Abstract
The central nervous system plays an important role in the regulation of energy balance and glucose homeostasis mainly via controlling the autonomic output to the visceral organs. The autonomic output is regulated by hormones and nutrients to maintain adequate energy and glucose homeostasis. Insulin action is mediated via insulin receptors (IR) resulting in phosphorylation of insulin receptor substrates (IRS) inducing activation of downstream pathways. Furthermore, insulin enhances transient receptor potential vanilloid type 1 (TRPV1) mediated currents. Activation of the TRPV1 receptor increases excitatory neurotransmitter release in autonomic centers of the brain, thereby impacting energy and glucose homeostasis. The aim of this study is to determine co-expression of IRS2 and TRPV1 receptors in the paraventricular nucleus of the hypothalamus (PVN) and dorsal motor nucleus of the vagus (DMV) in the mouse brain as well as expression of IRS2 and TRPV1 receptors at liver-related preautonomic neurons pre-labeled with a trans-neural, viral tracer (PRV-152). The data indicate that IRS2 and TRPV1 receptors are present and co-express in the PVN and the DMV. A large portion (over 50%) of the liver-related preautonomic DMV and PVN neurons expresses IRS2. Moreover, the majority of liver-related DMV and PVN neurons also express TRPV1 receptors, suggesting that insulin and TRPV1 actions may affect liver-related preautonomic neurons.
Keywords: paraventricular nucleus, dorsal motor nucleus of the vagus, IRS2, TRPV1, liver, PRV-152
1. Introduction
A large component of glucose metabolism and energy homeostasis is controlled by the central nervous system (CNS) (Sandoval et al., 2008; Zsombok and Smith, 2009), mainly through regulation of the activity of the autonomic nervous system (ANS) (Oomura, 1980; Pocai et al., 2005c; Undeland et al., 1998). The ANS directly controls visceral organ functions including hepatic glucose production (HGP). The paraventricular nucleus (PVN) of the hypothalamus incorporates signals from many different brain areas, including a variety of hypothalamic nuclei involved in the maintenance of energy and glucose homeostasis (e.g., arcuate nucleus, suprachiasmatic nucleus, dorso-medial hypothalamic nucleus etc.). The unique functioning of the PVN provides the integration of neuronal and humoral metabolic signals and organizes the autonomic and neuroendocrine outflow (Swanson and Sawchenko, 1980; Yi et al., 2010). The PVN directs liver functions through the sympathetic pathway (IML - celiac ganglia – liver) and parasympathetic (DMV – liver) innervations (Berthoud et al., 2004; Buijs et al., 2003; Yi et al., 2010), and plays a pivotal role in the regulation of HGP. Furthermore, both lesion and chemical stimulation studies support direct PVN involvement in plasma glucose control (Gunion et al., 1989; Ionescu et al., 1989; Leibowitz et al., 1988). Blockade of GABA receptors in the PVN or stimulation of NMDA receptors increases plasma glucose concentration via sympathetic activation of the liver (Kalsbeek et al., 2004). In addition, the dorsal vagal complex (DVC), containing the dorsal motor nucleus of the vagus (DMV) plays a pivotal role in the central regulation of the parasympathetic nervous system. Activation of DVC neurons lowers glucose production (Lam et al., 2010), and intact vagal efferents to the liver are necessary for the adequate suppression of hepatic gluconeogenesis (Lam et al., 2010; Pocai et al., 2005a; Pocai et al., 2005c), indicating that activation of the DVC triggers the parasympathetic brain-liver axis to decrease glucose production.
Adequate insulin signaling in the CNS is required for regulation of food intake (Baskin et al., 1999; Bruning et al., 2000), energy homeostasis, and glucose metabolism (Obici et al., 2002; Plum et al., 2005; Schwartz and Porte, 2005). Administration of insulin into the third ventricle reduces HGP due to inhibition of gluconeogenesis (Bruning et al., 2000; Pocai et al., 2005a; Pocai et al., 2005c), further indicating that central neuronal activity also controls glucose homeostasis. These studies suggest that insulin action is in the arcuate nucleus; however, neurons from many hypothalamic nuclei, including insulin sensitive neurons as well as neurons containing energy and glucose homeostasis related neuropeptides, project to the preautonomic PVN neurons (van den Hoek et al., 2008). These preautonomic PVN neurons are responsible for the integration of neural and humoral signals, thereby controlling sympathetic/parasympathetic activity of the visceral organs (Oomura, 1980; Undeland et al., 1998). Activation of insulin receptors causes phosphorylation of insulin receptor substrates (IRS) and activates downstream pathways (Plum et al., 2005; Plum et al., 2006). Among the four insulin receptor substrates (IRS 1-4), IRS2 appears to be the key mediator of the central regulation of energy homeostasis via the PI3K signal transduction pathway (Choudhury et al., 2005; Kubota et al., 2004; Lin et al., 2004; Masaki et al., 2004). However, despite the importance of the central insulin-insulin receptor substrate (IRS) signaling pathway in the autonomic regulation of HGP, the location and co-localization of IRS2, a key IRS with liver-related PVN neurons, is not known.
TRPV1 (transient receptor potential vanilloid type 1), a member of the transient receptor potential (TRP) family (Venkatachalam and Montell, 2007), is a ligand-gated, nonselective cation channel with high Ca2+ permeability that can be activated by physical and chemical stimulation (Starowicz et al., 2007; Szallasi et al., 2007; Tominaga and Tominaga, 2005). TRPV1 is widely expressed in many tissues related to glucose homeostasis (Starowicz et al., 2008a; Starowicz et al., 2008b) as well as in the peripheral and central nervous system, including the hypothalamus and brainstem (Cristino et al., 2006; Derbenev et al., 2006; Li et al., 2004; Mezey et al., 2000). Activation of TRPV1 receptors increases excitatory neurotransmitter release at PVN neurons (Li et al., 2004), including liver-related PVN neurons (Zsombok et al., 2010), indicating that TRPV1 regulates the activity of preautonomic liver-related PVN neurons, and thus, further suggests that the autonomic nervous system plays an important role in the regulation of HGP (Kalsbeek et al., 2004; Yi et al., 2010). Moreover, insulin and insulin-like growth factors enhance TRPV1-mediated currents in heterologous expression systems and cultured DRG cells (Van Buren et al., 2005), suggesting that insulin has the potential to further increase TRPV1-mediated activation of the neurons thereby increasing HGP through the sympathetic nervous system or decreasing HGP via the parasympathetic nervous system. However, the distribution of TRPV1 receptors associated with liver-related neurons or the distribution of TRPV1 versus IRS2 in the autonomic centers of the brain is unclear.
In this present study, we test the hypothesis that IRS2 and TRPV1 receptors are expressed in liver-related preautonomic PVN and DMV neurons. Our data indicate that IRS2 and TRPV1 receptors are present and co-express in the PVN and DMV and liver-related preautonomic neurons express IRS2 and TRPV1.
2. Results
TRPV1 and IRS2 co-expression in the PVN of hypothalamus and in the DMV of brainstem
TRPV1 and IRS2 immunofluorescence studies were carried out in male CD1 mice (n=6) to determine expression of TRPV1 and IRS2 in hypothalamic and brainstem slices.
A typical example of the distribution of IRS2 and TRPV1 and the summary of IRS2 and TRPV1 immunostaining within the PVN and brainstem is shown in Fig. 1 and in Table 1. Prominent IRS2 labeling was present in the DMV and the nucleus of cranial nerve 12 (12N, hypoglossal). IRS2 labeling was more abundant in the DMV compared to TRPV1 immunofluorescent labeling (Fig. 1A-F). The majority (66.6±8.9 %, range 38-95%) of the IRS2 immunopositive neurons showed TRPV1 co-expression in the DMV (Fig. 1D-F). To test the specificity of the TRPV1 and IRS2 staining, we performed staining without a primary or without a secondary antibody. In both cases, staining was not observed.
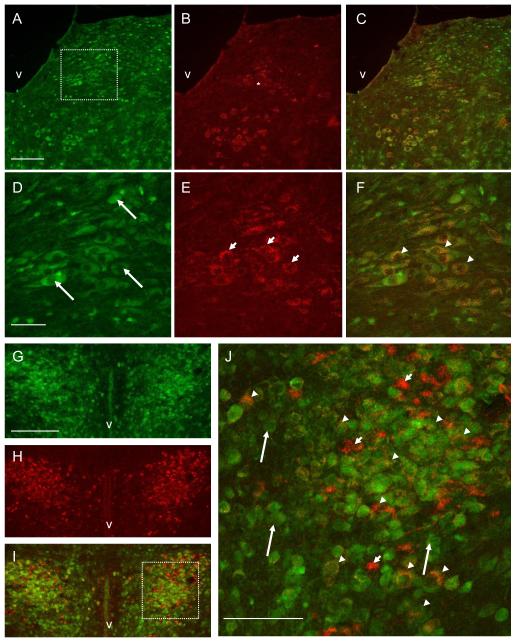
Figure 1.
Photomicrographs showing IRS2 (green) and TRPV1 (red) immunoreactivity in DMV and PVN neurons. A: IRS2 immunoreactivity in DMV. B: TRPV1 immunopositivity in the same section as in panel A. C: Dual labeling showing co-localization of IRS2 (green) and TRPV1 (red) in the same section as in panel A,B. D: Higher magnification of boxed DMV area shown in panel A illustrating IRS2 immunostaining in DMV. E: TRPV1 immunopositivity in the same section as in panel D. F: Dual labeling showing co-localization of IRS2 and TRPV1. G: IRS2 immunostaining in PVN. H: TRPV1 immunopositivity in the same section as in panel G. I: Double labeling showing co-localization of IRS2 (green) with TRPV1 (red). J: Higher magnification of boxed area shown in panel I illustrates double labeling in the PVN. The neurons indicated with arrows show IRS2 immunoreactivity; the neurons indicated with short arrows show TRPV1 immunopositivity; the neurons indicated with arrowheads reveal co-localization of IRS2 with TRPV1. V: 4th ventricle A-C, 3rd ventricle G-I. Scale bars=150μm in A-C and G-I; 50μm in D-F; 75μm in J.
Table 1.
Summary of IRS2 and TRPV1 immunostaining
| Brain area | IRS-2 immunoreactivity | TRPV1 immunoreactivity |
Co-expression of IRS-2 and TRPV1 |
|---|---|---|---|
| Brainstem | |||
| Dorsal motor nucleus of the vagus (DMV) |
XXX | XX | XX |
| Nucleus tractus solitarii | XX | X | X |
| Hypoglossal nucleus | XX | XXX | XXX |
|
Paraventricular nucleus
of the hypothalamus (PVN) |
|||
| Anterior part | XX | XX | X |
| Dorsal Cap (DC) | XX | XXX | XXX |
| Ventral parvocellular subnucleus (PaV) |
XX | XXX | XXX |
| Posterior parvocellular subnucleus (PaPo) |
XX | XXX | XX |
| Posterior magnocellular | X | XX | XX |
X, few; XX, moderate; XXX, abundant
Our experiments revealed abundant IRS2 immunopositive staining in the ventral parvocellular (PaV), posterior magnocellular, dorsal cap (DC), and medial parvocellular (PaMP) and posterior parvocellular (PaPo) subnuclei of the PVN (Fig. 1G, 3G). A moderate number of IRS2 neurons were observed in the anterior PVN, and only a few neurons were detected in the periventricular areas. TRPV1 immunostaining was observed mainly in the DC, PaV, posterior magnocellular, and PaPo areas of the PVN. Fewer neurons were detected in the anterior parvo- and magnocellular hypothalamus and periventricular areas. Double fluorescent labeling was used to detect co-expression of TRPV1 and IRS2. We detected abundant TRPV1 and IRS2 co-expression in the DC, posterior magnocellular and PaV, where the majority (>50%) of the IRS2 immunopositive neurons showed TRPV1 positivity.
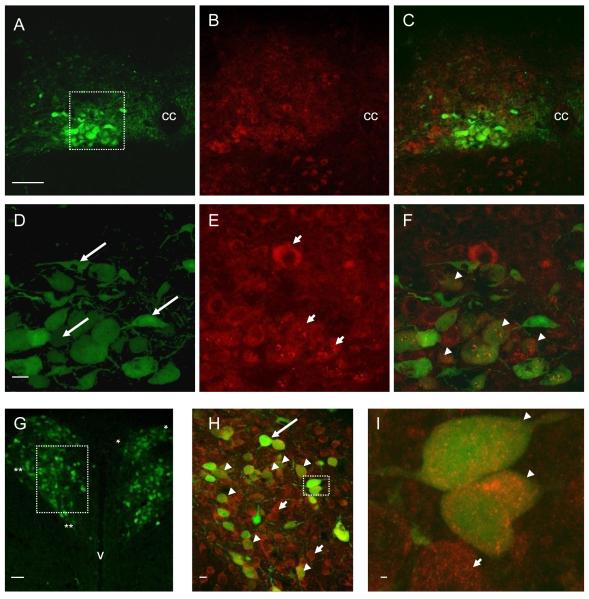
Figure 3.
Photomicrographs showing identified liver-related neurons and IRS2 immunoreactivity in mouse DMV and PVN following PRV-152 inoculation of the liver. A: Expression of enhanced green fluorescent protein (EGFP) in DMV neurons after inoculation of the liver with the transsynaptic, retrograde viral label, PRV-152 (green). B: IRS2 immunoreactivity in the same section as in panel A. C: Image of dual-labeled PRV-152 (green) and IRS2 (red) immunofluorescent staining. D: Higher magnification of boxed area in panel A illustrating liver-related DMV neurons pre-labeled with PRV-152. E: IRS2 immunoreactivity in the same section as in panel D. F: Image of dual-labeled PRV-152 (green) and IRS2 (red) staining in DMV. G: Expression of EGFP in PVN neurons after 96 h inoculation of the liver with PRV-152 (green); dorsal cap area between asterisks; PaV between double asterisks. H: Higher magnification of boxed area in panel G illustrating dual-labeled PRV-152 (green) and IRS2 (red) staining in PVN. I: Higher magnification of boxed area in panel H illustrating dual labeling. The neurons indicated with arrows show liver-related neurons pre-labeled with PRV-152; the neurons indicated with short arrows illustrate IRS2 immunoreactivity; the arrowhead indicates liver-related neurons showing IRS2 immunoreactivity. Scale bars = 100μm in A-C; 20μm in D-F; 50μm in G; 10μm in H; 1000nm in I. CC: central canal; V: 3rd ventricle.
Identification of liver-related mouse DMV and PVN neurons with PRV-152
A retrogradely transported pseudorabies virus-152 (PRV-152), a PRV construct isogenic with PRV Bartha, which expresses enhanced green fluorescent protein (EGFP), was used to identify liver-related neurons in the DMV and PVN. A set of experiments was conducted to determine the time course of PRV-152 labeling in mice to reveal sufficient labeling of preautonomic liver-related neurons in the DMV and PVN. Labeling in the brainstem and hypothalamus was examined between 24 and 120 h post-inoculation (24, 48, 72, 96 and 120 h). Primary PRV-152 infection was followed stepwise by infection of neurons in the brainstem and preautonomic neurons in the PVN. At 24 and 48 h post-inoculation (n=2 and n=3), we did not see EGFP labeling in either the brainstem or the PVN. The infection of DMV neurons occurred in the brainstem at 72 h after inoculation of the liver with PRV-152 (n=5). Few preautonomic neurons were detected at this time point in the brainstem, but there was no EGFP labeling in the PVN. At 96 h post-inoculation, we observed abundant EGFP labeling in the brainstem and a moderate number of liver-related neurons in the PVN (n=13). At 120 h, strong EGFP labeling was detected in both the DMV and the PVN (n=3). The infection stage following inoculation of the liver with PRV-152 in mice was consistent with previously published results using PRV Bartha in rats (Buijs et al., 2003).
TRPV1 or IRS2 expression at liver-related DMV and PVN neurons
Fluorescence immunochemistry for TRPV1 or IRS2 was carried out on hypothalamic and brainstem sections of male CD1 mice (n=7 for TRPV1, n=6 for IRS2) containing liver-related preautonomic neurons pre-labeled with PRV-152 to determine expression of TRPV1 or IRS2 at liver-related preautonomic neurons.
TRPV1 expression at liver-related neurons
Abundant TRPV1 immunopositivity was observed at liver-related DMV neurons (Fig. 2A-E). TRPV1 immunopositive staining was detected throughout the DMV independently from the distance of the bregma. 65±11% of liver-related DMV neurons showed TRPV1 immunopositivity (Fig. 2E).
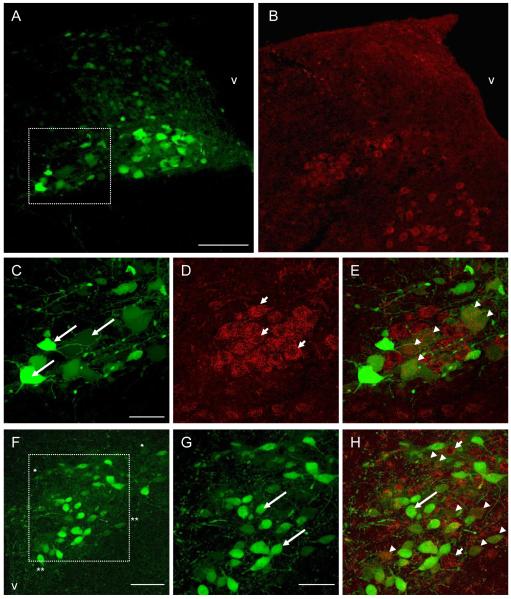
Figure 2.
Photomicrographs showing identified liver-related neurons and TRPV1 receptor immunoreactivity in mouse DMV and PVN following PRV-152 inoculation of the liver. A: Expression of enhanced green fluorescent protein (EGFP) in the DMV after inoculation of the liver with the transsynaptic, retrograde viral label, PRV-152 (green). B: TRPV1 immunoreactivity (red) in the same section as in panel A. C: Higher magnification of boxed area in panel A illustrating liver-related DMV neurons pre-labeled with PRV-152. D: TRPV1 immunoreactivity in the same section as in panel C. E: Image of dual-labeled PRV-152 (green) and TRPV1 (red) immunofluorescent staining. F: Expression of EGFP in PVN neurons after inoculation of the liver with PRV-152 (green), dorsal cap area between asterisks; PaV between double asterisks. G: Higher magnification of boxed area in panel F illustrating liver-related PVN neurons. H: Image of dual-labeled PRV-152 (green); and TRPV1 (red) staining in the PVN. The neurons indicated with arrows show liver-related neurons pre-labeled with PRV-152; the neurons indicated with short arrows illustrate TRPV1 immunoreactivity; arrowheads indicate liver-related neurons showing TRPV1 immunoreactivity. Scale bars = 150μm in A-B; 50μm in C-E; 75μm in F; 50μm in G-H. V: 4th ventricle in A-B; 3rd ventricle in F.
In the PVN of the hypothalamus TRPV1 expression was revealed among liver-related preautonomic neurons. 70.5±9% of the liver-related neurons showed TRPV1 immunopositivity in the PaV. Liver-related PVN neurons also showed TRPV1 immunostaining in the DC and PaPo areas of PVN.
IRS2 expression at liver-related neurons
IRS2 expression was revealed among liver-related preautonomic neurons both in the DMV and PVN (Fig. 3). Our data demonstrate that 72.2±3.5% (range 59.2-85%) of liver-related neurons showed IRS2 immunopositivity in the DMV.
In the PVN, we have detected IRS2 expression among liver-related preautonomic neurons. 84±9% of the liver-related neurons showed IRS2 immunopositivity, mainly in the DC and PaV subnuclei (Fig. 3).
3. Discussion
In this study, we report the distribution of IRS2 and TRPV1 receptors in the two main autonomic centers of the brain. Three major findings have emerged from this investigation: 1) IRS2 and TRPV1 are co-expressed in the DMV and PVN neurons; 2) liver-related DMV and PVN neurons express IRS2, and 3) liver-related DMV and PVN neurons express TRPV1 receptors.
TRPV1 and IRS2 co-expression
Our study shows that IRS2 and TRPV1 receptors appear to be co-expressed in DMV neurons in the brainstem and PVN neurons of the hypothalamus. Although immunohistochemical distribution of TRPV1 or IRS2 in mouse brains has been shown previously (Cristino et al., 2006, Pardini et al., 2006), this is the first study demonstrating TRPV1 receptor co-expression with IRS2 in the same neurons. Insulin signaling via the IRS2-PI3K pathway plays a pivotal role in the central regulation of energy homeostasis via regulation of the sympathetic and parasympathetic nervous system (Oomura, 1980; Sandoval et al., 2008; Undeland et al., 1998). In our experiments commercially available antibodies were used, and IRS2 or TRPV1 staining was not observed when sections were incubated without primary or secondary antibodies. Furthermore, the specificity of the antibodies has been previously determined by Western blot analysis and immunostaining (Guo et al., 1999; Pardini et al., 2006). Our data are consistent with previous findings showing strong IRS2 staining in the CNS, including both the PVN and the DMV (Pardini et al., 2006). Moreover, we extended this previous work by reporting IRS2 expression in many PVN subnuclei, particularly in preautonomic areas. The subnuclear localization of IRS2 is closely aligned with the distribution of insulin receptors in the PVN, as shown in a recent work (Cassaglia et al., 2011), further indicating that neurons containing insulin receptor and IRS2 immmunoreactivity are located in areas of the brain that are crucial for the control of food intake and autonomic function. Our data also revealed that more than 50% of IRS2 positive neurons co-express TRPV1 receptors both in the DMV and the PVN. Previous observations from heterologous expression systems and cultured dorsal root ganglion cells indicated that insulin and insulin-like growth factors enhance TRPV1-mediated currents (Van Buren et al., 2005). Furthermore, insulin is capable of increasing TRPV1 receptor sensitivity by activation of the PI3K-PKC pathway (Zsombok et al., 2008; Zsombok, 2010). Therefore, the synergy of TRPV1 and IRS2 can play a role in the regulation of energy homeostasis and glucose balance by altering synaptic activity, in a similar manner to insulin’s effect on GABAA and NMDA receptors (Skeberdis et al., 2001). In order to prove this, functional studies are required to evaluate the role of IRS2/TRPV1 signaling in the regulation of HGP.
PRV-152 technique
By using PRV-152, we identified liver-related neurons in the DMV of the brainstem and the PVN of the hypothalamus. This approach allowed us to determine expression of TRPV1 receptors or IRS2 at liver-related preautonomic neurons. Our results further support the viral fluorescent trans-neuronal tracer, PRV-152 as a useful tool for labeling a specific neuronal population that have direct or indirect projections to the peripheral organs (Buijs et al., 2003; Card, 1998; Enquist et al., 1994). PRV-152 is used to study neurons of the CNS that have projections to the peripheral organs including the stomach, kidney, liver, spleen (Buijs et al., 2003; Cano et al., 2001; Cano et al., 2004; Card et al., 1993; Enquist et al., 1994; Smith et al., 2000; Williams et al., 2007). This technique requires careful timing of survival to determine the location of all the areas that showed neurons (Buijs et al., 2003). Despite the rich blood supply of the liver previous studies already demonstrated the specificity of the PRV in terms of liver injection; however, it should be pointed out that the fraction of liver neurons inoculated has not been revealed, neither by our study nor by previous studies (Buijs et al., 2003). The fluoro-gold tracing studies in combination with PRV injection; combination of hepatic sympathectomy or parasympathectomy and application of PRV showed convincing supporting evidences regarding the specificity of this technique (Buijs et al., 2003; Card, 1998; Kalsbeek et al., 2004), however the results has to be interpreted carefully. Furthermore, questions regarding the specificity of PRV have repeatedly been raised and extensive research was conducted to determine the amount, the specificity, the timing and other aspects of the virus and can be found in numerous articles addressing these concerns (Card et al., 1993; Card, 1998; Ch’ng et al., 2007). In our experiments, we carefully controlled the amount and site of injection to obtain a reproducible infection rate and pattern. Moreover, our study is consistent with previous findings in rats (Buijs et al., 2003), indicating that PRV-152 appears in the brainstem neurons 3 days after inoculation of the liver and in the PVN after 4 days. However, despite the specific cellular labeling, which results in positive identification of liver-related preautonomic DMV and PVN neurons, we were not able to distinguish between pre-sympathetic and pre-parasympathetic neurons in the PVN. Even with these limitations, PRV-152 has proven to be valuable in characterizing liver-related neurons in the brainstem and hypothalamus, providing us the opportunity to investigate co-localization of liver-related neurons with receptors or neurotransmitters involved in the central regulation of liver homeostasis.
TRPV1 and IRS2 expression in liver-related neurons
Two major areas of the brain, the PVN of the hypothalamus and the DMV of the brainstem, are thought to play a pivotal role in the central regulation of energy homeostasis via regulation of the sympathetic and parasympathetic nervous system (Oomura, 1980; Sandoval et al., 2008; Undeland et al., 1998). Our observations revealed TRPV1 and IRS2 expression in liver-related preautonomic neurons indicating that insulin and TRPV1 signaling have the potential to govern liver function through the autonomic nervous system. Insulin action is mediated via insulin receptors, resulting in phosphorylation of insulin receptor substrates, inducing activation of downstream pathways (Bruning et al., 2000; Pardini et al., 2006; Plum et al., 2005; Plum et al., 2006). Insulin action in the brain is critical for activation of the sympathoadrenal response as well as, glucose counterregulation and inhibition of hepatic gluconeogenesis (Bruning et al., 2000; Fisher et al., 2005; Obici et al., 2002; Plum et al., 2006; Pocai et al., 2005b; Pocai et al., 2005c), suggesting that circulating insulin activates neuronal circuits required to maintain energy and glucose homeostasis. Our results, showing IRS2 immunopositivity of liver-related preautonomic PVN and DMV neurons, provide anatomical background for the regulation of hepatic glucose production through the autonomic nervous system. In the current study, we cannot differentiate between sympathetic and parasympathetic liver-related PVN neurons, as we mentioned earlier, although the existence of IRS2 immunopositive neurons in the DMV indicates parasympathetic involvement at the level of the brainstem.
Our data also showed that TRPV1 receptors expressed at liver-related preautonomic neurons suggest that activation of TRPV1 receptors regulates the activity of preautonomic neurons (Zsombok et al., 2010). The role of peripheral TRPV1 regarding diabetes is established (Razavi et al., 2006; Starowicz et al., 2008a; Suri and Szallasi, 2008; Szallasi et al., 2007); however, its central role, despite the widespread expression in the CNS, is still unclear (Cristino et al., 2006; Mezey et al., 2000). General expression of TRPV1 receptors in the brain were described in previous studies (Cristino et al., 2006; Mezey et al., 2000; Roberts et al., 2004), however these investigation were not able to distinguish pre-autonomic neurons in the CNS. Nevertheless, our data revealed TRPV1 expression in liver-related preautonomic neurons. Moreover, our observations of TRPV1 expression among liver-related neurons provides anatomical background to our functional observations that TRPV1 receptor agonist increase excitatory post-synaptic currents to liver-related PVN neurons (Zsombok et al., 2010) indicating that TRPV1 has the potential to regulate HGP. Central TRPV1 receptors can be directly activated by its endogenous ligands, the endovanilloids, arachidonic acid metabolites such as anandamide, or NADA (Starowicz et al., 2008a; Van Der Stelt and Di Marzo, 2004). Enhancement in the level of endogenous ligands has been reported in obese and diabetic patients and in animals, indicating systemic upregulation (Matias et al., 2006; Matias and Di Marzo, 2007; Matias et al., 2008).
In conclusion, our findings revealed IRS2 and TRPV1 expression at liver-related preautonomic DMV and PVN neurons providing anatomical background for both insulin signaling and TRPV1 activation, which potentially can contribute to the central control of the liver function. However, to establish the role of TRPV1 receptors in the regulation of HGP requires further functional investigation.
4. Experimental Procedures
Animals
Male (4-8 week old) CD1 mice (Harlan, Indianapolis, IN) were used for these experiments. The animals were housed in the vivarium under 12 h light-dark cycles with food and water available ad libitum. Experiments were performed following the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by Tulane University’s Animal Care and Use Committee. All efforts were made to minimize the number of animals employed and their suffering.
Injection of PRV-152
A retrogradely transported viral vector isogenic with PRV Bartha strain that reports EGFP (PRV-152; generously supplied by Dr L. W. Enquist, Princeton University) was used to identify liver-related DMV and PVN neurons (Davis et al., 2003; Glatzer et al., 2003; Smith et al., 2000). Under isoflurane anesthesia, a small transverse incision was made to expose the liver for injection under direct vision. Three injections (2 μl each) of PRV-152 at a titer of 1 × 108 plaque forming units (p.f.u.) ml−1 was made into the median lobe of the liver using a 5 μl Hamilton syringe fitted with a 26 gauge needle. Each injection site was immediately sealed with one drop of “liquid bandage” to prevent leakage of the virus. The animals were then maintained in a biosafety level 2 facility for up to 120 h post-injection, where they were allowed to recover. To minimize discomfort during and after PRV-152 infection, the animals were treated with buprenorphine (0.02 mg/kg). PRV infects terminal fields in the liver and selectively labels preautonomic central neurons retrogradely and trans-synaptically. Because it does not spread in axons or glial cells, the construct does not move orthogradely or non-specifically from axons to other structures (Ch’ng et al., 2007). The labeling sequence and timing have been characterized for this pathway including the initial appearance of fluorescent labeling in the brainstem and PVN, at about ~72 hours post-inoculation.
Immunohistochemistry
Immunohistochemical localization of TRPV1 and IRS2 was performed in a similar way to previous descriptions (Cristino et al., 2006; Pardini et al., 2006). After 96 h of PRV-152 inoculation of the liver, the mice were anesthetized with ketamine and xylane (0.99 mg/kg) and perfused transcardially with 0.15 M sodium phosphate buffer (pH 7.4) followed by 4% paraformaldehyde in 0.15 M sodium phosphate buffer. The brains were removed, post-fixed for 2 h in the same fixative, rinsed in 0.01 M phosphate-buffered saline (PBS, pH 7.4), immersed in 30% sucrose in PBS until they equilibrated, and sectioned at 20 μm (brainstem) and 30 μm (hypothalamus) with a cryostat. After several rinses in PBS, floating sections were immersed in guinea pig anti-TRPV1 antibody (Novus Biologicals Alpha Diagnostic Inc.) in PBS (1:1000; 24 h at 4°C) with 0.1% Triton X-100 and 0.1% normal goat serum or with rabbit polyclonal anti-IRS2 (1:300, 24 h at 4°C). After several rinses in PBS, sections were treated with a fluorescence-conjugated (AlexaFluor 593; Molecular Probes, Eugene, OR, USA) goat anti-guinea pig secondary antibody or goat anti-rabbit secondary antibody (IgG; 1:200; 2 h at room temperature), followed by more rinsing in PBS.
For double immunohistochemical staining, the sections were pre-incubated for 1 h in 10% normal goat serum followed by incubation for 24 h with a cocktail of anti-TRPV1 (1:1000) and anti-IRS2 (1:300) primary antibodies in 0.01M PBS containing 1% NGS and 0.5% Triton-X 100 at 4°C. Slices were then washed with PBS three times for 10 minutes and incubated for 2 h in goat anti-rabbit IgG (Alexa Fluor 488) 1:500; then they were washed several times and incubated for 2 h in goat anti-guinea pig IgG (Alexa Fluor 594) 1:200 at room temperature. Sections were washed and mounted onto charged slides (Superfrost Plus; Fisher Scientific, Pittsburgh, PA, USA), air dried, covered in an anti-oxidant medium (Vectashield, Vector Laboratories), and coverslipped.
To identify immunohistochemical co-localization of TRPV1, IRS2 and liver-related neurons a scanning laser confocal microscope (Leica TCS NT SP) was used. Double labeled neurons were counted by using Adobe Photoshop (version 7.0). These data were obtained from at least 6-8 sections.
Highlights.
 IRS2 and TRPV1 co-expressed in the hypothalamus and brainstem.
IRS2 and TRPV1 co-expressed in the hypothalamus and brainstem. Liver-related preautonomic neurons express IRS2 in the PVN and DMV.
Liver-related preautonomic neurons express IRS2 in the PVN and DMV. Liver-related preautonomic neurons express TRPV1 receptors in the PVN and DMV.
Liver-related preautonomic neurons express TRPV1 receptors in the PVN and DMV. TRPV1 and IRS2 could contribute to central autonomic control of the liver.
TRPV1 and IRS2 could contribute to central autonomic control of the liver.
Acknowledgments
We thank Dr. Lynn Enquist (Princeton University) for the PRV-152 and Drs. N. R. Kreisman and P. J. Kadowitz for their valuable comments on the manuscript. This work was supported by NIH-HLBI R21HL091293 and R21HL091293-01A1S1 for A.V.D.; and AHA 10GRNT4540000 and NIH-2K12HD043451 for A.Z.
Abbreviations
- DC
dorsal cap
- DMV
dorsal motor nucleus of the vagus
- DVC
dorsal vagal complex
- IRS2
insulin receptor substrate 2
- NADA
N-arachidonoyl-dopamine
- PaPo
posterior parvocellular subluclei
- PaV
ventral parvocellular subnuclei
- PI3K
phosphatidylinositol 3-kinase
- PRV-152
pseudorabies virus-152
- PVN
paraventricular nucleus
- TRP
transient receptor potential
- TRPV1
transient receptor potential vanilloid type 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference list
- Baskin DG, Lattemann D. Figlewicz, Seeley RJ, Woods SC, Porte D, Jr., Schwartz MW. Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res. 1999;848:114–23. doi: 10.1016/s0006-8993(99)01974-5. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil. 2004;16(Suppl 1):28–33. doi: 10.1111/j.1743-3150.2004.00471.x. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–5. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Buijs RM, la Fleur SE, Wortel J, Van Heyningen C, Zuiddam L, Mettenleiter TC, Kalsbeek A, Nagai K, Niijima A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J Comp Neurol. 2003;464:36–48. doi: 10.1002/cne.10765. [DOI] [PubMed] [Google Scholar]
- Cano G, Sved AF, Rinaman L, Rabin BS, Card JP. Characterization of the central nervous system innervation of the rat spleen using viral transneuronal tracing. J Comp Neurol. 2001;439:1–18. doi: 10.1002/cne.1331. [DOI] [PubMed] [Google Scholar]
- Cano G, Card JP, Sved AF. Dual viral transneuronal tracing of central autonomic circuits involved in the innervation of the two kidneys in rat. J Comp Neurol. 2004;471:462–81. doi: 10.1002/cne.20040. [DOI] [PubMed] [Google Scholar]
- Card JP, Rinaman L, Lynn RB, Lee BH, Meade RP, Miselis RR, Enquist LW. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport, and pathogenesis. J Neurosci. 1993;13:2515–39. doi: 10.1523/JNEUROSCI.13-06-02515.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP. Practical considerations for the use of pseudorabies virus in transneuronal studies of neural circuitry. Neurosci Biobehav Rev. 1998;22:685–94. doi: 10.1016/s0149-7634(98)00007-4. [DOI] [PubMed] [Google Scholar]
- Cassaglia PA, Hermes SM, Aicher SA, Brooks VL. Insulin acts in the arcuate nucleus to increase lumbar sympathetic nerve activity and baroreflex function in rats. J Physiol. 2011;589:1643–62. doi: 10.1113/jphysiol.2011.205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch’ng TH, Spear PG, Struyf F, Enquist LW. Glycoprotein D-independent spread of pseudorabies virus infection in cultured peripheral nervous system neurons in a compartmented system. J Virol. 2007;81:10742–57. doi: 10.1128/JVI.00981-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury AI, Heffron H, Smith MA, Al-Qassab H, Xu AW, Selman C, Simmgen M, Clements M, Claret M, Maccoll G, Bedford DC, Hisadome K, Diakonov I, Moosajee V, Bell JD, Speakman JR, Batterham RL, Barsh GS, Ashford ML, Withers DJ. The role of insulin receptor substrate 2 in hypothalamic and beta cell function. J Clin Invest. 2005;115:940–50. doi: 10.1172/JCI24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–15. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- Davis SF, Williams KW, Xu W, Glatzer NR, Smith BN. Selective enhancement of synaptic inhibition by hypocretin (orexin) in rat vagal motor neurons: implications for autonomic regulation. J Neurosci. 2003;23:3844–54. doi: 10.1523/JNEUROSCI.23-09-03844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbenev AV, Monroe MJ, Glatzer NR, Smith BN. Vanilloid-mediated heterosynaptic facilitation of inhibitory synaptic input to neurons of the rat dorsal motor nucleus of the vagus. J Neurosci. 2006;26:9666–72. doi: 10.1523/JNEUROSCI.1591-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist LW, Miselis RR, Card JP. Specific infection of rat neuronal circuits by pseudorabies virus. Gene Ther. 1994;1(Suppl 1):S10. [PubMed] [Google Scholar]
- Fisher SJ, Bruning JC, Lannon S, Kahn CR. Insulin signaling in the central nervous system is critical for the normal sympathoadrenal response to hypoglycemia. Diabetes. 2005;54:1447–51. doi: 10.2337/diabetes.54.5.1447. [DOI] [PubMed] [Google Scholar]
- Glatzer NR, Hasney CP, Bhaskaran MD, Smith BN. Synaptic and morphologic properties in vitro of premotor rat nucleus tractus solitarius neurons labeled transneuronally from the stomach. J Comp Neurol. 2003;464:525–39. doi: 10.1002/cne.10831. [DOI] [PubMed] [Google Scholar]
- Gunion MW, Tache Y, Rosenthal MJ, Miller S, Butler B, Zib B. Bombesin microinfusion into the rat hypothalamic paraventricular nucleus increases blood glucose, free fatty acids and corticosterone. Brain Res. 1989;478:47–58. doi: 10.1016/0006-8993(89)91476-5. [DOI] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–58. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Ionescu E, Coimbra CC, Walker CD, Jeanrenaud B. Paraventricular nucleus modulation of glycemia and insulinemia in freely moving lean rats. Am J Physiol. 1989;257:R1370–6. doi: 10.1152/ajpregu.1989.257.6.R1370. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, La Fleur S, Van Heijningen C, Buijs RM. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J Neurosci. 2004;24:7604–13. doi: 10.1523/JNEUROSCI.5328-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Tobe K, Yano W, Suzuki R, Ueki K, Takamoto I, Satoh H, Maki T, Kubota T, Moroi M, Okada-Iwabu M, Ezaki O, Nagai R, Ueta Y, Kadowaki T, Noda T. Insulin receptor substrate 2 plays a crucial role in beta cells and the hypothalamus. J Clin Invest. 2004;114:917–27. doi: 10.1172/JCI21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam CK, Chari M, Su BB, Cheung GW, Kokorovic A, Yang CS, Wang PY, Lai TY, Lam TK. Activation of N-methyl-D-aspartate (NMDA) receptors in the dorsal vagal complex lowers glucose production. J Biol Chem. 2010;285:21913–21. doi: 10.1074/jbc.M109.087338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz SF, Sladek C, Spencer L, Tempel D. Neuropeptide Y, epinephrine and norepinephrine in the paraventricular nucleus: stimulation of feeding and the release of corticosterone, vasopressin and glucose. Brain Res Bull. 1988;21:905–12. doi: 10.1016/0361-9230(88)90025-1. [DOI] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan HL. VR1 receptor activation induces glutamate release and postsynaptic firing in the paraventricular nucleus. J Neurophysiol. 2004;92:1807–16. doi: 10.1152/jn.00171.2004. [DOI] [PubMed] [Google Scholar]
- Lin X, Taguchi A, Park S, Kushner JA, Li F, Li Y, White MF. Dysregulation of insulin receptor substrate 2 in beta cells and brain causes obesity and diabetes. J Clin Invest. 2004;114:908–16. doi: 10.1172/JCI22217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki T, Chiba S, Noguchi H, Yasuda T, Tobe K, Suzuki R, Kadowaki T, Yoshimatsu H. Obesity in insulin receptor substrate-2-deficient mice: disrupted control of arcuate nucleus neuropeptides. Obes Res. 2004;12:878–85. doi: 10.1038/oby.2004.106. [DOI] [PubMed] [Google Scholar]
- Matias I, Gonthier MP, Orlando P, Martiadis V, De Petrocellis L, Cervino C, Petrosino S, Hoareau L, Festy F, Pasquali R, Roche R, Maj M, Pagotto U, Monteleone P, Di Marzo V. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab. 2006;91:3171–80. doi: 10.1210/jc.2005-2679. [DOI] [PubMed] [Google Scholar]
- Matias I, Di Marzo V. Endocannabinoids and the control of energy balance. Trends Endocrinol Metab. 2007;18:27–37. doi: 10.1016/j.tem.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Matias I, Petrosino S, Racioppi A, Capasso R, Izzo AA, Di Marzo V. Dysregulation of peripheral endocannabinoid levels in hyperglycemia and obesity: Effect of high fat diets. Mol Cell Endocrinol. 2008;286:S66–78. doi: 10.1016/j.mce.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Mezey E, Toth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci U S A. 2000;97:3655–60. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–82. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- Oomura Y. Input-output organization of the hypothalamus relating to food intake behavior. In: Morgane PJ, Panskepp J, editors. Handbook of the Hypothalamus. Vol. 2: Physiology of the Hypothalamus. Marcel Dekker; New York: 1980. pp. 557–620. 2. [Google Scholar]
- Pardini AW, Nguyen HT, Figlewicz DP, Baskin DG, Williams DL, Kim F, Schwartz MW. Distribution of insulin receptor substrate-2 in brain areas involved in energy homeostasis. Brain Res. 2006;1112:169–78. doi: 10.1016/j.brainres.2006.06.109. [DOI] [PubMed] [Google Scholar]
- Plum L, Schubert M, Bruning JC. The role of insulin receptor signaling in the brain. Trends Endocrinol Metab. 2005;16:59–65. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Plum L, Belgardt BF, Bruning JC. Central insulin action in energy and glucose homeostasis. J Clin Invest. 2006;116:1761–6. doi: 10.1172/JCI29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005a;434:1026–31. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- Pocai A, Morgan K, Buettner C, Gutierrez-Juarez R, Obici S, Rossetti L. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes. 2005b;54:3182–9. doi: 10.2337/diabetes.54.11.3182. [DOI] [PubMed] [Google Scholar]
- Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab. 2005c;1:53–61. doi: 10.1016/j.cmet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Razavi R, Chan Y, Afifiyan FN, Liu XJ, Wan X, Yantha J, Tsui H, Tang L, Tsai S, Santamaria P, Driver JP, Serreze D, Salter MW, Dosch HM. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127:1123–35. doi: 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]
- Roberts JC, Davis JB, Benham CD. [3H]Resiniferatoxin autoradiography in the CNS of wild-type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Res. 2004;995:176–83. doi: 10.1016/j.brainres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Sandoval D, Cota D, Seeley RJ. The integrative role of CNS fuel-sensing mechanisms in energy balance and glucose regulation. Annu Rev Physiol. 2008;70:513–35. doi: 10.1146/annurev.physiol.70.120806.095256. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Porte D., Jr. Diabetes, obesity, and the brain. Science. 2005;307:375–9. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- Skeberdis VA, Lan J, Zheng X, Zukin RS, Bennett MV. Insulin promotes rapid delivery of N-methyl-D- aspartate receptors to the cell surface by exocytosis. Proc Natl Acad Sci U S A. 2001;98:3561–6. doi: 10.1073/pnas.051634698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BN, Banfield BW, Smeraski CA, Wilcox CL, Dudek FE, Enquist LW, Pickard GE. Pseudorabies virus expressing enhanced green fluorescent protein: A tool for in vitro electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits. Proc Natl Acad Sci U S A. 2000;97:9264–9. doi: 10.1073/pnas.97.16.9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starowicz K, Nigam S, Di Marzo V. Biochemistry and pharmacology of endovanilloids. Pharmacol Ther. 2007;114:13–33. doi: 10.1016/j.pharmthera.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Starowicz K, Cristino L, Di Marzo V. TRPV1 receptors in the central nervous system: potential for previously unforeseen therapeutic applications. Curr Pharm Des. 2008a;14:42–54. doi: 10.2174/138161208783330790. [DOI] [PubMed] [Google Scholar]
- Starowicz KM, Cristino L, Matias I, Capasso R, Racioppi A, Izzo AA, Di Marzo V. Endocannabinoid dysregulation in the pancreas and adipose tissue of mice fed with a high-fat diet. Obesity (Silver Spring) 2008b;16:553–65. doi: 10.1038/oby.2007.106. [DOI] [PubMed] [Google Scholar]
- Suri A, Szallasi A. The emerging role of TRPV1 in diabetes and obesity. Trends Pharmacol Sci. 2008;29:29–36. doi: 10.1016/j.tips.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–7. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6:357–72. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Tominaga T. Structure and function of TRPV1. Pflugers Arch. 2005;451:143–50. doi: 10.1007/s00424-005-1457-8. [DOI] [PubMed] [Google Scholar]
- Undeland KA, Hausken T, Gilja OH, Aanderud S, Berstad A. Gastric meal accommodation studied by ultrasound in diabetes. Relation to vagal tone. Scand J Gastroenterol. 1998;33:236–41. doi: 10.1080/00365529850170784. [DOI] [PubMed] [Google Scholar]
- Van Buren JJ, Bhat S, Rotello R, Pauza ME, Premkumar LS. Sensitization and translocation of TRPV1 by insulin and IGF-I. Mol Pain. 2005;1:17. doi: 10.1186/1744-8069-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoek AM, van Heijningen C, Schroder-van der Elst JP, Ouwens DM, Havekes LM, Romijn JA, Kalsbeek A, Pijl H. Intracerebroventricular administration of neuropeptide Y induces hepatic insulin resistance via sympathetic innervation. Diabetes. 2008;57:2304–10. doi: 10.2337/db07-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Stelt M, Di Marzo V. Endovanilloids. Putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur J Biochem. 2004;271:1827–34. doi: 10.1111/j.1432-1033.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KW, Zsombok A, Smith BN. Rapid inhibition of neurons in the dorsal motor nucleus of the vagus by leptin. Endocrinology. 2007;148:1868–81. doi: 10.1210/en.2006-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi CX, la Fleur SE, Fliers E, Kalsbeek A. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim Biophys Acta. 2010;1802:416–31. doi: 10.1016/j.bbadis.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Zsombok A, Derbenev AV, Bhaskaran MD, Smith BN. Plasticity of synaptic input to the brainstem in a model of type 1 diabetes. SfN. 2008;481:423. [Google Scholar]
- Zsombok A, Smith BN. Plasticity of central autonomic neural circuits in diabetes. Biochim Biophys Acta. 2009;1792:423–31. doi: 10.1016/j.bbadis.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsombok A. Plasticity of synaptic input to liver-related hypothalmic neurons in a model of type 1 diabetes. 2010. EB 2010.
- Zsombok A, Gao H, Derbenev AV. TRPV1 Receptor Plasticity in the Paraventricular Nucleus of the Hypothalamus in Type 1 Diabetic Mice. Diabetes. 2010;59:A379. [Google Scholar]