Abstract
Objectives:
To study and compare the changes in nuclear and cellular size, shape and nuclear–cytoplasmic ratio of the cells in the basal layer of oral leukoplakia and well-differentiated oral squamous cell carcinoma (SCC) with normal buccal mucosa, using computer-aided image analysis in tissue sections.
Study design:
This was a retrospective study conducted on tissue sections on a total number of 70 cases to determine the various morphometric parameters. The data collected in this study were analyzed statistically by computing descriptive statistics, viz., percentage, mean, standard deviation, standard error of mean, 95% confidence interval for mean. The difference in the control and study groups for various diagnostic variables was compared by means of analysis of variance (ANOVA), Student’s t-test for independent samples, wherever applicable. Mann–Whitney U-test and Kruskal–Wallis test were used where the data were found to be asymmetrical and the standard deviations were also different. The results were considered statistically significant whenever P ≤ 0.05.
Results:
Our results were significant for the morphometric parameter, size. The values of nuclear perimeter and area, cellular perimeter and area increased gradually from the normal buccal mucosa to leukoplakia, reaching the highest value in SCC. There was statistically significant difference in the nuclear and cellular areas to differentiate between leukoplakia and squamous cell carcinoma. Two variables which were used to study the shape, “form perimeter (PE)” and “contour index (CI)”, showed significant difference between normal buccal mucosa and leukoplakia and between normal buccal mucosa and SCC. The morphometric parameter, nuclear–cytoplasmic ratio, in our results showed an increase in leukoplakia and SCC compared to normal buccal mucosa, but the difference was not significant between leukoplakia and SCC.
Conclusion:
The morphometric parameter, size, was useful to differentiate between normal, potentially malignant leukoplakia and SCC.
Keywords: Image analysis, leukoplakia, morphometry, parameters
INTRODUCTION
Oral squamous cell carcinoma (SCC) is recognized as the most common malignant epithelial neoplasm of the oral cavity, resulting from genetic damage, leading to uncontrolled cell proliferation of damaged cells.[1] In the course of its progression, visible changes are taking place at the cellular level (atypical) and at the resultant tissue level (epithelial dysplasia).[2] The sum total of these physical and morphological alterations is of diagnostic and prognostic relevance and is designated as precancerous changes. Oral leukoplakia is the best known precursor lesion. Earlier, clinical investigations had shown the rate of malignant change in leukoplakia ranging between 3% and 6%.[3] The latter studies suggest a malignant transformation potential of 4%, and is higher in specific clinical subtypes or phases of leukoplakia.[4] In two studies from India carried out by Gupta et al. and Silvennan et al., annual malignant transformation rates of 0.3% and 0.06%, respectively, have been reported.[5] The dilemma in managing patients with potentially malignant oral lesions and field change is of deciding which mucosal lesions or areas will progress to carcinoma.[6] From the vast literature on molecular markers in oral precancer, no reliable prognostic markers for risk assessment in putatively premalignant lesions have emerged.[7] However, histopathologically assessed severity of oral epithelial dysplasia (OED) is considered as the “gold standard” for the prediction of malignant transformation of precancerous lesions.[8]
Dysplasia comprises a loss in the uniformity of the individual cells as well as a loss in their architectural orientation. Dysplastic cells exhibit considerable pleomorphism (variation in the size and shape) and often possess hyperchromatic nuclei which are abnormally large for the size of the cell. The nuclear–cytoplasmic ratio increases from 1:4 to 1:1 at the expense of the cytoplasmic volume.[2]
There is, therefore, a substantial need to improve the histologic assessment of epithelial dysplasia. Pathologists differ in the emphasis they place on particular histopathologic features, and the interpretation of dysplasia varies from one pathologist to another. The wide variation between the observers in the subjective evaluation has been commented on by several authors and more objective means of evaluation are constantly sought for.[9–13] The interest then turned toward applying sophisticated technique of computer-assisted morphometry to investigate the cellular and nuclear changes in correlation with the histological behavior of the lesions.[14] It is a method of assessing the computerized images of histological stained sections. Several variables observable in microscopic images are amenable to morphometric analysis. The results have been more reliable, objective and reproducible.
The present study was conducted to observe the morphological features like cell area (CA), nuclear area (NA), cell perimeter (CP), nuclear perimeter (NP), and to assess the shape and nuclear–cytoplasmic ratio (N/C) in the basal cell layer in normal buccal mucosa, leukoplakia and SCC, and to see if any consistent changes in these features existed between them. The study group comprised a total of 70 cases, of which 10 cases were of normal buccal mucosa (control), 30 cases were of leukoplakia and 30 cases were of well-differentiated SCC.
MATERIALS AND METHODS
This retrospective morphometric study was conducted on tissue sections obtained from the biopsy tissue specimens received and from the cases retrieved from archives of Department of Oral Pathology and Microbiology, V. S. Dental College and Hospital, Bangalore.
The control group comprised normal buccal mucosa (10 cases) from healthy adult individuals, irrespective of age and with no habits. The biopsies were obtained from the patients with their consent, from the Department of Oral Surgery, V. S. Dental College and Hospital.
The study group comprised collection of 30 paraffin blocks of cases of oral leukoplakia of the buccal mucosa and 30 paraffin blocks of cases diagnosed with well-differentiated oral SCC of buccal mucosa.
Inclusion and exclusion criteria
The study group comprised the diagnosed cases, irrespective of age and sex, reported from the Department of Oral Pathology, V. S. Dental College and Hospital.
Clinically diagnosed cases of leukoplakia confirmed histologically as epithelial dysplasia [based on WHO grading of epithelial dysplasia (1978)] were only included in the study. Mild, moderate and severe dysplastic cases were all considered since our study included only the basal cell layer of the dysplastic epithelium.
Oral SCC cases were selected irrespective of the etiological factors, and only histologically well-differentiated oral SCC cases were included (based on Broder’s grading system).
For every case, the most representative areas were selected from the sections which could be subjected to the morphometric analysis in the basal cell layer.
Tissue sections of 5 mm thickness were cut using a soft tissue microtome from formalin-fixed, paraffin-embedded tissue blocks. The sections obtained were stained with Harris’s Hematoxylin and Eosin. The stained sections were observed under a microscope for confirmation of the diagnosis by two separate examiners.
Morphometric technique
After reviewing, the sections were further subjected to morphometric analysis. For morphometric analysis, images were captured using a three-chip CCD camera attached to a trinocular research microscope with a 100× objective. The final image captured on the monitor had a magnification of 1000×. For each section, the selected field included representative cells with largest cells in the basal layer where distinct cellular and nuclear outlines were seen avoiding areas of basal cell hyperplasia and overlapping cells. Histologically identifiable non-keratinocytes such as melanocytes showing clear cell change and inflammatory cells, as well as cells showing degenerative changes, and those undergoing mitoses were not measured. The images were classified, transferred and stored in the computer.
The actual measurements of the morphometric parameters were done using the image analyzer software Image-Proexpress (Media Cybernetics, Silver Spring, MD, USA) after accurate calibration was done using a stage micrometer. No corrections for section thickness or Holmes effect were applied so that the measurements represented the morphological image as seen.
Details of Morphometric Parameters Studied [Figures 1 and 2]
Figure 1.
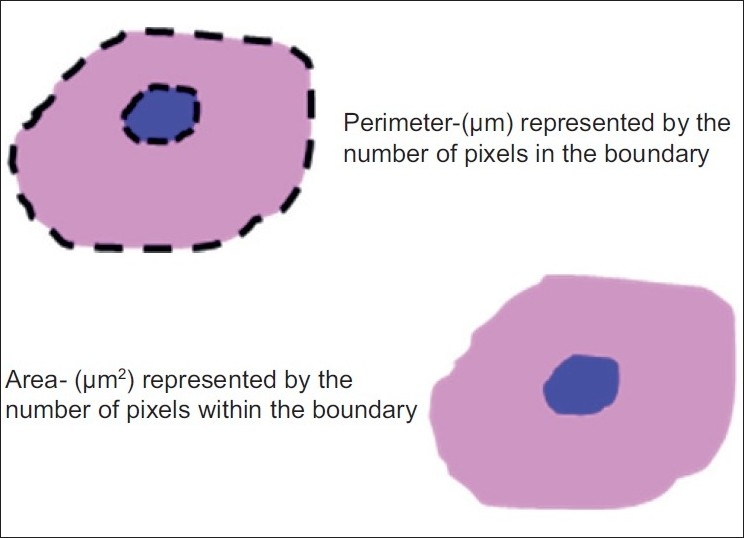
Illustrations and formulas of parameter Size
Figure 2.
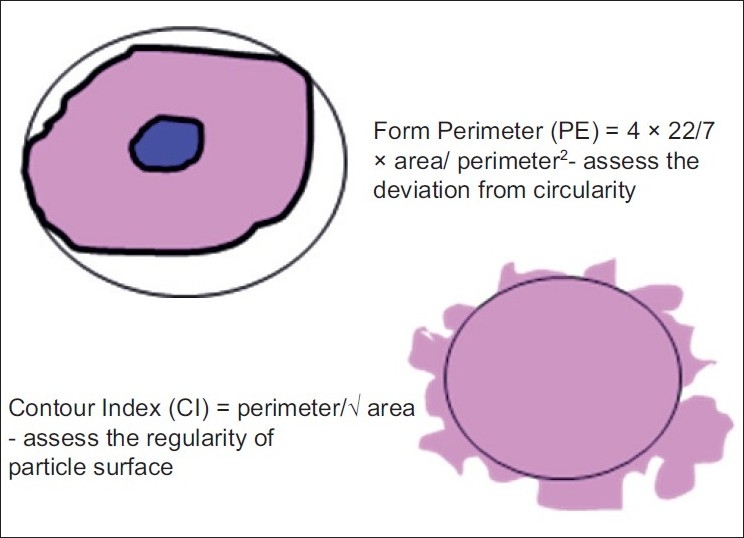
Illustrations and formulas of parameter Shape
1. Size
The size of the cell and its nucleus was measured with the area and perimeter.
Cell perimeter and nuclear perimeter
It was measured in microns. For measurement, the cell and the nuclear outline were traced and the software automatically calculated the cell and nuclear perimeter (number of boundary pixels detected, converted to micrometer); 5–7 largest cells with clear outline were selected from each field.
Cell area and nuclear area
It was measured in square microns when the perimeter was traced; the software automatically calculated the CA (number of pixels detected, converted to micrometer2).
From the CP, CA, NP and NA, the other morphometric parameters i.e., shape and nuclear–cytoplasmic ratio, were calculated.
2. Shape
The shape was measured using two variables.
-
“Form perimeter (PE)” which assesses the deviation from circularity. Using standard formula, a circle has a value of 1 and an ellipse or an irregular structure is less than 1.
Cellular form PE=4 × 22/7 × C area/C perimeter2
Nuclear form PE=4 × 22/7 × N area/N perimeter2
-
Contour index (CI) was the other shape measuring variable used to measure the regularity of the particle surface. A value of 3.54 is obtained for a circle by using standard formula. This formula was applied to the cellular and nuclear values.
Cellular CI=C perimeter/√C area
Nuclear CI=N perimeter/√N area
Higher values are obtained with the increase in the indentations or convolutions of the outline.
3. N:C ratio
The nuclear–cytoplasmic ratio was the other parameter which was calculated by using the formula
N:C ratio=N area/(C area – N area).
All the measurements were saved in Microsoft excel for further statistical analysis.
RESULTS
Approximately 7000 measurements were carried out [Table 1] and the results were then represented as mean area and perimeter [Table 2] and the data collected in this study were analyzed statistically by computing descriptive statistics, viz., percentage, mean, standard deviation, standard error of mean, 95% confidence interval for mean values obtained in microns as shown in the tables for various morphometric variables. The differences in the control group and study groups for various diagnostic variables were compared by means of analysis of variance (ANOVA) and Student’s t-test for independent samples, wherever applicable, and are shown in Tables 3–7.
Table 1.
Morphometric measurements carried out
| Total sections: 10 normal buccal mucosa + 30 leukoplakia + 30 oral SCC | 70 sections |
| Number of microscopic fields per section | 5–10 fields |
| Cells per field (basal layer) | 5–7 cells |
| Total cells per section | [(5–7 cells) × (5–10 fields)] |
| Total cells measured | 3500 approx. |
| Total nuclei measured | 3500 approx. |
| Total number of measurements | 7000 approx. |
Table 2.
Morphometry summary data
| Leukoplakia |
Well-differentiated SCC |
Normal buccal mucosa |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell |
Nucleus |
Cell |
Nucleus |
Cell |
Nucleus |
|||||||
| Area | Perimeter | Area | Perimeter | Area | Perimeter | Area | Perimeter | Area | Perimeter | Area | Perimeter | |
| 1 | 128.92 | 51.056 | 64.651 | 35.488 | 155.34 | 61.765 | 81.568 | 53.127 | 70.549 | 31.89 | 25.14 | 18.444 |
| 2 | 128.43 | 49.719 | 62.042 | 34.333 | 148.45 | 56.874 | 78.433 | 49.043 | 73.145 | 29.133 | 20.056 | 15.177 |
| 3 | 122.01 | 49.092 | 58.432 | 31.654 | 154.23 | 52.457 | 68.649 | 44.678 | 70.389 | 28.256 | 21.558 | 15.667 |
| 4 | 120.57 | 47.886 | 60.324 | 32.423 | 137.12 | 50.396 | 79.634 | 39.545 | 72.266 | 31.138 | 32.961 | 19.706 |
| 5 | 130.42 | 48.654 | 62.348 | 33.865 | 154.48 | 63.743 | 79.363 | 41.16 | 82.639 | 32.584 | 26.461 | 18.353 |
| 6 | 118.24 | 42.123 | 49.184 | 29.958 | 165.24 | 66.785 | 82.043 | 52.787 | 85.763 | 31.181 | 26.177 | 16.896 |
| 7 | 120.43 | 42.433 | 56.645 | 25.643 | 178.24 | 70.613 | 85.433 | 46.121 | 82.629 | 32.022 | 30.203 | 21.116 |
| 8 | 134.26 | 48.955 | 72.432 | 38.423 | 175.32 | 58.953 | 67.789 | 49.325 | 83.811 | 32.268 | 32.233 | 20.762 |
| 9 | 119.54 | 41.564 | 69.763 | 35.312 | 148.23 | 56.786 | 72.433 | 47.228 | 68.433 | 24.543 | 27.798 | 18.412 |
| 10 | 121.43 | 51.044 | 64.959 | 34.569 | 143.46 | 44.867 | 68.244 | 34.489 | 88.976 | 31.263 | 27.59 | 17.836 |
| 11 | 115.43 | 44.108 | 52.639 | 31.432 | 147.43 | 55.856 | 64.246 | 30.226 | ||||
| 12 | 114.62 | 40.948 | 55.234 | 27.538 | 154.05 | 48.732 | 81.942 | 38.772 | ||||
| 13 | 134.43 | 54.533 | 64.675 | 36.101 | 138.19 | 46.257 | 69.187 | 33.923 | ||||
| 14 | 124.43 | 55.665 | 60.326 | 34.426 | 139.43 | 42.601 | 80.516 | 37.231 | ||||
| 15 | 112.43 | 42.243 | 51.235 | 32.243 | 146.43 | 43.476 | 68.278 | 36.452 | ||||
| 16 | 133.94 | 51.607 | 65.787 | 35.914 | 173.23 | 55.787 | 83.343 | 47.306 | ||||
| 17 | 118.46 | 42.264 | 55.235 | 29.343 | 139.54 | 54.68 | 76.439 | 35.069 | ||||
| 18 | 134.54 | 53.569 | 58.886 | 37.585 | 171.34 | 57.054 | 72.433 | 39.211 | ||||
| 19 | 116.54 | 45.765 | 50.234 | 30.342 | 157.43 | 51.299 | 77.343 | 39.913 | ||||
| 20 | 126.64 | 51.718 | 51.432 | 45.581 | 155.43 | 50.309 | 82.456 | 41.105 | ||||
| 21 | 121.54 | 43.947 | 65.199 | 35.753 | 143.46 | 57.46 | 68.743 | 32.456 | ||||
| 22 | 114.33 | 45.433 | 56.178 | 33.126 | 166.25 | 56.598 | 68.346 | 35.343 | ||||
| 23 | 128.43 | 44.432 | 56.991 | 32.764 | 164.97 | 51.897 | 82.264 | 34.236 | ||||
| 24 | 114.96 | 46.277 | 61.614 | 32.412 | 165.69 | 49.136 | 72.428 | 39.253 | ||||
| 25 | 132.77 | 49.575 | 77.286 | 38.096 | 163.24 | 47.981 | 81.246 | 40.197 | ||||
| 26 | 131.82 | 49.211 | 60.576 | 35.078 | 137.24 | 46.753 | 68.234 | 37.163 | ||||
| 27 | 118.43 | 47.887 | 56.432 | 32.324 | 146.86 | 47.684 | 68.471 | 30.324 | ||||
| 28 | 112.34 | 54.333 | 44.232 | 53.88 | 143.56 | 47.006 | 75.839 | 37.163 | ||||
| 29 | 122.45 | 55.601 | 54.324 | 29.543 | 150.48 | 52.032 | 61.433 | 46.126 | ||||
| 30 | 130.55 | 56.796 | 62.784 | 32.654 | 143.9 | 42.895 | 67.343 | 33.896 | ||||
Table 3.
Analysis of variance of nuclear and cytoplasmic areas
| Area | Source of variation | Sum of squares | df | Mean square | F ratio | P value |
|---|---|---|---|---|---|---|
| Nucleus | Between groups | 17,064.426 | 2 | 8532.213 | 193.509 | <0.0001 |
| Within groups | 2954.169 | 67 | 44.092 | |||
| Total | 20,018.595 | 69 | ||||
| Cell | Between groups | 46,560.968 | 2 | 23,280.484 | 240.092 | <0.0001 |
Table 7.
Analysis of variance of nuclear–cytoplasmic ratio
| Source of variation | Sum of squares | df | Mean square | F ratio | P value |
|---|---|---|---|---|---|
| Between groups | 1.383 | 2 | 0.692 | 21.862 | <0.0001 |
| Within groups | 2.120 | 67 | 0.032 | ||
| Total | 3.503 | 69 |
Table 4.
Statistical inference based on independent Student’s t-test
| Area | Diagnostic groups | t value | df | P value |
|---|---|---|---|---|
| Nucleus | Leukoplakia and normal buccal mucosa | 13.509 | 38 | <0.0001 |
| SCC and normal buccal mucosa | 20.875 | 38 | <0.0001 | |
| Leukoplakia and SCC | 8.403 | 58 | <0.0001 | |
| Cytoplasm | Leukoplakia and normal buccal mucosa | 17.015 | 38 | <0.0001 |
| SCC and normal buccal mucosa | 18.367 | 38 | <0.0001 | |
| Leukoplakia and SCC | 11.869 | 58 | <0.0001 |
Table 5.
Analysis of variance of nuclear and cytoplasmic “form PE”
| CPE | Source of variation | Sum of squares | df | Mean square | F ratio | P value |
|---|---|---|---|---|---|---|
| Nucleus | Between groups | 1.290 | 2 | 0.645 | 27.693 | <0.0001 |
| Within groups | 1.560 | 67 | 0.023 | |||
| Total | 2.850 | 69 | ||||
| Cell | Between groups | 1.189 | 2 | 0.594 | m30.114 | <0.00m01 |
| Within groups | 1.323 | 67 | 0.020 | |||
| Total | 2.511 | 69 |
Table 6.
Analysis of variance of nuclear and cytoplasmic contour index (CI)
| CI | Source of variation | Sum of squares | df | Mean square | F ratio | P value |
|---|---|---|---|---|---|---|
| Nucleus | Between groups | 9.847 | 2 | 4.924 | 9.681 | <0.0001 |
| Within groups | 34.077 | 67 | 0.509 | |||
| Total | 43.924 | 69 | ||||
| Cell | Between groups | 6.308 | 2 | 3.154 | 18.122 | <0.0001 |
| Within groups | 11.660 | 67 | 0.174 | |||
| Total | 17.968 | 69 |
Mann–Whitney U-test and Kruskal–Wallis test were used where the data were found to be asymmetrical. The results were considered statistically significant whenever P≤0.05.
The results showed that the nuclear area and perimeter increased steadily from normal buccal mucosa to leukoplakia and reached the highest value in well-differentiated SCC. The mean values of the whole cell showed similar trends with increased values of cellular area and perimeter from normal buccal mucosa to leukoplakia to well-differentiated SCC [Figure 3].
Figure 3.
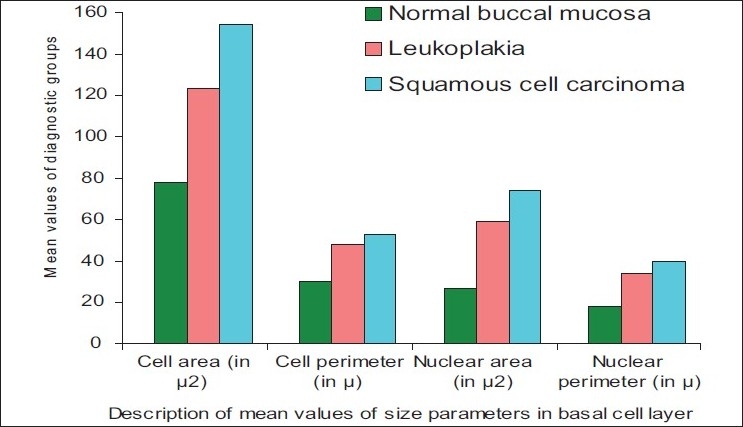
Bar graph showing the description of mean values in the basal cell layer
There was increase in the mean N:C ratio between normal buccal mucosa and leukoplakia and between normal buccal mucosa and SCC [Figure 4].
Figure 4.
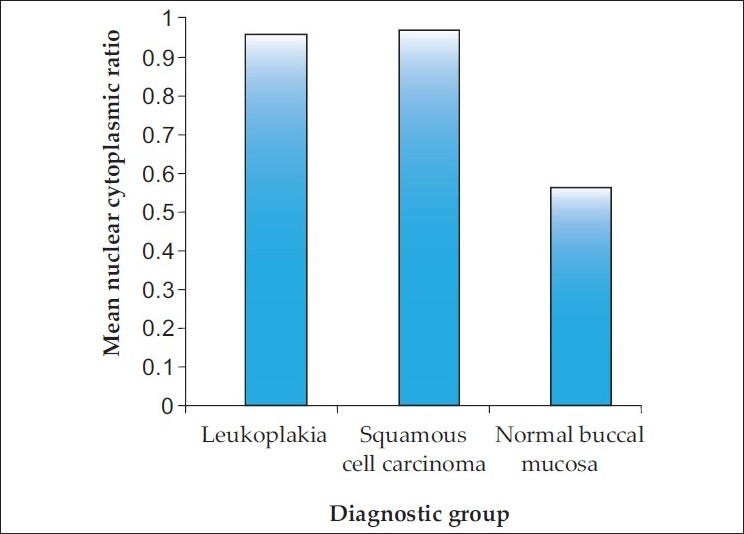
Bar graph showing the mean N:C ratio in the diagnostic groups
The nonparametric Mann–Whitney U-test was used to evaluate the independent pairwise difference between the diagnostic groups of nuclear “form PE”. It was observed that there was a highly statistical significance between normal buccal mucosa and leukoplakia (Z=4.46, P<0.0001) as well as between normal buccal mucosa and SCC (Z=4.56, P<0.0001). However, there was no statistically significant result between leukoplakia and SCC (Z=1.36, P>0.174) for nuclear “form PE”. The results were similar for cellular “form PE”. The Kruskal–Wallis test was used to test the changes in more than two independent groups. Both in nuclear “form PE” and cellular “form PE”, the Kruskal–Wallis test showed that the diagnostic groups were independently distributed (χ2=29.934, P<0.0001).
The Mann–Whitney test showed statistical significance in nuclear “CI” in the groups between normal buccal mucosa and leukoplakia (Z=4.46, P<0.0001), and normal buccal mucosa and SCC (Z=4.56, P<0.0001). There was no statistical significance in nuclear “CI” between leukoplakia and SCC (Z=1.36, P>0.174). For cellular “CI” also, the results were similar.
However, it could be observed by applying the Kruskal–Wallis test that the N:C ratios between the three diagnostic groups were independently distributed (χ2=22.17; df=2, P<0.0001)
DISCUSSION
Oral SCC is the most common malignant neoplasm arising from the mucosal epithelium of the oral cavity. While its incidence is relatively low in the western countries, there are some important exceptions to this trend. In the Indian subcontinent and in other parts of Asia, it remains one of the most common forms of cancer.[15] This is a paradoxical finding since most cases are preceded by readily detectable mucosal change, most often red or white patches.[16] The diagnosis of precancers is primarily based on clinical morphology and its grading on histology (dysplasia). The presence of epithelial dysplasia may be even more important in predicting the malignant development than the clinical characteristics.
Three major problems, however, are attached to the importance of epithelial dysplasia in predicting the malignant development:
The diagnosis is essentially subjective.
Not all lesions exhibiting dysplasia will eventually become malignant.
Carcinoma can develop from lesions in which epithelial dysplasia was not diagnosed in previous biopsies.
There is, therefore, a substantial need to improve the histologic assessment of epithelial dysplasia. Of late, interest has turned toward applying sophisticated technique of computer-assisted morphometry to investigate the cellular and nuclear changes in correlation with the histological behavior of the lesions. It is a method of assessing the computerized images of histological stained sections. The results have been more reliable, objective and reproducible.
Many investigators have evaluated the use of nuclear morphometry for grading and for predicting prognosis in esophageal, laryngeal, renal, bladder, breast, prostrate and colonic carcinomas. Attempts have also been made to apply morphometry to normal oral epithelium and also in various oral mucosal diseases like oral submucous fibrosis, leukoplakia, lichen planus, SCC, epithelial dysplasia, and traumatic keratosis.
Boysen and Reith[17] in their study on nasal mucosa in nickel workers have also reported a progressive increase in basal cellular and nuclear areas from pseudostratified columnar epithelium, through metaplastic epithelium, to their highest values in dysplastic epithelium. It could be observed in our study also that there was a steady increase in the basal cellular and nuclear size dimensions from normal buccal mucosa to leukoplakia lesions, indicating more biologic activity in the nucleus and the cytoplasm.
Eveson and MacDonald[18,19] observed that after the application of carcinogens to hamster cheek pouch epithelium, there was progressive increase both in size (represented by mean cell area) and number of progenitor cells. Similar changes have been described in the epithelia after application of turpentine, chemical carcinogens, and also during the menstrual cycle. These findings support our observation of increase in cellular area in the progenitor cells.
Saku and Sato[20] reported an increase in the proportion of the cells in the hyperdiploid to the hypertetraploid range in oral leukoplakia. In our study, the NA was almost twice as large in leukoplakia compared to the normal buccal mucosa.
Shabana et al.[14] studied the size and shape of the cells in the basal cell layer of the oral epithelium in 100 specimens from oral mucosa by using an interactive image analysis system (IBAS-1). Four groups of white lesions (traumatic keratosis, lichen planus, leukoplakia, and a “risk group”) in addition to two control groups (normal mucosa and SCC) were studied retrospectively. The results showed a progressive increase in the dimensions (area, perimeter, and maximum diameter) of the nuclei from normal mucosa through traumatic keratosis, lichen planus, leukoplakia and the “risk group” to carcinoma, with considerable differences. The nucleus in SCC was twice as large as in normal mucosa. A substantial increase in the dimensions of both the cell and the nucleus was found in the “risk group”. The nuclear:cytoplasmic ratio, contrary to what might have been anticipated in risk lesions, did not show considerable differences between the diagnostic groups. Furthermore, it was slightly decreased in the “risk group” compared with the normal mucosa. The shape factors (form PE and CI) seemed to be less helpful in the identification of the “risk group”. They concluded that the size of the basal cell and its nucleus can be of diagnostic value for lesions with a high risk of malignant transformation.
Our results were significant for the morphometric parameter, size. The values of NP and NA, CP and CA increased gradually from the normal buccal mucosa to leukoplakia, reaching the highest value in SCC. There was statistically significant difference in the nuclear and cellular areas to differentiate between leukoplakia and SCC. The morphometric parameter, size, was hence useful to differentiate between normal, potentially malignant leukoplakia and the malignant SCC. Two variables which were used to study the shape, “form PE” and “CI”, showed significant difference between normal buccal mucosa and leukoplakia and between normal buccal mucosa and SCC. However, this parameter was of no use to differentiate between leukoplakia and SCC. In our study, the mean±SD (mean) of N:C ratio was 0.941±0.182, whereas in the study by Shabana et al., the mean±SD (mean) was 0.809±0.215. The N:C ratio was slightly higher in our study denoting more NA compared to the cytoplasmic area. It can be observed that though there was a significant difference statistically in the N:C ratio between normal buccal mucosa and SCC, it was not seen between leukoplakia and SCC.
Cowpe et al.[21] found that tissues undergoing malignant transformation typically showed a reduction in CA before the reduction in NA, using semiautomatic image analysis techniques. They also suggested that samples of healthy mucosa from the same patient provide the best control. In an earlier study, they demonstrated that exfoliative cytology is capable of detecting malignant changes, through estimation of NA/CA using the planimeter method in Papanicolaou-stained smears.[22]
Truelson et al.[23] noted that nuclear DNA content and nuclear area were better indicators of the biologic aggressiveness of cancer in laryngeal cancers. In the study by Jin et al.,[24] the mean±SD of N:C ratio of islands of invasive SCC was 0.650±0.207. Even though their value increased from that of dysplastic epithelium (0.558±0.101), there was no significant difference statistically between the two groups. They concluded that this parameter was not useful to distinguish between premalignant and malignant lesions. Our results showed an increase in N:C ratio of the oral basal cells in leukoplakia and SCC compared to normal buccal mucosa, but the difference was not significant between leukoplakia and SCC.
Ramaesh et al.[25,26] used cytomorphometric techniques to assess nuclear diameter (ND) and cytoplasmic diameter (CD) in normal oral mucosa, in dysplastic lesions and in SCCs. They found that CD was highest in normal mucosa, lower in dysplastic lesions, and lowest in SCCs. By contrast, ND was the lowest in normal mucosa, higher in dysplastic lesions, and highest in SCCs. These studies suggested that reduced nuclear size and increased cytoplasm size are useful early indicators of malignant transformation, and thus, exfoliative cytology is of value for monitoring clinically suspected lesions and for early detection of malignancy.
Mollaoglu et al.[27] used an advanced imaging system, Seescan TV image analysis system (TVIAS), and could successfully identify dysplastic and malignant cells in oral smears containing large amounts of normal cells, on the basis of both integrated optical density (IOD) and NA values. There was a significant increase (P<0.001) in mean IOD for nuclei in smears from dysplastic lesions and carcinomas as compared with normal smears of 50 Feulgen-stained nuclei which were measured in smears collected from normal oral mucosa (n=6), lesions displaying epithelial dysplasia (n=5) and invasive SCC (n=5).
Zo Pektas et al,[28] used cytomorphometric measurements and nuclear Feulgen DNA content (DNA ploidy) analysis via oral brush biopsy in oral mucosal smears (n=44) from patients (n=22) presenting with various oral lesions using a cytobrush immediately before biopsy. They concluded that cytomorphometric analysis via oral brush biopsy, a valuable adjunct to biopsy for identification of premalignant and early stage cancerous oral lesions, is a rapid and minimally invasive procedure with high specificity and sensitivity rates, requiring no topical or local anesthetic.
Natarajan et al.[29] attempted an objective and reproducible evaluation of mitotic activity and nuclear morphometric factors in their study of 30 patients of oral SCC with a view to predicting local relapse and survival. Various nuclear parameters and volume-corrected mitotic index were calculated and compared with the recurrence and death of the study group (n=30), and the effectiveness of the M/V index in predicting the biological behavior and the outcome of oral SCC patients was determined.
Observations drawn from our study and few suggestions
More quantitative morphometric parameters like volume densities, texture analysis, state of nucleus described subjectively as coarse, spotty, dispersed or clumped have to be analyzed to determine the relationship of these atypical features to subsequent malignant transformation.
Other quantifiable features of epithelial dysplasia, already defined, have to be assessed to know whether they have greater or lesser diagnostic significance using image analysis.
Epidemiological studies have shown that candidal leukoplakia and proliferative verrucous leukoplakia have greater malignant potential. Further studies regarding these pathological groups are necessary to substantiate this claim.
- Rigorous assessments have to be performed using multivariate statistics to check the validity of the results obtained from large quantitative data.
- To summarize, the objectives of this morphometric analyses were to study the a) size, b) shape, and c) N:C ratio of the basal cells and nuclei in normal mucosa, leukoplakia and well-differentiated SCC using image analysis. The results have revealed the following:The morphometric parameter size was useful to differentiate basal cells in normal mucosa, leukoplakia and SCC.
- The morphometric parameter shape was useful to differentiate between normal mucosa and leukoplakia but not between leukoplakia and SCC.
- The N:C ratio parameter was useful to differentiate between normal mucosa and leukoplakia but not between leukoplakia and SCC.
In conclusion, the increase in basal cellular and nuclear size in leukoplakia has shown it to be more prone for malignant change, and therefore, their measurements may provide an objective means for the assessment of epithelial dysplasia and to predict their malignant potential. Techniques of image analysis offer an opportunity to quantify the nuclear and cell changes associated with malignancy and provide an objective basis for grading dysplasia and tumors. Overall, these results have shown that the quantitative histomorphometric techniques can detect features that may be overlooked by routine histological examination.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Scully C. Oncogenes, onco-suppressors, carcinogenesis and oral cancer. Br Dent J. 1992;173:53–9. doi: 10.1038/sj.bdj.4807936. [DOI] [PubMed] [Google Scholar]
- 2.Rajendran R. Oral leukoplakia (leukokeratosis): Compilation of facts and figures. J Oral Maxillofac Pathol. 2004;8:58–68. [Google Scholar]
- 3.Wright J, O’Brien JC. Premalignancy, Oral Cancer: Clinical and Pathological Considerations. In: Boca Raton., editor. Florida: CRC Press Inc; 1988. pp. 45–8. [Google Scholar]
- 4.Rajendran R, Sivapathasundharam B. 5th ed. Amsterdam: Elsvier; 2006. Shafer’s Text book of oral pathology. In Benign and malignant tumors of the oral cavity; pp. 121–30. [Google Scholar]
- 5.Neville BW, Damm DD, Allen CM, Bouquot JE. 2nd ed. Philadelphia, PA: Saunders; 2002. Oral and maxillofacial pathology; pp. 337–69. [Google Scholar]
- 6.Scully C, Sudbe J, Speight PM. Progress in determining the malignant potential of oral lesions. J Oral Pathol Med. 2003;32:251–6. doi: 10.1034/j.1600-0714.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 7.Warnakulasuriya S. Lack of molecular markers to predict malignant potential of oral pre-cancer. J Pathol. 2000;190:407–9. doi: 10.1002/(SICI)1096-9896(200003)190:4<407::AID-PATH546>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 8.Warnakulasuriya S. Histological grading of oral epithelial dysplasia: Revisited. J Pathol. 2001;194:294–7. doi: 10.1002/1096-9896(200107)194:3<294::AID-PATH911>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 9.Kramer IR, Lucas RB, EL Labban N, Lister LA. Computer-aided study on tissue changes on Oral keratosis and lichen planus and a analysis of care groupings by subjective and objective criteria. Br J Cancer. 1970;24:407–26. doi: 10.1038/bjc.1970.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pindborg JJ, Reibel J, Holmstrup P. Subjectivity in evaluating oral epithelial dysplasia, carcinoma in situ and initial carcinoma. J Oral Pathol. 1985;14:698–708. doi: 10.1111/j.1600-0714.1985.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 11.Abbey LM, Kaugars GE, Gunsolley JC, Burns JC, Page DG. Intraexaminer and interexaminer reliability in the diagnosis of oral epithelial dysplasia. Oral Surg Oral Med Oral Pathol Radiol Endod. 1995;80:189–91. doi: 10.1016/s1079-2104(05)80201-x. [DOI] [PubMed] [Google Scholar]
- 12.Karabulut A, Reibel J, Therkildoen MH, Practorius J, Nielsen HW. Observer variability in the histologic assessment of oral premalignant lesions. J Oral Pathol Med. 1995;24:198–200. doi: 10.1111/j.1600-0714.1995.tb01166.x. [DOI] [PubMed] [Google Scholar]
- 13.Brothwell DJ, Lewis DW, Bradley G, Leong I, Jordan RC. Observer agreement in the grading of oral epithelial dysplasia. Community Dent Oral Epidemiol. 2003;31:300–5. doi: 10.1034/j.1600-0528.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- 14.Shabana AH, Labban NG, Lee KW. Morphometric analysis of basal cell layer in oral premalignant white lesions and squamous cell carcinoma. J Clin Pathol. 1987;40:454–8. doi: 10.1136/jcp.40.4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore SR, Johnson NW, Pierce AM, Wilson DF. The epidemiology of mouth cancer: A review of global incidence. Oral Dis. 2000;6:65–74. doi: 10.1111/j.1601-0825.2000.tb00104.x. [DOI] [PubMed] [Google Scholar]
- 16.Subdo J, Reith A. Which putatively pre-malignant oral lesions become oral cancers? J Oral Pathol Med. 2003;32:63–70. doi: 10.1034/j.1600-0714.2003.00054.x. [DOI] [PubMed] [Google Scholar]
- 17.Boysen M, Rieth A. A morphometric model for light microscopic analysis of metaplastic, dysplastic and carcinomatous alterations of the nasal mucosa in nickel workers. Pathol Res Pract. 1980;166:362–71. doi: 10.1016/S0344-0338(80)80140-3. [DOI] [PubMed] [Google Scholar]
- 18.Eveson JW, MacDonald DG. Quantitative histological changes during early experimental carcinogenesis in the hamster cheek pouch. Br J Dermatol. 1978;98:639–44. doi: 10.1111/j.1365-2133.1978.tb03582.x. [DOI] [PubMed] [Google Scholar]
- 19.Eveson JW, Mac Donald DG. Hamster tongue carcinogenesis.Quantitative morphologic aspects of preneoplastic epithelium. J Oral Pathol. 1981;10:332–41. doi: 10.1111/j.1600-0714.1981.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 20.Saku T, Sato E. Prediction of malignant change in oral precancerous lesions by DNA cytofluorometry. J Oral Pathol. 1983;12:90–102. [Google Scholar]
- 21.Cowpe JG, Longmore RB, Green MW. Quantitative exfoliative cytology of abnormal oral mucosal smears. J R Soc Med. 1988;81:509–13. doi: 10.1177/014107688808100905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cowpe JG, Longmore RB, Green MW. Quantitative exfoliative cytologyof normal oral squames: An age, site and sex related survey. J R Soc Med. 1985;78:995–1004. doi: 10.1177/014107688507801204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Truelson JM. DNA content and histologic growth pattern correlate with prognosis in patients with advanced squamous cell carcinoma of the larynx. Cancer. 1992;70:56–62. doi: 10.1002/1097-0142(19920701)70:1<56::aid-cncr2820700110>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 24.Jin Y, Yang LJ, White FH. Preliminary assessment of the epithelial nuclear-cytoplasmic ratio and nuclear volume density in human palatal lesions. J Oral Pathol Med. 1995;24:261–5. doi: 10.1111/j.1600-0714.1995.tb01179.x. [DOI] [PubMed] [Google Scholar]
- 25.Ramaesh T, Mendis BR, Ratnatunga N, Thattil RO. Cytomorphotometric analysis of squames obtained from normal oral mucosa and lesions of oral leukoplakia and squamous cell carcinoma. J Oral Pathol Med. 1998;27:83–6. doi: 10.1111/j.1600-0714.1998.tb02099.x. [DOI] [PubMed] [Google Scholar]
- 26.Ramaesh T, Mendis BR, Ratnatunga N, Thattil RO. Diagnosis of oral premalignant and malignant lesions using cytomorphometry. Odontostomatol Trop. 1999;22:23–8. [PubMed] [Google Scholar]
- 27.Mollaoglu N, Cowpe JG, Potts AJ. Sensitivity of Seescan TV image analysis in discriminating between normal, dysplastic and malignant oral smears. Anal Quant Cytol Histol. 2000;22:218–22. [PubMed] [Google Scholar]
- 28.Pektas Z, Keskin A, Günhan O, Karsliogğlu Y. Evaluation of Nuclear Morphometry and DNA Ploidy Status for Detection of Malignant and Premalignant Oral Lesions: Quantitative Cytologic Assessment and Review of Methods for Cytomorphometric Measurements. J Oral Maxillofac Surg. 2006;64:628–35. doi: 10.1016/j.joms.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Natarajan S, Mahajan S, Boaz K. and Thomas George: Morphometric Analysis of Nuclear Features and Volume-Corrected Mitotic Index in the Prognosis of Oral Squamous Cell Carcinoma. Oral Sci Int. 2009;6:85–94. [Google Scholar]


