Abstract
Motor neuron disease (MND) may present as an isolated lower motor neuron (LMN) disorder. Although the significance of pathological 43 kDa transactive responsive sequence DNA binding protein (TDP-43) for amyotrophic lateral sclerosis (ALS) was appreciated only recently, the topographical distribution of TDP-43 pathology in MND clinically isolated to the LMN versus normal controls (COs) is only incompletely described. Therefore, we performed longitudinal clinical evaluation and retrospective chart review of autopsied patients diagnosed with isolated LMN disease. Cases with a disease duration over 4 years were designated as progressive muscular atrophy (PMA), and those with a more rapid course as MND/LMN. Immunohistochemistry was employed to identify neuronal and glial TDP-43 pathology in the central nervous system (CNS) in patients and COs. We examined 19 subjects including six patients (i.e., four with MND/LMN and two with PMA) and 13 COs. All patients showed significant TDP-43 linked degeneration of LMNs, and five cases showed a lesser degree of motor cortex degeneration. Additional brain areas were affected in varying degrees, ranging from predominantly brainstem pathology to significant involvement of the whole CNS including neocortical and limbic areas. Pathological TDP-43 was present only rarely in the CO group. We conclude that MND limited to the LMN and PMA is part of a disease continuum that includes ALS and FTLD-TDP, all of which are characterized by widespread TDP-43 pathology. Hence, we suggest that the next revision of the El Escorial criteria for the diagnosis of ALS include MND patients with disease clinically limited to the LMN and PMA as variants of ALS, which like classical ALS, are TDP-43 proteinopathies.
Keywords: Pathological 43 kDa transactive responsive sequence DNA binding protein, Motor neuron disease clinically limited to the lower motor neuron, Progressive muscular atrophy
Introduction
The various clinical forms of motor neuron disease (MND) are defined by symptoms and clinical signs. Amyotrophic lateral sclerosis (ALS), the prototypical form of MND, is characterized by the combination of progressive upper motor neuron (UMN) and lower motor neuron (LMN) signs and symptoms. Some individuals may show isolated LMN features at disease onset and develop UMN abnormalities over time. This is referred to as LMN onset ALS. In contrast, some patients may initially present with UMN features but develop LMN abnormalities as the disease progresses. This is called UMN onset ALS. By consensus individuals who continue to manifest clinically isolated UMN signs over 4 years of disease progression are considered to have the primary lateral sclerosis (PLS) variant of MND [13]. By analogy, it is reasonable to consider individuals who have clinically isolated LMN abnormalities over 4 years of disease progression to have the progressive muscular atrophy (PMA) variant of MND. Other features not typically considered to accompany the UMN and/or LMN features of ALS such as frontotemporal dementia (FTD), sensory signs, cerebellar signs, supranuclear ocular motility disturbance, extrapyramidal signs, or autonomic insufficiency characterize the ALS-Plus syndrome.
Pathological 43-kDa transactive responsive DNA-binding protein (TDP-43) has been identified as the major disease protein in ALS and frontotemporal lobar degeneration (FTLD) with ubiquitin positive inclusions with or without MND [27]. This significant biological advance has linked ALS to FTLD through TDP-43 pathology that may be widespread in the central nervous system (CNS) thereby creating a new concept of TDP-43 proteinopathy as a disease continuum that encompasses ALS and FTLD. Pathological TDP-43 inclusions in the CNS appear to be diagnostic of these disorders and may underlie mechanisms of neurodegeneration in ALS and FTLD. While the functions of TDP-43 are poorly understood, TDP-43 is a ubiquitously expressed, highly conserved nuclear protein that appears to be involved in transcriptional repression and alternative splicing, and may act as a scaffold for nuclear bodies through interactions with survival motor neuron proteins. Two reports on purported PLS or FTLD-PLS showed cortical and brainstem TDP-43 pathology to highly variable degrees ranging from widespread hippocampal and neocortical TDP-43 to no detectable TDP-43 lesions [8, 19]. We have previously reported a single individual with ALS-Plus characterized by progressive UMN and LMN disease, FTD, extrapyramidal findings and supranuclear ocular motility disturbance reminiscent of progressive supranuclear palsy who demonstrated diffuse TDP-43 pathology [23]. Previous autopsy studies of PMA demonstrated LMN pathology with a significant number of individuals also manifesting UMN pathology [18, 21, 31, 33].
As ALS becomes redefined as a multisystem CNS disorder, it is imperative to more completely pathologically characterize each of the ALS variants. While the whole CNS distribution of degeneration linked to pathological TDP-43 in ALS has been well documented, the whole CNS distribution of TDP-43 pathology in the setting of MND clinically isolated to the LMN or PMA in comparison to controls (COs) is incompletely described [28], and was, therefore, investigated in this study.
Materials and methods
Study subjects
All patients with the clinical diagnosis of MND (as determined by authors LFM and LBE) followed at the ALS Center at Pennsylvania Hospital are enrolled in a database. This database was queried for patients with MND isolated to the LMN as defined by (1) the presence of LMN signs of weakness with atrophy and possibly accompanying fasciculations and (2) the lack of UMN signs of increased reflexes, pathologic reflexes, spasticity, and pseudobulbar affect throughout their entire clinical course, who had also donated brain and spinal cord for research at the Center for Neurodegenerative Disease Research (CNDR) at the University of Pennsylvania between 1990 and 2009. COs without any known major neurologic or psychiatric conditions were enrolled from the CNDR database. Retrospective clinical chart review was performed for historical and clinical data.
To screen for TDP-43 pathology in these patients, salient CNS areas of the pyramidal (and extrapyramidal) system, i.e., LMNs (spinal cord/medulla), motor cortex, striatum/lentiform nucleus, and midbrain were immunostained with anti-TDP-43 antibodies. In addition, other subcortical and cortical brain areas were examined as available (in one case material was more restricted, see Results sections). For the CO group, selected brain areas (“central axis of the CNS”) including spinal cord, medulla, midbrain, striatum/lentiform nucleus, and motor cortex were examined. Data on TDP-43 pathology in these brain areas are scarce, as opposed to our earlier published data on TDP-43 pathology in the limbic/cortical system of control/elderly individuals [12].
Immunohistochemistry (IHC)
The study subjects were fully examined by routine neuropathologic diagnostic methods, as previously described [11, 26, 27, 32]. Briefly, small blocks of freshly dissected tissues from multiple CNS areas were fixed in 10% neutral buffered formalin or 70% ethanol with 150 mM NaCl, paraffin-embedded, and cut into 6 µm sections. Sections were subjected to IHC using the avidin–biotin complex detection method (Vectastain ABC kit, Vector Laboratories, Burlingame, CA, USA) with 3,3-diaminobenzidine (DAB) as the chromogen. The following primary antibodies were used: mouse anti-paired helical filament-1 monoclonal antibody (mAb; a gift of Peter Davies, PhD; 1:1,000), mouse anti-ubiquitin mAb (1510; Chemicon International Inc, Temecula, CA, USA; 1:100,000), mouse anti-TDP-43 mAb (TDP 171; generated in the CNDR, Philadelphia, PA, USA; 1:40,000), rabbit polyclonal anti-TDP-43 (Proteintech Group Inc, Chicago, IL, USA; 1:4,500), rat anti-phosphorylated TDP-43 mAb (S409/410 [26]; 1:500), mouse anti-α-synuclein mAb (Syn303; generated in CNDR, Philadelphia, PA, USA; 1:4,000), mouse anti-human leukocyte antigen-DR mAb (DakoCytomation, Glostrup, Denmark; 1:5,000), and anti-myelin basic protein (1:10,000). Sections stained for ubiquitin, TDP-43, and human leukocyte antigen-DR were pre-treated by boiling in citrate antigen unmasking solution (Vector Laboratories Inc, Burlingame, CA, USA; 1:100) using a microwave, and those stained for α-synuclein were pretreated with 80% formic acid. TDP-43 inclusions were assessed based on morphologies and distribution in a given brain area as described elsewhere [4, 11]. Positive controls were human disease CNS tissue sections with known pathological reactivity to the antibody in question, and they were included in every IHC staining procedure as described [11, 26, 27, 32]. Furthermore, normal nuclear staining in unaffected regions of CNS sections served as an “internal control” for each slide stained for the pan TDP-43 antibodies. Digital images were obtained using an Olympus BX 51 (Tokyo, Japan) microscope using a digital camera-DP71 (Olympus, Orangeburg, NY, USA), and DP manager (Olympus, Orangeburg, NY, USA).
Evaluation of pathological TDP-43
TDP-43 pathology was rated on a 5-point ordinal scale (0, none; 1; rare/minor; 2, mild; 3, moderate; 4, severe/numerous) by some of the authors (FG, MP, JQT). We chose the assessment of pathology by means of an ordinal scale rather than by using numeric image analysis based quantification tools, as the former acknowledges the sequential nature of stages of increasing severity, ultimately corresponding to a spread of pathology throughout a given section or the brain as shown for all major neurodegenerative diseases. In fact, ordinal data provide information about a relation of severity stages rather than being a measurement acknowledging that one stage follows continuously into the other, which, therefore, represent sequential classes rather than values on a numerical scale.
Statistical analyses
The data were analyzed using Statistical Package for the Social Sciences (SPSS, Inc., Chicago, IL, USA). The “ average” (and “spread”) of data on patient characteristics was estimated by calculating the median (and 25th to 75th percentiles). For group comparison the Mann–Whitney U test were used. The significance level for all comparisons was set at the 0.05 level. All statistical tests applied were two-sided.
Results
Study subjects’ clinical characteristics and diagnosis
We examined 19 subjects including six patients and 13 COs. All patients were male, whereas there were five males and eight females in the CO group. The median age of disease onset in the patients was 62.5 years (54.8–73.3), and the median disease duration from onset to death was 34.0 months (25.8–69.3). Two patients (#1 and #2) had a disease duration greater than 4 years (62 and 91 months) and would meet the usual definition of PMA. The four patients (#3–6) with MND isolated to the LMN and faster disease progression (i.e., from onset to death: 29, 16, 30, and 38 months respectively) were referred to as MND/LMN. Furthermore, the median age at death was 60 years (58–80.0) in the MND/LMN/PMA group and 70.0 years (60.5–79.5) in the CO group; this difference, however, did not reach statistical significance (p = 0.579). One patient (#6) had a family history of ALS in a maternal first cousin. Mutations in the TDP-43 (TARDBP, entire coding region), superoxide dismutase-1 (SOD-1, entire coding region), fused in sarcoma (FUS, exons 14 and 15), valosin-containing protein (exons 3, 5–10, 13–14) genes were not detected in this patient. No patients had a family history of FTD. All of the patients had limb onset, and all patients died of respiratory failure. None of the patients had evidence of executive dysfunction. Two patients (#1 and #5) had mild memory impairment.
Histologic findings
Significant TDP-43 pathology comprising neuronal and glial cytoplasmic inclusions, pathological cellular processes (dystrophic cellular processes or axonal swellings), roundish neuropil grains, and diffuse, dot-like, cytoplasmic staining (“pre-inclusions”), in combination with the absence of nuclear TDP-43 immunoreactivity was found in LMNs and the brainstem of all cases with MND/LMN or PMA (Figs. 1, 2; Table 1). This was the same “morphological range” of TDP-43 pathology as previously reported in ALS, ALS with dementia, FTLD-MND, and FTLD-TDP [4, 10–12]. The dot- or dash-like cytoplasmic TDP-43 immunoreactivity was seen by both the phosporylation-dependent (i.e., S409/410) and phosphorylation-independent antibodies (i.e., 171 TDP, and weaker by the rabbit polyclonal anti-TDP-43) (Fig. 1). Notably, since the phosporylation-dependent antibody does not stain normal TDP-43, all of the immunoreactivity detected by this antibody reflects TDP-43 pathology. Pathological TDP-43 was also present in the CO group rarely and comprised scant subpial lesions in the medulla oblongata of two cases (compare reference [12] where this morphological pattern, albeit to a higher degree, has been published previously by our group). TDP-43 pathology in CNS areas other than LMN/brainstem was present in all patients, in varying severity and topographical distribution (Figs. 2, 3; Table 1). Two patients with MND/LMN showed a significant involvement of the whole CNS including widespread neocortical and limbic areas (Fig. 3). In the two PMA cases, pathology was largely restricted to the brainstem (and motor cortex in one), and two MND/LMN patients had an intermediate position with brainstem and deep brain nuclei TDP-43 pathology. In one case (#4), no brainstem or deep brain nuclei tissues were available, but there was some TDP-43 pathology in the neocortex. In general, there was neuronal loss and gliosis in LMNs relative to the motor cortex. The two cases with significant cortical TDP-43 pathology, which was associated with varying degrees of neuronal loss and gliosis, were categorized as FTLD-TDP (formerly known as FTLD-U) type II according to Sampathu and Neumann [29]. The remainder of the cases with up to only mild neocortical pathology could not be categorized according to this scheme and are therefore best denoted as “unclassified”. No case showed unequivocal signs of long tract degeneration based on conventional myelin stains (i.e., Kluver–Barrera method). There were also no evident signs of demyelination based on myelin basic protein IHC in either the cases or CO subjects. Significant microglia proliferation was present in the spinal cord of the MND/LMN or PMA cases; in general, microglia infiltration was greater in the spinal cord than motor gyrus in the MND/LMN cases, with all cases showing robust microglia cells in the spinal cord but only two cases with significant microglia proliferation in the motor gyrus (Cases #5 and 6). In the CO group, microglia infiltration overall was less pronounced with significant microglia proliferation being present only in a subset of cases; however, the general trend towards a higher load of microglia cells in the spinal cord or descending tracts as compared to the motor cortex was present as well similar to the MND/LMN group. When looking at secondary tau pathology in the five cases with hippocampus, superior/midtemporal and frontal cortex available, the two cases with significant whole brain TDP43 pathology either had intermediate (Case #5) or high (Case #6) Braak stages; the remaining three (Cases #1–3) showed a more limited TDP-43 distribution and/or degree with low Braak stages. No α-synuclein pathology was found in any of the cases examined.
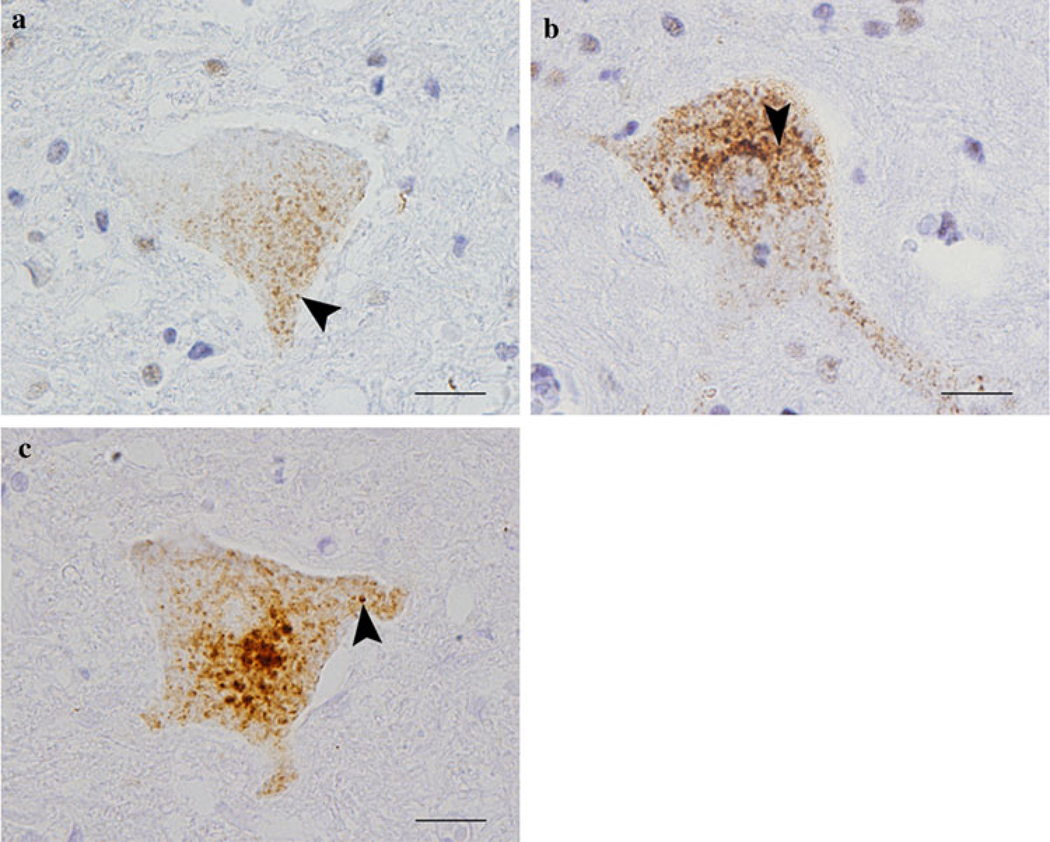
Fig. 1.
Dot- or dash-like cytoplasmic 43 kDa transactive responsive sequence DNA binding protein (TDP-43) pathology in a case of motor neuron disease (MND) clinically limited to lower motor neuron (LMN). Anti-TDP-43 immunohistochemistry using phosphorylation independent anti-TDP-43 antibodies in (a), i.e., commercial polyclonal anti-TDP-43, Proteintech Group Inc, as well as in (b), i.e., 171-anti-TDP-43, and the anti-phosphorylated S409/410 TDP-43 antibody in (c) showing dot-or dash-like inclusion formation in spinal cord lower motor neurons (examples denoted by arrows, bars 20 µm)
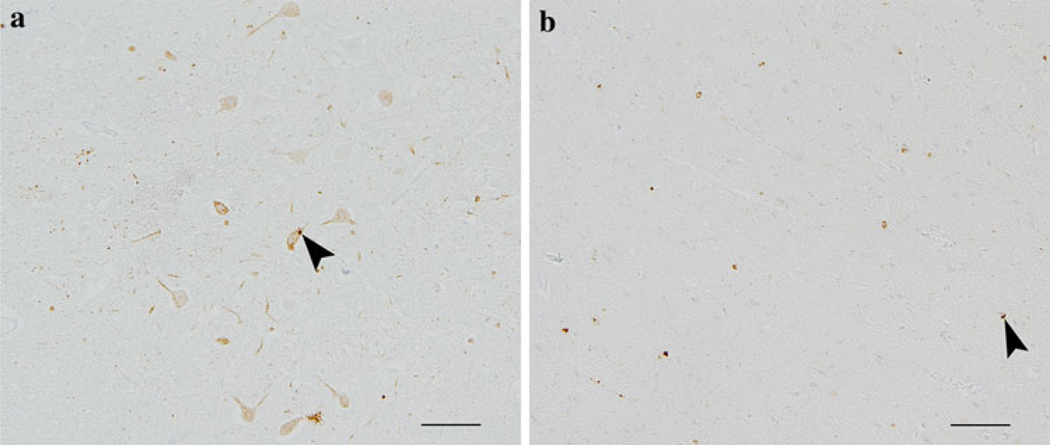
Fig. 2.
Lower and upper motor neuron 43 kDa transactive responsive sequence DNA binding protein (TDP-43) pathology in a patients with MND/LMN. Anti-TDP-43 immunohistochemistry using anti-phosphorylated S409/410 TDP-43 antibodies showing TDP-43 pathology (examples arrows) in the dorsal motor plate in the medulla oblongata (a) and motor cortex (b) (bars 100 µm). Since this antibody does not stain normal TDP-43, all of the immunoreactivity in brown reflects pathology
Table 1.
Histologic 43 kDa transactive responsive sequence DNA binding protein pathology findings in patients with progressive muscular atrophy (Cases #1 and 2) and motor neuron disease isolated to the lower motor neuron (Cases #3–6)
| (a) Spinal cord, brainstem, subcortex | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient # | Spi | Med | Pon | Mid | Bas | Tha | Cer | |||||||||
| Cer | Tho | Lum | Sac | Dor | Inf | Oth | Sub | Oth | Str | Len | Gre | Whi | Den | |||
| 1 | * | * | ||||||||||||||
| 2 | * | |||||||||||||||
| 3 | * | * | ||||||||||||||
| 4 | ||||||||||||||||
| 5 | * | |||||||||||||||
| 6 | * | |||||||||||||||
| (b) Cortex | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient # | Mot | Sen | Vis | Fro | Ang | Sup | Orb | |||||||
| Gre | Whi | Gre | Whi | Gre | Whi | Gre | Whi | Gre | Whi | Gre | Whi | Gre | Whi | |
| 1 | ||||||||||||||
| 2 | ||||||||||||||
| 3 | ||||||||||||||
| 4 | * | |||||||||||||
| 5 | ||||||||||||||
| 6 | ||||||||||||||
| Patient # | Cin | Tra | Hip | Amy | Per | ||||
|---|---|---|---|---|---|---|---|---|---|
| Gre | Whi | Gre | Whi | CA1 | Fas | Gre | Whi | ||
| 1 | |||||||||
| 2 | |||||||||
| 3 | ** | ||||||||
| 4 | |||||||||
| 5 | |||||||||
| 6 |
| None | Rare | Mild | Severe | Severe |
|---|---|---|---|---|
| Not done |
|---|
Spi spinal cord, Cer cervical, Tho thoracic, Lum lumbar, Sac sacral, Med medulla, Dor dorsal motor plate, Inf inferior olive, Oth other, Pon pons, Mid Midbrain, Sub subiculum, Bas basalganglia, Str striatum, Len lentiform, Tha thalamus, Cer cerebellum, Gre gray matter, Whi white matter, Den denate nucleus, Mo motor gyrus, Sen sensory cortex, Vis visual cortex, Fro frontal Gyrus, Ang angular gyrus, Sup superior-midtemporal gyurs, Orb orbitofrontal gyrus, Cin cingulate gyrus, Tra (trans-)entorhinal region, Hip hippocampus, CA1 CA1-subiculum, Fas Fascia dentata, Amy amygdala, Per Periamygdala
Focal,
perivascular
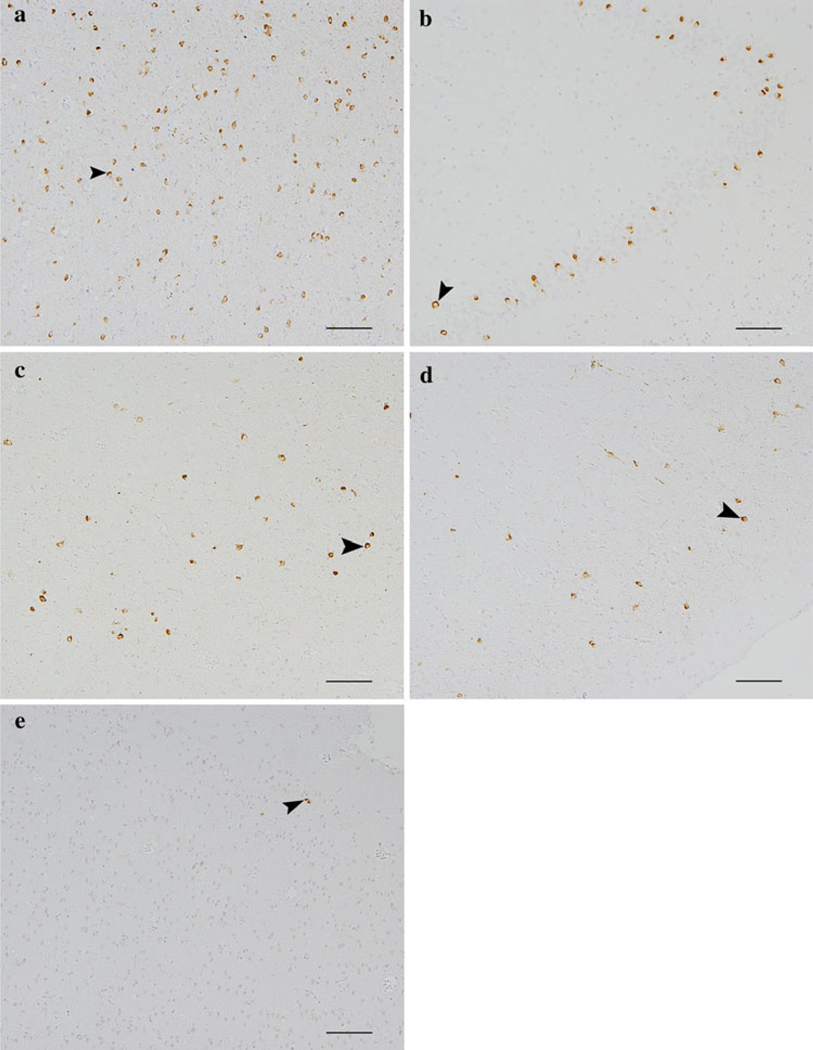
Fig. 3.
Cortical 43 kDa transactive responsive sequence DNA binding protein (TDP-43) pathology in a case of MND/LMN (same patient as in Fig. 1). Anti-TDP-43 immunohistochemistry using anti-phosphorylated S409/410 showing TDP-43 pathology (examples arrows) in the amygdala (a), dentate gyrus of the hippocampus (b), cingulate cortex (c), orbitofrontal cortex (d), and rarely visual cortex (e) (e.g., arrows) (bars 100 µm). As in Fig. 2, all of the immunoreactivity in brown here reflects pathology
Discussion
Our data add to the findings of others that MND clinically isolated to the LMN, including the variant of ALS known as PMA, is not a distinct nosological entity separate from ALS, but rather a clinical subtype of MND. Recently, it was demonstrated that patients with PMA frequently have long tract pathology and most have ubiquitinated inclusions typical of ALS suggesting that PMA has the pathology and pathophysiology of ALS irrespective of whether UMN signs are present clinically [18]. This may reflect the fact that as LMN disease progresses, the clinical identification of UMN disease may become impossible. Additionally, PMA is relentlessly progressive, and UMN involvement can eventually occur [21, 31, 33]. Because patients with PMA lack the required UMN features for the diagnosis of ALS, they are diagnosed with ‘suspected ALS’ according to classical El Escorial Criteria [5]. However, based on the data presented here, we suggest that the current El Escorial Criteria should be revised to include clinical PMA subjects in the same category as patients with clinical ALS [6].
Recent advances suggest ALS is a TDP-43 linked proteinopathy that not only affects the pyramidal motor system, but rather is also a multisystem neurodegenerative disorder with widespread distribution of TDP-43 pathology [10]. Despite this progress in redefining ALS as a multisystem neurodegenerative TDP-43 proteinopathy, there is still uncertainty regarding the classification of PMA, and data on whole CNS TDP-43 pathology in PMA are scant [28]. We here therefore determined the extent of TDP-43 pathology in the CNS of patients with MND/LMN and PMA versus COs, and found—in addition to TDP-43 linked LMN degeneration—various levels of extra-spinal TDP-43 pathology in the disease group. Our finding of a relatively higher degree of neurodegeneration in LMN as compared with UMN in PMA and MND/LMN is consistent with a recent report of PMA cases and ALS patients with a long disease duration [28]. Neurodegeneration was variably accompanied by microglia proliferation in our study, similar to published data [18]. The morphological range of TDP-43 positive inclusions in neurons and glia was indistinguishable as occurring in “classical” ALS cases [11]. Of note, cytoplasmic dash-like inclusion have been reported in brainstem motor nuclei neurons of ALS cases and have been said to stain only with phorsphorylation-dependent, but not phosphorylation-independent anti-TDP-43 antibodies [3]. We here show the presence of dot-like cytoplasmic immunoreactivity in the many different brain areas positive not only with the phosphorylation-dependent anti-TPD-43, but also with antibody (ies) that recognize the protein independent of its phosphorylation status. Similarly, dot-like inclusions, wavy or linear wisps, and granular cytoplasmic TDP-43 immunoreactivity have been reported by using the phosphorylation-independent polyclonal anti-TDP-43 antibody in a variety of CNS areas including the anterior horn motor neuron of ALS with or without dementia [25]. Taken together, these data suggest that aggregation of pathological TDP-43 does not necessarily depend upon phosphorylation.
Those cases with robust cortical pathology showed a FTLD-TDP subtype II according to Sampathu/Neumann et al. [29] consistent with previous reports that this subtype is the most MND-like form of FTLD-TDP [11]. Subtype II is characterized mainly by neuronal cytoplasmic ubiquitin/TDP-43 immunoreactivity. It appears that the mis-localization of TDP-43, a nuclear protein, to the cytoplasm is an early stage in a disease associated with short disease duration, as evidenced by the shorter survival or earlier disease onset of ALS or patients with MND combined with FTD as compared with FTLD-TDP without MND or FTLD-Tau [9, 15, 17, 20]. The finding that, despite significant LMN degeneration, five cases could not be categorized by subtype (hence called “unclassified”) owing either to the absence (one case) or the presence of only a low degree (four cases) of cortical pathology lends further support to the hypothesis that these cases might be in their earliest stage of pathology development, and would be projected to evolve into subtype II with longer disease duration or further disease progression. With the hypothesized continuation of TDP-43 mis-localization from the cytoplasm into cellular processes the other subtypes might emerge: subtype III is characterized by an abundance of short dystrophic profiles and (often ringform) cytoplasmic inclusions, and is associated with both motor neuron disease and dementia; subtype I features a predominance of long dystrophic profiles, has the longest disease duration, and is generally associated with cognitive/language impairment [11, 14, 22].
The finding of rare TDP-43 pathology “almost entirely” restricted to the LMN in spinocerebellar ataxia type 3 or Machado-Josephs disease, which is a CAG repeat disorder associated with neurodegeneration and inclusion pathology in multiple CNS areas, lends support to the idea that the LMNs are particularly susceptible for neurodegeneration [30]. We demonstrated recently that the earliest changes in cortical brain areas occur in the amygdala/periamygdaloid or hippocampus [12]. Neurodegenerative diseases have been classified according to their predominating pathological proteins. By analogy, similar patterns of early changes have been proposed for other pathological proteins including tau with the widely adopted Braak stages for Alzheimer’s disease [1], or also the scheme for Lewy body disorders as reflected by current consensus criteria [2, 24]. This argues that areas of the nervous system might be differentially susceptible to pathways leading to neurodegeneration associated with a variety of different pathological proteins. The occurrence of pathological TDP-43 in the amygdala or hippocampus of cases with progressive supranuclear palsy and corticobasal degeneration and in the amygdala or hippocampus/entorhinal cortex in Lewy body disease suggests that there might be a region-specific, rather than disease-specific, mechanism [34, 35]. Also, the synergistic interaction between tau, amyloid-β and α-synuclein as recently shown in a mammalian animal model could also apply to TDP-43 [7]. The two cases in the present study with significant whole CNS pathology showed the highest level of TDP-43 pathology in the mediotemporal lobe including amygdala and hippocampus; neocortical or association cortical TDP-43 pathology was robust, but somewhat less pronounced and primary cortical areas including visual, sensory and motor cortex were either spared or afflicted to a mild degree. Further, these two cases showed the highest level of the secondary tau pathology. This confirms previous work on TDP-43 pathology in elderly control subjects or patients with severe mental illness [12] or severe Alzheimer’s disease who show additional TDP-43 pathology [16]. The relative distribution of TDP-43 pathology follows a similar topographical distribution as neurofibrillary tau pathology as captured by the Braak stages [1]. This may indicate an intrinsic differential CNS vulnerability common for these proteins and/or an as yet to be identified mutually augmenting interaction of pathological proteins that eventually leads to their aggregation.
Conclusion
We conclude that MND/LMN or PMA is a TDP-43 proteinopathy similar to most cases of sporadic ALS. Furthermore, MND- related TDP-43 pathology might follow a sequentially additive pattern of development spreading from the spinal cord/brainstem into the CNS. This might reflect disease progression, thus representing the basis for the development of whole CNS pathological TDP-43 evolution schemata. The next revision of the El Escorial criteria for the diagnosis of ALS should include MND patients with disease clinically limited to the LMN and PMA as variants of ALS, that like classical ALS, are TDP-43 proteinopathies.
Acknowledgments
We thank the families of patients whose generosity made this research possible. Manuela Neumann, MD, provided the anti-phosphorylated TDP-43 antibody (S409/410). We thank our colleagues at the Center for Neurodegenerative Disease Research and Department of Psychiatry, University of Pennsylvania School of Medicine, for technical support and advice, particularly Theresa Schuck, BA, and John L. Robinson, BS. Supported by AG10124 and AG32953.
Contributor Information
Felix Geser, Department of Pathology and Laboratory Medicine, Center for Neurodegenerative Disease Research, Alzheimer’s Disease Core Center, Institute on Aging, University of Pennsylvania School of Medicine, Philadelphia, PA, USA; Department of Psychiatry, Psychosomatic Medicine and Psychotherapy, Goethe-University, Frankfurt am Main, Germany.
Beth Stein, Department of Neurology, Division of Neuromuscular Diseases, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY, USA.
Michael Partain, Department of Pathology and Laboratory Medicine, Center for Neurodegenerative Disease Research, Alzheimer’s Disease Core Center, Institute on Aging, University of Pennsylvania School of Medicine, Philadelphia, PA, USA.
Lauren B. Elman, Department of Neurology, University of Pennsylvania School of Medicine, Philadelphia, PA, USA
Leo F. McCluskey, Department of Neurology, University of Pennsylvania School of Medicine, Philadelphia, PA, USA
Sharon X. Xie, Department of Biostatistics and Epidemiology, University of Pennsylvania School of Medicine, Philadelphia, USA
Vivianna M. Van Deerlin, Department of Pathology and Laboratory Medicine, Center for Neurodegenerative Disease Research, Alzheimer’s Disease Core Center, Institute on Aging, University of Pennsylvania School of Medicine, Philadelphia, PA, USA
Linda K. Kwong, Department of Pathology and Laboratory Medicine, Center for Neurodegenerative Disease Research, Alzheimer’s Disease Core Center, Institute on Aging, University of Pennsylvania School of Medicine, Philadelphia, PA, USA
Virginia M.-Y. Lee, Department of Pathology and Laboratory Medicine, Center for Neurodegenerative Disease Research, Alzheimer’s Disease Core Center, Institute on Aging, University of Pennsylvania School of Medicine, Philadelphia, PA, USA
John Q. Trojanowski, Email: trojanow@mail.med.upenn.edu, Department of Pathology and Laboratory Medicine, Center for Neurodegenerative Disease Research, Alzheimer’s Disease Core Center, Institute on Aging, University of Pennsylvania School of Medicine, Philadelphia, PA, USA; Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, HUP, Maloney 3rd Floor, 36th and Spruce Streets, Philadelphia, PA 19104-4283, USA.
References
- 1.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sand-mann-Keil D, Rub U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages) J Neurol. 2002;249 Suppl 3:III:1–5. doi: 10.1007/s00415-002-1301-4. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Ludolph A, Thal DR, Del Tredici K. Amyotrophic lateral sclerosis: dash-like accumulation of phosphorylated TDP-43 in somatodendritic and axonal compartments of somatomotor neurons of the lower brainstem and spinal cord. Acta Neuropathol. 2010;120:67–74. doi: 10.1007/s00401-010-0683-0. [DOI] [PubMed] [Google Scholar]
- 4.Brandmeir NJ, Geser F, Kwong LK, Zimmerman E, Qian J, Lee VM, Trojanowski JQ. Severe subcortical TDP-43 pathology in sporadic frontotemporal lobar degeneration with motor neuron disease. Acta Neuropathol. 2008;115:123–131. doi: 10.1007/s00401-007-0315-5. [DOI] [PubMed] [Google Scholar]
- 5.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on motor neuron diseases/amyotrophic lateral sclerosis of the World Federation of Neurology Research Group on neuromuscular diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci. 1994;124 Suppl:96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 6.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 7.Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci. 2010;30:7281–7289. doi: 10.1523/JNEUROSCI.0490-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickson DW, Josephs KA, Amador-Ortiz C. TDP-43 in differential diagnosis of motor neuron disorders. Acta Neuropathol. 2007;114:71–79. doi: 10.1007/s00401-007-0234-5. [DOI] [PubMed] [Google Scholar]
- 9.Forman MS, Farmer J, Johnson JK, Clark CM, Arnold SE, Coslett HB, Chatterjee A, Hurtig HI, Karlawish JH, Rosen HJ, Van Deerlin V, Lee VM, Miller BL, Trojanowski JQ, Grossman M. Frontotemporal dementia: clinicopathological correlations. Ann Neurol. 2006;59:952–962. doi: 10.1002/ana.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geser F, Brandmeir NJ, Kwong LK, Martinez-Lage M, Elman L, McCluskey L, Xie SX, Lee VM, Trojanowski JQ. Evidence of multisystem disorder in whole-brain map of pathological TDP-43 in amyotrophic lateral sclerosis. Arch Neurol. 2008;65:636–641. doi: 10.1001/archneur.65.5.636. [DOI] [PubMed] [Google Scholar]
- 11.Geser F, Martinez-Lage M, Robinson J, Uryu K, Neumann M, Brandmeir NJ, Xie SX, Kwong LK, Elman L, McCluskey L, Clark CM, Malunda J, Miller BL, Zimmerman EA, Qian J, Van Deerlin V, Grossman M, Lee VM, Trojanowski JQ. Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Arch Neurol. 2009;66:180–189. doi: 10.1001/archneurol.2008.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geser F, Robinson JL, Malunda JA, Xie SX, Clark CM, Kwong LK, Moberg PJ, Moore EM, Van Deerlin VM, Lee VM, Arnold SE, Trojanowski JQ. Pathological 43-kDa transactivation response DNA-binding protein in older adults with and without severe mental illness. Arch Neurol. 2010;67:1238–1250. doi: 10.1001/archneurol.2010.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon PH, Cheng B, Katz IB, Pinto M, Hays AP, Mitsumoto H, Rowland LP. The natural history of primary lateral sclerosis. Neurology. 2006;66:647–653. doi: 10.1212/01.wnl.0000200962.94777.71. [DOI] [PubMed] [Google Scholar]
- 14.Grossman M, Wood EM, Moore P, Neumann M, Kwong L, Forman MS, Clark CM, McCluskey LF, Miller BL, Lee VM, Trojanowski JQ. TDP-43 pathologic lesions and clinical phenotype in frontotemporal lobar degeneration with ubiquitin-positive inclusions. Arch Neurol. 2007;64:1449–1454. doi: 10.1001/archneur.64.10.1449. [DOI] [PubMed] [Google Scholar]
- 15.Hodges JR, Davies R, Xuereb J, Kril J, Halliday G. Survival in frontotemporal dementia. Neurology. 2003;61:349–354. doi: 10.1212/01.wnl.0000078928.20107.52. [DOI] [PubMed] [Google Scholar]
- 16.Hu WT, Josephs KA, Knopman DS, Boeve BF, Dickson DW, Petersen RC, Parisi JE. Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathol. 2008;116:215–220. doi: 10.1007/s00401-008-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu WT, Seelaar H, Josephs KA, Knopman DS, Boeve BF, Sorenson EJ, McCluskey L, Elman L, Schelhaas HJ, Parisi JE, Kuesters B, Lee VM, Trojanowski JQ, Petersen RC, van Swieten JC, Grossman M. Survival profiles of patients with frontotemporal dementia and motor neuron disease. Arch Neurol. 2009;66:1359–1364. doi: 10.1001/archneurol.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ince PG, Evans J, Knopp M, Forster G, Hamdalla HH, Wharton SB, Shaw PJ. Corticospinal tract degeneration in the progressive muscular atrophy variant of ALS. Neurology. 2003;60:1252–1258. doi: 10.1212/01.wnl.0000058901.75728.4e. [DOI] [PubMed] [Google Scholar]
- 19.Josephs KA, Dickson DW. Frontotemporal lobar degeneration with upper motor neuron disease/primary lateral sclerosis. Neurology. 2007;69:1800–1801. doi: 10.1212/01.wnl.0000277270.99272.7e. [DOI] [PubMed] [Google Scholar]
- 20.Josephs KA, Knopman DS, Whitwell JL, Boeve BF, Parisi JE, Petersen RC, Dickson DW. Survival in two variants of tau negative frontotemporal lobar degeneration: FTLD-U vs. FTLD-MND. Neurology. 2005;65:645–647. doi: 10.1212/01.wnl.0000173178.67986.7f. [DOI] [PubMed] [Google Scholar]
- 21.Kim WK, Liu X, Sandner J, Pasmantier M, Andrews J, Rowland LP, Mitsumoto H. Study of 962 patients indicates progressive muscular atrophy is a form of ALS. Neurology. 2009;73:1686–1692. doi: 10.1212/WNL.0b013e3181c1dea3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackenzie IR, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, Perry RH, Neary D, Snowden JS, Mann DM. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol. 2006;112:539–549. doi: 10.1007/s00401-006-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCluskey LF, Elman LB, Martinez-Lage M, Van Deerlin V, Yuan W, Clay D, Siderowf A, Trojanowski JQ. Amyotrophic lateral sclerosis-plus syndrome with TAR DNA-binding protein-43 pathology. Arch Neurol. 2009;66:121–124. doi: 10.1001/archneur.66.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 25.Mori F, Tanji K, Zhang HX, Nishihira Y, Tan CF, Takahashi H, Wakabayashi K. Maturation process of TDP-43-positive neuronal cytoplasmic inclusions in amyotrophic lateral sclerosis with and without dementia. Acta Neuropathol. 2008;116:193–203. doi: 10.1007/s00401-008-0396-9. [DOI] [PubMed] [Google Scholar]
- 26.Neumann M, Kwong LK, Lee EB, Kremmer E, Flatley A, Xu Y, Forman MS, Troost D, Kretzschmar HA, Trojanowski JQ, Lee VM. Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol. 2009;117:137–149. doi: 10.1007/s00401-008-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 28.Nishihira Y, Tan CF, Hoshi Y, Iwanaga K, Yamada M, Kawachi I, Tsujihata M, Hozumi I, Morita T, Onodera O, Nishizawa M, Kakita A, Takahashi H. Sporadic amyotrophic lateral sclerosis of long duration is associated with relatively mild TDP-43 pathology. Acta Neuropathol. 2009;117:45–53. doi: 10.1007/s00401-008-0443-6. [DOI] [PubMed] [Google Scholar]
- 29.Sampathu DM, Neumann M, Kwong LK, Chou TT, Micsenyi M, Truax A, Bruce J, Grossman M, Trojanowski JQ, Lee VM. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol. 2006;169:1343–1352. doi: 10.2353/ajpath.2006.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan C-F, Yamada M, Toyoshima Y, Yokoseki A, Miki Y, Hoshi Y, Kaneko H, Ikeuchi T, Onodera O, Kakita A, Takahashi H. Selective occurrence of TDP-43-immunoreactive inclusions in the lower motor neurons in Machado-Joseph disease. Acta Neuropathol. 2009;118:553–560. doi: 10.1007/s00401-009-0552-x. [DOI] [PubMed] [Google Scholar]
- 31.Tsuchiya K, Sano M, Shiotsu H, Akiyama H, Watabiki S, Taki K, Kondo H, Nakano I, Ikeda K. Sporadic amyotrophic lateral sclerosis of long duration mimicking spinal progressive muscular atrophy exists: additional autopsy case with a clinical course of 19 years. Neuropathology. 2004;24:228–235. doi: 10.1111/j.1440-1789.2004.00546.x. [DOI] [PubMed] [Google Scholar]
- 32.Uryu K, Nakashima-Yasuda H, Forman MS, Kwong LK, Clark CM, Grossman M, Miller BL, Kretzschmar HA, Lee VM, Trojanowski JQ, Neumann M. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol. 2008;67:555–564. doi: 10.1097/NEN.0b013e31817713b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van den Berg-Vos RM, Visser J, Kalmijn S, Fischer K, de Visser M, de Jong V, de Haan RJ, Franssen H, Wokke JH, Van den Berg LH. A long-term prospective study of the natural course of sporadic adult-onset lower motor neuron syndromes. Arch Neurol. 2009;66:751–757. doi: 10.1001/archneurol.2009.91. [DOI] [PubMed] [Google Scholar]
- 34.Yokota O, Davidson Y, Arai T, Hasegawa M, Akiyama H, Ishizu H, Terada S, Sikkink S, Pickering-Brown S, Mann DM. Effect of topographical distribution of alpha-synuclein pathology on TDP-43 accumulation in Lewy body disease. Acta Neuropathol. 2010;120:789–801. doi: 10.1007/s00401-010-0731-9. [DOI] [PubMed] [Google Scholar]
- 35.Yokota O, Davidson Y, Bigio EH, Ishizu H, Terada S, Arai T, Hasegawa M, Akiyama H, Sikkink S, Pickering-Brown S, Mann DM. PhosphorylatedTDP-43 pathology and hippocampal sclerosis in progressive supranuclear palsy. Acta Neuropathol. 2010;120:55–66. doi: 10.1007/s00401-010-0702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]