from thirst to hunger. Q J Exp Psychol B 42: 413–431 [PubMed] [Google Scholar]
- Dickinson A, Balleine BW 1994. Motivational control over goal-directed action. Anim Learn Behav 22: 1–18 [Google Scholar]
- Dickinson A, Smith J, Mirenowicz J 2000. Dissociation of Pavlovian and instrumental incentive learning under dopamine antagonists. Behav Neurosci 114: 468–483 [DOI] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuh A, Willuhn I, Akers CA, Clinton SM, Phillips PEM, Akil H 2011. A selective role for dopamine in stimulus-reward learning. Nature 469: 53–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR 2009. The importance of proving the null. Psychol Rev 116: 439–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan JO, Walton ME, Phillips PE 2009. Dissociable cost and benefit encoding of future rewards by mesolimbic dopamine. Nat Neurosci 13: 25–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary N, Smith GP 1985. Pimozide decreases the positive reinforcing effect of sham fed sucrose in the rat. Pharmacol Biochem Behav 22: 787–790 [DOI] [PubMed] [Google Scholar]
- Genn RF, Higgs S, Cooper SJ 2003. The effects of 7-OH-DPAT, quinpirole and raclopride on licking for sucrose solutions in the non-deprived rat. Behav Pharmacol 14: 609–617 [DOI] [PubMed] [Google Scholar]
- Heath RG 1972. Pleasure and brain activity in man. Deep and surface electroencephalograms during orgasm. J Nerv Ment Dis 154: 3–18 [DOI] [PubMed] [Google Scholar]
- Higgs S, Cooper SJ 2000. The effect of the dopamine D2 receptor antagonist raclopride on the pattern of licking microstructure induced by midazolam in the rat. Eur J Pharmacol 409: 73–80 [DOI] [PubMed] [Google Scholar]
- Holland PC 2004. Relations between Pavlovian-instrumental transfer and reinforcer devaluation. J Exp Psychol Anim Behav Process 30: 104–117 [DOI] [PubMed] [Google Scholar]
- Kaplan JM, Roitman MF, Grill HJ 1995. Ingestive taste reactivity as licking behavior. Neurosci Biobehav Rev 19: 89–98 [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M 2001. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24: 97–129 [DOI] [PubMed] [Google Scholar]
- Leeb K, Parker L, Eikelboom R 1991. Effects of pimozide on the hedonic properties of sucrose: Analysis by the taste reactivity test. Pharmacol Biochem Behav 39: 895–901 [DOI] [PubMed] [Google Scholar]
- Lex A, Hauber W 2008. Dopamine D1 and D2 receptors in the nucleus accumbens core and shell mediate Pavlovian-instrumental transfer. Learn Mem 15: 483–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lex B, Hauber W 2009. The role of nucleus accumbens dopamine in outcome encoding in instrumental and Pavlovian conditioning. Neurobiol Learn Mem 93: 283–290 [DOI] [PubMed] [Google Scholar]
- Lopez HH, Ettenberg A 2002. Sexually conditioned incentives: Attenuation of motivational impact during dopamine receptor antagonism. Pharmacol Biochem Behav 72: 65–72 [DOI] [PubMed] [Google Scholar]
- Lovibond PF 1983. Facilitation of instrumental behavior by a Pavlovian appetitive conditioned stimulus. J Exp Psychol Anim Behav Process 9: 225–247 [PubMed] [Google Scholar]
- Ostlund SB, Wassum KM, Murphy NP, Balleine BW, Maidment NT 2011. Extracellular dopamine levels in striatal subregions track shifts in motivation and response cost during instrumental conditioning. J Neurosci 31: 200–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L, Leeb K 1994. Amphetamine-induced modification of quinine palatability: Analysis by the taste reactivity test. Pharmacol Biochem Behav 47: 413–420 [DOI] [PubMed] [Google Scholar]
- Pecina S, Berridge KC, Parker LA 1997. Pimozide does not shift palatability: Separation of anhedonia from sensorimotor suppression by taste reactivity. Pharmacol Biochem Behav 58: 801–811 [DOI] [PubMed] [Google Scholar]
- Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X 2003. Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. J Neurosci 23: 9395–9402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perona MT, Waters S, Hall FS, Sora I, Lesch KP, Murphy DL, Caron M, Uhl GR 2008. Animal models of depression in dopamine, serotonin, and norepinephrine transporter knockout mice: Prominent effects of dopamine transporter deletions. Behav Pharmacol 19: 566–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portenoy RK, Jarden JO, Sidtis JJ, Lipton RB, Foley KM, Rottenberg DA 1986. Compulsive thalamic self-stimulation: A case with metabolic, electrophysiologic and behavioral correlates. Pain 27: 277–290 [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Solomon RL 1967. Two-process learning theory: Relationships between Pavlovian conditioning and instrumental learning. Psychol Rev 74: 151–182 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC 1993. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18: 247–291 [DOI] [PubMed] [Google Scholar]
- Robinson S, Sandstrom SM, Denenberg VH, Palmiter RD 2005. Distinguishing whether dopamine regulates liking, wanting, and/or learning about rewards. Behav Neurosci 119: 5–15 [DOI] [PubMed] [Google Scholar]
- Rouder JN, Speckman PL, Sun D, Morey RD, Iverson G 2009. Bayesian t tests for accepting and rejecting the null hypothesis. Psychon Bull Rev 16: 225–237 [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM 2007. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 191: 461–482 [DOI] [PubMed] [Google Scholar]
- Thornton-Jones ZD, Kennett GA, Vickers SP, Clifton PG 2007. A comparison of the effects of the CB(1) receptor antagonist SR141716A, pre-feeding and changed palatability on the microstructure of ingestive behaviour. Psychopharmacology (Berl) 193: 1–9 [DOI] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W 2005. Adaptive coding of reward value by dopamine neurons. Science 307: 1642–1645 [DOI] [PubMed] [Google Scholar]
- Waelti P, Dickinson A, Schultz W 2001. Dopamine responses comply with basic assumptions of formal learning theory. Nature 412: 43–48 [DOI] [PubMed] [Google Scholar]
- Walton ME, Kennerley SW, Bannerman DM, Phillips PE, Rushworth MF 2006. Weighing up the benefits of work: Behavioral and neural analyses of effort-related decision making. Neural Netw 19: 1302–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Maidment NT, Balleine BW 2009. Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proc Natl Acad Sci 106: 12512–12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Cely IC, Balleine BW, Maidment NT 2011. Mu opioid receptor activation in the basolateral amygdala mediates the learning of increases but not decreases in the incentive value of a food reward. J Neurosci 31: 1583–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Chawla K, Sampson D, Sophokleous S, Muscat R 1988. Tests of functional equivalence between pimozide pretreatment, extinction and free feeding. Psychopharmacology (Berl) 95: 423–426 [DOI] [PubMed] [Google Scholar]
- Wise RA 2004. Dopamine, learning and motivation. Nat Rev Neurosci 5: 483–494 [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP 1989. Brain dopamine and reward. Annu Rev Psychol 40: 191–225 [DOI] [PubMed] [Google Scholar]
- Wise RA, Spindler J, Legault L 1978. Major attenuation of food reward with performance-sparing doses of pimozide in the rat. Can J Psychol 32: 77–85 [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC 2000. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: Enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci 20: 8122–8130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Zhuang X, Balleine BW 2006. Instrumental learning in hyperdopaminergic mice. Neurobiol Learn Mem 85: 283–288 [DOI] [PubMed] [Google Scholar]