Abstract
The use of immunotherapy for Alzheimer’s Disease (AD) has traditionally focused on the amyloid-β (Aβ) peptide and has shown great potential in both animal and human studies. However an emerging body of work has begun to concentrate on tau and to develop immunization protocols designed to decrease tau pathology in AD and other tauopathies. This commentary will discuss the use of immunotherapy for AD, focusing on tau immunotherapy in the context of recent reports on the use of tau phospho-peptides in transgenic models of tau pathology.
The Challenge of Alzheimer Disease Therapeutics - A role for immunotherapy against new targets
Alzheimer disease (AD) is the seventh most prevalent cause of death in the US and the leading cause of dementia, affecting more than 5 million Americans and 26 million worldwide. Without an effective therapy it is estimated that the number of patients with AD will duplicate by the year 2050 (Maslow, 2010). The cognitive impairment in patients with AD are closely associated with loss of synapses and the formation of neurofibrillary tangles (NFT) in the neocortex and limbic system (DeKosky and Scheff, 1990, DeKosky, et al., 1996, Klucken, et al., 2003, Spires-Jones, et al., 2009, Terry, et al., 1991).
The two major pathological findings in patients with AD are extracellular plaques formed mainly of the amyloid β (Aβ) peptide, (Selkoe, 1989, Selkoe, 1990, Selkoe, 1993) and intracellular NFTs, which contain hyperphosphorylated tau (Grundke-Iqbal, et al., 1986, Kosik, et al., 1986, Wood, et al., 1986). Several lines of investigation support the view that increasing levels of amyloid-β 1–42 (Aβ1–42), the proteolytic product of amyloid precursor protein (APP) metabolism, might be centrally involved in the pathogenesis of AD (Selkoe, 1989, Selkoe, 1990, Selkoe, 1993, Sisodia and Price, 1995). It has been proposed that in AD, progressive accumulation of Aβ might be involved in the mechanisms underlying NFT formation and synaptic loss (Mucke, et al., 2000, Perez, et al., 2008, Pham, et al., 2010, Ribe, et al., 2005). The mechanisms through which accumulation of Aβ and other APP metabolites might lead to synaptic damage and neurodegeneration are under investigation. More specifically, the potential role of neurotoxic Aβ oligomers has emerged as a topic of considerable interest in recent years (Glabe, 2005, Klein, 2002, Klein, et al., 2001, Walsh and Selkoe, 2004).
Most therapeutic approaches for AD have been focused at reducing Aβ accumulation by decreasing APP metabolism by blocking the β or γ secretases (Arbel and Solomon, 2007, Arbel, et al., 2005, Dovey, et al., 2001, Martone, et al., 2009, Richter, et al., 2010, Tomita and Iwatsubo, 2006), by preventing aggregation (Klein, et al., 2001, Wisniewski and Sadowski, 2008) or promoting clearance (Eckman and Eckman, 2005).
In recent years it has been reported that elderly AD patients express auto-antibodies against Aβ (Du, et al., 2001) and tau (Rosenmann, et al., 2006) suggesting that the immune system is capable of mounting a response against the pathological forms of these proteins. In this context a number of groups have conducted studies aimed at inducing or enhancing this immune response. To date, immunotherapeutic approaches to AD have mostly targeted Aβ as it is a secreted protein that can be found in plasma and CSF and is easily accessible to circulating antibodies. Immunotherapy has utilized antibodies against Aβ, generated following vaccination or introduced passively, which function by promoting clearance and reducing aggregation of this peptide (Lemere and Masliah, 2010). In the last decade, Aβ immunotherapy has progressed from preclinical studies in transgenic mouse models of AD to clinical trials in humans (Bard, et al., 2000, DeMattos, et al., 2001, Kokjohn and Roher, 2009, Vellas, et al., 2009).
Clinical trials of Aβ immunotherapy have investigated both active and passive immunization protocols and have shown varying degrees of success. The first immunotherapeutic approach to reach the clinical trail stage was an active immunization protocol using Elan Pharmaceuticals AN1792 antibody. A number of positive features of this trial included the ease of administration and the prospect of life-long immunity, however this trial was halted in 2002 when a small number of trial participants reported adverse side effects (Kokjohn and Roher, 2009), these effects have since been linked to the choice of adjuvant and will be discussed later. Subsequent clinical trials have included active immunization with CAD-106 (Novartis), a peptide vaccine that contains a short N-terminal fragment of Aβ which reportedly does not induce the T-cell response observed with AN-1792 (Lemere and Masliah, 2010). Results from this trail report no significant differences between CSF Aβ levels and MRI whole brain volumes between treated and placebo patients (Winblad, et al., 2009). A number of clinical trials of active immunization are still ongoing, these include the Merck V950 antibody, a peptide also based on the N-terminal region of Aβ and the Elan/Wyeth Pharmaceutical Aβ immuno-conjugate AAC-001, where a fragment of Aβ is attached to a carrier protein, though this trial was suspended in 2008 when a patient developed skin lesions it is now recruiting again for a Phase II trial.
A number of passive immunization approaches have also reached clinical trial stage including the Phase II Elan/Wyeth antibody Bapineuzumab trial, which showed side effects such as vascular edema in the high dose cohort (2.0mg/kg) resulting in this dose being excluded from the Phase III trial (Black, et al., Kerchner and Boxer, Laskowitz and Kolls). Bapineuzumab has also been reported to reduce cortical PiB retention in AD patients (Rinne, et al.). Another passive immunization approach was the humanized monocolonal antibody Solanezumab from Eli Lilly, which was also well tolerated at lower doses and showed a dose-dependent increase in CSF and plasma levels of Aβ (Siemers, et al.). The results from these ongoing trials will shed more light on the safety, efficacy and feasibility of immunotherapeutic approaches to AD and will no doubt be carefully monitored by researchers hoping to develop other novel antibodies for AD immunotherapy.
In the last few years some groups have investigated the possibility of utilizing immunotherapy to target NFTs composed of abnormally phosphorylated tau, the other neuropathological hallmark of AD. Two recent studies have shown that immunization against phosphorylated forms of tau might be effective at reducing NFT pathology in vivo and slowing the progression of behavioral deficits in transgenic mouse models of AD. The current study by Boimel et al (Boimel, et al., 2010) expands on previous studies of tau immunotherapy by demonstrating that antibodies against a number of phosphorylated tau forms can reduce NFT formation, in addition Boimel and colleagues address the issue of safety with regards to the efficacy of the chosen antigen and the antigen/adjuvant choice and combination.
The current paper by Boimel and colleagues (Boimel, et al., 2010) joins a relatively short list of studies that have concentrated on tau immunotherapy (Table 1). In the next section we discuss the findings of this important report by Boimel et al and how it compares with other reports.
Table 1.
Studies Investigating Tau Immunization
| ANIMAL MODEL | ANTIGEN/ IMMUNOGEN |
ADJUVANT | IMMUNIZATION | OUTCOME | REFERENCE |
|---|---|---|---|---|---|
| C57Bl/6 | 50µg recombinant human tau protein (i.p) | CFA and PT | ACTIVE | Anti-tau antibodies detected in serum however immunized animals displayed severe inflammation, axonal injury and loss. | (Rosenmann et al., 2006) |
| P301L Tau | Tau peptide 379–408, phosphorylated at Ser396/404 (s.c) | Alum | ACTIVE | Anti-tau antibodies detected in CNS bound to pathological forms of tau. Reductions of aggregates and improved behavioral outcome | (Asuni et al., 2007) |
| Human tau PS1 | Tau peptide 379–408, phosphorylated at Ser396/404 (s.c) | Alum | ACTIVE | Reductions of aggregates and improved cognitive performance | (Sigurdsson et al., 2008) |
| K257T/P301S double mutant “NFT-pathology model” AND K257T/P301S double mutant - exposed to EAE induced CNS inflammation “Enhanced NFT-pathology model” | Tau peptides: 195–213, phosphorylated at 202/205 207–212, phosphorylated at 212/214 224–238, phosphorylated at 231 (all i.p) | CFA and PT | ACTIVE | Anti-tau antibodies detected in serum and CNS, reduced tau aggregation, no adverse immune reaction (encephalitogenicity) | (Boimel et al., 2010) |
i.p - intraperitoneally; CFA - Complete Freund Adjuvant; PT- Pertussis Toxin; s.c - subcutaneously; NFT - neurofibrillary tangles; EAE - experimental autoimmune encephalomyelitis
Immunotherapy against tau in the treatment of Tauopathies
Tau is a primarily axonal microtubule-associated protein whose function is to stabilize the microtubule structure allowing the efficient transport of cargo such as cell organelles from the cell body along the axons. The activity of tau is tightly regulated by its phosphorylation state, with too much or too little phosphorylation adversely affecting the ability of tau to bind to the microtubules and its propensity to aggregate into fibrillar forms and tangles (Goedert, 2005, Hanger, et al., 2009, Johnson and Stoothoff, 2004, Mandelkow, et al., 2007, Mandelkow, et al., 1996). Hyperphosphorylated forms of tau have been reported in AD where it aggregates into the NFTs characteristic of AD neuropathology (Binder, et al., 2005, Goedert, et al., 2006). NFTs containing phospho-tau are also found in other neurodegenerative disorders including fronto-temporal dementia (FTD), Progressive supranuclear palsy, Corticobasal degeneration and Pick's disease (Bugiani, et al., 1999, Frank, et al., 2008, Lee, et al., 2001, Spires-Jones, et al., 2009). Extensive study by a number of groups has identified and characterized the particular phospho-epitopes associated with tau misfolding and aggregation and the presence of these epitopes has been confirmed in human brains (Goedert, 2005, Goedert, et al., 2006, Grundke-Iqbal, et al., 1986, Hasegawa, et al., 1992, Kosik, et al., 1986).
Unlike Aβ which is a predominantly extracellular protein, tau and the hyperphosphorylated forms of tau are intracellular and have traditionally thought to be inaccessible to antibodies. However the recent demonstration by Masliah et al (Masliah, et al., 2005) that aggregates of α-synuclein, an intracellular synaptic protein that accumulates in the brains of patients with Parkinson’s Disease and AD, were reduced following active immunization with recombinant α-synuclein in a transgenic mouse model, showed that intracellular proteins could also be potential targets of immunization.
A number of recent reports on Aβ pathology have reported effects on tau pathology both in triple transgenic mice (Oddo, et al., 2004) and in some AD patients (Masliah, et al., 2005, Serrano-Pozo, et al., 2010). The relatively small degree of tau clearance seen in these studies generated interest in the development of tau immunization protocols to specifically target pathological form of tau.
In 2007 Asuni et al demonstrated that active immunization with the tau peptide 379–408, phosphorylated at serine residues 396 and 404 (Tau379–408: Ser396/404) was effective reducing the levels of tau aggregates in the brain of P301L transgenic mouse model of tauopathy (Asuni, et al., 2007). These particular epitopes were chosen as they were known to be pathological forms of tau found in AD brains and had been reported to increase the fibrillogenic nature of tau increasing its propensity to assemble into paired helical filaments (PHFs). Asuni and colleagues showed that active immunization with the Tau379–408: Ser396/404 peptide was capable of inducing antibodies against the phosphorylated forms of tau and in the immunized mice the reduction in tau aggregation was accompanied by an amelioration of the sensori-motor deficits associated with tau pathology (Asuni, et al., 2007). The applicability of the Tau379–408: Ser396/404 peptide was subsequently confirmed by this group in another model of tangle pathology (M146L PS1 (presenilin) transgenic mice) where it was shown to improve cognitive performance in the immunized mice (Sigurdsson, et al., 2008).
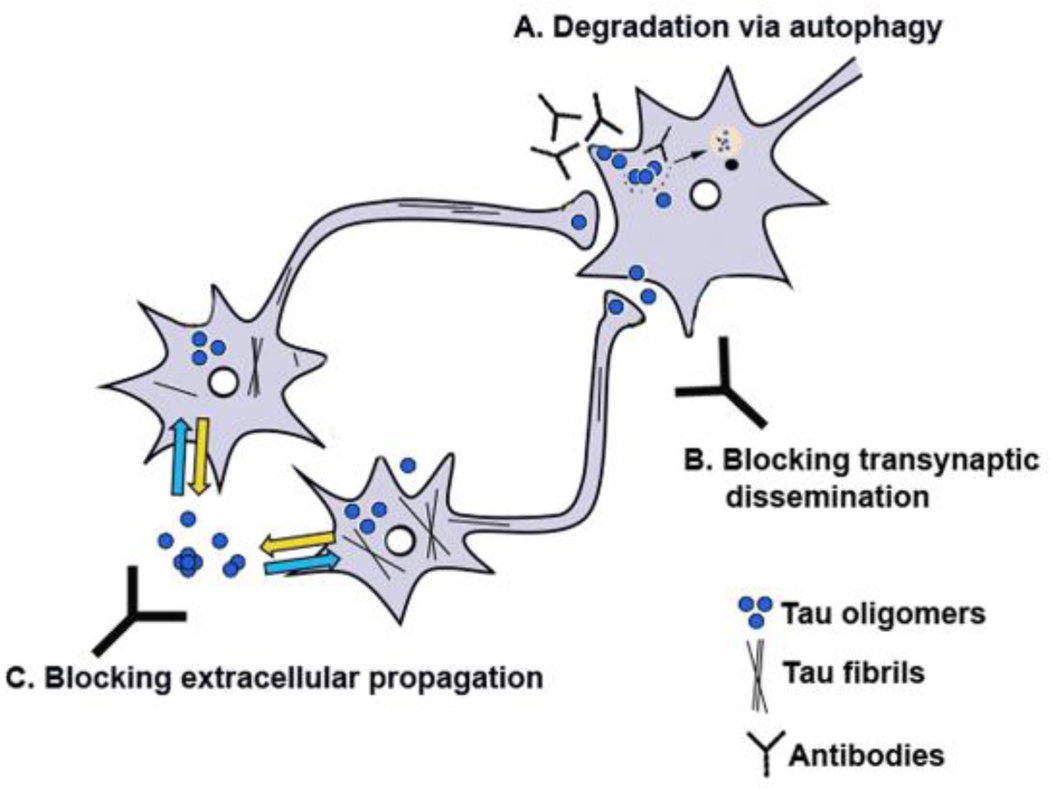
The present study by Boimel et al (Boimel, et al., 2010) also utilized an active immunization protocol, however they injected the mice with a different combination of three separate peptides: Tau195–213: Phospho202/205, Tau207–220: Phospho212/214 and Tau 224–238: Phospho231, all of which have been associated with AD and tau-related pathology (Binder, et al., 2005, Goedert, 2005, Mandelkow, et al., 2007, Mandelkow, et al., 1996). Boimel and colleagues show a significant reduction in the levels of NFT in the CNS in the immunized mice, which they discuss, may occur via lysosomal mechanisms. A similar mechanism involving autophagocytic clearance has been implicated in α-synuclein immunization (Masliah, et al., 2005) (Figure 1).
Figure 1. Mechanisms of tau aggregate clearance by antibodies.
Immunotherapy might reduce the pathology associated with the accumulation of tau aggregates either by promoting internalization of the antibody antigen complex and degradation via autophagy (A) or by preventing trans-synaptic dissemination of the aggregates and synaptic toxicity (B) or by cloaking the process of inter-neuronal transmission of excreted Tau aggregates (C).
When considering the efficacy of tau-based immunotherapy approaches, the exact mechanisms by which the antibody finds and interacts with tau and how they affect the clearance of tau has been a topic of much debate. As mentioned tau is an intracellular protein, however interactions between tau and the plasma membrane have been reported (Brandt, et al., 1995) and phosphorylation has been proposed to regulate this interaction (Maas, et al., 2000). Antibodies against tau may bind this tau, or other cell-surface receptors and be internalized via endocytosis. Though the exact mechanisms to be clarified, once endocytosed the anti-tau IgG may now be exposed to the intracellular tau. The clearance of pathological forms of tau has been reported to require an intact ubiquitin-proteasome pathway (David, et al., 2002, Oddo, et al., 2004), therefore it is plausible that the endocytosed anti-tau IgG may be able to interact with pathological tau conformers targeted for degradation, consistent with the results from Boimel and collegeagues and with the α-synuclein immunization studies (Masliah, et al., 2005).
Taken together the studies by Asuni et. al., 2007 and Boimel et. al., 2010 demonstrate the therapeutic potential of active immunization with tau peptides phosphorylated at sites associated with the pathogenic misfolding and aggregation of tau in AD and other tauopathies and demonstrate the innate ability of the immune system to develop antibodies against these pathological forms of tau. However, given that there are multiple phosphorylation sites on tau (Goedert, et al., 1995, Hanger, et al., 2009, Mandelkow, et al., 1993, Mandelkow and Mandelkow, 1993, Pevalova, et al., 2006), many of which have been reported to have pathological consequences, the identification and selection of the most therapeutically relevant phospho-epitopes requires further research. Additional studies are also necessary to evaluate the safety risks associated with a tau immunotherapy approaches. Unlike Aβ, tau is not found in the cerebro-vasculature so the risk of microhemorrhage upon immunotherapeutic intervention is slight, though the removal of tau tangles may lead to unforeseen complications. Also, given that the activity levels of tau are tightly regulated by its phosphorylation status, particular care should be taken to ensure that the immunotherapy approaches target specifically the pathological forms of phospho-tau allowing physiologically phosphorylated tau to continue to function. It is most practicable that these vaccine safety concerns be addressed in tau transgenic animals, rather than controls in order to allow all aspects of the interaction between the anti-tau IgG and pathological tau forms to be investigated.
EXPERIMENTAL AND SAFETY CONSIDERATIONS
In addition to the demonstration of NFT reduction by their chosen peptide antigens, Boimel et al also address the issue of adjuvant choice and encephalitogenicity, an important consideration given the concerns over the safety of immunotherapies. The importance of the safety of immunotherapeutic approaches to disease was highlighted by the early suspension of the trial of a vaccination with synthetic pre-aggregated Aβ1–42 (AN 1792) due to the development of meningoencephalitis in a small subset of immunized AD patients (Kokjohn and Roher, 2009).
The safety of vaccines can be influenced by the choice and combination of antigen and adjuvant. The antigen in a vaccine is the molecule that is recognized by the immune system and to which the immune system mounts a response, whilst adjuvants are agents introduced into the vaccine to enhance this immune response (Glenn and O'Hagan, 2007).
With regards to the choice of antigen, previous work by Boimel and colleagues (Rosenmann 2006) had demonstrated that immunization with recombinant, unphosphorylated human tau into wildtype (C57Bl/6) mice results in encephalomyelitis and actually increased NFT pathology, this they discussed was due to the use of the unphosphorylated forms rather than pathologically phosphorylated forms of tau as an antigen, a conclusion bolstered by the positive results with the use of a variety of different phospho-tau peptides as antigens in the current study by Boimel et al and previous studies (Asuni, et al., 2007, Boimel, et al., 2010, Sigurdsson, et al., 2008).
The choice of adjuvant has long been a source of debate, with the key issue being the balance between an adjuvant that would suitably enhance the activity of the immune system following immunization without causing an adverse immune reaction. The importance of adjuvant choice was underscored by the suspension of the AN-1792 trial, which was subsequently suspected to be due to the choice of adjuvant (QS21), which may have been due to increased T cell activity and infiltration in a subset of immunized patients (Cribbs, et al., 2003), although it should be noted that other reports have suggested that the meningoencephalitis observed in the AN-1792 trial may have been related to the carboxy terminal region of Aβ (Pride, et al., 2008). It is important to note that QS21 is widely and safely used in other vaccinations including those for HIV (Sasaki, et al., 1998) and malaria (Bojang, 2006). The most common types of adjuvants currently used in vaccines approved for human use are aluminium salts, which have many years of experimental and clinical use to attest to their safety (Baylor, et al., 2002). However a number of animal studies use complete Freund’s adjuvant (CFA) combined with pertussis toxin (PT) to induce an immune response. The initial study by Asuni et al used alum as an adjuvant as they report its effects to be milder that those associated with the use of CFA/PT and their study showed no adverse immune reactions (Asuni, et al., 2007).
Interestingly the previous study by Boimel and colleagues used the CFA/PT protocol in addition to the unphosphorylated tau antigen (Rosenmann, et al., 2006) therefore there had been some debate as to the role played by this adjuvant choice in the adverse immune reactions involved - however in the current study Boimel et al demonstrate that the use of the CFA/PT protocol this time in combination with the phospho-tau peptides did not result in the encephalomyelitis observed in their previous study (Boimel, et al., 2010). This suggests that the severe immune reaction seen in their previous paper was indeed related to the choice of antigen, the unphosphorylated tau, rather that the choice of adjuvant. The currently ongoing clinical trial with ACC-001 will be have groups that are given ACC-001 ± QS-21, and a QS-21 alone group, the results from these groups should also provide some information as to the safety of adjuvant choice.
Taken as a whole these studies highlight the importance of the choice of antigen used and demonstrate the efficacy and safety of phospho-tau peptides in combination with the CFA/PT immunization protocol in animal studies.
CONCLUSIONS AND FUTURE DIRECTIONS
Immunotherapeutic approaches to the treatment of AD, whether aimed at Aβ or tau, show real promise and are prime candidates for further research, though it is possible that a combination immunotherapy approach may be more beneficial than either approach alone. Furthermore, passive immunization protocols for tau could also be considered as an alternative to the active immunization approaches reported thus far. In addition to providing an important new therapeutic avenue for AD, these tau immunotherapy studies confirm the validity and relevance of immunization protocols aimed at intracellular proteins.
As with any new therapy, safety considerations need to be carefully addressed in the development of any immunotherapy protocol and given the differences in the immune systems of mice and humans it may be prudent that this work be carried out in more extensively in non-human primates before introduction to human patients.
However, the benefit from the development of effective vaccines to AD is likely to spur on many researchers especially as the development of effective and safe tau vaccines may be of potential use not only for AD but also for other tauopathies such as FTD.
ACKNOWLEDGMENTS
This work was supported by NIH grants AG18840, AG022074, AG03197, AG10435, NS057096
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arbel M, Solomon B. A novel immunotherapy for Alzheimer's disease: antibodies against the beta-secretase cleavage site of APP. Curr Alzheimer Res. 2007;4:437–445. doi: 10.2174/156720507781788792. [DOI] [PubMed] [Google Scholar]
- Arbel M, Yacoby I, Solomon B. Inhibition of amyloid precursor protein processing by beta-secretase through site-directed antibodies. Proc Natl Acad Sci U S A. 2005;102:7718–7723. doi: 10.1073/pnas.0502427102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27:9115–9129. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Baylor NW, Egan W, Richman P. Aluminum salts in vaccines--US perspective. Vaccine. 2002;20 Suppl 3:S18–S23. doi: 10.1016/s0264-410x(02)00166-4. [DOI] [PubMed] [Google Scholar]
- Binder LI, Guillozet-Bongaarts AL, Garcia-Sierra F, Berry RW. Tau, tangles, and Alzheimer's disease. Biochim Biophys Acta. 2005;1739:216–223. doi: 10.1016/j.bbadis.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Black RS, Sperling RA, Safirstein B, Motter RN, Pallay A, Nichols A, Grundman M. A single ascending dose study of bapineuzumab in patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 24:198–203. doi: 10.1097/WAD.0b013e3181c53b00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boimel M, Grigoriadis N, Lourbopoulos A, Haber E, Abramsky O, Rosenmann H. Efficacy and safety of immunization with phosphorylated tau against neurofibrillary tangles in mice. Exp Neurol. 2010;224:472–485. doi: 10.1016/j.expneurol.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Bojang KA. RTS,S/AS02A for malaria. Expert Rev Vaccines. 2006;5:611–615. doi: 10.1586/14760584.5.5.611. [DOI] [PubMed] [Google Scholar]
- Brandt R, Leger J, Lee G. Interaction of tau with the neural plasma membrane mediated by tau's amino-terminal projection domain. J Cell Biol. 1995;131:1327–1340. doi: 10.1083/jcb.131.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugiani O, Murrell JR, Giaccone G, Hasegawa M, Ghigo G, Tabaton M, Morbin M, Primavera A, Carella F, Solaro C, Grisoli M, Savoiardo M, Spillantini MG, Tagliavini F, Goedert M, Ghetti B. Frontotemporal dementia and corticobasal degeneration in a family with a P301S mutation in tau. J Neuropathol Exp Neurol. 1999;58:667–677. doi: 10.1097/00005072-199906000-00011. [DOI] [PubMed] [Google Scholar]
- Cribbs DH, Ghochikyan A, Vasilevko V, Tran M, Petrushina I, Sadzikava N, Babikyan D, Kesslak P, Kieber-Emmons T, Cotman CW, Agadjanyan MG. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. Int Immunol. 2003;15:505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DC, Layfield R, Serpell L, Narain Y, Goedert M, Spillantini MG. Proteasomal degradation of tau protein. J Neurochem. 2002;83:176–185. doi: 10.1046/j.1471-4159.2002.01137.x. [DOI] [PubMed] [Google Scholar]
- DeKosky S, Scheff S. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann.Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW, Styren SD. Structural correlates of cognition in dementia: quantification and assessment of synapse change. Neurodegeneration. 1996;5:417–421. doi: 10.1006/neur.1996.0056. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovey HF, John V, Anderson JP, Chen LZ, de Saint Andrieu P, Fang LY, Freedman SB, Folmer B, Goldbach E, Holsztynska EJ, Hu KL, Johnson-Wood KL, Kennedy SL, Kholodenko D, Knops JE, Latimer LH, Lee M, Liao Z, Lieberburg IM, Motter RN, Mutter LC, Nietz J, Quinn KP, Sacchi KL, Seubert PA, Shopp GM, Thorsett ED, Tung JS, Wu J, Yang S, Yin CT, Schenk DB, May PC, Altstiel LD, Bender MH, Boggs LN, Britton TC, Clemens JC, Czilli DL, Dieckman-McGinty DK, Droste JJ, Fuson KS, Gitter BD, Hyslop PA, Johnstone EM, Li WY, Little SP, Mabry TE, Miller FD, Audia JE. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem. 2001;76:173–181. doi: 10.1046/j.1471-4159.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- Du Y, Dodel R, Hampel H, Buerger K, Lin S, Eastwood B, Bales K, Gao F, Moeller HJ, Oertel W, Farlow M, Paul S. Reduced levels of amyloid beta-peptide antibody in Alzheimer disease. Neurology. 2001;57:801–805. doi: 10.1212/wnl.57.5.801. [DOI] [PubMed] [Google Scholar]
- Eckman EA, Eckman CB. Abeta-degrading enzymes: modulators of Alzheimer's disease pathogenesis and targets for therapeutic intervention. Biochem Soc Trans. 2005;33:1101–1105. doi: 10.1042/BST20051101. [DOI] [PubMed] [Google Scholar]
- Frank S, Clavaguera F, Tolnay M. Tauopathy models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115:39–53. doi: 10.1007/s00401-007-0291-9. [DOI] [PubMed] [Google Scholar]
- Glabe CC. Amyloid accumulation and pathogensis of Alzheimer's disease: significance of monomeric, oligomeric and fibrillar Abeta. Subcell Biochem. 2005;38:167–177. doi: 10.1007/0-387-23226-5_8. [DOI] [PubMed] [Google Scholar]
- Glenn GM, O'Hagan DT. Adjuvants: progress, regress and pandemic preparedness. Expert Rev Vaccines. 2007;6:651–652. doi: 10.1586/14760584.6.5.651. [DOI] [PubMed] [Google Scholar]
- Goedert M. Tau gene mutations and their effects. Mov Disord. 2005;20 Suppl 12:S45–S52. doi: 10.1002/mds.20539. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Spillantini MG, Crowther RA, Cohen P, Vanmechelen E, Probst A, Gotz J, Burki K. Tau protein in Alzheimer's disease. Biochem Soc Trans. 1995;23:80–85. doi: 10.1042/bst0230080. [DOI] [PubMed] [Google Scholar]
- Goedert M, Klug A, Crowther RA. Tau protein, the paired helical filament and Alzheimer's disease. J Alzheimers Dis. 2006;9:195–207. doi: 10.3233/jad-2006-9s323. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanger DP, Anderton BH, Noble W. Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol Med. 2009;15:112–119. doi: 10.1016/j.molmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Morishima-Kawashima M, Takio K, Suzuki M, Titani K, Ihara Y. Protein sequence and mass spectrometric analyses of tau in the Alzheimer's disease brain. J Biol Chem. 1992;267:17047–17054. [PubMed] [Google Scholar]
- Johnson GV, Stoothoff WH. Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci. 2004;117:5721–5729. doi: 10.1242/jcs.01558. [DOI] [PubMed] [Google Scholar]
- Kerchner GA, Boxer AL. Bapineuzumab. Expert Opin Biol Ther. 10:1121–1130. doi: 10.1517/14712598.2010.493872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein WL. Abeta toxicity in Alzheimer's disease: globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem Int. 2002;41:345–352. doi: 10.1016/s0197-0186(02)00050-5. [DOI] [PubMed] [Google Scholar]
- Klein WL, Krafft GA, Finch CE. Targeting small Abeta oligomers: the solution to an Alzheimer's disease conundrum? Trends Neurosci. 2001;24:219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- Klucken J, McLean PJ, Gomez-Tortosa E, Ingelsson M, Hyman BT. Neuritic alterations and neural system dysfunction in Alzheimer's disease and dementia with Lewy bodies. Neurochem Res. 2003;28:1683–1691. doi: 10.1023/a:1026061021946. [DOI] [PubMed] [Google Scholar]
- Kokjohn TA, Roher AE. Antibody responses, amyloid-beta peptide remnants and clinical effects of AN-1792 immunization in patients with AD in an interrupted trial. CNS Neurol Disord Drug Targets. 2009;8:88–97. doi: 10.2174/187152709787847315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowitz DT, Kolls BJ. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 74:2026. doi: 10.1212/WNL.0b013e3181e03844. author reply 2026–2027. [DOI] [PubMed] [Google Scholar]
- Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Lemere CA, Masliah E. Can Alzheimer disease be prevented by amyloid-beta immunotherapy? Nat Rev Neurol. 2010;6:108–119. doi: 10.1038/nrneurol.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas T, Eidenmuller J, Brandt R. Interaction of tau with the neural membrane cortex is regulated by phosphorylation at sites that are modified in paired helical filaments. J Biol Chem. 2000;275:15733–15740. doi: 10.1074/jbc.M000389200. [DOI] [PubMed] [Google Scholar]
- Mandelkow E, von Bergen M, Biernat J, Mandelkow EM. Structural principles of tau and the paired helical filaments of Alzheimer's disease. Brain Pathol. 2007;17:83–90. doi: 10.1111/j.1750-3639.2007.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow EM, Biernat J, Drewes G, Steiner B, Lichtenberg-Kraag B, Wille H, Gustke N, Mandelkow E. Microtubule-associated protein tau, paired helical filaments, and phosphorylation. Ann N Y Acad Sci. 1993;695:209–216. doi: 10.1111/j.1749-6632.1993.tb23054.x. [DOI] [PubMed] [Google Scholar]
- Mandelkow EM, Mandelkow E. Tau as a marker for Alzheimer's disease. Trends Biochem Sci. 1993;18:480–483. doi: 10.1016/0968-0004(93)90011-b. [DOI] [PubMed] [Google Scholar]
- Mandelkow EM, Schweers O, Drewes G, Biernat J, Gustke N, Trinczek B, Mandelkow E. Structure, microtubule interactions, and phosphorylation of tau protein. Ann N Y Acad Sci. 1996;777:96–106. doi: 10.1111/j.1749-6632.1996.tb34407.x. [DOI] [PubMed] [Google Scholar]
- Martone RL, Zhou H, Atchison K, Comery T, Xu JZ, Huang X, Gong X, Jin M, Kreft A, Harrison B, Mayer SC, Aschmies S, Gonzales C, Zaleska MM, Riddell DR, Wagner E, Lu P, Sun SC, Sonnenberg-Reines J, Oganesian A, Adkins K, Leach MW, Clarke DW, Huryn D, Abou-Gharbia M, Magolda R, Bard J, Frick G, Raje S, Forlow SB, Balliet C, Burczynski ME, Reinhart PH, Wan HI, Pangalos MN, Jacobsen JS. Begacestat (GSI-953): a novel, selective thiophene sulfonamide inhibitor of amyloid precursor protein gamma-secretase for the treatment of Alzheimer's disease. J Pharmacol Exp Ther. 2009;331:598–608. doi: 10.1124/jpet.109.152975. [DOI] [PubMed] [Google Scholar]
- Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, Seubert P, Games D, Kirby L, Schenk D. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005;64:129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Adame A, Alford M, Crews L, Hashimoto M, Seubert P, Lee M, Goldstein J, Chilcote T, Games D, Schenk D. Effects of alpha-synuclein immunization in a mouse model of Parkinson's disease. Neuron. 2005;46:857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Maslow K. 2010 Alzheimer's disease facts and figures. Alzheimers Dement. 2010;6:158–194. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Perez M, Moran MA, Ferrer I, Avila J, Gomez-Ramos P. Phosphorylated tau in neuritic plaques of APP(sw)/Tau (vlw) transgenic mice and Alzheimer disease. Acta Neuropathol. 2008;116:409–418. doi: 10.1007/s00401-008-0420-0. [DOI] [PubMed] [Google Scholar]
- Pevalova M, Filipcik P, Novak M, Avila J, Iqbal K. Post-translational modifications of tau protein. Bratisl Lek Listy. 2006;107:346–353. [PubMed] [Google Scholar]
- Pham E, Crews L, Ubhi K, Hansen L, Adame A, Cartier A, Salmon D, Galasko D, Michael S, Savas JN, Yates JR, Glabe C, Masliah E. Progressive accumulation of amyloid-beta oligomers in Alzheimer's disease and in amyloid precursor protein transgenic mice is accompanied by selective alterations in synaptic scaffold proteins. FEBS J. 2010;277:3051–3067. doi: 10.1111/j.1742-4658.2010.07719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pride M, Seubert P, Grundman M, Hagen M, Eldridge J, Black RS. Progress in the active immunotherapeutic approach to Alzheimer's disease: clinical investigations into AN1792-associated meningoencephalitis. Neurodegener Dis. 2008;5:194–196. doi: 10.1159/000113700. [DOI] [PubMed] [Google Scholar]
- Ribe EM, Perez M, Puig B, Gich I, Lim F, Cuadrado M, Sesma T, Catena S, Sanchez B, Nieto M, Gomez-Ramos P, Moran MA, Cabodevilla F, Samaranch L, Ortiz L, Perez A, Ferrer I, Avila J, Gomez-Isla T. Accelerated amyloid deposition, neurofibrillary degeneration and neuronal loss in double mutant APP/tau transgenic mice. Neurobiol Dis. 2005;20:814–822. doi: 10.1016/j.nbd.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Richter L, Munter LM, Ness J, Hildebrand PW, Dasari M, Unterreitmeier S, Bulic B, Beyermann M, Gust R, Reif B, Weggen S, Langosch D, Multhaup G. Amyloid beta 42 peptide (A{beta}42)-lowering compounds directly bind to A{beta} and interfere with amyloid precursor protein (APP) transmembrane dimerization. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1003026107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, Mathis CA, Blennow K, Barakos J, Okello AA, Rodriguez Martinez de Liano S, Liu E, Koller M, Gregg KM, Schenk D, Black R, Grundman M. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 9:363–372. doi: 10.1016/S1474-4422(10)70043-0. [DOI] [PubMed] [Google Scholar]
- Rosenmann H, Grigoriadis N, Karussis D, Boimel M, Touloumi O, Ovadia H, Abramsky O. Tauopathy-like abnormalities and neurologic deficits in mice immunized with neuronal tau protein. Arch Neurol. 2006;63:1459–1467. doi: 10.1001/archneur.63.10.1459. [DOI] [PubMed] [Google Scholar]
- Rosenmann H, Meiner Z, Geylis V, Abramsky O, Steinitz M. Detection of circulating antibodies against tau protein in its unphosphorylated and in its neurofibrillary tangles-related phosphorylated state in Alzheimer's disease and healthy subjects. Neurosci Lett. 2006;410:90–93. doi: 10.1016/j.neulet.2006.01.072. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Sumino K, Hamajima K, Fukushima J, Ishii N, Kawamoto S, Mohri H, Kensil CR, Okuda K. Induction of systemic and mucosal immune responses to human immunodeficiency virus type 1 by a DNA vaccine formulated with QS-21 saponin adjuvant via intramuscular and intranasal routes. J Virol. 1998;72:4931–4939. doi: 10.1128/jvi.72.6.4931-4939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. Amyloid β protein precursor and the pathogenesis of Alzheimer's disease. Cell. 1989;58:611–612. doi: 10.1016/0092-8674(89)90093-7. [DOI] [PubMed] [Google Scholar]
- Selkoe D. Amyloid β-protein deposition as a seminal pathogenic event in AD: an hypothesis. Neurobiol.Aging. 1990;11:299. [Google Scholar]
- Selkoe D. Physiological production of the β-amyloid protein and the mechanisms of Alzheimer's disease. Trends Neurosci. 1993;16:403–409. doi: 10.1016/0166-2236(93)90008-a. [DOI] [PubMed] [Google Scholar]
- Serrano-Pozo A, William CM, Ferrer I, Uro-Coste E, Delisle MB, Maurage CA, Hock C, Nitsch RM, Masliah E, Growdon JH, Frosch MP, Hyman BT. Beneficial effect of human anti-amyloid-beta active immunization on neurite morphology and tau pathology. Brain. 2010;133:1312–1327. doi: 10.1093/brain/awq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemers ER, Friedrich S, Dean RA, Gonzales CR, Farlow MR, Paul SM, Demattos RB. Safety and changes in plasma and cerebrospinal fluid amyloid beta after a single administration of an amyloid beta monoclonal antibody in subjects with Alzheimer disease. Clin Neuropharmacol. 33:67–73. doi: 10.1097/WNF.0b013e3181cb577a. [DOI] [PubMed] [Google Scholar]
- Sigurdsson EM, Quartermain D, Boutajangout A. Tau immunotherapy prevents cognitive decline and clears pathological tau in a tangle mouse model. Alzheimer's Dementia 4 T191. 2008 doi: 10.1523/JNEUROSCI.4363-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisodia S, Price D. Role of the beta-amyloid protein in Alzheimer's disease. FASEB J. 1995;9:366–370. doi: 10.1096/fasebj.9.5.7896005. [DOI] [PubMed] [Google Scholar]
- Spires-Jones TL, Stoothoff WH, de Calignon A, Jones PB, Hyman BT. Tau pathophysiology in neurodegeneration: a tangled issue. Trends Neurosci. 2009;32:150–159. doi: 10.1016/j.tins.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Terry R, Masliah E, Salmon D, Butters N, DeTeresa R, Hill R, Hansen L, Katzman R. Physical basis of cognitive alterations in Alzheimer disease: synapse loss is the major correlate of cognitive impairment. Ann.Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Tomita T, Iwatsubo T. gamma-secretase as a therapeutic target for treatment of Alzheimer's disease. Curr Pharm Des. 2006;12:661–670. doi: 10.2174/138161206775474206. [DOI] [PubMed] [Google Scholar]
- Vellas B, Black R, Thal LJ, Fox NC, Daniels M, McLennan G, Tompkins C, Leibman C, Pomfret M, Grundman M. Long-term follow-up of patients immunized with AN1792: reduced functional decline in antibody responders. Curr Alzheimer Res. 2009;6:144–151. doi: 10.2174/156720509787602852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Oligomers on the brain: the emerging role of soluble protein aggregates in neurodegeneration. Protein Pept Lett. 2004;11:213–228. doi: 10.2174/0929866043407174. [DOI] [PubMed] [Google Scholar]
- Winblad B, Minthon L, Floesser A, Imbert G, Dumortier T, He Y, Maguire P, Karlsson M, Ostlund H, Lundmark J, Orgogozo J, Graf A, Andreasen N. Results of the first-in-man study with the active Ab immunotherapy CAD-106 in Alzheimer Patients. Alzheimers Dementia. 2009;5:P113–P114. O112-105-105. [Google Scholar]
- Wisniewski T, Sadowski M. Preventing beta-amyloid fibrillization and deposition: beta-sheet breakers and pathological chaperone inhibitors. BMC Neurosci. 2008;9 Suppl 2:S5. doi: 10.1186/1471-2202-9-S2-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Mirra SS, Pollock NJ, Binder LI. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau) Proc Natl Acad Sci U S A. 1986;83:4040–4043. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]