Abstract
The μ and δ types of opioid receptors form heteromers that exhibit pharmacological and functional properties distinct from those of homomeric receptors. To characterize these complexes in the brain, we generated antibodies that selectively recognize the μ-δ heteromer and blocked its in vitro signaling. With these antibodies, we showed that chronic, but not acute, morphine treatment caused an increase in the abundance of μ-δ heteromers in key areas of the central nervous system that are implicated in pain processing. Because of its distinct signaling properties, the μ-δ heteromer could be a therapeutic target in the treatment of chronic pain and addiction.
Introduction
Morphine, the choice analgesic in the treatment of chronic pain, elicits its effects through opioid receptors. Repeated or continuous use of morphine leads to the development of tolerance and physical dependence. Opioid receptors are members of the G protein-coupled receptor (GPCR) superfamily characterized by the presence of seven transmembrane regions. To date, three subtypes of the opioid receptor have been identified: μ, δ, and κ. Functional and physical interactions between these receptor subtypes have been noted (1–5). Heteromerization between μ and δ opioid receptors leads to distinct receptor pharmacology in that low non-signaling doses of δ receptor ligands (agonists and antagonists) can potentiate the binding and signaling of μ receptor agonists, an effect not seen in cells expressing only μ receptor homomers (6,7). In addition, while homomers of μ or δ opioid receptors signal via pertussin toxin sensitive inhibitory G-proteins, Gαi, studies show that the μ–δ heteromer either couples to a pertussis toxin insensitive G-protein, Gαz (8), or exhibits a switch in receptor coupling from G-protein to β-arrestin-2 (9,10). In addition, μ–δ heteromerization could play a role in morphine-mediated analgesia since studies with KO animals show that the analgesic effects of morphine are mediated via μ receptors (11) and interestingly, low doses of δ receptor antagonists can potentiate morphine-mediated analgesia (7). For these reasons, μ-δ heteromers are considered to be a choice target for the development of new therapies to treat chronic pain (12). However, relatively little information is available about the biochemical and signaling properties of the endogenous heteromers and their regulation under pathological conditions, mainly due to the lack of appropriate tools to study heteromers in situ.
In the case of GPCRs, antibodies have been used as tools for receptor characterization, as reagents for their purification and tissue localization, and as probes for mapping their functional domains (13). Thus, we reasoned that heteromer-specific antibodies would be a useful tool to study endogenous heteromers in tissue, to probe their regulation in situ, and to delineate the mechanisms of regulation. Using a subtractive immunization strategy (13–16) in which antibody-producing cells to unwanted antigens are eliminated through cyclophosphamide treatment, leading to the enrichment of cells producing antibodies to the desired antigen (in this case, a region shared by the heteromer), we generated μ-δ heteromer-selective antibodies. Using these heteromer-selective antibodies, we show that conditions that lead to the development of morphine tolerance correlate with increased abundance of the μ–δ heteromer in regions of the brain involved in pain perception. This suggests that the μ–δ heteromer could play a role in the development of morphine tolerance. Because the μ–δ heteromer exhibits unique pharmacology in that non-signaling doses of δ receptor ligands can potentiate μ receptor-mediated binding and signaling as well as morphine antinociception (6–10), these results identify this heteromer as a target for the development of new therapeutics in the treatment of chronic or neuropathic pain.
Results
Generation of μ–δ heteromer-selective antibodies
We used a subtractive immunization strategy (14) to generate antibodies that selectively recognize the endogenous μ–δ heteromer but do not recognize either μ or δ receptors (table S1). Mice were first made tolerant to unwanted epitopes on membrane proteins by the simultaneous administration of human embryonic kidney (HEK) 293 cell membranes and cyclophosphamide, which causes the destruction of antibody generating activated B cells (14–16). Once a low titer to HEK293 membrane proteins was achieved, mice were immunized with membranes from HEK293 cells coexpressing μ-δ receptors (fig. S1A). The spleens of mice with high antibody titers were used to generate monoclonal antibodies. The supernatants from the resultant hybridoma clones were screened with HEK293 membranes alone, membranes from cells expressing only μ or δ receptors, and membranes from cells coexpressing both μ and δ receptors. This led to the identification of various antibody-secreting clones (table S1), including the 1E12D1 clone that gave a high signal with membranes from cells coexpressing μ and δ receptors, but not with membranes from cells expressing only μ or δ receptors (table S1).
The 1E12D1 antibody-secreting clone recognized an epitope in cells coexpressing μ and δ receptors, but not in cells coexpressing μ or δ receptors in combination with other family A GPCRs, and in membranes from wild-type animals, but not from animals lacking μ or δ receptors (fig. S1B & Fig. 1A). Preincubation of the antibody with membranes from HEK293 cells expressing both μ and δ receptors, but not those from cells expressing the receptors individually, substantially decreased the recognition of an epitope in SK-N-SH cells, presumably the endogenous μ and δ receptors (fig. S1C). In addition, the μ–δ heteromer antibody exhibited maximal recognition when μ and δ receptors were expressed at a 1:1 ratio, but not 5:1 or 1:5 ratio (fig. S1D). Furthermore, the antibody recognized wild-type μ and δ receptors better than μ–δ chimeric constructs (table S2). These results indicate that the 1E12D1 monoclonal antibody exhibited μ–δ heteromer selectivity. Using these heteromer-selective antibodies, we could isolate μ–δ heteromers from cells expressing recombinant μ-δ receptors as well as endogenous μ-δ heteromers from primary dorsal root ganglion (DRG) neurons (fig. S1E).
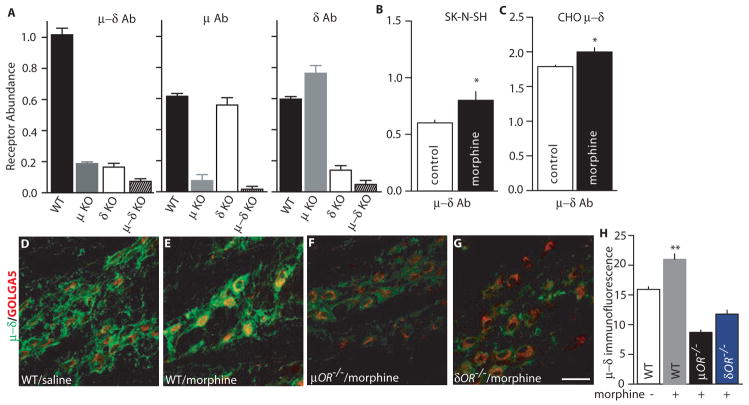
Fig. 1.
Detection of μ–δ heteromers using heteromer-selective monoclonal antibodies. (A) Receptor abundance was determined in cortical membranes from wild-type, μ knockout (KO), δ KO, or μ–δ double KO mice with monoclonal antibodies to μ–δ, μ, or δ receptors by ELISA. (B–C) Cells endogenously (B) or stably (C) coexpressing μ–δ receptors were treated without or with morphine (1 μM) for 48 hours. Heteromer abundance was determined by ELISA with μ–δ heteromer-selective antibodies. Results are means ± SEM (n = 3 experiments). *p < 0.05. (D–G) Repeated morphine treatment increased μ–δ heteromer immunoreactivity in the medial nucleus of the trapezoid body (MNTB). Immunoreactivity in μ KO or δ KO mice after morphine treatment was below the detection limits. (H) Individual neuronal profiles were outlined (n = 15–20/group) and μ–δ heteromer immunoreactivity, as assessed by mean optical density (O.D.), was determined. Morphine treatment increased μ–δ heteromer immunoreactivity in MNTB neurons from wild-type mice (p < 0.01 compared to untreated control), but not in those from μ or δ KO mice.
Increased abundance of endogenous μ–δ heteromers after chronic morphine treatment
Because chronic opiate treatment has been previously reported to lead to increased opioid receptor abundance (17), we examined the effect of chronic morphine treatment on the heteromerization and subcellular distribution of μ and δ opioid receptors in cultured DRG neurons. Immunofluorescence labeling with antibodies to μ and δ opioid receptors in cultured DRG neurons showed μ-δ colocalization in neurons following vehicle or prolonged morphine treatment (fig. S2A). Increased abundance and colocalization was detected at the plasma membrane as well as in intracellular compartments. Quantification of the extent of colocalization of the two receptors demonstrated significantly increased probability of μ andδ heteromer formation in morphine-treated DRG neurons compared to vehicle-treated controls (fig. S2B) in all cell sizes. Chronic, but not acute, treatment led to a significant increase in μ-δ heteromer immunoreactivity that is also seen by ELISA (fig. S2B and C).
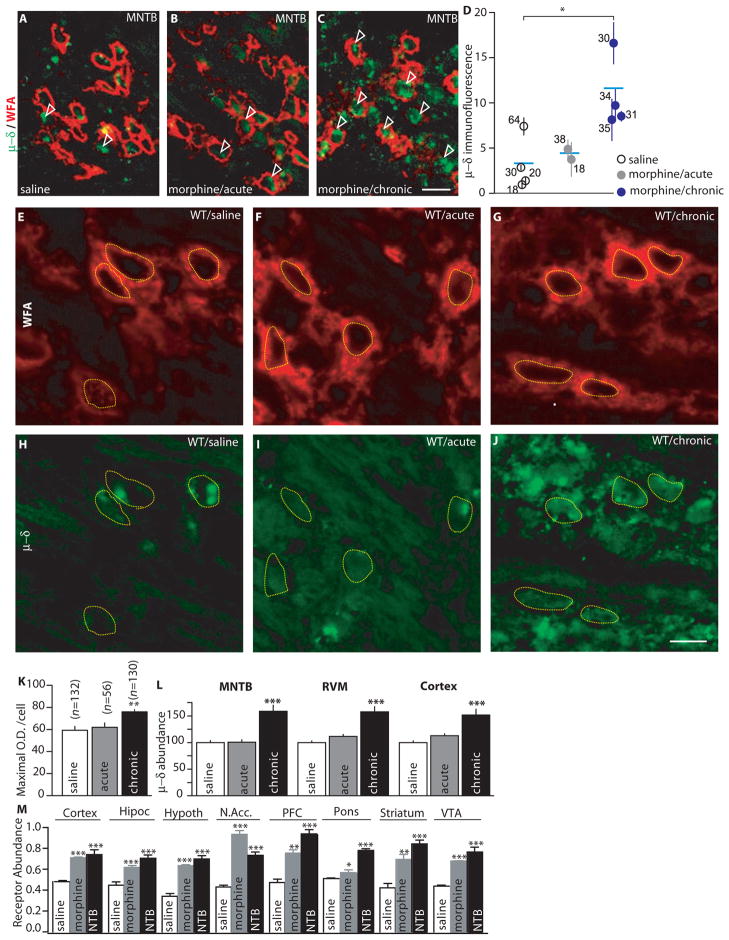
Next, we determined whether chronic morphine treatment triggers the formation of μ–δ heteromers using a chronic escalating dose of morphine administration paradigm (18) that leads to the development of antinociceptive tolerance. Immunohistochemistry using the μ–δ heteromer-selective antibody revealed increased μ–δ heteromer immunoreactivity after morphine administration in the medial nucleus of the trapezoid body (MNTB), an auditory relay nucleus, (Fig. 1, D–G) and the rostral ventral medulla (RVM), a key relay nucleus of pain perception (fig. S3, A to E), relative to saline treatment in wild-type mice. A search through the Allen Brain Atlas (www.brainatlas.org/), a genome-wide map of gene expression in the mouse brain created by high-throughput in situ hybridization analysis, shows that both μ and δ receptor mRNAs are abundant in these nuclei. μ–δ heteromer immunofluorescence in MTNB neurons was higher in wild-type mice than in mice lacking either μ or δ receptors (Fig. 1, D to H). In MTNB neurons μ–δ heteromer immunoreactivity was detected in putative glycinergic neurons that were positive to parvalbumin staining, a calcium binding protein that is expressed in neurons that use either GABA and/or glycine as neurotransmitters (19–21) (fig. S3, F to I). A single (acute) administration of morphine (30 min treatment) did not significantly alter the abundance of μ–δ heteromer immunoreactivity in either the MNTB or RVM as examined by immunohistochemistry (Fig. 2, A to K) or ELISA (Fig. 2L). In contrast, chronic escalating morphine treatment led to significant μ–δ heteromer formation as indicated by both the significantly increased density and intensity of μ–δ heteromer immunoreactivity in MNTB neurons (Fig. 2, A to K). Within MNTB the immunoreactivity was localized to glycinergic neurons ensheated in perineuronal nets, a specialized form of extracellular matrix that provides highly active neurons with a well-hydrated strongly anionic microenvironment that facilitates fast-spiking neuronal activity and the rapid transport of cations (21) (Fig. 2, A to K). Overall, our results indicate that chronic morphine treatment selectively increases μ–δ heteromer assembly in brainstem neurons.
Fig. 2.
Chronic morphine treatment induces μ–δ heteromer abundance in different brain regions. (A–D) Chronic (5 days of escalating dose regime), but not acute (30 min), morphine treatment significantly increased μ–δ heteromer immunoreactivity in perineuronal net-bearing MNTB neurons detected by Wisteria floribunda agglutinin (WFA). Arrowheads indicate neurons bearing μ–δ immunoreactivity ensheated by perineuronal nets. Scale bars = 25 μm. (D) represents the mean grey-scale optical density measured within individual neurons of 2–4 mice. (E–K) Quantitative analysis of the maximal fluorescence intensity of μ–δ immunoreactivity in individual perineuronal net-bearing neurons of the MNTB after acute or chronic morphine treatment. Numbers in parentheses indicate the number of neurons. **p <0.01 (L) ELISA with membranes from the MNTB, RVM, or cortex of mice shows that a 5 day chronic escalating dose of morphine but not a single dose of morphine (acute, 10 mg/kg) or saline significantly increased μ–δ receptor abundance. Results are means ± SEM (n = 3 experiments). *p<0.05; ***p < 0.001 compared to saline. (M) ELISA with membranes from different mice brain regions shows that treatment with morphine (5 mg/kg), or NTB (0.1 mg/kg) for 9 days significantly increased μ–δ receptor abundance. Results are means ± SEM (n=3 experiments). *p<0.05; **p<0.01; ***p<0.001.
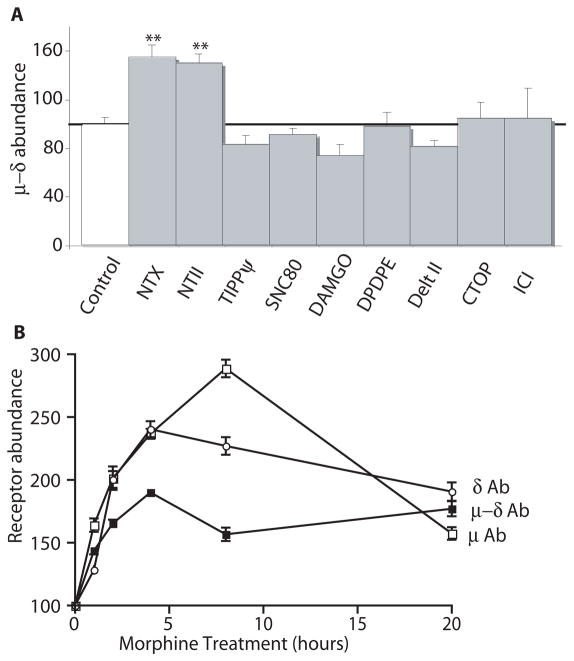
To quantify the morphine-induced increase in abundance of μ–δ heteromers, we carried out ELISA assays with brain membranes from saline and morphine-treated animals. Animals treated chronically with morphine showed increased heteromer abundance in the cortex, MNTB, and RVM (Fig. 2L), as well as in the hypothalamus, nucleus accumbens, and ventral tegmental area, compared to saline-treated controls (Fig. 2M). We had previously shown that δ antagonists could potentiate morphine analgesia. Therefore we examined whether chronic naltriben (NTB), an alkaloid δ antagonist, enhances μ–δ heteromer abundance. Similar to chronic morphine treatment, chronic treatment with naltriben (NTB), an alkaloid δ antagonist, caused increased μ–δ heteromer abundance in different brain regions (Fig. 2M). In addition, treatment of cells expressing recombinant or native μ and δ receptors with morphine for 48 hours significantly increased the abundance of the μ–δ heteromer (Fig. 1B, and fig. S4B). Thus, a component of the morphine-mediated up-regulation of μ–δ heteromer formation can be recapitulated in cell culture systems. Therefore, we used CHO cells expressing recombinant μ and δ receptors and SK-N-SH cells expressing native μ and δ receptors to investigate if μ–δ heteromer up-regulation was induced only by morphine. Treatment of the cell line for 48 hours with alkaloid but not peptide ligands (either antagonists or agonists) increased μ–δ heteromer abundance (Fig. 3A). The time course analysis in SK-N-SH cells revealed that this increase was seen within 2 hours of treatment and peaked around 8 hours (Fig. 3B). This increase was also seen in cells expressing recombinant receptors, thereby suggesting a post-translational mode of regulation of the heteromer.
Fig. 3.
Chronic alkaloid treatment increases μ–δ heteromer abundance. (A) CHO cells stably expressing μ and δ receptors were treated with the indicated ligands (1 μM) for 48 hours and μ–δ heteromer abundance was determined by ELISA. Results are means ± SEM (n=3–4 experiments). **p<0.01. μ–δ abundance refers to ELISA results with the μ–δ heteromer selective antibody (B) Morphine-treated (1 μM) SK-N-SH cells were probed by ELISA using monoclonal antibodies to μ, δ, or μ–δ receptors. Results are means ± SEM (n=3–4 experiments).
Inhibition of agonist binding and signaling by the μ–δ heteromer-selective antibody
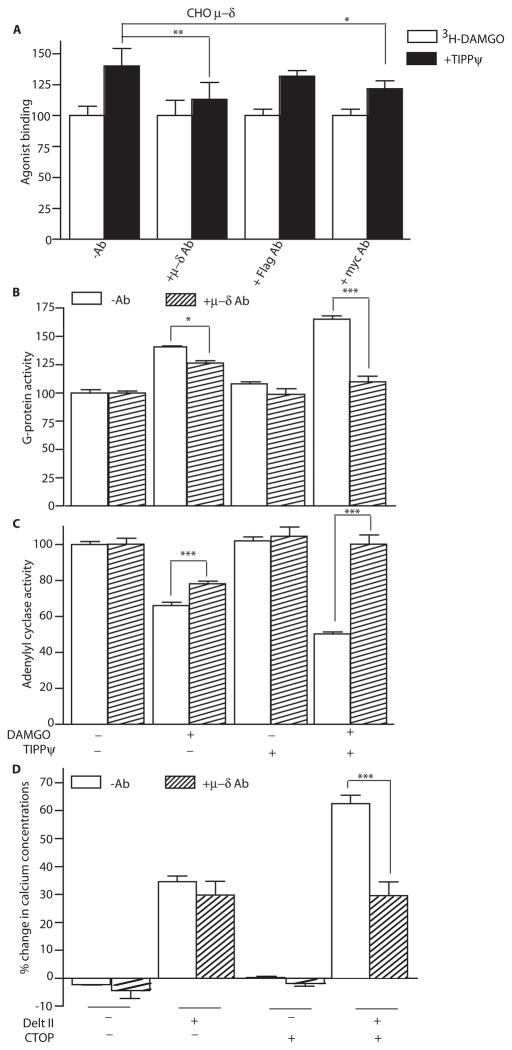
We examined the ability of the μ–δ heteromer-selective antibody to selectively inhibit the ability of low doses of the δ antagonist, TIPPψ [(2S)-2-[[(2S)-2-[[(3S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]-3, 4-dihydro-1H-isoquinolin-3-yl]methylamino]-3-phenylpropanoyl]amino]-3-phenylpropanoic acid], to potentiate the binding and signaling of the μ agonist, DAMGO (Tyr-D-Ala-Gly-MePhe-Gly-ol) (6,7). The μ–δ heteromer-selective antibody significantly decreased δ antagonist-mediated increases in the binding of μ agonist (Fig. 4A and fig. S5A). We used the [35S]GTPγS binding and adenylyl cyclase activity assays to measure the effect of the heteromer-selective antibody on receptor downstream signaling events. Activation of opioid receptors leads to exchange of GTP for GDP at the associated G-protein (Gαi) subunit; this can be measured using a radiolabeled non-hydrolyzable analog of GTP, [35S]GTPγS. This exchange, in turn, leads to the activation of the Gαi subunit followed by inhibition of adenylyl cyclase activity and consequently decreases in intracellular cAMP levels. Using these signaling assays, we find that the heteromer antibody significantly blocked the δ antagonist-mediated increases in μ receptor agonist-mediated signaling in membranes from wild-type animals, but not those from animals lacking μ or δ receptors (Fig. 4, B and C, and fig. S5, B to D).
Fig.4.
μ–δ heteromer-selective antibody blocks heteromer-mediated increases in binding and signaling. (A) CHO cells coexpressing Flag-tagged μ and Myc-tagged δ receptors were pre-treated with either the μ–δ heteromer-selective antibody or monoclonal antibodies to Flag or Myc. Binding of the μ receptor agonist [3H]DAMGO (10 nM) to cells was measured in the absence or presence of δ receptor antagonist TIPPψ (10 nM). Results are means ± SEM (n=3 experiments). (B and C) Mouse cortical membranes were preincubated without or with μ–δ heteromer antibodies and G-protein activity, as assessed by [35S]GTPγS binding (expressed as G-protein activity), (B) or adenylyl cyclase activity (C) in response to DAMGO (1 μM) was determined in the absence or presence of TIPPψ (10 nM). Results are means ± SEM (n=3 experiments). (D) HEK293 cells coexpressing chimeric G16/Gi3 and μ and δ opioid receptors were preincubated without or with μ–δ heteromer antibodies, then treated with the δ opioid agonist deltorphin II (1 μM) in the absence or presence of the μ opioid antagonist CTOP (10 nM) and intracellular Ca2+ concentrations were determined. Results are means ± SEM (n=3 experiments). *p<0.05; **p<0.01; ***p<0.001
We have previously shown that μ receptor antagonists can potentiate δ agonist binding and signaling in cells co-expressing μ and δ receptors (6). Therefore, we examined whether the heteromer-selective antibody could block μ antagonist-mediated increases in δ agonist signaling. For this we used an assay where cells are transfected with μ and δ receptors along with a chimeric G16/Gi3 protein. The activation of this chimeric G-protein following binding of a receptor agonist results in a sequence of events that leads to increases in intracellular Ca2+ concentrations that can be measured using a Ca2+ binding dye. Using this assay we find that the heteromer-selective antibody is able to block μ–δ heteromer-mediated (deltorphin II, a δ agonist, in the presence of CTOP, a μ antagonist) increases in intracellular Ca2+ concentrations in cells coexpressing these receptors (Fig. 4D). These results indicate that our μ–δ heteromer antibody exhibits receptor heteromer selectivity and can be used to probe μ–δ heteromer-mediated effects.
Discussion
Various in vitro studies have demonstrated that GPCRs heteromerize altering their pharmacological and functional properties (1–5). However, the evaluation of the in situ distribution of heteromers, and their functional relevance and modulation under pathological conditions, has been hampered by the lack of appropriate tools. We generated heteromer-selective antibodies using a subtractive immunization strategy in which cyclophosphamide was used to kill cells that generated antibodies to undesired antigens (in this case, HEK293 membranes), thereby increasing the exposure of the desired antigen (μ–δ heteromers) to antibody-producing cells. Our heteromer-selective antibody allowed the detection and isolation of μ–δ heteromers and blocked heteromer-mediated signaling. Thus, a subtractive immunization strategy could be used to generate antibodies against other GPCR heteromers and enable studies examining the abundance and regulation of receptor multimers in normal and pathological states.
The heteromer-selective antibody generated in this study blocked heteromer-mediated ligand binding and signaling and hence could be used to determine the biological contribution of heteromers compared to homomers following receptor activation. These findings are relevant to opiate action because the relative ratio of μ–δ heteromers to μ homomers could play a role in modulating the response to an opiate, and previous studies have shown that the heteromer transduces signaling through a pathway that is distinct from that of receptor homomers (9). μ-δ receptors display decreased G-protein coupling and signaling compared to μ homomers and exhibit a β-arrestin–mediated μ receptor response (9). This, in turn, leads to changes in the spatio-temporal dynamics of signaling as seen with phosphorylation of mitogen-activated protein kinase. In this assay μ homomers exhibited peak phosphorylation at 3–5 min and the activated kinase localized primarily to the nucleus while μ heteromers exhibited peak phosphorylation at 15 min and the activated kinase was retained in the cytoplasm (9). This led to the differential activation of transcription factors (9) that could be responsible for the differences in gene expression that occur with the development of morphine tolerance. Thus, the switch to the “β-arrestin–dependent” signaling cascade could contribute to the changes in morphine response that underlie tolerance. In addition, the absence of morphine tolerance in animals lacking δ receptors (23) or in animals with reduced surface abundance of δ receptors due to deletion of the preprotachykinin gene (24) is consistent with a requirement of μ–δ heteromer mediated signaling in the development of morphine tolerance. Furthermore, mouse knock-outs lacking β-arrestin-2 or δ receptors do not develop morphine tolerance (23, 25). Thus, μ–δ heteromers through their association with β-arrestin2 could underlie the development of tolerance to morphine. Although our data showing increased μ–δ heteromer abundance following chronic morphine administration would support this notion further studies are needed to examine how interactions between β-arrestin2 and μ–δ heteromers contribute to the development of morphine tolerance.
We found that μ–δ heteromer abundance was increased in the RVM following chronic morphine treatment. The RVM, a brain region containing both μ and δ opioid receptor mRNAs, is involved in antinociception through facilitation of the descending inhibitory pain pathways. Malfunction in this circuitry is thought to play a role in neuropathic pain, a condition characterized by the presence of hyperalgesia (supersensitivity to painful stimuli) and tactile allodynia (painful sensation by normally non-painful stimuli) (26, 27). Therefore, if neuropathic pain increases the likelihood of μ–δ heteromer formation in the RVM, this could account for the lack of analgesic potency of morphine in the treatment of neuropathic pain (28), because the μ–δ heteromer signals through a β-arrestin-2 (9) and studies have shown that functional deletion of β-arrestin-2 gene in mice leads to a potentiation as well as prolongation of the analgesic effects of morphine (29). Therefore further studies are needed to examine the effects of neuropathic pain on μ–δ heteromers in the RVM. Glycinergic neurons of the MNTB, an auditory relay nucleus, also showed increased μ–δ heteromer abundance following chronic morphine administration. However, not much is known about the role of these receptors in auditory processing. Because chronic morphine administration causes increased μ–δ heteromer abundance in the MNTB, studies are needed to examine the role of these receptors in both acute and chronic pain states.
We observe co-localization of μ and δ receptors in cultured DRG neurons that is increased upon prolonged treatment with morphine. A recent study with mice with a knock-in of δ opioid receptor tagged with EGFP (δEGFP) used antibodies against GFP and μ receptors and showed that δEGFP colocalizes with μ opioid receptors in less than 5% of DRG neurons (30). This degree of colocalization could be an underestimate because these mice have increased abundance of δ opioid receptors (31), and the GFP antibody exhibits higher avidity for GFP than the μ antibody does towards μ receptors. This may result in an overestimation of δ opioid receptor receptor abundanve selective to μ receptors. In addition, the GFP tag at the C-terminus increases cell surface localization of the δ opioid receptor (32). When taken together with the evidence that increased abundance of δ opioid receptor attenuates the maturation of the μ opioid receptor (33), these results suggest that the low degree of colocalization between δEGFP and the μ opioid receptor (30) could be due to alterations in δ opioid receptor maturation. Thus, as supported by our immunostaining data μ and δ opioid receptors may colocalize in DRGs, as well as in other regions of the brain.
In summary, we report the generation of a μ–δ heteromer-selective antibody that enabled us to examine chronic morphine treatment-mediated up-regulation of μ–δ heteromers in endogenous tissue. The subtractive immunization strategy used in the generation of the μ–δ heteromer-selective antibodies could be used to generate antibodies selective for other GPCR heteromers. This would help studies examining the role of GPCR heteromers in physiological and pathophysiological conditions.
Materials and Methods
ELISAs
ELISAs were carried out as previously described (34) either using cells (2 × 105 cells/well) expressing individual receptors, or cells coexpressing μ and δ opioid receptors, that were treated with 1 or 10 μM of different opioid ligands for 0 to 72 hours, cultured DRG neurons, or with membranes (10 μg) prepared from different brain regions of wild type, μ KO, δ KO, μ–δ double KO mice, or from mice that were treated either acutely or with escalating doses of morphine. Data obtained with μ–δ heteromer-selective antibodies in ELISA assays has been expressed as μ–δ abundance in the figures.
Binding assays
CHO or SK-N-SH cells (2 × 105 cells/well) coexpressing μ and δ opioid receptors were incubated with 5 μg antibodies for 10 min at room temperature (RT), then with [3H]DAMGO (10 nM) in the presence of antibodies and in the absence or presence of TIPPψ (10 nM) (35).
GTPγS binding assays
Membranes (10 μg) were incubated with 5 μg antibodies for 10 min at RT, then with 1 μM DAMGO, antibodies, and 10 nM TIPPψ and [35S]GTPγS binding was determined as described (7).
Adenylyl cyclase assays
Membranes (10 μg) were incubated with 5 μg antibodies for 10 min at RT, then with 1 μM DAMGO in the absence or presence of 10 nM TIPPψ and in the presence of the antibodies. Adenylyl cyclase activity was determined as described (36, 37).
Gai16-facilitated Ca2+ release
CHO cells coexpressing a chimeric G16/Gi3, μ, and δ opioid receptors were plated into poly-L-lysine coated 96-well clear bottom plates (40,000 cells/well). The next day, the growth media was removed, and the cells were washed twice in HBSS buffer containing 20 mM HEPES. Cells were incubated with Fluo-4 NW calcium dye (3 μM in 100 μl) for 1 hour at 37°C. The cells were preincubated for 30 min without or with 5 μg antibodies. Deltorphin II (1 μM) or CTOP (10 nM) were added to the wells (in the presence of the antibodies) by the robotic arm of the FLEX Station and intracellular Ca2+ concentrations was measured for 300 seconds at excitation 494 nm and emission 516 nm.
Immunoprecipitation and Western blotting
CHO cells coexpressing HA-tagged μ and Flag-tagged δ receptors or DRGs with endogenous μ and δ receptors [isolated from embryonic rat pups at E16 (38)] were immunoprecipitated using the μ–δ heteromer-selective antibody as described (7). μ and δ receptors were detected in the immunoprecipitates using polyclonal antibodies to the epitope tags (CHO cells) or to the individual receptors (DRGs). The δ receptor antibodies were from Chemicon or Proteimax while the μ receptor antibodies were a gift from Dr. T. Cote (USUHS).
Colocalization of μ, δ, and μ-δ receptors in DRG neurons
Dorsal root ganglion (DRG) neurons from adult rats were grown in culture for 4 days. They were then treated with either vehicle (saline) or morphine (10 μM, 48 hours) prior to fixation with 4% paraformaldehyde in 0.1 M phosphate buffer for 15 min at 37C. Immunocytochemical labeling for μ and δ opioid receptors was accomplished with antibodies against the μ receptor (Neuromics) and the δ receptor (Alamone) and Alexa 488- and 594-conjugated secondary antibodies, respectively. Photomicrographs were captured by a Leica TCS SP2 multi-photon confocal microscope (63× primary magnification; Leica Microsystems) and images were acquired and digitized for quantitative analysis with Leica Confocal Software.
Animal studies
Animal studies were carried out according to protocols approved by the Mount Sinai School of Medicine Animal Care and Use Committee. Mice lacking μ opioid (35) (n = 6) or δ opioid receptors (36) (n = 6) were back-crossed onto a C57Bl6/J background, bred, and maintained as described (39, 40). Wild-type littermates (n = 8) were used as controls in pharmacological challenge experiments. Morphine was systemically administered according to a chronic intermittent escalating-dose protocol (from 10 mg/kg on day 1 to 100 mg/kg on day 5) or acutely (a single injection of 10 mg/kg). For tissue collection, each mouse brain was placed in a mouse brain mold (Braintree Sci) and the brainstem was cornonally sliced at 1mm thickness. The ventral portion of the 1 mm slice located between −4.60mm and −5.60mm relative to Bregma included the MNTB and RVM. Using a 14-gauge tissue needle (Scientific Commodities), bilateral MNTB and midline RVM regions were punched, pooled, and stored at −80°C until used. For immunohistochemistry, animals were perfused with ice-cold 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4) that was preceded by a short pre-rinse with physiological saline. Tissues were cryoprotected in 30% sucrose in PB.
Immunohistochemistry
Coronal sections of wild-type and knock-out mice treated or not with morphine were prepared on a cryostat microtome at a 16 μm thickness and thaw-mounted onto fluorescence-free glass slides. Sections at identical anterior-posterior coordinates from wild-type and μ KO or δ KO mice treated or not with drug were mounted onto each glass slide to allow simultaneous histochemical processing. Similar simultaneous tissue processing was performed when comparing the effects of acute and chronic morphine treatments, with sections spanning the MTNB and RVM from a control, acute or chronic morphine-treated mouse mounted onto each glass slide. Specimens were rinsed in phosphate-buffered saline (PBS, 0.01 M; pH 7.4), blocked in PBS containing 10% normal donkey serum and 0.3% Triton X-100, and incubated in diluted (> 1:4,000) supernatant from the 1E12D1 hybridoma secreting clone (expressing monoclonal μ–δ heteromer-selective antibody) that was diluted in PBS 0.3% Triton X-100 and 0.1% NaN3 at 4°C for 16–24 hours. Immunoreactivity was visualized using the tyramide signal amplification system (TSA-Plus; NEN Life Science Products): sections were washed in TNT buffer (0.1 M Tris-HCl (pH 7.5); 0.15 M NaCl; 0.05% Tween 20) for 10 min, followed by 30 min incubation with horseradish peroxidase-conjugated donkey anti-mouse IgG (1:500, Jackson ImmunoResearch). After three washes in TNT, sections were exposed to fluorescein- or carbocyanine (Cy) 3-conjugated biotinyl-tyramide diluted in amplification diluent (1:100) for 15 min at RT, and thereafter washed twice in TNT buffer. After single-staining to reveal μ-δ heteromers, sections were repeatedly washed in PBS and exposed to rabbit anti-golgin subfamily A5 (GOLGA5; 1:1,000; Atlas Antibodies) (39–41) or goat anti-parvalbumin antibodies (1:1,1000; SWant); biotinylated Wisteria floribunda agglutinin (WFA) was used to visualize perineuronal nets (20 μg/ml; Sigma) (38) overnight at 4C. After repeated rinses in TNT buffer, immunoreactivities were revealed by using Cy2-, Cy3- or Cy5-conjugated donkey anti-rabbit or anti-goat antibodies (1:300, Jackson). WFA binding was revealed by Cy2-tagged streptavidin (1:5,000; Jackson).
Specimens were imaged with a confocal laser-scanning microscope (Model 510, Zeiss) equipped with appropriate excitation and emission filters for maximum separation of Cy2 (505–530 nm, band-pass), Cy3 (560–610 nm, band-pass) and Cy5 signals (>650 nm, long-pass). Images were taken by using identical pinhole, detector gain and offset, and amplification gain settings to allow direct comparisons of degree of immunoreactivity, which was measured from primary unmodified confocal laser-scanning microscope images using NIH ImageJ optical software (version 1.41). The mean grey-scale optical density and the maximum of pixel intensity were measured within individual neurons obtained from n = 2–4 animals per treatment group. Results were analyzed using one-way ANOVA design with Bonferroni post-hoc correction. Digital images were color-coded for optimal visualization of signals and underwent linear optimization of brightness and contrast using Adobe Photoshop CS3 (Adobe Systems).
Supplementary Material
Fig. S1. Generation and characterization of μ–δ heteromer-selective antibodies.
Fig. S2. Subcellular distribution and colocalization of μ and δ opioid receptors.
Fig. S3. Chronic morphine treatment induces increased μ–δ heteromer immunoreactivity in the brainstem.
Fig. S4. Chronic morphine treatment increases μ–δ heteromer abundance.
Fig. S5. μ–δ heteromer selective antibodies block heteromer-mediated binding and signaling.
Table S1. Characterization of select hybridoma clones.
Table S2. Heteromer-selective antibody recognition using chimeric μ–δ constructs.
Acknowledgments
We thank Dr. T. Cote (USUHS) for the gift of polyclonal antibodies to μ opioid receptors; Prof. B. Kieffer (IGBMC) for the gift of μ and δ knock-out animals; Prof. J. Pintar for the gift of bains from μ, δ, and μ–δ knock-out animals; Amira Prodhen for help with processing the tissue and Dr. Khatuna Gagnidze for help in preparation of primary DRG cultures. This work was supported in part by NIH grants DA023214 (T.H.), DC08301 (E.M.), DC06696 (E.M.), DC07984 (E.M.), T32GM062754 (I.B.), DA019521 (L.A.D.), DA08863(L.A.D.), GM071558 (L.A.D.) and CIHR and CRC (C.C.) and by the Scottish Universities Life Science Alliance (T.H.), EMBO Young Investigator Program (T.H.). Jan Mulder is an Alzheimer’s Research Trust fellow.
Footnotes
Author contributions: A.G. generated heteromer selective monoclonal antibodies and carried out ELISA and binding assays; J.M. carried out immunohistochemistry studies with animal tissue; I.G. carried out western blot analysis, signaling assays, data analysis and helped in manuscript preparation; R.R. helped with signaling assays and critically reviewed the manuscript; I.B. carried out ELISA assays with MNTB and RVM membranes; E.O. examined effect of morphine on heteromer levels in DRG cultures; M.L. carried out RT-PCR studies; E.M. helped in intracellular calcium assays; M.J. helped in experiments examining effect of morphine on heteromer levels in DRG cultures; C.M.C. designed and analysed immunofluorescence experiments with DRGs; T.H. designed and analyzed immunohistochemistry data with animals; and L.A.D. initiated this project, designed the experiments and wrote the manuscript
Conflicts of interest: None.
References and Notes
- 1.Milligan G. G protein-coupled receptor dimerisation: molecular basis an relevance to function. Biochim Biophys Acta. 2007;1768:825–835. doi: 10.1016/j.bbamem.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 2.Prinster SC, Hague C, Hall RA. Heterodimerization of G protein-coupled receptors: specificity and functional significance. Pharmacol Rev. 2005;57:289–298. doi: 10.1124/pr.57.3.1. [DOI] [PubMed] [Google Scholar]
- 3.Maggio R, Novi F, Scarselli M, Corsini GU. The impact of G-protein-coupled receptor hetero-oligomerization on function and pharmacology. FEBS J. 2005;272:2939–294. doi: 10.1111/j.1742-4658.2005.04729.x. [DOI] [PubMed] [Google Scholar]
- 4.Terrillon S, Bouvier M. Roles of G-protein coupled receptor dimerization. EMBO Rep. 2004;5:30–34. doi: 10.1038/sj.embor.7400052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rios CD, Jordan BA, Gomes I, Devi LA. G-protein-coupled receptor dimerization: modulation of receptor function. Pharmacol Ther. 2001;92:71–87. doi: 10.1016/s0163-7258(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 6.Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. J Neurosci. 2000;20:RC110 . doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci U S A. 2004;101:5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, O’Dowd BF. Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J Biol Chem. 2000;275:26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- 9.Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J. 2007;21:2455–2465. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rozenfeld R, Devi LA. Receptor heterodimerization and drug discovery. Trends Pharmacol Sci. 2010;31:124–130. doi: 10.1016/j.tips.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kieffer BL. Opioids: first lessons from knockout mice. Trends Pharmacol Sci. 1999;20:19–26. doi: 10.1016/s0165-6147(98)01279-6. [DOI] [PubMed] [Google Scholar]
- 12.Rozenfeld R, Décaillot FM, Ijzerman AP, Devi LA. Heterodimers of G protein-coupled receptors as novel and distinct drug targets. Drug Discovery Today: Therapeutic Strategies. 2006;3:437–443. [Google Scholar]
- 13.Gupta A, Heimann AS, Gomes I, Devi LA. Antibodies against G-protein coupled receptors: novel uses in screening and drug development. Comb Chem High Throughput Screen. 2008;11:463–7. doi: 10.2174/138620708784911465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salata RA, Malhotra IJ, Hampson RK, Ayers DF, Tomich CS, Rottman FM. Application of an immune-tolerizing procedure to generate monoclonal antibodies specific to an alternate protein isoform of bovine growth hormone. AnalyticalBiochemistry. 1992;207:142–149. doi: 10.1016/0003-2697(92)90515-9. [DOI] [PubMed] [Google Scholar]
- 15.Sleister HM, Rao AG. Strategies to generate antibodies capable of distinguishing between proteins with >90% amino acid identity. J Immunol Methods. 2001;252:121–129. doi: 10.1016/s0022-1759(01)00346-5. [DOI] [PubMed] [Google Scholar]
- 16.Sleister HM, Rao AG. Subtractive immunization: a tool for the generation of discriminatory antibodies to proteins of similar sequence. J Imuunol Methods. 2002;261:213–220. doi: 10.1016/s0022-1759(01)00567-1. [DOI] [PubMed] [Google Scholar]
- 17.Lucido AL, Morinville A, Gendron L, Stroh T, Beaudet A. Prolonged morphine treatment selectively increases membrane recruitment of delta-opioid receptors in mouse basal ganglia. J Mol Neurosci. 2005;25:207–14. doi: 10.1385/JMN:25:3:207. [DOI] [PubMed] [Google Scholar]
- 18.Décaillot FM, Che FY, Fricker LD, Devi LA. Peptidomics of Cpefat/fat mouse hypothalamus and striatum: effect of chronic morphine administration. J Mol Neurosci. 2006;28:277–284. doi: 10.1385/JMN:28:3:277. [DOI] [PubMed] [Google Scholar]
- 19.Laing I, Todd AJ, Heizmann CW, Schmidt HH. Subpopulations of GABAergic neurons in laminae I–III of rat spinal dorsal horn defined by coexistence with classical transmitters, peptides, nitric oxide synthase or parvalbumin. Neuroscience. 1994;61:123–32. doi: 10.1016/0306-4522(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 20.Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antal M, Polgár E, Chalmers J, Minson JB, Llewellyn-Smith I, Heizmann CW, Somogyi P. Different populations of parvalbumin- and calbindin-D28k-immunoreactive neurons contain GABA and accumulate 3H-D-aspartate in the dorsal horn of the rat spinal cord. J Comp Neurol. 1991;314:114–124. doi: 10.1002/cne.903140111. [DOI] [PubMed] [Google Scholar]
- 22.Härtig W, Singer A, Grosche J, Brauer K, Ottersen OP, Brückner G. Perineuronal nets in the rat medial nucleus of the trapezoid body surround neurons immunoreactive for various amino acids, calcium-binding proteins and the potassium channel subunit Kv3.1b. Brain Res. 2001;899:123–133. doi: 10.1016/s0006-8993(01)02211-9. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Y, King M, Schuller A, Nitsche J, Reidl M, Elde RP, Unterwald E, Pasternak GW, Pintar GWJE. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 24.Guan JS, Xu ZZ, Gao H, He SQ, Ma GQ, Sun T, Wang LH, Zhang ZN, Lena I, Kitchen I, Elde R, Zimmer A, He C, Pei G, Bao L, Zhang X. Interaction with vesicle luminal protachykinin regulates surface expression of delta-opioid receptors and opioid analgesia. Cell. 2005;122:619–631. doi: 10.1016/j.cell.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- 26.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 27.Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain. 2002;100:1–6. doi: 10.1016/s0304-3959(02)00368-8. [DOI] [PubMed] [Google Scholar]
- 28.Przewlocki R, Przewlocka B. Opioids in neuropathic pain. Curr Pharm Des. 2005;11:3013–25. doi: 10.2174/1381612054865055. [DOI] [PubMed] [Google Scholar]
- 29.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 30.Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–59. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scherrer G, Tryoen-Tóth P, Filliol D, Matifas A, Laustriat D, Cao YQ, Basbaum AI, Dierich A, Vonesh JL, Gavériaux-Ruff C, Kieffer BL. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci U S A. 2006;103:9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang HB, Guan JS, Bao L, Zhang X. Distinct subcellular distribution of delta-opioid receptor fused with various tags in PC12 cells. Neurochem Res. 2008;33:2028–2034. doi: 10.1007/s11064-008-9678-9. [DOI] [PubMed] [Google Scholar]
- 33.Décaillot FM, Rozenfeld R, Gupta A, Devi LA. Maturation and cell surface targeting of μ-δ heterodimers by RTP4. Proc Natl Acad Sci U S A. 2008;105:16045–16050. doi: 10.1073/pnas.0804106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta A, Décaillot FM, Gomes I, Tkalych O, Heimann AS, Ferro ES, Devi LA. Conformation state-sensitive antibodies to G-protein-coupled receptors. J BiolChem. 2007;282:5116–5124. doi: 10.1074/jbc.M609254200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomes I, Filipovska J, Devi LA. Opioid receptor oligomerization. Detection and functional characterization of interacting receptors. Methods Mol Med. 2003;84:157–183. doi: 10.1385/1-59259-379-8:157. [DOI] [PubMed] [Google Scholar]
- 36.Heimann AS, Gomes I, Dale CS, Pagano RL, Gupta A, de Souza LL, Luchessi AD, Castro LM, Giorgi R, Rioli V, Ferro ES, Devi LA. Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc Natl Acad Sci U S A. 2007;104:20588–20593. doi: 10.1073/pnas.0706980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Unterwald EM, Cox BM, Kreek MJ, Cote TE, Izenwasser S. Chronic repeated cocaine administration alters basal and opioid-regulated adenylyl cyclase activity. Synapse. 1993;15:33–38. doi: 10.1002/syn.890150104. [DOI] [PubMed] [Google Scholar]
- 38.Breit A, Gagnidze K, Devi LA, Lagacé M, Bouvier M. Simultaneous activation of the delta opioid receptor (deltaOR)/sensory neuron-specific receptor-4 (SNSR-4) hetero-oligomer by the mixed bivalent agonist bovine adrenal medulla peptide 22 activates SNSR-4 but inhibits deltaOR signaling. Mol Pharmacol. 2006;70:686–696. doi: 10.1124/mol.106.022897. [DOI] [PubMed] [Google Scholar]
- 39.Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dollé P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 40.Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gavériaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- 41.Mulder J, Wernérus H, Shi TJ, Pontén F, Hober S, Uhlén M, Hökfelt T. Systematically generated antibodies against human gene products: high throughput screening on sections from the rat nervous system. Neuroscience. 2007;146:1689–1703. doi: 10.1016/j.neuroscience.2007.02.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Generation and characterization of μ–δ heteromer-selective antibodies.
Fig. S2. Subcellular distribution and colocalization of μ and δ opioid receptors.
Fig. S3. Chronic morphine treatment induces increased μ–δ heteromer immunoreactivity in the brainstem.
Fig. S4. Chronic morphine treatment increases μ–δ heteromer abundance.
Fig. S5. μ–δ heteromer selective antibodies block heteromer-mediated binding and signaling.
Table S1. Characterization of select hybridoma clones.
Table S2. Heteromer-selective antibody recognition using chimeric μ–δ constructs.