Abstract
Seasonal breeding in the temperate zone is a dramatic example of a naturally occurring change in physiology and behaviour. Cues that predict periods of environmental amelioration favourable for breeding must be processed by the brain so that the appropriate responses in reproductive physiology can be implemented. The neural integration of several environmental cues converges on discrete hypothalamic neurons in order to regulate reproductive physiology. Gonadotrophin-releasing hormone-1 (GnRH1) and Kisspeptin (Kiss1) neurons in avian and mammalian species, respectively, show marked variation in expression that is positively associated with breeding state. We applied the constancy/contingency model of predictability to investigate how GnRH1 and Kiss1 integrate different environmental cues to regulate reproduction. We show that variation in GnRH1 from a highly seasonal avian species exhibits a predictive change that is primarily based on contingency information. Opportunistic species have low measures of predictability and exhibit a greater contribution of constancy information that is sex-dependent. In hamsters, Kiss1 exhibited a predictive change in expression that was predominantly contingency information and is anatomically localized. The model applied here provides a framework for studies geared towards determining the impact of variation in climate patterns to reproductive success in vertebrate species.
Keywords: photoperiod, gonadotrophin-releasing hormone-1, luteinizing hormone releasing hormone, Kisspeptin, starling, hamster
1. Introduction
Seasonal reproduction is a very successful strategy employed by individuals in many vertebrate species to maximize fitness in highly variable environments [1,2]. There are several seasonal and/or environmental cues that animals can rely on to drive changes in their reproductive state [3–5]. The development of a framework to categorize environmental stimuli into either ultimate and/or proximate factors greatly facilitated studies designed to study seasonal reproduction [6]. Ultimate factors are cues and events that impact on the annual reproductive cycle in a way that result ultimately in variation in reproductive fitness. One consequence of these ultimate factors (e.g. food availability, the timing of predator arrival, etc.) is that they shape the optimal period as to when young should be produced. Proximate factors are environmental cues that individuals actually process and respond to in order to regulate reproductive processes from the onset to the termination of breeding [6].
The annual change in day length is a highly predictable seasonal pattern and provides a reliable signal to time reproduction [7,8]. In less-predictable habitats like the tropics, local environmental cues are more variable and many animals generally adopt an opportunistic strategy and breed when conditions are optimal (a time which is not necessarily consistent from year to year; [9,10]). Moreover, under certain ecological circumstances, animals inhabiting a higher latitude and/or altitude exhibit breeding periods that are tied to the irregular availability of nutrient resources and as a result also engage in an opportunistic strategy [11,12]. Given these different patterns of reproduction, it is clear that animals must rely on the temporal integration of a number of different environmental cues to fine-tune the timing of reproduction [13,14].
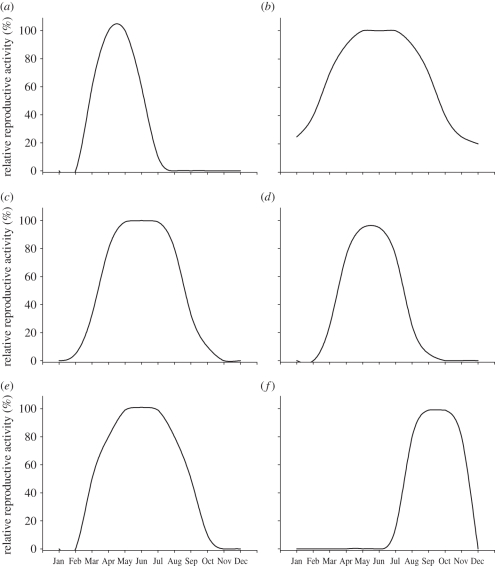
In general, temperate zone species have evolved neuroendocrine responses that respond to either the vernal increase in daylength; the autumnal decrease in daylength, or both in order to time reproduction (figure 1; [7,8]). In vertebrate species, the neuroendocrine system is primarily governed by discrete hypothalamic peptides that are critical for regulating reproduction [15]. Indeed, the neural circuitry underlying seasonal reproduction is considerably different between avian and mammalian species [16,17]. However, gonadotrophin-releasing hormone 1 (GnRH1) is a highly conserved peptide that connects the brain with peripheral endocrine responses by regulating the release of gonadotrophins; luteinizing hormone and follicular stimulating hormone from the pituitary gland [15,18,19]. Across the vast majority of avian and mammalian species, environmental cues are integrated at multiple levels ultimately converging on and regulating GnRH1 neurons and/or terminals [16]. In mammals, GnRH function is regulated primarily via release [20] while it is at the level of GnRH1 gene transcription and release in avian species [21,22]. A novel neuropeptide, Kisspeptin (Kiss1), was identified in mammalian species and subsequently shown to stimulate GnRH1 release [23,24]. To date, Kiss1 has not been identified in avian species [25]. It is well established that there are profound changes in the number of GnRH1 cells in avian species (reviewed in [21]) and Kiss1 in mammalian species [26,27] across the breeding state and is dependent on photoperiodic experience. Even though GnRH1 and Kiss1 have different anatomical locations and neural connectivity, the marked annual variation in both GnRH1 and Kiss1 expression serves the same functional outcome; to time reproduction with environmental conditions favourable for the survival of the developing offspring.
Figure 1.
Representative graphs depicting the seasonal change in breeding patterns. Most temperate zone species time reproduction to occur during the spring and summer periods and the annual change in day length provides a predictive cue for future conditions. (a) European starling, (b) white-winged crossbill, (c) pine siskin, (d) common redpoll, (e) syrian hamster, (f) ewe. Data were adapted from [43,66–70].
It was previously shown that a reliable environmental cue can be mathematically predictable and subsequently used for timing a future event (i.e. reproduction; [28]). Information theory is a branch of probability and statistics and has been applied to univariate or multivariate, discrete or continuous, as well as qualitative or quantitative measures [29]. One application of information theory has been to understand the periodic cycles in physical and biological phenomena using the concept of predictability [28,30]. There are two components that contribute to the overall predictability of an environment: constancy (C, the environment is predictable because it is always the same) and contingency (M, the environment is predictable in the degree of change from season to season). The constancy/contingency model was initially applied to demonstrate the predictability of seasonal flowering and fruiting in plants [30] and subsequently applied to avian gonadal cycles [31]. The vertebrate hypothalamus is vital for the neuroendocrine control of gonadal cycles and coordinating a series of essential seasonal changes in other physiological processes. The goal of the present paper was to apply the concepts of predictability, constancy and contingency developed by Colwell [30] to address how these measures contribute to variation in the function of two key neuropeptides: (i) GnRH1 in avian species, and (ii) Kiss1 in mammalian species. The aim of this paper is to demonstrate mathematically that environmental signals, primarily the change in photoperiod, are integrated by specific neuropeptide systems in birds and mammals to optimize the timing of reproduction.
2. Measurement of predictability, constancy and contingency
The methods described here can be applied to any phenomenon known to occur on a periodic or cyclical time scale (or space) which can be scored for at least two states. The data are presented in the form of a frequency matrix and the measurements are based on the Shannon information statistics with t columns (times within a cycle) and s rows (states of the phenomenon). Here, Nij is the number of cycles for which the phenomenon was in state i at time j. The row (Eq. 1), column (Eq. 2) and grand total (Eq. 3) for the frequency matrices are then easily calculated (electronic supplementary material, tables S1 and S2 for equations and examples; [28,30]). Next, given that predictability is the opposite of uncertainty, we can use the uncertainty with respect to time, space and the interaction to formulate values used to compute predictability (P; Eq. 7), constancy (C; Eq. 8) and contingency (M; Eq. 9). Electronic supplementary material, table S2 provides an example to demonstrate how the three values can be used to statistically describe different reproductive cycles in animal species. The predictability of a given cycle is maximal when there is complete certainty, this occurs when there is only one non-zero value in each column (electronic supplementary material, table S2; species a and b). When predictability is at its minimum, all states are equiprobable at all times (electronic supplementary material, table S2; species c). Constancy is maximized when all row totals but one are zero (electronic supplementary material, table S2; species a) and minimal when all row totals are equal (electronic supplementary material, table S2; species c). Contingency is maximized when the number of non-zero for each state and each row approaches one; while minimized when all states at different times are homogeneous with one another (electronic supplementary material, table S2; species b). According to these statistical definitions, the sum of constancy and contingency equals the predictability of the periodic phenomenon [30].
Statistical significance values can be computed for the predictability, constancy and contingency values using a G-statistic [32,33]. G approximates the χ2 distribution and is applicable as a test for goodness of fit with the same number of degrees of freedom as would be used for a χ2 test. For constancy, to test the hypothesis that the row totals are not equal (C = 0), we use (Eq. 10) with s−1 degrees of freedom and compared against a χ2 distribution. The prediction here is that the lower the variation across row totals, the higher is the probability for observing a significant contribution of constancy. Next, we compute the G-statistic for contingency using (Eq. 11) with (s − 1)(t − 1) degrees of freedom. This significance test determines that contingency is not significantly different from zero (M = 0), but that the deviation from homogeneity of the columns (time) is minimal. Here the prediction is that the greater variation within a column across the different time (t) points would lead to a significant contribution of contingency. The obtained p-value does not describe the significance of the value of constancy or contingency, but provides a value to show whether they contribute significantly to the overall predictability. To determine whether the predictability is significant, we can simply add the GC and GM [30]. This value is then compared with the χ2 distribution using t(s − 1) for the degrees of freedom.
Wingfield and colleagues [14,31] have devised an environmental information factor (Ie) that provides a method to determine the types of environmental information that an individual requires to successfully time reproduction. Ie is calculated by simply taking the ratio of the value for contingency/constancy (M/C). This provides an accurate indicator of the way in which organisms may integrate initial predictive (i.e. photoperiod) and/or supplementary cues (i.e. rainfall, temperature, etc. [31]). This theoretical model suggests that when Ie is very low (close to 0), little or no environmental information is necessary to time reproduction given that environmental conditions are always suitable for breeding. When Ie is low (<1) then constancy information predominates and contingency is less. In this environment, initial predictive information primarily regulates reproduction. When both constancy and contingency are equal then both initial predictive and supplementary information are equally integrated. Here, the initial predictive information generally prevails and animals are more probably seasonal breeders at higher latitudes. When Ie is high (>1), the contribution of contingency is greater than constancy and supplementary information would predominate, although initial predictive cues can still provide important information. As the breeding season begins, animals require more information from both initial predictive and supplementary information need to be integrated. Animals that have very high Ie values require a more opportunistic strategy in order to cope with unpredictable environments [14,31].
This theoretical approach provides a valuable means to examine how closely related populations and/or species respond to environmental cues. Indeed, the brain is the primary integrator of environmental information and regulator of reproduction. Herein, we applied these methods to determine the predictability of neuroendocrine function by creating frequency matrices for GnRH1 in avian species and Kiss1 in mammalian species. The results presented here reveal that specific variations in neuronal populations are intimately tied to seasonal breeding.
3. Seasonal reproduction in avian species
There are three components that underly the photoperiodic response in birds: (i) an encephalic photoreceptor linked to a circadian clock to measure day length; (ii) the GnRH1 system in the anterior/preoptic area (POA) of the hypothalamus; and (iii) the peripheral endocrine system [16,34]. For a number of avian species, the GnRH1 neuronal system exhibits a profound change in the expression of gnrh1 mRNA [22,35] and GnRH1 protein (see [16,21] for reviews) in response to changes in day length. In many temperate zone birds, the vernal increase in daylength stimulates the hypothalamo-pituitary-gonadal axis (HPG) leading to increase in GnRH1 neurons and/or release resulting in gonadal recrudescence; the breeding state is referred to as the photostimulated state [7]. Prolonged exposure to long day lengths results in a gradual involution in reproductive physiology rendering the birds in a non-breeding state termed photorefractory [36]. Only exposure to short day lengths reinstate the physiological responsiveness to the photoinducibility of long day lengths, and birds in this state are termed photosensitive [7].
The European starling (Sturnus vulgaris) has been a valuable model system for studying the photoperiodic effects on the neuroendocrine control of reproduction. Specifically, starlings exhibit a profound change in GnRH1 cell numbers in the POA and it provides a reliable marker of reproductive state (figure 1; [22]). Photosensitive starlings placed on long day lengths in the laboratory or the vernal increase in photoperiod leads to a dramatic increase in GnRH1 protein content [37] and mRNA expression [22,35]. Subsequent exposure to long day lengths and the onset of photorefractoriness is characterized by a marked decrease in GnRH1 [7,35,38]. Herein, we applied the models developed by Colwell [30] to address the contribution of contingency and/or constancy information to the overall predictability in the change in GnRH1 neuronal expression and corresponding changes in the gonadal cycle.
To conduct the analysis, a different matrix was required when compared with the example given previously. Let t (times in the cycle) = breeding cycle (different physiological points across the annual reproductive cycle), and let s (states) = either the variation in GnRH1 (the binned values that span the obtained range of GnRH1 values) or in the case of peripheral physiology gonadal state (binned values that span the obtained testis volume). Colwell [30] argued that a continuous variable can be determined using the methods described above. Six states were required to equally bin the values for both GnRH1 and gonadal volume so that the minimal number of states (i) within the range observed for each variable were obtained. Thus, six was the value used to represent s in all the equations outlined in electronic supplementary material, table S1. As the data presented here are real data collected from starlings, we can compute a G-statistic for predictability, constancy and contingency, and determine the significance levels to compare the GnRH1 optical density (OD) with values obtained from testicular volumes.
Table 1 shows the results of applying the contingency/constancy model to the change in GnRH1 mRNA expression and gonadal state (i.e. testicular volume) in starlings that were photostimulated (i.e. breeding) and subsequently analysed during the onset of photorefractoriness (i.e. non-breeding). The time points used in this study are representative of the late winter when starlings are photosensitive (i.e. pre-breeding; short day) and then placed on long day lengths (16 L : 8 D) to simulate the vernal increase in photoperiod for three to five weeks (i.e. breeding; long day (LD) three weeks, LD four weeks, LD five weeks). After seven and nine weeks of exposure to long day lengths, the gradual onset of photorefractoriness occurs (i.e. non-breeding LD seven weeks, LD nine weeks) that is representative of starlings during early summer and is maintained until early winter. The model reveals that the proportion of predictability in the variation in GnRH1 mRNA expression is predominantly owing to contingency information. After conducting the statistical analysis, we found that the change is highly significant and that only contingency information contributes significantly and not constancy information. By contrast, the variation in gonadal state is significant for predictability and both contingency and constancy information contribute. The greater contribution of constancy information for gonadal state is intriguing in that they suggest a hierarchical integration of environmental information, specifically, that contingency and constancy information are integrated at different levels in the HPG axis. The difference is most probably owing to variation in the ability of environmental cues to regulate GnRH1 transcription/translation and GnRH1 release that has been shown to occur across the reproductive cycle [37]. Given, that the Ie values obtained for GnRH1 and gonadal state were greater than one, Wingfield and colleagues [31] suggest that starlings should integrate initial predictive cues as well as supplementary cues. This hypothesis is supported by evidence demonstrating that the photo-induced increase in GnRH1 function [39,40] and testicular volume [41,42] is modulated by the social context and temperature.
Table 1.
Frequency matrices for predictability, constancy and contingency across the photo-induced change in GnRH1 cell numbers and peripheral physiology across the reproductive cycle in European starlings. (OD, optical density; SD, short day; LD, long day. Data taken from Stevenson et al. [35].)
| SD (8 L : 16 D) | LD 3 weeks | LD 4 weeks | LD 5 weeks | LD 7 weeks | LD 9 weeks | |
|---|---|---|---|---|---|---|
| GnRH OD | ||||||
| 0–2 | 2 | 1 | 0 | 0 | 2 | 6 |
| 2.1–4 | 2 | 1 | 2 | 2 | 1 | 2 |
| 4.1–6 | 2 | 1 | 3 | 2 | 2 | 0 |
| 6.1–8 | 2 | 3 | 1 | 0 | 1 | 0 |
| 8.1–10 | 0 | 0 | 0 | 2 | 1 | 0 |
| 10.1–12 | 0 | 2 | 2 | 2 | 1 | 0 |
| P = 0.26; C = 0.03; M = 0.23; C/P = 14%; M/P = 86%; Ie = 7.66; GM = 39.5; p < 0.05; GC = 10.2; p > 0.05; GP = 49.8; p < 0.001 | ||||||
| gonad volume | ||||||
| 0–200 | 8 | 0 | 0 | 1 | 3 | 8 |
| 201–400 | 0 | 2 | 3 | 1 | 3 | 0 |
| 401–600 | 0 | 3 | 1 | 3 | 1 | 0 |
| 601–800 | 0 | 1 | 2 | 3 | 0 | 0 |
| 801–1000 | 0 | 2 | 1 | 0 | 1 | 0 |
| 1001–1200 | 0 | 0 | 1 | 0 | 0 | 0 |
| P = 0.62; C = 0.14; M = 0.47; C/P = 23%; M/P = 76%; Ie = 3.35; GM = 61.7; p < 0.001; GC = 29.5; p < 0.001; GP = 90.7; p < 0.001 | ||||||
Indeed, the general pattern of seasonal breeding in temperate species consists of tying reproduction to the spring and summer periods, however a number of species are opportunistic and rapidly initiate breeding in the presence of an abundant food supply [3]. Opportunistic species provides an alternative means to investigate the neuroendocrine control of reproduction owing to the critical importance of food cues to successfully time the breeding period. White-winged crossbills (Loxia leucoptera) have been observed to breed throughout the year and rapidly engage in copulatory behaviours in the presence of seeds (figure 1; [43]). Interestingly, white-winged crossbills do not exhibit the seasonal plasticity in GnRH1 that is characteristic of many temperate passeriformes [44,45]. The current hypothesis is that the low variability in GnRH1 observed in white-winged crossbills is important to maintain a physiological responsiveness to unpredictable food resources [45].
We have applied the contingency/constancy model to analyse the predictability of the change in GnRH1 cell number in male and female white-crowned crossbill studied by MacDougall-Shackleton et al. ([44]; table 2). The birds were collected at three distinct points across the annual reproductive cycle (January, May and October). In this paper, male white-winged crossbills did not show a significant change in GnRH1 cell numbers across the year, however, females exhibited a significant increase in May when compared with male crossbills [44]. We found high levels of predictability in males and females with a greater percentage contributed from constancy information in males, whereas females had relatively equal contribution from both contingency and constancy information (table 2). As expected, the significance values obtained for the overall predictability, contingency and constancy information are considerably low, which is probably owing to the opportunistic breeding strategy [14,31]. This pattern was also apparent when the model was applied to another study that investigated the neuroendocrine responses in avian species which show variations in the degree of reproductive flexibility (electronic supplementary material, table S3; [45]). Interestingly, GnRH1 cell numbers are significantly predictable and constancy information contributes significantly to the overall effect in both males and females. The current hypothesis is that opportunistic breeders maintain a tonic level of GnRH1 activation on the pituitary in order to rapidly respond to favourable environmental conditions [44,45]. We suggest that the significant levels of predictability indicated that a greater number of GnRH1 cells are required to provide a constant innervation of the median eminence. As a result, environmental cues act at the level of the median eminence to regulate the release of gonadotrophins from the pituitary.
Table 2.
Frequency matrices for the number of GnRH1 cells from an opportunistic breeder. (The matrices compare the different values obtained for predictability, constancy and contingency between male and female white-winged crossbills. Data taken from MacDougall-Shackleton et al. [44].)
| males |
females |
||||||
|---|---|---|---|---|---|---|---|
| cell numbers | January | May | October | cell numbers | January | May | October |
| 0–100 | 0 | 0 | 1 | 0–100 | 0 | 0 | 0 |
| 101–200 | 2 | 2 | 3 | 101–200 | 1 | 0 | 2 |
| 201–300 | 2 | 2 | 0 | 201–300 | 3 | 1 | 2 |
| 301–400 | 0 | 0 | 0 | 301–400 | 0 | 3 | 0 |
| P = 0.53; C = 0.35; M = 0.17; C/P = 67%; M/P = 32%; Ie = 0.47; GM = 5.71; p = 0.45; GC = 11.9; p < 0.01; GP = 17.6; p < 0.05 | P = 0.56; C = 0.25; M = 0.31; C/P = 44%; M/P = 56%; Ie=1.25; GM = 10.4; p = 0.11; GC = 8.3; p < 0.05; GP = 18.7; p < 0.05 | ||||||
When we investigated the role of different environmental cues on the GnRH1 system, we found that all birds had Ie values that were considerably low. Wingfield and colleagues [31] proposed that animals with Ie values of less than one should rely predominantly on initial predictive cues with a minor role of supplementary cues. However, data collected from continual breeders (i.e. rock doves) are well below one and the authors proposed that body condition, age and/or endogenous rhythms could potentially affect the timing of breeding [31]. These species also inhabit an environment in which there is a reliable supply of nutrients, as such we propose that the low Ie values observed for GnRH1 cell numbers in males is owing to tonic activation required to rapidly initiate a breeding state. Interestingly, females were found to have Ie values suggesting that initial predictive cues are integrated to time reproduction and that responsiveness to supplementary cues fine-tune the breeding periods. Taken together, the data suggest that the extent to which opportunistic breeders rely on environmental cues to time breeding at the level of the GnRH1 cell is minimal and sex-dependent. Furthermore, the contingency/constancy model applied here provides mathematical support that opportunistic breeders maintain a tonic level of GnRH1 activation, but the release of gonadotrophins may be tied to nutrient availability.
4. Seasonal reproduction in mammalian species
Indeed, variation in the GnRH1 expression discussed above is unique to avian species and few studies have shown a similar pattern in mammalian species [46–48]. However, these observations appear to be the exception rather than the rule. Recently, a family of neuropeptides encoded by the Kiss1 gene and the cognate receptors were identified and subsequently demonstrated to be essential for the onset of puberty and reproductive function in mammalian species [23,24]. Recent evidence has demonstrated that Kiss1 can act directly on GnRH1 cells and is critical for stimulating GnRH1 secretion in mammals [27,49–51]. Kiss1 immunoreactivity has been observed in a number of hypothalamic regions with cell bodies predominantly located in the anteroventral periventricular nucleus (AvPv) and arcuate nucleus (Arc; [27,49]). Importantly, Kiss1 has been shown to exhibit a large degree of variation across the annual reproductive cycle in a number of mammals [27,49,52]. Given that GnRH1 cell numbers are relatively stable across the mammalian reproductive cycle, the dynamic changes in Kiss1 cells over the reproductive cycle that have been identified to date suggest that the dataset from this peptide make it a good candidate to model seasonal changes in the brain of mammals as we did with GnRH1 in birds.
The Syrian hamster (Mesocricetus auratus) is an excellent model species for studies geared towards investigating the neuroendocrine control of reproduction. Syrian hamsters can reproduce constantly when maintained on a photoperiod equal to or greater than 12.5 h, anything less than 12 h results in gonadal regression [53]. However, after prolonged exposure to short day lengths (approximately four to five months), Syrian hamsters become refractory to the short day length leading to gonadal recrudescence [54]. Kiss1 mRNA expression in Syrian hamsters varies considerably across the breeding cycle and is associated with greater cell number expression during the breeding season [26,55,56]. In addition to hamsters, the seasonal breeding cycle in ewes is also associated with marked variation in Kiss1 [57,58].
In order to conduct an analysis analogous to that reported above for GnRH1, we let t (times in the cycle) = breeding cycle (different physiological points across the annual reproductive cycle), and let s (states) = the number of observed Kiss1 neurons (the binned values that span the obtained range of Kiss1 values). Table 3 shows data from hamsters held on long and short day photoperiods and collected when in the respective breeding and non-breeding conditions [26]. Kiss1 neurons in both the AvPv and Arc show significant predictability across the photoperiodic conditions. Interestingly, the relative contributions of constancy and contingency differ between the two populations. The AvPv shows a significant contribution from both contingency and constancy, whereas the Arc population appears to be primarily dependent on information derived from constancy. It does appear that the two populations show different patterns with a greater proportion of the overall predictability in the AvPv (60% for contingency) and Arc (59% constancy). The different patterns observed between the two populations may be the result of the different effects of sex steroid feedback in regulating Kiss1 in hamsters [59]. The Ie values obtained from Kiss1 expression in the AvPv is within the range suggesting that both initial predictive and supplementary information are integrated, whereas the Arc has Ie values associated with initial predictive information.
Table 3.
Frequency matrices for predictability, constancy and contingency values obtained for Kiss1 cell numbers in Syrian hamsters from two different points across the reproductive cycle. (All values were obtained from Greives et al. [26].)
| AvPv |
Arc |
||||
|---|---|---|---|---|---|
| no. of Kiss1 cells | LD | SD | no. of Kiss1 cells | LD | SD |
| 0–20 | 0 | 9 | 0–20 | 2 | 0 |
| 21–40 | 3 | 4 | 21–40 | 2 | 6 |
| 41–60 | 2 | 0 | 41–60 | 5 | 2 |
| 61–80 | 1 | 0 | 61–80 | 1 | 0 |
| 81–100 | 1 | 0 | 81–100 | 1 | 2 |
| 101–120 | 2 | 0 | 101–120 | 0 | 0 |
| >120 | 2 | 0 | >120 | 0 | 3 |
| P = 0.42; C = 0.17; M = 0.25; C/P = 40%; M/P = 60%; Ie=1.47; GM = 23.5; p < 0.001; GC = 16.9; p < 0.01; GP = 40.4; p < 0.001 | P = 0.31; C = 0.18; M = 0.13; C/P = 59%; M/P = 41%;Ie=0.72; GM = 11.9; p > 0.05; GC = 18.2; p < 0.01; GP = 30.2; p < 0.005 | ||||
5. Summary and conclusions
The application of information theory models permits the formulation of values that provide specific information on how an individual or population will respond to an environment and which environmental cues they may use to time seasonal reproduction. Here, we present data from avian and mammalian species to show that specific neuropeptides integrate either initial predictive cues and/or supplementary cues to successfully time reproduction. The findings show that (i) environmental cues are integrated by GnRH1 cells and the release of GnRH1 indicated by the change in gonadal measures; (ii) variation in breeding strategies are reflected in the degree of predictability; and (iii) the integration of environmental cues by Kiss1 may be anatomically localized.
One striking discovery revealed by the contingency/constancy model was the observation of different Ie values between male and female white-winged crossbills. These findings imply that female crossbills integrate a larger number of environmental cues to time reproduction when compared with male crossbills. It is generally assumed that the photoperiodic control of reproduction is similar between the sexes; however, the integration of supplementary cues can vary considerably between the two which is most likely a result of different selection pressures [60,61]. One hypothesis is that the neural integration of supplementary cues involves a number of ‘processing stages’ and the balance between inhibitory and stimulatory gating leads to the different degrees of physiological responsiveness between males and females. Supplementary cues are perceived through complex neural processes involving a number of telencephalic regions that are susceptible to different neuromodulatory systems before the cues can influence the function of the GnRH system. In males it appears that supplementary cues are readily transduced leading to the change in responsiveness, whereas females may require the action of various neuromodulatory systems (e.g. sex steroids) to shift the responsiveness across a number of brain regions that in turn facilitate the probability and intensity of responding to supplementary cues [60]. This is generally supported by the fact that males readily exhibit full gonadal recrudescence when photostimulated, whereas females require a number of additional supplementary cues (i.e. food availability, behavioural interactions, etc.) in order to attain full gonadal development [62]. The different degrees to which the sexes integrate environmental signals may markedly constrain the timing of reproduction in different ways in males and females and potentially could lead to a detrimental effect by mismatching when reproduction occurs.
The concept that the neural integration of environmental cues includes a number of processing stages is also supported by the observation of increasing values of predictability and Ie at different levels of the HPG axis. One common theme across the examples provided in the present paper is that the integration of environmental cues occurs at multiple levels. Specifically, environmental cues can influence the neuropeptidergic cells directly by regulating mRNA and protein synthesis as well as the release of gonadotropins indicated by variation in gonadal state. The GnRH1 neurons in vertebrates are the final output of complex synaptic networks that control reproduction, and environmental cues can act at the level of the GnRH1 cells and/or terminals in avian [7,16] and mammal species [8,63]. The data presented here provide mathematical support for the neural integration of biologically significant signals. Moreover, we encourage the computation of Ie values from future studies across different levels of the HPG axis in order to provide a means to identify at what step in the circuitry the variation supplementary cues influence reproductive physiology and in turn will facilitate the ability to delineate the circuitry underlying the environmental control of seasonal reproduction.
It is important to note that a greater sample number of columns (t) significantly increase the resolution of predictability, constancy and contingency. A limitation in the data presented here is the lack of studies that have collected data from species at a number of time points in the annual cycle. Indeed, the resolution for determining significant contributions is low owing to the vast majority of studies collecting animals during the breeding and non-breeding seasons, which only allow the analysis between two points in the annual cycle. Clearly, the breeding season potentially spans many months, increasing the number of observations of months with measures of hypothalamic peptide values would significantly increase the resolution and provide more accurate predictability, constancy and contingency values. A second limitation of the application of the contingency/constancy model used here is that the data from two examples were taken from laboratory held animals. Ideally, collecting wild animals from several points across the annual cycle is most beneficial and would address both limitations. However, this approach can be technically challenging owing to yearly variation in local conditions as well as raising concerns over the number of animals collected across the year(s).
Predictability in brain switches in response to photoperiod raises interesting questions regarding an individual and/or populations ability to respond to climate changes. Caro and colleagues [64,65] have recently shown that two populations of Corsican blue tits (Parus caeruleus) reside at the same latitude but exhibit a one month difference in the onset of breeding. The shift in the timing of breeding appears to be mediated by a change in the responsiveness of female blue tits from one population to adapt to a change in the availability of food required to raise the offspring [64,65]. As a result, it appears that a greater emphasis on the integration of supplementary short-term cues in Corsican blue tits are required to adjust with the developing changes in local environmental conditions. Thus, the prediction here is that natural selection would favour animals that exhibit greater flexibility in the GnRH1 system directly in avian species or indirectly via Kiss1 in mammals. Taken together, the data presented here showed that a specific neuronal population located in the hypothalamus are highly responsive to environmental cues, are predictive of reproductive state and are essential to maintain flexibility in order for the individual to cope with changes in local climates.
Acknowledgements
We would like to thank Drs Timothy Greives, Gregory Demas, Lance Kriegsfeld, Tom Hahn, Scott MacDougall-Shackleton and Maria Pereyra for contributing data for the analyses. The work was supported by a pre-doctoral grant NSERC-PGSD 334570 to T.J.S. and NIH/NINDS RO1467 to G.F.B.
References
- 1.Murton R. K., Westwood N. J. 1977. Avian breeding cycles, p. 594 London, UK: Oxford University Press [Google Scholar]
- 2.Bronson F. H. 1989. Seasonal strategies. In Mammalian reproductive biology, pp. 23–59 Chicago, IL: The University of Chicago Press [Google Scholar]
- 3.Lack D. 1968. Ecological adaptations for breeding in birds. London, UK: Methuen [Google Scholar]
- 4.Marshall A. J. 1970. Environmental factors other than light involved in the control of sexual cycles in birds and mammals. In La photoregulation de la Reproduction Chez les Oiseaux et les Mammiferes (eds Benoit J., Assenmacher I.), pp. 53–64 Paris, France: Centre National de la Recherche Scientifique [Google Scholar]
- 5.Immelmann K. 1973. Role of the environment in reproduction as a source of predictive information. In Breeding biology of birds. (ed. Farner D. S.), pp. 121–147 Washington, DC: National Academy of Sciences [Google Scholar]
- 6.Baker J. R. 1938. The evolution of breeding seasons. In Evolution (ed. de Beer G. R.), pp. 161–177 London, UK: Oxford University Press [Google Scholar]
- 7.Dawson A., King V. M., Bentley G. E., Ball G. F. 2001. Photoperiodic control of seasonality in birds. J. Biol. Rhyth. 16, 365–380 10.1177/074873001129002079 (doi:10.1177/074873001129002079) [DOI] [PubMed] [Google Scholar]
- 8.Goldman B. D. 2001. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J. Biol. Rhyth. 16, 263–283 10.1177/074873001129001980 (doi:10.1177/074873001129001980) [DOI] [PubMed] [Google Scholar]
- 9.Hau M. 2001. Timing of breeding in variable environments: tropical birds as model systems. Horm. Behav. 40, 281–290 10.1006/hbeh.2001.1673 (doi:10.1006/hbeh.2001.1673) [DOI] [PubMed] [Google Scholar]
- 10.Wikelski M., Hau M., Robinson D. W., Wingfield J. C. 1999. Seasonal endocrinology of tropical passerines: a comparative approach. In Proc. 22nd Int Ornithol. Congr. (eds Adams N., Slotow R.), pp. 1223–1241 Durban, Johannesburg, South Africa: Birdlife South Aftica [Google Scholar]
- 11.Hahn T. P., Boswell T., Wingfield J. C., Ball G. F. 1997. Temporal flexibility in avian reproduction. In Current ornithology (eds Nolan V., Ketterson E. D., Thompson C. F.), pp. 39–80 New York, NY: Plenum [Google Scholar]
- 12.Hahn T. P. 1998. Reproductive seasonality in an opportunistic breeder, the red crossbill (Loxia curvirostra). Ecology 79, 2365–2375 [Google Scholar]
- 13.Wingfield J. C. 1988. Changes in reproductive function of free living birds in direct response to environmental perturbations. In Processing of environmental information in vertebrates (ed. Stetson M. H.), pp. 121–148 Berlin, Germany: Springer [Google Scholar]
- 14.Wingfield J. C., Jacobs J. D., Tramontin A. D., Perfito N., Meddle S., Maney D. L., Soma K. 2000. Toward an ecological basis of hormone-behavior interactions in reproduction of birds. In Reproduction in context (eds Wallen K., Schneider J. E.), pp. 85–129 Cambridge, MA: The MIT Press [Google Scholar]
- 15.Herbison A. E. 2006. Physiology of the gonadotropin-releasing hormone neuronal network. In Knobil and Neill's physiology of reproduction (ed. Neill J. D.), pp. 1415–1482, 3rd edn New York, NY: Raven Press [Google Scholar]
- 16.Ball G. F. 1993. The neural integration of environmental information by seasonally breeding birds. Am. Zool. 33, 185–199 [Google Scholar]
- 17.Goodman R. L., Jansen H. T., Billings H. J., Coolen L. M., Lehman M. N. 2010. Neural systems mediating seasonal breeding in the ewe. J. Neuroendocrinol. 22, 674–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharp P. J., Talbot R. T., Main G. M., Dunn I. C., Fraser H. M., Huskisson N. S. 1990. Physiological roles of chicken LHRH-I and –II in the control of gonadotrophin release in the domestic chicken. J. Endocrinol. 124, 291–299 10.1677/joe.0.1240291 (doi:10.1677/joe.0.1240291) [DOI] [PubMed] [Google Scholar]
- 19.Silverman A. J., Livne I., Witkin J. W. 1994. The gonadotropin-releasing hormone (GnRH), neuronal system: immunocytochemistry and in situ hybridization. In The physiology of reproduction (eds Knobil E., Neill J. D.), pp. 1683–1709 New York, NY: Raven Press [Google Scholar]
- 20.Lehman M. N., Goodman R. L., Karsch F. J., Jackson G. L., Berriman S. J., Jansen H. T. 1997. The GnRH system of seasonal breeders: anatomy and plasticity. Brain Res. Bull. 44, 445–457 10.1016/S0361-9230(97)00225-6 (doi:10.1016/S0361-9230(97)00225-6) [DOI] [PubMed] [Google Scholar]
- 21.Ball G. F., Hahn T. P. 1997. GnRH neuronal systems in birds and their relation to the control of seasonal reproduction. In GnRH neurons: gene to behavior (eds Parhar I. S., Sakuma Y.), pp. 325–342 Tokyo, Japan: Brain Shuppan [Google Scholar]
- 22.Stevenson T. J., Bernard D. J., Ball G. F. 2009. Photoperiodic condition is associated with region-specific expression of GNRH1 mRNA in the preoptic area of the male starling. Biol. Reprod. 81, 674–680 10.1095/biolreprod.109.076794 (doi:10.1095/biolreprod.109.076794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Roux N., Genin E., Carel J. C., Matsuda F., Chaussain J. L., Milgrom E. 2003. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl Acad. Sci. USA 100, 10 972–10 976 10.1073/pnas.1834399100 (doi:10.1073/pnas.1834399100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Funes S., Hedrick J. A., Vassileva G., Markowitz L., Abbondanzo S., Golovko A. 2003. The KiSS1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem. Biophys. Res. Commun. 312, 1357–1363 10.1016/j.bbrc.2003.11.066 (doi:10.1016/j.bbrc.2003.11.066) [DOI] [PubMed] [Google Scholar]
- 25.Tobari Y., Iigima N., Tsunekawa K., Osugi T., Okanoya K., Tsutsui K., Ozawa H. 2010. Identification of gonadotropin-inhibitory hormone in the zebra finch: peptide isolation, cDNA cloning and brain distribution. Peptides 31, 816–826 10.1016/j.peptides.2010.01.015 (doi:10.1016/j.peptides.2010.01.015) [DOI] [PubMed] [Google Scholar]
- 26.Greives T. J., Mason A. O., Scotti M. A. L., Levine J., Ketterson E. D., Kriegsfeld L. J., Demas G. E. 2007. Environmental control of kisspeptin: implications for seasonal reproduction. Endocrinology 148, 1158–1166 10.1210/en.2006-1249 (doi:10.1210/en.2006-1249) [DOI] [PubMed] [Google Scholar]
- 27.Simonneaux V., Ansel L., Revel F. G., Klosen P., Pevet P., Mikkelsen J. D. 2009. Kisspeptin and the seasonal control of reproduction in hamsters. Peptides 30, 146–153 10.1016/j.peptides.2008.06.006 (doi:10.1016/j.peptides.2008.06.006) [DOI] [PubMed] [Google Scholar]
- 28.Cohen D. 1966. Optimizing reproduction in a randomly varying environment. J. Theor. Biol. 12, 1–14 10.1016/0022-5193(66)90188-3 (doi:10.1016/0022-5193(66)90188-3) [DOI] [PubMed] [Google Scholar]
- 29.Kullback S. 1959. Information theory and statistics, p. 395 New York, NY: Wiley [Google Scholar]
- 30.Colwell R. K. 1974. Predictability, constancy, and contingency of periodic phenomena. Ecology 55, 1148–1153 10.2307/1940366 (doi:10.2307/1940366) [DOI] [Google Scholar]
- 31.Wingfield J. C., Hahn T. P., Levin R., Honey P. 1992. Environmental predictability and control of gonadal cycles in birds. J. Exp. Zool. 261, 214–231 10.1002/jez.1402610212 (doi:10.1002/jez.1402610212) [DOI] [Google Scholar]
- 32.Sokal R. R., Rohlf F. J. 1981. Biometry, pp. 691–778 San Francisco, CA: Freeman [Google Scholar]
- 33.Zar J. H. 1984. Biostatistical analysis, pp. 40–79 Englewood Cliffs, NJ: Prentice-Hall [Google Scholar]
- 34.Follett B. K. 1984. Birds. In ‘Marshall's’ physiology of reproduction, vol. 1 (ed. Lamming G. E.), pp. 283–350 Edinburgh, UK: Longman Green [Google Scholar]
- 35.Stevenson T. J., Lynch K. S., Lamba P., Ball G. F., Bernard D. J. 2009. Cloning of gonadotropin-releasing hormone 1 complementary DNAs in songbirds facilitates dissection of mechanisms mediating seasonal changes in reproduction. Endocrinology 150, 1826–1833 10.1210/en.2008-1435 (doi:10.1210/en.2008-1435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicholls T. J., Goldsmith A. R., Dawson A. 1988. Photorefractoriness in birds and comparison with mammals. Physiol. Rev. 68, 133–176 [DOI] [PubMed] [Google Scholar]
- 37.Dawson A., Goldsmith A. R. 1997. Changes in gonadotropin-releasing hormone in the preoptic area and median eminence of starlings during the recovery of photosensitivity and during photostimulation. J. Reprod. Fertility 111, 1–6 10.1530/jrf.0.1110001 (doi:10.1530/jrf.0.1110001) [DOI] [PubMed] [Google Scholar]
- 38.Dawson A., Goldsmith A. R., Nicholls T. J., Follett B. K. 1986. Endocrine changes associated with the termination of photorefractoriness by short day lengths and thyroidectomy in starlings. J. Endocrinol. 110, 73–79 10.1677/joe.0.1100073 (doi:10.1677/joe.0.1100073) [DOI] [PubMed] [Google Scholar]
- 39.Dawson S., Sharp P. J. 2010. Seasonal changes in concentrations of plasma LH and prolactin associated with the advance in the development of photorefractoriness and molt by high temperature in the starling. Gen. Comp. Endocrinol. 167, 122–127 10.1016/j.ygcen.2010.02.004 (doi:10.1016/j.ygcen.2010.02.004) [DOI] [PubMed] [Google Scholar]
- 40.Stevenson T. J., Ball G. F. 2009. Anatomical localization of the effects of reproductive state, castration, and social milieu on cells immunoreactive for gonadotropin-releasing hormone 1 in male European starlings. J. Comp. Neurol. 517, 146–155 10.1002/cne.22159 (doi:10.1002/cne.22159) [DOI] [PubMed] [Google Scholar]
- 41.Burger J. W. 1947. On the relation of day length to the phases of testicular involution and inactivity of the spermatogenic cycle of the European starling. J. Exp. Zool. 195, 259–268 10.1002/jez.1401050207 (doi:10.1002/jez.1401050207) [DOI] [PubMed] [Google Scholar]
- 42.Burger J. W. 1953. The effect of photic and psychic stimuli on the reproductive cycle of the male starling, Sturnus vulgaris. J. Exp. Zool. 124, 227–239 10.1002/jez.1401240203 (doi:10.1002/jez.1401240203) [DOI] [Google Scholar]
- 43.Benkman C. W. 1992. White-winged crossbill. In The birds of North America, vol. 27 (eds Poole A., Gill F.), pp. 1–20 Philadelphia, PA: The Academy of Natural Sciences [Google Scholar]
- 44.MacDougall-Shackleton S. A., Deviche P. J., Crain R. D., Ball G. F., Hahn T. P. 2001. Seasonal changes in brain GnRH immunoreactivity and song control nuclei volumes in an opportunistically breeding songbird. Brain Behav. Evol. 58, 38–48 10.1159/000047260 (doi:10.1159/000047260) [DOI] [PubMed] [Google Scholar]
- 45.Pereyra M. E., Sharbaugh S. M., Hahn T. P. 2005. Interspecific variation in photo-induced GnRH plasticity among normadic cardueline finches. Brain Behav. Evol. 66, 35–49 10.1159/000085046 (doi:10.1159/000085046) [DOI] [PubMed] [Google Scholar]
- 46.Pickard G. E., Silverman A. J. 1979. Effects of photoperiod on hypothalamic luteinizing hormone releasing hormone in the male hamster. J. Endocrinol. 83, 421–428 10.1677/joe.0.0830421 (doi:10.1677/joe.0.0830421) [DOI] [PubMed] [Google Scholar]
- 47.Ronchi E., Krey L. C., Pfaff D. W. 1992. Steady state analysis of hypothalamic GnRH mRNA levels in male Syrian hamsters: influences of photoperiod and androgen. Neuroendocrinology 55, 146–155 10.1159/000126109 (doi:10.1159/000126109) [DOI] [PubMed] [Google Scholar]
- 48.Urbanski H. F., Doan A., Pierce M. 1991. Immunocytochemical investigation of luteinizing hormone-releasing hormone neurons in Syrian hamsters maintained under long or short days. Biol. Reprod. 44, 687–692 10.1095/biolreprod44.4.687 (doi:10.1095/biolreprod44.4.687) [DOI] [PubMed] [Google Scholar]
- 49.Clarke I. J., Smith J. T., Caraty A., Goodman R. L., Lehman M. N. 2009. Kisspeptin and seasonality in sheep. Peptides 30, 154–163 10.1016/j.peptides.2008.08.022 (doi:10.1016/j.peptides.2008.08.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colledge W. H. 2008. Kisspeptins and GnRH neuronal signaling. Trends Endocrinol. Metab. 20, 115–121 [DOI] [PubMed] [Google Scholar]
- 51.Popa S. M., Clifton D. K., Steiner R. A. 2008. The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Ann. Rev. Physiol. 70, 213–238 10.1146/annurev.physiol.70.113006.100540 (doi:10.1146/annurev.physiol.70.113006.100540) [DOI] [PubMed] [Google Scholar]
- 52.Greives T. J., Kriegsfeld L. J., Bentley G. E. 2008. Recent advances in reproductive neuroendocrinology: a role for RFamide peptides in seasonal reproduction. Proc. R. Soc. B 275, 1943–1951 10.1098/rspb.2008.0433 (doi:10.1098/rspb.2008.0433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elliot J. A. 1976. Circadian rhythms and photoperiodic time measurement in mammals. Fed. Proc. 35, 2339–2346 [PubMed] [Google Scholar]
- 54.Elliot J. A., Goldman B. D. 1981. Seasonal reproduction: photoperiodism and biological clocks. In Neuroendocrinology of reproduction (ed. Adler N. T.), pp. 377–423 New York, NY: Plenum Press [Google Scholar]
- 55.Mason A. O., Greives T. J., Scotti M. A. L., Levine J., Frommeyer S., Ketterson E. D., Demas G. E., Kriegsfeld L. J. 2007. Suppression of kisspeptin expression and gonadotropic axis sensitivity following exposure to inhibitory day lengths in female Siberian hamsters. Horm. Behav. 52, 492–498 10.1016/j.yhbeh.2007.07.004 (doi:10.1016/j.yhbeh.2007.07.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Revel F. G., Saboureau M., Masson-Pevet M., Pevet P., Mikkelsen J. D., Simonneaux V. 2006. Kisspeptin mediates the photoperiodic control of reproduction in hamsters. Curr. Biol. 16, 1730–1735 10.1016/j.cub.2006.07.025 (doi:10.1016/j.cub.2006.07.025) [DOI] [PubMed] [Google Scholar]
- 57.Smith J. T., Coolen L. M., Kriegsfeld L. J. 2008. Variation in Kisspeptin and RFamide-Related Peptide (RFRP) expression and terminal connections to Gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology 149, 5770–5782 10.1210/en.2008-0581 (doi:10.1210/en.2008-0581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wagner G. C., Johnston J. D., Clarke I. J., Lincoln G. A., Hazlerigg D. G. 2008. Redefining the limits of day length responsiveness in a seasonal mammal. Endocrinology 149, 32–39 10.1210/en.2007-0658 (doi:10.1210/en.2007-0658) [DOI] [PubMed] [Google Scholar]
- 59.Greives T. J., Humber S. A., Goldstein A. N., Scottie M. A., Demas G. E., Kriegsfeld L. J. 2008. Photoperiod and testosterone interact to drive seasonal changes in kisspeptin expression in Siberian hamsters. J. Neuroendocrinol. 12, 1339–1347 10.1111/j.1365-2826.2008.01790.x (doi:10.1111/j.1365-2826.2008.01790.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ball G. F., Ketterson E. D. 2008. Sex differences in the response to environmental cues regulating seasonal reproduction in birds. Phil. Trans. R. Soc. B 363, 231–246 10.1098/rstb.2007.2137 (doi:10.1098/rstb.2007.2137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prendergast B. J. 2005. Internalization of seasonal time. Horm. Behav. 48, 502–511 10.1016/j.yhbeh.2005.05.013 (doi:10.1016/j.yhbeh.2005.05.013) [DOI] [PubMed] [Google Scholar]
- 62.Farner D. S., Wilson A. C. 1957. A quantitative examination of testicular growth in the white-crowned sparrow. Biol. Bull. 113, 254–267 10.2307/1539083 (doi:10.2307/1539083) [DOI] [PubMed] [Google Scholar]
- 63.Campbell R. E., Suter K. J. 2010. Redefining the gonadotrophin-releasing hormone neurone dendrite. J. Neuroendocrinol. 22, 650–658 [DOI] [PubMed] [Google Scholar]
- 64.Caro S. P., Balthazart J., Thomas D. W., Lacroix A., Chastel O., Lambrechts M. M. 2005. Endocrine correlates of the breeding asynchrony between two Corsican populations of blue tits (Parus caeruleus). Gen. Comp. Endocrinol. 140, 52–60 10.1016/j.ygcen.2004.09.016 (doi:10.1016/j.ygcen.2004.09.016) [DOI] [PubMed] [Google Scholar]
- 65.Caro S. P., Lambrechts M. M., Chastel O., Sharp P. J., Thomas D. W., Balthazart J. 2006. Simultaneous pituitary–gonadal recrudescence in two Corsican populations of male blue tits with asynchronous breeding dates. Horm. Behav. 50, 347–360 10.1016/j.yhbeh.2006.03.001 (doi:10.1016/j.yhbeh.2006.03.001) [DOI] [PubMed] [Google Scholar]
- 66.Dawson A. 1983. Plasma gonadal steroids levels in wild starlings during the annual cycle and in relation to the stages of breeding. Gen. Comp. Endocrinol. 49, 286–294 10.1016/0016-6480(83)90146-6 (doi:10.1016/0016-6480(83)90146-6) [DOI] [PubMed] [Google Scholar]
- 67.Dawson W. R. 1997. Pine siskin (Carduelis pinus). In The birds of North America, vol. 280 (eds Poole A., Gill F.), pp. 1–24 Philadelphia, PA: The Academy of Natural Sciences [Google Scholar]
- 68.Knox A. G., Lowther P. E. 2000. Common redpoll (Carduelis flammea). In The birds of North America, vol. 78 (eds Poole A., Gill F.), pp. 1–23 Philadelphia, PA: The Academy of Natural Sciences [Google Scholar]
- 69.Bronson F. H., Heideman P. D. 1994. Seasonal regulation of reproduction in mammals. In The physiology of reproduction, vol. 2 (eds Knobil E., Neill J. D.), pp. 541–583, 2nd edn New York, NY: Raven Press [Google Scholar]
- 70.Goodman R. L. 1994. Neuroendocrine control of the ovine estrous cycle. In The physiology of reproduction (eds Knobil E., Neill J. D.), pp. 659–710, 2nd edn New York, NY: Raven Press [Google Scholar]