Abstract
OBJECTIVES
To investigate how changes in frailty status and mortality risk relate to baseline frailty state, mobility performance, age and sex.
SETTING
The Yale Precipitating Events Project, a cohort study based in New Haven CT.
PARTICIPANTS
754 community-dwelling people, aged 70+ years at baseline followed-up at 18, 36 and 54 months.
METHODS
Frailty status, assessed at 18-month intervals, was defined by a frailty index, as the number of deficits in 36 health variables. Mobility was defined as the time in seconds on the rapid gait test, where participants walked back and forth over a 20-ft course as quickly as possible. Multi-state transition probabilities were calculated with baseline frailty, mobility, age and sex estimated by Poisson and logistic regressions in survivors and those who died, respectively.
RESULTS
In multivariable analyses, baseline frailty status and age were significantly associated with changes in frailty status and the risk of death, while mobility was significantly associated with the former, but not with mortality. At all values of the frailty index, compared to those with poor mobility, participants with better mobility were more likely to remain stable or to improve. For example, at 54 months, 20.6% (95% confidence interval (CI) =16–25.2) of participants with poor mobility had the same or fewer deficits compared to 32.4% (95% CI=27.9–36.9) of those with better mobility.
CONCLUSION
A multi-state transition model effectively measured the probability of changes in frailty status and in the risk of death. Mobility, age and baseline frailty were significant factors in frailty state transitions.
Keywords: frailty, Frailty Index, mortality, aging, mobility, multistate model
INTRODUCTION
Aging is associated with an increasing vulnerability to adverse health outcomes, including death. The state of increased risk known as frailty can be quantified using a frailty index (FI).1–3 The FI measures so-called health deficits (e.g., symptoms, signs, diseases, disabilities or laboratory abnormalities) usually presented as a count of deficits present in an individual divided by the total amount of deficits considered. The more deficits that individuals have, the more frail they are likely to be.1–6 Calculated like this, the FI has shown replicable properties, including a prevalence that consistently increases exponentially with age and is strongly related to the risk of institutionalisation, death,2–8 health service use,9 and worsening health.10–12 An FI can be created using any sufficiently large deficit count, following a few standard procedures, and so can be operationalized readily in clinical and epidemiological databases.13 For these reasons, the FI is an integrative trait suitable for quantitative analyses, including dynamic and recurrent frailty state transitions. 10–12
Changes in health states, such as transitions between different states of mobility and function14–18 are of considerable clinical and public health interest. Although, on average, the number of deficits that people accumulate increases with age, this average masks important variability, including improvement, as reflected by fewer deficits. This improvement depends on both the prior level of frailty10–12 and on several factors, not the least of which is age.14,16,19–21
In general, investigations of health transitions model only a small number of states (e.g., between being non-disabled, disabled, severely disabled, or dead). Isolating the effect of risk factors when considering more than a few states is difficult, because the number of possible outcomes increases exponentially as the number of states increases. To allow many states and many factors to be analyzed without loss of power, a method has been introduced, whereby transitions between any numbers of states in an integrative variable can be represented by a multi-state model.3,10–12 The model has a small number of interpretable parameters, which can be estimated in relation to other variables.22. This multivariable stochastic model of frailty state transitions has been used, for example, in various Canadian health surveys to study the effects of exercise, sex and age on cognition22,23 and frailty.24
Mobility is a major item in many frailty definitions1,25–27 and appears to predict incidentfrailty.28 The objective of the current study was to investigate how changes in frailty status and mortality risk depend on baseline mobility performance, age and sex. A further aim was to demonstrate the use of this stochastic model of frailty state transitions in evaluating how one clinically important item (mobility) can be considered in relation to the large number of variables that are included in the frailty index
METHODS
Sample
The Yale Precipitating Events Project (PEP) is a cohort study based in New Haven CT. As detailed elsewhere,15,29 754 community-dwelling, English speaking, non-disabled persons, aged 70 years or older, with a life expectancy and plans to stay nearby for >12 months, were enrolled. Comprehensive home-based assessments were completed at baseline and every 18 months. Here, data from the baseline and 18-month, 36-month and 54-month reassessments were used to define frailty transitions in relation to the baseline frailty state, defined by the baseline deficit count. The deficit count was computed for all participants at baseline. Thirty, 36 and 40 people refused interview or had incomplete data to compute deficit counts at 18, 36 and 54 months follow-up respectively. Forty-nine participants had died before the 18 month assessment. The number of decedents was 98 at 36 months and 166 at 54 months.
Definition of frailty states
The baseline and follow-up frailty states are defined by the frailty index (FI), being the number of deficits present at any assessment, divided by the total number of deficits considered. The FI reported here was modified to evaluate the effect of mobility as an independent variable, so that instead of counting the 40 deficits reported previously from this cohort,13 only 36 were counted. Two of the variables excluded here are mobility measures (walking a 20-ft course at one’s usual pace and as quickly as possible). Instead of being included in the FI, walking a 20-ft course as quickly as possible is treated as an independent variable, signifying poor mobility. The two other variables that were excluded (“stayed in bed at least half the day due to health in last month” and “cut down on usual activity in last month”) were not available at follow-up assessments. The remaining FI deficits consisted of self reports of health attitudes, disability in Activities Daily Living (ADL) and Instrumental ADL as well as measures of cognition, physical performance (e.g. impaired grip strength), co-morbidity, and depression. For ease of understanding, both the absolute deficit count, and its corresponding FI score (i.e. the deficit count divided by 36) are reported where appropriate. Note that each deficit count defines a discrete frailty state, e.g. state 0 defines a participants with 0 deficits, state 1 signifies 1 deficit (=FI of 0.03) and so on.
Definition of mobility status
Mobility status was assessed as the time in seconds on the rapid gait test, in which participants walked back and forth over the 20-ft course as quickly as possible. This was employed both as a continuous variable and as a dichotomous variable, with a cut-point of > 10 seconds denoting poor mobility (coded as "1"). Otherwise, participants were considered to have good mobility (coded as "0").
Data Analysis
Transitions in frailty states were operationalized as changes in the deficit counts. The probability of transitioning between baseline and follow-up frailty states was analysed using a multi-state transition model.10–12,23 A more detailed description of the model is presented in the Appendix: Supporting Materials available in online version. Age and mobility were considered as continuous covariates This model estimates the probabilities of all transitions, i.e. death, and for survivors, improvement, stability and decline. For each follow-up period, the parameters of the model were estimated using a Generalized Linear Model (GLM). This allowed an evaluation of the effects of covariates (baseline frailty status, age, sex, and mobility) on transitions in frailty states of survivors (Poisson regression) and the effects of these covariates on mortality (logistic regression). All calculations were performed using the most recent version of the SPSS (PASW-18).
RESULTS
At baseline, the participants’ mean age was 78 years; 64% were women. The mean number of deficits at baseline was 6.3 (i.e. FI= 0.17), which gradually increased to 7.9 deficits (FI=0.22) at the 54 month reassessment (Table 1). The percentage of participants with poor mobility did not change significantly over the follow-up periods. Forty-nine participants (6.5%) died at 18 months, 98 (13%) had died by 36 months and 166 (22%) by 54 months. Table 2A and Table 2B provide the results of the multivariate analyses for the changes in frailty states and mortality, respectively. The regression coefficients indicate that the FI at baseline had the strongest influence on frailty transitions and mortality, as its regression coefficients are an order of magnitude higher than they are for mobility and age. Sex had no independent influence on the changes in the number of deficits (Table 2A) but was strongly associated with the risk of death (Table 2B). In contrast, mobility had no association with death. Interestingly, when the mortality model included only mobility, it was significantly associated with mortality (Appendix available in online version, Table S1) but when baseline frailty was added to the model, mobility no longer appeared to be significant.
Table 1.
Baseline and follow-up characteristics of the sample.
| Characteristic | Baseline (n=754) |
Survivors to 18 months (n=675) |
Decedents up to 18 months (n=49) |
Survivors To 36 months (n=620) |
Decedents up to 36 months (n=98) |
Survivors to 54 months (n=548) |
Decedents up to 54 months (n=166) |
|---|---|---|---|---|---|---|---|
| Age, mean ±SD | 78.4 ±5.1) | 79.7 ±5.1 | 80.9 ±6.1 | 80.9 ±5 | 80.7 ±5.6 | 82.2 ±4.9 | 80.7 ±5.5 |
| Female, n (%) | 487 (64.5) | 444 (65.7) | 24 (49) | 415 (66.9) | 48 (49) | 374 (68.2) | 90 (54.2) |
| Frailty Index (count), mean±SD | 6.3 ±4.1 | 6.9 ±4.4 | 8.7 ±5.1 | 7.8 ±4.8 | 8.5 ±4.6 | 7.9 ±5.3 | 8.6 ±4.5 |
| Frailty Index (ratio), mean±SD | 0.17 ±0.11 | 0.19 ±0.12 | 0.24 ±0.14 | 0.22 ±0.13 | 0.24 ±0.13 | 0.22 ±0.15 | 0.24 ±0.13 |
| Poor Mobility* n (%) | 323 (42.8) | 281 (41.6) | 29 (59.2) | 273 (44.0) | 55 (56.1) | 262 (47.8) | 102 (61.4) |
Poor mobility was defined as a slow rapid pace walk (>10 sec)
Table 2.
| A. Parameter estimates and their 95% confidence intervals for Poisson regression | |||
|---|---|---|---|
| Covariate | Regression coefficient for Poisson model in survivors | ||
| 18-month follow-up | 36-month follow-up | 54-month follow-up | |
| (Intercept) | 0.21 (−0.28, 0.71)* | 0.43 (−0.13, 0.99)* | 0.32 (−0.38, 1.01)* |
| Frailty Index count at baseline | 0.84 (0.77, 0.89) | 0.89 (0.82, 0.96) | 0.88 (0.79, 0.96) |
| Age† | 0.08 (0.04, 0.11) | 0.10 (0.05, 0.14) | 0.18 (0.14, 0.23) |
| Sex | 0.23 (−0.13, 0.57)* | 0.36 (−0.05, 0.78)* | −0.23 (−0.68, 0.19)* |
| Mobility | 0.08 (0.02, 0.14) | 0.11 (0.04, 0.18) | 0.12 (0.03, 0.21) |
| B. Parameter estimates and their 95% confidence intervals for logistic regression | |||
|---|---|---|---|
| Covariate | Regression coefficients for logistic regression for mortality | ||
| 18-month follow-up | 36-month follow-up | 54-month follow-up | |
| (Intercept) | −4.56 (−5.51,−3.62) | −3.95 (−4.66,−3.23) | −3.81 (−4.45, −3.17) |
| Frailty Index count at baseline | 0.14 (0.07, 0.23) | 0.15 (0.08, 0.21) | 0.16 (0.11, 0.21) |
| Age† | 0.07 (0.01, 0.13) | 0.07 (0.03, 0.12) | 0.08 (0.04, 0.12) |
| Sex | 0.79 (0.17, 1.41) | 0.87 (0.41, 1.34) | 0.86 (0.45, 1.26) |
| Mobility | 0.01 (−0.07, 0.05)* | 0.01 (−0.05, 0.04)* | 0.01 (−0.01, 0.07)* |
indicates statistically insignificant estimates (p>0.05)
age is entered as the difference between the age at baseline and 70 (minimal age of the sample).
Mobility and age can be also used as dichotomised variables, simplifying the illustration of difference in transitions in frailty states. The density distributions of all transitions between the different states showed some similarities between the effects of age (Appendix available in online version, Figure S2, Panel A) and mobility (Figure S2, Panel B). In all panels, there is an evident shift to the right of the curves represented by older participants (Panel A, solid curves) and participants with poor mobility (Panel B. solid curves).
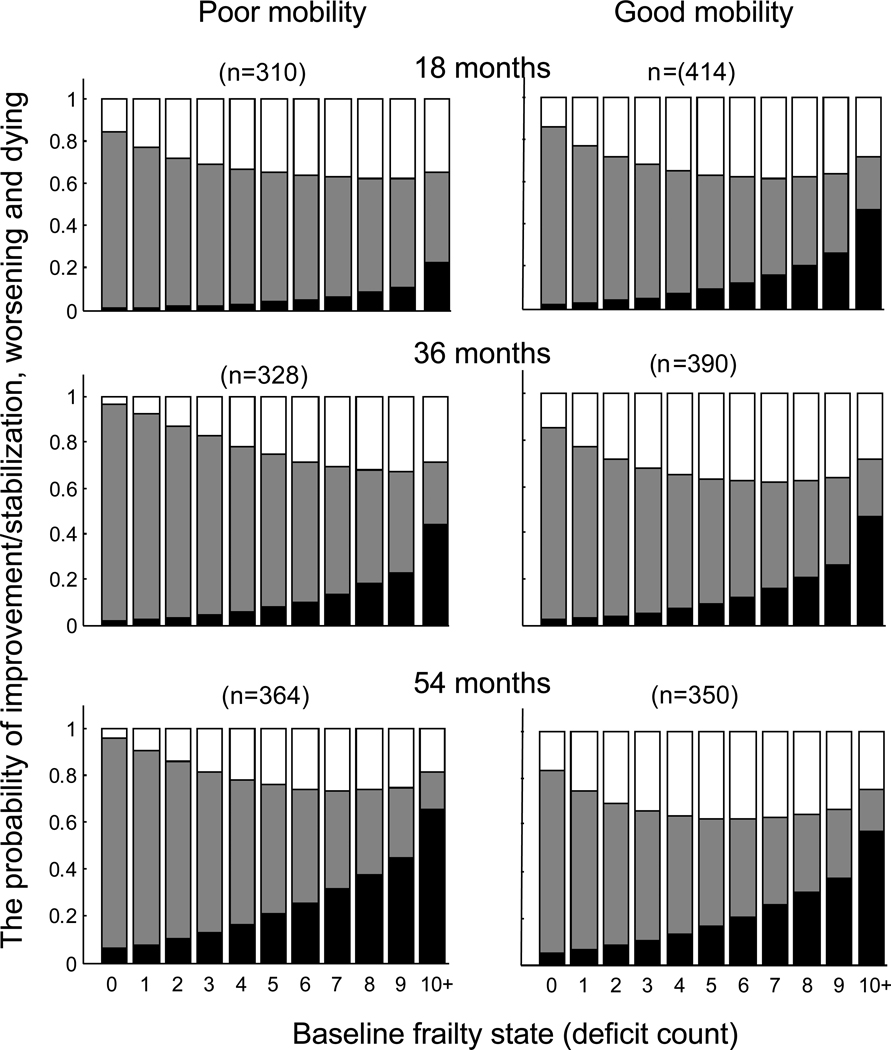
Figure 1 shows changes in frailty status (deficit count) in relation to baseline mobility for three consecutive follow-up assessments. The probability of improvement or stability (indicated in white) was significantly higher for participants with better baseline mobility, especially at 18 months of follow-up. Although the probability of death increased over the follow-up period, the differences in mortality between participants with better and worse mobility at baseline were not significant. Over the first 18 months, more participants who had poor mobility at baseline died (9.3%, CI= 6.0–12.6) than did those with better baseline mobility (4.7%, 95% CI= 2.7–6.7). Similarly, fewer participants with poor mobility remained at the same FI (13%, 95% CI=9.2–16.8) than did those who had better mobility (23.5%, 95% CI=19.4–27.6). Improvement in frailty status (i.e. a lower FI) was seen in similar proportions in participants with poor baseline mobility (27.0%, 95% CI=22.0–32.0) and in those with better baseline mobility, 28.5% (95% CI=24.2–32.8).
Figure 1.
Changes in frailty status (deficit count) in relation to baseline mobility for three consecutive follow-up assessments. The probability of improvement or stability is indicated in white, worsening is shown in grey, and probability of death is shown in black. The probabilities of the outcomes among participants with poor mobility (>10 sec on rapid gait test) are shown at the left panels; the probabilities of the outcomes among participants with good mobility are presented at the right panels. The upper panels show the transition probabilities after the first 18 months, the middle panels displays the probabilities of transitions between baseline and 36 months and the bottom panel shows the probabilities between baseline and 54 months. The last state (10+) refers to the “collapsed states” for those who had 10 deficits and over (<8%).
By 36 months, more participants who had poor mobility at baseline died (17.3%, 95% CI= 13–21.6) as compared to those with better baseline mobility (10.2%, 95% CI= 7.3–13.1). Similarly, fewer participants with poor mobility remained at the same FI (10%, 95% CI=6.6–13.4) as compared to those who had better baseline mobility (22.1%, 95% CI= 18.1–26.1). Improvement in frailty status (i.e. a lower FI) was seen in similar proportions of participants with poor baseline mobility (14%, 95% CI=10.1–17.9) and with better baseline mobility, 14.1% (95% CI= 10.8–17.4).
Similar results were observed at 54 months. More participants who had poor mobility at baseline died (32.3%, 95% CI= 27–37.6) than did those with better baseline mobility (15.2%, 95% CI= 11.8–18.6). Similarly, fewer participants with poor mobility remained at the same FI (7.6%, 95% CI=4.6–10.6) as compared to those who had better mobility (16.1%, 95% CI=12.6–19.6). As compared to earlier follow-up evaluations, improvement in frailty status (i.e. a lower FI) was seen less often, both in participants with poor baseline mobility (13%, 95% CI=9.2–16.8) and in those with better baseline mobility, 16.3% (95% CI= 12.8–19.8).
DISCUSSION
The main finding of the current study is that a stochastic model of frailty state transitions allowed both the evaluation of the average change in frailty states, and simultaneous estimation of the probability of improving, remaining stable, worsening, and dying. Of relevance to clinicians and researchers are that most changes in frailty states occurred gradually, and despite a background of average decline, some participants improved; age and mobility were significantly associated with transitions in frailty states. Significantly more participants with good mobility did not worsen, as compared to those with poor mobility. By contrast, mobility and sex were significantly related to mortality, but not with frailty state transitions. Although effect sizes increased as time passed, similar relationships between frailty transitions (i.e. the chance of increasing or decreasing the deficit counts) and mortality, and age, sex and mobility were seen for each of the intervals from baseline to 54 months. In short, we see mobility and frailty as inextricably linked, with clear interactions; our data likely reflect that frail people move less, and that people who move less become (even) more frail. From a systems perspective, both mobility and frailty are state variables – i.e. are high order, integrative variables that reflect the function of many systems, including the cardiovascular, musculoskeletal, neurological, respiratory, etc.
Our data contribute to the literature on frailty and how it changes with age. In contrast to other transition models,14,19,30 the model presented here is not limited to a few states. This stochastic model of frailty state transitions quantifies the chance of not just greater frailty, (which is the most common case, and which is represented by an increasing number of deficits) but also models mortality, stabilization and improvement as competing events and calculates probabilities for each of these outcomes.3,10–12 In this stochastic model of frailty state transitions, a large number of baseline states are represented by a small number of readily interpretable parameters. In this way, the approach employed here allows quantification of the probability of improvement in health. Fluctuation in mobility and disability states is well described14,16,19–21 and improvement is of increasing interest.12,17,18 The frailty index describes improvement from any frailty state, except the zero state, from which, by definition no improvement is possible (i.e. it is not possible to have fewer than zero deficits at follow-up.) Even so, improvement in clinically relevant items that were not measured by the frailty index is possible, such as improvement in exercise tolerance. As frailty is potentially modifiable,27 the model can be used to assess factors that may contribute to improvement. For example, a similar transition model quantified the effect of exercise on transitions in frailty states during a 5-year follow-up period.24
Our data must be interpreted with caution. This is a secondary analysis, so prospective confirmation is required, and a larger sample size would allow additional subgroups of interest to be studied (e.g. persons with social vulnerability). In addition, the analytical method is comparatively new, introduced only in 2006.10 Nonetheless, it has been replicated in various settings, including with measures other than the Frailty Index, and the model is being further developed. (See Supplementary Materials for additional, formal notation and references.) Here, too, we have been able to demonstrate how the model can include continuous variables, and not just dichotomous ones as covariates. Of note, all the terms that were significant in dichotomized models were also significant in the continuous models. Similarly, other terms that were not significant in the dichotomized model were significant in the continuous models.
An additional limitation is that the model relates risk only to the baseline state, which obviously can change over time, and as the model illustrates, can show improvement. Clearly too, the closer in time the information is, the more refined any prediction will become. A further and related caution is that interpreting these results in relation to frailty depends on the frailty states being well represented by the deficit count. Recalling that the essence of frailty is the vulnerability to adverse outcomes, several studies show that deficit accumulation is strongly associated with the risk of dying, being institutionalized, using health care services,1–12 and having fewer, the same, or more frailty deficits at follow-up.10–12
It might be argued that frailty is not just correlated with mobility, but operationally inseparable from it. Empirically, this is not so. Although mobility was not associated with mortality independently of frailty, it was associated with the transition in frailty states. Even there, however, the contribution of mobility to frailty is comparatively small, as can be illustrated by an example, applying the parameter estimates from Table 2. For any group of participants, the overall change in the value of the frailty index is driven largely by the starting value of the frailty index. Consider the 36 month outcomes, which are arithmetically convenient. Most change in the frailty index will come from the starting value of the frailty index, which will be multiplied by 0.89 for each starting deficit. The frailty index will also increase by 0.1 deficit for every year older that a person is from age 70 (the youngest age at baseline). Mobility has about the same impact as age: for every second to walk 20 feet in the rapid gait test, the frailty index at 36 months will increase by 0.1 deficits. Because the minimum recorded time was 4 seconds, mobility status adds at least 0.4 deficits. In short, applying the coefficients from the continuous model in Table 2B, a 77 year old man who had 6 deficits at baseline (this is the median baseline deficit count) and who took 10 seconds to walk twenty feet (this is also the median baseline value) would, on average, have 13 deficits after 3 years. Of these 7 additional deficits acquired over 3 years, 1 would be gained in association with age, 1 in association with mobility and the remaining 5 in association with the starting frailty index.
The approach presented here illustrates how the effect of items of particular interest can be evaluated. Mobility is clinically important, readily discernable, influences health outcomes and is a key prognostic feature in relation to frailty. 28,31 Quantitative models that can isolate its impact in relation to a large number of other states allow better understanding of the complexity of frailty.
Supplementary Material
ACKNOWLEDGMENTS
These analyses were supported by a grant from the Canadian Institutes of health research (MOP-64169) of which Dr. Mitnitski is a principal investigator). Dr. Fallah is supported by a postdoctoral fellowship from the Alzheimer’s Society of Canada. Samuel Searle was supported by the George Mattar Summer Studentship. Dr. Rockwood is supported by the Dalhousie Medical Research Foundation as the Kathryn Allen Weldon Chair in Alzheimer Research. The Participating Events Project study was supported by grants R37AG17560 and R01AG022993 from the National Institute on Aging. Dr Gill is the recipient of a Midcareer Investigator Award (K24AG021507) in Patient-Oriented Research from the National Institute on Aging.
Sponsor’s Role: None
Footnotes
Conflict of Interest:
None of the authors have any conflicts of interest to declare.
Author Contributions:
NF, SDS, AM: analysis, writing, review.
TMG, EAG: data collection, review.
KR: conceptualization, writing, review.
REFERENCES
- 1.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Scientific World Journal. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–2189. doi: 10.1111/j.1532-5415.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 3.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol Biol Sci Med Sci. 2007;62A:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 4.Goggins WB, Woo J, Sham A, et al. Frailty index as a measure of biological age in a Chinese population. J Gerontol A Biol Sci Med Sci. 2005;60:1046–1051. doi: 10.1093/gerona/60.8.1046. [DOI] [PubMed] [Google Scholar]
- 5.Kulminski AM, Arbeev KG, Ukraintseva SV, et al. Changes in health status among participants of the Framingham Heart Study from the 1960s to the 1990s: application of an index of cumulative deficits. Ann Epidemiol. 2008;18:696–701. doi: 10.1016/j.annepidem.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu D, Dupre ME, Sautter J, et al. Frailty and mortality among Chinese at advanced ages. J Gerontol B Psychol Sci Soc Sci. 2009;64:279–289. doi: 10.1093/geronb/gbn009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulminski A, Yashin A, Arbeev K, et al. Cumulative index of health disorders as an indicator of aging-associated processes in the elderly: Results from analyses of the National Long Term Care Survey. Mech Ageing Dev. 2007;128:250–258. doi: 10.1016/j.mad.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kulminski AM, Ukraintseva SV, Culminskaya IV, et al. Cumulative deficits and physiological indices as predictors of mortality and long life. J Gerontol A Biol Sci Med Sci. 2008;63:1053–1059. doi: 10.1093/gerona/63.10.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones D, Song X, Mitnitski A, et al. Evaluation of a frailty index based on a comprehensive geriatric assessment in a population based study of elderly Canadians. Aging Clin Exp Res. 2005;17:465–471. doi: 10.1007/BF03327413. [DOI] [PubMed] [Google Scholar]
- 10.Mitnitski A, Bao L, Rockwood K. Going from bad to worse: A stochastic model of transitions in deficit accumulation, in relation to mortality. Mech Ageing Dev. 2006;127:490–493. doi: 10.1016/j.mad.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Mitnitski A, Bao L, Skoog I, et al. A cross-national study of transitions in deficit counts in two birth cohorts: Implications for modeling ageing. Exp Gerontol. 2007;42:241–246. doi: 10.1016/j.exger.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Mitnitski A, Song X, Rockwood K. Improvement and decline in health status from late middle age: Modeling age-related changes in deficit accumulation. Experimental Gerontology. 2007;42:1109–1115. doi: 10.1016/j.exger.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Searle SD, Mitnitski A, Gahbauer EA, et al. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy SE, Dubin JA, Holford TR, et al. Transitions between states of disability and independence among older persons. Am J Epidemiol. 2005;161:575–584. doi: 10.1093/aje/kwi083. [DOI] [PubMed] [Google Scholar]
- 15.Gill TM, Allore HG, Hardy SE, et al. The dynamic nature of mobility disability in older persons. J Am Geriatr Soc. 2006;54:248–254. doi: 10.1111/j.1532-5415.2005.00586.x. [DOI] [PubMed] [Google Scholar]
- 16.Hardy SE, Allore HG, Guo Z, et al. The effect of prior disability history on subsequent functional transitions. J Gerontol A Biol Sci Med Sci. 2006;61:272–277. doi: 10.1093/gerona/61.3.272. [DOI] [PubMed] [Google Scholar]
- 17.Boyd CM, Ricks M, Fried LP, et al. Functional decline and recovery of activities of daily living in hospitalized, disabled older women: The Women's Health and Aging Study I. J Am Geriatr Soc. 2009;57:1757–1766. doi: 10.1111/j.1532-5415.2009.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seidel D, Jagger C, Brayne C, et al. CFAS Recovery in instrumental activities of daily living (IADLs): findings from the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Age Ageing. 2009;38:663–668. doi: 10.1093/ageing/afp128. [DOI] [PubMed] [Google Scholar]
- 19.Gill TM, Gahbauer EA, Allore HG, et al. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166:418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 20.Hardy SE, Allore HG, Guo Z, et al. Explaining the effect of gender on functional transitions in older persons. Gerontology. 2008;54:79–86. doi: 10.1159/000115004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gill TM, Guo Z, Allore HG. Subtypes of disability in older persons over the course of nearly 8 years. J Am Geriatr Soc. 2008;56:436–443. doi: 10.1111/j.1532-5415.2007.01603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Middleton LE, Mitnitski A, Fallah N, et al. Changes in cognition and mortality in relation to exercise in late life: a population based study. PLoS ONE. 2008;3:e3124. doi: 10.1371/journal.pone.0003124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitnitski A, Fallah N, Wu Y, et al. Changes in cognition during the course of eight years in elderly Japanese Americans: A multistate transition model. Ann Epidemiol. 2010;2:480–486. doi: 10.1016/j.annepidem.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Hubbard RE, Fallah N, Searle SD, et al. Impact of exercise in community-dwelling older adults. PLoS One. 2009;4:e6174. doi: 10.1371/journal.pone.0006174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 26.Rolfson DB, Majumdar SR, Tsuyuki RT, et al. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35:526–529. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pel-Littel RE, Schuurmans MJ, Emmelot-Vonk MH, et al. Frailty: defining and measuring of a concept. J Nutr Health Aging. 2009;13:390–394. doi: 10.1007/s12603-009-0051-8. [DOI] [PubMed] [Google Scholar]
- 28.Rothman MD, Leo-Summers L, Gill TM. Prognostic significance of potential frailty criteria. J Am Geriatr Soc. 2008;56:2211–2216. doi: 10.1111/j.1532-5415.2008.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gill TM, Desai MM, Gahbauer EA, et al. Restricted activity among community-living older persons: incidence, precipitants, and health care utilization. Ann Intern Med. 2001;135:313–321. doi: 10.7326/0003-4819-135-5-200109040-00007. [DOI] [PubMed] [Google Scholar]
- 30.Tyas SL, Salazar JC, Snowdon DA, et al. Transitions to Mild Cognitive Impairments, Dementia, and Death: Findings from the Nun Study. Am J Epidemiol. 2007;165:1231–1238. doi: 10.1093/aje/kwm085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis DHJ, Rockwood MRH, Mitnitski AB, et al. Impairments in mobility and balance in relation to frailty. Arch Gertontol Geriatr. 2010 Jul 31; doi: 10.1016/j.archger.2010.06.013. [Epub ahead of print] PMID: 20678816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.