Abstract
Objectives
Administration of eicosapentaenoic acid (EPA) and docosahexanoic acid (DHA), omega-3 fatty acids in fish oil, has been associated with improved patient outcomes in acute lung injury (ALI) when studied in a commercial enteral formula. However, fish oil has not been tested independently in ALI. We therefore sought to determine if enteral fish oil alone would reduce pulmonary and systemic inflammation in patients with ALI.
Design
Phase II randomized controlled trial.
Setting
Four North American medical centers.
Patients
Mechanically ventilated patients with ALI ≥ 18 years of age.
Interventions
Subjects were randomized to receive enteral fish oil (9.75g EPA and 6.75g DHA daily) or saline placebo for up to 14 days.
Measurements and Main Results
Bronchoalveolar lavage fluid (BALF) and blood were collected at baseline (day 0), day 4±1, and day 8±1. The primary endpoint was BALF interleukin (IL)-8 levels. Forty-one participants received fish oil and 49 received placebo. Enteral fish oil administration was associated with increased serum EPA concentration (p<0.0001). However, there was no significant difference in the change in BALF IL-8 from baseline to day 4 (p=0.37) or day 8 (p=0.55) between treatment arms. There were no appreciable improvements in other BALF or plasma biomarkers in the fish oil group compared to the control group. Similarly, organ failure score, ventilator-free days, ICU-free days, and 60-day mortality did not differ between the groups.
Conclusions
Fish oil did not reduce biomarkers of pulmonary or systemic inflammation in patients with ALI, and the results do not support the conduct of a larger clinical trial in this population with this agent. This experimental approach is feasible for proof of concept studies evaluating new treatments for ALI.
Keywords: Acute lung injury, randomized controlled trial, eicosapentaenoic acid, docosahexaenoic acid, acute respiratory distress syndrome, fish oils
INTRODUCTION
There are 200,000 US cases of acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) each year with a case fatality of 30–40%.(1,2) While low tidal volume ventilation improves survival, there are currently no pharmacologic treatments for ALI. Several recent studies in animals and humans, however, suggest that omega-3 fatty acids (Ω-3s) may be beneficial in ALI.(3–8)
ALI pathogenesis is thought to result from massive activation of the proinflammatory response, including release of inflammatory mediators such as the eiconsanoids.(9) The most pro-inflammatory eicosanoids are metabolized from arachidonic acid (AA) and include 2-series prostaglandins and thromboxanes and 4-series leukotrienes (Figure A in supplemental digital content).(10,11) They play a prominent role in fever, vascular permeability, vasodilatation, platelet aggregation, leukocyte adhesion, and cytokine production.(12–14)
Eicosapentaenoic acid (EPA) and docosahexanoic acid (DHA) are two essential Ω-3s found in fish oil (Figure A in supplemental digital content) that reduce inflammatory eicosanoid production through a variety of anti-inflammatory mechanisms.(11,15–18) Additionally, the novel anti-inflammatory mediators resolvins and protectins are derived from EPA and DHA.(19,20)
EPA and DHA have been combined with γ-linoleic acid (GLA, an Ω-6 fatty acid) and several antioxidants into a commercial high-fat low-carbohydrate enteral formula. In industry-sponsored animal studies of lung injury, this commercial formula had biologic and physiologic benefits.(3–5,21,22) Prior studies of enteral Ω-3s in critically ill humans have all used this commercial formula. Five small randomized controlled trials (RCTs) have been performed in patients with ALI and sepsis;(6–8,23,24) one is published only in abstract form and one is unpublished. The control groups in all 5 trials received a high-fat low-carbohydrate enteral formula isonitrogenous with the fish-oil containing formula but free of fish oil. A meta-analysis of these 5 trials suggested that the fish oil/GLA/antioxidant formula was associated with reduced mortality, ICU length of stay (LOS), and duration of mechanical ventilation.(25)
Interpretation of these data, however, is difficult because: 1) control groups received a high fat formula that is not standard of care in critically ill patients and may be pro-inflammatory and 2) independent effects of Ω-3s cannot be assessed because the feeding formula contains ingredients other than fish oil. Furthermore, because critically ill patients commonly receive <50% of prescribed caloric need enterally, inclusion of a pharmaconutrient in an enteral formula could result in inadequate delivery of the nutrient.(26) To determine if EPA and DHA have an independent therapeutic benefit in patients with ALI, we conducted a phase II RCT of enteral fish oil (alone and separate from any enteral feeding) versus placebo.
MATERIALS AND METHODS
Participants and Study Setting
A detailed methods description is in the supplemental digital content. Participants were enrolled from 2006–2008 at five North American centers. All Human Subjects Committees and a Data Safety Monitoring Board (DSMB) approved the trial, which was registered as NCT00351533. All intubated, mechanically ventilated patients >17 years of age meeting ALI criteria as defined by the American-European Consensus Conference(27) (ratio of partial pressure of arterial oxygen to fraction of inspired oxygen [PaO2/FiO2] ≤300, chest radiograph demonstrating bilateral infiltrates consistent with pulmonary edema, and no evidence of left heart failure causing the infiltrates) were eligible for enrollment. Table 1 lists exclusion criteria.
Table 1.
Exclusion Criteria
|
Study Design and Intervention
After informed consent and within 48 hours of ALI onset, participants were randomized in a 1:1 ratio to receive enteral fish oil (7.5cc every 6 hours equalling 9.75g EPA and 6.75g DHA daily) or 0.9% saline. Computer-generated random allocation in permuted blocks of four was performed by Investigational Drug Services at Harborview Medical Center in Seattle, WA, and randomization was stratified by study site and the presence of trauma. Sequentially numbered, opaque, sealed envelopes were sent to each site. After enrolling a new patient, research pharmacists at each site opened the next envelope in the sequence.
We used a concentrated liquid fish oil (Ultimate Omega, Nordic Naturals) manufactured in a single batch. Independent analysis before study start found the expected concentration of EPA and DHA and no heavy metal contaminants. Both fish oil and placebo were dispensed in opaque brown syringes by research pharmacists, and the exterior of all syringes was brushed with fish oil to preserve blinding. Only research pharmacists were aware of group assignment. Study participants and personnel remained blinded throughout the trial.
Patients received study drug for 14 days or until death, ICU discharge, or 48 hours of unassisted breathing, whichever occurred first. All subjects were ventilated according to local protocols mimicking the ARDS Network low tidal volume ventilation protocol.(28) Enteral or parenteral feeding was at the discretion of treating clinicians.
Procedures and Outcomes
Bronchoalveolar lavage fluid (BALF) and plasma were obtained at study entry (day 0) and on days 4±1 and 8±1. The 2nd and 3rd BALs did not occur if participants were extubated or did not meet safety criteria for bronchoscopy. Serum was collected on day 5 for EPA and DHA measurement. Adverse events were monitored daily.
BALF interleukin (IL)-8 was chosen as our primary endpoint because it is the dominant neutrophil chemoattractant in ALI(29) and is decreased in patients receiving low tidal volume ventilation,(30) an intervention known to improve survival in ALI.(28) Additionally, in the only prior trial of the fish oil-containing enteral formula to perform BALs, patients in the intervention group had reduced BALF IL-8 compared to the control group.(6) Secondary outcomes included BALF IL-6, leukotriene B4 (LTB4), monocyte chemotactic protein-1 (MCP-1) and neutrophil count; plasma IL-8, LTB4, IL-6, vonWillebrand Factor (vWF), and surfactant protein-D (SP-D); lung compliance; and oxygenation. IL-6 activates leukocytes, affects the acute phase response, and levels correlate with ALI severity.(31) LTB4 is also a potent neutrophil chemoattractant produced by alveolar macrophages,(32) and its production is presumably directly inhibited by Ω-3s. MCP-1 is a monocyte chemoattractant, and increasing levels have been associated with both worse lung injury and persistent ALI.(33) SP-D is a biomarker of alveolar epithelial injury and vWF is a marker of endovascular injury; both have been associated with increased mortality in ALI.(34–38)
Although the trial was not powered to detect differences in clinical outcomes, we measured organ failure with the Multiple Organ Dysfunction Score (MODS),(39) ventilator-free and ICU-free days during the first 28 days(40), hospital LOS, and hospital and 60-day mortality.
Measurements
Biomarker assays were performed in Seattle, WA. IL-8, IL-6, and MCP-1 concentrations were determined using Luminex bead-based immunoassays (R&D Systems).(41) LTB4 was measured by enzyme immunoassay (Cayman Chemical) after solid phase extraction.(42) SP-D and vWF were measured by enzyme-linked immunosorbent assays (BioVendor and Diagnostica Stago). For serum EPA and DHA measurement, lipids were extracted using methods described previously,(43) and phospholipids were separated by thin layer chromatography.(44) Fatty acid methyl esters were prepared using methods of Lepage(45) and separated with gas chromatography.
Sample Size and Statistical Analyses
The primary outcome was a 50% or greater reduction in BALF IL-8 in the intervention compared with the control group, measured as the change from baseline to day 4±1 and day 8±1. With α=0.05 and β=0.2 (80% power), we calculated that 26 patients per group were needed if participants underwent two BALs. Because low tidal volume ventilation is now standard, we assumed biomarker levels would lower than historical data. We therefore conservatively estimated that our study required 45 patients per group. The DSMB performed interim safety analyses after 30 and 60 enrolled patients.
Baseline characteristics were analyzed with t-, chi-square, and Fisher’s exact tests. As biomarker data are commonly nonparametric, we used Mann-Whitney U tests to compare changes from baseline to day 4 and to day 8. Additionally, given the longitudinal nature of the biomarker data, we also used mixed model linear regression analyses with log-transformation of inflammatory biomarker data and random intercepts and slopes for each patient.
Clinical outcomes were analyzed in an intent-to-treat fashion. Worst MODS,(39) ventilator-free days, ICU-free days, and hospital LOS were analyzed with t-tests and Mann Whitney U tests. Hospital and 60-day mortality were compared with chi-square tests. Planned a priori subgroup analyses were performed on participants whose ALI risk factor was trauma versus other. All analyses were performed with SAS version 9.2 (Cary, North Carolina). Reported p-values are two-sided and are uncorrected for multiple comparisons.
RESULTS
Participants
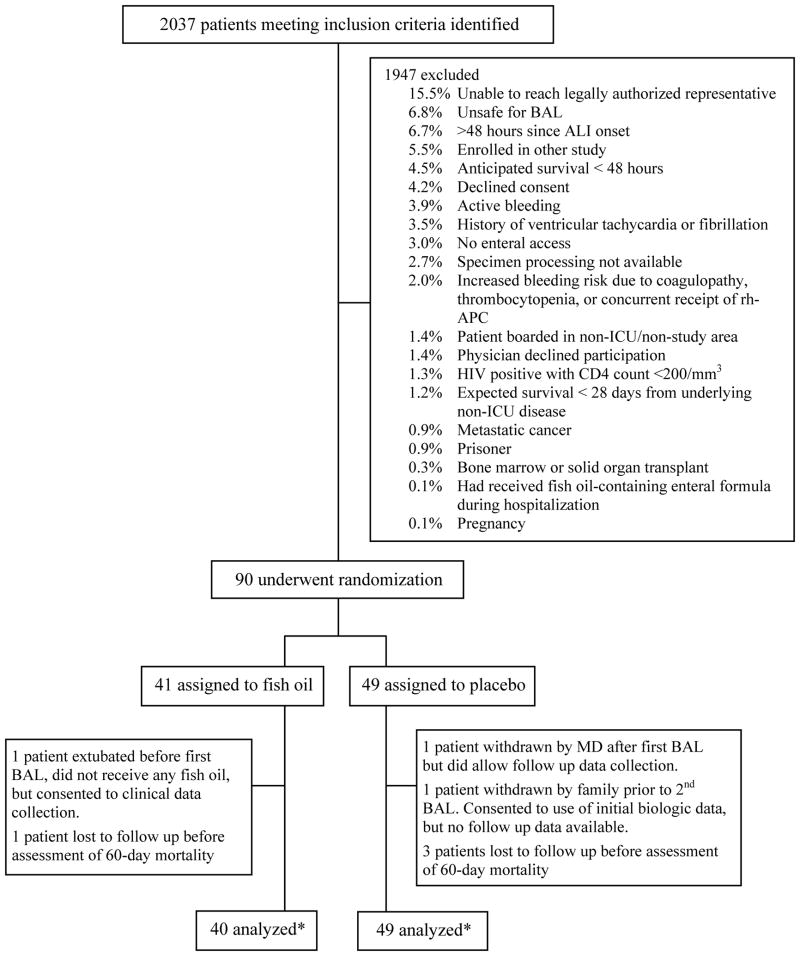
During the enrollment period, 2037 patients met initial screening criteria. Of these, 1947 were excluded for reasons shown in Figure 1. Forty-one patients were randomized to receive fish oil and 49 to receive placebo. One patient in the fish oil group was extubated before the first BAL was performed, did not receive any fish oil, and declined further participation but did allow collection of clinical follow up data. One patient in the placebo group underwent the first BAL but was subsequently withdrawn by his/her physician. This patient did not receive any placebo doses but did consent to collecting further clinical data. Another patient in the placebo group was withdrawn by his/her family prior to the second BAL because the decision had been made to remove life-sustaining therapies; no laboratory or clinical data were collected beyond that time. One patient in the fish oil group and three patients in the placebo group were lost to follow up between hospital discharge and day 60.
Figure 1.
Diagram of Participant Flow and Randomization. Patients may have had more than one reason for exclusion. *Biologic data available on 40 participants in the fish oil group but some clinical data are available on all 41 patients. In the placebo group, biologic data are available on all 49 patients and clinical follow up data are available in 48 patients with the exception of 60-day mortality which was available in 45 patients.
Baseline Characteristics
Demographic and physiologic characteristics of the two groups were well-balanced at baseline (Table 2). The groups were similar with regard to age, gender, race, ethnicity, admission diagnosis, Acute Physiology and Chronic Health Evaluation (APACHE) II score (46), PaO2/FiO2 ratio, tidal volume, plateau pressure, and positive end expiratory pressure (PEEP). However, patients receiving fish oil more commonly had aspiration as an ALI risk factor (29.3% versus 10.2%, p=0.02) and had a higher Lung Injury Score (2.8±0.5 versus 2.4±0.7).(47)
Table 2.
Baseline Participant Characteristics
| Fish oil | Placebo | p-value | |
|---|---|---|---|
| n=41 | n=49 | ||
|
| |||
| Age (years) a | 49.0±16.5 | 50.7±16.5 | 0.63 |
|
| |||
| Male gender, n (%)b | 24 (58.5) | 33 (67.3) | 0.38 |
|
| |||
| Race, n (%)b | |||
| African-American | 2 (4.9) | 1 (2.0) | 0.59 |
| American Indian | 0 (0) | 2 (4.1) | 0.50 |
| Asian | 1 (2.4) | 1 (2.0) | 1.00 |
| Pacific Islander | 0 (0) | 3 (6.1) | 0.25 |
| White | 38 (92.7) | 42 (85.7) | 0.30 |
|
| |||
| Ethnicity, n (%)b | |||
| Hispanic | 2 (4.9) | 1 (2.0) | 0.59 |
| Non-Hispanic | 39 (95.1) | 45 (91.8) | 0.53 |
| Unknown | 0 (0) | 3 (6.1) | 0.25 |
|
| |||
| ALI risk factor, n (%) (not mutually exclusive) b | |||
| Trauma | 17 (41.5) | 19 (38.8) | 0.80 |
| Shock | 16 (39.0) | 20 (40.8) | 0.86 |
| Aspiration | 12 (29.3) | 5 (10.2) | 0.02 |
| Sepsis | 7 (17.1) | 7 (14.3) | 0.72 |
| Pneumonia | 6 (14.6) | 6 (12.2) | 0.74 |
| Massive transfusion | 1 (2.4) | 1 (2.0) | 1.00 |
| Moderate transfusion | 3 (7.3) | 5 (10.2) | 0.72 |
| Overdose | 1 (2.4) | 2 (4.1) | 1.00 |
| Near drowning | 0 (0) | 1 (2.0) | 1.00 |
| Inhalation injury | 1 (2.4) | 0 (0) | 0.46 |
|
| |||
| Admission Diagnosis, n (%)b | |||
| Trauma | 17 (41.5) | 17 (34.7) | 0.51 |
| Sepsis | 6 (14.6) | 7 (14.3) | 0.96 |
| Pneumonia | 3 (7.3) | 6 (12.2) | 0.50 |
| Respiratory failure | 5 (12.2) | 4 (8.2) | 0.73 |
| ALI/ARDS | 3 (7.3) | 2 (4.1) | 0.66 |
| Intracranial hemorrhage | 1 (2.4) | 4 (8.2) | 0.37 |
| Other | 6 (14.6) | 9 (18.3) | 0.78 |
|
| |||
| APACHE II Scorea | 22.8±7.3 | 21.1±5.9 | 0.23 |
|
| |||
| PaO2/FiO2 a | 154.7±46.5 | 172.5±65.3 | 0.15 |
|
| |||
| Tidal volume (mL/kg predicted body weight) a | 7.0±1.3 | 7.6±1.7 | 0.10 |
|
| |||
| Plateau pressure (cm H20) a | 25.5±6.1 | 23.8±5.1 | 0.19 |
|
| |||
| PEEP (cm H20) a | 9.4±3.5 | 8.9±4.3 | 0.59 |
|
| |||
| Lung Injury Score(47) a | 2.8±0.5 | 2.4±0.7 | 0.008 |
Data presented as mean ± standard deviation; analysis with Student’s t test.
Analysis with chi square or Fischer’s exact test.
Abbreviations: ALI = acute lung injury; ARDS = acute respiratory distress syndrome; APACHE = Acute Physiologic and Chronic Health Evaluation; PaO2/FiO2 = ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen; PEEP = positive end expiratory pressure.
Protocol Compliance
Participants in the intervention and placebo groups received a median of 29 (IQR 16–48) and 36 doses (IQR 17–55), respectively. Patients receiving at least 7 days of study drug numbered 26/41 (63.4%) in the fish oil group and 32/49 (65.3%) in the placebo group (p=0.85). Ten of 41 (24.4%) participants receiving fish oil and 19/49 (38.8%) receiving placebo completed the full 14 days of study drug (p=0.15), with study drug being stopped due to death, unassisted breathing for ≥48 hours, or ICU discharge as per protocol. There were 37 total individual doses (mean 0.90 per participant) missed in the active group and 35 missed (mean 0.71 per participant) in the placebo group. Reasons for missed doses included: 1) having procedure out of ICU, 2) not receiving any enteral intake (NPO), 3) emesis, 4) nursing error, and 5) pharmacy error. In the fish oil and placebo groups, respectively, 30/41 (73.2%) and 36/49 (73.5%) patients underwent at least 2 BALs (p=0.97). Fifteen of 41 patients (36.6%) receiving fish oil and 26/49 (53.1%) receiving placebo (p=0.12) underwent all three BALs.
Participants receiving fish oil had higher serum EPA and DHA levels on study day 5 than did participants receiving placebo. Median serum EPA was 31.9 mg/L (IQR 24.1–59.7) in the fish oil group and 2.4 mg/L (IQR 1.5–6.3) in the placebo group (p<0.0001). Median DHA concentration was 24.1 mg/L (IQR 15.8–39.8) versus 12.8 mg/L (IQR 9.1–17.9) in the fish oil and placebo groups, respectively (p=0.0009).
Outcomes
Biomarker Outcomes
There was no statistically significant difference in the change in BAL IL-8 from baseline to day 4 or from baseline to day 8 between the fish oil and placebo groups (Table 3). Likewise, there were no significant differences in changes between BAL IL-6, MCP, and neutrophil count on either day 4 or day 8. We did find that BAL LTB4 concentration increased from baseline to day 4 in the fish oil group and decreased in the placebo group (p=0.04 without adjustment for multiple comparisons). See Table A in the supplemental digital content for BALF biomarker values.
Table 3.
Change in Bronchoalveolar Lavage Fluid Biomarker Measurements (pg/mL)
| Change from Day 0 to Day 4 | p valuec | Change from Day 0 to Day 8 | p valued | |||
|---|---|---|---|---|---|---|
| Fish Oil (n=30) | Placebo (n=36) | Fish Oil (n=15) | Placebo (n=27) | |||
| IL-8 a | 43 (-656-604) | -241 (-807-349) | 0.37 | -531 (-1308-94) | -298 (-870-397) | 0.55 |
| IL-6 a | -24 (-340-51) | -200 (-692-0) | 0.16 | -52 (-898-0) | -198 (-865- -17) | 0.84 |
| MCP-1 a | -150 (-1812-93) | -226 (-752- -38) | 0.79 | -402 (-1690- -41) | -670 (-1057- -151) | 0.74 |
| LTB4 a | 6 (-1-14) | -2 (-26-9) | 0.04 | 0 (-2-33) | 16 (0-50) | 0.38 |
| PMNb | -3 (-19-12) | -10 (-21-7) | 0.34 | -4 (-48-8) | -10 (-56-15) | 0.84 |
Data are presented as median (interquartile range).
IL-8, IL-6, MCP-1, and LTB4 data are presented as pg/mL.
PMN data are presented as number of neutrophils per high power field.
p-value for comparison of change from baseline to day 4 between groups using Mann-Whitney U test
p-value for comparison of change from baseline to day 8 between groups using Mann-Whitney U test
Similarly, there were no appreciable differences in plasma IL-8, IL-6, SP-D, and vWF between the two groups when comparing changes between baseline to day 4 and baseline to day 8 (Table 4). Again, we found that plasma LTB4 increased from baseline to day 4 in the fish oil group and decreased in the placebo group (p=0.002). See Table B in the supplemental digital content for plasma biomarker values.
Table 4.
Change in Plasma Biomarker Measurements
| pg/mL | Change from Day 0 to Day 4 | p valuea | Change from Day 0 to Day 8 | p valueb | ||
|---|---|---|---|---|---|---|
| Fish Oil (n=30) | Placebo (n=36) | Fish Oil (n=15) | Placebo (n=27) | |||
| IL-8 | 0 (-8-0) | 0 (-13-0) | 0.89 | -1 (-17-0) | 0 (-15-2) | 0.71 |
| IL-6 | -33 (-162- -13) | -78 (-163- -29) | 0.27 | -83 (-174-14) | -78 (-163- -29) | 0.86 |
| SP-D | 25 (-5-78) | 36 (1-139) | 0.20 | 26 (-12-78) | 17 (-36-68) | 0.89 |
| LTB4 | 21 (0-32) | -6 (-35-7) | 0.002 | 10 (3-40) | 6 (-22-14) | 0.08 |
| vWF | 15 (-103-158) | 68 (21-206) | 0.11 | 116 (-9-235) | 43 (-21-164) | 0.34 |
Data are presented as median (interquartile range).
p-value for comparison of change from baseline to day 4 between groups using Mann-Whitney U test
p-value for comparison of change from baseline to day 8 between groups using Mann-Whitney U test
As secondary analyses, we also performed longitudinal analyses using linear regression mixed modeling with log-transformed biomarker levels and random intercepts and slopes for each participant (data not shown). No differences were found in any BAL or plasma biomarkers.
We performed planned a priori subgroup analyses for participants whose ALI risk factor was trauma and those with other risk factors. BALF and plasma biomarker levels for trauma and non-trauma patients are shown in Tables C and D in the supplemental digital content. There were no differences in changes in BALF inflammatory mediator levels between the two groups within either trauma or non-trauma patients. However, BALF IL-8 levels were generally greater in trauma than non-trauma patients. When plasma biomarkers were examined, we found that changes in LTB4 between baseline and day 4 were significantly different in both trauma and non-trauma subgroups, with greater increases in those subgroups randomized to fish oil than to placebo. Similarly, the increase in plasma vWF between baseline and day 4 was also greater in trauma patients receiving fish oil than in trauma patients receiving placebo.
Cointerventions and Physiologic Outcomes
In this study, enteral feeding was determined by treating clinicians. Participants in the fish oil and placebo groups began receiving enteral feeding 1.8±1.2 and 1.9±1.3 days after ICU admission, respectively (p=0.65). Mean caloric intake during the study’s first week was 7362±3800 kcal in the fish oil group and 7495±3831 kcal in the placebo group (p=0.87). Because propofol is delivered in a lipid carrier, we also recorded its use. Median volume of propofol received during the first week was 62mL (IQR 0–555mL) and 233mL (IQR 0–980mL) in the fish oil and placebo groups, respectively (p=0.18).
Results of physiologic outcomes are shown in Table 5. There were no appreciable differences in tidal volume per kg predicted body weight, oxygenation, PEEP, or plateau pressure between the groups at day 4 or day 8. We did find that patients in the placebo group had improved total thoracic compliance on day 8 (p=0.048), without adjustment for multiple comparisons.
Table 5.
Physiologic Outcomes
| Variable (mean±SD) | Day 4 | Day 8 | ||||
|---|---|---|---|---|---|---|
| Fish Oil | Placebo | p valuea | Fish Oil | Placebo | p valuea | |
| Vt/kg PBW | 6.8±1.3 | 6.6±1.6 | 0.86 | 6.5±1.8 | 6.6±1.1 | 0.27 |
| PaO2/FiO2 | 215.4±92.8 | 174.0±63.4 | 0.06 | 197.6±63.4 | 205.4±74.9 | 0.76 |
| PEEP (cm H20) | 8.4±4.2 | 8.8±3.0 | 0.61 | 9.1±5.0 | 8.6±3.4 | 0.70 |
| Plateau pressure (cm H20) | 24.9±7.1 | 22.8±4.9 | 0.19 | 25.3±5.7 | 21.3±7.2 | 0.11 |
| Compliance | 31.8±10.1 | 36.3±20.1 | 0.26 | 27.9±10.9 | 41.7±27.3 | 0.048 |
p-value for comparison between fish oil and placebo groups using t-tests.
Abbreviations: Vt/kg PBW = tidal volume per kilogram predicted body weight; PaO2/FiO2 = ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen; PEEP = positive end expiratory pressure.
Clinical Outcomes
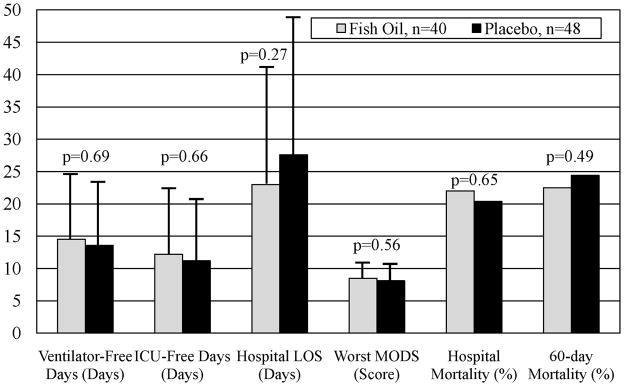
Hospital mortality (Figure 2) was 22.0% in the fish oil group and 20.4% in the placebo group (p=0.65). We also assessed 60-day mortality (Figure 2) but were unable to contact one patient in the fish oil group (lost to follow up) and four patients in the placebo group (one patient withdrawn and three patients lost to follow up). Among the 85 patients with available data, mortality was 22.5% in the fish oil group and 24.4% in the placebo group (p=0.49).
Figure 2.
Results of Clinical Outcomes. Abbreviations: LOS = length of stay and MODS = Multiple Organ Dysfunction Score. Data for ventilator- and ICU-free days, hospital LOS, and MODS are shown as means with error bars depicting standard deviation. Mortality data are shown as percent.
Ventilator-free and ICU-free days through day 28, hospital LOS, and worst MODS through day 28 are shown in Figure 2. There were no significant differences in these outcomes between the two treatment arms.
Adverse Events
There were 14 severe adverse events (SAEs) in the fish oil group and 15 SAEs in the placebo group (Table 6), none of which were determined to be related to the study. There were no severe adverse bleeding events in the fish oil group. One patient in the placebo group developed a retroperitoneal hematoma.
Table 6.
Severe Adverse Events
| SAE name | Fish Oil | Placebo |
|---|---|---|
| Atrial Fibrillation with RVR | 2 | 1 |
| Renal failure requiring renal replacement therapy | 2 | 2 |
| Bilateral pleural effusions & pericardial effusion | 1 | 0 |
| Critical hypoxemia (not associated with BAL) | 1 | 2 |
| Thrombocytopenia | 0 | 1 |
| Fulminant Liver Failure | 1 | 0 |
| Right eye blindness (after prolonged prone surgery) | 1 | 0 |
| Falls leading to hospital readmission | 0 | 1 |
| Aspiration with reintubation | 0 | 2 |
| Pneumothorax | 1 | 2 |
| Nosocomial sepsis | 1 | 1 |
| PEA arrest | 1 | 0 |
| Erythema multiforme minor | 0 | 1 |
| Delayed spinal cord contusion and injury | 1 | 0 |
| Severe hypotension | 1 | 1 |
| Pericardial tamponade | 1 | 0 |
| Retroperitoneal Hematoma | 0 | 1 |
| Total | 14 | 15 |
DISCUSSION
With prior studies suggesting that enteral formulas containing Ω-3s may benefit patients with ALI and sepsis,(6–8) this phase II double-blind placebo controlled RCT was designed to determine the efficacy of enteral fish oil alone in reducing pulmonary and systemic inflammation in patients with ALI. Although we demonstrated that enteral Ω-3s are easily delivered apart from enteral feeding, absorbed well, and safe in critically ill patients, we did not find a decrease in markers of pulmonary or systemic inflammation. Enteral fish oil did not lead to a clear decrease in any BALF or plasma inflammatory biomarkers, including our primary endpoint IL-8. Our analyses found that LTB4 increased in the fish oil group and decreased in the placebo group. The reasons for these LTB4 changes are not clear, and these results should be interpreted with caution because we did not adjust for multiple comparisons. Additionally, we did not find any differences in organ failure, ventilator-free days, ICU-free days, or mortality.
There are several possible explanations for why our results conflict with prior literature. First, our study is the first investigation of Ω-3s in ALI patients to administer a true inert placebo to the control group. While prior studies of fish oil-containing formulas used an isonitrogenous high-fat formula as a control, we administered enteral saline to our control group. Therefore, it may be that a high-fat control enteral formula in the prior studies was actually harmful, thus allowing the results to appear as though the omega-3 formula was efficacious. Second, it is possible that Ω-3s are not the beneficial agent, but rather other ingredients in the fish oil-containing formulas, such as GLA or antioxidants. Third, perhaps a combination of ingredients is actually beneficial instead of a single agent. Fourth, there are no dosing data on Ω-3s in this population. Therefore, although the dose we used is approximately 25% greater than the EPA dose received by participants in the prior studies of the commercial enteral formula, we cannot be sure that it was the optimal dose. Fifth, we delivered the fish oil as a small 7.5cc bolus every 6 hours while prior studies delivered it continuously in enteral feeding. Sixth, our study population was substantially different from that of other ALI clinical trials (28,48) in that a high proportion of patients had trauma as their ALI risk factor. This feature occurred because the site enrolling the most participants is an urban level 1 trauma center. Because patients with trauma-associated ALI have less severe endothelial and alveolar epithelial lung injury, and improved clinical outcomes, compared with patients with sepsis or another non-trauma ALI risk factor,(37) it is possible that our intervention designed to reduce pulmonary inflammation was less effective in trauma patients. Indeed, there was a suggestion in our data that BALF levels of IL-8 among trauma patients receiving fish oil increased between baseline and day 4, while levels decreased in both trauma patients receiving placebo and in non-trauma patients receiving either fish oil or placebo. Additionally, increases in plasma vWF, a marker of endothelial injury,(38) between baseline and day 4 were greater in trauma patients receiving fish oil than in those receiving placebo.
However, perhaps most important is the setting of our trial in an era of relatively improved care for ALI patients. Levels of both BALF and plasma inflammatory mediators in this RCT were substantially decreased, even several fold, compared with prior studies in similar ALI patients.(30,49,50) These decreased levels may be explained by different populations or assay methods between studies. However, a more likely explanation is that various processes of care have lead to reduced lung inflammation in patients with ALI, especially in the era of low tidal volume ventilation. Survival of ALI patients has improved over the past decades.(2,51,52) Reasons for this increased survival are unknown but may be due to improved overall care, including early antibiotic administration, early goal-directed fluid resuscitation, and stress ulcer prophylaxis.(53–55) Furthermore, low tidal volume ventilation, an intervention that decreases both lung and systemic inflammation as well as mortality,(28,30,56) is now standard of care in patients with ALI. If all of these interventions have resulted in reduced BALF and plasma inflammatory biomarkers, conducting appropriate phase II clinical trials with these biologic endpoints then becomes more difficult and requires much larger sample sizes. Furthermore, while it is known that low tidal volume ventilation decreases pulmonary inflammation, it is unclear if other interventions such as Ω-3s are also able to further reduce lung inflammation in the setting of lung protective ventilation. The prior trial by Gadek and colleagues found that ALI patients receiving the commercial enteral formula had decreased BALF IL-8 compared to patients receiving the control formula.(6) However, that study was conducted before lung protective ventilation was standard of care, and baseline and subsequent levels of BALF biomarkers in that study were substantially greater than in our trial.
There are some limitations to our trial. First, because our population has a high proportion of trauma patients, and as is true in many critical care RCTs, many screened patients were excluded as shown in Figure 1, generalizability of our results may be limited. Second, the number of patients in our trial was modest (n=90). Therefore, we had statistical power to detectonly a relatively large difference in the primary endpoint, BALF IL-8, and in other inflammatory mediators. Thus, our results cannot exclude a small to moderate beneficial effect of fish oil on lung inflammation.
Our results are somewhat consistent with the OMEGA trial, a large phase III RCT conducted by the NHLBI ARDS Network to investigate an enteral supplement (delivered twice daily) containing EPA, DHA, GLA, and antioxidants in patients with ALI (this trial remains unpublished, but results were presented at the 2009 American Thoracic Society International Conference in San Diego, California(57)). The control group in that study received an isocaloric amount of a standard enteral feeding formula; thus, calories received were the same between the two groups, but not fat or protein. The primary endpoint of OMEGA was ventilator-free days, and the planned sample size was 1000. This RCT was stopped for futility in March 2009 after accrual of 272 patients. There was no difference between the intervention and control groups with regard to ventilator-free days, ICU-free days, or mortality at 60 days. Our study is different than OMEGA in that we used fish oil alone rather than a cocktail containing several ingredients and we used a saline placebo, but it is interesting that both our study and OMEGA found no benefit while prior studies of Ω-3s had demonstrated improved outcomes with the enteral formula containing fish oil.
CONCLUSIONS
In conclusion, this phase II RCT of enteral fish oil in patients with ALI did not demonstrate a decrease in pulmonary or systemic inflammatory mediators. Similarly, we did not find a decrease in ventilator-free days, ICU-free days, organ failure, or mortality. Although our findings contradict prior studies that suggest benefit of an enteral formula containing fish oil, they are consistent with those of a phase III RCT recently stopped for lack of efficacy. Ω-3s have many anti-inflammatory properties and future research is needed to determine why results of various studies over the past decade, including ours, are discordant.
Supplementary Material
Acknowledgments
The research was performed at the University of Washington, University of Vermont, University of Toronto, and St. Alphonsus Regional Medical Center in Boise, Idaho.
Source of Support:
This research was supported by an American Thoracic Society/Acute Respiratory Distress Syndrome Foundation Award, an American Society for Parenteral and Enteral Nutrition Rhoads Research Foundation Award, and National Institutes of Health Grants 1P50HL073996, 8K12RR023265, 5P20RR015557. Nordic Naturals also donated the fish oil used in this trial but had no input into study design, study conduct, data management, or publication.
The authors received funding from NIH, ATS/ARDS Foundation, and A.S.P.E.N.
Footnotes
No reprints will be ordered.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Stapleton RD, Wang BM, Hudson LD, et al. Causes and timing of death in patients with ARDS. Chest. 2005;128:525–532. doi: 10.1378/chest.128.2.525. [DOI] [PubMed] [Google Scholar]
- 3.Mancuso P, Whelan J, DeMichele SJ, et al. Dietary fish oil and fish and borage oil suppress intrapulmonary proinflammatory eicosanoid biosynthesis and attenuate pulmonary neutrophil accumulation in endotoxic rats. Crit Care Med. 1997;25:1198–1206. doi: 10.1097/00003246-199707000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Palombo JD, DeMichele SJ, Boyce PJ, et al. Metabolism of dietary alpha-linolenic acid vs. eicosapentaenoic acid in rat immune cell phospholipids during endotoxemia. Lipids. 1998;33:1099–1105. doi: 10.1007/s11745-998-0311-x. [DOI] [PubMed] [Google Scholar]
- 5.Palombo JD, DeMichele SJ, Boyce PJ, et al. Effect of short-term enteral feeding with eicosapentaenoic and gamma-linolenic acids on alveolar macrophage eicosanoid synthesis and bactericidal function in rats. Crit Care Med. 1999;27:1908–1915. doi: 10.1097/00003246-199909000-00032. [DOI] [PubMed] [Google Scholar]
- 6.Gadek JE, DeMichele SJ, Karlstad MD, et al. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Enteral Nutrition in ARDS Study Group. Crit Care Med. 1999;27:1409–1420. doi: 10.1097/00003246-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Singer P, Theilla M, Fisher H, et al. Benefit of an enteral diet enriched with eicosapentaenoic acid and gamma-linolenic acid in ventilated patients with acute lung injury. Crit Care Med. 2006;34:1033–1038. doi: 10.1097/01.CCM.0000206111.23629.0A. [DOI] [PubMed] [Google Scholar]
- 8.Pontes-Arruda A, Aragao AM, Albuquerque JD. Effects of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit Care Med. 2006;34:2325–2333. doi: 10.1097/01.CCM.0000234033.65657.B6. [DOI] [PubMed] [Google Scholar]
- 9.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 10.Palombo JD, Lydon EE, Chen PL, et al. Fatty acid composition of lung, macrophage and surfactant phospholipids after short-term enteral feeding with n-3 lipids. Lipids. 1994;29:643–649. doi: 10.1007/BF02536099. [DOI] [PubMed] [Google Scholar]
- 11.Yaqoob P, Pala HS, Cortina-Borja M, et al. Encapsulated fish oil enriched in alpha-tocopherol alters plasma phospholipid and mononuclear cell fatty acid compositions but not mononuclear cell functions. Eur J Clin Invest. 2000;30:260–274. doi: 10.1046/j.1365-2362.2000.00623.x. [DOI] [PubMed] [Google Scholar]
- 12.Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest. 2001;108:15–23. doi: 10.1172/JCI13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bulger EM, Maier RV. Lipid mediators in the pathophysiology of critical illness. Crit Care Med. 2000;28:N27–36. doi: 10.1097/00003246-200004001-00004. [DOI] [PubMed] [Google Scholar]
- 14.Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990;323:645–655. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- 15.Calder PC. Polyunsaturated fatty acids, inflammation, and immunity. Lipids. 2001;36:1007–1024. doi: 10.1007/s11745-001-0812-7. [DOI] [PubMed] [Google Scholar]
- 16.Obata T, Nagakura T, Masaki T, et al. Eicosapentaenoic acid inhibits prostaglandin D2 generation by inhibiting cyclo-oxygenase-2 in cultured human mast cells. Clin Exp Allergy. 1999;29:1129–1135. doi: 10.1046/j.1365-2222.1999.00604.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee TH, Menica-Huerta JM, Shih C, et al. Characterization and biologic properties of 5,12-dihydroxy derivatives of eicosapentaenoic acid, including leukotriene B5 and the double lipoxygenase product. J Biol Chem. 1984;259:2383–2389. [PubMed] [Google Scholar]
- 18.Goldman DW, Pickett WC, Goetzl EJ. Human neutrophil chemotactic and degranulating activities of leukotriene B5 (LTB5) derived from eicosapentaenoic acid. Biochem Biophys Res Commun. 1983;117:282–288. doi: 10.1016/0006-291x(83)91572-3. [DOI] [PubMed] [Google Scholar]
- 19.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nature immunology. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 20.Singer P, Shapiro H, Theilla M, et al. Anti-inflammatory properties of omega-3 fatty acids in critical illness: novel mechanisms and an integrative perspective. Intensive Care Med. 2008;34:1580–1592. doi: 10.1007/s00134-008-1142-4. [DOI] [PubMed] [Google Scholar]
- 21.Palombo JD, DeMichele SJ, Lydon EE, et al. Rapid modulation of lung and liver macrophage phospholipid fatty acids in endotoxemic rats by continuous enteral feeding with n-3 and gamma-linolenic fatty acids. Am J Clin Nutr. 1996;63:208–219. doi: 10.1093/ajcn/63.2.208. [DOI] [PubMed] [Google Scholar]
- 22.Mancuso P, Whelan J, DeMichele SJ, et al. Effects of eicosapentaenoic and gamma-linolenic acid on lung permeability and alveolar macrophage eicosanoid synthesis in endotoxic rats. Crit Care Med. 1997;25:523–532. doi: 10.1097/00003246-199703000-00024. [DOI] [PubMed] [Google Scholar]
- 23.Moran V, Grau T, Lorenzo Ad, et al. Effect of an enteral feeding with eicosapentaenoic and gamma-linoleic acids on the outcome of mechanically ventilated critically ill septic patients. Crit Care Med. 2006;34:A70. (Abstract) [Google Scholar]
- 24.Miller A, Ziad S. Efficacy of enteral nutrition with EPA, GLA and antioxidants in patients with ARDS and MODS: a prospective multi-center randomized controlled trial. Unpublished data, see http://www.criticalcarenutrition.com/docs/cpg/4.1bfish%20oils_FINAL.pdf.
- 25. [Accessed on October 1, 2010.];Canadian Critical Care Nutrition Clinical Practice Guidelines 4.1(b). Composition of enteral nutrition: fish oils. Available at: http://www.criticalcarenutrition.com/docs/cpg/4.1bfish%20oils_FINAL.pdf.
- 26.Heyland DK, Schroter-Noppe D, Drover JW, et al. Nutrition support in the critical care setting: current practice in canadian ICUs--opportunities for improvement? JPEN J Parenter Enteral Nutr. 2003;27:74–83. doi: 10.1177/014860710302700174. [DOI] [PubMed] [Google Scholar]
- 27.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 28.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 29.Goodman RB, Pugin J, Lee JS, et al. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev. 2003;14:523–535. doi: 10.1016/s1359-6101(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 30.Ranieri VM, Suter PM, Tortorella C, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. Jama. 1999;282:54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 31.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202:145–156. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- 32.Martin TR, Pistorese BP, Chi EY, et al. Effects of leukotriene B4 in the human lung. Recruitment of neutrophils into the alveolar spaces without a change in protein permeability. J Clin Invest. 1989;84:1609–1619. doi: 10.1172/JCI114338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodman RB, Strieter RM, Martin DP, et al. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154:602–611. doi: 10.1164/ajrccm.154.3.8810593. [DOI] [PubMed] [Google Scholar]
- 34.Ware LB, Matthay MA, Parsons PE, et al. Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2007;35:1821–1828. doi: 10.1097/01.CCM.0000221922.08878.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ware LB, Eisner MD, Thompson BT, et al. Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med. 2004;170:766–772. doi: 10.1164/rccm.200310-1434OC. [DOI] [PubMed] [Google Scholar]
- 36.Eisner MD, Parsons P, Matthay MA, et al. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax. 2003;58:983–988. doi: 10.1136/thorax.58.11.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calfee CS, Eisner MD, Ware LB, et al. Trauma-associated lung injury differs clinically and biologically from acute lung injury due to other clinical disorders. Crit Care Med. 2007;35:2243–2250. doi: 10.1097/01.ccm.0000280434.33451.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ware LB, Conner ER, Matthay MA. von Willebrand factor antigen is an independent marker of poor outcome in patients with early acute lung injury. Crit Care Med. 2001;29:2325–2331. doi: 10.1097/00003246-200112000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30:1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 41.Haegens A, Barrett TF, Gell J, et al. Airway epithelial NF-kappaB activation modulates asbestos-induced inflammation and mucin production in vivo. J Immunol. 2007;178:1800–1808. doi: 10.4049/jimmunol.178.3.1800. [DOI] [PubMed] [Google Scholar]
- 42.Debley JS, Hallstrand TS, Monge T, et al. Methods to improve measurement of cysteinyl leukotrienes in exhaled breath condensate from subjects with asthma and healthy controls. The Journal of allergy and clinical immunology. 2007;120:1216–1217. doi: 10.1016/j.jaci.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 43.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. The Journal of biological chemistry. 1957;226:497–509. [PubMed] [Google Scholar]
- 44.Schlierf G, Wood P. Quantitative Determination of Plasma Free Fatty Acids and Triglycerides by Thin-Layer Chromatography. Journal of lipid research. 1965;6:317–319. [PubMed] [Google Scholar]
- 45.Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. Journal of lipid research. 1986;27:114–120. [PubMed] [Google Scholar]
- 46.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 47.Murray JF, Matthay MA, Luce JM, et al. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 48.Wheeler AP, Bernard GR, Thompson BT, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 49.Park WY, Goodman RB, Steinberg KP, et al. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:1896–1903. doi: 10.1164/ajrccm.164.10.2104013. [DOI] [PubMed] [Google Scholar]
- 50.Pacht ER, DeMichele SJ, Nelson JL, et al. Enteral nutrition with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants reduces alveolar inflammatory mediators and protein influx in patients with acute respiratory distress syndrome. Crit Care Med. 2003;31:491–500. doi: 10.1097/01.CCM.0000049952.96496.3E. [DOI] [PubMed] [Google Scholar]
- 51.Phua J, Badia JR, Adhikari NK, et al. Has mortality from acute respiratory distress syndrome decreased over time?: A systematic review. Am J Respir Crit Care Med. 2009;179:220–227. doi: 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]
- 52.Erickson SE, Martin GS, Davis JL, et al. Recent trends in acute lung injury mortality: 1996–2005. Crit Care Med. 2009;37:1574–1579. doi: 10.1097/CCM.0b013e31819fefdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hudson LD. New therapies for ARDS. Chest. 1995;108:79S–91S. doi: 10.1378/chest.108.2_supplement.79s. [DOI] [PubMed] [Google Scholar]
- 54.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 55.Cook DJ, Reeve BK, Guyatt GH, et al. Stress ulcer prophylaxis in critically ill patients. Resolving discordant meta-analyses. Jama. 1996;275:308–314. [PubMed] [Google Scholar]
- 56.Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. discussion 230–232. [DOI] [PubMed] [Google Scholar]
- 57.The NHLBI ARDS Network. Trial of omega-3 fatty acid, gamma-linolenic acid and antioxidant supplemention in the management of acute lung injury (OMEGA). Results presented at: American Thoracic Society International Conference; May, 18, 2009; San Diego, California. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.