Abstract
The adenosine A2A receptor (A2AR) is highly enriched in the striatum where it is uniquely positioned to integrate dopaminergic, glutamatergic, and other signals to modulate cognition. Although previous studies support the hypothesis that A2AR inactivation can be pro-cognitive, analyses of A2AR's effects on cognitive functions have been restricted to a small subset of cognitive domains. Furthermore, the relative contribution of A2ARs in distinct brain regions remains largely unknown. Here, we studied the regulation of multiple memory processes by brain region-specific populations of A2ARs. Specifically, we evaluated the cognitive impacts of conditional A2AR deletion restricted to either the entire forebrain (i.e., cerebral cortex, hippocampus, and striatum, fb-A2AR KO) or to striatum alone (st-A2AR KO) in recognition memory, working memory, reference memory, and reversal learning. This comprehensive, comparative analysis showed for the first time that depletion of A2AR-dependent signaling in either the entire forebrain or striatum alone is associated with two specific phenotypes indicative of cognitive flexibility—enhanced working memory and enhanced reversal learning. These selective pro-cognitive phenotypes seemed largely attributed to inactivation of striatal A2ARs as they were captured by A2AR deletion restricted to striatal neurons. Neither spatial reference memory acquisition nor spatial recognition memory were grossly affected, and no evidence for compensatory changes in striatal or cortical D1, D2, or A1 receptor expression was found. This study provides the first direct demonstration that targeting striatal A2ARs may be an effective, novel strategy to facilitate cognitive flexibility under normal and pathologic conditions.
Adenosine modulates neurotransmission and synaptic function in the central nervous system (e.g., de Mendonca and Ribeiro 1994, 1997; Sebastiao and Ribeiro 1996) and is increasingly thought to play an important role in learning and memory (e.g., Fredholm et al. 2005; Boison 2007; Yee et al. 2007). Adenosine A2A receptors (A2ARs) are uniquely positioned in the brain to modulate cognitive functions that depend on the complex integration of dopaminergic, glutamatergic, and other neuronal signals. They are abundantly expressed in the striatum and are largely found on post-synaptic striatopallidal medium spiny neurons (MSNs) of the “indirect” pathway (e.g., Schiffmann et al. 1991a,b; Rosin et al. 1998; Rebola et al. 2005) where they co-localize and interact with receptors of other neurotransmitter and neuromodulatory systems to influence synaptic plasticity and behavior (Ferre et al. 2007; Wei et al. 2011). Specifically, striatal A2ARs interact antagonistically with dopamine D2 receptors (D2Rs) (Hillion et al. 2002; Canals et al. 2003) and oppose N-methyl-D-aspartate receptor (NMDAR) function (Norenberg et al. 1998; Wirkner et al. 2000; Gerevich et al. 2002). In contrast, striatal A2ARs may also interact synergistically with metabotropic glutamate 5 receptors (mGlu5Rs) (Ferre et al. 2002; Kachroo et al. 2005) and cannabinoid CB1 receptors (CB1Rs) (Tebano et al. 2009; Lerner et al. 2010). Moreover, A2AR activity on MSNs has been shown to critically modulate long-term potentiation (LTP) at cortico-accumbal synapses (d'Alcantara et al. 2001) and spike-timing-dependent LTP at glutamatergic synapses onto striatopallidal MSNs (Shen et al. 2008b), a process thought to subserve learning and memory (Bliss and Collingridge 1993). Outside the striatum, A2ARs are also weakly expressed in neurons in the hippocampus and the cortex (Cunha et al. 1994; Dixon et al. 1996; Rosin et al. 1998). Such extra-striatal A2ARs similarly interact with other receptors such as mGlu5Rs (Rodrigues et al. 2005; Tebano et al. 2005). Within the hippocampus, A2ARs appear to be essential for LTP at mossy fiber-CA3 (Rebola et al. 2008) and CA3 → CA1 (Fontinha et al. 2009) synapses. A2ARs are also found at pre-synaptic terminals in cortical afferent neurons projecting onto striatal MSNs (Rosin et al. 2003; Rebola et al. 2005), and these strategically located cortical A2ARs can modulate glutamate release in the striatum (Rebola et al. 2005; Ciruela et al. 2006). Hence, A2ARs are uniquely positioned to fine-tune, at the circuitry level, the complex integration of dopaminergic, glutamatergic, and other neuronal signals that underlie cognitive processes including learning and memory. In transgenic models, A2AR overexpression impaired short-term object recognition memory and working memory (Gimenez-Llort et al. 2007), whereas A2AR inactivation enhanced spatial recognition memory (Wang et al. 2006) and working memory (Zhou et al. 2009). The latter might suggest that suppression of A2AR activity could be pro-cognitive as A2AR inactivation also counteracted age-related and pathologic memory loss in rodents (Prediger et al. 2005a,b,c; Dall'Igna et al. 2007).
However, existing studies have been limited in scope, with analyses restricted to a small subset of cognitive domains. A comprehensive characterization of A2AR's effects on particular cognitive functions is lacking. Furthermore, the relative contribution of A2ARs within distinct brain regions to these A2AR-dependent cognitive outcomes has only begun to be recognized. Yu et al. (2009) recently identified a critical role for striatal A2ARs in habit formation during instrumental learning, a finding that is consistent with the well-known striatal locus for habit formation (Packard and Knowlton 2002). The relevance of these abundant striatal A2ARs to higher cognitive processes such as spatial learning, which is typically attributable to hippocampus and association cortices (for review, see Aggleton et al. 2000; Kesner 2009; Save and Poucet 2009), however, has not yet been explored. Despite their relatively low expression levels, hippocampal and cortical A2ARs have been shown to modulate important synaptic functions (like LTP, as mentioned previously) thought to critically underlie learning and memory (Bliss and Collingridge 1993; Lynch 2004). These extra-striatal A2ARs might therefore also regulate such higher cognitive functions. A functional dissociation between striatal and extra-striatal (i.e., cortical and hippocampal) A2ARs in the regulation of behavior is highlighted by our recent study suggesting that they exert opposite control over dopamine- and glutamate/NMDAR-mediated psychomotor activity (Shen et al. 2008a).
The present study builds upon this initial finding and aims to dissect the complex, complementary contributions of striatal and cortical A2ARs to higher cognitive functions. We assessed multiple learning and memory domains with brain region specification of A2AR's effects by determining the cognitive impacts of conditional deletion of Adora2a (A2AR) restricted to forebrain (i.e., cortex, hippocampus, and striatum, fb-A2AR KO) (Bastia et al. 2005) or to striatum (st-A2AR KO) (Shen et al. 2008a) in recognition memory, working memory, reference memory, and reversal learning. This comparison allowed us to dissociate the effects of striatal A2ARs from extra-striatal (but within forebrain) A2ARs on specific cognitive processes. This comprehensive assessment revealed two previously underappreciated phenotypes of improved cognitive flexibility following targeted brain regional A2AR deletion. Specifically, deleting A2ARs in forebrain neurons led to a selective enhancement of working memory and reversal learning. Restricting the deletion to A2ARs only in striatal neurons still captured these same pro-cognitive phenotypes, thus suggesting that striatal A2AR inactivation alone is sufficient to enhance working memory and reversal learning. Targeting striatal A2ARs may therefore represent a novel approach for facilitating cognitive flexibility underlying effective goal-directed behavior when environmental demands or conditions change.
Results
Validation of brain region-specific A2AR deletion restricted to forebrain or striatum
Two separate conditional A2AR knockout mouse lines with targeted A2AR deletion in either the entire forebrain (i.e., fb-A2AR KO, Camk2a-cre(+)-Adora2aflox/flox) or striatum only (i.e., st-A2AR KO, Dlx5/6-cre(+)-Adora2aflox/flox) were generated using the Cre-loxP strategy. This standard strategy uses promoters with brain region-specific activity to drive the localized expression of the Cre protein, which subsequently recombines/deletes the particular gene sequence flanked by loxP sequences. In this study, the Camk2a promoter or the Dlx5/6 enhancer elements was used to produce Cre-mediated A2AR deletion restricted to the forebrain or striatum, respectively.
Fb-A2AR KO and st-A2AR KO mice have been characterized for their selective A2AR deletion in the forebrain (i.e., the cortex, the hippocampus, and the striatum) (Bastia et al. 2005; Yu et al. 2008) or exclusively in striatal (Shen et al. 2008a) neurons, as shown in our previous studies. To further characterize the Cre-mediated effects on A2AR expression in different brain regions in fb-A2AR KO and st-A2AR KO mice, we determined Cre expression by X-gal staining of LacZ in a Rosa26 reporter transgenic line, PCR analysis of Cre-mediated A2AR deletion in brain, and 3H-ZM241385 (a selective A2AR antagonist) radioligand binding of A2AR density in the brain.
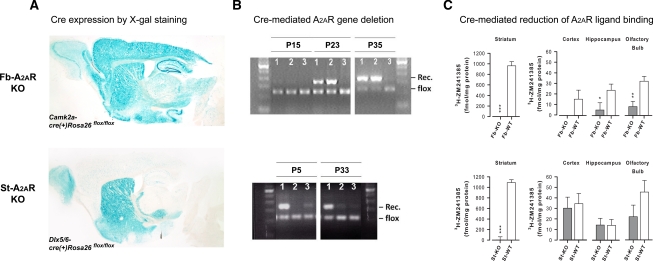
X-gal staining revealed high levels of Cre recombination in the cortex and the hippocampus and moderate to high levels in the striatum of Camk2a-cre(+)Rosa26flox/flox mice (Fig. 1A, upper panel). This expression pattern is consistent with our previous detection of Cre-mediated A2AR deletion in fb-A2AR KO mice by PCR analysis and by 3H-SCH58261 radioligand binding assay (Yu et al. 2008). In contrast, in Dlx5/6-cre(+)Rosa26flox/flox mice, Cre recombination was robust and localized mainly to dorsal and ventral striatum, although sparse recombination was detected in extra-striatal brain regions such as the hippocampus and the cortical mantle (Fig. 1A, lower panel). This pattern of scattered and weak staining in extra-striatal brain regions did not appear to include the principal excitatory neurons in which A2ARs are found, but might represent instead GABA-ergic interneurons (Batista-Brito et al. 2008).
Figure 1.
Forebrain- or striatum-specific A2AR KO is selective. (A) X-gal staining for brain regions of Cre expression demonstrates strong staining throughout the entire forebrain (i.e., cortex, hippocampus, and striatum) in Camk2a-cre(+)Rosa26flox/flox mice (upper panel). X-gal staining also shows strong staining throughout the entire striatum (that is largely absent in the cortex or the hippocampus) in Dlx5/6-cre(+)Rosa26flox/flox mice (lower panel). (B) Postnatal developmental time course of Cre recombination and deletion of the “floxed” A2AR allele. Representative PCR analysis of genomic DNA isolated from (1) the striatum, (2) the cortex, and (3) the cerebellum of fb-A2AR KO mice at the various developmental stages (P15, P23, and P35) demonstrates a forebrain-specific pattern of A2AR deletion in the striatum and the cortex, but not in the cerebellum beginning around P23 (upper panel). Similar PCR analysis of (1) the striatum, (2) the cortex, and (3) the hippocampus from st-A2AR KO mice at different developmental stages indicates a striatum-specific pattern of A2AR deletion in the striatum, but not in the cortex or the hippocampus as early as P5 (lower panel). (C) 3H-ZM241385 (selective A2AR antagonist) radioligand binding to quantify Cre-mediated loss of A2AR expression in the striatum, the cortex, the hippocampus, and the olfactory bulb in fb-A2AR KO mice and st-A2AR KO mice. 3H-ZM241385 binding is reduced in all forebrain regions examined in fb-A2AR KO mice (upper panel), but only in the striatum of st-A2AR KO mice (lower panel). n = 5–7 per group. Mean ± SEM are plotted. *P = 0.06, **P < 0.01, ***P < 0.0001, KO vs. WT.
To evaluate the time course of Cre-mediated A2AR deletion, we performed PCR analysis of genomic DNA extracted from the striatum, the cortex, the hippocampus, and/or the cerebellum of fb-A2AR KO or st-A2AR KO mice. As shown in Figure 1B (upper panel) Cre-mediated recombination was first detected at postnatal day 23 in fb-A2AR KO mice in the striatum and the cortex, but not in the cerebellum, thus demonstrating the forebrain specificity of the A2AR deletion at the DNA level. In contrast, in st-A2AR KO mice, Cre-mediated recombination was evident as early as postnatal day 5 (the earliest postnatal day examined) in the striatum, but was largely absent in the cortex or the hippocampus (Fig. 1B, lower panel), thereby confirming striatum-specific A2AR deletion. In both fb-A2AR KO and st-A2AR KO mice, it appeared that the recombination might be incomplete as shown by the persistence of the flox band.
As Cre activity may not directly reflect the extent of A2AR protein loss, 3H-ZM241385 radioligand binding assays were also conducted to quantify A2AR protein expression in the striatum, the cortex, the hippocampus, and the olfactory bulb of fb-A2AR KO and st-A2AR KO mice (Fig. 1C). One-way ANOVA (n = 5–7 mice per group) of 3H-ZM241385 binding in fb-A2AR KO and fb-WT mice revealed significant loss of A2AR expression in the striatum (F(1,10) = 64.48, P < 0.0001) and the olfactory bulb (F(1,9) = 12.87, P < 0.01), as well as a trend of reduction in the hippocampus and the cortex (Fig. 1C, upper panel). These results are entirely consistent with the pattern previously reported using 3H-SCH58261 (another selective A2AR antagonist) as the radioligand (Yu et al. 2008). In contrast, analysis of st-A2AR KO and st-WT mice (n = 6–7 per group) demonstrated that A2AR expression was completely lost in the striatum (F(1,11) = 177.47, P < 0.0001), but preserved in the cortex and the hippocampus (F's < 1), as well as olfactory bulb (P = 0.16) of st-A2AR KO mice (Fig. 1C, lower panel). Thus, forebrain-specific or striatum-specific A2AR deletion was also achieved at the protein level in fb-A2AR KO and st-A2AR KO mice, respectively.
In summary, analyses of the spatial and temporal patterns of Cre-recombination and Cre-mediated A2AR deletion at both the genomic and receptor binding levels in both knockout mouse lines collectively demonstrated that fb-A2AR KO had achieved selective loss of A2ARs in the entire forebrain (i.e., cortex, hippocampus, and striatum) beginning at postnatal day 23, whereas st-A2AR KO had achieved a highly selective loss of A2ARs in the striatum only (but not in the cortex or the hippocampus) as early as postnatal day 5. Thus, both fb-A2AR KO and st-A2AR KO mice shared a common feature of deficient A2AR expression in the striatum, although they differed in the timing of deletion. In fb-A2AR KO mice, as expected, this regional deletion extended from striatum to also include the cortex and the hippocampus and it occurred later.
Lack of compensatory changes in adenosine A1 or dopamine D1 and D2 receptors
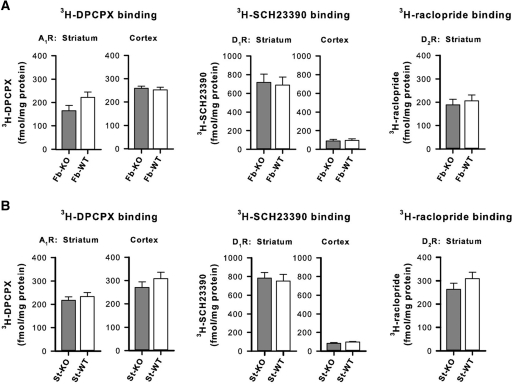
A2ARs are closely linked to the adenosinergic (i.e., A1Rs) and dopaminergic (i.e., D1Rs and D2Rs) receptor systems (Fredholm et al. 2007). Compensatory changes in the expression of these receptors might affect behavior independently of A2AR deletion (Fredholm et al. 2005; El-Ghundi et al. 2007). We therefore performed quantitative assays of binding densities in striatal and/or cortical total membranes from fb-A2AR KO and st-A2AR KO mice for A1Rs (3H-DPCPX, a selective A1R antagonist), D1Rs (3H-SCH23390, a selective D1R antagonist), and D2Rs (3H-raclopride, a selective D2R antagonist). No significant changes emerged in the binding assays for A1Rs or D1Rs in both striatal and cortical membranes, or for D2Rs in striatal membranes from fb-A2AR KO and st-A2AR KO mice in comparison with their respective WT littermates (Fig. 2A,B). These data suggest that compensatory changes in A1R, D1R, or D2R expression were not a concern in our A2AR KO mouse models. However, we cannot exclude the possibility of compensatory shifts in cell surface expression of these receptors, G-protein-coupling, or other downstream signaling pathways.
Figure 2.
Forebrain- and striatum-specific A2AR KO is without compensatory changes in A1, D1, or D2 receptor levels. (A,B) Quantitative analysis of 3H-DPCPX (selective A1R antagonist), 3H-SCH23390 (selective D1R antagonist), and 3H-raclopride (selective D2R antagonist) in total membranes from the striatum and/or cortex of fb-A2AR KO and fb-WT mice (n = 3–6 per group) (A), and of st-A2AR KO and st-WT mice (n = 5–8 per group) (B). No differences in radioligand binding densities were demonstrated for these receptors (one-way ANOVA, P's > 0.13). Mean ± SEM are plotted.
Spatial recognition memory in the Y-maze is unaffected in both fb-A2AR KO and st-A2AR KO mice
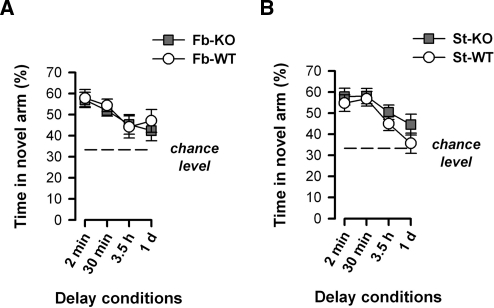
Recognition memory for distant spatial cues across a broad range of retention delays was evaluated in the nonaversive Y-maze test. Preferential exploration of the novel arm during the test phase performed 2 min, 30 min, 3.5 h, or 1 d after the sample phase was evaluated as an index of spatial recognition memory. A clear and comparable preference for the novel arm was apparent between genotypes in both forebrain and striatum cohorts, with all groups showing the expected decline in preference with increasing delay between sample and test phases (Fig. 3A,B).
Figure 3.
Fb-A2AR KO and st-A2AR KO mice show normal spatial recognition memory in the Y-maze. To evaluate memory for distant spatial cues, mice were first allowed 5 min to explore the start and familiar arms of the maze (sample phase) and then returned to the maze after a delay period (2 min, 30 min, 3.5 h, and then 1 d) and given 3 min to explore these same two arms plus an additional, novel arm (test phase). Preference for the novel arm, expressed as the percentage of time spent in the novel arm [(time in novel arm/time in all arms) × 100%], during each test phase was used to index spatial recognition memory. (A) Fb-A2AR KO and fb-WT mice showed a similar preference for the novel arm, which progressively weakened at a comparable rate with increasing retention demands. (B) St-A2AR KO and st-WT mice also showed a strong and comparable preference for the novel arm that gradually declined at a similar rate toward chance performance upon increasing the delay interval. Values depicted are mean ± SEM. Chance level = 33.33%, dashed line.
These interpretations were supported by separate 2 × 2 × 4 (Genotype × Sex × Delays) ANOVAs of the percent time spent in the novel arm, each of which only yielded a significant effect of Delays (fb-A2AR KO, F(3,63) = 3.92, P < 0.05; st-A2AR KO, F(3,81) = 7.99, P < 0.001). An additional analysis of the time spent in each arm using separate 2 × 2 × 4 × 3 (Genotype × Sex × Delays × Arms) ANOVAs further confirmed the overall preference for the novel arm (fb-A2AR KO, F(2,42) = 71.19, P < 0.001; st-A2AR KO, F(2,54) = 93.73, P < 0.001) and its dependency on the delay (Delays × Arms interaction: fb-A2AR KO, F(6,126) = 2.80, P < 0.05; st-A2AR KO, F(6,162) = 5.91, P < 0.001). It was therefore concluded that spatial recognition across a range of retention delays was not significantly altered by loss of forebrain or striatal A2ARs. This conclusion was strengthened by supplementary analyses of the distance traveled in each arm, which yielded a parallel pattern of results, thus arguing against any potential confound of locomotor activity on the time measure. Similarly, novel arm preference during the test phase was not confounded by any difference in arm exposure during the sample phase: familiar arm exploration was highly comparable between genotypes across each delay condition following either fb-A2AR KO or st-A2AR KO.
Spatial working memory performance is facilitated in both fb-A2AR KO and st-A2AR KO mice
Visually guided escape behavior
Initial training using the visible cued platform yielded no evidence of any genotype difference (Fig. 4A). All mice acquired the swimming and escape response, with performance improving over the four trials performed across two consecutive days, thus demonstrating their ability to utilize the local visual cues to locate the escape platform. A 2 × 2 × 4 (Genotype × Sex × Trials) ANOVA of escape latency confirmed the presence of a significant trials effect (fb-A2AR KO, F(3,75) = 20.78, P < 0.001; st-A2AR KO, F(3,87) = 22.57, P < 0.001). An identical pattern of results was obtained in the separate analysis of path length (Trials effect: fb-A2AR KO, F(3,75) = 18.25, P < 0.001; st-A2AR KO, F(3,87) = 22.01, P < 0.001), which is consistent with the impression that swim speed remained relatively stable and comparable between KO and WT mice [mean swim speed: fb-A2AR KO: 14.47 ± 0.49 cm/sec, fb-WT: 15.57 ± 0.58 cm/sec, F(1,25) = 2.09, P = 0.16; st-A2AR KO: 16.13 ± 0.67 cm/sec, st-WT: 17.57 ± 0.71 cm/sec, F(1,29) = 2.17, P = 0.151]. An identical analysis of these parameters during the visible cue task also yielded a similar pattern of results reflecting similar performance and behaviors among st-A2AR KO and st-WT mice of the second striatal cohort (Experiment set II, data not shown). Thus, neither the motor nor motivational component of the water-maze escape task was significantly affected in fb-A2AR KO or st-A2AR KO mice.
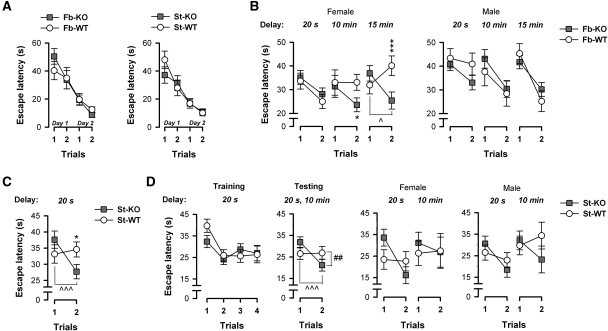
Figure 4.
Fb-A2AR KO (female only) and st-A2AR KO mice demonstrate normal visually guided escape behavior but improved spatial working memory. Mice were first pretrained in the water maze for up to two consecutive days (two trials per day) to swim directly to a visible platform for escape. Working memory was subsequently evaluated by examining over blocks of 4 d the improvement in escape latency (s) from trial 1 to 2 to reach a hidden platform whose location changed every day. (A) Both fb-A2AR KO (left panel) and st-A2AR KO (right panel) mice showed a comparable reduction in escape latency with each trial compared to fb-WT and st-WT mice, respectively. This indicates that A2AR deletion did not alter motivation or sensory and motor capabilities required to successfully learn and execute an escape onto a visible platform. (B,C) Experiment set I: The forebrain cohort was tested over three blocks corresponding to a 20-sec, 10-min, or 15-min delay between trials. The striatal cohort was tested in a single block at the minimal delay of 20 sec. Among the female mice (B, left panel), clear evidence for a delay-dependent enhancement of working memory was observed in fb-A2AR KO mice: these mice demonstrated a consistent and marked reduction in escape latency from trial 1 to 2 at all delays, whereas fb-WT mice only readily showed such improvement at the shorter delay, suggesting improved working memory capabilities in these female fb-A2AR KO mice. In contrast, among the male mice (B, right panel), fb-A2AR KO and fb-WT mice performed remarkably similarly, showing comparable improvements in escape latency from trial 1 to 2 at all delays. St-A2AR KO mice, like female fb-A2AR KO mice, readily showed improvement across the two trials, whereas st-WT mice did not (C). (D) Experiment set II: A separate striatal cohort was first trained for 4 d using a four-trial-per-day training protocol to facilitate learning and ensure mastery of the matching rule. Mice were then returned in the testing phase to a two-trial-per-day protocol as in Experiment set I and tested at 20-sec and 10-min delays. St-A2AR KO and st-WT mice demonstrated comparable improvement from trial 1 to 2 during training, but as the task demands increased during testing, st-A2AR KO mice again continued to out-perform st-WT mice, an effect that was not sex-dependent. Despite this lack of sex dependency in st-A2AR KO mice, the data are further plotted split by sex in order to provide a parallel comparison with the enhanced working memory phenotype in fb-A2AR KO mice, which did show a sex effect. Values depicted are mean ± SEM. *P < 0.05, ***P < 0.005, KO vs. WT. ∧P < 0.02, ∧∧∧P < 0.005, trial 1 vs. 2 in A2AR KO mice; ##P < 0.01, Genotype × Trials interaction.
Working memory performance in fb-A2AR KO mice
In this test, improved escape performance from trial 1 to 2 provides a measure of working memory (Hodges et al. 1995). The working memory task taxes the application of a daily matching-to-sample rule where the animal's experience on trial 1 (i.e., when the mouse has located the novel unknown position of a hidden platform) is used to guide the subsequent search for an identically located escape platform on trial 2 on a given day. Hence, only the same day's trial 1 experience is effective in guiding search performance during trial 2 in the working memory task. Initial analysis of both escape latency and path length by separate 2 × 2 × 3 × 4 × 2 (Genotype × Sex × Delays × Days × Trials) ANOVAs yielded a significant four-way interaction (Genotype × Sex × Delays × Trials interaction: escape latency: F(2,46) = 3.57, P < 0.05; path length: F(2,46) = 3.19, P = 0.05), which was suggestive of a sex-dependent genotype effect (see Fig. 4B). This was investigated by additional analyses restricted to either sex alone, which clearly demarcated the presence of a genotype effect on working memory performance in the female but not in the male sex. It is worth noting that the phenotypic difference in working memory observed between male and female fb-A2AR KO mice is unlikely attributable to a differential loss of forebrain A2ARs in these mice as X-gal staining showed a similar Cre-expression pattern between male and female fb-A2AR KO mice (data not shown).
Significant Genotype × Trials (escape latency: F(1,12) = 7.61, P < 0.05; path length: F(1,12) = 4.58, P = 0.05) and Genotype × Delays × Trials (escape latency: F(2,24) = 0.057; path length: F(2,24) = 3.76, P < 0.05) interactions were evident in the females, but clearly absent in the males (F's < 1). As illustrated in Figure 4B (left panel), these interaction terms, which were unique to the females, arose because fb-WT mice showed the expected delay-dependent deterioration of performance, whereas fb-A2AR KO mice continued to show a clear improvement in escape during trial 2 relative to trial 1, regardless of the delay. Forebrain A2AR deletion thus led to a delay-dependent improvement of working memory performance: fb-A2AR KO and fb-WT mice were highly comparable at the minimal delay, but diverged only when the increasing retention demand of the delay effectively reduced performance in fb-WT mice to chance level. Post-hoc pair-wise comparisons confirmed that female fb-A2AR KO and fb-WT mice never significantly differed from each other during trial 1 (P's > 0.30). A clear difference, however, was detected during trial 2 under the delay conditions of 10 min (P < 0.05) and 15 min (P < 0.005), and only fb-A2AR KO mice demonstrated significant performance improvement from trial 1 to 2 at the longest delay (P < 0.02). This genetic knockout effect on working memory performance was completely absent in the males (Fig. 4B, right panel).
Working memory performance in st-A2AR KO mice
Experiment set I
Striatal A2AR KO similarly led to a facilitation of working memory performance (Fig. 4C), but this effect was (1) already evident at the minimal delay condition when st-WT mice failed to consistently improve performance from trial 1 to 2 and (2) comparable between sexes, i.e., not sex-dependent. Separate 2 × 2 × 4 × 2 (Genotype × Sex × Days × Trials) ANOVAs of the two performance measures yielded a significant Genotype × Trials interaction (escape latency: F(1,29) = 6.80, P < 0.05; path length: F(1,29) = 6.41, P < 0.05) that was independent of sex (Genotype × Sex × Trials interaction: escape latency: F(1,29) = 1.55, P = 0.22; path length: F(1,29) = 1.15, P = 0.29). Post-hoc comparisons indicated the presence of a significant improvement from trial 1 to 2 in st-A2AR KO mice (P < 0.005), but not in st-WT mice (P = 0.64), and performance on trial 2 differed significantly between genotypes (P < 0.05) despite comparable performance on trial 1 (P = 0.15).
Experiment set II
The lack of evidence of effective working memory function among st-WT mice in Experiment set I raised the possibility that st-WT mice might have failed to master the matching rule of the task, thus leaving open the interpretation that the performance enhancement in st-A2AR KO mice might not represent an enhancement in working memory ability. To exclude this alternative interpretation, we conducted a separate experiment in a separate cohort of st-A2AR KO and st-WT mice using a slightly modified training protocol to ensure satisfactory learning performance in the st-WT controls (see Materials and Methods).
To capture the key element of working memory, in particular, we focused our analyses to the first two trials of each day. As depicted in Figure 4D (first panel), both st-A2AR KO and st-WT mice similarly demonstrated a clear improvement in trial 2 compared to trial 1 when matching-to-sample learning was promoted by using the four-trial-per-day training protocol in conjunction with a minimal retention load of 20 sec. This impression was confirmed by separate 2 × 2 × 4 × 2 (Genotype × Sex × Days × Trials) ANOVAs of the two performance measures. As expected, all mice demonstrated significant improvement from trial 1 to 2 (main effect of Trials: escape latency: F(1,13) = 20.55, P < 0.001; path length: F(1,13) = 7.71, P < 0.05), which did not differ between st-A2AR KO and st-WT mice (Genotype × Trials interaction: escape latency: F(1,13) = 1.28, P = 0.28; path length: F(1,13) = 2.60, P = 0.13). These results confirmed that both st-A2AR KO and st-WT mice were able to learn the procedures and acquire the day-dependent matching rule of the task.
Following initial training with the four-trial-per-day training protocol in which performance was comparable between genotypes, mice were then returned to a two-trial-per-day testing protocol as in Experiment set I. As shown in Figure 4D (last three panels), performance began to diverge again with st-A2AR KO mice (but not st-WT mice) showing significant improvement from trial 1 to 2. This genotype effect was evident with the short ITI (20-sec delay) and persisted as the retention load was increased with the extended ITI (10-min delay) in both the females and males. Thus, this outcome was entirely consistent with that in Experiment set I in which st-A2AR KO mice consistently out-performed st-WT mice in both sexes. A 2 × 2 × 2 × 4 × 2 (Genotype × Sex × Delay × Days × Trials) ANOVA of escape latency confirmed this observation yielding only a main effect of Trials (F(1,13) = 8.12, P < 0.05) and a significant Genotype × Trials interaction (F(1,13) = 9.18, P < 0.01), which were both independent of the delay intervals (Delay × Trials interaction: F(1,13) = 3.26, P = 0.09; Genotype × Delay × Trials interaction: F < 1) and of sex (Sex × Trials and Genotype × Sex × Trials interactions: F's < 1). Post-hoc comparisons indicated a significant improvement in escape latency from trial 1 to 2 in st-A2AR KO mice (P < 0.005), but not in st-WT mice (P = 0.92) despite their having demonstrated similar baseline performance on trial 1 (P = 0.11).
Collectively, these data from both sets of experiments indicate that st-A2AR KO mice consistently showed improved spatial working memory compared to st-WT mice, and this was not due to st-WT mice failing to acquire the matching rule. The fact that Experiment set II successfully replicated the phenotype previously observed in Experiment set I also strongly argues against any critical impact of prior trainings in the first striatal cohort on the original observation of working memory enhancement because the two striatal cohorts differed substantially in their respective experimental history. Moreover, unlike the phenotype that had emerged in fb-A2AR KO mice, the phenotype of enhanced working memory in st-A2AR KO mice was demonstrably independent of sex in both Experiment set I and Experiment set II (see Fig. 4D, last two panels).
Reference memory acquisition performance is unaffected in both fb-A2AR KO and st-A2AR KO mice
In contrast to working memory, when the platform is fixed in a constant position across days as in the reference memory procedure, the gradual accumulation of a relevant memory trace connecting the fixed location with the opportunity to escape from the water contributes to (1) performance improvement over days and (2) development of a search preference for the relevant spatial location.
Across the 10 d of acquisition training, all groups showed a gradual improvement in their escape performance. This was evidenced by a reduction in escape latency (Fig. 5A,D) and path length, with KO and WT mice demonstrating near-identical performance levels at the end of training. Separate 2 × 2 × 10 × 2 (Genotype × Sex × Days × Trials) ANOVAs of both performance measures in the two cohorts yielded a highly significant Days effect (fb-A2AR KO: escape latency: F(9,225) = 6.33, P < 0.001; path length: F(9,225) = 10.27, P < 0.001; st-A2AR KO: escape latency: F(9,243) = 2.58, P < 0.01; path length: F(9,243) = 3.35, P < 0.001). Neither the main effect of Genotype nor its interactions approached statistical significance. Thus, A2A receptor knockout in the forebrain or the striatum did not significantly affect acquisition performance.
Figure 5.
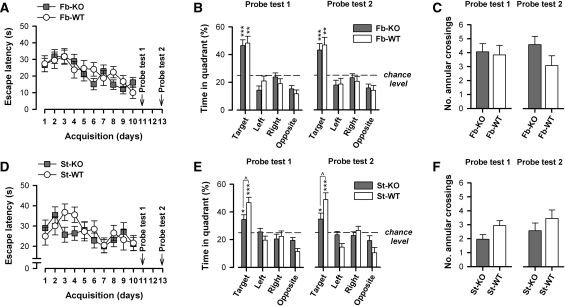
Reference memory performance is spared in fb-A2AR KO and st-A2AR KO mice. Mice were trained for 10 consecutive days (two trials per day) to acquire the fixed spatial location of a hidden escape platform. Following acquisition, two probe tests were conducted on days 11 (Probe test 1) and 13 (Probe test 2) during which the escape platform was removed from the pool and the spatial search pattern of mice was observed for 60 sec. (A) Fb-A2AR KO mice, like fb-WT mice, demonstrated a steady decline in escape latency with each training day, showing highly comparable escape performance by the end of acquisition training (day 10). (B) Both fb-A2AR KO and fb-WT mice showed a strong and similar search preference for the spatial location of the escape platform (i.e., target quadrant) in both probe tests. (C) Search accuracy defined by the number of annular crossings was comparable between fb-A2AR KO and fb-WT mice. (D) Similarly, st-A2AR KO and st-WT mice showed gradual performance improvement during acquisition, achieving near-identical performance at the end of training (day 10). (E) Both st-A2AR KO and st-WT mice demonstrated an above-chance level search preference for the target quadrant in both probe tests; however, this preference was weaker in st-A2AR KO mice. (F) St-A2AR KO and st-WT mice did not significantly differ in the number of annular crossings, indicating comparable search accuracy in both probe tests. Values depicted are mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 vs. chance level (=25%, dashed line). ∧P < 0.05, st-A2AR KO vs. st-WT.
Post-acquisition probe test performance is reduced in st-A2AR KO but not fb-A2AR KO mice
Spatial search pattern in Probe tests 1 and 2
Two probe tests, in which the platform was removed from the maze, were conducted 24 and 72 h following the conclusion of acquisition training to assess the strength and retention of the spatial search preference that had developed as a result of acquisition training. The two tests yielded parallel outcomes, suggesting stable retention of the spatial preference (Fig. 5B,E). Comparison between fb-A2AR KO and fb-WT mice indicated that both groups showed a strong preference for the quadrant area (i.e., target quadrant) centering on the platform's location and comparable search accuracy of the precise platform location during acquisition training (Fig. 5B,C). In contrast, this preference, albeit still above chance level in st-A2AR KO mice, was significantly weaker in comparison with that in st-WT mice (Fig. 5E). These interpretations were confirmed by separate 2 × 2 × 4 (Genotype × Sex × Quadrants) ANOVAs of the percent time spent per quadrant in each probe test. The analyses comparing fb-A2AR KO and fb-WT mice only yielded a main effect of Quadrants (Probe test 1: F(3,75) = 28.69, P < 0.001; Probe test 2: F(3,75) = 18.42, P < 0.001). In contrast, the analyses comparing st-A2AR KO and st-WT mice revealed in addition, a significant Genotype × Quadrants interaction in both probe tests (Probe test 1: F(3,81) = 3.18, P < 0.05; Probe test 2: F(3,81) = 3.76, P < 0.05). This was attributable to different target quadrant preference between genotypes as indicated by post-hoc pair-wise comparisons (P < 0.05). One-sample t-tests further confirmed that all groups displayed a target quadrant preference significantly exceeding that expected by chance, i.e., >25% (P's < 0.05).
Normal memory retention and temporal search pattern during probe testing in st-A2AR KO mice
Differences in the amount of time spent in the target quadrant during probe testing can reflect differences in memory retention as well as in temporal search patterns. Therefore, we performed a set of additional analyses to test whether the observed relative performance impairment on Probe tests 1 and 2 in st-A2AR KO mice might be attributable to impaired memory retention or an altered temporal search pattern during probe testing in st-A2AR KO mice. First, to address whether st-A2AR KO mice differed in their ability to retain the memory for the spatial location of the platform, we examined trial 1 performance during acquisition training, which can indicate memory retention from the previous day's training. Separate 2 × 2 × 10 (Genotype × Sex × Days) ANOVAs of trial 1 escape latency and path length did not yield any significant Genotype effect (F's < 1) or its interactions (Genotype × Days interaction: escape latency: F(9,243) = 1.34, P = 0.24; path length: F(9,243) = 1.38, P = 0.22), a result that is consistent with the impression based on the overall analysis in the acquisition phase (see Fig. 5D). To further clarify this issue, we also analyzed the number of annular crossings (i.e., the frequency at which the swim path crossed the former spatial location of the platform) and the latency of the first annular crossing in each probe test to provide both, respectively, an index of search accuracy and an estimate of what the escape latency would have been if the platform were present. Separate two-way (Genotype × Sex) ANOVAs of the number of annular crossings (Fig. 5F) or the latency of the first annular crossing (data not shown) in each of the two probe tests did not reveal any significant differences between st-A2AR KO and st-WT mice. Together with the above-mentioned findings that trial 1 performance across acquisition days showed gradual improvement and was indistinguishable between genotypes, these data suggest that st-A2AR KO mice were not consistently impaired in their ability to retain the memory of the precise spatial location of the escape platform compared to st-WT mice.
Second, to address whether a different temporal search pattern (e.g., possibly reflective of enhanced within-session extinction) in st-A2AR KO mice could account for the apparent probe test deficit reported previously, we examined the temporal profile (across successive 15-sec bins) of the preference for the target quadrant in each probe test. Separate 2 × 2 × 4 (Genotype × Sex × Bins) ANOVAs of the percent time spent in the target quadrant suggested that the preference was relatively stable over time and without evidence for extinction in both st-A2AR KO and st-WT mice (Bins effect and Genotype × Bins interaction: F's < 1), but consistent with the initial probe test analyses comparing all four quadrants (see Fig. 5E), this preference was reduced in st-A2AR KO mice (main effect of Genotype: Probe test 1: F(1,27) = 5.42, P < 0.05; Probe test 2: F(1,27) = 4.71, P < 0.05). Therefore, the probe test performance deficit observed in st-A2AR KO mice was not associated with a difference in their temporal search pattern in the target quadrant during probe testing, when actual escape onto the platform was not available. This suggests that enhanced extinction of the target quadrant preference is an unlikely explanation for their weaker probe test performance. The exact reason for the weaker performance in Probe tests 1 and 2 in st-A2AR KO mice remains unclear but may stem from a weakening of habit formation (see Discussion) rather than from an impairment in reference memory retention or acquisition as such.
Reversal phase learning occurs more rapidly in both fb-A2AR KO and st-A2AR KO mice
Reversal learning began 4 d after the end of acquisition on experimental day 14 (see Fig. 6). This lasted for 4 d in the forebrain cohort when fb-WT mice showed a clear preference for the new target quadrant in Probe test 3. This preference, however, was not observed in st-WT mice, and thus, reversal training continued for another 4 d in the striatal cohort. By the end of this additional training, st-WT mice had exhibited a clear preference for the new target quadrant. This pattern of results aligned with the impression obtained from the working memory test, which suggested that the fb-WT mice tended to learn more quickly in general relative to the st-WT mice.
Figure 6.
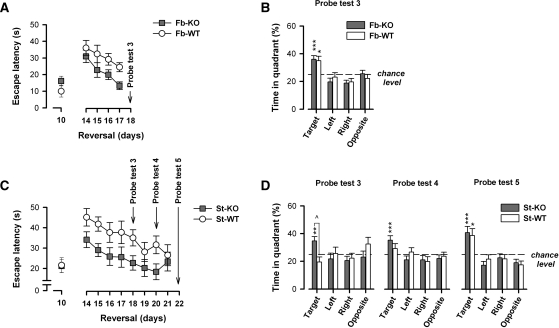
Faster reversal learning in fb-A2AR KO and st-A2AR KO mice. Reversal learning began once the mice had learned the fixed position of the hidden escape platform during acquisition. It lasted for 4 d (days 14–17) in the forebrain cohort and for 8 d (days 14–21) in the striatal cohort. During reversal, the location of the platform was shifted 180° to the opposite quadrant, and mice were required to learn the new escape location. Probe tests were conducted 24 h after a training session to evaluate the progress of reversal learning on day 18 (Probe test 3) in both cohorts, and additionally on days 20 and 22 (Probe tests 4 and 5) in the striatal cohort only. (A) Fb-A2AR KO mice were less affected by the shift in platform location as indicated by faster escape latencies during the reversal phase. This effect appeared more pronounced with additional reversal training as demonstrated by a divergence in escape latencies. (B) Both fb-A2AR KO and fb-WT mice exhibited a comparable search preference for the new target quadrant after 4 d of reversal training. (C) St-A2AR KO mice also escaped more quickly throughout reversal learning compared to st-WT mice, indicating that they were less disrupted by the sudden change in platform location. (D) St-A2AR KO mice also demonstrated a strong preference for the new target quadrant in all three probe tests. This preference was significantly greater in st-A2AR KO mice during Probe test 3 compared to that in st-WT mice. A significant target preference was not observed in st-WT mice until the end of reversal training (i.e., Probe test 5). Values depicted are mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.005 vs. chance level (=25%, dashed line). ∧P < 0.05, st-A2AR KO vs. st-WT.
Fb-A2AR KO facilitated reversal learning performance without affecting probe test performance
The change of platform location (from day 10 to day 14) disrupted the efficiency of locating the escape platform in fb-A2AR KO and fb-WT mice. To gauge this reversal effect, analyses contrasting the last acquisition day and the first reversal day were performed, which yielded only a main effect of Days (three-way ANOVA: escape latency: F(1,25) = 34.99, P < 0.001; path length: F(1,25) = 34.91, P < 0.001). Although the initial impact of reversal appeared comparable between fb-A2AR KO and fb-WT mice, their performance appeared to diverge as reversal training continued (Fig. 6A).
Indeed, fb-A2AR KO mice rapidly improved over the 4 d of reversal training, achieving a performance level (i.e., on day 17) that was comparable with that at the end of acquisition (i.e., on day 10). In contrast, fb-WT mice performed relatively poorly during the reversal phase. Separate 2 × 2 × 4 × 2 (Genotype × Sex × Days × Trials) ANOVAs of escape latency and path length yielded similar patterns of results, although the latency measure appeared to be more powerful in detecting a genotypic difference. Significant effects of Genotype (escape latency: F(1,25) = 4.39, P < 0.05; path length: F(1,25) = 3.92, P = 0.06) and of Days (escape latency: F(3,75) = 10.02, P < 0.001; path length: F(3,75) = 9.49, P < 0.001) were found.
A probe test conducted to evaluate the pattern of spatial search on day 18 yielded highly comparable outcomes between fb-A2AR KO and fb-WT mice (Fig. 6B). After 4 d of reversal training, both groups had developed an overall preference for the new target quadrant that significantly exceeded chance level (P's < 0.01) with limited evidence of any residual preference for the former (now opposite) target quadrant. A 2 × 2 × 4 (Genotype × Sex × Quadrants) ANOVA of the percent time spent in each quadrant only yielded a highly significant Quadrants effect (F(3,75) = 10.64, P < 0.001).
St-A2AR KO facilitated reversal learning and showed a less persistent preference for the previously acquired platform location during probe testing
The impact of relocating the escape platform impaired performance as expected (Fig. 6C). Analyses contrasting the last acquisition day and the first reversal day confirmed the impact of reversal on both performance measures, yielding a significant Days effect (escape latency: F(1,27) = 25.13, P < 0.001; path length: F(1,27) = 19.36, P < 0.001). There was no statistical support that the initial impact of reversal differed between st-A2AR KO and st-WT mice (Genotype × Days interaction: escape latency: F(1,27) = 2.02, P = 0.17; path length: F < 1).
However, st-A2AR KO mice out-performed st-WT mice over the course of reversal learning. Separate 2 × 2 × 8 × 2 (Genotype × Sex × Days × Trials) ANOVAs of the two performance measures yielded similar outcomes, although escape latency was again more powerful in detecting a genotypic difference. Significant effects of Genotype (escape latency: F(1,27) = 7.49, P = 0.01; path length: F(1,27) = 3.78, P = 0.06) and of Days (escape latency: F(7,189) = 4.64, P < 0.001; path length: F(7,189) = 3.54, P < P < 0.01) emerged, but their interaction was not significant (F's < 1). Thus, the statistical outcomes resembled those obtained in the forebrain cohort although the two experiments differed in the number of training days.
To allow an effective assessment of the competition between any residual preference for the previous platform location (i.e., during acquisition) and the newly acquired preference for the platform location used during reversal training, three probe tests (Probe tests 3, 4, and 5) were performed over the course of reversal training. The first of these probe tests was performed after 4 d of reversal training (just before reversal training on day 5). St-A2AR KO mice already showed a preference for the new target quadrant during this probe test, whereas st-WT mice still exhibited an overall preference for the previous target quadrant used during acquisition (see Probe test 3) (Fig. 6D). This impression was confirmed by the emergence of a near-significant Genotype × Quadrants interaction (F(3,81) = 2.70, P = 0.05) in a 2 × 2 × 4 (Genotype × Sex × Quadrants) ANOVA of percent time spent per quadrant. The same interaction term achieved clear statistical significance in a separate 2 × 2 × 2 ANOVA contrasting solely the percent time spent between the (diagonally opposite) new and previous target quadrants (F(1,27) = 5.76, P = 0.025). This analysis provides a meaningful and specific assessment of the expression of the reversal effect by focusing on the search behavior in the previously reinforced quadrant and the presently (newly) reinforced quadrant in exclusion of the never-reinforced quadrants. Post-hoc pair-wise comparisons further confirmed that st-A2AR KO mice already preferred the new target quadrant more than did st-WT mice on the first reversal probe test (Probe test 3, P < 0.05).
By the second and third probe tests in the reversal phase, however, performance between st-A2AR KO and st-WT mice had become increasingly comparable (see Probe tests 4 and 5) (Fig. 6D). In st-WT mice, preference for the new target quadrant continued to increase as their preference for the previous target quadrant waned. On the other hand, performance across these three probe tests was relatively stable in st-A2AR KO mice. Separate 2 × 2 × 4 (Genotype × Sex × Quadrants) ANOVAs of the percent time spent per quadrant revealed only a main effect of Quadrant in these two probe tests (Probe test 4: F(3,81) = 4.02, P = 0.01; Probe test 5: F(3,81) = 12.81, P < 0.001) without any evidence for genotypic differences. The latter result was in agreement with the analysis of annular crossings variables (data not shown). Additional one-sample t-tests confirmed that target quadrant preference exceeded chance level in all probe tests in st-A2AR KO mice (P's < 0.006), but st-WT mice only achieved that in the final probe test (P < 0.02). Increasing preference for the new target quadrant across the three probe tests in st-WT mice (F(2,24) = 5.88, P < 0.01), but not in st-A2AR KO mice (F(2,30) = 1.77, P = 0.20), was confirmed by a repeated measures ANOVA that directly compared preference for the new target quadrant across the three probe tests.
Discussion
This study provides the first direct demonstration that targeted inactivation of A2ARs on intrinsic striatal neurons is sufficient to facilitate two forms of cognitive-behavioral flexibility—working memory and reversal learning. These phenotypes arose largely in the absence of any gross or persistent effects on other behavioral parameters, such as spatial reference memory acquisition or spatial recognition memory. They were also free from compensatory changes in D1, D2, or A1 receptor expression in the striatum or the cortex. Moreover, comparative analysis of fb-A2AR KO and st-A2AR KO revealed largely similar phenotypes of enhanced working memory and reversal learning, leading to the conclusion that A2AR inactivation in the striatum (rather than in the cortex or the hippocampus) plays a predominant role in the expression of cognitive flexibility by A2ARs. Thus, targeting striatal A2ARs alone may be sufficient to facilitate effective behavioral adaptations in response to changing environmental contingencies. Striatal A2ARs might therefore represent an attractive, novel strategy to restore cognitive flexibility in neuropsychiatric conditions, such as Parkinson's disease, schizophrenia, or related neuropsychiatric disorders in which striatal dysfunction is implicated.
Selective inactivation of striatal neuronal A2ARs is sufficient to enhance working memory
Working memory captures important elements of cognitive flexibility, notably the capacity to maintain or update information held online and select appropriate behavioral responses in accordance to shifting positive and negative stimulus-response contingencies (Goldman-Rakic 1995; Marie and Defer 2003; Dalley et al. 2004). A2AR deletion on striatal neurons facilitated spatial working memory performance as evidenced by consistent trial 1-to-2 improvement in st-A2AR KO but not st-WT mice in two independent experiments (i.e., Experiment sets I and II). We observed similar results in female fb-A2AR KO mice, but this difference was absent in the males. This sex-dependent phenotypic difference is unlikely attributable to a differential loss of forebrain A2ARs between sexes given that Cre expression did not reveal any such sex difference (data not shown). Although the precise origin or mechanism underlying this sex-dependent working memory phenotype in fb-A2AR KO mice is presently unclear, it is worth noting that global A2AR knockout has previously been reported to influence other brain-relevant processes in a sex-dependent manner, including body temperature regulation (Yang et al. 2009) and ethanol sensitivity and consumption (Naassila et al. 2002). Interestingly, estrogen has been shown to modify the expression of A2AR transcript in several different brain regions (Ribeiro et al. 2009); thus, one might venture to speculate a degree of sex hormonal influence over A2AR-dependent phenotypes. Here, because the expression of the working memory phenotype was only dependent on sex in the forebrain but not striatal A2AR knockout mouse line, any such modulation by sex hormones might be more critical and/or more likely occurring in areas outside the striatum (e.g., in the cortex and/or the hippocampus where A2ARs were deleted in fb-A2AR KO but spared in st-A2AR KO mice). Regardless of the precise mechanism underlying the sex-dependent effect unique to fb-A2AR KO mice, it does not undermine the major finding that working memory enhancement can be induced by A2AR inactivation (which led to a similar overall impact between the two conditional A2AR knockout lines) and that striatal A2AR inactivation alone is sufficient to produce this selective enhancement, which incidentally was not significantly modified by sex.
Improved working memory in st-A2AR KO and female fb-A2AR KO mice unlikely reflects ineffective mastery of the matching rule by their respective controls: both control groups clearly demonstrated successful task performance when the cognitive load was minimal (i.e., Experiment set II training in the striatal cohort under the initial four-trial-per-day protocol and 20-sec delay in the forebrain cohort). Thus, performance between knockout and control only diverged when task difficulty increased (see Fig. 4B,D). In contrast, spatial recognition memory and reference memory acquisition were largely unaffected by A2AR inactivation in either knockout line. This pattern of outcomes closely resembles that seen following global A2AR inactivation in mice (Zhou et al. 2009), which also selectively enhanced working memory without affecting reference memory learning. Conversely, overexpression of A2ARs in the brain impaired working memory performance, but also did not affect reference memory function in transgenic rats (Gimenez-Llort et al. 2007). The selectivity of these findings is consistent with the view that striatal function is not critical to forming spatial representations as such (Packard and McGaugh 1992; McDonald et al. 2008; Berke et al. 2009).
The novel finding of our study is that selective inactivation of striatal A2ARs alone was sufficient to reproduce the pro-cognitive phenotypes resulting from A2AR deletion extending to the entire forebrain. An earlier transgenic study suggested that A2ARs in the cortex and the hippocampus modulate working memory (Gimenez-Llort et al. 2007), which is consistent with A2AR's well-documented functional effects on neuronal plasticity at the cortico-striatal (Schiffmann et al. 2007) and the hippocampal mossy fiber-CA3 (Rebola et al. 2008) pathways and with the identification of cortical regions (e.g., prefrontal and parietal cortices), in particular as the key structures subserving effective working memory based on electrophysiology and imaging (Goldman-Rakic 1995; Rowe et al. 2000).
Our comparative analysis, however, revealed a largely similar pro-cognitive phenotype in both fb-A2AR KO and st-A2AR KO mice. This profile was similar both in the type and selectivity of the cognitive functions that were enhanced, as well as in the magnitude of the observed cognitive enhancements, particularly in the case of working memory. This strongly suggests that striatal A2ARs (not cortical or hippocampal A2ARs) play a critical role in A2AR-dependent modulation of cognition. Extending the deletion of A2ARs beyond the striatum to include the hippocampus and the cortex (i.e., extra-striatal) as in fb-A2AR KO mice did not produce any phenotypes that were distinguishable from those already present in st-A2AR KO mice. Thus, striatal A2AR activity appears to assume a more prominent influence on these cognitive functions compared with extra-striatal cortical/hippocampal A2AR activity. The exact contribution of cortical/hippocampal A2ARs to cognition, however, remains to be defined, e.g., through the use of cortex- and/or hippocampal-specific A2AR KO mouse models. Last, it would be prudent to point out that there are extra-striatal regions where A2ARs were apparently deleted (e.g., hypothalamus) in both A2AR KO mouse lines examined. The possible loss of A2ARs in hypothalamus, where expression is normally low, might be predicted to affect performance via its control of arousal rather than of learning as such. Given that striatal A2ARs represent the majority of the A2ARs commonly lost between both knockout lines (due to its shear number), it is both reasonable and parsimonious to infer that striatal A2ARs play a key role in yielding the observed working memory enhancement.
Striatal D2R activity is important for effective working memory as shown in animals and humans (e.g., Kellendonk et al. 2006; Mehta et al. 2008). Inactivation of striatal A2ARs might potentiate striatal dopaminergic signaling via D2Rs to produce the working memory enhancement, given the well-documented antagonistic A2AR–D2R interaction in striatopallidal MSNs of the “indirect” pathway (Ferre et al. 1997; Fredholm et al. 2007). In keeping with this notion, we have recently shown that st-A2AR KO potentiated the motor-stimulant effect induced by dopaminergic stimulation (Shen et al. 2008a). Interestingly, a form of striatal LTD (i.e., eCB-LTD) that is dependent on endocannabinoid release and D2R activation (Kreitzer and Malenka 2005, 2007) was found to be restricted to this very same population of striatopallidal MSNs (Kreitzer and Malenka 2007). Moreover, this critical release of endocannabinoids from striatal MSNs was previously reported to require dopamine release in conjunction with up-state-dependent activation of mGlu5Rs and L-type calcium channels (Kreitzer and Malenka 2005). Consistent with this observation, D2R, mGlu5R, and Cav1.3 stimulation was shown to induce LTD using a protocol capable of eliciting spike-timing-dependent plasticity (STDP) (Shen et al. 2008b). Although the impact of post-synaptic striatal A2AR activity on striatal LTD has not yet been directly examined, concomitant stimulation of A2ARs and D2Rs shifted the striatopallidal MSN plasticity response from that of a D2R-induced LTD response to a LTP response instead (Shen et al. 2008b). It is noteworthy that activation of A2ARs, in the setting of FGFR co-activation, has also been demonstrated to promote LTP at these cortico-striatopallidal synapses (Flajolet et al. 2008), and suppression of A2ARs by pharmacologic blockade or by global A2AR deletion has been shown to impair LTP at these synapses (d'Alcantara et al. 2001; Shen et al. 2008b). Therefore, it is possible that A2AR deficiency in the striatal “indirect” pathway MSNs modifies working memory by modulating dopamine-dependent signaling and plasticity, LTD, and/or LTP in the striatum.
A2AR modulation of NMDAR current at post-synaptic striatal neurons (Norenberg et al. 1998), as well as A2AR heterodimerization and functional interaction with mGlu5Rs (Ferre et al. 2002), further suggest that striatal A2AR activity may also modify working memory by modulating striatal glutamatergic signaling. The modification of the striatal function potentially through dopaminergic and/or glutamatergic mechanisms by striatal A2ARs might be expected to alter the processing of cortical information entering the striatum, as well as striatal projections to efferent targets such as the prefrontal cortex (Simpson et al. 2010). The latter is supported by the recent demonstration that manipulation of striatal D2R activity is sufficient to modify the prefrontal cortical function and working memory (Kellendonk et al. 2006).
Selective inactivation of striatal neuronal A2ARs enhances reversal learning
Reversal learning is an accepted measure of cognitive flexibility because efficient task performance requires the ability to rapidly switch from one response pattern to a conflicting one in response to the complete reversal of environmental contingencies between the relevant action and goal. This study provides the first demonstration that striatal A2AR inactivation facilitates spatial reversal learning largely without affecting spatial reference memory acquisition or recognition memory. Both fb-A2AR KO and st-A2AR KO reversed faster, as evidenced by their efficient escape performance during reversal training (fb-A2AR KO and st-A2AR KO) and the earlier emergence of a spatial bias for the novel target quadrant across successive probe tests (st-A2AR KO). Thus, compared to their respective control groups, these knockout mice more effectively and more rapidly redirected their search behavior in response to the relocation of the escape platform from its initial constant position. This outcome might reflect that fb-A2AR KO and st-A2AR KO mice were more ready to inhibit their previously acquired (but no longer effective) response and extend their search to alternative areas, thereby allowing them to establish the newly adaptive response more quickly than their controls. This phenotype cannot, however, be solely attributed to poorer initial learning of the original platform location because st-A2AR KO and st-WT mice performed comparably during acquisition, achieving near-identical performance on the last training day, and demonstrated equivalent search accuracy and above-chance performance during probe testing (see Fig. 5D,F). This interpretation is reinforced by the observation of a similar enhanced reversal learning phenotype in fb-A2AR KO mice that had clearly demonstrated normal probe test performance.
Although the water-maze reversal procedure differs from reversal learning based on the two-alternative forced choice paradigm by the fact that there were areas of the maze that were consistently not associated with the escape platform across both acquisition and reversal, it is still highly effective in taxing the ability to suppress or inhibit the previously reinforced response. As mentioned previously, the reversal learning phenotype may reflect a facilitation of the ability to suppress a learned response in general. This possibility is strengthened by our recent demonstration showing that st-A2AR KO mice remained sensitive to the devaluation of a reinforcer's incentive value when overtraining had instilled a resistance in the controls whose responding had become habitual (Yu et al. 2009). Similarly, st-A2AR KO mice were also demonstrably more responsive to an “omission” procedure, whereby lever pressing was discouraged by lowering the frequency and increasing the delay of food delivery (Yu et al. 2009). Consistent with this finding is the evidence suggesting that the striatum plays an important role in active response suppression (e.g., Zandbelt and Vink 2010).
All together, it is apparent that facilitated reversal learning and cognitive flexibility (observed in this study) may come at the expense of weakened habit formation (as shown in Yu et al. 2009). The balance and trade-off between the two strategies of behavioral control might therefore be effectively modulated by striatal A2ARs. For example, under conditions in which persistence is maladaptive and unproductive, the promotion of alternative behavioral responses might lead to a more rapid adaptation to changing environmental contingencies, leading to ”enhanced” performance outcomes. Such conditions were prominent in both the reversal test in the present study as well as in the instrumental tests reported by Yu et al. (2009). One interpretation of these findings is that st-A2AR KO mice remained goal-directed with a corresponding weakening of habit formation. The latter may imply that habit formation depends on the induction of A2AR-dependent striatal LTP (Lovinger 2010) as LTP in the striatum is reduced in global A2AR KO mice (d'Alcantara et al. 2001; Shen et al. 2008b).
Neural circuits in several different brain regions including the prefrontal cortex, the hippocampus, the amygdala, and the striatum are collectively involved in reversal learning. Genetic knockout studies have implicated glutamatergic and dopaminergic signaling in reversal learning, but have not determined the relative contributions of cortical vs. subcortical receptors. The similar enhanced reversal phenotype in both knockout mouse lines argues that modulating A2AR signaling in the striatum alone is sufficient for reversal learning enhancement and suggests that striatal A2ARs play a critical role in modulating this form of cognitive flexibility. This interpretation agrees with converging evidence that the striatum is pivotal in reversal learning (Bellebaum et al. 2008; Clarke et al. 2008).
Our finding of enhanced reversal learning, without disrupting acquisition during water-maze testing, is reminiscent of the impaired reversal learning phenotype in mutant mice with glutamatergic signaling deficiency including mGlu5R (Xu et al. 2009), NMDA receptor subunit NR2A (Bannerman et al. 2008), and AMPA receptor subunit GluR-A (Bannerman et al. 2003) knockout mice and of the enhanced phenotype in forebrain neuron-specific glycine transporter (GlyT1) knockout mice (Singer et al. 2009). These gene knockout studies suggest that the potentiation of glutamatergic and/or NMDAR-mediated signaling may be linked to the enhancement of reversal learning. In light of A2AR's functional antagonism with NMDARs (Wirkner et al. 2004) in the striatum, the enhanced reversal phenotype might also be mediated by heightened striatal NMDAR/glutamatergic signaling resulting from deficient striatal A2AR activity.
Notwithstanding, D2Rs are also important for reversal learning. In humans, reversal performance correlated positively with D2R binding in the caudate nucleus (Clatworthy et al. 2009), whereas pharmacologic D2R blockade in nonhuman primates (Lee et al. 2007), genetic D2R deletion in mice (Kruzich et al. 2006; De Steno and Schmauss 2009), or striatal D2R overexpression in mice (Kellendonk et al. 2006) impaired reversal learning. Thus, A2AR's interaction with D2Rs offers another mechanism whereby striatal A2ARs may influence reversal learning.
Last, recent evidence has shown that A2AR antagonism can increase levels of the retrograde endocannabinoid messenger, 2-arachidonoylglycerol, in the striatum and facilitate endocannabinoid-dependent LTD at excitatory synapses onto post-synaptic striatopallidal MSNs (Lerner et al. 2010). Moreover, the motor stimulating effect of A2AR antagonism, which is known to occur through a blockade of post-synaptic striatal A2ARs (Shen et al. 2008a), was markedly attenuated by global CB1-receptor antagonism or knockout (Lerner et al. 2010), thus highlighting the cross-talk between post-synaptic striatal A2ARs and endocannabinoid-CB1 receptor pathways. Along with evidence that pharmacologic manipulation (Hill et al. 2006) or genetic deletion (Varvel and Lichtman 2002) of CB1 receptors can impair reversal performance in rodents, one might speculate that suppression of striatal A2AR activity may also facilitate reversal learning by potentiating endocannabinoid-CB1 receptor signaling in excitatory (e.g., cortical glutamatergic) synapses in the striatum.
Conclusion
In summary, we provide the first direct demonstration that A2AR inactivation on intrinsic striatal neurons where A2AR expression is most abundant is sufficient to selectively enhance working memory performance and facilitate reversal learning. This suggests that striatal A2ARs may be an effective, novel target to enhance cognition under physiological conditions. As A2AR antagonists are in clinical phase II–III trials for Parkinson's disease and cognitive inflexibility is a core cognitive disturbance in neuropsychiatric disorders including Parkinson's disease and schizophrenia, A2AR antagonists might ameliorate these associated cognitive deficits. Our results support an important role for the striatum in cognition (Simpson et al. 2010) and lend a partial explanation for the prominent cognitive changes associated with neuropathologic disorders, like Huntington's disease and Parkinson's disease, where neuronal degeneration and loss is largely restricted to the striatum, without significant pathologic changes in the cortex.
Materials and Methods
Approval from the Institutional Animal Care and Use Committee at Boston University School of Medicine and the Zurich Cantonal Veterinarian Office had been previously granted for all experiments conducted in Boston and Zurich, respectively. They adhered to the NIH Guide for the Care and Use of Laboratory Animals (1982), the Swiss Federal Law and Ordinance on Animal Protection, and European Council Directive 86/609/EEC (1986).
Generation of forebrain-specific and striatum-specific A2AR knockout mice
Two conditional A2AR knockout mouse lines with brain region-specific deletion of a critical region (i.e., exon 2) of Adora2a, the gene encoding the adenosine A2A receptor (A2AR), were generated using the Cre/loxP strategy. This particular strategy uses a region-specific promoter to drive the expression of Cre, which subsequently mediates the deletion of a gene flanked by loxP sites. Although we refer to our engineered mouse lines as gene “knockout” to maintain consistency with our previous reports using these engineered mouse lines, our model might more precisely be considered a gene “knockdown” as Cre expression and/or Cre-mediated recombination of Adora2a may be incomplete (see Results).
Forebrain-specific A2AR knockout mice (fb-A2AR KO, Camk2a-cre(+)-Adora2a flox/flox, congenic C57BL/6 genetic background) were generated and genotyped as previously detailed (Bastia et al. 2005). Briefly, Camk2a-cre(+) mice (L7ag#13 line, C57BL/6 genetic background) (Dragatsis and Zeitlin 2000) and Adora2aflox/flox mice (mixed Sv129 × C57BL/6 genetic background) were independently backcrossed to C57BL/6 mice for 10 generations at the Laboratory of Dr. Michael Schwarzschild (Massachusetts General Hospital) and then interbred to generate congenic fb-A2AR KO mice and their WT littermates.
Striatum-specific A2AR knockout mice (st-A2AR KO, Dlx5/6-cre(+)-Adora2aflox/flox, of a mixed FVB × C57BL/6 genetic background) were generated and genotyped as previously described (Shen et al. 2008a). Briefly, Dlx5/6-cre(+) transgenic mice in a FVB genetic background were provided (Ohtsuka et al. 2008) and cross-bred to Adora2aflox/flox mice in a congenic C57BL/6 genetic background. A Dlx5/6 intron regulatory element drove the embryonic, striatal neuron-specific Cre-mediated deletion of the “floxed” allele (Zerucha et al. 2000; Ghanem et al. 2003; Ohtsuka et al. 2008).
In earlier pilot studies, both Adora2aflox/flox mice (i.e., without the cre transgene) and Adora2a–/– mice (i.e., with Camk2a-cre or Dlx5/6-cre transgene, but without floxed allele) responded similarly to amphetamine. Thus, for this study, we used only the Adora2aflox/flox mice for our “wild-type” littermate control groups (fb-WT or st-WT for the fb-A2AR KO or st-A2AR KO lines, respectively).
Generation of Camk2a-cre(+)Rosa26flox/flox and Dlx5/6-cre(+)Rosa26flox/flox reporter mice and X-gal staining of Cre expression in brain
Rosa26 (R26R) reporter mice (B6.129S4.Gt(ROSA)26Sortm1Sor/J, Jackson Laboratories, Bar Harbor, ME) were crossed with Camk2a-cre(+) (fb-A2AR KO line) or Dlx5/6-cre(+) (st-A2AR KO line) transgenic mice to generate Camk2a-cre(+)Rosa26flox/flox or Dlx5/6-cre(+)Rosa26flox/flox mice for visualization of Cre expression in the brain by X-gal staining.
Briefly, naive mice were anesthetized with tribromoethanol (Avertin), transcardially perfused with ice-cold phosphate buffered saline (PBS) followed by 2% paraformaldehyde. The brains were removed in toto, post-fixed overnight, and then cryoprotected in a sucrose solution (10%–20%–30%) until processing. Parasagittal sections of 50 µm were incubated in X-gal solution (5 mM K4Fe(CN)6 · 3H2O, 5 mM K3Fe(CN)6, 2 mM MgCl2, 1 mg/mL X-gal (5-bromo-4-chloro-3-indoyl-β-D-galactoside, Invitrogen) in PBS and 2.5% dimethyl formamide) for 30 min at 37°C.
PCR analysis of Cre-mediated recombination and A2AR deletion in various brain regions during postnatal development
PCR was conducted on striatal, cortical, and cerebellar tissue isolated from fb-A2AR KO and fb-WT mice at postnatal days 15, 23, and 35 and on striatal, cortical, and hippocampal tissue isolated from st-A2AR KO and st-WT mice at postnatal days 5 and 33. Whole tissue samples were isolated from each hemisphere, and PCR on extracted genomic DNA was conducted according to the procedure described in full previously (Bastia et al. 2005).
Total membrane binding assessment of adenosine and dopamine receptors in various brain regions
Total membranes were prepared from the striatum, the cortex, the hippocampus, and/or the olfactory bulb, and single-point saturation binding assays were performed in duplicate to quantify total A2AR, A1R, D1R, and D2R levels as described earlier (Dewar et al. 1989; Lidow et al. 1989; Cunha et al. 1996; Lopes et al. 2004; Houchi et al. 2005; Rebola et al. 2005) with slight modifications. Each 300-μL binding assay, consisting of assay buffer (50 µL), radioligand (50 µL), and membranes (200 µL, 100–200 µg), was incubated at room temperature (or 30°C for D1R) for 1 h (or 2 h for A1R). The radioligands and final concentrations were: (1) A2AR: 3H-ZM241385 (3 nM, s.a. 27.4 Ci/mmol, American Radiolabled Chemicals, Inc.); (2) A1R: 3H-DPCPX (2 nM, s.a. 111.6 Ci/mmol, GE Healthcare); (3) D1R: 3H-SCH23390 (3 nM, s.a. 73.1 Ci/mmol, PerkinElmer); and (4) D2R: 3H-raclopride (5 nM, s.a. 82.8 Ci/mmol, PerkinElmer). Nonspecific binding was determined using 2 µM xanthine amine congener (A2AR and A1R), 5 µM fluphenazine dihydrochloride (D1R), or 300 µM (S)-(−)-sulpiride (D2R) (Sigma).
Behavioral evaluation
Experiment set I (fb-A2AR KO and st-A2AR KO mice)
Two cohorts of mice, referred to hereon as the “forebrain” and “striatal cohorts,” were used exclusively for behavioral evaluations. The forebrain cohort comprised 17 fb-A2AR KO mice (nine male and eight female) and 13 fb-WT mice (six male and seven female), and the striatal cohort comprised 17 st-A2AR KO mice (nine male and eight female) and 17 st-WT mice (seven male and 10 female). They were bred at Boston University School of Medicine (Boston, MA, USA) and transported to ETH-Zurich (Schwerzenbach, Switzerland) 1 mo before experimentation began, when they were 3.5–4.5 mo old. All mice were individually housed with ad libitum food and water in a temperature- and humidity-controlled vivarium maintained under a 12-h reversed light–dark cycle (lights on at 7 pm). Behavioral testing was always conducted in the dark phase.
A total of 11 tests were conducted and their chronologic sequence is listed in Supplemental Table 1, Experiment set I. This within-subjects approach was essential to assess potential confounding neurologic changes and other nonspecific effects. It also facilitated comparisons across tests and avoided excessive animal use. To minimize transfer effects that might complicate data interpretation, the tests were ordered in terms of the severity of experimental stress that animals might incur. The present report includes results derived from the (1) Y-maze test of spatial recognition and (2) water-maze tests of visual discrimination, working memory, reference memory, and reversal learning. Results of the remaining tests (i.e., elevated plus maze test of anxiety, open field and home cage activity, prepulse inhibition test of sensorimotor gating, and Pavlovian fear conditioning) are to be reported elsewhere (CJ Wei, P Singer, D Boison, J Feldon, BK Yee, and JF Chen, in prep.).
Experiment set II (st-A2A R KO mice)
A separate, independent striatal cohort comprising nine st-A2AR KO mice (five male and four female) and eight st-WT mice (five male and three female) was subsequently tested in a follow-up experiment to further examine working memory performance in the water maze. These mice had previously undergone active avoidance testing followed by conditioned taste aversion testing (P Singer, CJ Wei, JF Chen, and BK Yee, unpubl.). The testing sequence of the five total behavioral tests along with the subject number are detailed in Supplemental Table 1, Experiment set II.
Spatial recognition
This was assessed by measuring the spontaneous preference for novel over familiar places using a Y-maze as fully described before (Pietropaolo et al. 2009). Briefly, mice were placed in the maze in a randomly selected start arm and allowed to explore one other arm (familiar arm). After 5 min, mice were removed from the maze for a variable duration (2 min, 30 min, 3.5 h, or 1 d) and then re-introduced to the maze for an additional 3 min during which they could explore all three maze arms. Preferential exploration of the never-visited novel arm during this test phase was indexed by the following ratio: (time spent in novel arm/time spent in all three arms) × 100%. A novel testing room with unique distal spatial cues was used for each delay condition, and a minimum of 2 d separated successive tests. Video tracking was performed by Ethovision (version 3.1, Noldus Technology).
Water maze
Experiment set I (fb-A2AR KO and st-A2AR KO mice)
The apparatus has been fully described before (Yee et al. 2007; Singer et al. 2009). Three tests were conducted: visible cue task, working memory task, and acquisition and reversal of reference memory. Each test was conducted in a distinct testing room with a unique set of distal spatial cues. In all tests, mice were given two trials per day to learn to escape from the water within 60 sec by climbing onto an escape platform (7-cm diameter) submerged 1 cm below the water surface, on which they remained for a 20-sec intertrial interval (ITI) before initiating the second trial. Platform placements were counterbalanced among subjects. A pseudorandomized, nonrepetitive, sequence of start positions was used. Video tracking was performed by Ethovision (version 3.1, Noldus Technology).
Visible cue task
This served to detect any confounding change in swimming or escape behavior. For two consecutive days, the location of the platform was made visible by a local cue placed directly above the escape platform and was varied between the two days.
Working memory task
Next, the escape platform stayed hidden and assumed a new position on each day, but remained unchanged across the two trials on the same day. Improvement from trial 1 to 2 provided a measure of working memory—the retrieval of the day-specific location of the escape platform learned from trial 1 (Hodges et al. 1995). St-A2AR KO and st-WT mice were evaluated over a block of 4 d, with a minimal ITI (i.e., 20 sec), which proved to be sufficiently difficult for st-WT mice. On the other hand, fb-A2AR KO and fb-WT mice were tested for additional two 4-d blocks to accommodate more extended ITIs: 10 min for the second block and 15 min for the final block. These additional training blocks allowed us to achieve a level of difficulty that also reduced the performance of fb-WT mice to chance level. The daily platform locations and start positions within a block were counterbalanced with a pseudorandom sequence.
Acquisition and reversal of reference memory
This commenced 6 or 14 d after the working memory test for the forebrain or striatal cohort, respectively. The platform now assumed a constant location throughout the acquisition phase of the experiment, which lasted for 10 consecutive days. The platform position was counterbalanced across groups. A 60-sec probe test (with the escape platform removed) was performed on days 11 and 13. Next, reversal learning began and lasted for 4 or 8 d in the respective forebrain or striatal cohorts. In this phase, the escape platform assumed a new constant location that was diagonally opposite to its former location. An initial probe test, performed on day 5, revealed successful reversal learning in fb-A2AR KO and fb-WT mice, which led to termination of the test. On the other hand, it was apparent that st-A2AR KO and st-WT mice required further training. To monitor the progress of reversal learning across the additional training days, two more probe tests were performed: one at the beginning of day 7 and another at the beginning of day 9. Testing was then terminated in this cohort at the end of the final probe test on day 9. Performance was indexed by escape latency and path length. Probe test performance was evaluated by the proportion of time spent in the target quadrant, where the platform was previously located, relative to the other three quadrants.
Experiment set II (st-A2A R KO mice)
As the performance enhancement in Experiment set I was observed against a background of weak st-WT control performance, additional visible and working memory tasks in Experiment set II were performed on a separate cohort of st-A2AR KO and st-WT mice to solidify the original interpretation of enhanced working memory in st-A2AR KO mice based on Experiment set I and to exclude the alternative interpretation that st-WT mice had failed to learn the matching rule. A four-trial-per-day training protocol was used to improve st-WT performance with the modifications described in the following.
Working memory task
Training: Mice were first trained for 4 d in the working memory task using four trials per day to facilitate learning and ensure that all mice could learn the procedures and the matching rule inherent to the working memory task.
Testing: Working memory testing proceeded for the next 8 d, as in Experiment set I, once effective working memory functioning (trial 1-to-2 improvement) during training was observed. Two delays (ITIs) of varying retention loads, 20 sec or 10 min, separated the two daily trials. Mice were tested for 4 d at each delay: 2 d at 20 sec, then 4 d at 10 min, and then 2 d more at 20 sec. This sequence ensured that comparison across the two delay conditions was not systemically confounded by overall training effects across the 8 d of testing.
Statistical analysis
Data from the two A2AR KO mouse lines (i.e., fb-A2AR KO or st-A2AR KO) and from the two experiments (i.e., Experimental sets I and II in the st-A2AR KO mouse line) were separately analyzed by parametric analysis of variance (ANOVA) of the appropriate design. Thus, all analyses involved comparisons between subjects that shared identical experimental histories. Between-subject factors included genotype (i.e., A2AR KO vs. WT) and sex (i.e., male vs. female) if present. Within-subjects factors such as trials, days, delays, and arms were included as required by the experimental design. Post-hoc pair-wise comparisons or restricted ANOVAs were performed to facilitate interpretation of higher-order interaction effects whenever appropriate. One-sample Students t-tests were used to gauge performance against chance level. All analyses were conducted using SPSS (Version 16) with a Type I error rate of 0.05.
Acknowledgments
This work is supported by NIH grants R01MH083973, RO1NS48995, RO1NS41083-07, and RO1DA19362; Department of Defense grant W81XWH-071-1-0012; a grant from the Bumpus Foundation; and ETH Zurich.
Footnotes
[Supplemental material is available for this article.]
References
- Aggleton JP, Vann SD, Oswald CJ, Good M 2000. Identifying cortical inputs to the rat hippocampus that subserve allocentric spatial processes: A simple problem with a complex answer. Hippocampus 10: 466–474 [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Deacon RM, Seeburg PH, Rawlins JN 2003. GluR-A-deficient mice display normal acquisition of a hippocampus-dependent spatial reference memory task but are impaired during spatial reversal. Behav Neurosci 117: 866–870 [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Niewoehner B, Lyon L, Romberg C, Schmitt WB, Taylor A, Sanderson DJ, Cottam J, Sprengel R, Seeburg PH, et al. 2008. NMDA receptor subunit NR2A is required for rapidly acquired spatial working memory but not incremental spatial reference memory. J Neurosci 28: 3623–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastia E, Xu YH, Scibelli AC, Day YJ, Linden J, Chen JF, Schwarzschild MA 2005. A crucial role for forebrain adenosine A(2A) receptors in amphetamine sensitization. Neuropsychopharmacology 30: 891–900 [DOI] [PubMed] [Google Scholar]
- Batista-Brito R, Machold R, Klein C, Fishell G 2008. Gene expression in cortical interneuron precursors is prescient of their mature function. Cereb Cortex 18: 2306–2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellebaum C, Koch B, Schwarz M, Daum I 2008. Focal basal ganglia lesions are associated with impairments in reward-based reversal learning. Brain 131 (Pt 3): 829–841 [DOI] [PubMed] [Google Scholar]
- Berke JD, Breck JT, Eichenbaum H 2009. Striatal versus hippocampal representations during win-stay maze performance. J Neurophysiol 101: 1575–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL 1993. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 361: 31–39 [DOI] [PubMed] [Google Scholar]
- Boison D 2007. Adenosine as a modulator of brain activity. Drug News Perspect 20: 607–611 [DOI] [PubMed] [Google Scholar]
- Canals M, Marcellino D, Fanelli F, Ciruela F, de Benedetti P, Goldberg SR, Neve K, Fuxe K, Agnati LF, Woods AS, et al. 2003. Adenosine A2A-dopamine D2 receptor-receptor heteromerization: Qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem 278: 46741–46749 [DOI] [PubMed] [Google Scholar]
- Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, et al. 2006. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci 26: 2080–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Robbins TW, Roberts AC 2008. Lesions of the medial striatum in monkeys produce perseverative impairments during reversal learning similar to those produced by lesions of the orbitofrontal cortex. J Neurosci 28: 10972–10982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatworthy PL, Lewis SJ, Brichard L, Hong YT, Izquierdo D, Clark L, Cools R, Aigbirhio FI, Baron JC, Fryer TD, et al. 2009. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J Neurosci 29: 4690–4696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA, Johansson B, van der Ploeg I, Sebastiao AM, Ribeiro JA, Fredholm BB 1994. Evidence for functionally important adenosine A2a receptors in the rat hippocampus. Brain Res 649: 208–216 [DOI] [PubMed] [Google Scholar]
- Cunha RA, Johansson B, Constantino MD, Sebastiao AM, Fredholm BB 1996. Evidence for high-affinity binding sites for the adenosine A2A receptor agonist [3H] CGS 21680 in the rat hippocampus and cerebral cortex that are different from striatal A2A receptors. Naunyn Schmiedebergs Arch Pharmacol 353: 261–271 [DOI] [PubMed] [Google Scholar]
- d'Alcantara P, Ledent C, Swillens S, Schiffmann SN 2001. Inactivation of adenosine A2A receptor impairs long term potentiation in the accumbens nucleus without altering basal synaptic transmission. Neuroscience 107: 455–464 [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW 2004. Prefrontal executive and cognitive functions in rodents: Neural and neurochemical substrates. Neurosci Biobehav Rev 28: 771–784 [DOI] [PubMed] [Google Scholar]
- Dall'Igna OP, Fett P, Gomes MW, Souza DO, Cunha RA, Lara DR 2007. Caffeine and adenosine A2a receptor antagonists prevent beta-amyloid (25–35)-induced cognitive deficits in mice. Exp Neurol 203: 241–245 [DOI] [PubMed] [Google Scholar]
- de Mendonca A, Ribeiro JA 1994. Endogenous adenosine modulates long-term potentiation in the hippocampus. Neuroscience 62: 385–390 [DOI] [PubMed] [Google Scholar]
- de Mendonca A, Ribeiro JA 1997. Adenosine and neuronal plasticity. Life Sci 60: 245–251 [DOI] [PubMed] [Google Scholar]
- De Steno DA, Schmauss C 2009. A role for dopamine D2 receptors in reversal learning. Neuroscience 162: 118–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar KM, Montreuil B, Grondin L, Reader TA 1989. Dopamine D2 receptors labeled with [3H]raclopride in rat and rabbit brains. Equilibrium binding, kinetics, distribution and selectivity. J Pharmacol Exp Ther 250: 696–706 [PubMed] [Google Scholar]
- Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC 1996. Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol 118: 1461–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragatsis I, Zeitlin S 2000. CaMKIIalpha-Cre transgene expression and recombination patterns in the mouse brain. Genesis 26: 133–135 [DOI] [PubMed] [Google Scholar]
- El-Ghundi M, O'Dowd BF, George SR 2007. Insights into the role of dopamine receptor systems in learning and memory. Rev Neurosci 18: 37–66 [DOI] [PubMed] [Google Scholar]
- Ferre S, Fredholm BB, Morelli M, Popoli P, Fuxe K 1997. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci 20: 482–487 [DOI] [PubMed] [Google Scholar]
- Ferre S, Karcz-Kubicha M, Hope BT, Popoli P, Burgueno J, Gutierrez MA, Casado V, Fuxe K, Goldberg SR, Lluis C, et al. 2002. Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: Implications for striatal neuronal function. Proc Natl Acad Sci 99: 11940–11945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Diamond I, Goldberg SR, Yao L, Hourani SM, Huang ZL, Urade Y, Kitchen I 2007. Adenosine A2A receptors in ventral striatum, hypothalamus and nociceptive circuitry implications for drug addiction, sleep and pain. Prog Neurobiol 83: 332–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajolet M, Wang Z, Futter M, Shen W, Nuangchamnong N, Bendor J, Wallach I, Nairn AC, Surmeier DJ, Greengard P 2008. FGF acts as a co-transmitter through adenosine A2A receptor to regulate synaptic plasticity. Nat Neurosci 11: 1402–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontinha BM, Delgado-Garcia JM, Madronal N, Ribeiro JA, Sebastiao AM, Gruart A 2009. Adenosine A2A receptor modulation of hippocampal CA3-CA1 synapse plasticity during associative learning in behaving mice. Neuropsychopharmacology 34: 1865–1874 [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM 2005. Adenosine and brain function. Int Rev Neurobiol 63: 191–270 [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chern Y, Franco R, Sitkovsky M 2007. Aspects of the general biology of adenosine A2A signaling. Prog Neurobiol 83: 263–276 [DOI] [PubMed] [Google Scholar]
- Gerevich Z, Wirkner K, Illes P 2002. Adenosine A2A receptors inhibit the N-methyl-D-aspartate component of excitatory synaptic currents in rat striatal neurons. Eur J Pharmacol 451: 161–164 [DOI] [PubMed] [Google Scholar]
- Ghanem N, Jarinova O, Amores A, Long Q, Hatch G, Park BK, Rubenstein JL, Ekker M 2003. Regulatory roles of conserved intergenic domains in vertebrate Dlx bigene clusters. Genome Res 13: 533–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Llort L, Schiffmann SN, Shmidt T, Canela L, Camon L, Wassholm M, Canals M, Terasmaa A, Fernandez-Teruel A, Tobena A, et al. 2007. Working memory deficits in transgenic rats overexpressing human adenosine A2A receptors in the brain. Neurobiol Learn Mem 87: 42–56 [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS 1995. Cellular basis of working memory. Neuron 14: 477–485 [DOI] [PubMed] [Google Scholar]
- Hill MN, Froese LM, Morrish AC, Sun JC, Floresco SB 2006. Alterations in behavioral flexibility by cannabinoid CB1 receptor agonists and antagonists. Psychopharmacology (Berl) 187: 245–259 [DOI] [PubMed] [Google Scholar]
- Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, Hansson A, Watson S, Olah ME, Mallol J, et al. 2002. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem 277: 18091–18097 [DOI] [PubMed] [Google Scholar]
- Hodges H, Sowinski P, Sinden JD, Netto CA, Fletcher A 1995. The selective 5-HT3 receptor antagonist, WAY100289, enhances spatial memory in rats with ibotenate lesions of the forebrain cholinergic projection system. Psychopharmacology (Berl) 117: 318–332 [DOI] [PubMed] [Google Scholar]
- Houchi H, Babovic D, Pierrefiche O, Ledent C, Daoust M, Naassila M 2005. CB1 receptor knockout mice display reduced ethanol-induced conditioned place preference and increased striatal dopamine D2 receptors. Neuropsychopharmacology 30: 339–349 [DOI] [PubMed] [Google Scholar]
- Kachroo A, Orlando LR, Grandy DK, Chen JF, Young AB, Schwarzschild MA 2005. Interactions between metabotropic glutamate 5 and adenosine A2A receptors in normal and parkinsonian mice. J Neurosci 25: 10414–10419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER 2006. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron 49: 603–615 [DOI] [PubMed] [Google Scholar]
- Kesner RP 2009. The posterior parietal cortex and long-term memory representation of spatial information. Neurobiol Learn Mem 91: 197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC 2005. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci 25: 10537–10545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC 2007. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature 445: 643–647 [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, Mitchell SH, Younkin A, Grandy DK 2006. Dopamine D2 receptors mediate reversal learning in male C57BL/6J mice. Cogn Affect Behav Neurosci 6: 86–90 [DOI] [PubMed] [Google Scholar]
- Lee B, Groman S, London ED, Jentsch JD 2007. Dopamine D2/D3 receptors play a specific role in the reversal of a learned visual discrimination in monkeys. Neuropsychopharmacology 32: 2125–2134 [DOI] [PubMed] [Google Scholar]
- Lerner TN, Horne EA, Stella N, Kreitzer AC 2010. Endocannabinoid signaling mediates psychomotor activation by adenosine A2A antagonists. J Neurosci 30: 2160–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Rakic P, Innis RB 1989. Dopamine D2 receptors in the cerebral cortex: Distribution and pharmacological characterization with [3H]raclopride. Proc Natl Acad Sci 86: 6412–6416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes LV, Halldner L, Rebola N, Johansson B, Ledent C, Chen JF, Fredholm BB, Cunha RA 2004. Binding of the prototypical adenosine A2A receptor agonist CGS 21680 to the cerebral cortex of adenosine A1 and A2A receptor knockout mice. Br J Pharmacol 141: 1006–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM 2010. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology 58: 951–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA 2004. Long-term potentiation and memory. Physiol Rev 84: 87–136 [DOI] [PubMed] [Google Scholar]
- Marie RM, Defer GL 2003. Working memory and dopamine: Clinical and experimental clues. Curr Opin Neurol 16 (Suppl 2): S29–S35 [DOI] [PubMed] [Google Scholar]
- McDonald RJ, King AL, Foong N, Rizos Z, Hong NS 2008. Neurotoxic lesions of the medial prefrontal cortex or medial striatum impair multiple-location place learning in the water task: Evidence for neural structures with complementary roles in behavioural flexibility. Exp Brain Res 187: 419–427 [DOI] [PubMed] [Google Scholar]
- Mehta MA, Montgomery AJ, Kitamura Y, Grasby PM 2008. Dopamine D2 receptor occupancy levels of acute sulpiride challenges that produce working memory and learning impairments in healthy volunteers. Psychopharmacology (Berl) 196: 157–165 [DOI] [PubMed] [Google Scholar]
- Naassila M, Ledent C, Daoust M 2002. Low ethanol sensitivity and increased ethanol consumption in mice lacking adenosine A2A receptors. J Neurosci 22: 10487–10493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenberg W, Wirkner K, Assmann H, Richter M, Illes P 1998. Adenosine A2A receptors inhibit the conductance of NMDA receptor channels in rat neostriatal neurons. Amino Acids 14: 33–39 [DOI] [PubMed] [Google Scholar]
- Ohtsuka N, Tansky MF, Kuang H, Kourrich S, Thomas MJ, Rubenstein JL, Ekker M, Leeman SE, Tsien JZ 2008. Functional disturbances in the striatum by region-specific ablation of NMDA receptors. Proc Natl Acad Sci 105: 12961–12966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ 2002. Learning and memory functions of the basal ganglia. Annu Rev Neurosci 25: 563–593 [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL 1992. Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: Further evidence for multiple memory systems. Behav Neurosci 106: 439–446 [DOI] [PubMed] [Google Scholar]
- Pietropaolo S, Sun Y, Li R, Brana C, Feldon J, Yee BK 2009. Limited impact of social isolation on Alzheimer-like symptoms in a triple transgenic mouse model. Behav Neurosci 123: 181–195 [DOI] [PubMed] [Google Scholar]
- Prediger RD, Batista LC, Takahashi RN 2005a. Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats. Involvement of adenosine A1 and A2A receptors. Neurobiol Aging 26: 957–964 [DOI] [PubMed] [Google Scholar]
- Prediger RD, Da Cunha C, Takahashi RN 2005b. Antagonistic interaction between adenosine A2A and dopamine D2 receptors modulates the social recognition memory in reserpine-treated rats. Behav Pharmacol 16: 209–218 [DOI] [PubMed] [Google Scholar]
- Prediger RD, Fernandes D, Takahashi RN 2005c. Blockade of adenosine A2A receptors reverses short-term social memory impairments in spontaneously hypertensive rats. Behav Brain Res 159: 197–205 [DOI] [PubMed] [Google Scholar]
- Rebola N, Canas PM, Oliveira CR, Cunha RA 2005. Different synaptic and subsynaptic localization of adenosine A2A receptors in the hippocampus and striatum of the rat. Neuroscience 132: 893–903 [DOI] [PubMed] [Google Scholar]
- Rebola N, Lujan R, Cunha RA, Mulle C 2008. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron 57: 121–134 [DOI] [PubMed] [Google Scholar]
- Ribeiro AC, Pfaff DW, Devidze N 2009. Estradiol modulates behavioral arousal and induces changes in gene expression profiles in brain regions involved in the control of vigilance. Eur J Neurosci 29: 795–801 [DOI] [PubMed] [Google Scholar]
- Rodrigues RJ, Alfaro TM, Rebola N, Oliveira CR, Cunha RA 2005. Co-localization and functional interaction between adenosine A2A and metabotropic group 5 receptors in glutamatergic nerve terminals of the rat striatum. J Neurochem 92: 433–441 [DOI] [PubMed] [Google Scholar]
- Rosin DL, Robeva A, Woodard RL, Guyenet PG, Linden J 1998. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J Comp Neurol 40: 163–186 [PubMed] [Google Scholar]
- Rosin DL, Hettinger BD, Lee A, Linden J 2003. Anatomy of adenosine A2A receptors in brain: Morphological substrates for integration of striatal function. Neurology 61 (11 Suppl 6): S12–S18 [DOI] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE 2000. The prefrontal cortex: Response selection or maintenance within working memory? Science 288: 1656–1660 [DOI] [PubMed] [Google Scholar]
- Save E, Poucet B 2009. Role of the parietal cortex in long-term representation of spatial information in the rat. Neurobiol Learn Mem 91: 172–178 [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Jacobs O, Vanderhaeghen JJ 1991a. Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: An in situ hybridization histochemistry study. J Neurochem 57: 1062–1067 [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Libert F, Vassart G, Vanderhaeghen JJ 1991b. Distribution of adenosine A2 receptor mRNA in the human brain. Neurosci Lett 130: 177–181 [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferre S 2007. Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol 83: 277–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiao AM, Ribeiro JA 1996. Adenosine A2 receptor-mediated excitatory actions on the nervous system. Prog Neurobiol 48: 167–189 [DOI] [PubMed] [Google Scholar]
- Shen HY, Coelho JE, Ohtsuka N, Canas PM, Day YJ, Huang QY, Rebola N, Yu L, Boison D, Cunha RA, et al. 2008a. A critical role of the adenosine A2A receptor in extrastriatal neurons in modulating psychomotor activity as revealed by opposite phenotypes of striatum and forebrain A2A receptor knock-outs. J Neurosci 28: 2970–2975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ 2008b. Dichotomous dopaminergic control of striatal synaptic plasticity. Science 321: 848–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EH, Kellendonk C, Kandel E 2010. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron 65: 585–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer P, Boison D, Mohler H, Feldon J, Yee BK 2009. Deletion of glycine transporter 1 (GlyT1) in forebrain neurons facilitates reversal learning: Enhanced cognitive adaptability? Behav Neurosci 123: 1012–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebano MT, Martire A, Rebola N, Pepponi R, Domenici MR, Gro MC, Schwarzschild MA, Chen JF, Cunha RA, Popoli P 2005. Adenosine A2A receptors and metabotropic glutamate 5 receptors are co-localized and functionally interact in the hippocampus: A possible key mechanism in the modulation of N-methyl-D-aspartate effects. J Neurochem 95: 1188–1200 [DOI] [PubMed] [Google Scholar]
- Tebano MT, Martire A, Chiodi V, Pepponi R, Ferrante A, Domenici MR, Frank C, Chen JF, Ledent C, Popoli P 2009. Adenosine A2A receptors enable the synaptic effects of cannabinoid CB1 receptors in the rodent striatum. J Neurochem 110: 1921–1930 [DOI] [PubMed] [Google Scholar]
- Varvel SA, Lichtman AH 2002. Evaluation of CB1 receptor knockout mice in the Morris water maze. J Pharmacol Exp Ther 301: 915–924 [DOI] [PubMed] [Google Scholar]
- Wang JH, Ma YY, van den Buuse M 2006. Improved spatial recognition memory in mice lacking adenosine A2A receptors. Exp Neurol 199: 438–445 [DOI] [PubMed] [Google Scholar]
- Wei CJ, Li W, Chen JF 2011. Normal and abnormal functions of adenosine receptors in the central nervous system revealed by genetic knockout studies. Biochim Biophys Acta 1808: 1358–1379 [DOI] [PubMed] [Google Scholar]
- Wirkner K, Assmann H, Koles L, Gerevich Z, Franke H, Norenberg W, Boehm R, Illes P 2000. Inhibition by adenosine A2A receptors of NMDA but not AMPA currents in rat neostriatal neurons. Br J Pharmacol 130: 259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirkner K, Gerevich Z, Krause T, Gunther A, Koles L, Schneider D, Norenberg W, Illes P 2004. Adenosine A2A receptor-induced inhibition of NMDA and GABAA receptor-mediated synaptic currents in a subpopulation of rat striatal neurons. Neuropharmacology 46: 994–1007 [DOI] [PubMed] [Google Scholar]
- Xu J, Zhu Y, Contractor A, Heinemann SF 2009. mGluR5 has a critical role in inhibitory learning. J Neurosci 29: 3676–3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JN, Chen JF, Fredholm BB 2009. Physiological roles of A1 and A2A adenosine receptors in regulating heart rate, body temperature, and locomotion as revealed using knockout mice and caffeine. Am J Physiol Heart Circ Physiol 296: H1141–H1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee BK, Singer P, Chen JF, Feldon J, Boison D 2007. Transgenic overexpression of adenosine kinase in brain leads to multiple learning impairments and altered sensitivity to psychomimetic drugs. Eur J Neurosci 26: 3237–3252 [DOI] [PubMed] [Google Scholar]
- Yu L, Shen HY, Coelho JE, Araujo IM, Huang QY, Day YJ, Rebola N, Canas PM, Rapp EK, Ferrara J, et al. 2008. Adenosine A2A receptor antagonists exert motor and neuroprotective effects by distinct cellular mechanisms. Ann Neurol 63: 338–346 [DOI] [PubMed] [Google Scholar]
- Yu C, Gupta J, Chen JF, Yin HH 2009. Genetic deletion of A2A adenosine receptors in the striatum selectively impairs habit formation. J Neurosci 29: 15100–15103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandbelt BB, Vink M 2010. On the role of the striatum in response inhibition. PLoS One 5: e13848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerucha T, Stuhmer T, Hatch G, Park BK, Long Q, Yu G, Gambarotta A, Schultz JR, Rubenstein JL, Ekker M 2000. A highly conserved enhancer in the Dlx5/Dlx6 intergenic region is the site of cross-regulatory interactions between Dlx genes in the embryonic forebrain. J Neurosci 20: 709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SJ, Zhu ME, Shu D, Du XP, Song XH, Wang XT, Zheng RY, Cai XH, Chen JF, He JC 2009. Preferential enhancement of working memory in mice lacking adenosine A2A receptors. Brain Res 1303: 74–83 [DOI] [PubMed] [Google Scholar]