Abstract
Planarians are flatworms that constitutively maintain adult tissues through cell turnover and can regenerate entire organisms from tiny body fragments. In addition to requiring new cells (from neoblasts), these feats require mechanisms that specify tissue identity in the adult. Critical roles for Wnt and BMP signaling in regeneration and maintenance of the body axes have been uncovered, among other regulatory factors. Available data indicate that genes involved in positional identity regulation at key embryonic stages in other animals display persisting regionalized expression in adult planarians. These expression patterns suggest that a constitutively active gene expression map exists for maintenance of the planarian body. Planarians therefore present a fertile ground for identification of factors regulating regionalization of the metazoan body plan and for study of the attributes of these factors that can lead to maintenance and regeneration of adult tissues.
Adult developmental biology
Tissue turnover and regeneration are widespread features of adult biology. Many questions can be asked about these adult processes that are conceptually similar to important questions for embryonic development. Where and what is the information for polarizing and regionalizing newly forming tissues? What guides differentiation paths? What is the source of cells for particular differentiated cell types? How are tissues formed in the right scale and proportions? For embryonic development we can hope to connect the dots from gametogenesis and fertilization to the formation of the body plan in a temporal series of events. For tissue maintenance, a different situation is encountered in which new cells are continuously integrated into an already developed body plan (or regionalized tissue) to replace aged differentiated cells. During this process tissue pattern must be maintained rather than developed de novo. How pattern is maintained long-term and propagated through the generations of cells involved in tissue turnover is poorly understood. In addition to tissue maintenance, missing tissues can be restored following injury in many animals [1]. In these cases, the body plan or any patterned tissue/organ, already exists at the time of injury. Programs must exist for re-development of the missing regions/parts following a limitless number of injury types and, unlike the case for embryonic development, with very different physical starting points for the process. How regeneration tailors new tissue production to match the identity and scale of missing tissues is largely mysterious.
In this review I focus on emerging data related to tissue identity determination in tissue maintenance and regeneration from planarians. A hypothesis that emerges from available data is that constitutive regional expression of developmental control genes provides a blueprint for maintenance -and perhaps in part, regeneration- of body parts.
Planarians as a model for study of tissue identity specification in adult biology
Planarians are famous for the ability to regenerate missing body parts [2] and can regenerate from tiny fragments containing roughly 10,000 or more cells [3]. Planarians are freshwater triclad (possessing an intestine with three main branches) flatworm species [4]. These animals have a complex internal anatomy with dozens of cell types organized in patterns along body axes (Figure 1A). Planarians have eyes, a brain with two cephalic ganglia, a diverse array of regionalized neuron types, musculature for turning, epidermis with ventral motile cilia for locomotion, a multibranched intestine (the gastrovascular system), protonephridia for waste removal, a centrally localized pharynx for feeding and defecation, and many other cell types [5]. Planarians maintain and regenerate, with exquisite control of proportions, all of these cell types, organs, and tissue patterns [2].
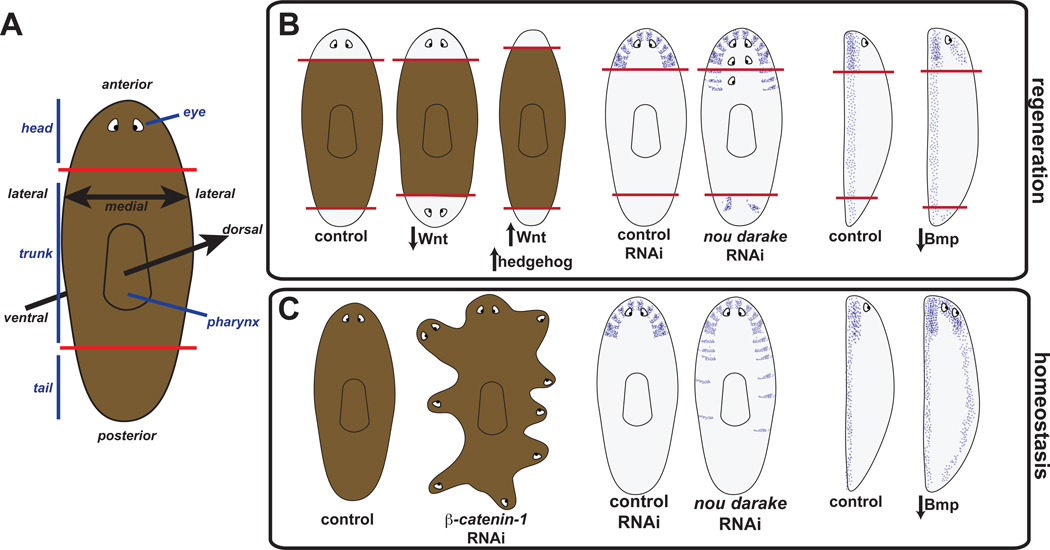
Figure 1. RNAi studies have identified genes required for guiding tissue identity in regeneration and tissue turnover in adult planarians.
(A) Schematic of planarian depicting visible body regions and animal axes. Red lines indicate amputation planes approximately utilized to generate animals cartooned in part B. (B) Left, decrease in Wnt signaling components, such as following RNAi of β-catenin-1, cause regeneration of two heads whereas increase in Wnt signaling (with APC RNAi) or increase in Hedgehog signaling (with patched RNAi) cause regeneration of two tails [41–44, 96, 97]. Middle and right, schematized phenotypes are assessed with anatomical markers using in situ hybridizations. Middle, nou darake RNAi causes regeneration of ectopic brain cells (purple) and eyes (adapted from [52]). Right, decrease in BMP signaling causes ventralization of regeneration blastemas (normally ventral neurons are depicted in purple) [56–58]. Side views are depicted with ventral to the left. Red lines indicate amputation planes, white areas depict regeneration blastemas and pigmented areas depict pre-existing tissues. (C) Left, β-catenin-1 RNAi causes intact animals to develop ectopic heads around the periphery. Middle, ectopic brain cells appear progressively more posterior in intact nou darake(RNAi) animals. Right, inhibition of BMP signaling components with RNAi causes intact animals to develop normally ventral structures (such as brain and nerve cords, purple) on their dorsal sides (see part B for references).
As members of the phylum Platyhelminthes, planarians are representatives of the Lophotrochozoa, a sister clade to the Ecdysozoa, which include arthropods (such as Drosophila melanogaster) and nematodes (such as Caenorhabditis elegans) [6]. Most animals with long life require mechanisms for extensive cell replacement and in some cases regrowth of missing body parts. In adult C. elegans and Drosophila, which are animals with short generation times, these aspects of adult life are either absent or limited to a particular tissue [7–9]. By contrast, the planarian Schmidtea mediterranea is highly suited for study of repair and replenishment of many tissues, processes fundamental to adult life in many animals [10].
Planarian regeneration involves new tissue outgrowth at the wound site (the blastema) [2]. However, small fragments do not produce all missing tissues, at original scale, by outgrowth at the wound. Indeed this could not be possible, because a fragment without mouth or brain must accomplish regeneration with existing materials and energy. Planarian regeneration, instead, is accomplished by a combination of blastema formation and changes in pre-existing tissues (often referred to as morphallaxis) [2, 11–13]. A critical source of new planarian tissues is a proliferative somatic cell population called neoblasts, which are of interest for molecular study of stem cell biology. Uninjured planarians undergo constant turnover of tissues, with aged differentiated cells replaced by neoblast progeny [2, 13–15]. Planarians take tissue turnover to the extreme, as they can grow and shrink depending upon nutrient status, involving cell number changes [16, 17]. The term adult could be deemed confusing for such an animal. In this review, the adult processes under discussion occur in animals that have developed the final and full complement of differentiated cell types, in proper proportions, and that are capable of reproduction.
Recent developments with molecular methods have helped make planarians a powerful molecular genetic system for studying tissue turnover and regeneration. For example, RNA interference (RNAi) methods work remarkably well for inhibiting planarian gene expression [18, 19] and can be used in screens [20]; labeling sites of gene expression with in situ hybridizations is robust [21, 22]; and the neoblasts can be labeled with BrdU, antibodies, or RNA probes [14, 20, 23], isolated using flow cytometry [24], and their differentiation can be assessed [14, 23, 25–27]. These emerging attributes of planarians as an experimental system have been reviewed elsewhere [10, 28, 29]. I also direct readers to other reviews that focus on the potential of planarians for stem cell research (not discussed here) [2, 30–32], as well as other reviews on patterning and regeneration [33, 34] as resources.
RNAi phenotypes and the specification of adult tissue identity
With complete genome sequence and well-developed RNAi methods in hand, it has become possible to identify numerous planarian phenotypes. Genes described in this review were identified using RNAi screening and study of genes homologous to developmental control genes from other organisms. Numerous RNAi phenotypes affect the nuts and bolts of the regeneration process, such as the action of neoblasts [20, 23, 26, 27, 32, 35–39]. Here, by contrast, I highlight genes that are not required for regeneration per se (regeneration in general or for regeneration of individual cell types), but that are required for specification of regional tissue identity in regeneration. These genes are associated with dramatic RNAi phenotypes including homeotic-like changes (replacement of one body part/region with that of another), aberrations in regionalized regeneration of a tissue type with respect to others, or failed regeneration of only certain body regions (Figure 1B). Many of these genes also have roles in maintenance, in addition to regeneration, of regional tissue identity that can be revealed under long-term RNAi conditions (in some cases following months of tissue turnover). Analysis of these genes reveals constitutive regional gene expression domains described below that provide spatial information guiding tissue replenishment.
Regeneration and maintenance of the anteroposterior axis
The primary body axis is polarized from head-to-tail and is commonly referred to as the anteroposterior (AP) axis. Planarians can regenerate an entire AP axis from nearly any body fragment, providing a robust assay for genes required for primary body axis regeneration and regionalization.
Head-versus-tail planarian tissue identity
A head or a tail can be regenerated at any transverse amputation plane, depending upon whether the wound site is anterior- or posterior-facing. How is the correct regeneration decision made (head versus tail)? This process, referred to as regeneration polarity, has been a classic problem for over a century [11, 40]. RNAi of the Wnt-signaling component β-catenin-1, followed by head and tail amputation, results in the dramatic regeneration phenotype of two-headed animals [41–43] (Figure 1B left). Similarly, RNAi of the Wnt gene wnt1 (previously named wntP-1, see Figure 2 legend) results in regeneration of two-headed animals [44, 45], a defect enhanced by inhibition of wntP-2 [45] (with proposed name wnt11-5 [46], see Figure 2 legend). Consistent with these data, RNAi of wntless, which encodes a protein required for Wnt secretion [47–49], results in regeneration of two-headed planarians [44]. By contrast, inhibition of the APC gene, which encodes a β-catenin inhibitor, results in the opposite phenotype of regeneration of two tails [42] (Figure 1B). β-catenin-1 inhibition in intact planarians undergoing tissue turnover results in a striking body plan transformation with heads appearing around the body periphery [41–43] (Figure 1C). These RNAi animals lose a body axis, nearing radial symmetry about an intact dorsal-ventral axis. These data suggest that a switch-like process occurs at wounds, with Wnt signaling promoting tail regeneration and absence of Wnt signaling leading to head regeneration, and that Wnt signaling also promotes maintenance of the polarized primary axis during tissue turnover.
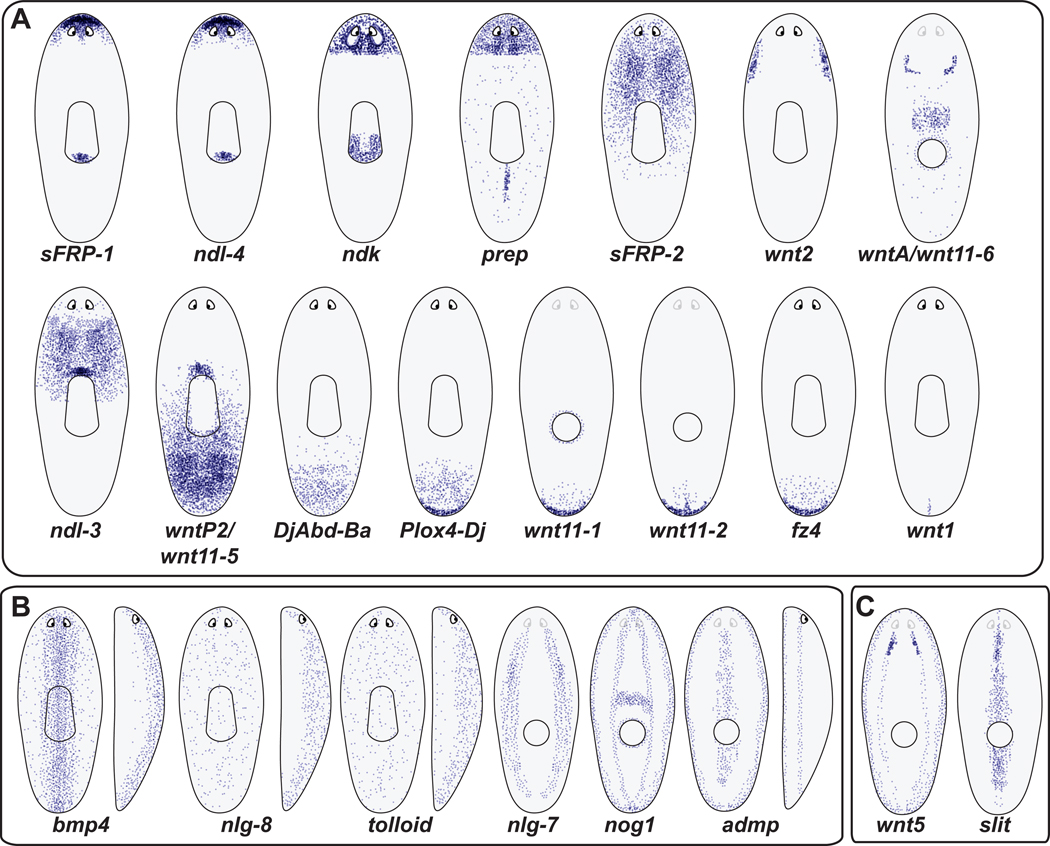
Figure 2. A persistent, adult gene expression map regulates maintenance and regeneration of the planarian body plan.
Cartoon depiction of gene expression domains (purple spots, individual cells) for genes expressed in a regional manner. These genes are selected candidate position-regulatory factors, for which the expression domain is simply regional, as opposed to matching the distribution of a known differentiated cell type. Patterns are approximations based upon primary data referenced within the body text (see text for details and references). (A) Gene expression domains distributed along the anteroposterior axis. Patterns are roughly arranged from most anterior (top, left) to most posterior (bottom, right). Some animals are displayed with a dorsal view (entire pharynx depicted as a central, oblong structure). Other animals are depicted from a ventral view, with the mouth visible as a circle and the eyes lighter. Wnt names are challenging because of difficulty with orthology assessment [41, 46, 104]: wnt1 is former wntP-1; wnt2 was previously named as wnt2-1; orthology for several wnt genes is still as yet unresolved: wnt11-2, wntP-4 (wnt11-3), wntP-2 (wnt11-5), and wntA (wnt11-6). (B) Gene expression domains that display strong dorsal-ventral asymmetry are depicted. In addition to dorsal and ventral views, some side views are provided. In these instances ventral is to the left and dorsal is to the right (recognized with an eye depicted anterior, right). C. Gene expression domains that are polarized mediolaterally. wnt5 expression is also largely ventral.
Numerous other genes must be involved in AP axis regeneration, but few additional phenotypes have been described. The planarian prep gene is required for normal head regeneration, providing one example [50]. prep encodes a TALE-class homeodomain protein; these proteins can act as co-factors for Hox proteins and have other roles [51]. prep RNAi anterior blastemas do not produce cephalic ganglia and fail to express normal anterior makers (but do not become tails). This phenotype suggests that Prep has a role in promoting anterior tissue identity during head regeneration.
Establishment of axis pattern by restriction of a tissue domain within the AP axis
Study of the planarian nou darake gene revealed another phenotype class that implicates the gene in adult tissue/body-plan regulation. In this case, the regionalization of only particular tissues is affected. nou darake (ndk - "brains everywhere" in japanese) encodes an FGF-receptor-like protein homologous to vertebrate FGFRL proteins [52]. Unlike normal FGF receptors, Nou Darake and FGFRL proteins lack a cytoplasmic kinase domain [53]; available data suggest Nou Darake inhibits FGF signaling [52]. nou darake RNAi causes the striking phenotype of posterior brain expansion during regeneration and homeostatic tissue turnover in the adult (ectopic brain cells and eyes extend from the head into more posterior regions) [52] (Figure 1B–C). Not all head cells were expanded in these RNAi animals, indicating a tissue-specific effect. How nou darake acts mechanistically, within the pattern of the AP axis, to accomplish proper brain regionalization awaits elucidation. As was the case for Wnt signaling, study of nou darake reveals a process acting in regeneration and during tissue maintenance.
Regeneration and maintenance of the dorsal-ventral and mediolateral axes
The back-to-belly, or dorsal-ventral (DV), axis is orthogonal to the AP axis and present in animals with bilateral symmetry. Establishment of pattern along this axis frequently involves BMP signaling [54]. Medial-to-lateral tissue polarization (often referred to as the mediolateral (ML) axis) is also present in animals with bilateral symmetry.
BMP signaling and dorsal-ventral polarity
Bmp proteins are secreted signaling ligands that activate Smad proteins, which mediate transcriptional pathway output [55]. RNAi of BMP pathway genes bmp4, smad1, or smad4 leads to similar phenotypes involving ventralized, medially indented blastemas (ventral cell types such as neurons and motile cilia regenerate dorsally) [56–58] (Figure 1B right). The Bmp-family gene Admp promotes BMP signaling in vertebrates [59, 60], and a planarian admp gene is required for regeneration of cells at the midpoint between DV poles, and promotes bmp4 activity [61, 62]. BMP signaling can be inhibited by a variety of signaling antagonists, such as Noggin proteins. Planarian noggin-related genes [63, 64] are categorized as "noggin" (nog) or "noggin-like" (nlg) (nlg genes possess an insertion in the noggin domain-encoding region) [64]. Simultaneous inhibition of nog1 and nog2 results in a dorsalized phenotype [61]. Together, these results indicate BMP signaling promotes dorsal and inhibits ventral tissue regeneration.
RNAi of BMP signaling components in intact animals leads to a dramatic transformation of the body plan with ventral cell types (such as neurons and motile cilia) steadily appearing dorsally [56, 57] (Figure 1C). These animals are able ultimately to glide on their backs or bellies and have a near radial symmetry, with loss of the dorsoventral axis and maintenance of an anteroposterior axis. These data indicate that polarity of the two orthogonal body axes (AP and DV) are constitutively maintained by conserved signaling pathways (Wnt for AP and BMP for DV) and that regeneration of axis polarity requires these axis-maintenance pathways.
Maintenance and regeneration of mediolateral tissue pattern
When BMP pathway components are inhibited and animals are amputated sagittally, regeneration completely fails [56, 57]. Therefore, genes involved in DV tissue polarization are also required for lateral regeneration for poorly understood reasons. RNAi studies have also implicated additional genes (slit, wnt5, and admp) in regeneration and maintenance of ML tissue pattern. Slit is an extracellular, leucine-rich-repeat protein that mediates midline repulsion of axons during nervous system development in many animals [43, 65]. In planarians, slit RNAi results in medial collapse of nervous system and intestine during regeneration [66]; slit maintains existing ML pattern as well [66]. RNAi of planarian wnt5 (homologous to Wnts that mediate non-canonical Wnt signaling) results in nervous system thickening [44] and can cause appearance of ectopic pharynges laterally [46]. wnt5 RNAi [46] and admp RNAi [62] cause ectopic lateral expression of slit, and slit RNAi causes expansion of wnt5 expression into medial regions [46]. Together these observations indicate roles for BMP signaling, Wnt5, and Slit in maintenance and regeneration of ML pattern. The AP, DV, and ML phenotypes described indicate that signaling pathways affect very broad body axis regions in planarians to provide regulatory control of regeneration and tissue turnover.
A regionalized expression map for the body plan persists in adult planarians
The phenotypes described above identify key factors that guide regeneration and tissue turnover. Many of these genes are expressed during normal adult life (Figure 2). A central thesis of this review is that these expression patterns reveal a molecular blueprint for the body plan that persists in the adult, described below (Figure 3). The genes described are expressed in discrete body-axis regions, and in some cases mRNA-expression levels are graded with position [34]. Many of these expression patterns are regional in dispersed subepidermal cells as opposed to being regional simply by occurring within a known localized organ system. For some genes, regional expression corresponds to very large body domains whereas other genes are restricted in expression to a few cells. A number of these genes have not been directly implicated in tissue-pattern maintenance with a homeostasis RNAi phenotype, but are presented here because of homology and expression pattern.
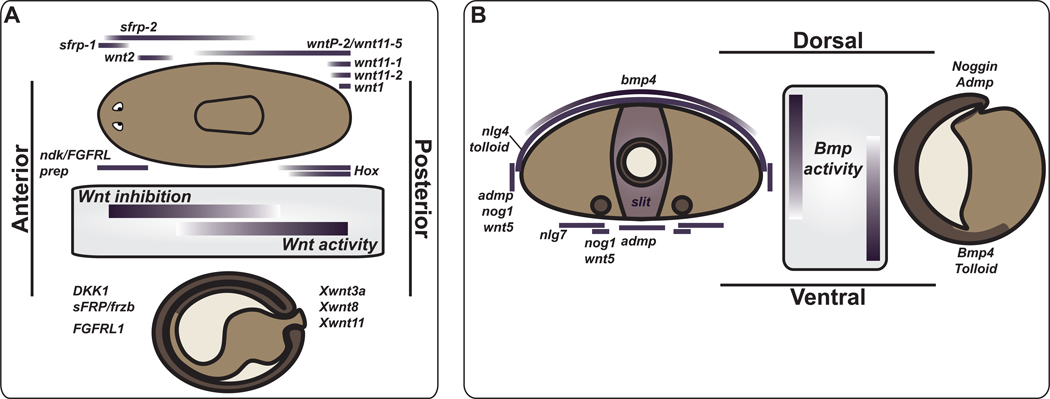
Figure 3. A regulatory map for metazoan body plans.
Comparison of gene expression related to the planarian body plan to body plan development during embryogenesis in Xenopus. (A) Wnt signaling in control of the primary, anteroposterior, axis and (B) BMP signaling in control of the dorsoventral axis (see text for references). (A) AP axis comparison between an intact planarian and neurula-stage Xenopus embryo. The planarian is depicted with a dorsal view, the Xenopus embryo is a sagittal view with dorsal up. Wnt inhibition in the anterior and Wnt expression in the posterior are present in both cases. FGFRL expression in the head region is also observed, as is regional expression of Hox genes (see text). (B) BMP signaling is active on the dorsal side of planarians and on the prospective ventral side of Xenopus gastrula-stage embryos. A transverse section of a planarian is depicted with the central hole representing a gut branch and the ventral circles representing nerve cords. The Xenopus embryo is depicted as a sagittal section. Admp and Noggin expression spatially oppose the site of Bmp expression in both cases. A proposed axis inversion in the evolution of deuterostomes could explain the opposite orientation in protostomes and chordates.
Regionalized expression domains for the anteroposterior axis
Location of active Wnt signaling can be approximated by the location of expression of Wnt ligands or pathway antagonists. Assessment of nuclear β-catenin protein location provides a more direct pathway read-out, but has not yet been accomplished in planarians. There are nine identified planarian Wnt genes. The two Wnt genes implicated in regeneration polarity, wnt1 and wntP-2 (wnt11-5) are expressed in the posterior of intact planarians, with wnt1 expressed in only a few cells at the tail tip and wntP-2 (wnt11-5) expressed in a posterior-to-anterior gradient extending for the majority of the head-to-tail length of the animal [41] (Figure 2A).
Other Wnt genes have intriguing AP-regionalized expression domains as well (wnt2: laterally in the anterior; wntA/wnt11-6: posterior end of the cephalic ganglia; wnt11-1 and wnt11-2: at the posterior pole) [41, 46] (Figure 2A). Frizzled proteins are Wnt receptors, and the frizzled-4 (fz4) gene is also expressed at the posterior pole (Figure 2A). There are three candidate Wnt signaling antagonists described, sFRP-1, sFRP-2, and sFRP-3 [41, 42, 46]. sFRP-1 is expressed at the anterior planarian pole [41, 42]. sFRP-2, while absent from the anterior-most region, is expressed in an anterior-to-posterior gradient that extends for half or more of the length of the animal [46] (Figure 2A). sFRP-3 is expressed in the head and broadly along the primary axis [46]. Overall, these expression patterns are most consistent with varying degrees of Wnt signaling activity existing along the AP axis, with posterior activity and anterior inhibition (Figure 3A).
Additional regional expression domains along the AP axis have been revealed from examination of multiple nou darake-like genes, Hox genes, and the prep gene (Figure 2A). nou darake expression domains suggest anterior restriction of FGF signaling [52, 67]. prep is expressed in animal heads, with weaker expression elsewhere [50]. Finally, at least some Hox genes, which are famous for regional AP expression across the animal kingdom [68], are expressed regionally in adult planarians, emanating from the posterior [69, 70]. Taken together, described expression patterns reveal a complex, spatially overlapping set of gene expression domains, defining possible regulatory regions of the AP axis (Figures 2A, 3A).
Expression domains for the dorsoventral and mediolateral axes
The BMP-pathway signaling ligand bmp4 is expressed on the dorsal side of animals, strongest medially and extending laterally in a graded fashion, which should approximate the site of pathway activity [71] (Figure 2B). Other BMP pathway components, such as admp, tolloid, noggin, and noggin-like genes, are expressed in unique DV-polarized domains [56, 61, 62, 64] (Figure 2B). Many expression domains that are DV-polarized also occur in different ML positions and additional ML-polarized gene expression domains have also been identified (Figure 2B, C). slit is expressed medially, throughout the dorsal-ventral axis, consistent with its role in mediolateral patterning [66] (Figure 2C). admp displays a medial expression domain on the ventral side [61, 62] and the nlg7 gene is expressed in two ventral domains flanking the ventral medial-most zone [72] (Figure 2B). The genes wnt2, wnt5, admp, and nog1 have lateral expression domains (in addition to other expression locations) [44, 61, 62, 72] (Figure 2A–C). Overall, a diversity of broad DV and ML-polarized, constitutively active, expression domains have been revealed (Figures 2B, C and 3B), with BMP signaling appearing maximally active at the dorsal midline.
Comparison of the adult planarian gene expression map to embryogenesis in other organisms
Embryos pass through key stages during which regionalized gene expression controls development of differentiated cells in proper positions [73–76]. Establishment of the body plan during embryogenesis occurs in many stages and study of body plan regulation can therefore be challenging. For example, some genes have early roles in an embryo that precede an axial patterning role; gene perturbation experiments can thus result in early defects that obscure later roles. Xenopus laevis β-catenin, for instance, promotes establishment of Spemann's organizer, which impacts DV-axis development, but later, β-catenin is important for AP-axis patterning [77, 78]. Furthermore, early embryonic events can vary dramatically in animals. However, despite numerous differences between early embryos in the Bilateria, it is clear that some prominent patterning similarities exist, most famously illustrated by the regional action of Hox genes [68]. Planarians, with constant, easy to visualize gene expression in the adult therefore present a straightforward and attractive venue for identification and study of conserved factors that regulate metazoan body plans.
The constitutive expression domains of position-regulatory genes in planarians bear striking resemblance to expression domains of homologous genes at key embryonic stages in other organisms [75, 79, 80]. Wnt signaling in the posterior and Wnt inhibition in the anterior has emerged as a prominent and widespread feature of primary body axis patterning in animals [79] (Figure 3A). For example, posterior Wnt-pathway activity and/or anterior pathway inhibition occurs during AP patterning of Xenopus neuroectoderm [75, 78, 81–83], in mouse embryos [82, 84], and in C. elegans [85–87], among numerous other examples. Although further investigations are needed, Hox genes are regionally expressed on the AP axis in planarians [69, 70], as is the case in most bilaterians. Finally, a Xenopus homolog of nou darake, XFGFRL1/Xndk is expressed at the early neurula stage in an anterior region across multiple germ layers, similar to the head expression of planarian nou darake (Figure 3A) [88].
Polarization of the dorsoventral axis by BMP signaling, as is observed in planarians, occurs in frogs, fish, flies, and other animals, and has emerged as one of the central tenets of animal development (Figure 3B) [75, 89–91]. The opposite orientation of Bmp activity (Bmp active on the dorsal side in Drosophila and planarians, or on the ventral side in vertebrates) reflects a probable dorsal-ventral axis inversion that occurred during the evolution of the deuterostomes [54]. Furthermore, the expression of planarian noggin and admp genes, spatially opposing the location of bmp and tolloid expression, is reminiscent of vertebrate axis molecular architecture (Figure 3B) [59, 60, 77]. The similarities in gene expression in adult planarians and during embryogenesis in other organisms identify candidate gene expression domains underlying body plans in a large number of animals; similar roles for these genes in body plan development were possibly present in the ancestor of the Bilateria [75, 79, 80]. Prominent in this proposal is the BMP system for the DV axis and Wnt signaling for the AP axis (Figure 3A, B).
Regeneration: specification of tissue identity and restoration of tissue pattern
Planarian regeneration is confronted with essentially unlimited potential "starting points. " A reasonable hypothesis is that common regeneration programs exist that can handle the diversity of injuries that occur. Identifying and understanding such programs is a major challenge of regeneration research. Neoblasts in the tail can regenerate head cells and vice versa, consistent with regional information for regeneration being housed outside of the neoblasts. Regenerative cells (neoblasts and neoblast progeny cells) therefore probably interpret surroundings, such as the regionally expressed patternregulatory factors described here, for regeneration decisions.
Does regeneration act by simply re-scaling existing activity gradients to fit the dimensions of a now smaller tissue fragment, by regeneration-specific programs, or by a combination of both types of processes [45, 92, 93]? One example that has conceptual similarities to adult regeneration involves study of embryonic regulation in Xenopus, in which dorsal halves of bisected embryos produce a normal frog. This process is proposed to involve re-scaling of BMP-signaling expression domains involving inherent BMP-signaling circuitry attributes, without need to invoke any regeneration-specific components [60, 77, 93–95]. Unexpectedly, however, the wnt1 gene (normally expressed at the posterior pole) is induced at essentially all planarian wound types [45]. wnt1 expression is required for initiation of a posterior-specific gene expression program [45]. These observations suggest that wounds have input into re-setting the AP axis and that the programs that result in body plan regeneration may involve regeneration-specific steps.
Additional conditions cause regeneration of two heads or tails and should prove to be important for understanding axis regeneration. Hedgehog (Hh) signaling promotes planarian tail regeneration [96–98] (Figure 1B), and can impact wnt1-expression levels at wounds [96, 97]. No homeostatic AP axis regionalization phenotype has been reported for Hh pathway perturbation. It will therefore be intriguing to investigate a candidate regeneration-specific role for Hh signaling. Simultaneous inhibition of three innexin genes in the planarian Dugesia japonica results in the regeneration of heads in place of tails, suggesting a gap junction-mediated process is important in regeneration polarity [99]. Finally, modulation of Ca2+ influx also can disrupt regeneration polarity [100].
Numerous other genes display expression changes during regeneration that, in contrast to the wound-site expression for wnt1, roughly reflect their expression domains in intact animals. For example, wntP-2, sFRP-1, and Hox genes all turn on with time at only posterior-facing or anterior-facing wounds during regeneration, similar (in orientation) to their normal polarized expression in homeostasis [41, 42, 44–46, 69, 70]. Aspects of the gene expression program occurring at posterior-facing wounds can run, at least in part, in the absence of neoblasts, such as wnt1 wound-site expression [45], wntP-2 (wnt11-5) expression at posterior-facing wounds [45], and sFRP-1, wnt2, and sFRP-2 expression at anterior-facing wounds [46]. This indicates that wound-site expression changes occur in differentiated tissues and, in part, independently from blastema formation. Following injury, it is not always the case that regional expression for a gene is missing. By contrast, some expression domains remain but out of proportion for the size of the new fragment and rapid re-scaling occurs. For example, wntP-2 expression in amputated tail fragments rapidly shrinks to the tail tip [45], with differentiated tissues becoming present in new signaling environments as a consequence of amputation [46] being a potential factor for morphallaxis.
BMP signaling provides a second case study for examination of regional expression domain regeneration. Following parasagittal amputation, lateral fragments induce bmp4 expression, which ultimately resolves to a new midline in pre-existing tissue [56, 71]. Induction of bmp4 expression in these fragments occurs even in the absence of neoblasts, indicating (as is the case for early AP regeneration) that changes in expression domains of DV regulatory factors can occur independently from blastema formation [56]. How bmp4 expression is induced and resolves to a new midline remains unknown. Additional mysteries abound; for example, admp expression initially abates near wounds and later returns [62].
These data represent the beginning of investigation into programs of sequential gene expression acting at wounds for regeneration. The re-establishment of proper expression domain scale and position during regeneration, for the myriad of factors with normal regionalized expression (Figure 2), will be a fascinating process to investigate. It will also be important to determine whether genes exist that are expressed only transiently during regeneration with important roles in formation of pattern, similar to transient steps in embryogenesis. In addition, some genes could prove to have one role during homeostatic maintenance of the body plan and a different role during regeneration.
Concluding remarks and future perspectives
Identifying and understanding the functions of regulatory factors that specify position in the metazoan body plan is one of the most fundamental goals of developmental biology. Regionalized gene expression domains in adult planarians present a static snapshot of the body plan, with striking similarities to the expression positions of important regulatory factors in many animals (Figure 3A, B). Because of this attribute, key factors that regulate positional tissue identity in the metazoan body plan could continue to be identified by studying planarian tissue maintenance and regeneration.
Planarians present the opportunity to study the key properties of the regulatory circuitry involved in regional specification that make this particular circuitry so successful for allowing tissue turnover and regeneration. First, expression domains in adult planarian tissues must be stable, in contrast to embryos, where development-regulatory factors can be transiently expressed. Second, gene expression domains can change in scale, while roughly maintaining proportions, as is seen during growth or de-growth. Characterizing the mechanisms that allow the adult gene expression map to scale with body size could be relevant to understanding how similar genes act in embryogenesis. For example, some embryos have changed size during the evolution of closely related species, or, for some species, embryos grow during development. Furthermore, regions of cells can expand or move in embryogenesis. In these cases, regulatory systems must be capable of scaling to accommodate changing dimensions [93, 101]. Some patterning mechanisms may prove to be adept at this task. Finally, there exists in planarians the capacity to regenerate the patterns of gene expression. Naturally, investigation of this process is of interest for understanding regeneration. In addition, understanding pattern regeneration in adult planarians may also prove relevant for understanding the remarkable ability of embryos to develop normally in the face of physical or environmental perturbation [102].
Adult tissue replenishment is important for life in most animals, and some animals have robust regenerative abilities. Whether constitutive expression of developmental control genes occurs and promotes tissue maintenance and regeneration in other adult animals is underexplored. Hox genes are continuously regionally expressed in vertebrate skin, presumably for maintaining skin positional information [103]. It will be important to more thoroughly investigate the adult expression domains of position-regulatory factors in regenerative and non-regenerative tissues in multiple organisms. Continued study of the identity and attributes of genes constitutively expressed in adult planarians should prove important to understanding in general how animals maintain and regenerate adult tissues.
Acknowledgements
I thank the Reddien Lab for comments and discussion, and Tom DiCesare and Irving Wang for illustration assistance. P.W.R. is an early career scientist of the Howard Hughes Medical Institute, and acknowledges support by NIH R01GM080639, ACS RSG-07-180-01-DDC, and the Keck Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sánchez Alvarado A. Regeneration in the Metazoans: Why does it happen? BioEssays. 2000;22:578–590. doi: 10.1002/(SICI)1521-1878(200006)22:6<578::AID-BIES11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 2.Reddien PW, Sánchez Alvarado A. Fundamentals of planarian regeneration. Ann. Rev. Cell Dev. Bio. 2004;20:725–757. doi: 10.1146/annurev.cellbio.20.010403.095114. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery J, Coward S. On the minimal size of a planarian capable of regeneration. Trans. Am. Microsc. Soc. 1974;93(3):386–391. [PubMed] [Google Scholar]
- 4.Egger B, Gschwentner R, Rieger R. Free-living flatworms under the knife: past and present. Dev Genes Evol. 2007;217(2):89–104. doi: 10.1007/s00427-006-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyman LH. The Invertebrates: Platyhelminthes and Rhynchocoela The acoelomate bilateria. Vol. II. New York: McGraw-Hill Book Company Inc.; 1951. [Google Scholar]
- 6.Adoutte A, et al. The new animal phylogeny: reliability and implications. Proc Natl Acad Sci USA. 2000;97(9):4453–4456. doi: 10.1073/pnas.97.9.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439(7075):470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 8.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439(7075):475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 9.Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4(1):49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sánchez Alvarado A. Planarian regeneration: its end is its beginning. Cell. 2006;124(2):241–245. doi: 10.1016/j.cell.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Morgan TH. Experimental studies of the regeneration of Planaria maculata. Arch. Entw. Mech. Org. 1898;7:364–397. [Google Scholar]
- 12.Agata K, et al. Intercalary regeneration in planarians. Dev Dyn. 2003;226(2):308–316. doi: 10.1002/dvdy.10249. [DOI] [PubMed] [Google Scholar]
- 13.Pellettieri J, et al. Cell death and tissue remodeling in planarian regeneration. Dev Biol. 2010;338(1):76–85. doi: 10.1016/j.ydbio.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newmark P, Sánchez Alvarado A. Bromodeoxyuridine specifically labels the regenerative stem cells of planarians. Dev. Biol. 2000;220(2):142–153. doi: 10.1006/dbio.2000.9645. [DOI] [PubMed] [Google Scholar]
- 15.Pellettieri J, Alvarado AS. Cell turnover and adult tissue homeostasis: from humans to planarians. Annu Rev Genet. 2007;41:83–105. doi: 10.1146/annurev.genet.41.110306.130244. [DOI] [PubMed] [Google Scholar]
- 16.Baguñà J, Romero R. Quantitative analysis of cell types during growth, degrowth and regeneration in the planarians Dugesia mediterranea and Dugesia tigrina. Hydrobiologia. 1981;84:181–194. [Google Scholar]
- 17.Oviedo NJ, Newmark PA, Sánchez Alvarado A. Allometric scaling and proportion regulation in the freshwater planarian Schmidtea mediterranea. Dev. Dyn. 2003;226(2):326–333. doi: 10.1002/dvdy.10228. [DOI] [PubMed] [Google Scholar]
- 18.Sánchez Alvarado A, Newmark PA. Double-stranded RNA specifically disrupts gene expression during planarian regeneration. Proc. Natl. Acad. Sci. 1999;96:5049–5054. doi: 10.1073/pnas.96.9.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newmark PA, et al. Ingestion of bacterially expressed double-stranded RNA inhibits gene expression in planarians. Proc. Natl. Acad. Sci. 2003;100:11861–11865. doi: 10.1073/pnas.1834205100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddien PW, et al. Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Dev. Cell. 2005;8(5):635–649. doi: 10.1016/j.devcel.2005.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Umesono Y, Watanabe K, Agata K. A planarian orthopedia homolog is specifically expressed in the branch region of both the mature and regenerating brain. Develop. Growth Differ. 1997;39:723–727. doi: 10.1046/j.1440-169x.1997.t01-5-00008.x. [DOI] [PubMed] [Google Scholar]
- 22.Pearson BJ, et al. Formaldehyde-based whole-mount in situ hybridization method for planarians. Dev Dyn. 2009;238(2):443–450. doi: 10.1002/dvdy.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo T, Peters AH, Newmark PA. A Bruno-like gene is required for stem cell maintenance in planarians. Dev Cell. 2006;11(2):159–169. doi: 10.1016/j.devcel.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi T, et al. Isolation of planarian X-ray-sensitive stem cells by fluorescence-activated cell sorting. Dev Growth Differ. 2006;48(6):371–380. doi: 10.1111/j.1440-169X.2006.00876.x. [DOI] [PubMed] [Google Scholar]
- 25.Eisenhoffer GT, Kang H, Sánchez Alvarado A. Molecular analysis of stem cells and their descendants during cell turnover and regeneration in the planarian Schmidtea mediterranea. Cell Stem Cell. 2008;3(3):327–339. doi: 10.1016/j.stem.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scimone ML, Meisel J, Reddien PW. The Mi-2-like Smed-CHD4 gene is required for stem cell differentiation in the planarian Schmidtea mediterranea. Development. 2010;137:1231–1241. doi: 10.1242/dev.042051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wenemoser D, Reddien PW. Planarian regeneration involves distinct stem cell responses to wounds and tissue absence. Dev Biol. 2010;344(2):979–991. doi: 10.1016/j.ydbio.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newmark PA, Sánchez Alvarado A. Not your father's planarian: a classic model enters the era of functional genomics. Nature Reviews Genetics. 2002;3:210–219. doi: 10.1038/nrg759. [DOI] [PubMed] [Google Scholar]
- 29.Saló E, et al. Planarian regeneration: achievements and future directions after 20 years of research. Int J Dev Biol. 2009;53(8–10):1317–1327. doi: 10.1387/ijdb.072414es. [DOI] [PubMed] [Google Scholar]
- 30.Aboobaker AA. Planarian stem cells: a simple paradigm for regeneration. Trends Cell Biol. 2011 doi: 10.1016/j.tcb.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Sánchez Alvarado A. Stem cells and the Planarian Schmidtea mediterranea. C R Biol. 2007;330(6–7):498–503. doi: 10.1016/j.crvi.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shibata N, Rouhana L, Agata K. Cellular and molecular dissection of pluripotent adult somatic stem cells in planarians. Dev Growth Differ. 2010;52(1):27–41. doi: 10.1111/j.1440-169X.2009.01155.x. [DOI] [PubMed] [Google Scholar]
- 33.Forsthoefel DJ, Newmark P. Emerging patterns in planarian regeneration. Current Opinion in Genetics and Development. 2009;19:412–420. doi: 10.1016/j.gde.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adell T, Cebria F, Salo E. Gradients in planarian regeneration and homeostasis. Cold Spring Harb Perspect Biol. 2010;2(1):a000505. doi: 10.1101/cshperspect.a000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reddien PW, et al. SMEDWI-2 is a PIWI-like protein that regulates planarian stem cells for regeneration and homeostasis. Science. 2005;310:1327–1330. doi: 10.1126/science.1116110. [DOI] [PubMed] [Google Scholar]
- 36.Pearson BJ, Sánchez Alvarado A. A planarian p53 homolog regulates proliferation and self-renewal in adult stem cell lineages. Development. 2010;137(2):213–221. doi: 10.1242/dev.044297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solana J, Lasko P, Romero R. Spoltud-1 is a chromatoid body component required for planarian long-term stem cell self-renewal. Dev Biol. 2009;328(2):410–421. doi: 10.1016/j.ydbio.2009.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oviedo NJ, Levin M. smedinx-11 is a planarian stem cell gap junction gene required for regeneration and homeostasis. Development. 2007;134(17):3121–3131. doi: 10.1242/dev.006635. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez-Taboada E, et al. Smed-SmB, a member of the LSm protein superfamily, is essential for chromatoid body organization and planarian stem cell proliferation. Development. 2010;137(7):1055–1065. doi: 10.1242/dev.042564. [DOI] [PubMed] [Google Scholar]
- 40.Morgan TH. "Polarity" considered as a phenomenon of gradation of materials. J. Exp. Zool. 1905;2:495–506. [Google Scholar]
- 41.Petersen CP, Reddien PW. Smed-betacatenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science. 2008;319(5861):327–330. doi: 10.1126/science.1149943. [DOI] [PubMed] [Google Scholar]
- 42.Gurley KA, Rink JC, Sánchez Alvarado A. Beta-catenin defines head versus tail identity during planarian regeneration and homeostasis. Science. 2008;319(5861):323–327. doi: 10.1126/science.1150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iglesias M, et al. Silencing of Smed-betacatenin1 generates radial-like hypercephalized planarians. Development. 2008;135(7):1215–1221. doi: 10.1242/dev.020289. [DOI] [PubMed] [Google Scholar]
- 44.Adell T, et al. Smed-Evi/Wntless is required for beta-catenin-dependent and -independent processes during planarian regeneration. Development. 2009;136(6):905–910. doi: 10.1242/dev.033761. [DOI] [PubMed] [Google Scholar]
- 45.Petersen CP, Reddien PW. A wound-induced Wnt expression program controls planarian regeneration polarity. Proc Natl Acad Sci U S A. 2009;106(40):17061–17066. doi: 10.1073/pnas.0906823106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gurley KA, et al. Expression of secreted Wnt pathway components reveals unexpected complexity of the planarian amputation response. Dev Biol. 2010;347(1):24–39. doi: 10.1016/j.ydbio.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Banziger C, et al. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125(3):509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 48.Bartscherer K, et al. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125(3):523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 49.Goodman RM, et al. Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development. 2006;133(24):4901–4911. doi: 10.1242/dev.02674. [DOI] [PubMed] [Google Scholar]
- 50.Felix DA, Aboobaker AA. The TALE class homeobox gene Smed-prep defines the anterior compartment for head regeneration. PLoS Genet. 2010;6(4):e1000915. doi: 10.1371/journal.pgen.1000915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moens CB, Selleri L. Hox cofactors in vertebrate development. Dev Biol. 2006;291(2):193–206. doi: 10.1016/j.ydbio.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 52.Cebrià F, et al. nou-darake, a novel gene related to FGF receptors is involved in restricting brain tissues to the head region of planarians. Nature. 2002;419(6907):620–624. doi: 10.1038/nature01042. [DOI] [PubMed] [Google Scholar]
- 53.Wiedermann M, Trueb B. Characterization of a novel protein (FGFRL1) from human cartilage related to FGF receptors. Genomics. 2000;69:275–279. doi: 10.1006/geno.2000.6332. [DOI] [PubMed] [Google Scholar]
- 54.DeRobertis E, Sasai Y. A common plan for dorsoventral patterning in Bilateria. Nature. 1996;380(6569):37–40. doi: 10.1038/380037a0. [DOI] [PubMed] [Google Scholar]
- 55.ten Dijke P, Hill CS. New insights into TGF-β-Smad signalling. Trends Biochem Sci. 2004;29(5):265–273. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 56.Reddien PW, et al. BMP signaling regulates the dorsal planarian midline and is needed for asymmetric regeneration. Development. 2007;134(22):4043–4051. doi: 10.1242/dev.007138. [DOI] [PubMed] [Google Scholar]
- 57.Molina MD, Saló E, Cebria F. The BMP pathway is essential for re-specification and maintenance of the dorsoventral axis in regenerating and intact planarians. Dev Biol. 2007;311(1):79–94. doi: 10.1016/j.ydbio.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 58.Orii H, Watanabe K. Bone morphogenetic protein is required for dorso-ventral patterning in the planarian Dugesia japonica. Dev Growth Differ. 2007;49(4):345–349. doi: 10.1111/j.1440-169X.2007.00931.x. [DOI] [PubMed] [Google Scholar]
- 59.Moos M, Jr, Wang S, Krinks M. Anti-dorsalizing morphogenetic protein is a novel TGF-beta homolog expressed in the Spemann organizer. Development. 1995;121(12):4293–4301. doi: 10.1242/dev.121.12.4293. [DOI] [PubMed] [Google Scholar]
- 60.Reversade B, De Robertis EM. Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell. 2005;123(6):1147–1160. doi: 10.1016/j.cell.2005.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Molina MD, et al. Noggin and noggin-like genes control dorsoventral axis regeneration in planarians. Curr Biol. 2011;21(4):300–305. doi: 10.1016/j.cub.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 62.Gavino MA, Reddien PW. A Bmp/Admp regulatory circuit controls maintenance and regeneration of dorsal-ventral polarity in planarians. Curr Biol. 2011;21(4):294–299. doi: 10.1016/j.cub.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ogawa K, et al. Induction of a noggin-like gene by ectopic DV interaction during planarian regeneration. Dev Biol. 2002;250(1):59–70. doi: 10.1006/dbio.2002.0790. [DOI] [PubMed] [Google Scholar]
- 64.Molina MD, Saló E, Cebria F. Expression pattern of the expanded noggin gene family in the planarian Schmidtea mediterranea. Gene Expr Patterns. 2009;9(4):246–253. doi: 10.1016/j.gep.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 65.Ypsilanti AR, Zagar Y, Chedotal A. Moving away from the midline: new developments for Slit and Robo. Development. 2010;137(12):1939–1952. doi: 10.1242/dev.044511. [DOI] [PubMed] [Google Scholar]
- 66.Cebria F, et al. Regeneration and maintenance of the planarian midline is regulated by a slit orthologue. Dev Biol. 2007;307(2):394–406. doi: 10.1016/j.ydbio.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rink JC, et al. Planarian Hh signaling regulates regeneration polarity and links Hh pathway evolution to cilia. Science. 2009;326(5958):1406–1410. doi: 10.1126/science.1178712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68(2):283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- 69.Orii H, et al. The planarian HOM/HOX homeobox genes (Plox) expressed along the anteroposterior axis. Dev Biol. 1999;210(2):456–456. doi: 10.1006/dbio.1999.9275. [DOI] [PubMed] [Google Scholar]
- 70.Nogi T, Watanabe K. Position-specific and non-colinear expression of the planarian posterior (Abdominal-B-like) gene. Dev Growth Differ. 2001;43(2):177–184. doi: 10.1046/j.1440-169x.2001.00564.x. [DOI] [PubMed] [Google Scholar]
- 71.Orii H, et al. Molecular cloning of bone morphogenetic protein (BMP) gene from the planarian Dugesia japonica. Zool. Science. 1998;15:871–877. [Google Scholar]
- 72.Molina MD, Salo E, Cebria F. The BMP pathway is essential for re-specification and maintenance of the dorsoventral axis in regenerating and intact planarians. Dev Biol. 2007;311(1):79–94. doi: 10.1016/j.ydbio.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 73.Wolpert L. Positional information and pattern formation in development. Dev Genet. 1994;15(6):485–490. doi: 10.1002/dvg.1020150607. [DOI] [PubMed] [Google Scholar]
- 74.Niehrs C. Regionally specific induction by the Spemann-Mangold organizer. Nat Rev Genet. 2004;5(6):425–434. doi: 10.1038/nrg1347. [DOI] [PubMed] [Google Scholar]
- 75.Niehrs C. On growth and form: a Cartesian coordinate system of Wnt and BMP signaling specifies bilaterian body axes. Development. 2010;137(6):845–857. doi: 10.1242/dev.039651. [DOI] [PubMed] [Google Scholar]
- 76.Wolpert L. Positional information and patterning revisited. J Theor Biol. 2011;269(1):359–365. doi: 10.1016/j.jtbi.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 77.De Robertis EM. Spemann's organizer and self-regulation in amphibian embryos. Nat Rev Mol Cell Biol. 2006;7(4):296–302. doi: 10.1038/nrm1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kiecker C, Niehrs C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development. 2001;128(21):4189–4201. doi: 10.1242/dev.128.21.4189. [DOI] [PubMed] [Google Scholar]
- 79.Petersen CP, Reddien PW. Wnt signaling and the polarity of the primary body axis. Cell. 2009;139(6):1056–1068. doi: 10.1016/j.cell.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 80.De Robertis EM. Wnt signaling in axial patterning and regeneration: lessons from planaria. Sci Signal. 2010;3(127):pe21. doi: 10.1126/scisignal.3127pe21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Christian JL, Moon RT. Interactions between Xwnt-8 and Spemann organizer signaling pathways generate dorsoventral pattern in the embryonic mesoderm of Xenopus. Genes Dev. 1993;7(1):13–28. doi: 10.1101/gad.7.1.13. [DOI] [PubMed] [Google Scholar]
- 82.Glinka A, et al. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391(6665):357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 83.Schohl A, Fagotto F. Beta-catenin, MAPK and Smad signaling during early Xenopus development. Development. 2002;129(1):37–52. doi: 10.1242/dev.129.1.37. [DOI] [PubMed] [Google Scholar]
- 84.Mannini L, et al. Two msh/msx-related genes, Djmsh1 and Djmsh2, contribute to the early blastema growth during planarian head regeneration. Int J Dev Biol. 2008;52(7):943–952. doi: 10.1387/ijdb.072476lm. [DOI] [PubMed] [Google Scholar]
- 85.Herman MA, et al. The C. elegans gene lin-44, which controls the polarity of certain asymmetric cell divisions, encodes a Wnt protein and acts cell nonautonomously. Cell. 1995;83(1):101–110. doi: 10.1016/0092-8674(95)90238-4. [DOI] [PubMed] [Google Scholar]
- 86.Coudreuse DY, et al. Wnt gradient formation requires retromer function in Wnt-producing cells. Science. 2006;312(5775):921–924. doi: 10.1126/science.1124856. [DOI] [PubMed] [Google Scholar]
- 87.Pan CL, et al. Multiple Wnts and frizzled receptors regulate anteriorly directed cell and growth cone migrations in Caenorhabditis elegans. Dev Cell. 2006;10(3):367–377. doi: 10.1016/j.devcel.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 88.Hayashi S, et al. Expression patterns of Xenopus FGF receptor-like 1/noudarake in early Xenopus development resemble those of planarian nou-darake and Xenopus FGF8. Dev Dyn. 2004;230(4):700–707. doi: 10.1002/dvdy.20040. [DOI] [PubMed] [Google Scholar]
- 89.Holley SA, et al. A conserved system for dorsal-ventral patterning in insects and vertebrates involving sog and chordin. Nature. 1995;376(6537):249–253. doi: 10.1038/376249a0. [DOI] [PubMed] [Google Scholar]
- 90.De Robertis EM, Sasai Y. A common plan for dorsoventral patterning in Bilateria. Nature. 1996;380(6569):37–40. doi: 10.1038/380037a0. [DOI] [PubMed] [Google Scholar]
- 91.Khokha MK, et al. Depletion of three BMP antagonists from Spemann's organizer leads to a catastrophic loss of dorsal structures. Dev Cell. 2005;8(3):401–411. doi: 10.1016/j.devcel.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 92.Meinhardt H. Models for the generation of the embryonic body axes: ontogenetic and evolutionary aspects. Curr Opin Genet Dev. 2004;14(4):446–454. doi: 10.1016/j.gde.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 93.Ben-Zvi D, et al. Scaling of the BMP activation gradient in Xenopus embryos. Nature. 2008;453(7199):1205–1211. doi: 10.1038/nature07059. [DOI] [PubMed] [Google Scholar]
- 94.Spemann H. Embryonic development and induction. New Haven: Yale University Press; 1938. [Google Scholar]
- 95.Cooke J. Scale of body pattern adjusts to available cell number in amphibian embryos. Nature. 1981;290(5809):775–778. doi: 10.1038/290775a0. [DOI] [PubMed] [Google Scholar]
- 96.Yazawa S, et al. Planarian Hedgehog/Patched establishes anterior-posterior polarity by regulating Wnt signaling. Proc Natl Acad Sci U S A. 2009;106(52):22329–22334. doi: 10.1073/pnas.0907464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rink JC, et al. Planarian Hh signaling regulates regeneration polarity and links Hh pathway evolution to cilia. Science. 2009;326(5958):1406–1410. doi: 10.1126/science.1178712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Glazer AM, et al. The Zn finger protein Iguana impacts Hedgehog signaling by promoting ciliogenesis. Dev Biol. 2010;337(1):148–156. doi: 10.1016/j.ydbio.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oviedo NJ, et al. Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration. Dev Biol. 2010;339(1):188–199. doi: 10.1016/j.ydbio.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nogi T, et al. A novel biological activity of praziquantel requiring voltage-operated Ca2+ channel beta subunits: subversion of flatworm regenerative polarity. PLoS Negl Trop Dis. 2009;3(6):e464. doi: 10.1371/journal.pntd.0000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barkai N, Shilo BZ. Robust generation and decoding of morphogen gradients. Cold Spring Harb Perspect Biol. 2009;1(5):a001990. doi: 10.1101/cshperspect.a001990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Waddington C. Canalization of development and the inheritance of acquired characters. Nature. 1942;150:563–565. [Google Scholar]
- 103.Wang KC, Helms JA, Chang HY. Regeneration, repair and remembering identity: the three Rs of Hox gene expression. Trends Cell Biol. 2009;19(6):268–275. doi: 10.1016/j.tcb.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kobayashi C, et al. Wnt signaling is required for antero-posterior patterning of the planarian brain. Dev Biol. 2007;306(2):714–724. doi: 10.1016/j.ydbio.2007.04.010. [DOI] [PubMed] [Google Scholar]