Abstract
Offspring provisioning is commonly referenced as the most important influence on men's and women's foraging decisions. However, the provisioning of other adults may be equally important in determining gender differences in resource choice, particularly when the goals of provisioning offspring versus others cannot be met with the acquisition of the same resources. Here, we examine how resources vary in their expected daily energetic returns and in the variance or risk around those returns. We predict that when available resources impose no trade-off between risk and energy, the targets of men's and women's foraging will converge on high-energy, low-risk resources that allow for the simultaneous provisioning of offspring and others. However, when minimizing risk and maximizing energy trade-off with one another, we expect men's foraging to focus on provisioning others through the unreliable acquisition of large harvests, while women focus on reliably acquiring smaller harvests to feed offspring. We test these predictions with foraging data from three populations (Aché, Martu and Meriam). The results uphold the predictions, suggesting that men's and women's foraging interests converge when high-energy resources can be reliably acquired, but diverge when higher-energy resources are associated with higher levels of risk. Social factors, particularly the availability of alloparental support, may also play a major role.
Keywords: gender division of labour, central place provisioning, risk, variance, human behavioural ecology, hunter–gatherers
1. Introduction
Offspring provisioning among humans is particularly prolonged, extensive and cooperative, and has been implicated as an important influence on the gender division of foraging labour, the formation of nuclear families, food sharing and many other uniquely human traits [1–3]. However, foragers frequently target resources that provide returns exceeding the requirements of feeding self and dependents [4–6]. Because humans are social central-place foragers, often without access to storage, acquired foods are routinely distributed to other non-dependent group members in what can be called social provisioning. Social provisioning may shape resource acquisition decisions as much as offspring provisioning, and while it may offer no direct somatic benefits like feeding self, or immediate reproductive benefits like feeding offspring, feeding others may lead to delayed benefits in kind [7], or indirect social [8] or reproductive [9,10] benefits. If offspring and social provisioning trade off with each other, and if men and women evaluate the costs and benefits of offspring versus social provisioning differently, then this trade-off may influence gender differences in foraging strategies. While some suggest that offspring provisioning is more important in shaping men's and women's foraging decisions [11], others highlight the importance of social provisioning [12]. We ask whether gender differences in resource choice can be explained through a consideration of both offspring and social provisioning and how they trade off in different ecological and social contexts.
The relative amounts of energy and risk that available resources provide may be particularly important in shaping this trade-off [13,14]. Sometimes, targeting resources that maximize energy returns per foraging trip will provide harvests large enough to satisfy both offspring provisioning and social provisioning goals. However, as Hawkes [13] pointed out, if resources providing higher daily returns are associated with higher variance owing to acquisition failure, foragers will experience a trade-off between minimizing daily acquisition risk and maximizing energetic returns [15]. High variance is generally bad for offspring provisioning as the cost of shortfalls may be higher than the potential benefits of exceeding the mean expected return; such costs may include reduced offspring growth rates or chronic undernourishment [16]. While acquisition variance could be ameliorated through relying on the income of other foragers for provisioning (e.g. sharing), it might not if acquisition success and failure covary between foragers, if there are consistent differences in skill or effort (e.g. free riders), or if failure is much more likely than success, leading to many days without a harvest. On the other hand, variance may be less costly and even beneficial for social provisioning goals. The costs of shortfall are relatively low because non-dependent adults are less affected by scarcity than dependents and are able to forage for themselves. The benefits of acquiring a larger-than-average harvest are relatively high, as such bonanzas can be shared more widely to others outside the family. In environments where there are many high-energy options with low or predictable variance, social and offspring provisioning goals could be met with the same suite of resources. Where maximizing energy returns leads to greater variance owing to unpredictable search or pursuit failure, which is frequently the case [17], the goals of social and offspring provisioning come into conflict, and we should expect those who perceive higher payoffs from offspring provisioning to invest more effort in low-variance resources, and others who favour social provisioning to invest more in attempting to acquire high-energy, high-variance resources.
How foragers solve this trade-off and allocate their time to resources that minimize risk or maximize energy will depend on whether they gain greater benefits from investments in direct offspring provisioning relative to the benefits gained (and opportunities forgone) from investments in social provisioning. Gender is likely to be an important influence on the way individuals solve the risk–energy trade-off: men and women tend to consistently allocate their time differently, especially with respect to household work and parental care [6,18]. We also expect other individual characteristics (such as age, marital status and the level of offspring need), as well as social factors (such as cooperative caretaking, political systems, marriage patterns and post-marital residence) to be important in influencing (and being influenced by) the balance of effort to social and offspring provisioning strategies for both men and women. In the interest of parsimony, however, we begin with the simplest model, asking to what extent trade-offs between strategies that minimize risk and those that maximize energy predict the relative production of men and women across foraging activities in three contemporary populations: Aché, Martu and Meriam.
2. Background and methods
Aché live in the forests of Eastern Paraguay and were full-time foragers until about the mid-1970s. In the early 1980s, Aché were living mostly in mission settlements, but returned to the forest frequently for short foraging bouts or extended trips during which they relied heavily on foraged foods. Aché have a heavily male-biased foraging economy, with women contributing 13 per cent of acquired resources [19]. Aché data examined here come from published sources that detail Aché foraging decisions over nine extended foraging trips [4,9,20–24]. Martu live in the arid deserts of northern Western Australia where they maintained a full-time foraging lifestyle until the mid-1960s. Today Martu reside in remote settlements within their native title and frequently forage for wild resources. Martu have a female-biased foraging economy, with women bringing in the majority of total kilocalories on average [25]. Data examined here come out of an ongoing project and the current sample was collected from 2000 to 2009 [16,25,26]. Meriam live on islands in the eastern Torres Strait in permanent settlements, many of which were occupied prior to European contact. Subsistence is mixed, consisting of maritime foraging practices and part-time horticulture. Meriam gendered production is seasonally variable; men produce more and contribute more to household consumption when turtles are nesting, but women contribute more outside of turtle nesting season. Data examined here were collected from 1994 to 1999 [27–30]. While each of these populations has some access to alternative resources available from horticulture, agriculture or market exchange, men and women within the same population still make very different decisions while foraging and it is these decisions that we examine here. Of populations for which quantitative foraging data have been collected, these are the only groups with available data on mean harvest size in resource type or foraging activity, daily or per-bout variance in expected harvest size (risk), and the proportion of calories acquired by men and women of that resource type.
As a measure of harvest size, we use the mean energy (kcals) expected on a single foraging trip for each resource type. Risk is calculated as the coefficient of variation (CV) in mean energy. For Aché, mean energy and risk are calculated per family per day (in tables 5 and 7 [24]); for Martu and Meriam, they are calculated per individual foraging bout. The proportion of calories acquired by Meriam and Martu women in each foraging activity was calculated using the sum of all calories acquired by women in each foraging activity; similar measures for Aché were taken from Hawkes (fig. 3 in [5]). Resources for which data on any of these variables were unattainable were excluded.
All data are presented in the electronic supplementary material, table S1. To test our predictions, we examine the proportion of kilocalories women contribute relative to men for each resource as a function of risk and energy in a series of generalized linear models with a distribution (binomial) and link function (logit) appropriate to proportional data [31]. Each value is weighted based on the observed sample size (electronic supplementary material, table S1). Each set of models is evaluated by Akaike information criterion (AIC) values (which should be minimized [32]) and likelihood ratios (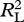 ; which should be maximized [33]). Data analysis was performed in R 2.10.1 [34].
; which should be maximized [33]). Data analysis was performed in R 2.10.1 [34].
3. Predictions
Offspring provisioning strategies should favour resources that provide an average harvest near a threshold requirement (feeding self and dependents) while minimizing variance. Social provisioning strategies should favour resources that maximize the quantity of energy (harvest size) acquired without much attention to variance. Resources that can be both reliably acquired and provide a large average harvest size (i.e. resources for which no risk–energy trade-off exists) should meet both offspring and social provisioning goals. According to this model, resources that are neither reliably acquired nor provide a large harvest should generally be ignored. We ask whether the trade-off between risk and harvest size influences the relative productivity of men and women in foraging activities associated with different energy–variance combinations. While individuals are likely to incorporate both high- and low-variance resources into their optimal diet breadth, the balance of effort to one or the other should be influenced by social and offspring provisioning goals. If men are more likely to give greater emphasis to social provisioning than women, then women should contribute more to low-variance resources with less attention to energy, while men should contribute more to high-energy resources with less attention to variance.
4. Results
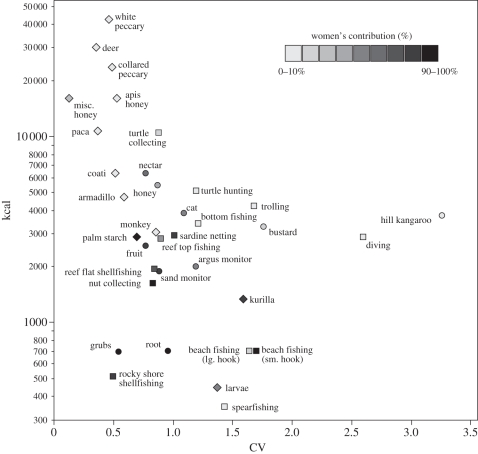
Figure 1 plots risk and harvest size for each foraging group with each point categorized by the proportion of total kilocalories contributed by women (see also figure 2; and electronic supplementary material, figures S1 and S2). To test our predictions, we examine these data in a series of generalized linear models to determine if the risk and energy for each resource predict women's contribution relative to men. Examining all three groups combined, the models confirm our predictions, showing that women's direct contribution is lower and men's higher for resource types with higher energy and/or greater levels of risk (table 1: models 1–3; electronic supplementary material, table S2). Our predictions are also confirmed when examining each group independently (table 1; figure 3; and electronic supplementary material, figure S3); however, the relative importance of risk versus energy differs for each group.
Figure 1.
Resource/hunt types plotted by their risk (CV of mean energy) and mean energy (kcal, on a log axis) per foraging bout; Aché (diamonds), Martu (circles) and Meriam (squares). Resources are categorized by the percentage contributed by women relative to men, in 10 per cent intervals. Data are presented in the electronic supplementary material, table S1.
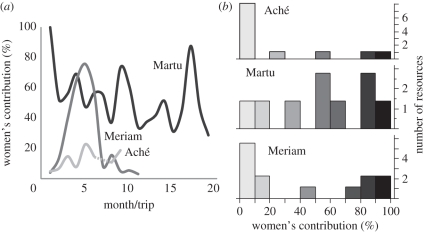
Figure 2.
(a) Percentage of total kilocalories women contribute relative to men per trip (Aché [19]) or month (Martu and Meriam). (b) Frequency distribution of resource/hunt types by the percentage of women contribute relative to men.
Table 1.
Model results examining the proportion women contribute to each resource/hunt type relative to men as a function of energy (kcal) and risk (CV). The best model for each dataset is displayed in bold. See electronic supplementary material, table S2 for extended information on each model. Data are presented in the electronic supplementary material, table S1.
| no. | variables | AIC | 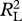 |
|---|---|---|---|
| populations combined | |||
| 1 | kcal + CV | 789.53 | 0.55* |
| 2 | kcal | 1147.60 | 0.32* |
| 3 | CV | 1514.00 | 0.10* |
| Aché | |||
| 4 | kcal + CV | 197.23 | 0.35* |
| 5 | kcal | 206.71 | 0.31* |
| 6 | CV | 207.41 | 0.31* |
| Martu | |||
| 7 | kcal + CV | 104.94 | 0.91 * |
| 8 | kcal | 294.14 | 0.62* |
| 9 | CV | 139.69 | 0.86* |
| Meriam | |||
| 10 | kcal + CV | 336.35 | 0.24 * |
| 11 | kcal | 377.36 | 0.12* |
| 12 | CV | 420.54 | 0.02** |
*p < 0.0001.
** p < 0.001.
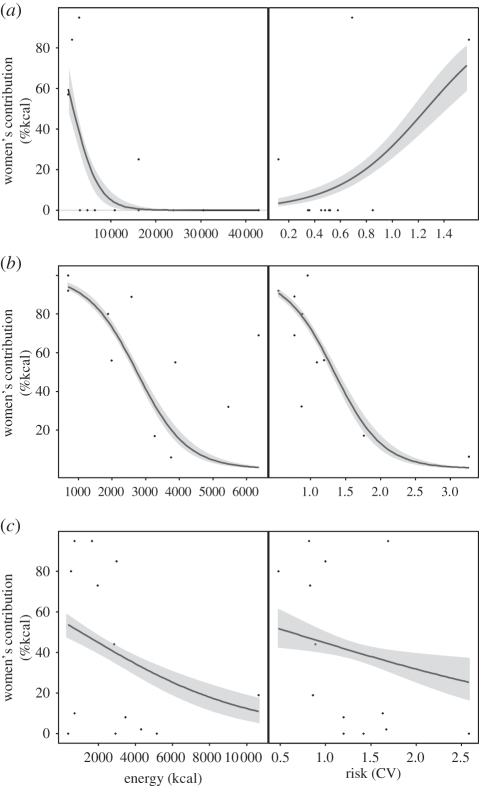
Figure 3.
Results of generalized linear models examining the effect of energy (kcal) and risk (CV) on women' s relative contributions to each resource acquired by (a) Aché, (b) Martu and (c) Meriam. Each point represents a resource, as in figure 1. See table 1 and electronic supplementary material, table S2 for model details. Combined model results are shown in electronic supplementary material, figure S3.
Aché women's proportional contribution across resources declines when energy increases, but actually increases with risk (table 1: models 4–6; figure 3a). Aché resources seem to impose no trade-off between risk and harvest size, thus Aché men are able to focus on high-energy resources without experiencing higher levels of risk. While Aché women contribute more to two relatively higher variance resources than men (kurilla and larvae), the absolute level of risk for these resources is quite low, especially in comparison with the other populations (electronic supplementary material, table S1).
As predicted, Martu women's contribution declines significantly as a function of increasing risk and energy (table 1: models 7–9; figure 3b; and electronic supplementary material, figure S3). With the resources available to Martu, risk and energy covary, thus imposing a strong risk–energy trade-off between offspring and social provisioning. The models show that risk alone is more important than energy alone in predicting Martu women's contribution to each resource, suggesting that Martu women are relatively risk-averse and Martu men risk-neutral or risk-prone.
As with Martu, Meriam women's contribution to resources declines when either risk or energy increases (table 1: models 10–12; figure 3c; and electronic supplementary material, figure S3). While energy alone appears more important than risk in predicting Meriam women's relative contribution, risk may be more important outside of turtle nesting season when men hunt turtle out at sea with higher levels of risk (discussed more below).
Aché men and women are relatively risk-indifferent because acquisition risk for all resources is generally low. In contrast, the resources available to Martu impose a serious trade-off between risk and energy, leading to risk sensitivity on the part of women and risk indifference by men. Meriam appear intermediate between these two extremes depending on the season, with men (and women) able to maximize energy with low risk in turtle nesting season.
The differences between each population can be characterized both by the balance of relative production (figure 2a) and the degree of overlapping resource choice (figure 2b). The suite of resource types in the Aché environment is heavily biased towards high-energy, low-variance items. While out on these foraging treks, the Aché division of labour is male-dominated, with the majority of resources taken almost exclusively by men, few taken only by women and several taken by both men and women (figure 2b). The outcome is a low relative production of women (13 per cent averaged across trips; figure 2a). The Martu environment provides mainly small to moderate harvest sizes with low variance, while nearly all high-energy resources (except honey) are high-variance. The result is an overlapping division of labour, with very few resources exclusive to one gender or the other, but more resources are taken by women overall (figure 2b), leading to a high relative production by women (64%). The Meriam environment contains the widest variety of resource types, including prey types in each energy–variance combination. Meriam have the most rigid division with the strongest bimodality and only a few overlapping resources (figure 2b). Here, women contribute 44 per cent to subsistence production outside the turtle nesting season, but only 6 per cent within it, and a total of 27 per cent averaged across all months (figure 2a).
5. Discussion
Within each population, differences in women's and men's relative contribution to foraging activities are patterned by risk–energy trade-offs: women have high contributions in low-variance resource types and men have high contributions in very high-energy resource types that are both high and low risk. Differences between each population appear to be a function of the types of resources available to each population and where those resources fall on the risk–energy continuum. These results suggest the following hypotheses: women tend to contribute more to offspring provisioning and perhaps more to overall production when there are strong trade-offs between harvest size and risk (Martu; Meriam outside the turtle nesting season), and that men contribute more extensively to offspring provisioning when they have access to large, low-variance resource types, and thus face few trade-offs between offspring and social provisioning (Aché; Meriam in turtle nesting season).
As we suggested at the outset, there are likely to be important social factors that interact to influence the extent to which women focus on risk minimization, accepting smaller harvests, and men on harvest maximization, accepting more risk. These factors are likely to shift men's and women's strategies along a continuum of possible combinations of social and offspring provisioning allocations. At one end of this continuum, men's and women's resource choices appear to converge with one another, while at the other end they diverge.
(a). ‘Convergent’ divisions of labour
We expect that the resources men and women target will overlap when habitats contain many resources that provide high-energy harvests with low risk. Here, we expect a division of labour in which men and women work together to procure resources that maximize daily harvest size while simultaneously supplying their dependent offspring with a reliable flow of resources. Whether or not women are also involved in acquiring high-energy, low-risk resources (or work to support acquisition) may depend on the availability of alloparental care.
Investment in offspring generally trades off with other activities: nursing limits the duration of foraging bouts [35], and carrying offspring decreases foraging efficiency by increasing load constraints and decreasing mobility [36]. The degree to which an individual can draw on support systems including intra- [1] and inter-generational care [37,38] should buffer childcare constraints, and thus alter the trade-off between risk minimization and energy maximization. When social support is low, and mothers are unable to rely on others to co-nurse, care for an infant during foraging bouts or specialize in alternative foraging tasks, women's foraging decisions should be more sensitive to childcare trade-offs. Under such circumstances, we expect that men should be more crucial in offspring provisioning either by bringing in large harvests for everyone (including children) or by forming cooperative productive units [11,39].
Of the three populations examined here, the Aché seem to have the most convergent division of labour. While out on these forest treks, Aché women's tasks frequently appear to be centred on the logistical support of men's hunting [21]. This is similar to the gender division of labour characterizing foraging strategies in the circumpolar north [40], where women's logistical support allows for men's specialized acquisition of large prey. This does not, however, mean Aché men do not gain benefits from social provisioning, but because there is no trade-off between risk and energy, men are able to simultaneously satisfy both foraging goals with acquisition of the same resources.
With more convergent divisions of labour, the extent to which women's foraging supports offspring provisioning should vary according to the opportunities for social support. If provisioner support networks are extensive, a mother's trade-off between childcare and foraging is reduced because others will be available to assist in direct childcare while mothers are out foraging; with increased support, we expect that women will be more involved in the acquisition of resources that provide large harvests with relatively low risk. A mother's overall risk aversion may also decline as others will frequently help acquire resources on a reliable basis to meet children's provisioning needs.
Aché caregiver networks have been characterized as relatively depauperate [22], especially given the lack of much grandmaternal investment owing to the high mortality of older women; this may explain why women are not often directly involved in hunting and subsequently rely more on men's income. In contrast to the Aché, the more extensive caregiver networks available to Agta women in northeastern Luzon may explain why despite similarities in the overall environment, Agta women actively participate in acquiring large prey [41].
Risk sensitivities might be further reduced in environments where resources are homogenously distributed. In such environments, travel and search time are shared for all resources. Thus, a forager attempting to maximize harvest size could preferentially target larger prey, but after several failed attempts could switch to pursuing smaller, lower-utility, less risky prey without having to move to a different patch or activity type. This may be the case with Aché foraging, where resource distribution is relatively homogeneous and hunts are successful on 37 to 63 per cent of days [42].
(b). ‘Divergent’ divisions of labour
The targets of women's and men's foraging should diverge and appear conflictive when resources with the potential to provide a larger harvest are also associated with higher levels of risk. In such circumstances, women's resource acquisition could be more important than men's for the daily provisioning of children, and at the extreme women's foraging may even subsidize men's risk-prone foraging. For example, Martu men's hill kangaroo hunting provides a larger harvest than women's sand monitor hunting, but succeeds too infrequently to provide a consistent income; thus women focus on the reliable acquisition of sand monitors to provision self and offspring [17,26]. This may also be the case in Tanzania, where Hadza men successfully acquire large game only once out of every 29 hunter days [43] and women provide more than 60 per cent of foraged foods and produce more than men across their lifespan [44].
However, the gender division of labour is flexible and dynamic: men and women are likely to respond sensitively to the needs of offspring and to each other. We expect that when offspring provisioning benefits are high and opportunity costs low, men should shift their focus to low-variance resources when and where they become available. For example, turtle hunting among Meriam is associated with moderate variance and is most frequently undertaken by unmarried men; turtle collecting during the nesting season is more reliable and is undertaken more by married men [28]. During the nesting season, turtle is an important resource for household provisioning, and although women do not often participate in their acquisition, they are involved in planning and supporting nesting turtle acquisition and in the butchery and distribution of meat that follows. Similarly, Hadza men switch from targeting large unreliable prey to smaller reliable prey when their wives are pregnant [45], and Martu men switch from hunting kangaroo to hunting monitor lizards when older (unpublished data in possession of the authors).
The targets of women's and men's foraging probably diverge more in patchier environments where large prey hunting success is rare. When environments are heterogeneous and resources of different utility occur in different spatial locations, a forager must first decide what patch to target. Patch dispersion and the number of different resource types in each patch will generally mediate this trade-off. But as patches become more dispersed and/or the complement of prey within them declines, foragers will face greater trade-offs between choosing patches associated with large harvest size and those associated with lower acquisition risk. Such patchiness may affect acquisition risk among !Kung (Ju/'hoansi) in Botswana [46,47]; where hunters fail on 73 per cent of all hunting days [42] and about 70 per cent of calories come from gathered resources [46].
Expanded social networks may also influence men's and women's allocations to social and offspring provisioning. More divergent divisions of labour may generate a need for (and be supported by) greater reliance on alloparenting, provisioning networks and intergenerational transfers. Expanded social networks may also change the nature of the relative benefits attainable through investing in social provisioning. Larger social networks provide a forager with a larger pool of potential recipients and a larger audience, leading to higher payoffs for returning with large quantities of food to distribute widely, such as gaining higher status, deference in decision-making and perhaps greater social support [8,29,30,47]; these in turn may provide indirect benefits to existing offspring, including increased offspring survivorship [10].
6. Conclusion
Our model predicts variability in the division of foraging labour as a function of the structure of local environments. Particularly important are the resources available to foragers and how those resources vary in their expected daily energetic returns and the variance associated with those returns. This influences whether there are trade-offs between social and offspring provisioning, which tend to be solved differently by men and women. The model we propose is an interactive one, positing dynamic flows between social structure, foraging decisions and the environment. When risk and energy trade off, gender differences may be mediated by the interaction between risk and social support; in such circumstances, the targets of men's and women's foraging tend to diverge. When risk and energy do not trade off, gender differences may be mediated by the interaction between childcare compatibility and social support; when this is the case, the targets of men's and women's foraging will tend to converge.
Here, we have shown that hunting and gathering are not unitary phenomena; both vary along the axes of risk and energy. Depending on the risk–energy combinations of available resources, foragers may have to make tough decisions about the costs and benefits of offspring versus social provisioning. While recent research has tended to emphasize either offspring or social provisioning as the primary explanation for patterns in men's and women's foraging, we show that it is crucial to understand both [11,12] and how they interact with each other. Ultimately, whether the goals of offspring or social provisioning trade off with one another depends on how local environments structure the trade-off between risk (variance) and energy (harvest size). Because risk and energy differences are not static across or even within environments, we expect these trade-offs to change over time and across space as a function of the availability of resources, both ecological and social. As such, we suggest that the gender division of foraging labour emerges through complex but predictable socioecological interactions and the individual decisions that result. Viewing the gender division of labour in this way may better account for the variability observed in men's and women's foraging decisions in diverse environments.
Acknowledgements
We would like to thank Martu and Meriam for their hospitality and patience. We also thank our colleagues who have worked with Aché and the Aché with whom they spent a great amount of time. Thanks to Bobbi Low and the anonymous referees whose thoughtful comments have greatly improved this paper. The Meriam project was funded by the NSF (SBR-9616096, SBR-9616887), AIATSIS and the Leakey Foundation. The Martu project is funded by the NSF (GRFP, DDIG BCS-0915380 and BCS-0127681, -0314406 and -0850664) and Stanford University.
References
- 1.Hrdy S. B. 2009. Mothers and others: the evolutionary origins of mutual understanding. Cambridge, MA: Harvard University Press [Google Scholar]
- 2.Kaplan H., Hill K., Lancaster J., Hurtado A. M. 2000. A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185 (doi:10.1002/1520-6505(2000)9:4<156::AID-EVAN5>3.0.CO;2-7) [DOI] [Google Scholar]
- 3.Kramer K. 2005. Maya children: helpers at the farm. Cambridge, MA: Harvard University Press [Google Scholar]
- 4.Hawkes K. 1991. Showing off: tests of an hypothesis about men's foraging goals. Ethol. Sociobiol. 12, 29–54 10.1016/0162-3095(91)90011-E (doi:10.1016/0162-3095(91)90011-E) [DOI] [Google Scholar]
- 5.Hawkes K. 1993. Why hunter–gathers work: an ancient version of the problem of public goods. Curr. Anthropol. 34, 341–361 10.1086/204182 (doi:10.1086/204182) [DOI] [Google Scholar]
- 6.Marlowe F. W. 2005. Hunter–gatherers and human evolution. Evol. Anthropol. 14, 54–67 10.1002/evan.20046 (doi:10.1002/evan.20046) [DOI] [PubMed] [Google Scholar]
- 7.Gurven M. 2004. To give and to give not: the behavioral ecology of human food transfers. Behav. Brain Sci. 27, 543–583 [Google Scholar]
- 8.Bliege Bird R., Smith E. A. 2005. Signaling theory, strategic interaction, and symbolic capital. Curr. Anthropol. 46, 221–248 10.1086/427115 (doi:10.1086/427115) [DOI] [Google Scholar]
- 9.Kaplan H., Hill K. 1985. Hunting and reproductive success among male Ache foragers: preliminary results. Curr. Anthropol. 26, 131–133 10.1086/203235 (doi:10.1086/203235) [DOI] [Google Scholar]
- 10.von Rueden C., Gurven M., Kaplan H. 2010. Why do men seek status? Fitness payoffs to dominance and prestige. Proc. R. Soc. B. 278, 2223–2232 10.1098/rspb.2010.2145 (doi:10.1098/rspb.2010.2145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurven M., Hill K. 2009. Why do men hunt? A re-evaluation of ‘man the hunter’ and the sexual division of labor. Curr. Anthropol. 50, 51–74 10.1086/595620 (doi:10.1086/595620) [DOI] [PubMed] [Google Scholar]
- 12.Hawkes K., O'Connell J. F., Coxworth J. E. 2010. Family provisioning is not the only reason men hunt: a comment on Gurven and Hill. Curr. Anthropol. 51, 259–264 10.1086/651074 (doi:10.1086/651074) [DOI] [Google Scholar]
- 13.Hawkes K. 1990. Why do men hunt? Some benefits for risky strategies. In Risk and uncertainty in tribal and peasant economies (ed. Cashdan E.), pp. 145–166 Boulder, CO: Westview Press [Google Scholar]
- 14.Jochim M. A. 1988. Optimal foraging and the division of labor. Am. Anthropol. 90, 130–136 10.1525/aa.1988.90.1.02a00100 (doi:10.1525/aa.1988.90.1.02a00100) [DOI] [Google Scholar]
- 15.Bird R. B. 1999. Cooperation and conflict: the behavioral ecology of the sexual division of labor. Evol. Anthropol. 8, 65–75 (doi:10.1002/(SICI)1520-6505(1999)8:2<65::AID-EVAN5>3.0.CO;2-3) [DOI] [Google Scholar]
- 16.Hawkes K., O'Connell J. F., Blurton Jones N. G. 1997. Hadza women's time allocation, offspring provisioning and the evolution of long postmenopausal life spans. Curr. Anthropol. 38, 551–577 10.1086/204646 (doi:10.1086/204646) [DOI] [Google Scholar]
- 17.Bird D. W., Bliege Bird R., Codding B. F. 2009. In pursuit of mobile prey: Martu foraging and Archaeofaunal interpretation. Am. Antiquity 74, 3–29 [Google Scholar]
- 18.Hewlett B. S., Lamb M. E. 2005. Hunter–gatherer childhoods: evolutionary, developmental and cultural perspectives. New Brunswick, NJ: Transaction Publishers [Google Scholar]
- 19.Hurtado A., Hawkes K., Hill K., Kaplan H. 1985. Female subsistence strategies among Ache hunter–gatherers of eastern Paraguay. Hum. Ecol. 13, 1–28 10.1007/BF01531086 (doi:10.1007/BF01531086) [DOI] [Google Scholar]
- 20.Hawkes K., Hill K., O'Connell J. F. 1982. Why hunters gather: optimal foraging and the Aché of Eastern Paraguay. Am. Ethnol. 9, 379–398 10.1525/ae.1982.9.2.02a00100 (doi:10.1525/ae.1982.9.2.02a00100) [DOI] [Google Scholar]
- 21.Hawkes K., Kaplan H., Hill K., Hurtado A. M. 1987. Ache at the settlement: contrasts between farming and foraging. Hum. Ecol. 15, 133–161 10.1007/BF00888378 (doi:10.1007/BF00888378) [DOI] [Google Scholar]
- 22.Hill K., Hurtado A. M. 1996. Aché life history: the ecology and demography of a foraging people. New York, NY: Aldine de Gruyter [Google Scholar]
- 23.Hill K., Kaplan H., Hawkes K., Hurtado M. 1987. Foraging decisions among Aché hunter-gatherers: new data and implications for optimal foraging models. Ethol. Sociobiol. 8, 1–36 10.1016/0162-3095(87)90055-0 (doi:10.1016/0162-3095(87)90055-0) [DOI] [Google Scholar]
- 24.Kaplan H., Hill K. 1985. Food sharing among Ache foragers: tests of explanatory hypotheses. Curr. Anthropol. 26, 223–245 10.1086/203251 (doi:10.1086/203251) [DOI] [Google Scholar]
- 25.Bliege Bird R., Codding B. F., Bird D. W. 2009. Determinants of gendered foraging and production inequalities among Martu. Hum. Nat. Int. Biosci. 20, 105–129 10.1007/s12110-009-9061-9 (doi:10.1007/s12110-009-9061-9) [DOI] [PubMed] [Google Scholar]
- 26.Bliege Bird R., Bird D. W. 2008. Why women hunt: risk and contemporary foraging in a Western Desert Aboriginal community. Curr. Anthropol. 49, 655–693 10.1086/587700 (doi:10.1086/587700) [DOI] [PubMed] [Google Scholar]
- 27.Bliege Bird R. 2007. Fishing and the sexual division of labor among the Meriam. Am. Anthropol. 109, 442–451 10.1525/aa.2007.109.3.442 (doi:10.1525/aa.2007.109.3.442) [DOI] [Google Scholar]
- 28.Bliege Bird R., Bird D. W. 1997. Delayed reciprocity and tolerated theft: the behavioral ecology of food sharing strategies. Curr. Anthropol. 38, 49–78 10.1086/204581 (doi:10.1086/204581) [DOI] [Google Scholar]
- 29.Bliege Bird R., Smith E. A., Bird D. W. 2001. The hunting handicap: costly signaling in male foraging strategies. Behav. Ecol. Sociobiol. 50, 9–19 10.1007/s002650100338 (doi:10.1007/s002650100338) [DOI] [Google Scholar]
- 30.Smith E. A., Bliege Bird R. 2000. Turtle hunting and tombstone opening: public generosity as costly signaling. Evol. Hum. Behav. 21, 245–261 10.1016/S1090-5138(00)00031-3 (doi:10.1016/S1090-5138(00)00031-3) [DOI] [PubMed] [Google Scholar]
- 31.Faraway J. J. 2006. Extending the linear model with R: generalized linear, mixed effects and nonparametric regression models. New York, NY: Chapman and Hall [Google Scholar]
- 32.Akaike H. 1974. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 19, 716–723 10.1109/TAC.1974.1100705 (doi:10.1109/TAC.1974.1100705) [DOI] [Google Scholar]
- 33.Menard S. 2002. Applied logistic regression analysis, 2nd edn. Thousand Oaks, CA: Sage Publications [Google Scholar]
- 34.R Development Core Team 2009. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 35.Hurtado A., Hill K., Kaplan H., Hurtado I. 1992. Tradeoffs between female food acquisition and child care among Hiwi and Ache foragers. Hum. Nat. Int. Biosci. 3, 185–216 10.1007/BF02692239 (doi:10.1007/BF02692239) [DOI] [PubMed] [Google Scholar]
- 36.Blurton Jones N. G. 1986. Bushman birth spacing: a test for optimal interbirth intervals. Ethol. Sociobiol. 7, 91–105 10.1016/0162-3095(86)90002-6 (doi:10.1016/0162-3095(86)90002-6) [DOI] [Google Scholar]
- 37.Hawkes K., O'Connell J. F., Blurton Jones N. G., Alvarez H., Charnov E. L. 1998. Grandmothering, menopause, and the evolution of human life histories. Proc. Natl Acad. Sci. USA 95, 1336–1339 10.1073/pnas.95.3.1336 (doi:10.1073/pnas.95.3.1336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sear R., Mace R. 2008. Who keeps children alive? A review of the effects of kin on child survival. Evol. Hum. Behav. 29, 1–18 10.1016/j.evolhumbehav.2007.10.001 (doi:10.1016/j.evolhumbehav.2007.10.001) [DOI] [Google Scholar]
- 39.Hill K., Hurtado A. M. 2009. Cooperative breeding in South American hunter–gatherers. Proc. R. Soc. B 276, 3863–3870 10.1098/rspb.2009.1061 (doi:10.1098/rspb.2009.1061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jarvenpa R., Brumbach H. J. 2006. Circumpolar lives and livelihood: a comparative ethnoarchaeology of gender and subsistence. Lincoln, NE: University of Nebraska Press [Google Scholar]
- 41.Goodman M. J., Griffin P. B., Estioko-Griffin A. A., Grove J. S. 1985. The compatibility of hunting and mothering among the Agta hunter–gatherers of the Philippines. Sex Roles 12, 1199–1209 10.1007/BF00287829 (doi:10.1007/BF00287829) [DOI] [Google Scholar]
- 42.Hill K., Kintigh K. 2009. Can anthropologists distinguish good and poor Hunters? Implications for hunting hypotheses, sharing conventions, and cultural transmission. Curr. Anthropol. 50, 369–377 10.1086/597981 (doi:10.1086/597981) [DOI] [Google Scholar]
- 43.Hawkes K., O'Connell J. F., Blurton Jones N. G. 1991. Hunting income patterns among the Hadza: big game, common goods, foraging goals and the evolution of the human diet. Phil. Trans. R. Soc. Lond. B 334, 243–251 10.1098/rstb.1991.0113 (doi:10.1098/rstb.1991.0113) [DOI] [PubMed] [Google Scholar]
- 44.Marlowe F. W. 2010. The Hadza hunter–gatherers of Tanzania. Berkeley, CA: University of California Press [Google Scholar]
- 45.Marlowe F. W. 2003. A critical period for provisioning by Hadza men: implications for pair bonding. Evol. Hum. Behav. 24, 217–229 10.1016/S1090-5138(03)00014-X (doi:10.1016/S1090-5138(03)00014-X) [DOI] [Google Scholar]
- 46.Lee R. B. 1979. The !Kung San: men, women and work in a foraging society. Cambridge, UK: Cambridge University Press [Google Scholar]
- 47.Wiessner P. 2002. Hunting, healing, and hxaro exchange: a long-term perspective on !Kung (Ju/'hoansi) large-game hunting. Evol. Hum. Behav. 23, 407–436 10.1016/S1090-5138(02)00096-X (doi:10.1016/S1090-5138(02)00096-X) [DOI] [Google Scholar]