Abstract
Recent studies in the Northern Hemisphere have shown that songbirds living in noisy urban environments sing at higher frequencies than their rural counterparts. However, several aspects of this phenomenon remain poorly understood. These include the geographical scale over which such patterns occur (most studies have compared local populations), and whether they involve phenotypic plasticity or microevolutionary change. We conducted a field study of silvereye (Zosterops lateralis) vocalizations over more than 1 million km2 of urban and rural south-eastern Australia, and compared possible effects of urban noise on songs (which are learned) and contact calls (which are innate). Across 14 paired urban and rural populations, silvereyes consistently sang both songs and contact calls at higher frequencies in urban environments. Syllable rate (syllables per second) decreased in urban environments, consistent with the hypothesis that reflective structures degrade song and encourage longer intervals between syllables. This comprehensive study is, to our knowledge, the first to demonstrate varied adaptations of urban bird vocalizations over a vast geographical area, and to provide insight into the mechanism responsible for these changes.
Keywords: acoustic interference, calls, silvereye, song, urban noise, Zosterops lateralis
1. Introduction
Effective communication between individuals may be mediated by behavioural, physiological and environmental factors, each of which can exert selective pressures on the sending and receiving of information. The ‘acoustic adaptation hypothesis’ [1] proposes that animals alter or adapt their acoustic communication patterns in response to environmental factors such as background noise and physical structures, in order to reduce degradation and/or to be better heard above background noise [2]. Populations of the same species occupying acoustically different habitats are thus expected to adapt their vocalizations to broadcast with greater efficiency. Consistent with this hypothesis, Wiley [3] demonstrated that both frequency and temporal patterns in songs of 120 North American oscine species differed according to habitat type.
Urban habitats represent new and often extreme acoustic environments for wild animals. Songbirds are excellent study subjects for investigating the effects of urban noise on acoustic communication. Birds are among the most ubiquitous and conspicuous faunal elements of cities, and acoustic communication is central to many aspects of their biology, including territory tenure, mate selection and antagonistic interactions [2]. These varied applications of song must remain effective in urban habitats, making song highly vulnerable to external selection pressures that may affect its transmission. In addition to having high levels of background noise, urban areas also differ considerably from natural environments in their physical properties. Tall buildings act as reflective surfaces, which reverberate and distort song [4], causing repeated song elements to be blended or masked [5]. Whether a given species persists or even thrives in urban habitats will thus depend critically on whether or not it can adapt acoustically to the novel physical elements in its environment, and the presence of human-generated noise. Depending on the function of a particular vocalization, high fidelity of transmission may be more important in some instances, whereas in other contexts, the need to be heard over high levels of noise may become more important than the need to convey exact information through the subtleties of the song. For example, if song elements are repeated more slowly, or are separated by longer intervals, then birds in urban areas may be able to send and receive acoustic information without high levels of degradation [6].
Vocal learning in passerines may allow them to change their song not only over evolutionary time, but also within their lifetimes to suit the novel acoustic environments. This makes them ideal for field studies investigating adaptations and changes in response to urban noise [7]. There has been burgeoning interest in the effects of urban noise on acoustic communication in birds in the past few years, but previous studies have been conducted predominantly in Europe and North America. Overall, while bird population density and species diversity tend to decrease with increased proximity to traffic noise [8–11], species that are able to remain in areas with high levels of anthropogenic noise show evidence of diverse adaptations. For example, European robins (Erithacus rubecula) tend to sing at certain times of day to avoid noise masking [12]. Nightingales (Luscinia megarhynchos) and zebra finches (Taeniopygia guttata) increase song volume in noisier areas [13,14], and great tits (Parus major) and grey shrike-thrushes (Colluricincla harmonica) modify their song frequencies to avoid masking effects of the background urban noise [9,15]. There is also evidence that birds can adapt their songs to improve propagation through environments with structural impediments. For instance, great tits sing songs with longer spaced intervals in forests versus open areas to reduce degradation [16]. Recent studies have shown that some urban birds sing more ‘hurried’ songs in cities [15], while others sing fewer notes per song in urban environments [17].
While there is now little doubt that songs sung by urban populations of a particular bird species often differ in one or more attributes from those sung by rural populations (e.g. [18,19]), the mechanism underlying these differences remains unclear. One view is that these differences are the result of individual phenotypic plasticity. This hypothesis is supported by a recent study by Halfwerk & Slabbekoorn [20], which showed that individual great tits sang higher or lower songs in their repertoire depending on the background noise. Nevertheless, differences in songs from urban and rural habitats may not be due exclusively to phenotypic plasticity. For instance, acoustic differences could simply be a consequence of genetic adaptations to the local environment. Such an explanation is consistent with the observed changes in urban anuran calls, which are considered to be innate [21].
A potentially fruitful approach for evaluating the relative influences of phenotypic plasticity versus microevolutionary change in vocalization characteristics is to evaluate the effect of urban noise on both songs and calls. Unlike songs, passerine contact calls are sung by both males and females in all species, and are used throughout the year regardless of breeding status. Calls are also generally shorter and simpler than songs. Although some plasticity in call structure has been identified, they are generally considered to be much less flexible than song, and remain static throughout an individual's lifetime [22]. Thus, if calls as well as songs display predictable variation in relation to urban noise, this suggests that such responses result from evolutionary changes, rather than phenotypic plasticity.
The aim of this study was to determine whether there are differences in silvereye (Zosterops lateralis) song and/or calls between urban and rural populations across a large geographical area. A comparison between urban and rural great tits found that frequency shifts to avoid masking occur in cities in Europe [15], but the phenomenon depends heavily on species identity [9,23]. Urban noise may mask lower frequencies in birds' songs, and therefore silvereyes in cities may sing at higher frequencies to avoid this masking. Since different song elements may convey different types of information [24], and environmental properties can affect a variety of song characteristics, we may observe change in some elements but not others. Therefore, we also investigated any potential urban effects on syllable rate, predicting that silvereyes may attempt to reduce signal degradation by slowing syllable rate in urban areas.
2. Methods
(a). Study species
Silvereyes (Z. lateralis) are widely distributed native Australian passerines, common in both urban and rural environments. Each male sings a unique song as a territorial or sexual advertisement [25]. Both songs and calls of silvereyes fall within the frequency range of approximately 2–6 kHz ([26]; personal observation of previous song analysis). The lower range is within the masking effects of urban noise (approx. 1–4 kHz; [27]). This may make lower notes more difficult to hear in urban environments, and favour upward shifts in frequency within the normal range of silvereye song to avoid this masking. In addition, silvereye song is comprised of a number of discreet elements or syllables sung in rapid succession. Hence, there is also potential for slowing down or creating space between these syllables in closed environments to reduce degradation.
(b). Study sites
The study sites were paired urban and rural locations in distinct geographical areas around Australia as follows: Melbourne, Victoria (Darebin Parklands and Lerderderg State Park); Adelaide, South Australia (Glenalta and Coorong National Park); Sydney, New South Wales (Poulton Park and Munghorn Gap Nature Reserve); Grafton, New South Wales (Susan Island and Lamington National Park); Brisbane, Queensland (Kingfisher Park and Mount Coot-Tha State Forest); Hobart, Tasmania (Seven Mile Beach/Hobart Airport and Mount Wellington Reserve) and Canberra, ACT (Australian National Botanic Gardens and Namadgi National Park). All of these sites have breeding, resident populations of silvereyes.
(c). Field methods
Between September 2009 and February 2010 (silvereye breeding season, to ensure resident populations), silvereyes were caught in mistnets during the early morning over the course of 2–8 days. Each captured individual was fitted with an Australian Bird and Bat Banding Scheme (ABBBS) aluminium-numbered band, along with three colour bands for later identification while singing in the field. Individuals were also weighed and their morphometrics measured before immediate re-release. During subsequent days, we relocated banded individuals and recorded their songs and calls with Marantz Professional PMD660 Solid State recorders and Sennheiser ME67 directional microphones. The recording sampling rate was 48 kHz. We recorded songs and calls from four to nine individuals per study site, with a total of 81 individuals, recording complete dawn choruses and any singing bouts and contact calling between dawn and 12:00 h at every site.
Within each site, we took sound level readings at 10 separate locations, each 20 m apart. Readings were taken for 1 min at each location at 6:00 h, 9:00 h and 12:00 h using a Lutron SL-4001 Sound Level Meter. We used a slow response measurement with ‘A’ weighting. These readings were then combined to determine the average levels of background noise during the dawn chorus and morning singing period at each study site.
(d). Sound analysis
We used the program RavenPro 1.4 (Cornell Lab of Ornithology, 2009) to generate spectrograms of all recorded songs and calls. Recordings were analysed aurally as well as visually and silvereye vocalizations were identified by shape, energy and timbre, and separated from background noises present in recordings. We discarded recordings for which background noise was so high that it could have obscured low-frequency song elements (less than 1% of all recordings). We determined the minimum frequency, peak frequency (frequency with highest amplitude used in the song) and syllable rate (number of syllables per second) in each vocalization. Syllables were defined as one or more distinct notes that always occurred together [28]. These analyses were performed blind to the identity of the bird and the site. Average values were calculated for each individual.
(e). Statistical analysis
We used a Bayesian framework for our statistical analyses, with an emphasis on effect sizes and precision. This framework is well suited for analysing hierarchical models that include random and fixed effects [29], such as those used in this study. Our regression models included flat (uninformative) priors to reflect an absence of prior information, giving results that are numerically similar to those based on maximum-likelihood estimation.
We produced a linear regression model using OpenBUGS [30,31] to estimate the effect of background noise on the lowest frequency values, peak frequencies, song duration and syllable rate (syllables s−1) of songs and calls. This model also included a random site effect and a random ‘pair’ effect to account for variation in song owing to the geographical location of each pair of sites. We discarded the first 100 000 samples as a burn-in and checked for convergence. We used a 95% CI, estimating the mean and standard deviation from 200 000 samples from the posterior distribution.
3. Results
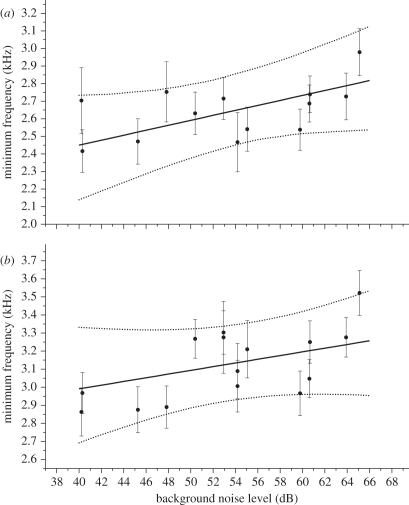
Urban habitats had a mean background noise level of 9.0 dB higher than rural habitats (95% CI: 2.09, 15.94; electronic supplementary material table S4). We found no important effect of noise or urban habitat on peak frequencies in either song or calls in silvereyes (electronic supplementary material table S3). However, we did find that urban habitats predicted higher lowest frequencies in song (mean 195 Hz; 95% CI: −63 Hz, 462 Hz), and to a lesser extent in calls (mean 90Hz; 95% CI: −223 Hz, 423 Hz). We also found a consistent effect of noise on lowest frequencies in song, with a predicted increase of 13.4 Hz dB−1 of noise (95% CI: −5 Hz, 31 Hz), and calls, with a predicted increase of 11.4 Hz dB−1 of noise (95% CI: −7 Hz, 28 Hz; figure 1 and electronic supplementary material, table S1). Overall, this represents a proportional increase in song frequency of 0.74 dB−1 of noise, resulting in a 14.15 per cent (348 Hz) increase over the range of data. For calls, this increase was calculated at 0.53% dB−1 of noise, or a total increase in frequency of 9.7 per cent (288 Hz). On average, the frequency range of songs was 225 Hz narrower (95% CI: −673, 215) in urban areas than in rural areas. However, noise did not seem to have a consistent effect on the frequency range of song, and the frequency range of calls did not change with noise.
Figure 1.
Minimum frequency of silvereye (a) song and (b) calls versus the noise level at a site. Solid lines, predicted relationship; dashed lines, 95% CI. The dots are the mean of the posterior distribution of song frequency at each site, and the error bars are s.d. of the posterior distribution (equivalent to the s.e.).
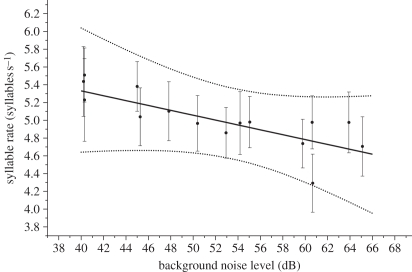
Neither urban habitat nor noise had an important effect on song duration. Silvereyes sang at a slower syllable rate in urban than in rural habitats (mean −0.476 syllables s−1; 95% CI: −1.36, 0.432). Syllable rate also decreased with an increase in noise level (mean −0.03 syllables s−1 dB−1; 95% CI: −0.089, 0.031), with a predicted reduction of 0.75 syllables s−1 between the quietest and the noisiest sites (figure 2 and electronic supplementary material, table S2).
Figure 2.
The effect of background noise on syllable rate (syllables s−1) in silvereye song. The dots are the mean of the posterior distribution of song frequency at each site, and the error bars are s.d. of the posterior distribution (equivalent to the s.e.). Solid lines, predicted relationship; dashed lines, 95% CI.
4. Discussion
(a). Song frequencies
As expected, both noise and urbanized habitats predicted an upward shift of lowest frequencies in song. Urban noise is generated predominantly by traffic (ground and air) at around 1–4 kHz. Silvereyes, therefore, may be singing at higher frequencies to avoid the masking effects of this urban noise. The effect observed translated to an approximate increase of 195 Hz in city environments, or a 13.4 Hz rise in frequency per decibel of background noise, giving a total increase of approximately 350 Hz between the quietest and the noisiest sites. This is a similar effect size to that observed in previous studies [15,17]. Importantly, our analyses did not show a notable effect of geographical location (‘pair effect’) on song. Thus, the effects we observed are less likely to be due to dialect formation in different geographical locations and more likely to represent consistent adaptation among unrelated populations. Additionally, we did not find a difference in body mass between urban and rural birds (mean −0.197; 95% CI: −0.712, 0.377). It is therefore unlikely that differences in song are the result of changes in body condition caused by the stressful urban environment (e.g. [25]).
Nemeth & Brumm [32] found that the increase in song frequency observed in city-dwelling birds leads to a relatively small increase in the distance over which a song can be detected in urban noise. Using data on great tits and blackbirds, they predicted increases of around 10 and 20 per cent in linear and areal communication distances, respectively, with a 200 Hz shift in song frequency. This is substantially smaller than the 50 per cent increase in linear communication distance obtained by increasing the amplitude of the signal by 5.2 dB (as seen in urban nightingales; [13]). Consequently, Nemeth & Brumm [32] argue that frequency shifts in urban noise may not be adaptive. However, if frequency shifts occur in response to a selection pressure (low-frequency urban noise), and the resulting increase in communication distance leads to an increase in reproductive success, then these shifts would be adaptive. There are currently no published data on the breeding success of birds with high- and low-frequency songs in noisy urban environments, so this question remains open.
Our study included some rural areas that, while less noisy than their geographically matched urban counterparts, were nevertheless noisier than some urban areas in other geographical locations. For instance, windy or beachside rural habitats (e.g. Coorong National Park) had higher levels of background noise than some city habitats (e.g. Australian National Botanic Gardens). The acoustic adaptation hypothesis predicts that noise (both biotic and abiotic, natural or anthropogenic) can act as a strong selection pressure on birdsong, and indeed many species have been shown to alter their songs in specific types of natural habitats (Reviewed in [2]). However, noise in natural environments covers a wide frequency range [33] and therefore may not exert a strong directional selection pressure for higher frequencies across multiple geographical locations in the same way as urban noise, which is consistent between cities, and is almost exclusively concentrated in the lower frequency bands.
(b). Call frequencies
It is often hypothesized that songbirds change the frequencies of their song in urban environments because they have the ability to plastically modify their song; for instance, by learning a new song or changing their repertoires according to habitat [15,34]. However, frequency shifts in urban environments have also been observed in taxa that do not learn their vocalizations, such as non-passerine birds [23], and the southern brown tree frog (Litoria ewingii; [21]). We found that not only songs, but also contact calls were higher in urban environments. Calls are not subject to a learning process like song, but are rather developed innately from begging vocalizations [35].
The changes observed in contact calls in urban environments suggest that vocalizations may be changing from an early age. Tree swallows (Tachycineta bicolor) subject to urban noise levels as nestlings raise the frequency of their begging calls [36]. If urban nestling silvereyes are adjusting their calls in a similar way, this may affect the development of their contact calls as adults. Another possibility is that there is some genetic adaptation for either higher frequency calls, or for higher call plasticity in urban birds. Calls are predominantly innate, although there is evidence that plasticity is advantageous in many songbird species [22]. The extent to which calls might be able to change throughout an individual's lifetime is poorly understood and depends heavily on species and context. Individuals in several species are able to converge their calls to a common structure when in a social group, but this process does not affect all types of calls [22]. Our findings lend support to the idea that urban habitats directly influence the evolution of vocalizations by selecting for genetic changes in vocal plasticity or ability.
(c). Syllable rate
While many studies have focused on frequency changes in song in urban habitats, relatively few have investigated the impact of the physical properties of cities on song structure [4]. We found that syllable rate decreased in urban or noisy habitats. Slabbekoorn & den Boer-Visser [15] previously found that great tits sang shorter, faster songs in cities, suggesting that urban habitats might be considered more ‘open’ than forested habitats. We also found that song duration was unaffected by habitat, similar to a study on dark-eyed juncos (Junco hyemalis; [37]). However, urban silvereyes sang fewer syllables s−1 and fewer syllables per song. This is consistent with Nemeth & Brumm's [17] finding that blackbirds sang fewer notes per song in cities. One hypothesis is that buildings and urban areas may act like canyons, degrading intricate syllables through reverberation, and blending separate song elements [4]. By increasing the temporal separation of syllables, silvereyes may be able to communicate these syllables more effectively. However, not all sites used in our study were surrounded by tall buildings, so this may not provide a complete explanation.
It seems reasonable to expect that under urban conditions, information contained in faster or more complex songs may be lost because the syllables are not only degraded by the physical properties of the environment, but may also be masked by high levels of noise. Song and syllable complexity are important indicators of male attractiveness and/or quality in many birds [2]. High syllable rates may be energetically or physiologically costly to maintain (e.g. [38]), and thus individuals might reduce the cost and increase the effectiveness of their vocalizations by slowing songs down and in the process, communicate song complexity more effectively.
Unfortunately, we do not yet know the implications of such findings for the attractiveness of male birds to potential mates, or for breeding success in noisy urban habitats. The aspects of silvereye song that are important to conspecifics (e.g. frequency range and syllable rate) are not yet known, making it difficult to predict whether the changes observed would affect female preference or other interactions. Since frequency range may be limited by physical or physiological constraints, a wider bandwidth in song may indicate a higher quality individual [2]. This may also be the case for birds that sing at a higher syllable rate, and indeed time intervals between song elements are important recognition cues for indigo buntings Passerina cyanea [39,40]. Additionally, many species are sensitive to frequency changes in conspecifics, such as male field sparrows, Spizella pusilla [41].
A study on great tits has shown that conspecifics from urban or rural environments may prefer individuals that sing familiar songs [34]. This could mean that urban and rural populations are diverging as a result of changes in vocalizations. Future studies will focus on the effectiveness of urban and rural songs in environments where noise and reverberation are present, using controlled experiments. In this way, we might test if the correlations observed in this field study between urban noise and the frequency of vocalizations are indeed causal.
We conclude that silvereyes in Australia are adjusting their vocalizations—both their learned songs and their innate calls—to be heard more effectively in noisy urban environments. To do this, they are both raising frequencies and singing slower songs. Both of these changes may have wider implications for mate choice and sexual selection in urban populations of this species.
Acknowledgements
All capturing and handling procedures were undertaken with the approval of the following agencies: Animal Ethics Committee at the University of Melbourne, Director-General's Animal Care and Ethics Committee at the NSW Department of Primary Industries, and Wildlife Ethics Committee at the SA Department for Environment and Heritage.
We thank Jeremy Kruckel for field assistance, Graham Fry, Alan Leishman, Greg Clancy, Andrew Tennant, David Paton, David Williams, Eric Woehler, Richard Fuller and Alan Fletcher for assistance in locating and banding, the Australian National Botanic Gardens (ACT), Namadgi National Park (ACT), Glenorchy City Council (Tas), Brisbane City Council (Qld), Susan Island Trust (NSW), Munghorn Gap Nature Reserve (NSW), Camden Airport (NSW), Kogarah City Council (NSW), Darebin City Council (Vic) and Darebin Parklands Association (Vic) for permission to conduct work on their lands. We also thank Michael McCarthy for help with data analysis. This research was funded by a Holsworth Wildlife Research Endowment, and by Birds Tasmania.
References
- 1.Morton E. S. 1975. Ecological sources of selection on avian sounds. Am. Nat. 109, 17–34 10.1086/282971 (doi:10.1086/282971) [DOI] [Google Scholar]
- 2.Catchpole C. K., Slater P. J. B. 2008. Bird song: biological themes and variations, 2nd edn. Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.Wiley R. H. 1991. Associations of song properties with habitats for territorial oscine birds of eastern North America. Am. Nat. 138, 973–993 10.1086/285263 (doi:10.1086/285263) [DOI] [Google Scholar]
- 4.Warren P. S., Katti M., Ermann M., Brazel A. 2006. Urban bioacoustics: its not just noise. Anim. Behav. 71, 491–502 10.1016/j.anbehav.2005.07.014 (doi:10.1016/j.anbehav.2005.07.014) [DOI] [Google Scholar]
- 5.Wiley R. H., Richards D. G. 1982. Adaptations for acoustic communication in birds: sound transmission and signal detection. In Acoustic communication in birds (eds Kroodsma D. E., Miller E. H.), pp. 131–181 New York, NY: Academic Press [Google Scholar]
- 6.Richards D. G., Wiley R. H. 1980. Reverberations and amplitude fluctuations in the propogation of sound in a forest: implications for animal communication. Am. Nat. 115, 381–399 10.1086/283568 (doi:10.1086/283568) [DOI] [Google Scholar]
- 7.Slabbekoorn H., Ripmeester E. A. P. 2008. Birdsong and anthropogenic noise: implications and applications for conservation. Mol. Ecol. 17, 72–83 10.1111/j.1365-294X.2007.03487.x (doi:10.1111/j.1365-294X.2007.03487.x) [DOI] [PubMed] [Google Scholar]
- 8.Francis C. D., Ortega C. P., Cruz A. 2009. Noise pollution changes avian communities and species interactions. Curr. Biol. 19, 1415–1419 10.1016/j.cub.2009.06.052 (doi:10.1016/j.cub.2009.06.052) [DOI] [PubMed] [Google Scholar]
- 9.Parris K. M., Schneider A. 2009. Impacts of traffic noise and traffic volume on birds of roadside habitats. Ecol. Soc. 14, 29 [Google Scholar]
- 10.Peris S. J., Pescador M. 2004. Effects of traffic noise on passerine populations in Mediterranean wooded pastures. Appl. Acoust. 65, 357–366 10.1016/j.apacoust.2003.10.005 (doi:10.1016/j.apacoust.2003.10.005) [DOI] [Google Scholar]
- 11.Reijnen R., Foppen R., Braak C. T., Thissen J. 1995. The effects of car traffic on breeding bird populations in woodland. III. Reduction of density in relation to the proximity of main roads. J. Appl. Ecol. 32, 187–202 10.2307/2404428 (doi:10.2307/2404428) [DOI] [Google Scholar]
- 12.Fuller R. A., Warren P. H., Gaston K. J. 2007. Daytime noise predicts nocturnal singing in urban robins. Biol. Lett. 3, 368–370 10.1098/rsbl.2007.0134 (doi:10.1098/rsbl.2007.0134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brumm H. 2004. The impact of environmental noise on song amplitude in a territorial bird. J. Anim. Ecol. 73, 434–440 10.1111/j.0021-8790.2004.00814.x (doi:10.1111/j.0021-8790.2004.00814.x) [DOI] [Google Scholar]
- 14.Cynx J., Lewis R., Tavel B., Tse H. 1998. Amplitude regulation of vocalizations in noise by a songbird, Taeniopygia guttata. Anim. Behav. 56, 107–113 10.1006/anbe.1998.0746 (doi:10.1006/anbe.1998.0746) [DOI] [PubMed] [Google Scholar]
- 15.Slabbekoorn H., den Boer-Visser A. 2006. Cities change the songs of birds. Curr. Biol. 16, 2326–2331 10.1016/j.cub.2006.10.008 (doi:10.1016/j.cub.2006.10.008) [DOI] [PubMed] [Google Scholar]
- 16.Hunter M. L., Krebs J. R. 1979. Geographical variation in the song of the great tit (Parus major) in relation to ecological factors. J. Anim. Ecol. 48, 759–785 10.2307/4194 (doi:10.2307/4194) [DOI] [Google Scholar]
- 17.Nemeth E., Brumm H. 2009. Blackbirds sing higher-pitched songs in citie: adaptation to habitat acoustics or side-effect of urbanization? Anim. Behav. 78, 637–641 10.1016/j.anbehav.2009.06.016 (doi:10.1016/j.anbehav.2009.06.016) [DOI] [Google Scholar]
- 18.Barrett L. 2009. Songs and the city. Anim. Behav. 78, 1279–1280 10.1016/j.anbehav.2009.10.019 (doi:10.1016/j.anbehav.2009.10.019) [DOI] [Google Scholar]
- 19.Slabbekoorn H., Peet M. 2003. Birds sing at a higher pitch in urban noise. Nature 424, 267. 10.1038/424267a (doi:10.1038/424267a) [DOI] [PubMed] [Google Scholar]
- 20.Halfwerk W., Slabbekoorn H. 2009. A behavioural mechanism explaining noise-dependent frequency use in urban birdsong. Anim. Behav. 78, 1301–1307 10.1016/j.anbehav.2009.09.015 (doi:10.1016/j.anbehav.2009.09.015) [DOI] [Google Scholar]
- 21.Parris K. M., Velik-Lord M., North J. M. A. 2009. Frogs call at a higher pitch in traffic noise. Ecol. Soc. 14, 25 [Google Scholar]
- 22.Marler P. 2004. Bird calls: their potential for behavioral neurobiology. Ann. N.Y. Acad. Sci. 1016, 31–44 10.1196/annals.1298.034 (doi:10.1196/annals.1298.034) [DOI] [PubMed] [Google Scholar]
- 23.Hu Y., Cardoso G. C. 2010. Which birds adjust the frequency of vocalizations in urban noise? Anim. Behav. 79, 863–867 10.1016/j.anbehav.2009.12.036 (doi:10.1016/j.anbehav.2009.12.036) [DOI] [Google Scholar]
- 24.Gil D., Gahr M. 2002. The honesty of bird song: multiple constraints for multiple traits. Trends Ecol. Evol. 17, 133–141 10.1016/S0169-5347(02)02410-2 (doi:10.1016/S0169-5347(02)02410-2) [DOI] [Google Scholar]
- 25.Barnett C. A., Briskie J. V. 2007. Energetic state and the performance of dawn chorus in silvereyes (Zosterops lateralis). Behav. Ecol. Sociobiol. 61, 579–587 10.1007/s00265-006-0286-x (doi:10.1007/s00265-006-0286-x) [DOI] [Google Scholar]
- 26.Robertson B. C. 1996. Vocal mate recognition in a monogamous, flock-forming bird, the silvereye, Zosterops lateralis. Anim. Behav. 51, 303–311 10.1006/anbe.1996.0030 (doi:10.1006/anbe.1996.0030) [DOI] [Google Scholar]
- 27.Skiba R. 2000. Possible ‘rain call’ selection in the chaffinch (Fringilla coelebs) by noise intensity—an investigation of a hypothesis. J. Ornithol. 141, 160–167 10.1046/j.1439-0361.2000.00071.x (doi:10.1046/j.1439-0361.2000.00071.x) [DOI] [Google Scholar]
- 28.Stewart K. A., MacDougall-Shackleton E. A. 2008. Local song elements indicate local genotypes and predict physiological condition in song sparrows Melospiza melodia. Biol. Lett. 4, 240–242 10.1098/rsbl.2008.0010 (doi:10.1098/rsbl.2008.0010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark J. S. 2005. Why environmental scientists are becoming Bayesians. Ecol. Lett. 8, 2–15 10.1111/j.1461-0248.2004.00702.x (doi:10.1111/j.1461-0248.2004.00702.x) [DOI] [Google Scholar]
- 30.McCarthy M. A. 2007. Bayesian methods for ecology. Cambridge, UK: Cambridge University Press [Google Scholar]
- 31.Spiegelhalter D., Thomas A., Best N., Lunn D. 2006. OpenBUGS user manual version 2.20. Cambridge, UK: MRC Biostatistics Unit [Google Scholar]
- 32.Nemeth E., Brumm H. 2010. Birds and anthropogenic noise: are urban songs adaptive? Am Nat. 176, 465–475 10.1086/656275 (doi:10.1086/656275) [DOI] [PubMed] [Google Scholar]
- 33.Bucur V. 2006. Urban forest acoustics. Berlin, Germany: Springer [Google Scholar]
- 34.Mockford E. J., Marshall R. C. 2009. Effects of urban noise on song and response behaviour in great tits. Proc. R. Soc. B 276, 2979–2985 10.1098/rspb.2009.0586 (doi:10.1098/rspb.2009.0586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jurisevic M. A. 1999. Structural change of begging vocalisations and vocal repertoires in two hand-raised Australian passerines, the little raven Corvus mellori and white-winged chough Corcorax melanorhamphos. Emu 99, 1–8 10.1071/MU99001 (doi:10.1071/MU99001) [DOI] [Google Scholar]
- 36.Leonard M. L., Horn A. G. 2008. Does ambient noise affect growth and begging call structure in nestling birds? Behav. Ecol. 19, 502–507 10.1093/beheco/arm161 (doi:10.1093/beheco/arm161) [DOI] [Google Scholar]
- 37.Slabbekoorn H., Yeh P., Hunt K. 2007. Sound transmission and song divergence: a comparison of urban and forest acoustics. Condor 109, 67–78 10.1650/0010-5422(2007)109[67:STASDA]2.0.CO;2 (doi:10.1650/0010-5422(2007)109[67:STASDA]2.0.CO;2) [DOI] [Google Scholar]
- 38.Oberweger K., Goller F. 2001. The metabolic cost of birdsong production. J. Exp. Biol. 204, 3379–3385 [DOI] [PubMed] [Google Scholar]
- 39.Ballentine B., Hyman J., Nowicki S. 2004. Vocal performance influences female response to male bird song: an experimental test. Behav. Ecol. 15, 163–168 10.1093/beheco/arg090 (doi:10.1093/beheco/arg090) [DOI] [Google Scholar]
- 40.Emlen S. T. 1972. An experimental analysis of the parameters of bird song eliciting species recognition. Behaviour 41, 130–171 10.1163/156853972X00248 (doi:10.1163/156853972X00248) [DOI] [Google Scholar]
- 41.Nelson D. A. 1998. External validity and experimental design: the sensitive phase for song learning. Anim. Behav. 56, 487–491 10.1006/anbe.1998.0805 (doi:10.1006/anbe.1998.0805) [DOI] [PubMed] [Google Scholar]