Abstract
Determinants of contemporary patterns of diversity, particularly those spanning extensive latitudinal gradients, are some of the most intensely debated issues in ecology. Recently, focus has shifted from a contemporary environmental perspective to a historical one in an attempt to better understand the construction of latitudinal gradients. Although the vast majority of research on historical mechanisms has focused on tropical niche conservatism (TNC), other historical scenarios could produce similar latitudinal gradients. Herein, I formalize predictions to distinguish between two such historical processes—namely time for speciation (TFS) and TNC—and test relative support based on diversity gradients of New World bats. TFS and TNC are distinctly spatial and environmental mechanisms, respectively. Nonetheless, because of the way that environmental characteristics vary spatially, these two mechanisms are hard to distinguish. Evidence provided herein suggests that TNC has had a more important effect than TFS in determining diversity gradients of New World bats. Indeed, relative effects of different historical mechanisms, as well as relative effects of historical and contemporary environmental determinants, are probably context-dependent. Future research should move away from attempting to identify the mechanism with primacy and instead attempt to understand the particular contexts in which different mechanisms have greater influence on diversity gradients.
Keywords: bats, biodiversity, historical process, latitudinal diversity gradient, time for speciation, tropical niche conservatism
1. Introduction
The holy grail of ecology is a comprehensive understanding of the mechanistic basis of diversity gradients that are, with few exceptions, ubiquitous characteristics of the biota through both space and time [1,2]. Classical theory has relied primarily on contemporary environmental determinants to explain diversity gradients [2]. Nonetheless, recently, historical processes such as differential rates of speciation and extinction predicted under metabolic theory [3], variation in the amount of time for speciation (TFS) [4] and niche conservatism [5] have come to the limelight and have provided alternative directions that have rapidly expedited the understanding of the formation of diversity gradients.
Tropical niche conservatism (TNC) has gained perhaps the most popular focus regarding historical explanations of latitudinal gradients of diversity. This hypothesis describes how counteracting interactions between phylogenetic niche conservatism and niche evolution create gradients of diversity [5]. Niche evolution allows taxa to enter new environmental regimes but phylogenetic niche conservatism constrains diversification because many aspects of the niche are inherited from their ancestor. When effects of niche conservatism are greater than effects of niche evolution, the geographical expansion of the entire clade through cladogenesis is slowed, and species differentially accumulate towards the centre of origin and not the geographical periphery. A common test of TNC is to examine significant correlations between environmental and phylogenetic characteristics such as average taxon age or degree of derivedness of taxa in an assemblage with latitude [6–13], with a few making these associations directly to environmental conditions [6,11,14,15].
What makes TNC so attractive is the explicit, testable predictions that can be generated from the theory, in particular those focusing on how higher climatic variability in higher latitude environments drives niche evolution. For taxa of tropical origin, climatic variability that increases with latitude probably represents an important selective regime limiting distribution and abundance [5]. Variability is particularly pronounced for temperature, which demonstrates a monotonic increase with latitude across the globe [16]. Moreover, winter freezing temperatures, while dependent on other environmental variables (especially elevation), generally begin around the edge of the tropics (approx. 23.5° latitude). Cold temperatures and freezing tolerance are major selective agents for numerous plant [17] and animal [18] taxa, and can determine species composition and diversity of communities [19]. Thus, TNC makes three hierarchical predictions that can be used to infer its influence on diversity gradients: (i) climatic gradients drive diversification and are thus related to phylogenetic gradients of the age of taxa and amount of evolutionary novelty; (ii) temperature is more related to gradients of phylogenetic characteristics than precipitation or productivity; and (iii) variability of temperatures is more related to phylogenetic characteristics than magnitude of temperature.
Recently, it has been suggested that ecologists in particular may have too frequently attributed phylogenetic signal to phylogenetic niche conservatism and that in general these effects need to be differentiated [20]. From an ecological context, phylogenetic signal represents correlation between phylogenetic and ecological similarity. In contrast, the mark of phylogenetic niche conservatism is when species are more ecologically similar than expected based on their phylogenetic relationships [20]. This extends logically to an analogue in terms of latitudinal gradients of diversity. Specifically, phylogenetic signal to patterns of species composition that vary latitudinally should be the null expectation and not the expectation of TNC. TFS [4] should lead to a gradient in phylogenetic signal in the absence of niche conservatism. Although described explicitly by Hennig's progression rule long ago [21], such a null expectation has been overlooked in the recent diversity gradient literature. Given sufficient time, diversification from a particular place of origin should result in (i) a pattern of phylogenetic signal and (ii) a species richness gradient. To this end, if most larger taxa (e.g. orders or families) are of tropical origin, then a simple Brownian motion of diversification should result in the development of a diversity gradient through time, and demonstration of geographical gradients in phylogenetic characteristics such as age or derivedness are not sufficient to support a hypothesis that TNC drives diversity gradients.
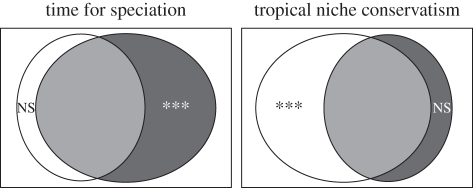
Because both competing hypotheses (TNC, TFS) generate latitudinal gradients in both species richness and phylogenetic characteristics of taxa of tropical origin, distinguishing between mechanisms is not possible based on the examination of latitudinal effects alone. Nonetheless, two mutually exclusive quantitative predictions provide an elegant means of distinguishing TNC from that expected under cladogenesis alone. TNC is an explicit environmental mechanism, whereas TFS is an explicit spatial mechanism. Distinguishing between these two mechanisms is complicated by the fact that environmental characteristics such as temperature, precipitation and productivity form gradients and are thus to some degree inherently spatial. Nonetheless, variation in environmental characteristics is not purely spatial and the mismatch between environmental and spatial gradients can be used to determine the relative likelihood that TNC and TFS contribute to contemporary gradients in biodiversity. If TFS is in operation and the primary driver of diversity gradients, then the diversification process should be diffuse and its signature should be primarily spatial. Alternatively, if the TNC hypothesis is in operation and the primary driver of diversity gradients, then the signature of diversification should be primarily environmental. Operationally, because environmental characteristics tend to form gradients, variation in phylogenetic characteristics accounted for by variation correlated between spatial and environmental descriptors should be substantive. Nonetheless, as the correlation between environmental and spatial characteristics is typically only moderate, variation remains that may be accounted for separately and independently by spatial or environmental characteristics. This variation accounted for uniquely by spatial characteristics or uniquely by environmental characteristics represents the tool to distinguish between these two competing hypotheses (figure 1).
Figure 1.
Conceptual distinction between two competing historical explanations of diversity gradients based on variation accounted for by environmental and spatial variables. In both cases, the rectangle represents the total amount of variation in phylogenetic characteristics of taxa. White circles represent variation accounted for by environmental characteristics, whereas black circles represent variation accounted for by spatial characteristics. Portion of white circle not overlapping with black circle represents unique environmental variation not related to spatial characteristics. Portion of black circle not overlapping with white circle represents unique spatial variation not related to the environment. Area of overlap represents shared variation explained by both suites of variables. The TFS hypothesis is a purely spatial mechanism and proceeds by a diffusive process. Thus, there should only be spatial signature to diversification. Nonetheless, because environmental characteristics inherently form gradients, variation in species richness and phylogenetic characteristics will be related to the environment to some degree and both environmental characteristics and spatial characteristics account for variation. Because this is a purely spatial process, spatial characteristics will account for more variation than environmental characteristics. Moreover, variation accounted for by environment will be only that correlated with spatial characteristics. Spatial characteristics will uniquely account for significant variation, whereas environmental characteristics will not. In contrast, the TNC hypothesis is a purely environmental mechanism that proceeds by evolution of species ecological niches. Thus, variation in phylogenetic characteristics should be most related to environmental characteristics. Nonetheless, again because environmental characteristics inherently form gradients, variation in phylogenetic characteristics will be related to spatial characteristics to some degree. Because this is a purely environmental process, environmental characteristics will account for more variation than spatial characteristics. Moreover, that variation accounted for by spatial characteristics will be only that correlated with environment. Environmental characteristics will uniquely account for significant variation in phylogenetic characteristics, whereas spatial characteristics will not.
If TFS creates gradients in species richness and phylogenetic characteristics, then the only variation accounted for by environmental characteristics will be that shared with spatial characteristics. Pure environmental variation will be non-significant. Variation accounted for by spatial gradients unrelated to environmental characteristics will be highly significant. In contrast, if TNC creates gradients in species richness and phylogenetic characteristics, then the only variation accounted for by spatial characteristics will be that shared with environmental characteristics. Pure spatial variation will be non-significant. Variation accounted for by environmental gradients unrelated to spatial characteristics will be highly significant.
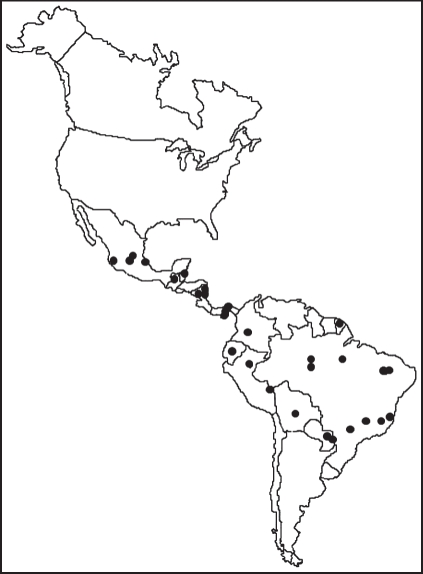
Bats represent an ideal group of organisms with which to distinguish effects of TFS and TNC. In the New World, bats are exceedingly ecologically [22] and phylogenetically [23] diverse and species-rich. In particular, the family of New World leaf-nosed bats (Phyllostomidae) makes an ideal monophyletic taxon from which to contrast these two hypotheses. The clade has had a dynamic distribution in the New World for approximately the last 35 Myr [24–26]. Moreover, there is considerable phylogenetic signal to the geographical distribution of taxa [10], allowing for powerful historical reconstruction of the diversification of this clade. The oldest known fossils of this family are from the Miocene of Colombia [27], suggesting a tropical origin to the family. This group comprises 55 genera and 160 species [28] that exhibit strong latitudinal diversity gradients [29–32], which determines much of the gradient for all bats in the New World (figure 2).
Figure 2.
Locations of 29 New World phyllostomid assemblages used to test historical biogeographic models.
2. Materials and methods
(a). Estimates of phylogenetic diversity of assemblages
Species composition and phylogenetic characteristics of 29 phyllostomid assemblages distributed throughout the Neotropics comes from Stevens [10]. One site (Zabelitas, Colombia) was omitted because of anomalous corresponding climate data. Values of average and standard deviation of relative ages of taxa and average and standard deviation of the degree of derivedness of taxa within each assemblage came from the same source. Relative age of a taxon was estimated using the terminal branch length representing the amount of sequence divergence between a taxon and its most recent putative ancestor [11,33,34]. Assemblages containing taxa with short average terminal branch lengths can be considered to be composed of species that are relatively younger than assemblages containing taxa with longer terminal branch lengths on average. Derivedness was estimated based on the amount of sequence divergence that has resulted in a taxon as estimated by the total branch length between a taxon and the root of the phyllostomid tree (i.e. root distance [6,8,9,15,35]). This estimates how much evolution has given rise to a particular taxon. Assemblages containing species with a long average root distance represent taxa that are more diverged from the common ancestor of Phyllostomidae than assemblages containing species that have small root distances on average. Predictions from both TNC and TFS are that average age should decrease, average derivedness should increase and the standard deviation of both characteristics should decrease with latitude. Tree was that presented in Baker et al. [36] and based on concatenated RAG2 nuclear gene and mitochondrial genes (12S rRNA, tRNAVAL and 16S RNA). Inferred phylogenetic relationships were based on a Bayesian analysis implemented in MrBayes 2.01 [37] using a general time-reversible model with allowances for a gamma distribution of rate variation and for the proportion of variant sites (GTR + Γ + I) based on Model-Test [38]. Much statistical support for this phylogeny existed across nodes. Eighty-two per cent of clades received posterior probabilities greater than 0.95 and 76 per cent of clades received posterior probabilities of 1 [36]. Scleronycteris ega was omitted from one assemblage and Lichonycteris obscura was omitted from four assemblages because these two monotypic genera were not represented in the cladogram.
(b). Environmental and spatial characteristics of assemblages
Temperature, precipitation and productivity are all important limiting factors of bat distribution and abundance [39–41]. Ten environmental variables estimating these characteristics for each locale were extracted from rasters provided by Hijmans et al. [42]. Variables to characterize temperature regime were (i) annual mean, (ii) mean monthly range, (iii) seasonality, (iv) maximum in the warmest month, (v) minimum in the coldest month and (vi) annual range. Variables to characterize precipitation regime were (i) total, (ii) total in wettest month, (iii) total in driest month and (iv) seasonality. Initially, I included a measure of annual primary productivity from Imhoff et al. [43]. This variable exhibited very high degrees of collinearity with precipitation and temperature, and I subsequently decided to restrict analyses to primary environmental gradients. To reduce dimensionality, principal component analysis was performed on temperature and precipitation datasets separately. I used the broken-stick stopping rule [44] to select informative principal components (PCs).
Spatial configuration of sites was based on latitudinal and longitudinal coordinates. Spatial relationships among assemblages were estimated based on principal coordinates of neighbourhood matrices [45] and all eigenvectors with positive eigenvalues (PCNMs) were retained for subsequent inferential analyses. Inclusion of PCNMs into regression analyses has the added benefit of providing information on the strength of environmental gradients while maintaining type 1 error rate at α = 0.05 given spatial autocorrelation [46]. Mathematical properties of PCNMs make interpretation fairly straightforward. PCNMs represent orthogonal waves of spatial variation whose wavelengths range across all scales of a particular data structure [47]. Variance corresponds to a scale at which spatial structure is defined by a particular PCNM (positive eigenvector) [48]. Moreover, as eigenvectors are ordered sequentially in terms of the magnitude of their variance, PCNMs range from those that reflect broad coarse spatial variation (first few PCNMs) to those that capture variation in very fine-scaled spatial structure (last few PCNMs).
(c). Inferential analyses
I performed a redundancy analysis [49], in which PCNMs were independent variables and environmental PCs were dependent variables, to evaluate how much variation in environmental characteristics was shared with spatial characteristics. This also provides information on how much environmental variation is non-spatial and hence the amount of potential variation that can be used to distinguish between TNC and TFS hypotheses. I then examined abilities of TNC (environmental determinants) and TFS (spatial determinants) separately to account for phylogenetic characteristics based on stepwise multiple regression performed in SPSS 9.0. Importance of environmental or spatial variables was judged based on (i) whether a particular variable entered the regression model, and then (ii) magnitude of the partial coefficient of determination (r2) for each independent variable in the model. To distinguish relative effects of TNC and TFS, I conducted another stepwise multiple regression analysis for each phylogenetic characteristic in which all variables (environmental PCs and PCNMs) were entered as independent variables. The same aforementioned criteria were used to interpret the relative importance of spatial and environmental variables, and hence the relative degree to which TNC or TFS was related to phylogenetic diversity gradients (figure 3).
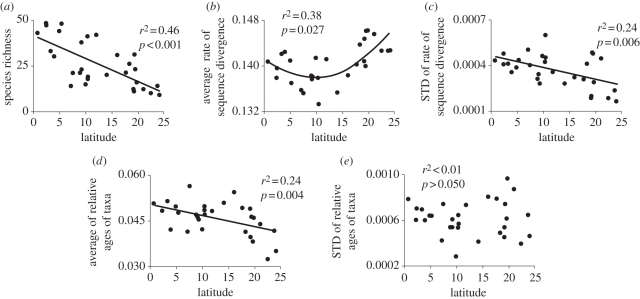
Figure 3.
Latitudinal gradients of diversity and phylogenetic characteristics of New World leaf-nosed bat assemblages (redrawn from Stevens [10]). STD, standard deviation.
3. Results
(a). Environmental characterization
The temperature dataset generated two informative PCs that accounted for 91 per cent of variation among sites. Variables reflecting temperature variability and seasonality were most related to the first PC, whereas variables reflecting magnitude were most related to the second PC (electronic supplementary material, appendix). The precipitation dataset generated two informative PCs that accounted for 96 per cent of the variation among sites. The first PC was most related to the amount of precipitation, whereas the second PC was most related to the relative variation in precipitation (electronic supplementary material, appendix). Substantive spatial autocorrelation was detected in climatic variables indicating correlated distribution of spatial and environmental variables. Redundancy analysis indicated that the suite of PCNMs accounted for approximately 45 per cent (p ≪ 0.001) of variation in climatic variation across the 29 bat assemblages. Nonetheless, 55 per cent of the environmental variation was non-spatial and represents substantive independent environmental signal from which to contrast effects of TNC and TFS.
(b). Evidence supporting tropical niche conservatism
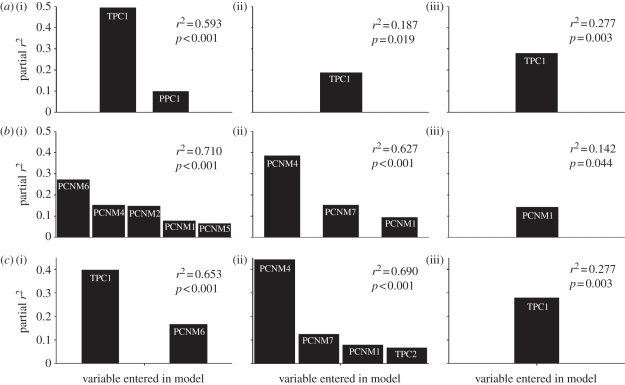
Environmental characteristics accounted for significant amounts of variation in all phylogenetic characteristics except the standard deviation of relative ages of taxa (figure 4). Aspects of temperature were important predictors in all significant regressions. Only for average amount of sequence divergence were both aspects of temperature and precipitation significant predictors. In this case, the first temperature PC (TPC1) was the variable that loaded first into the regression model and this variable accounted for much more unique variation in the average rate of sequence divergence than the first precipitation PC (PPC1). Variability of temperature (TPC1) was an important predictor in all significant regressions, whereas the magnitude of temperature (TPC2) never entered into climatic models. To this end, all predictions are upheld and provide support that TNC has at least contributed to gradients of phylogenetic characteristics in the Phyllostomidae. In particular, environmental characteristics are significant predictors of phylogenetic characteristics. Aspects of temperature are better predictors than aspects of precipitation. Finally, seasonality of temperature is a better predictor of phylogenetic characteristics than magnitude of temperature.
Figure 4.
Results of stepwise multiple regressions between phylogenetic characteristics (dependent variables) and environmental and spatial variables, analysed separately (a,b) and in combined analyses (c) to examine relative contributions of TNC and TFS mechanisms. No variables significantly accounted for variation in standard deviation of age in any analysis and these are not included in the figure. TPC1, first principal component based on temperature and representing variation in seasonality; TPC2, second principal component based on temperature and representing variation in magnitude; PCNM-1 to PCNM-7, first to seventh PCNM (principal coordinates of neighbour matrices) axes. (a,b,c)(i) Average rate, (ii) standard deviation of rate and (iii) average age for (a) tropical niche conservatism, (b) time for speciation and (c) competing mechanisms.
(c). Evidence supporting time for speciation
Seven positive eigenvectors were generated from PCNM analysis. Spatial characteristics accounted for significant amounts of variation in all phylogenetic characteristics except the standard deviation of relative ages of taxa (figure 4). Amount of variation accounted for by regression models ranged from 14 to 71 per cent.
(d). Relative contributions of tropical niche conservatism and time for speciation
Based on the inspection of individual multiple regression results, spatial variables tended to account for more variation in phylogenetic characteristics than did environmental variables. Nonetheless, variability of temperature was always the most important environmental variable, whereas which spatial variables were important in predicting the phylogenetic characteristics was more variable.
Abilities of spatial and environmental variables to predict aspects of phylogenetic characteristics were mixed (figure 4). Temperature variability accounted for significant variation over and beyond that shared with spatial variables for the average amount of sequence divergence and the average relative age of taxa. In areas of high seasonality of temperature, taxa were younger and more diverged than in more tropical areas with low temperature variability. Spatial variables were significant predictors in some cases as well but not as frequently as for climatic variables. Spatial variables improved the predictability of the average amount of sequence divergence and were the most important predictors of the standard deviation of sequence divergence. Results involving average amount of sequence divergence and average age of taxa provide support for TNC, whereas those involving standard deviation of sequence divergence provide support for TFS.
4. Discussion
Contemporary perspectives on the construction and maintenance of diversity gradients increasingly integrate effects of historical and contemporary processes [50]. Although examinations of relationships with contemporary environmental conditions fill the classical literature on diversity gradients, contemporary climate offers only partial explanation of contemporary patterns of diversity. Indeed, contemporary diversity had to result from diversification in the past and differences in diversity along gradients, at least in part, must be owing to differences in speciation or extinction either in absolute rate or in the amount of time in which such processes have been in operation [5].
Typically, investigators have relied on latitudinal variation in phylogenetic characteristics of assemblages to identify TNC [6–15]. Nonetheless, TNC may not be the only historical mechanism creating spatial gradients in phylogenetic characteristics, nor is it the most parsimonious. Because of the diffusive nature of the diversification process, even in the absence of environmental drivers, latitudinal gradients cannot distinguish between phylogenetic signal produced simply by TFS and that of TNC when attempting to determine the mechanistic basis of the diversity gradient of tropical taxa. Strong tests of TNC should require that environmental conditions account for more variation in phylogenetic characteristics than does spatial structure, which would be common to both TFS and TNC processes.
(a). Relative support for tropical niche conservatism and time for speciation
Contemporary diversity of New World leaf-nosed bats has a strong historical component, whereby the youngest and most derived taxa occur at the geographical extremes of the distribution of this taxon and change systematically with latitude [10]. Deeper examination demonstrated that both climatic variation consistent with TNC and spatial variation consistent with TFS account for significant amounts of variation in phylogenetic characteristics of taxa. Moreover, spatial variables consistently accounted for more variation than environmental variables in the three phylogenetic characteristics that exhibited gradients when analyses focused on those two suites of variables separately. Importance of spatial variables diminished in combined analyses, whereby fewer spatial variables accounted for significant unique variation in phylogenetic characteristics. Also, in only one regression did spatial variables load first and account for the most unique variation. The loss of spatial variables in combined analyses probably reflects the complex environmental gradients affecting phylogenetic characteristics. Even in the combined analysis there was no clear indication that one mechanism was fully supported while the other was fully rejected. Nonetheless, aspects of temperature, in particular its seasonality, appear to indicate that it is the single most important variable associated with phylogenetic characteristics. Although this conclusion is not ideally strong, it does appear as if there is more support for TNC than TFS.
(b). Is the tropical niche conservatism hypothesis operational?
Indeed, historical processes are important contributors to diversity of Phyllostomidae, and probably many higher taxa of tropical origin. What is less clear is the degree to which TNC has contributed to these gradients. One major weakness of testing determinants of latitudinal gradients is that most mechanisms make exactly the same qualitative predictions, namely an increase in species richness towards the equator [51]. The strength of TNC is that it makes additional predictions regarding phylogenetic characteristics of taxa and how they should vary along latitudinal as well as environmental gradients. Here, I have conducted an even stronger test by requiring variation explained by environment to be greater than that produced by TFS. Data presented here suggest that TNC has been more influential than TFS, but tests competing both of these putative mechanisms are difficult and may not be operational in many circumstances. Variation in seasonality is strongly spatial, primarily because of the axial tilt of Earth, which determines the angle of solar radiation as well as seasonality in the amount received [52]. This variation increases with latitude. To this end, in many cases there may not be sufficient non-spatial (and, perhaps more importantly, non-latitudinal) variation in temperature to distinguish between environmental and spatial effects.
(c). Are historical mechanisms equally important across taxa?
Although effects of TNC on diversity gradients are becoming better appreciated and are probably common in many taxa, the degree of importance is probably idiosyncratic [53], for at least three reasons. First, clade age may contribute to idiosyncrasy of the effects of TNC. Effects of history are contingent on the particular temporal range of a clade and this should affect not only the magnitude of the historical effect but its overall effect relative to other processes. For example, because of the diversity dependence of diversification rates [54], historical processes will be more important determinants of diversity patterns in younger than older clades because young clades are still at the accumulation phase of the clade diversity curve [55]. Thus, one form of historical idiosyncrasy will be proximity to the extinction–speciation equilibrium and how that determines the rate of diversification.
Second, there is spatial variation in the place of origin and diversification of clades, and different geographical positions translate into different environmental regimes under which clades diversify. Thus, degree to which a taxon is ‘tropical’ should determine the degree of niche conservatism for constant tropical environments and also determine the degree to which seasonality affects distribution and abundance [53]. Thus, the reason why TNC appears to be at least contributing to diversity gradients in Phyllostomidae is that it is one of the youngest bat clades [25] that may have not yet met the speciation–extinction threshold and probably has an equatorial centre of origin. Nonetheless, Phyllostomidae is only one large clade of organisms in general and of mammals in particular. Other large clades such as rodents and dog-like carnivores exhibit quite different patterns of diversification [53]. Moreover, Phyllostomidae is only one of 18 families of bats [56]. Bat families are highly variable in terms of diversity, distribution and age. Such variation within larger clades of organisms could potentially provide many insights into understanding the particular context that enhances the effects of TNC.
Third, taxa probably exhibit clade-specific extinction and speciation dynamics. We still do not have a good understanding of the relative contributions of extinction and speciation to diversification rate. Speciation rates are more readily estimated than extinction rates [57]. Nonetheless, at least some of the decrease in species richness towards higher latitudes in some taxa may result from accelerated extinction, whether it be local or global. For example, climatic variability at higher latitudes could cause the outright extinction of some clades over the millennia. Nonetheless, another form of extinction that may be equally important but much harder to detect is the local extinction that could result from contraction of species ranges during times of climatic change [58]. Greater focus on how the process of extinction affects diversity gradients and variation among taxa regarding these rates will greatly enhance our understanding of historical processes.
(d). Multiple determinants of latitudinal gradients
Traditionally, ecologists and biogeographers alike have interpreted latitudinal gradients in diversity in light of responses to contemporary environmental gradients. Nonetheless, a body of recent research is developing that suggests historical processes such as those addressed here may be as important as (or more important than) contemporary climate [6,8,59] in determining diversity gradients. Climate may be important, but it may be environmental effects through millennia that form the majority of the climatic effect and not as much those transpiring over the most recent time periods. Climate and history probably interact. Historical idiosyncrasy of diversification of particular clades combined with other non-environmental effects (such as geometric constraints on diversification) suggest that explanations based on single processes may be of limited utility in attempting to understand the mechanistic basis to latitudinal gradients [51]. Ecologists have become frustrated by a lack of ability to identify ‘all-encompassing theories [that] make the contingencies go away’ (p. 453 in [60]). Nonetheless, because of contingency it is likely that there is no all-encompassing mechanism, but a complex mix of primary and secondary mechanisms that shift in relative importance depending on historical contingency, geographical position, environmental regime or intrinsic characteristics of the clade of interest (i.e. rate of speciation, generation time, life-history characteristics). Consensus may be closer than we think, but it may be more useful to think in terms of consensus on the group of most important mechanisms and the circumstances defining their relative importance as opposed to consensus on the most important mechanism.
(e). Final consideration
A last consideration is dynamics. Results presented here point out the importance of temperature on diversity gradients, which is common across many taxa [61]. Temperature regimes have been variable across the millennia and are perhaps the environmental variable most frequently predicted to change given future climate models. Indeed, change in temperature regimes across contemporary times is noticeably translated into changes in the geographical distribution of many taxa [58,62–65]. Accordingly, diversity in many groups and the resultant diversity gradients corresponding to their diversification appear sensitive to global climate change in the present. To this end, understanding relative effects of temperature on diversity gradients and dynamics is of both basic and applied significance if we are to understand the construction of diversity gradients and their dynamics in a changing world.
Acknowledgements
I was funded by the NSF-USA (DEB-1020890) and Louisiana Board of Regents (LEQSF-2006-09, NSF(2009)-PFUND-139) while producing this manuscript. J. Tello provided climatic data from online raster files.
References
- 1.Hillebrand H. 2004. On the generality of the latitudinal diversity gradient. Am. Nat. 163, 192–211 10.1086/381004 (doi:10.1086/381004) [DOI] [PubMed] [Google Scholar]
- 2.Willig M. R., Kaufman D. M., Stevens R. D. 2003. Latitudinal gradients in biodiversity: pattern, process, scale, and synthesis. Ann. Rev. Ecol. Evol. Syst. 34, 273–309 10.1146/annurev.ecolsys.34.012103.144032 (doi:10.1146/annurev.ecolsys.34.012103.144032) [DOI] [Google Scholar]
- 3.Allen A. P., Gillooly J. F. 2006. Assessing latitudinal gradients in speciation rates and biodiversity at the global scale. Ecol. Lett. 9, 947–954 10.1111/j.1461-0248.2006.00946.x (doi:10.1111/j.1461-0248.2006.00946.x) [DOI] [PubMed] [Google Scholar]
- 4.Stephens P. R., Wiens J. J. 2003. Explaining species richness from continents to communities: the time-for-speciation effect in emydid turtles. Am. Nat. 161, 112–128 10.1086/345091 (doi:10.1086/345091) [DOI] [PubMed] [Google Scholar]
- 5.Wiens J. J., Donoghue M. J. 2004. Historical biogeography, ecology and species richness. Trend. Ecol. Evol. 19, 639–644 [DOI] [PubMed] [Google Scholar]
- 6.Algar A. C., Kerr J. T., Currie D. J. 2009. Evolutionary constraints on regional faunas: whom, but not how many. Ecol. Lett. 12, 57–65 10.1111/j.1461-0248.2008.01260.x (doi:10.1111/j.1461-0248.2008.01260.x) [DOI] [PubMed] [Google Scholar]
- 7.Diniz J. A. F., Rangel T. F. L. V. B., Bini L. M., Hawkins B. A. 2007. Macroevolutionary dynamics in environmental space and the latitudinal diversity gradient in New World birds. Proc. R. Soc. B 274, 43–52 10.1098/rspb.2006.3712 (doi:10.1098/rspb.2006.3712) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawkins B. A., Diniz J. A. F., Jaramillo C. A., Soeller S. A. 2007. Climate, niche conservatism and the global bird diversity gradient. Am. Nat. 170, S16–S27 10.1086/519009 (doi:10.1086/519009) [DOI] [PubMed] [Google Scholar]
- 9.Hawkins B. A., Diniz J. A. F., Jaramillo C. A., Soeller S. A. 2006. Post-eocene climate change, niche conservatism, and the latitudinal gradient of New World birds. J. Biogeogr. 33, 770–778 10.1111/j.1365-2699.2006.01452.x (doi:10.1111/j.1365-2699.2006.01452.x) [DOI] [Google Scholar]
- 10.Stevens R. D. 2006. Historical processes enhance patterns of diversity along latitudinal gradients. Proc. R. Soc. B 273, 2283–2289 10.1098/rspb.2006.3596 (doi:10.1098/rspb.2006.3596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiens J. J., Sukumaran J., Pyron R. A., Brown R. M. 2009. Evolutionary and biogeographic origins of high tropical diversity in old world frogs (Ranidae). Evolution 63, 1217–1231 10.1111/j.1558-5646.2009.00610.x (doi:10.1111/j.1558-5646.2009.00610.x) [DOI] [PubMed] [Google Scholar]
- 12.Wiens J. J. 2007. Global patterns of diversification and species richness in amphibians. Am. Nat. 170, S86–S106 10.1086/519396 (doi:10.1086/519396) [DOI] [PubMed] [Google Scholar]
- 13.Wiens J. J., Graham C. H., Moen D. S., Smith S. A., Reeder T. W. 2006. Evolutionary and ecological causes of the latitudinal diversity gradient in hylid frogs: treefrog trees unearth the roots of high tropical diversity. Am. Nat. 168, 579–596 10.1086/507882 (doi:10.1086/507882) [DOI] [PubMed] [Google Scholar]
- 14.Hawkins B. A. 2010. Multiregional comparison of the ecological and phylogenetic structure of butterfly species richness gradients. J. Biogeogr. 37, 647–656 10.1111/j.1365-2699.2009.02250.x (doi:10.1111/j.1365-2699.2009.02250.x) [DOI] [Google Scholar]
- 15.Hawkins B. A., DeVries P. J. 2009. Tropical niche conservatism and the species richness gradient of North American butterflies. J. Biogeogr. 36, 1698–1711 10.1111/j.1365-2699.2009.02119.x (doi:10.1111/j.1365-2699.2009.02119.x) [DOI] [Google Scholar]
- 16.Vazquez D. P., Stevens R. D. 2004. The latitudinal gradient in niche breadth: concepts and evidence. Am. Nat. 164, E1–E19 10.1086/421445 (doi:10.1086/421445) [DOI] [PubMed] [Google Scholar]
- 17.Thomashow M. F. 1999. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Ann. Rev. Plant Phys. Plant Mol. Biol. 50, 571–599 10.1146/annurev.arplant.50.1.571 (doi:10.1146/annurev.arplant.50.1.571) [DOI] [PubMed] [Google Scholar]
- 18.Storey K. B., Storey J. M. 1988. Freeze tolerance in animals. Phys. Rev. 68, 27–84 [DOI] [PubMed] [Google Scholar]
- 19.Wittman S. E., Sanders N. J., Ellison A. M., Jules E. S., Ratchford J. S., Gotelli N. J. 2010. Species interactions and thermal constraints on ant community structure. Oikos 119, 551–559 10.1111/j.1600-0706.2009.17792.x (doi:10.1111/j.1600-0706.2009.17792.x) [DOI] [Google Scholar]
- 20.Losos J. B. 2008. Phylogenetic niche conservatism, phylogenetic signal and the relationships between phylogenetic relatedness and ecological similarity among species. Ecol. Lett. 11, 995–1003 10.1111/j.1461-0248.2008.01229.x (doi:10.1111/j.1461-0248.2008.01229.x) [DOI] [PubMed] [Google Scholar]
- 21.Hennig W. 1966. Phylogenetic systematics. Urbana, IL: University of Illinois Press [Google Scholar]
- 22.Stevens R. D., Cox S. B., Willig M. R., Strauss R. E. 2003. Patterns of functional diversity across an extensive environmental gradient: vertebrate consumers, hidden treatments, and latitudinal trends. Ecol. Lett. 6, 1099–1108 10.1046/j.1461-0248.2003.00541.x (doi:10.1046/j.1461-0248.2003.00541.x) [DOI] [Google Scholar]
- 23.Jones K. E., Purvis A., MacLarnon A., Bininda-Emonds O. R. P., Simmons N. B. 2002. A phylogenetic supertree of the bats (Mammalia: Chiroptera). Biol. Rev. 77, 223–259 10.1017/S1464793101005899 (doi:10.1017/S1464793101005899) [DOI] [PubMed] [Google Scholar]
- 24.Davalos L. M. 2007. Short-faced bats (Phyllostomidae: Stenodermatina): a Caribbean radiation of strict frugivores. J. Biogeogr. 34, 364–375 10.1111/j.1365-2699.2006.01610.x (doi:10.1111/j.1365-2699.2006.01610.x) [DOI] [Google Scholar]
- 25.Teeling E. C., Springer M. S., Madsen O., Bates P., O'Brien S. J., Murphey W. J. 2005. A molecular phylogeny for bats illuminates biogeography and the fossil record. Science 307, 580–584 10.1126/science.1105113 (doi:10.1126/science.1105113) [DOI] [PubMed] [Google Scholar]
- 26.Velazco P. M., Patterson B. D. 2008. Phylogenetics and biogeography of the broad-nosed bats, genus Plyattyrrhinus (Chiroptera: Phyllostomidae). Mol. Phylogenet. Evol. 49, 749–759 10.1016/j.ympev.2008.09.015 (doi:10.1016/j.ympev.2008.09.015) [DOI] [PubMed] [Google Scholar]
- 27.Savage D. E. 1951. A Miocene phyllostomatid bat from Colombia, South America. Univ. Calif. Pub. Geol. Sci. 28, 357–365 [Google Scholar]
- 28.Wilson D. E., Reeder D. M. (eds) 2005. Mammal species of the world, 3rd edn Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 29.Lyons S. K., Willig M. R. 1999. A hemispheric assessment of scale dependence in latitudinal gradients of species richness. Ecology 80, 2483–2491 10.1890/0012-9658(1999)080[2483:AHAOSD]2.0.CO;2 (doi:10.1890/0012-9658(1999)080[2483:AHAOSD]2.0.CO;2) [DOI] [Google Scholar]
- 30.Stevens R. D. 2004. Untangling latitudinal richness gradients at higher taxonomic levels: familial perspectives on the diversity of New World bat communities. J. Biogeogr. 31, 665–674 10.1111/j.1365-2699.2003.01042.x (doi:10.1111/j.1365-2699.2003.01042.x) [DOI] [Google Scholar]
- 31.Stevens R. D., Willig M. R. 2002. Geographical ecology at the community level: perspectives on the diversity of New World bats. Ecology 83, 545–560 10.1890/0012-9658(2002)083[0545:GEATCL]2.0.CO;2 (doi:10.1890/0012-9658(2002)083[0545:GEATCL]2.0.CO;2) [DOI] [Google Scholar]
- 32.Willig M. R., Selcer K. W. 1989. Bat species density gradients in the New World: a statistical assessment. J. Biogeogr. 16, 189–195 10.2307/2845093 (doi:10.2307/2845093) [DOI] [Google Scholar]
- 33.Kooistra W. H. C. F., Medlin L. K. 1996. Evolution of the diatoms (bacillariophyta). 4. Reconstruction of their age from small subunit rRNA coding regions and the fossil record. Mol. Phylogenet. Evol. 6, 391–407 10.1006/mpev.1996.0088 (doi:10.1006/mpev.1996.0088) [DOI] [PubMed] [Google Scholar]
- 34.Moen D. S., Smith S. A., Wiens J. J. 2009. Community assembly through evolutionary diversification and dispersal in Middle American treefrogs. Evolution 63, 3228–3247 10.1111/j.1558-5646.2009.00810.x (doi:10.1111/j.1558-5646.2009.00810.x) [DOI] [PubMed] [Google Scholar]
- 35.Kerr J. T., Currie D. J. 1999. The relative importance of evolutionary and environmental controls on broad-scale patterns of species richness in North America. Ecoscience 6, 329–337 [Google Scholar]
- 36.Baker R. J., Hoofer S. R., Porter C. A., Van Den Bussche R. A. 2003. Diversification among New World leaf-nosed bats: an evolutionary hypothesis and classification inferred from digenomic congruence of DNA sequence. Occas. Pap. Mus. Texas Tech. Univ. 230, 1–32 [Google Scholar]
- 37.Huelsenbeck J. P., Ronquist F. 2001. MrBayes: Bayesian inference of phylogeny. Bioinformatics 17, 754–755 10.1093/bioinformatics/17.8.754 (doi:10.1093/bioinformatics/17.8.754) [DOI] [PubMed] [Google Scholar]
- 38.Posada D., Crandall K. A. 1998. ModelTest: testing the model of DNA substitution. Bioinformatics 14, 817–818 10.1093/bioinformatics/14.9.817 (doi:10.1093/bioinformatics/14.9.817) [DOI] [PubMed] [Google Scholar]
- 39.Patten M. A. 2004. Correlates of species richness in North American bat families. J. Biogeogr. 31, 975–985 10.1111/j.1365-2699.2004.01087.x (doi:10.1111/j.1365-2699.2004.01087.x) [DOI] [Google Scholar]
- 40.Tello J. S., Stevens R. D. 2010. Multiple environmental determinants of species richness: energy, heterogeneity, and seasonality. Ecography 33, 796–808 10.1111/j.1600-0587.2009.05991.x (doi:10.1111/j.1600-0587.2009.05991.x) [DOI] [Google Scholar]
- 41.Ulrich W., Sachanowicz K., Michalak M. 2007. Environmental correlates of species richness of European bats (Mammalia: Chiroptera). Acta Chiropterol. 9, 347–360 10.3161/1733-5329(2007)9[347:ECOSRO]2.0.CO;2 (doi:10.3161/1733-5329(2007)9[347:ECOSRO]2.0.CO;2) [DOI] [Google Scholar]
- 42.Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G., Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climate 25, 1965–1978 10.1002/joc.1276 (doi:10.1002/joc.1276) [DOI] [Google Scholar]
- 43.Imhoff M. L., et al. 2004. Global patterns in human consumption net primary productivity. Nature 429, 870–873 10.1038/nature02619 (doi:10.1038/nature02619) [DOI] [PubMed] [Google Scholar]
- 44.Jackson D. A. 1993. Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology 74, 2204–2214 10.2307/1939574 (doi:10.2307/1939574) [DOI] [Google Scholar]
- 45.Borcard D., Legendre P. 2002. All-scale analysis of ecological data by means of principal coordinates of neighbor matrices. Ecol. Model. 153, 51–68 10.1016/S0304-3800(01)00501-4 (doi:10.1016/S0304-3800(01)00501-4) [DOI] [Google Scholar]
- 46.Peres-Neto P. R., Legendre P. 2010. Estimating and controlling for spatial structure in the study of ecological communities. Glob. Ecol. Biogeogr. 19, 174–184 10.1111/j.1466-8238.2009.00506.x (doi:10.1111/j.1466-8238.2009.00506.x) [DOI] [Google Scholar]
- 47.Jones M. M., Tuomisto H., Borcard D., Legendre P., Clarck D. B., Olivas C. C. 2008. Explaining variation in tropical plant community composition: influence of environmental and spatial data quality. Oecologia 155, 593–604 10.1007/s00442-007-0923-8 (doi:10.1007/s00442-007-0923-8) [DOI] [PubMed] [Google Scholar]
- 48.Legendre P., et al. 2009. Partitioning beta diversity in a subtropical broad-leaved forest of China. Ecology 90, 663–674 10.1890/07-1880.1 (doi:10.1890/07-1880.1) [DOI] [PubMed] [Google Scholar]
- 49.Legendre P., Legendre L. 1998. Numerical ecology, 2nd edn. Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 50.Currie D. J., et al. 2004. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett. 7, 1121–1134 10.1111/j.1461-0248.2004.00671.x (doi:10.1111/j.1461-0248.2004.00671.x) [DOI] [Google Scholar]
- 51.Gotelli N. J., et al. 2009. Patterns and causes of species richness: a general simulation model for macroecology. Ecol. Lett. 12, 873–886 10.1111/j.1461-0248.2009.01353.x (doi:10.1111/j.1461-0248.2009.01353.x) [DOI] [PubMed] [Google Scholar]
- 52.Williams G. E. 1993. History of the Earth's obliquity. Earth-Sci. Rev. 34, 1–45 10.1016/0012-8252(93)900004-Q (doi:10.1016/0012-8252(93)900004-Q) [DOI] [Google Scholar]
- 53.Buckley L. B., et al. 2010. Phylogeny, niche conservatism and the latitudinal diversity gradient in mammals. Proc. R. Soc. B 277, 2131–2138 10.1098/rspb.2010.0179 (doi:10.1098/rspb.2010.0179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weir J. T. 2006. Divergent timing and patterns of species accumulation in lowland and highland Neotropical birds. Evolution 60, 842–855 [PubMed] [Google Scholar]
- 55.Rabosky D. L. 2009. Ecological limits and diversification rate: alternative paradigms to explain variation in species richness among clades and regions. Ecol. Lett. 12, 735–743 10.1111/j.1461-0248.2009.01333.x (doi:10.1111/j.1461-0248.2009.01333.x) [DOI] [PubMed] [Google Scholar]
- 56.Simmons N. B. 2005. Order Chiroptera. In Mammal species of the world (eds Wilson D. E., Reeder D. M.), 3rd edn, pp. 312–531 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 57.Rabosky D. L., Lovette I. J. 2008. Explosive evolutionary radiations: decreasing speciation or increasing extinction through time? Evolution 62, 1866–1875 10.1111/j.1558-5646.2008.00409.x (doi:10.1111/j.1558-5646.2008.00409.x) [DOI] [PubMed] [Google Scholar]
- 58.Lyons S. K. 2003. A quantitative assessment of the range shifts of Pleistocene mammals. J. Mammal 84, 385–402 (doi:10.1644/1545-1542(2003)084<0385:AQAOTR>2.0.CO;2) [DOI] [Google Scholar]
- 59.Kozak K. H., Graham C. H., Wiens J. J. 2008. Integrating GIS-based environmental data into evolutionary biology. Trends. Ecol. Evol. 23, 141–148 [DOI] [PubMed] [Google Scholar]
- 60.Vellend M., Orrock J. L. 2010. Ecological and genetic models of diversity. In The theory of island biogeography revisited (eds Losos J. B., Ricklefs R. E.), pp. 439–461 Princeton, NJ: Princeton University Press [Google Scholar]
- 61.Field R., et al. 2009. Spatial species-richness gradients across scales: a meta-analysis. J. Biogeogr. 36, 132–147 10.1111/j.1365-2699.2008.01963.x (doi:10.1111/j.1365-2699.2008.01963.x) [DOI] [Google Scholar]
- 62.Forister M. L., McCall A. C., Sanders N. J., Fordyce J. A., Thorne J. H., O'Brien J., Waetjen D. P., Shapiro A. M. 2010. Compounded effects of climate change and habitat alteration shift patterns of butterfly diversity. Proc. Natl Acad. Sci. USA 107, 2088–2092 10.1073/pnas.0909686107 (doi:10.1073/pnas.0909686107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sorte C. J. B., Williams S. L., Carlton J. T. 2010. Marine range shifts and species introductions: comparative spread rates and community impacts. Glob. Ecol. Biogeogr. 19, 303–316 10.1111/j.1466-8238.2009.00519.x (doi:10.1111/j.1466-8238.2009.00519.x) [DOI] [Google Scholar]
- 64.Thomas C. D. 2010. Climate, climate change and range boundaries. Div. Distr. 16, 488–495 10.1111/j.1472-4642.2010.00642.x (doi:10.1111/j.1472-4642.2010.00642.x) [DOI] [Google Scholar]
- 65.Zuckerberg B., Woods A. M., Porter W. F. 2009. Poleward shifts in breeding bird distributions in New York State. Glob. Change Biol. 15, 1866–1883 10.1111/j.1365-2486.2009.01878.x (doi:10.1111/j.1365-2486.2009.01878.x) [DOI] [Google Scholar]