Abstract
Coevolution between antagonistic species has produced instances of exquisite mimicry. Among brood-parasitic cuckoos, host defences have driven the evolution of mimetic eggs, but the evolutionary arms race was believed to be constrained from progressing to the chick stage, with cuckoo nestlings generally looking unlike host young. However, recent studies on bronze-cuckoos have confounded theoretical expectations by demonstrating cuckoo nestling rejection by hosts. Coevolutionary theory predicts reciprocal selection for visual mimicry of host young by cuckoos, although this has not been demonstrated previously. Here we show that, in the eyes of hosts, nestlings of three bronze-cuckoo species are striking visual mimics of the young of their morphologically diverse hosts, providing the first evidence that coevolution can select for visual mimicry of hosts in cuckoo chicks. Bronze-cuckoos resemble their own hosts more closely than other host species, but the accuracy of mimicry varies according to the diversity of hosts they exploit.
Keywords: brood parasitism, visual mimicry, avian vision, cuckoo, coevolution
1. Introduction
Mimicry is a tactic commonly deployed by brood parasites to deceive their hosts. For example, social insect parasites reproduce the hydrocarbon signatures of their hosts [1,2], cuckoos lay eggs that closely resemble those of their hosts [3–5] and nestling cuckoos mimic the begging calls of host young [6–9]. Experimental studies have demonstrated that mimicry in these cases has evolved in response to sophisticated recognition systems on the part of the host [10–12].
Among brood-parasitic cuckoos, the exquisite mimicry of host eggs is in stark contrast to the general lack of resemblance between parasite and host young [13,14]. The failure of cuckoos to mimic host young has been attributed to constraints on the evolution of recognition systems in hosts at the nestling stage [15,16]. However, recent studies have provided novel evidence of cuckoo chick discrimination by hosts [11,17–19]. Chick rejection appears to be particularly prevalent among hosts of the Australian bronze-cuckoos (Chalcites spp.) [11,18,19]. Here we test whether the evolution of cuckoo chick discrimination by hosts of Australian bronze-cuckoos has reciprocally selected visual mimicry of host young by cuckoos.
The Chalcites bronze-cuckoos are obligate brood parasites endemic to Australasia [20–22]. They parasitize taxonomically distant passerine hosts, primarily belonging to the Acanthizidae and Maluridae. Soon after hatching, the Chalcites cuckoo nestling evicts all host offspring from the nest, thus imposing a high fitness cost upon its hosts. Selection therefore favours hosts that resist parasitism in the first place, by mounting defences against the cuckoo. However, unlike hosts of the common cuckoo [23], Chalcites cuckoo hosts lack robust defences at the egg stage of the breeding cycle [11,24–26], probably owing to constraints on detection of the mimetic or cryptic cuckoo eggs [11,27,28], and instead mount their primary line of resistance at the chick stage [11,18,19,29]. Chalcites hosts reject the alien chick within a few days of hatching either by abandoning it [11,29] or by grasping the parasitic chick and flinging it from the nest [18,19].
In theory, chick rejection by hosts should select reciprocal counter-strategies in the cuckoo nestling to evade detection, just as egg rejection by hosts has driven the evolution of cuckoo eggs that mimic the host clutch [23]. Whether chick rejection actually has selected cuckoo nestlings that resemble host young remains unclear, however. Field experiments have shown that nestlings that look different from host young are more likely to be rejected by host parents [11], but whether this has yielded mimetic cuckoo chicks is harder to determine. Quantifying the extent of mimicry is not straightforward because the sensory systems of the target audience are commonly quite unlike our own [30–32]. For birds, models of avian visual processing [33,34] offer a solution to the problem because they effectively enable us to ‘see’ objects as though through a bird's eyes and thereby allow us to measure the accuracy of any potential mimicry [12]. Here we apply these techniques to quantify the extent of host mimicry in nestlings belonging to three species of Chalcites cuckoo: Horsfield's bronze-cuckoo C. basalis (primarily parasitizes Malurus hosts); shining bronze-cuckoo C. lucidus plagosus (primarily parasitizes Acanthiza hosts); and little bronze-cuckoo C. minutillus (primarily parasitizes Gerygone hosts). We demonstrate that bronze-cuckoo nestlings are striking visual mimics of their hosts, and that the accuracy of mimicry varies according to the diversity of hosts they exploit.
2. Material and methods
(a). Study species and sites
We studied three species of bronze-cuckoos at five sites in Australia:
— Little bronze-cuckoos were studied at two sites: C. m. minutillus in Darwin, Northern Territory (Leanyer Swamp 130°54′ E, 12°22′ S; Casuarina Coastal Reserve 130°52′ E, 12°21′ S; Ludmilla Creek 130°51′ E, 12°25′ S) in 2005, and C. m. russatus in Cairns, north-eastern Australia (Cairns Botanic Gardens area 145°44′ E, 16°55′ S) in 2007. The primary host at both sites was the large-billed gerygone Gerygone magnirostris. Nestlings of the two cuckoo subspecies were morphologically similar but differed in one respect: C. m. minutillus sported significantly more down on the back (mean ± s.e. = 17.5 ± 2.3 plumules) than C. m. russatus (mean ± s.e. = 0.4 ± 0.3 plumules; number of C. m. minutillus (4/4) versus C. m. russatus (0/7) with greater than 2 plumules on back, Fisher's exact test, p = 0.003).
— Shining bronze-cuckoos C. lucidus were studied in Campbell Park, Canberra, south-eastern Australia (149°9′ E, 35°16′ S) from 1999 to 2009, where their primary hosts were yellow-rumped thornbills Acanthiza chrysorrhoa. Nestling down was either absent or restricted to very short, fine filaments on the head. Nestlings exhibited two skin colour morphs: yellow and black [11]. Black nestlings (n = 4/20 nestlings, probably all offspring of the same pair) were observed only in 2001, before spectral analyses commenced, and data presented here are from yellow chicks. The two colour morphs may be indicative of distinct populations or subspecies of the shining bronze-cuckoo, which parasitize hosts with different skin colours. Similar subspecific differences in bronze-cuckoo nestling morphology that match that of their respective hosts have been documented previously for two subspecies of the shining bronze-cuckoo (white natal down, white rictal flange, pink and grey skin in C. l. lucidus [35,36]; and sparse or no down, yellow rictal flange, yellow skin in C. l. plagosus, this study and [37]) and two subspecies of the little bronze-cuckoo (C. m. minutillus and C. m. barnardi [38]; see §4).
— Horsfield's bronze-cuckoos, C. basalis, were from two sites: Campbell Park, Canberra, south-eastern Australia (149°9′ E, 35°16′ S) from 1999 to 2009, where they parasitized superb fairy-wrens Malurus cyaneus, and Mornington Wildlife Sanctuary, the Kimberley, western Australia (126°6′ E, 17°31′ S) from 2006 to 2007, where they parasitized purple-crowned fairy-wrens M. coronatus. Data collection at Mornington Wildlife Sanctuary differed from that at the other sites (see electronic supplementary material) so it was not included in visual modelling analyses. Horsfield's bronze-cuckoos lacked nestling down.
(b). Field methods
Nests were located either by following adults during nest-building or incubation, or, in the case of G. magnirostris, by searching along creek lines where nests are suspended above the water. To minimize loss of data owing to high predation rates, some nests of M. cyaneus and A. chrysorrhoa were protected with a large, dome-shaped cage throughout the incubation and nestling period, which excluded large predators but had sufficiently large mesh to allow access by the host adults. Small predators could still access nests, but overall caging reduced predation rates from 66 to 28 per cent [11]. Spectral reflectance of nestlings was measured in the field, a few metres from the nest. A single host or cuckoo nestling was removed from the nest for approximately 5 mins for measurement and then returned to the nest. In cool conditions, the nestling was placed on an insulated hot water bottle or in a woollen sock. One randomly selected host nestling from each brood was used for measurement. Measurements were made before the appearance of ‘pin’ feathers on the body (up to 8 days after hatching). Spectral reflectance (300–700 nm) of the skin of cuckoo and host nestlings was quantified in the field using an Ocean Optics (Dunedin, FL, USA) USB2000 spectrometer with illumination by a PX-2 pulsed xenon lamp and an S2000 spectrometer with illumination by a PX-1 pulsed xenon lamp. We used a narrow-ended UV-VIS unidirectional reflectance probe, held at a constant 45 degrees to the surface by a small sleeve with a bevelled edge. We measured the reflectance of the chicks at four points on the back (front left, front right, rear left, rear right) and four points on the flange (two left, two right). Measurements were recorded at 2 nm intervals from 300 to 700 nm, expressed relative to a Spectralon 99 per cent white reflectance standard (Labsphere, Congleton, UK). Nest irradiance (‘ambient’ light) spectra were calculated using a cosine-corrected spectrometer (integration time 5000) and a 600 × 2 optical fibre, from measurements taken between 09.30 and 16.30.
(c). Calculations of volume in tetrahedral colour space
To calculate the level of colour variation among and within the cuckoos and hosts, we analysed the total volume of avian colour space that they occupied; a larger volume equates to greater variation as individuals with more differing appearances are more separated in colour space. We first calculated the predicted photon catches for the four avian single cones, using the reflectance and irradiance spectra and the spectral sensitivity of an avian visual system (see below), followed by transforming the four standardized single cone catches into three (X, Y, Z) coordinates in tetrahedral colour space [33]. We then calculated a minimum convex polygon containing all points corresponding to the chick back colours [39]. We used this data to calculate the volume occupied by the three cuckoo species and by each cuckoo–host pair. However, comparison of the volume occupied by the three cuckoo species versus each cuckoo–host pair could be influenced by the unequal sample sizes for the three host species (greater sample sizes may have larger volumes). Therefore, we randomly re-sampled the data for the three cuckoo species to a sample size equal to each cuckoo–host pair. This was repeated 500 times for each comparison with the three sets of cuckoo–host pairs, and the average volume occupied by the three cuckoo species was then calculated and used for comparison with the cuckoo–host pair. In addition to the above calculations, we also checked the robustness of the above approach with an additional measure of colour variation. This comprised, for a given dataset (i.e. all cuckoos combined, or specific cuckoo–host pairs), calculating the Euclidean distance in tetrahedral colour space between each chick colour and every other chick colour, followed by taking the overall mean. This calculates the average difference in bird colour space between individuals in a given sample. Although the results were less marked, this analysis confirmed the findings of the volume analysis, with the average distance between all cuckoos larger than the average distance for any cuckoo–host pair: all cuckoos combined occupied 67, 22 and 9 per cent more colour space than that occupied by the cuckoo–host pairs for shining, Horfield's and little bronze-cuckoos respectively.
(d). Visual modelling
We used a log form of a model of avian visual processing, based on evidence that noise arising in the photoreceptors limits the discrimination ability of the observer [34,40] (see electronic supplementary material for detailed methods). The model predicts when an observer, such as a bird, should be able to discriminate between two objects based on colour (chromatic variation) [34] or luminance (‘perceived lightness’) [41]. The model produces an output in terms of just noticeable differences (‘jnds’). In high light levels, a jnd of less than 1.00 means the observer is unable to discriminate between two objects; values between 1.00 and 3.00 mean two objects are hard to discriminate except under good viewing conditions; and values above this should be discriminable. However, these thresholds are relevant to high light level models, and when modelling dark conditions the values vary in relation to a parameter that estimates noise at low light levels (set at a flux of 103 photons per integration time here, as in [28]). Thus, absolute jnd values are not precise, but they are standardized units of discrimination relative to one another. The key factor for this study is that increasing jnd values indicate that two objects (chick signals) are less similar (poorer mimicry).
(e). Statistical methods
We used a generalized linear model (GLM) with a binomial distribution and logit link function to test whether the incidence of nestling down (present or absent) in cuckoos and their hosts was best explained by (i) host species or (ii) whether the individual was a cuckoo or a host. For analyses of colour and luminance reported in the text we used Vorobyev–Osorio contrasts generated using the blue tit visual system and a ‘d65’ daylight illuminant (a standard daylight irradiance spectrum). Shapiro–Wilk W-tests were performed on the distribution of the residuals to check assumptions of normality, and data were log-transformed or non-parametric tests were used if necessary. We used matching model analyses to determine the extent to which each bronze-cuckoo species resembled their own hosts versus host young with other skin colours. First, we compared each individual cuckoo nestling in the analysis with all the host young of a given species to calculate a mean jnd, unique to each cuckoo chick. These means were the unit of analysis in the statistics. The factor was host species (large-billed gerygone, superb fairy-wren, yellow-rumped thornbill), and the mean contrasts between an individual cuckoo chick and each of the three species of host chicks were matched. After finding a significant effect of host species in each analysis, we investigated which levels produced higher or lower responses using Tukey HSD multiple comparisons of the least square means predicted by the model. In one analysis (skin colour of little bronze-cuckoo) the data were not normally distributed, so a non-parametric Wilcoxon test was used. Analyses were conducted using JMP v. 6.0 (SAS Institute Inc., Cary, NC, USA).
(f). Statistical analyses using contrasts generated by the peafowl visual system
We repeated all the statistical analyses of colour and luminance reported in the text using the contrast values generated by the peafowl visual system rather than the blue tit visual system to account for differences that may exist between different types of avian visual system [32]. The results are qualitatively identical to those obtained using the blue tit visual system for all analyses (see electronic supplementary material).
3. Results
Despite their close relatedness [21], Chalcites cuckoo nestlings varied substantially between species in their appearance, and bore a striking resemblance to the young of their respective hosts (figures 1 and 2). Focusing first on nestling down feathers, we found that they were absent in Horsfield's bronze-cuckoos, vestigial in shining bronze-cuckoos and comprised plumules of multiple non-interlocking white barbs in little bronze-cuckoos. The presence of down in cuckoo nestlings was explained statistically by host species (GLM, 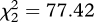 , p < 0.0001), indicating that cuckoo species were more similar to their hosts in this regard than they were to their congeners. The multi-barbed ‘fluffy’ down exhibited by little bronze-cuckoos is of particular interest, because although typical of nestling passerines, it is apparently unique among cuckoos [42].
, p < 0.0001), indicating that cuckoo species were more similar to their hosts in this regard than they were to their congeners. The multi-barbed ‘fluffy’ down exhibited by little bronze-cuckoos is of particular interest, because although typical of nestling passerines, it is apparently unique among cuckoos [42].
Figure 1.
Representative photographs and mean ± s.e. reflectance of the skin of nestling bronze-cuckoos (blue lines) and their hosts (pink lines). (a) Little bronze-cuckoo (n = 10) and large-billed gerygone (n = 12). (b) Shining bronze-cuckoo (n = 8) and yellow-rumped thornbill (n = 5). (c) Horsfield's bronze-cuckoo (n = 11; photo illustrates a pale individual) and superb fairy-wren (n = 17). (d) We also include a second host of Horsfield's bronze-cuckoo (n = 4; photo illustrates a dark individual), the purple-crowned fairy-wren M. coronatus (n = 8), to illustrate some of the variation in nestling colour among malurid hosts.
Figure 2.
Representative photographs and mean ± s.e. reflectance of the rictal flange of nestling bronze-cuckoos (blue lines) and their hosts (pink lines). (a) Little bronze-cuckoo (n = 4) and large-billed gerygone (n = 5). (b) Shining bronze-cuckoo (n = 7) and yellow-rumped thornbill (n = 5). (c) Horsfield's bronze-cuckoo (n = 6) and superb fairy-wren (n = 15).
Skin colour ranged from uniform black in little bronze-cuckoos to two-tone pink and grey in Horsfield's bronze-cuckoos and yellow in shining bronze-cuckoos. To measure the variation in skin colour between species, we modelled the predicted photon catches of an avian visual system using reflectance spectra of the nestling skin colour (see §2), and then plotted these values in avian tetrahedral colour space [33]. We then analysed the total volume encompassed in colour space [39] by the different cuckoos and hosts, representing the range of variation in avian-perceived colours (figure 3). Variation in nestling skin colour was greater among cuckoo species than within each cuckoo–host species pair; the volume occupied in avian colour space by all cuckoo species combined was 1.4 times greater than the volume occupied by the Horsfield's bronze-cuckoo and superb fairy-wren, 4.2 times greater than that occupied by the shining bronze-cuckoo and yellow-rumped thornbill, and 2.3 times greater than the volume occupied by the little bronze cuckoo and large-billed gerygone (controlling for differences in sample size; see §2 and figure 3). The relatively greater similarity between cuckoos and their hosts than between the closely related cuckoos allows us to discount the possibility that cuckoos resemble their hosts by chance or due to common ancestry, and provides strong evidence that nestling Chalcites cuckoo appearance has diversified under selection from hosts, unconstrained by phylogenetic history.
Figure 3.
(a) Distribution of host and cuckoo chick skin colours in avian colour space. Each point corresponds to an individual, with the points based on standardized single cone catch values transformed into three (X, Y, Z) coordinates in tetrahedral colour space [33]. HBC, Horsfield's bronze-cuckoo; SFW, superb fairy-wren; SBC, shining bronze-cuckoo; YRT, yellow-rumped thornbill; LBC, little bronze-cuckoo and LBG, large-billed gerygone. Cuckoos and their respective hosts have the same symbol outline colour. (b) A close-up view of the data points.
We also tested whether each cuckoo species was a better mimic of its own hosts than the other host species in the analysis. The perception of mimicry depends very much on the eye of the beholder [31,43], so we used avian visual modelling [33,34] to compare the appearance of cuckoo and host young as though through the eyes of a host species.
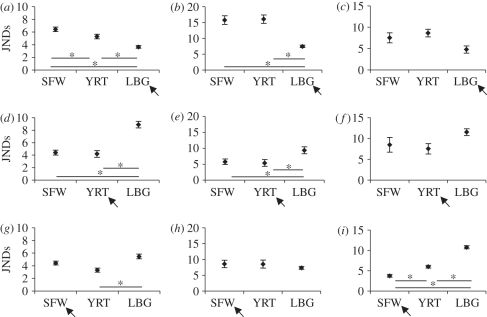
The little bronze-cuckoo, a specialist parasite of dark-skinned gerygone hosts [20], was an almost perfect match of its host in skin colour and luminance, and significantly different from the other host young in the analysis (figure 4a,b). The shining bronze-cuckoo shows intermediate host specificity, specializing on thornbill (Acanthiza spp.) hosts, but also secondarily exploiting fairy-wrens (M. cyaneus and M. splendens [20]). Shining bronze-cuckoos were significantly more similar to both thornbill and fairy-wren hosts than to gergyone hosts in skin colour, and to thornbill hosts than gergyone hosts in skin luminance (figure 4d,e). Horsfield's bronze-cuckoo is the least host-specific bronze-cuckoo. It specializes on fairy-wrens throughout its range, but secondarily exploits a range of other hosts including thornbills, robins (Petroica spp.), heathwrens (Hylacola sp.) and chats (Epthianura spp. [20]). Nestling skin colour varies among these hosts, including pink or grey in the malurids (figure 1c,d), and dark grey (e.g. flame robin Petroica phoenicea) and black (e.g. shy heathwren Hylacola cauta) in secondary hosts [44]. Horsfield's bronze-cuckoos' skin colour and luminance bore a similar degree of resemblance to pale-skinned fairy-wren and thornbill chicks and dark-skinned gerygone chicks, although they were slightly more similar to thornbills than to gerygones in colour (figure 4g). Horsfield's bronze-cuckoos probably achieve this ‘jack-of-all-trades’ visual mimicry by employing a two-tone skin colour of pink and grey (figure 1c,d), enabling them to look similar to both pale- (e.g. figure 1c) and dark-skinned (e.g. figure 1d) host young.
Figure 4.
The disparity (mean ± s.e.) between bronze-cuckoos and their own hosts versus other hosts in (a,d,g) skin colour, (b,e,h) skin luminance and (c,f,i) rictal flange colour. (a–c) Little bronze-cuckoo; (d–f) shining bronze-cuckoo; (g–i) Horsfield's bronze-cuckoo. SFW, superb fairy-wren; YRT, yellow-rumped thornbill; LBG, large-billed gerygone. The arrow indicates the primary host in each graph. The asterisks indicate Tukey HSD significance levels (p < 0.05) with the bars below indicating the two species being compared. JND = just noticeable difference; a smaller JND indicates better mimicry in the eyes of the host parent. JNDs were calculated using D65 light irradiance, under low light conditions, with the blue tit visual system. Sample sizes are given in figures 1 and 2.
The rictal flange colour of nestling bronze-cuckoos was more similar to their primary hosts than to non-hosts for all three cuckoo species (figure 4c,f,i). In this trait, Horsfield's bronze-cuckoos did not employ a ‘jack-of-all-trades’ strategy, but showed significantly more accurate mimicry of their primary hosts than other host species. There was no significant effect of host species on flange luminance for any cuckoo species.
4. Discussion
Recent studies have revealed a new stage in the coevolutionary arms race between cuckoos and their hosts: rejection of cuckoo nestlings by hosts [11,17–19]. Here we present evidence of a reciprocal adaptation in cuckoos: visual mimicry of host young by cuckoo nestlings. Nestlings of the three bronze-cuckoo species were striking visual mimics of the offspring of their hosts (figures 1 and 2), and more similar to their own hosts than to the other host species in the study (figures 3 and 4). Visual mimicry of host young has not been previously demonstrated in a cuckoo species that evicts the host young [13,45]. Some brood-parasitic nestlings that are reared alongside the host young are, to human eyes, similar in their appearance (eg. Vidua finches, screaming cowbirds Molothrus rufoaxillaris) [13,14,45]. However, it is unclear whether this similarity has evolved through coevolution in response to discrimination by hosts, or to exploit the pre-existing parent–offspring communication systems in order to facilitate competition with host young for food [14,45–47]. By contrast, coevolution between Chalcites cuckoos and their hosts almost certainly has selected for mimicry of host nestlings by cuckoos because experimental studies show that (i) hosts of Chalcites cuckoos can reject parasite nestlings [11,18,19]; (ii) chick rejection is a specific response to brood-parasitism, such that hosts show flexibility in their responses to nestlings depending on the risk of parasitism [29]; and (iii) non-mimetic nestlings suffer a survival cost [11]. Thus, our results provide novel evidence of a further escalation in the coevolutionary arms race between cuckoos and their hosts: the evolution of visual mimicry of host nestlings by cuckoos in response to rejection by hosts.
Together with two other recent studies [6,48], this study counters theoretical arguments that in both avian and insect brood-parasitic systems coevolution will not extend beyond the egg stage [16,49]. To date, the only other suggestion that rejection by hosts has selected for mimetic parasitic offspring in any brood-parasitic system comes from recent work on an insect social parasite. Here, female pupae of brood-parasitic slave-making ants Protomognathus americanus suffer high rates of rejection by their Temnothorax hosts, but male parasitic offspring usually avoid rejection, perhaps owing to visual and chemical mimicry of host young [48,50]. Furthermore, our evidence of visually mimetic cuckoo nestlings provides the final piece of evidence that visual mimicry is a tactic that has evolved in cuckoos in response to host defences at every stage of parasitism; cuckoos have evolved visual mimicry of avian predators in response to mobbing by hosts [51,52], egg mimicry in response to egg rejection by hosts [3–5] and chick mimicry in response to chick rejection by hosts (this study).
The strategy for mimicry differed between the three species according to the diversity of hosts they exploit. The specialist little bronze-cuckoo exhibited highly accurate visual mimicry of its gerygone hosts, including expression of a derived trait; multi-barbed white down that closely matches the down of its host, but is unique among cuckoos [53]. The shining bronze-cuckoo, which specializes on pale-skinned Acanthiza and Malurus hosts, occupied the area of overlap in avian colour space between its primary and secondary hosts (figure 3). The more generalist Horsfield's bronze-cuckoo employed two-tone pink–grey coloration that facilitated mimicry of a diversity of nestling skin colours. Similarly, common cuckoo host races lay eggs that mimic their respective hosts, but use more ‘average’ markings than their hosts, probably to facilitate the use of secondary hosts [5].
Our previous study revealed higher rates of rejection of shining bronze-cuckoo chicks than Horsfield's bronze-cuckoo chicks by superb fairy-wren hosts [11]. However, our results here reveal that the two cuckoo species are equally similar to superb fairy-wren chicks in skin colour (figure 4d,g). Additional cues used by superb fairy-wrens to discriminate shining bronze-cuckoos may include rictal flange colour, which does not resemble that of fairy-wrens (figure 4f,i), and nestling begging calls [6,11].
As well as resembling host young visually, bronze-cuckoo chicks are vocal mimics of host nestlings ([6,8,11]; N. E. Langmore 2010, unpublished data). Cross-fostering experiments reveal that the generalist Horsfield's bronze-cuckoo can facultatively adjust the structure of its begging call during the nestling period to match the notes produced by both Malurus and Acanthiza host young [6]. By contrast, the begging calls of the specialist shining bronze-cuckoo do not appear to vary according to the host that rears it [8]. The Horsfield's bronze-cuckoo's capacity for facultative vocal nestling mimicry, combined with its average mimicry of host nestling skin colour, facilitates exploitation of diverse hosts, without segregating into host-specific genetic races [6]. By contrast, where mimicry is highly accurate, as in the case of the little bronze-cuckoo and the shining bronze-cuckoo, annexation of new hosts could lead to diversifying selection, in which mimics diverge in appearance to resemble different models [54]. For example, although the offspring of the gerygone hosts of the little bronze-cuckoo typically display dark skin and white down as described in this study, in one host species (G. albogularis) offspring display pink skin and yellow down [38]. Remarkably, the subspecies of little bronze-cuckoo (C. m. barnardi) that parasitizes G. albogularis also produces offspring with pink skin and yellow down [38]. Selection by hosts for mimetic cuckoo nestlings could reinforce reproductive isolation among cuckoo populations that exploit different host species. This could explain why Horsfield's bronze-cuckoos are monotypic, whereas shining bronze-cuckoos comprise four subspecies and there are 10 or more subspecies of little bronze-cuckoo [42]. Furthermore, if cuckoo chick rejection by hosts proves to be more common than is currently thought, it might also explain the recent evidence that species richness is higher in parasitic cuckoos than non-parasitic cuckoos [55].
In general, our results challenge the classical view that evictor cuckoo nestlings are unlikely to be rejected by hosts and so are unlikely to coevolve visual mimicry of host young [13,16] because recognizing cuckoo chicks is too cognitively challenging for hosts [13,16]. In our view, the best explanation for the rarity of evictor cuckoo chick rejection and cuckoo chick mimicry centres on the success of other lines of host defence [13,14]. This has been termed ‘strategy blocking’ [56] and refers to the diminishing returns of later lines of defence if an earlier defence is successful. Unlike many other cuckoo hosts, Chalcites hosts do not reject cuckoo eggs [11,26], perhaps because these parasitic eggs are too cryptic [28] or mimetic [25] to detect. Consequently, their only remaining line of defence is to reject parasitic nestlings, which in turn drives the evolution of visually mimetic cuckoo nestlings.
Acknowledgements
Data were collected under the approval of the Australian National University Animal Experimentation Ethics Committee (Protocol Numbers F.BTZ.99.99 and F.BTZ.61.03), the Queensland Parks and Wildlife Service (permit numbers WITK04582707, WISP04740407), the Parks and Wildlife Commission of the Northern Territory (Permit number 26849), Environment ACT (Licence number LT2006229, LT2007266), the Australian Bird and Bat Banding Scheme, the Department of Conservation and Land Management, and the Australian Wildlife Conservancy.
We are very grateful to J. Grant, S. Hunt, R. Noske, E. Rosenfeld and B. Venables for assistance in the field, to J. Hemmi and K. Delhey for advice on spectrometry and to J. Welbergen for helpful comments on the manuscript. N.E.L. was supported by an ARC Australian Research Fellowship. R.M.K. was supported by a Royal Society University Research Fellowship. G.M. was supported by an ARC Discovery grant to N.E.L. and a Leverhulme grant to R.M.K. M.S. was supported by a Biotechnology and Biological Sciences Research Council David Phillips Fellowship (BB/G022887/1), and Churchill College, Cambridge. M.L.H. and A.P. were supported by the ‘Sonderprogramm zur Förderung hervorragender Wissenschaftlerinnen’ of the Max Planck Society. We are grateful to Environment ACT, the Parks and Wildlife Commission of the Northern Territory, Australian Wildlife Conservancy, Cairns City Council, and Queensland Parks and Wildlife Service for permission to work on the study sites.
References
- 1.Lenoir A., D'Ettoir P., Errard C. 2001. Chemical ecology and social parasitism in ants. Ann. Rev. Entomol. 46, 573–599 10.1146/annurev.ento.46.1.573 (doi:10.1146/annurev.ento.46.1.573) [DOI] [PubMed] [Google Scholar]
- 2.Strohm E., Kroiss J., Herzner G., Laurien-Kehnen C., Boland W., Schreier P., Schmitt T. 2008. A cuckoo in wolves' clothing? Chemical mimicry in a specialized cuckoo wasp of the European beewolf (Hymenoptera, Chrysididae and Crabronidae). Front. Zool. 5, 2. 10.1186/1742-9994-5-2 (doi:10.1186/1742-9994-5-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooke M. de. L., Davies N. B. 1988. Egg mimicry by cuckoos Cuculus canorus in relation to discrimination by hosts. Nature 335, 630–632 10.1038/335630a0 (doi:10.1038/335630a0) [DOI] [Google Scholar]
- 4.Starling M., Heinsohn R., Cockburn A., Langmore N. E. 2006. Cryptic gentes revealed in pallid cuckoos Cuculus pallidus using reflectance spectrophotometry. Proc. R. Soc. B 273, 1929–1934 10.1098/rspb.2006.3490 (doi:10.1098/rspb.2006.3490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoddard M. C., Stevens M. 2010. Pattern mimicry of host eggs by the common cuckoo, as seen through a bird's eye. Proc. R. Soc. B 277, 1387–1393 10.1098/rspb.2009.2018 (doi:10.1098/rspb.2009.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langmore N. E., Maurer G., Adcock G. J., Kilner R. M. 2008. Socially acquired host-specific mimicry and the evolution of host races in Horsfield's bronze-cuckoo Chalcites basalis. Evolution 62, 1689–1699 10.1111/j.1558-5646.2008.00405.x (doi:10.1111/j.1558-5646.2008.00405.x) [DOI] [PubMed] [Google Scholar]
- 7.McLean I. G., Waas J. R. 1987. Do cuckoo chicks mimic the begging calls of their hosts? Anim. Behav. 35, 1896–1897 10.1016/S0003-3472(87)80083-0 (doi:10.1016/S0003-3472(87)80083-0) [DOI] [Google Scholar]
- 8.Payne R. B., Payne L. L. 1998. Nestling eviction and vocal begging behaviors in the Australian glossy cuckoos Chrysococcyx basalis and C. lucidus. In Parasitic birds and their hosts. Studies in coevolution (eds Rothstein S. I., Robinson S. K.), pp. 152–169 Oxford, UK: Oxford University Press [Google Scholar]
- 9.Redondo T., Arias de Reyna L. 1988. Vocal mimicry of hosts by great spotted cuckoo Clamator glandiarius: further evidence. Ibis 130, 540–544 10.1111/j.1474-919X.1988.tb02720.x (doi:10.1111/j.1474-919X.1988.tb02720.x) [DOI] [Google Scholar]
- 10.Dronnet S., Simon X., Verhaeghe J. C., Rasmont P., Errard C. 2005. Bumblebee inquilinism in Bombus (Fernaldaepsithyrus) sylvestris (Hymenoptera, Apidae): behavioural and chemical analyses of host–parasite interactions. Apidologie 36, 59–70 10.1051/apido:2004070 (doi:10.1051/apido:2004070) [DOI] [Google Scholar]
- 11.Langmore N. E., Hunt S., Kilner R. M. 2003. Escalation of a coevolutionary arms race through host rejection of brood parasitic young. Nature 422, 157–160 10.1038/nature01460 (doi:10.1038/nature01460) [DOI] [PubMed] [Google Scholar]
- 12.Spottiswoode C. N., Stevens M. 2010. Visual modeling shows that avian host parents use multiple visual cues in rejecting parasitic eggs. Proc. Natl Acad. Sci. USA 107, 8672–8676 10.1073/pnas.0910486107 (doi:10.1073/pnas.0910486107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies N. B., Brooke M. D. E. L. 1988. Cuckoos versus reed warblers: adaptations and counteradaptations. Anim. Behav. 36, 262–284 10.1016/S0003-3472(88)80269-0 (doi:10.1016/S0003-3472(88)80269-0) [DOI] [Google Scholar]
- 14.Grim T. 2005. Mimicry versus similarity: which resemblances between brood parasites and their hosts are mimetic and which are not? Biol. J. Linn. Soc. 84, 69–78 10.1111/j.1095-8312.2005.00414.x (doi:10.1111/j.1095-8312.2005.00414.x) [DOI] [Google Scholar]
- 15.Lawes M. J., Marthews T. R. 2003. When will rejection of parasite nestlings by hosts of nonevicting avian brood parasites be favored? A misimprinting-equilibrium model. Behav. Ecol. 14, 757–770 10.1093/beheco/arg068 (doi:10.1093/beheco/arg068) [DOI] [Google Scholar]
- 16.Lotem A. 1993. Learning to recognize nestlings is maladaptive for cuckoo Cuculus canorus hosts. Nature 362, 743–745 10.1038/362743a0 (doi:10.1038/362743a0) [DOI] [Google Scholar]
- 17.Grim T. 2007. Experimental evidence for chick discrimination without recognition in a brood parasite host. Proc. R. Soc. B 274, 373–381 10.1098/rspb.2006.3731 (doi:10.1098/rspb.2006.3731) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato N. J., Tokue K., Noske R. A., Mikami O. K., Ueda K. 2010. Evicting cuckoo nestlings from the nest: a new anti-parasite behaviour. Biol. Lett. 6, 67–69 10.1098/rsbl.2009.0540 (doi:10.1098/rsbl.2009.0540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tokue K., Ueda K. 2010. Mangrove gerygones reject and eject little bronze-cuckoo hatchlings from parasitized nests. Ibis 152, 835–839 10.1111/j.1474-919X.2010.01056.x (doi:10.1111/j.1474-919X.2010.01056.x) [DOI] [Google Scholar]
- 20.Brooker M. G., Brooker L. C. 1989. Cuckoo hosts in Australia. Aust. Zool. Rev. 2, 1–67 [Google Scholar]
- 21.Christidis L., Boles W. 2008. Systematics and taxonomy of Australian birds. Melbourne, Australia: CSIRO Publishing [Google Scholar]
- 22.Higgins P. J. E. 1999. Handbook of Australian, New Zealand and Antarctic birds. Vol. 4. Parrots to Dollarbird. Melbourne, Australia: Oxford University Press [Google Scholar]
- 23.Davies N. B. 2000. Cuckoos, cowbirds and other cheats. London, UK: T. & A. D. Poyser [Google Scholar]
- 24.Brooker M. G., Brooker L. C. 1989. The comparative breeding behaviour of two sympatric cuckoos, Horsfield's bronze-cuckoo Chrysococcyx basalis and the shining bronze-cuckoo C. lucidus, in Western Australia: a new model for the evolution of egg morphology and host specificity in avian brood parasites. Ibis 131, 528–547 10.1111/j.1474-919X.1989.tb04789.x (doi:10.1111/j.1474-919X.1989.tb04789.x) [DOI] [Google Scholar]
- 25.Langmore N. E., Kilner R. M. 2009. Why do Horsfield's bronze-cuckoo Chalcites basalis eggs mimic those of their hosts? Behav. Ecol. Sociobiol. 63, 1127–1131 10.1007/s00265-009-0759-9 (doi:10.1007/s00265-009-0759-9) [DOI] [Google Scholar]
- 26.Langmore N. E., et al. 2005. The evolution of egg rejection by cuckoo hosts in Australia and Europe. Behav. Ecol. 16, 686–692 10.1093/beheco/ari041 (doi:10.1093/beheco/ari041) [DOI] [Google Scholar]
- 27.Langmore N. E., Kilner R. M. 2010. The co-evolutionary arms race between Horsfield's bronze-cuckoos and superb fairy-wrens. Emu 110, 32–38 10.1071/MU09032 (doi:10.1071/MU09032) [DOI] [Google Scholar]
- 28.Langmore N. E., Stevens M., Maurer G., Kilner R. M. 2009. Are dark cuckoo eggs cryptic in host nests? Anim. Behav. 78, 461–468 10.1016/j.anbehav.2009.06.003 (doi:10.1016/j.anbehav.2009.06.003) [DOI] [Google Scholar]
- 29.Langmore N. E., Cockburn A., Russell A. F., Kilner R. M. 2009. Flexible cuckoo chick-rejection rules in the superb fairy-wren. Behav. Ecol. 20, 978–984 10.1093/beheco/arp086 (doi:10.1093/beheco/arp086) [DOI] [Google Scholar]
- 30.Cheney K. L., Marshall N. J. 2009. Mimicry in coral reef fish: how accurate is this deception in terms of color and luminance? Behav. Ecol. 20, 459–468 10.1093/beheco/arp017 (doi:10.1093/beheco/arp017) [DOI] [Google Scholar]
- 31.Chittka L., Osorio D. 2007. Cognitive dimensions of predator responses to imperfect mimicry? PLoS Biol. 5, e339. 10.1371/journal.pbio.0050339 (doi:10.1371/journal.pbio.0050339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuthill I. C. 2006. Color perception. In Bird coloration, mechanisms and measurements, vol. 1 (eds Hill G. E., McGraw K. J.). Cambridge, MA: Harvard University Press [Google Scholar]
- 33.Endler J. A., Mielke P. W. J. 2005. Comparing color patterns as birds see them. Biol. J. Linn. Soc. 86, 405–431 10.1111/j.1095-8312.2005.00540.x (doi:10.1111/j.1095-8312.2005.00540.x) [DOI] [Google Scholar]
- 34.Vorobyev M., Osorio D. 1998. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. Lond. B 265, 351–358 10.1098/rspb.1998.0302 (doi:10.1098/rspb.1998.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gill B. J. 1998. Behavior and ecology of the shining cuckoo, Chrysococcyx lucidus. In Parasitic birds and their hosts: studies in coevolution (eds Rothstein S. I., Robinson S. K.). Oxford, UK: Oxford University Press [Google Scholar]
- 36.Gill B. J. 1982. Notes on the shining cuckoo (Chrysococcyx lucidus) in New Zealand. Notornis 29, 215–227 [Google Scholar]
- 37.Brooker M., Brooker L. 1986. Identification and development of the nestling cuckoos, Chrysococcyx basalis and C. lucidus plagosus, in Western Australia. Aust. Wildl. Res. 13, 197–202 10.1071/WR9860197 (doi:10.1071/WR9860197) [DOI] [Google Scholar]
- 38.McGill I. G., Goddard M. T. 1979. The little bronze cuckoo in New South Wales. Aust. Birds 14, 23–24 [Google Scholar]
- 39.Stoddard M. C., Prum R. O. 2008. Evolution of avian plumage color in a tetrahedral color space: a phylogenetic analysis of new world buntings. Am. Nat. 171, 755–776 10.1086/587526 (doi:10.1086/587526) [DOI] [PubMed] [Google Scholar]
- 40.Osorio D., Smith A. C., Vorobyev M., Buchanan-Smith H. M. 2004. Detection of fruit and the selection of primate visual pigments for color vision. Am. Nat. 164, 696–708 10.1086/425332 (doi:10.1086/425332) [DOI] [PubMed] [Google Scholar]
- 41.Siddiqi A., Cronin T. W., Loew E. R., Vorobyev M., Summers K. 2004. Interspecific and intraspecific views of color signals in the strawberry poison frog Dendrobates pumilio. J. Exp. Biol. 207, 2471–2485 10.1242/jeb.01047 (doi:10.1242/jeb.01047) [DOI] [PubMed] [Google Scholar]
- 42.Payne R. B. 2005. The cuckoos. Oxford, UK: Oxford University Press [Google Scholar]
- 43.Cuthill I. C., Bennett A. T. D. 1993. Mimicry and the eye of the beholder. Proc. R. Soc. Lond. B 253, 203–204 10.1098/rspb.1993.0103 (doi:10.1098/rspb.1993.0103) [DOI] [Google Scholar]
- 44.Higgins P., Peter J. M. 2002. Handbook of Australian, New Zealand and Antarctic birds. Vol. 6. Pardalotes to shrike-thrushes. Oxford, UK: Oxford University Press [Google Scholar]
- 45.Fraga R. M. 1998. Interactions of the parasitic screaming and shiny cowbirds (Molothrus rufoaxillaris and M. bonariensis) with a shared host, the bay-winged cowbird (M. badius). In Parasitic birds and their hosts (eds Rothstein S. I., Robinson S. K.), pp. 173–193 Oxford, UK: Oxford University Press [Google Scholar]
- 46.Hauber M. E., Kilner R. M. 2007. Coevolution, communication and host-chick mimicry in parasitic finches: who mimics whom? Behav. Ecol. Sociobiol. 61, 497–503 10.1007/s00265-006-0291-0 (doi:10.1007/s00265-006-0291-0) [DOI] [Google Scholar]
- 47.Schuetz J. G. 2005. Low survival of parasite chicks may result from their imperfect adaptation to hosts rather than expression of defenses against parasitism. Evolution 59, 2017–2024 [PubMed] [Google Scholar]
- 48.Achenbach A., Foitzik S. 2009. First evidence for slave rebellion: enslaved ant workers systematically kill the brood of their social parasite Protomognathus americanus. Evolution 63, 1068–1075 10.1111/j.1558-5646.2009.00591.x (doi:10.1111/j.1558-5646.2009.00591.x) [DOI] [PubMed] [Google Scholar]
- 49.Gladstone D. E. 1981. Why there are no ant slave rebellions? Am. Nat. 117, 779–781 10.1086/283759 (doi:10.1086/283759) [DOI] [Google Scholar]
- 50.Achenbach A., Witte V., Foitzik S. 2010. Brood exchange experiments and chemical analyses shed light on slave rebellion in ants. Behav. Ecol. 21, 948–956 10.1093/beheco/arq008 (doi:10.1093/beheco/arq008) [DOI] [Google Scholar]
- 51.Welbergen J. A., Davies N. B. 2009. Strategic variation in mobbing as a front line of defense against brood parasitism. Curr. Biol. 19, 235–240 10.1016/j.cub.2008.12.041 (doi:10.1016/j.cub.2008.12.041) [DOI] [PubMed] [Google Scholar]
- 52.Welbergen J. A., Davies N. B. In press A parasite in wolf's clothing: hawk mimicry reduces mobbing of cuckoos by hosts. Behav. Ecol. [Google Scholar]
- 53.Payne R. B. 1977. The ecology of brood parasitism in birds. Ann. Rev. Ecol. Syst. 8, 1–28 10.1146/annurev.es.08.110177.000245 (doi:10.1146/annurev.es.08.110177.000245) [DOI] [Google Scholar]
- 54.Jiggins C. D., Naisbitt R. E., Coe R. L., Mallet J. 2001. Reproductive isolation caused by colour pattern mimicry. Nature 411, 302–305 10.1038/35077075 (doi:10.1038/35077075) [DOI] [PubMed] [Google Scholar]
- 55.Krüger O., Sorenson M. D., Davies N. B. 2009. Does coevolution promote species richness in parasitic cuckoos? Proc. R. Soc. B 276, 3871–3879 10.1098/rspb.2009.1142 (doi:10.1098/rspb.2009.1142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Britton N. F., Planque R., Franks N. R. 2007. Evolution of defence portfolios in exploiter–victim systems. Bull. Math. Biol. 69, 957–988 10.1007/s11538-006-9178-5 (doi:10.1007/s11538-006-9178-5) [DOI] [PubMed] [Google Scholar]