Abstract
Chronic marijuana users (MJ Users) perform poorly on the Iowa Gambling Task (IGT), a complex decision-making task in which monetary wins and losses guide strategy development. This study sought to determine if the poor performance of MJ Users was related to differences in brain activity while evaluating wins and losses during the strategy development phase of the IGT. MJ Users (16) and Controls (16) performed a modified IGT in an MRI scanner. Performance was tracked and functional activity to early wins and losses was examined. While the MJ Users continued to perform poorly at the end of the task, there was no difference in group performance during the initial strategy development phase. During this phase, before the emergence of behavioral differences, Controls exhibited significantly greater activity in response to losses in the anterior cingulate cortex, medial frontal cortex, precuneus, superior parietal lobe, occipital lobe and cerebellum as compared to MJ Users. Furthermore, in Controls, but not MJ Users, the functional response to losses in the anterior cingulate cortex, ventral medial prefrontal cortex and rostral prefrontal cortex positively correlated with performance over time. These data suggest MJ Users are less sensitive to negative feedback during strategy development.
Keywords: human, cannabis, affect, emotion, imaging, functional MRI
1 Introduction
Marijuana is the most commonly used illegal drug in the United States and is known to influence multiple aspects of executive function including impulsivity (McDonald et al., 2003), attention (Fletcher et al., 1996; Pope et al., 2001), working memory (Miller and Branconnier 1983) and cognitive flexibility (Bolla et al., 2002; Lane et al., 2007). Importantly, chronic marijuana use is associated with deficits in decision-making that impair the ability to make advantageous decisions over time (Whitlow et al., 2004; Hermann et al., 2009). While the basis for this deficit has not been completely characterized, successful strategy development during normal decision-making involves the processing of positive and negative information, and using this information to guide future decisions towards achieving a goal (Sutton and Barto 1998; Dayan and Balleine 2002; Camerer 2003). Understanding the basis of poor strategy development in chronic marijuana users (MJ Users), therefore, may help explain their deficits in decision-making.
The Iowa Gambling Task (IGT) is a complex decision-making task (Bechara et al., 1994) that has been used to demonstrate deficits in current (Whitlow et al., 2004; Hermann et al., 2009) and abstinent heavy marijuana users (Bolla et al., 2005). To perform the task, participants begin selecting cards randomly from four card decks under ambiguous conditions. Each selection produces a monetary gain or win; however, some selections also result in a monetary loss. Over time, based on the wins and losses associated with each deck, two of the decks emerge as advantageous and two disadvantageous. The disadvantageous decks produce larger immediate gains but larger losses over time while the advantageous decks yield smaller immediate gains but smaller losses over time.
Performance on the IGT is typically measured by a Net Score which reflects the difference between selections allocated to advantageous and disadvantageous decks throughout the task. In the early phases of the task, as participants evaluate win and loss contingencies associated with deck choices, they develop decision-making strategies that are implemented in later phases of the task. In previous studies from our lab, no differences in Net Score between groups were observed in the early strategy development phase of the task as both Controls and MJ Users chose predominantly from disadvantageous decks (Whitlow et al., 2004). It was only after multiple exposures to win and, in particular, loss evaluation that Controls generally began shifting their selections to the smaller gain but smaller loss, advantageous decks. MJ Users, in contrast, generally failed to alter their selection patterns and continued to select from disadvantageous decks throughout the task. These data are consistent with other studies that suggest that the evaluation of, and response to, monetary wins and losses are crucial for the development of successful decision-making strategies and successful performance as measured by higher Net Scores (Bechara et al., 1994; Bolla et al., 2005; Lawrence et al., 2009). Therefore, the poor performance of MJ Users on this task suggests that their processing of wins and losses during the initial phases of the task may differ significantly from Controls resulting in ineffective strategy development.
Several imaging studies have focused on the role of wins and losses on performance throughout the IGT. In a cohort of healthy participants, comparing the evaluation of wins and losses across the entire task revealed that monetary wins produced greater activity in medial frontal brain areas whereas losses were associated with greater activity in lateral frontal regions (Tanabe et al., 2007). Lin et al. (2008) observed that the largest monetary losses on the task produced greater activity in medial frontal and parietal cortical regions, when compared to control events. Interestingly, MJ Users exhibit altered patterns of activation in many of these same brain regions while performing other executive function tasks (Quickfall and Crockford 2006; Chang and Chronicle 2007). While these studies demonstrate differences in the functional activity induced by wins and losses in healthy Controls, there have been no studies to date comparing win and loss outcomes in MJ Users who are known to perform poorly on the task, particularly during the critical strategy development phase of the task as participants acquire the foundations of their decision-making strategies. Previous imaging studies of MJ users during performance of the IGT task have been limited to abstinent users in whom performance was assessed with positron emission tomography which did not allow the distinction between various phases of the task, nor the evaluation of wins and losses separately (Bolla et al., 2005).
The purpose of this study, therefore, was to 1) isolate functional brain activity in Controls and MJ Users during win and loss evaluation in the strategy development phase of the IGT before differences in behavioral performance emerge and to 2) examine functional differences between groups during this sensitive period of the task. Finally, we sought to identify activity during the strategy development phase predictive of learning on the task. We hypothesized that MJ Users would display altered brain activity in response to evaluation during this strategy development phase of the task. Specifically, we expected MJ Users to show decreased activity during the evaluation of monetary losses.
2 Methods
2.1 Participants
Sixteen MJ Users and 16 age and gender matched non-marijuana smoking Controls were included in this study (see Table 1). All participants were right-handed. Following an initial phone screen, participants were invited into the laboratory and agreed to participate in procedures approved by the Wake Forest University School of Medicine Institutional Review Board. On the initial visit, participants provided urine samples to test for pregnancy and drug use and were administered the Structured Clinical Interview for DSM-IV (SCID; First 1997) as well as the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler 1999). Exclusion criteria included systemic diseases of the central nervous system, head trauma, neurological disorders, Axis-I psychiatric disorders (other than marijuana dependence for the MJ Users), abuse of substances other than nicotine, or an I.Q. of less than 80. MJ Users were required to test negative for illicit drugs other than marijuana and Controls were required to test negative for all illicit drugs. Participants who met all inclusion criteria were scheduled for a scan visit. MJ Users were asked to abstain from using marijuana starting at midnight the night before the scheduled scan visit.
Table 1.
Group Demographics
| Variable | Controls (N = 16) | MJ Users (N = 16) | ||
|---|---|---|---|---|
| Mean (± SD) | Mean (± SD) | t/X2Value | p | |
| Age (years) | 26.6 (6.1) | 26.4 (3.6) | 0.11 | n.s. |
| I.Q. | 115 (8.4) | 109.1 (13.6) | 1.47 | n.s. |
| Sex | 0.29 | n.s. | ||
| Male | 6 | 9 | ||
| Female | 10 | 7 | ||
| Cigarette Smokers | 12.5 % | 50 % | 5.24 | 0.022 |
| Alcohol AUDIT Score | 2.8 (2.0) | 4.2 (2.3) | 1.91 | n.s. |
| Caffeine (mg/day) | 96.9 (99.8) | 120.3 (119.5) | 0.60 | n.s. |
| Spielberger State Anxiety | 24.6 (5.6) | 26.1 (6.6) | 0.69 | n.s. |
| Beck’s Depression | 2.5 (4.0) | 3.4 (3.5) | 0.71 | n.s. |
| Marijuana Use: | ||||
| Age of onset (years) | 16.3 (2.1) | |||
| Years of Total Use | 9.6 (4.1) | |||
| Days per month | 29.4 (1.0) | |||
| Times per day | 2.1 (1.5) | |||
| Years at current use level | 4.5 (3.8) |
2.2 Procedure
On the scan visit, participants arrived at the testing center approximately three hours prior to the acquisition of their functional MRI scans. Participants provided urine samples to test for pregnancy and drug use and completed depression (Beck’s Depression Inventory) and anxiety (Speilberger Test of Anxiety) inventories. At no time did MJ Users report or overtly exhibit signs of marijuana withdrawal (Budney and Hughes 2006).
Approximately one hour before entering the scanner, participants were trained on the IGT using a standard laptop computer and button box. As part of the training, participants visually followed along as task instructions were read aloud by a study technician. Participants then completed a trial run of the IGT containing 8 gambling events and 2 control events in order to become familiar with the layout and timing of the task. During the trial run, monetary win and loss contingencies associated with deck selections were randomized in order to avoid strategy carryover to the scanner task. Participants were made aware of this distinction and performed an additional trial run if the initial run produced multiple mistimed events. Participants were informed that the individual who performed the best on the task would receive a fifty dollar bonus at the end of the study. Although previous studies have found no difference between smokers and nonsmokers on IGT performance (Lejuez et al., 2003; Harmsen et al., 2006), participants were given a 15 minute break with the opportunity to smoke a cigarette to avoid potential confounds of nicotine withdrawal on functional brain activity (Wang et al., 2007; Xu et al., 2007). Three participants in the MJ Users group took advantage of the opportunity to smoke a cigarette.
2.3 Iowa gambling task (IGT)
In the MRI scanner a modified version of the IGT was presented on MR compatible goggles, and responses were recorded on a button box positioned under the right hand. Before the task onset participants followed along as the task instructions were read aloud. As part of the instructions, emphasis was placed on the key role that monetary losses play in solving the task. The instructions were read as follows:
In front of you are four decks of cards: A, B, C, and D. When the game begins, you will see instructions to “Select a card…” for each turn. During each turn you have about 2 seconds to choose one card from any deck. You are free to switch from one deck to any other as often as you wish. Turns will last for varying lengths of time, so don’t be concerned if you do not receive instructions immediately following a card choice.
Each time you select a card you will win some money. Every so often, however, you will also lose some money. The goal of the game is to win as much money as possible and to avoid losing money. There is no way to figure out when you will lose money. All I can say is that some decks are worse than others. No matter how much you find yourself losing, you can still win the most if you stay away from the worst decks.
Occasionally, you will be prompted to select from a specific deck but you will neither win nor lose any money for that turn. Please treat the play money in this game as real money and any decision on what to do with it should be made as if you were using your own money. You will not know when the game will end. Please keep on playing until you are told to stop.
Once participants verbally acknowledged comprehension of the instructions the task was initiated with a 20 second countdown. Each participant performed three segments or runs of the IGT each consisting of 45 gambling events and 13 randomly inserted control events. The first run of the task was considered the early, strategy development phase and consisted of events 1 through 58. The second run consisted of events 59 through 116, and the third run encompassed events 117 through 174.
When the task started participants received instructions to “Select a Deck” for a fixed period of 2 seconds via a text box on the upper left-hand side of the task screen. During this period participants selected a card from one of four card decks labeled A, B, C and D by pressing buttons 1, 2, 3 or 4 on the button box, respectively. Immediately following the selection period, participants received feedback for a variable period of time jittered around 2 seconds. The length of evaluation events were jittered to correct for stimulus onset asynchrony and to assure adequate sampling of the HDR for imaging analyses. The end of each evaluation period signaled the onset of the next selection period. During evaluation, participants viewed the monetary gain and/or loss associated with their selection. Evaluation information alternated with task instructions in the text box on the upper left-hand side of the screen. Examples of win and loss evaluation events can be seen in Fig. 1. On the upper right-hand side of the screen an ongoing monetary score was updated following each deck selection. On selection control trials participants received instructions to select a card from a specific deck (e.g. “Select Deck B”). Directly following selection control events, participants viewed an evaluation control screen that contained the phrase “You Neither Win Nor Lose”. For all imaging analyses win and loss evaluation events were compared to evaluation control events.
Fig. 1.
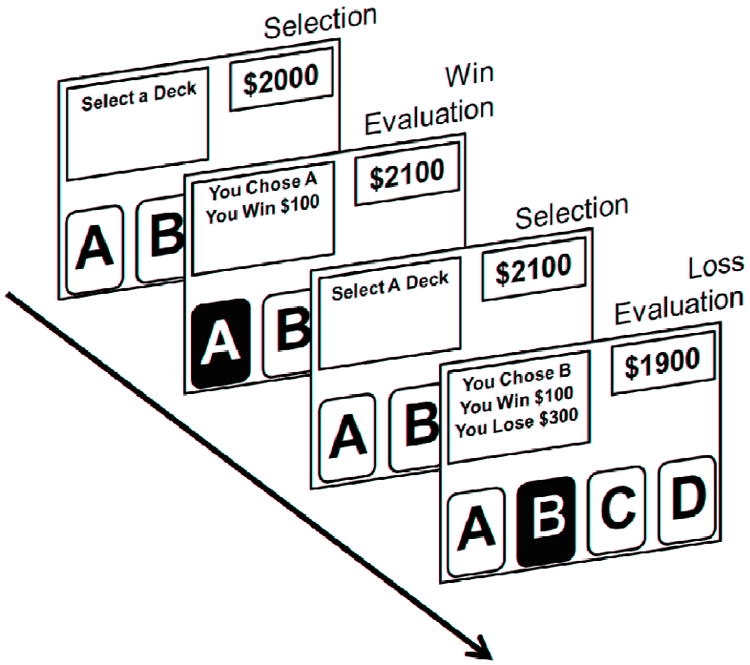
Iowa gambling task (IGT) event types. In this event-related functional MRI task, participants were instructed to “Select a Deck” for 2 seconds from one of four decks of cards (A,B,C,D) using an MR-compatible response box. Following each selection, participants evaluated feedback regarding the amount of money won and / or lost during that gambling event.
Selections from decks A and B resulted in immediate gains of $100 each with losses over time ranging from $250 to $1200 and were considered disadvantageous. Selections from decks C and D produced immediate gains of $50 with losses over time ranging from $50 to $250 and were considered advantageous. To ensure that the typical advantageous decision-making emerged in the control group following the strategy development phase of the task (RUN 1), the proportion of responses allocated to disadvantageous (A and B) and advantageous (C and D) decks were calculated for each of the three sections of the task (RUN 1, RUN 2 and RUN 3) and used to calculate individual Net Scores of performance.
2.4 Functional MRI data acquisition
Images were acquired on a 1.5T General Electric scanner with a birdcage-type standard quadrature head coil and an advanced nuclear magnetic resonance echoplanar system. Foam padding was used to limit head motion. High-resolution T1-weighted anatomical images (3D SPGR, TR=10 ms, TE=3 ms, voxel dimensions 1.0×1.0×1.5 mm, 256×256 voxels, 124 slices) were acquired for co-registration and normalization of functional images. A total of 162 co-planar functional images were acquired using a gradient echoplanar sequence (TR=2100 ms, TE=40 ms, voxel dimensions 3.75×3.75×5.0 mm, 64×64 voxels, 28 slices). The scanning planes were oriented parallel to the anterior–posterior commissure line and extended from the superior extent of motor cortex to the base of the cerebellum. Six volumes of data were acquired during the 20 second countdown period and immediately discarded to allow for equilibrium before selections began.
2.5 Statistical analyses
2.5.1 Demographics and behavior
Independent samples t-tests were used to compare groups on parametric demographic variables. Chi-square analyses were used to compare group differences in sex and the proportion of participants who were cigarette smokers. To examine advantageous and disadvantageous choices in each of the three sections (i.e. RUNS) of the task, a Net Score of performance was calculated for each individual in each section by subtracting the number of selections on advantageous decks from the number on disadvantageous decks. A positive Net Score reflected more advantageous deck selections, relative to disadvantageous selections, within that section of the task. To determine if the Net Score varied according to group a 2×3 mixed model ANOVA was conducted with between-subjects group factor (Controls and MJ Users) and within-subject RUN factor (1, 2 and 3). Independent and paired sample t-tests were used for post-hoc analyses accordingly with Bonferroni corrections. To determine if the number of event types experienced differed between groups during the strategy development phase of the task (RUN 1), a 2×2 ANOVA was conducted with between-subjects group factor (Controls and MJ Users) and within-subjects event type (win and loss evaluation). To examine improvement in task performance across the task, a Net Score Difference value was calculated for each individual. This value was calculated by subtracting each individual’s Net Score in the first section of the task from their Net Score in the last section of the task (Net Score Difference = RUN 3 Net Score minus RUN 1 Net Score). A positive Net Score Difference reflected improvement in performance across the task. An independent samples t-test was used to examine differences in Net Score Difference values between groups. All behavioral data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 11.5.
2.5.2 Functional MRI preprocessing and data analysis
To examine differences in the functional brain response to win and loss evaluation during the strategy development phase (RUN 1), each individual’s neural response to win evaluation, loss evaluation, and control evaluation events was isolated. The functional data from each participant were corrected for acquisition time (slice timing), realigned to the first volume (motion correction), normalized into a standardized neuroanatomical space (Montreal Neurological Institute brain template), smoothed using a Gaussian kernel of 8 mm, and high-pass filtered (128s) to remove low frequency noise. Inspection of motion correction revealed that all corrections were less than 2 mm. For each individual, a multiple linear regression analysis was performed. Regressors corresponded to time periods during which the participant 1) made deck selections or 2) viewed selection control events and then evaluated feedback of monetary 3) wins, 4) losses or 5) viewed evaluation control events. As the aim of this study was to investigate the neural responses during the evaluation of wins and losses, event times corresponding to selection events and selection control events were modeled to remove variance associated with periods but not included in further contrast maps. Evaluation conditions (win, loss and control) were modeled by convolving relevant evaluation times with a canonical hemodynamic response function. Trials in which no response was made were excluded from the analyses. For cigarette smokers, the reported average number of cigarettes smoked per day was treated as a nuisance variable and variance associated with this variable was covaried out of all functional imaging analyses, an approach used in other substance abuse studies (Bolla et al., 2005). First, for each individual statistical contrast maps of activity associated with all RUN 1 evaluation periods were made by comparing activity during all RUN 1 evaluation events to activity during all RUN 1 evaluation control events (win evaluation + loss evaluation > control evaluation). Next, for each individual, statistical contrast maps of RUN 1 wins (win evaluation > control evaluation) and RUN 1 losses (loss evaluation > control evaluation) were created. These contrast maps were compared between groups to find 1) differences between groups in all RUN 1 evaluation and 2) differences between groups specific to win and loss evaluation. For these between group comparisons, contrast maps were thresholded with a voxel-wise P value of 0.05 further adjusted at the cluster level (P <0.001, corrected) to reduce the chance of Type I error (Christakou et al., 2009; Hartstra et al., 2010). Lastly, in order to identify the relationship between RUN 1 evaluation event types (wins or losses) and performance on the IGT, within each group, the Net Score Difference measure (a measure of improvement across the task) was regressed with the whole brain functional response during RUN 1 win (win evaluation > control evaluation) and loss (loss evaluation > control evaluation) events. For these analyses, a voxel-wise P value of 0.01 was used with further adjustments at the cluster level (P <0.001, corrected). All imaging analyses were performed with SPM 5 (Wellcome Department of Imaging Neuroscience, London, UK) in the MATLAB 7.0 (Mathworks, Natick, MA) shell using an event-related model (Friston et al., 1998).
3 Results
3.1 Demographics
A description of study participants is shown in Table 1. MJ Users were 26.4 ± 3.6 years old (mean ± sd) and reported using marijuana 2.1 ± 1.5 times a day, 29.4 ± 1.0 days a month, for 9.6 ± 4.1 years. The average age of first marijuana use was 16.3 ± 2.1 years. Controls were 26.6 ± 6.1 years old and did not meet dependence criteria for any illegal drugs. Four of 16 members of the Control group reported previous marijuana use with use limited to fewer than 50 lifetime uses, occurring more than 2 years prior to the study. Four of 16 MJ Users met criteria for marijuana dependence. On the scanning day, all participants had negative urine screens for illegal substances (other than marijuana in MJ Users). All members of the MJ Users group tested positive for marijuana metabolites the day of scanning and reported a mean (± sd) abstinence from marijuana of 12.0 ± 2.9 hours (range = 8.5 − 16 hours). There were no significant differences between groups in depression scores on the Becks Depression Inventory, nor anxiety scores on the Spielberger Test of Anxiety at the time of scanning.
3.2 IGT behavioral performance
Behavioral performance on the IGT is shown in Fig. 2a. In Controls, behavioral performance improved across the three segments of the task, observed as a shift in the mean (± sd) Net Score (RUN 1 = −5.50 ± 14.2, RUN 2 = +3.06 ± 16.1, RUN 3 = +5.06 ± 20.2). Improvement was not observed in MJ Users (RUN 1 = −7.25 ± 7.3, RUN 2 = −3.38 ± 8.7, RUN 3 = −8.5 ± 11.4). There was a significant group × run interaction F(1,30) = 4.31, p = 0.04). Post hoc analysis revealed that during the final segment of the task Controls had significantly higher Net Scores as compared to MJ Users (RUN 3: Controls = +5.0 ± 14.2 vs. MJ Users = −8.5 ± 11.4) t(16) = 2.90, p = 0.01.
Fig. 2.
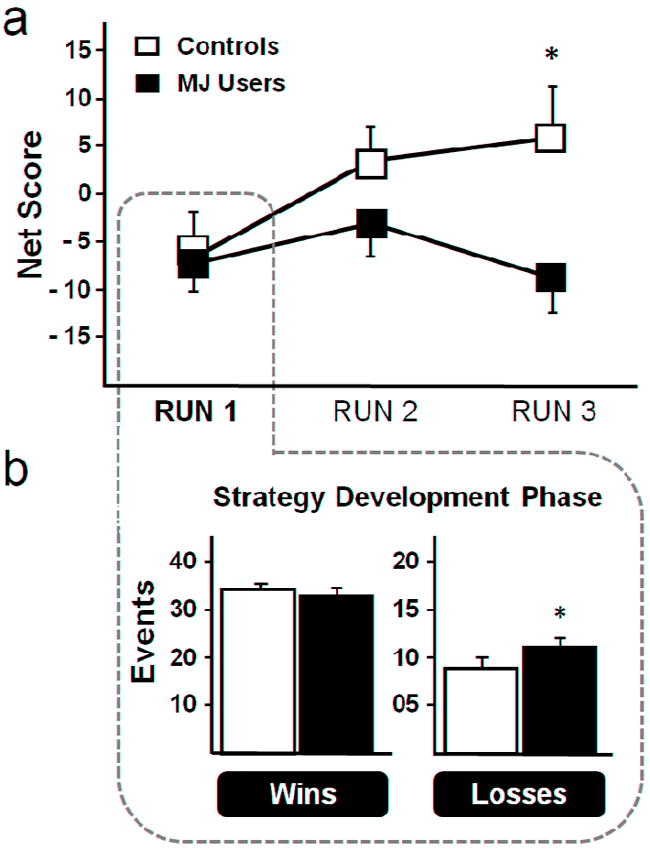
a) Behavioral Performance in three sections of the Iowa Gambling Task (174 trials). By the end of the task (RUN 3), Controls (unfilled) made significantly more advantageous deck selections compared to chronic marijuana users (MJ Users; filled). There was no difference in deck selections between groups during the strategy development phase (RUN 1). b) During the strategy development phase (RUN 1), MJ Users experienced more loss events than Controls. * = p < 0.05
During first segment of the task, the strategy development phase, groups did not differ in performance as measured by the Net Score (RUN 1: Controls = −5.50 ± 14.2 vs. MJ Users = −7.25 ± 7.3). During this segment, a significant group × event type interaction was observed F(1,30) = 7.73, p = 0.009. As shown in Fig 2b, there was no difference in the number of win events experienced between groups (Wins: Controls = 34.19 ± 1.1 vs. MJ Users = 33.13 ± 2.2). There was, however, a significant difference in the number of loss events experienced between groups, with MJ Users experiencing more loss events (11.50 ± 2.3), compared to Controls (9.13 ± 1.8) t(30) = 3.28, p = 0.003.
3.3 Functional imaging during strategy development
3.3.1 Combined evaluation events (wins and losses)
In order to elucidate the role of early evaluation events during strategy development, functional imaging analyses were restricted to the first segment of the task (i.e. RUN 1) before performance differences emerged between the two groups. Analyses were also restricted to the evaluation periods of this early phase as participants viewed feedback regarding the positive (wins) and negative (losses) consequences associated with their initial choices. Differences in brain activity between groups in response to all RUN 1 evaluation (win + loss) events can be seen in Fig. 3 and Table 2. Comparisons of activity between groups revealed that, compared to MJ Users, Controls had significantly greater activity during RUN 1 evaluation in several frontal brain regions. Controls displayed greater responses in clusters of activity coincident with the anterior cingulate cortex (Fig 3a, b[Y = 26, 34, 38]) and medial frontal cortex (Fig 3a, b[Y = 56, 54]). This activity also extended dorsally to include portions of the superior medial frontal cortex (Fig 3a, b[Y = 34, 38]). In contrast, there were no suprathreshold clusters where MJ Users had greater activity than Controls during RUN 1 evaluation (Table 2).
Fig. 3.
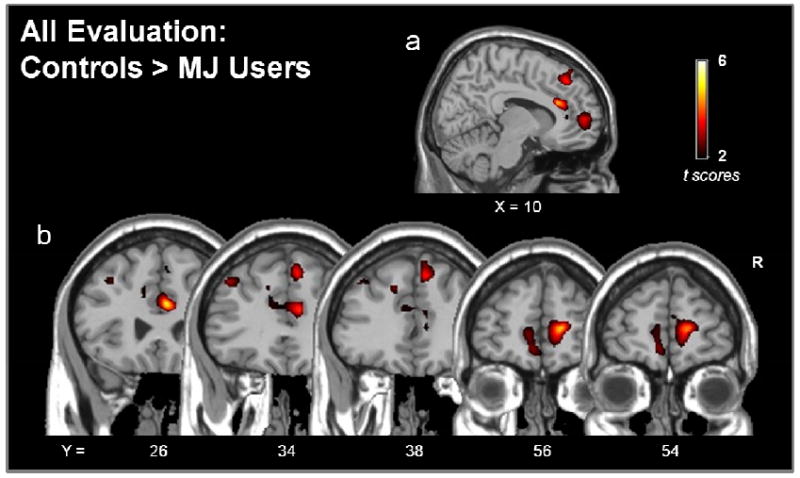
The difference in functional activity of Controls and chronic marijuana users (MJ Users) during all evaluation events (wins + losses) during the strategy development phase of the IGT (RUN 1). Images show clusters where activity was greater in Controls compared to MJ Users. There were no suprathreshold clusters where MJ Users had greater activity compared to Controls. Probability thresholds were set to p< 0.05 at the voxel-level and further corrected at the cluster level (p<.001, corrected).
Table 2.
Clusters of significant differences in BOLD signals during Win and Loss evaluation between Controls and MJ Users during strategy development (Run1) on the IGT
| Analysis | Side | Anatomical Regions | BA* | MNI Coordinates+ (x, y, z) | Maximum Voxel t-value | ||
|---|---|---|---|---|---|---|---|
| All Evaluation (Wins + Losses) | |||||||
| Controls > MJ Users | R | Anterior Cingulate Cortex | 24 | 6 | 26 | 22 | 4.53 |
| R | Medial Frontal Gyrus | 10 | 16 | 54 | 6 | 4.35 | |
| MJ Users > Controls | N/A | no suprathreshold cluster | |||||
| Win Evaluation | |||||||
| Controls > MJ Users | N/A | no suprathreshold cluster | |||||
| MJ Users > Controls | N/A | no suprathreshold cluster | |||||
| Loss Evaluation | |||||||
| Controls > MJ Users | R | Anterior Cingulate Cortex | 24 | 6 | 26 | 24 | 4.20 |
| L | Medial Frontal Gyrus | 9 | -12 | 38 | 34 | 3.92 | |
| R | Medial Frontal Gyrus | 8 | 8 | 34 | 46 | 3.71 | |
| R | Precuneus | 7 | 4 | -60 | 64 | 3.95 | |
| L | Cerebellum: Declive | -28 | -74 | -22 | 3.93 | ||
| R | Superior Parietal Lobe | 7 | 20 | -72 | 56 | 3.98 | |
| MJ Users > Controls | N/A | no suprathreshold cluster | |||||
BA, Brodmann areas. Listed areas correspond to location of the maximum voxel of activation and other BAs associated with the activity cluster.
MNI, Montreal Neurological Institute. Bolded regions and coordinates correspond to the maximum voxel of activity within the cluster followed by regions of local maxima within the cluster.
3.3.2 Differences according to evaluation event type (wins or losses)
Differences in activity between Controls and MJ Users was further examined according to specific event type, win evaluation (win events > control events) and loss evaluation (loss events > control events). These data can be seen in Fig. 4 and Table 2. There were no differences between groups in the activity observed during win evaluation. There were several areas, however, where activity during loss evaluation was greater in Controls, compared to MJ Users (Fig. 4). Portions of this activity were spatially coincident with differences observed when examining combined event types (wins + losses). For example, during loss evaluation, Controls displayed greater activity than MJ Users in the anterior cingulate cortex (Fig 4a, b[Y = 26, 34, 38]) and medial frontal cortex (Fig 4a, b[Y = 56, 54]). This activity also extended dorsally into the superior medial frontal cortex (Fig 4a, b[Y = 34, 38]) but also extended rostrally to include more prefrontal areas (Fig 4a, b[Y = 56, 54]). In addition, differences emerged that were not observed in the combined analysis. Compared to MJ Users, Controls showed greater activity during loss evaluation in clusters enveloping portions of the precuneus, posterior cingulate cortex and dorsal cerebellum as well as portions of the superior parietal lobe and occipital cortex (Fig. 4a; Table 2.).
Fig. 4.
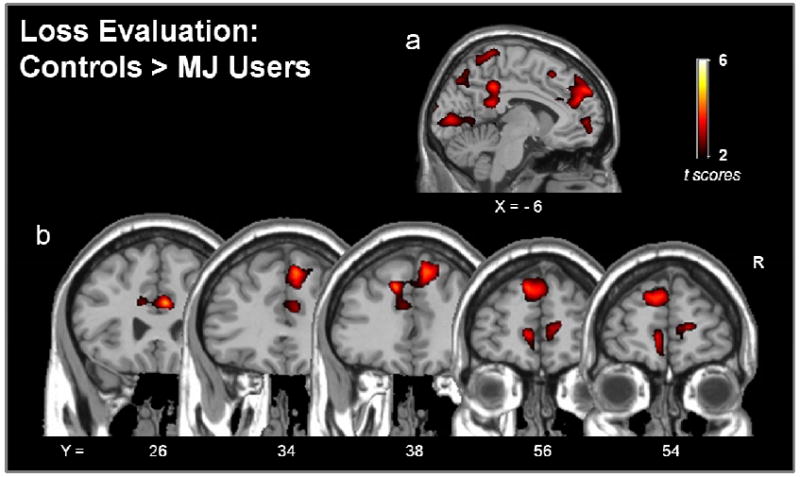
The difference in functional activity of Controls and chronic marijuana users (MJ Users) during monetary loss evaluation in the strategy development phase of the IGT (RUN 1). Images show clusters where activity was greater in Controls compared to MJ Users. There were no suprathreshold clusters where MJ Users had greater activity compared to Controls. There were also no differences between groups during win evaluation in this phase of the task. Probability thresholds were set to p< 0.05 at the voxel-level and further corrected at the cluster level (p<.001, corrected).
3.3.3 Loss evaluation and task performance
To characterize the relationship between early evaluation events and IGT performance, the Net Score Difference (Net Score: RUN 3 - RUN 1), a measure of improvement over the course of the task, was correlated with the functional response to RUN 1 win and loss evaluation events within each group. Consistent with the Net Score results (Fig. 2a), groups significantly differed in the Net Score Difference t(30) = 2.08, p = 0.04 (Fig. 5a). The mean (± sd) Net Score Difference in Controls (10.56 ± 19.5; range: −19 - +46) was significantly greater than that observed in MJ Users (−1.25 ± 11.8; range: −26 - +20). A regression of the Net Score Difference with the whole brain response during RUN 1 loss evaluation, revealed activity in Controls that was associated with future improvement in task performance. In Controls, but not MJ Users, the magnitude of response in the anterior cingulate cortex, ventral medial prefrontal cortex, and rostral prefrontal cortex during RUN 1 loss evaluation positively correlated with the Net Score Difference (Fig 5b). No relationship was observed between Net Score Difference and the response to RUN 1 win evaluation in either group. Together these data demonstrate that before behavioral differences emerge on the IGT, MJ Users have decreased responsivity to the monetary losses that aid strategy development.
Fig. 5.
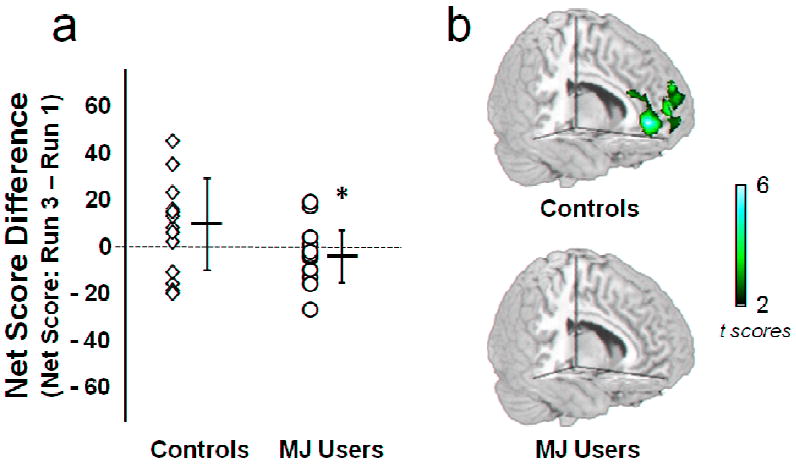
a) Improvement in performance across the Iowa Gambling Task (IGT) as measured by the Net Score Difference (Net Score: RUN 3 – RUN 1). Performance in Controls significantly improved over the course of the task, compared to chronic marijuana users (MJ Users). b) Net Score Difference correlated with whole brain functional activity during RUN 1 loss evaluation in Controls and MJ Users. Responses in the anterior cingulate cortex, ventral medial prefrontal cortex and rostral prefrontal cortex during RUN 1 loss evaluation predicted improvement in Controls, but not MJ Users. * = p < 0.05
4 Discussion
The results of the present study demonstrate that chronic marijuana users (MJ Users) perform poorly on the Iowa Gambling Task (IGT), failing to develop advantageous decision making strategies, thus confirming previous studies using similar tasks (Whitlow et al., 2004; Hermann et al., 2009). Furthermore, the present study extended these findings by showing that during the strategy development phase of the IGT, before the emergence of group differences in behavioral performance, the functional brain activity of MJ Users during evaluation is distinctly different from that of Controls. During early evaluation events (wins + losses) MJ Users had smaller BOLD responses than Controls in the anterior cingulate cortex, the ventral medial prefrontal cortex and portions of the superior medial frontal cortex. During the evaluation of monetary losses, MJ Users had less activity in these same areas as well as in the precuneus, posterior cingulate cortex, the superior parietal lobe, and portions of the dorsal cerebellum and occipital cortex, compared to Controls. Finally, correlating performance over the course of the task with BOLD activity during early loss evaluation revealed that the response to losses in the anterior cingulate cortex, ventral medial prefrontal cortex and rostral prefrontal cortex predicted improvement in Controls, whereas MJ Users showed no correlations. These data suggest that the failure of MJ Users to develop successful decision-making results from a relative insensitivity to the early monetary losses that aid strategy development and precede successful performance on the IGT.
Previous studies of healthy participants have shown that in the course of strategy development, positive and negative information guide future decisions towards achieving a goal (Sutton and Barto 1998; Dayan and Balleine 2002; Camerer 2003). This is also the case of the IGT (Bechara et al., 2005), where early monetary losses provide the incentive to shift to advantageous deck selections over time and early wins encourage continued selection on disadvantageous decks. Previously it was found that over the course of the task, activity in the inferior parietal lobe and medial frontal cortex is increased during evaluation, and the medial prefrontal cortex responds to the largest losses (Lin et al., 2008). Our data support these findings and extend them by highlighting the importance of the medial frontal cortex while processing early losses during the strategy development phase of the task. Furthermore, our data show that, relative to Controls, MJ Users have altered processing in this brain area.
The separation of all of the evaluation events (wins and losses) into specific event types (wins or losses) revealed that the response to monetary losses was critical for the differences in strategy development between Controls and MJ Users. This is supported by the absence of differences between groups in the functional response to early win events (Table 2). Furthermore, the differences that emerged during loss evaluation, particularly in the medial frontal lobe, were spatially coincident with the differences observed when all evaluation events, wins and losses, were considered together. Therefore, it appears that the key difference between MJ Users and Controls is in the evaluation of the negative information conveyed by losses, rather than in the evaluation of wins.
Reduced responses in the superior frontal gyrus during loss evaluation is consistent with previous reports examining evaluation of losses over the entire course of the task (Tanabe et al., 2007) . This compromised activity in MJ Users, as well as that observed in the precuneus and cingulate cortex, may represent altered attentional resources directed to monetary losses, similar to functional abnormalities observed in adults with attention-deficit disorder (Castellanos et al., 2008). Also, the functional relationship between the medial superior frontal gyrus and the precuneus has been shown to be time locked to attentional shifts between object features (Nagahama et al., 1999), suggesting that MJ Users may have an inability to shift attention between the various components of trials (i.e. selection vs. evaluation). This could also be the case for win events. However, during loss evaluation the additional information regarding the monetary loss represents an additional and necessary component that must be attended to in order to learn the task. It is possible that the lack of activity in these areas in MJ Users is associated failure to attend to this additional component. Failure to addend to this information would explain continued selections made on disadvantageous decks.
There are several potential explanations for diminished activity of MJ Users in the anterior cingulate cortex and ventral medial prefrontal cortex, as compared to Controls. This could reflect compromised error processing during loss evaluation events, a hypothesis suggested by others (Lin et al., 2008) and consistent with studies demonstrating that anterior cingulate activity increases in response to errors (Swick and Turken 2002). This may also reflect more general deficits in performance monitoring, as increases have also been observed under conditions where errors are likely to occur (Carter et al., 1998) and during violations of outcome expectancy (Oliveira et al., 2007). These reductions may reflect decreased motivation as a result of experiencing monetary losses (Martin-Soelch et al., 2009; Simoes-Franklin et al., 2009). Differences in motivation did not appear to be a significant contributing factor in the study, however, as all individuals completed the task, and groups did not differ in the number of omitted or “no response” events on the task. More importantly, on selection trials immediately following monetary loss events, both groups shifted selections away from loss producing decks more than 95% of the time. This suggests that monetary losses motivated changes in selection strategies in both groups. Finally, it has been hypothesized that lesions to these medial brain areas results in the inability to integrate affective information into executive functioning processes (Bechara 2004). This suggests that MJ Users may fail to incorporate the negative affective experience of early monetary losses into developing strategies to perform the task. While further studies are needed to explore these possibilities, it is clear from this study that MJ Users lack a functional response to early losses in the anterior cingulate, the ventral medial prefrontal cortex, and rostral prefrontal cortex that predicts improvement in Controls. This is evidence that altered processing to early losses in these areas is directly related to the inability of MJ Users to develop advantageous strategies on the task.
There are a growing number of reports that demonstrate abnormalities in affective processing in recreational cannabis users as well as long-term heavy marijuana users (Degenhardt et al., 2003; Wadsworth et al., 2006; Dorard et al., 2008; Skosnik et al., 2008; Gruber et al., 2009). For example, Gruber et al. (2009) recently demonstrated that MJ Users have altered responsivity to affective faces presented below the level of consciousness in the amygdala and the anterior cingulate cortex. The data from the current study extend these findings by showing deficits specific to negative affective information processing in MJ Users. This is consistent with the ability of cannabinoids to modulate the behavioral responses to aversive stimuli and the conditional associations between aversive events and the environment. For example, blocking normal or endogenous cannabinoid system function compromises learned escape behaviors (Varvel et al., 2005). Furthermore, blocking the cannabinoid system genetically (CB1-deficient mice) or pharmacologically (with a CB1 antagonist) increases the retention of fear memories in altered mice, compared to wild type mice (Marsicano et al., 2002). Conversely, and of particular relevance to this study, enhancing cannabinoid system function with CB1 receptor agonists, similar to THC, blocks the expression of fear memories as measured by fear-potentiated startle (Lin et al., 2006). These findings suggest that the functional insensitivity to aversive events observed in the current study may result from disrupted cannabinoid system function associated with heavy marijuana use. This is also consistent with human studies demonstrating that THC administration decreases the functional reactivity to social signals of threat in recreational users (Phan et al., 2008).
That MJ Users appear to have a blunted response to negative stimuli is consistent with studies demonstrating that the poor performance of cocaine users performing the IGT is related to less responsiveness to losses (Stout et al., 2004). Other studies have observed poor performance on the IGT in various drug abusing populations, however, these studies did not evaluate the specific role that early win and loss evaluation play in development of task performance (Bolla et al., 2003; Tucker et al., 2004; Vadhan et al., 2007; Acheson et al., 2009; Vadhan et al., 2009). Results from the present study suggest that MJ Users may be relatively insensitive to negative information as they first attempt to solve problems. This insensitivity may interfere with the important role that monetary losses play in facilitating successful strategy-development in the early phases of the IGT. As the aim of this study was to model experiences in day-to-day marijuana users, we tested individuals at a time between the offset of THC’s psychoactive effects (2-3 hrs) and the onset of marijuana withdrawal symptoms (1-3 days). It is not clear if the results observed here persist following prolonged abstinence, however, one study reported behavioral and neurofunctional changes in MJ Users performing the IGT can persist following 28 days of abstinence (Bolla et al., 2005).
The current study has some inherent limitations based on the task design and analysis that may warrant further investigation. All participants received the same monetary compensation for completion, with the exception of the best performer who received a fifty dollar bonus. Though participants were asked to “treat the play money in this game as real money” it is unclear if performance in MJ Users would vary as a function of motivation for various reward types while performing the task (e.g. the IGT monetary score reflecting real versus fictitious money), which has been observed in both control populations (Bowman and Turnbull 2003) and cocaine using populations (Vadhan et al., 2009). There were more cigarette smokers in the MJ Users group than the Controls group. Post-hoc analysis of the smokers (n = 8) and non-smokers (n = 8) in the MJ User group however revealed no difference in behavioral performance or brain activity during the task. This is congruent with other studies that have reported no difference in IGT performance in smokers and non-smokers (Lejuez et al., 2003; Harmsen et al., 2006). Furthermore, the number of cigarettes smoked per day was incorporated as a covariate in the imaging analyses similar to other imaging studies with significant differences in demographic variables between groups (Bolla et al., 2005). Finally, it is not possible to determine whether these results are the direct result of a history of heavy marijuana use or are the result of preexisting conditions including psychiatric disorders or genetic background.
To summarize, in the current study MJ Users failed to develop successful decision-making strategies on the IGT, relative to Controls. During the early, strategy development phase of the task, when performance did not differ between groups, MJ Users processed evaluation differently than Controls. Specifically, MJ Users showed reduced activity while evaluating monetary losses. Furthermore, MJ Users lacked a functional response to monetary losses in medial frontal brain areas that predicted task improvement in Controls. Since the early monetary losses on the IGT drives successful strategy development, the diminished response to losses in MJ Users may explain their inability to engage successful decision-making strategies on the task. These data suggest that MJ Users do not process negative information in the same manner as non-marijuana using Controls during ongoing decision-making. This may result in inefficient strategies used to solve problems. In light of the growing number of people reporting marijuana use disorder (Compton et al., 2004) an appreciation of the relationship between affective information processing and decision-making in chronic marijuana users may be clinically relevant. Understanding how marijuana influences the perception of what is “negative” may help explain continued marijuana use and aid in the development of effective strategies for the treatment of this disorder.
Acknowledgments
This work was supported by the National Institute of Drug Abuse grants DA007246 (MJW), DA020074 (LJP), and DA06634 (LJP). The authors thank, Mack D. Miller, Hilary R. Smith and Thomas J.R. Beveridge for their comments on this manuscript and Marla Torrence for her assistance in recruitment and processing of the participants.
Footnotes
None of the authors have any financial conflict of interest in the performance or publication of this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheson A, Robinson JL, Glahn DC, Lovallo WR, Fox PT. Differential activation of the anterior cingulate cortex and caudate nucleus during a gambling simulation in persons with a family history of alcoholism: studies from the Oklahoma Family Health Patterns Project. Drug Alcohol Depend. 2009;100:17–23. doi: 10.1016/j.drugalcdep.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. The role of emotion in decision-making: evidence from neurological patients with orbitofrontal damage. Brain and Cognition. 2004;55:30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. The Iowa Gambling Task and the somatic marker hypothesis: some questions and answers. Trends in Cognitive Sciences. 2005;9:159–62. doi: 10.1016/j.tics.2005.02.002. discussion 162-4. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–43. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–94. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. 2005;26:480–92. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Bowman CH, Turnbull OH. Real versus facsimile reinforcers on the Iowa Gambling Task. Brain and Cognition. 2003;53:207–10. doi: 10.1016/s0278-2626(03)00111-8. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR. The cannabis withdrawal syndrome. Current Opinion in Psychiatry. 2006;19:233–8. doi: 10.1097/01.yco.0000218592.00689.e5. [DOI] [PubMed] [Google Scholar]
- Camerer CF. Behavioural studies of strategic thinking in games. Trends in Cognitive Sciences. 2003;7:225–231. doi: 10.1016/s1364-6613(03)00094-9. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–9. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga-Barke EJ, Rotrosen J, Adler LA, Milham MP. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biological Psychiatry. 2008;63:332–7. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Chronicle EP. Functional imaging studies in cannabis users. Neuroscientist. 2007;13:422–32. doi: 10.1177/1073858406296601. [DOI] [PubMed] [Google Scholar]
- Christakou A, Brammer M, Giampietro V, Rubia K. Right ventromedial and dorsolateral prefrontal cortices mediate adaptive decisions under ambiguity by integrating choice utility and outcome evaluation. J Neurosci. 2009;29:11020–8. doi: 10.1523/JNEUROSCI.1279-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991-1992 and 2001-2002. Journal Of the American Medical Association. 2004;291:2114–21. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- Dayan P, Balleine BW. Reward, motivation, and reinforcement learning. Neuron. 2002;36:285–98. doi: 10.1016/s0896-6273(02)00963-7. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, Lynskey M. Exploring the association between cannabis use and depression. Addiction. 2003;98:1493–504. doi: 10.1046/j.1360-0443.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- Dorard G, Berthoz S, Phan O, Corcos M, Bungener C. Affect dysregulation in cannabis abusers: a study in adolescents and young adults. European Child & Adolescent Psychiatry. 2008;17:274–82. doi: 10.1007/s00787-007-0663-7. [DOI] [PubMed] [Google Scholar]
- First M. Users Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Clinical Version. American Psychiatric Publishing, Inc; 1997. [Google Scholar]
- Fletcher JM, Page JB, Francis DJ, Copeland K, Naus MJ, Davis CM, Morris R, Krauskopf D, Satz P. Cognitive correlates of long-term cannabis use in Costa Rican men. Archives of General Psychiatry. 1996;53:1051–7. doi: 10.1001/archpsyc.1996.01830110089011. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Rogowska J, Yurgelun-Todd DA. Altered affective response in marijuana smokers: An FMRI study. Drug and Alcohol Dependence. 2009;105:139–153. doi: 10.1016/j.drugalcdep.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen H, Bischof G, Brooks A, Hohagen F, Rumpf HJ. The relationship between impaired decision-making, sensation seeking and readiness to change in cigarette smokers. Addictive Behaviors. 2006;31:581–92. doi: 10.1016/j.addbeh.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Hartstra E, Oldenburg JF, Van Leijenhorst L, Rombouts SA, Crone EA. Brain regions involved in the learning and application of reward rules in a two-deck gambling task. Neuropsychologia. 2010;48:1438–46. doi: 10.1016/j.neuropsychologia.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Hermann D, Lemenager T, Gelbke J, Welzel H, Skopp G, Mann K. Decision Making of Heavy Cannabis Users on the Iowa Gambling Task: Stronger Association with THC of Hair Analysis than with Personality Traits of the Tridimensional Personality Questionnaire. European Addiction Research. 2009;15:94–98. doi: 10.1159/000189788. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Tcheremissine OV, Steinberg JL, Sharon JL. Response perseveration and adaptation in heavy marijuana-smoking adolescents. Addictive Behaviors. 2007;32:977–90. doi: 10.1016/j.addbeh.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Jollant F, O’Daly O, Zelaya F, Phillips ML. Distinct roles of prefrontal cortical subregions in the Iowa Gambling Task. Cerebral Cortex. 2009;19:1134–43. doi: 10.1093/cercor/bhn154. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Jones HA, Richards JB, Strong DR, Kahler CW, Read JP. The Balloon Analogue Risk Task (BART) differentiates smokers and nonsmokers. Experimental and Clinical Psychopharmacology. 2003;11:26–33. doi: 10.1037//1064-1297.11.1.26. [DOI] [PubMed] [Google Scholar]
- Lin CH, Chiu YC, Cheng CM, Hsieh JC. Brain maps of Iowa gambling task. BMC Neuroscience. 2008;9:72. doi: 10.1186/1471-2202-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Gean PW. Effects of intra-amygdala infusion of CB1 receptor agonists on the reconsolidation of fear-potentiated startle. Learn Mem. 2006;13:316–21. doi: 10.1101/lm.217006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–4. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C, Kobel M, Stoecklin M, Michael T, Weber S, Krebs B, Opwis K. Reduced response to reward in smokers and cannabis users. Neuropsychobiology. 2009;60:94–103. doi: 10.1159/000239685. [DOI] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, de Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28:1356–65. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- Miller LL, Branconnier RJ. Cannabis: effects on memory and the cholinergic limbic system. Psychological Bulletin. 1983;93:441–56. [PubMed] [Google Scholar]
- Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Sawamoto N, Toma K, Nakamura K, Hanakawa T, Konishi J, Fukuyama H, Shibasaki H. Transient neural activity in the medial superior frontal gyrus and precuneus time locked with attention shift between object features. Neuroimage. 1999;10:193–9. doi: 10.1006/nimg.1999.0451. [DOI] [PubMed] [Google Scholar]
- Oliveira FT, McDonald JJ, Goodman D. Performance monitoring in the anterior cingulate is not all error related: expectancy deviation and the representation of action-outcome associations. J Cogn Neurosci. 2007;19:1994–2004. doi: 10.1162/jocn.2007.19.12.1994. [DOI] [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, Onyewuenyi I, Popovska A, de Wit H. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. The Journal of Neuroscience. 2008;28:2313–9. doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Archives of General Psychiatry. 2001;58:909–15. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Quickfall J, Crockford D. Brain neuroimaging in cannabis use: a review. The Journal of Neuropsychiatry and Clinical Neurosciences. 2006;18:318–32. doi: 10.1176/jnp.2006.18.3.318. [DOI] [PubMed] [Google Scholar]
- Simoes-Franklin C, Hester R, Shpaner M, Foxe JJ, Garavan H. Executive function and error detection: The effect of motivation on cingulate and ventral striatum activity. Human Brain Mapping. 2009;31:458–69. doi: 10.1002/hbm.20879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skosnik PD, Park S, Dobbs L, Gardner WL. Affect processing and positive syndrome schizotypy in cannabis users. Psychiatry Research. 2008;157:279–82. doi: 10.1016/j.psychres.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Stout JC, Busemeyer JR, Lin A, Grant SJ, Bonson KR. Cognitive modeling analysis of decision-making processes in cocaine abusers. Psychonomic Bulletin and Review. 2004;11:742–7. doi: 10.3758/bf03196629. [DOI] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Reinforcement learning: an introduction. IEEE Transactions on Neural Networks. 1998;9:1054. [Google Scholar]
- Swick D, Turken AU. Dissociation between conflict detection and error monitoring in the human anterior cingulate cortex. Proc Natl Acad Sci U S A. 2002;99:16354–9. doi: 10.1073/pnas.252521499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Thompson L, Claus E, Dalwani M, Hutchison K, Banich MT. Prefrontal Cortex Activity is Reduced in Gambling and Nongambling Substance Users During Decision-Making. Human Brain Mapping. 2007;28:1276–86. doi: 10.1002/hbm.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KA, Potenza MN, Beauvais JE, Browndyke JN, Gottschalk PC, Kosten TR. Perfusion abnormalities and decision making in cocaine dependence. Biological Psychiatry. 2004;56:527–30. doi: 10.1016/j.biopsych.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Vadhan NP, Hart CL, Haney M, van Gorp WG, Foltin RW. Decision-making in long-term cocaine users: Effects of a cash monetary contingency on Gambling task performance. Drug and Alcohol Dependence. 2009;102:95–101. doi: 10.1016/j.drugalcdep.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadhan NP, Hart CL, van Gorp WG, Gunderson EW, Haney M, Foltin RW. Acute effects of smoked marijuana on decision making, as assessed by a modified gambling task, in experienced marijuana users. Journal of Clinical and Experimental Neuropsychology. 2007;29:357–64. doi: 10.1080/13803390600693615. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Anum E, Niyuhire F, Wise LE, Lichtman AH. Delta(9)-THC-induced cognitive deficits in mice are reversed by the GABA(A) antagonist bicuculline. Psychopharmacology (Berl) 2005;178:317–27. doi: 10.1007/s00213-004-1988-2. [DOI] [PubMed] [Google Scholar]
- Wadsworth EJ, Moss SC, Simpson SA, Smith AP. Cannabis use, cognitive performance and mood in a sample of workers. Journal of Psychopharmacology. 2006;20:14–23. doi: 10.1177/0269881105056644. [DOI] [PubMed] [Google Scholar]
- Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, Detre JA, Lerman C. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. The Journal of Neuroscience. 2007;27:14035–40. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) Manual. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Whitlow CT, Liguori A, Livengood LB, Hart SL, Mussat-Whitlow BJ, Lamborn CM, Laurienti PJ, Porrino LJ. Long-term heavy marijuana users make costly decisions on a gambling task. Drug and Alcohol Dependence. 2004;76:107–11. doi: 10.1016/j.drugalcdep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Simon S, Jarvik M, Olmstead R, Brody AL, Ernst M, London ED. Effect of cigarette smoking on prefrontal cortical function in nondeprived smokers performing the Stroop Task. Neuropsychopharmacology. 2007;32:1421–8. doi: 10.1038/sj.npp.1301272. [DOI] [PMC free article] [PubMed] [Google Scholar]


