Abstract
Obesity is associated with the metabolic syndrome, a significant risk factor for developing type 2 diabetes and cardiovascular diseases. Chronic low-grade inflammation occurring in the adipose tissue of obese individuals is causally linked to the pathogenesis of insulin resistance and the metabolic syndrome. Although the exact trigger of this inflammatory process is unknown, adipose tissue hypoxia, endoplasmic reticular stress, and saturated fatty acid–mediated activation of innate immune processes have been identified as important processes in these disorders. Furthermore, macrophages and T lymphocytes have important roles in orchestrating this immune process. Although energy restriction leading to weight loss is the primary dietary intervention to reverse these obesity-associated metabolic disorders, other interventions targeted at alleviating adipose tissue inflammation have not been explored in detail. In this regard, (n-3) PUFA of marine origin both prevent and reverse high-fat-diet–induced adipose tissue inflammation and insulin resistance in rodents. We provide an update on the pathogenesis of adipose tissue inflammation and insulin resistance in obesity and discuss potential mechanisms by which (n-3) PUFA prevent and reverse these changes and the implications in human health.
Introduction
Obesity is a major health problem in the United States and worldwide. It is associated with metabolic syndrome, which is characterized by hyperglycemia, abdominal obesity, hypertension, elevated plasma TG, and reduced plasma HDL cholesterol levels (1). Individuals with metabolic syndrome frequently exhibit proinflammatory and prothrombotic metabolic profiles and are at a higher risk for developing type 2 diabetes and cardiovascular disease. Recent evidence has causally linked obesity and increased adiposity to the pathogenesis of metabolic syndrome and type 2 diabetes. Furthermore, adipose tissue dysfunction and inflammation have been identified as major players in these disorders.
Although energy restriction leading to weight loss is a successful dietary intervention for improving obesity-associated metabolic disorders, other dietary interventions such as ones targeted at reducing adipose tissue inflammation, regardless of weight loss, have not been explored in detail. Long-chain (n-3) PUFA, namely EPA and DHA, have antiinflammatory properties (2). Moreover, they are well documented for reducing plasma TG (3, 4) and these fatty acids exhibit antiobesity effects on humans (5) and rodents (6).
Currently, the effect of EPA and DHA on insulin sensitivity is not well characterized. Whereas these fatty acids consistently prevent the development of insulin resistance associated with high-fat (7, 8) or high-sucrose (9) feeding in rodents, they do not significantly improve insulin sensitivity in individuals with type 2 diabetes (10–12). Nevertheless, some promising evidence suggests that EPA and DHA might help delay the progression of metabolic syndrome to type 2 diabetes (13). In this context, elucidating the mechanisms responsible for improvement of insulin sensitivity due to EPA and DHA might enhance the understanding of the pathophysiology of obesity-associated insulin resistance and identification of nutritionally relevant targets for the treatment of metabolic syndrome.
This review provides an update on the mechanistic aspects of the pathogenesis of adipose tissue inflammation and insulin resistance in obesity, followed by a summary of potential mechanisms by which EPA and DHA prevent and reverse these processes.
Current status of knowledge
Adipose tissue dysfunction in obesity
White adipose tissue is the major site for storage of excess energy in the body. It is composed of adipocytes, an extracellular matrix (ECM),8 vascular and neural tissues, and other cell types (14). These other cell types include preadipocytes, fibroblasts, stem cells, and immune cells such as macrophages and T lymphocytes. Adipose tissue secretes numerous bioactive peptides collectively known as adipokines (15, 16). Examples include hormones involved in energy and glucose homeostasis such as leptin, adiponectin, resistin, apelin, and visfatin; chemokines such as monocyte chemotactic protein (MCP)-1 and IL-8; other proinflammatory cytokines such as IL-6, IL-1, angiotensin-II, and TNF-α; and antiinflammatory cytokines such as IL-10 (Table 1). Thus, adipose tissue is a dynamic endocrine organ with major roles in energy balance, glucose homeostasis, blood pressure regulation, and immune function (17).
Table 1.
Major adipocytokines and their functions
| Adipokine | Physiological effects | Reference |
| Leptin | Reduces energy intake and increases expenditure, angiogenesis, and hematopoiesis, immune functions | (130) |
| Adiponectin | Improves insulin sensitivity, antiinflammatory, antiatherogenic, promotes fatty acid oxidation | (131) |
| Resistin | Promotes insulin resistance | (132) |
| Angiotensin-II/angiotensinogen | Vasoconstriction, sodium and water retention, increases blood pressure, proinflammatory, promotes insulin resistance and induces lipogenesis | (133–135) |
| MCP-1 | Promotes macrophage infiltration and insulin resistance, proinflammatory, chemotaxic | (136,137) |
| TNFα | Proinflammatory, promotes insulin resistance | (72) |
| PAI-1 | Prothrombotic, proinflammatory | (138) |
| IL-6 | Proinflammatory | (139) |
| IL-10 | Antiinflammatory | (140) |
| Visfatin | Insulin-mimetic actions, cell proliferation | (141) |
| Apelin | Promotes glucose uptake, angiogenesis | (142) |
| Retinol-binding protein-4 | Promotes insulin resistance | (143) |
| Vascular endothelial growth factor | Angiogenesis | (144) |
| Nerve growth factor | Neuronal development | (145) |
| IL-1 | Proinflammatory | (146) |
| IL-1 receptor antagonist | Antiinflammatory | (146) |
| Vaspin | Insulin-sensitizing effects | (147) |
| Omentin | Regulates insulin action | (148) |
| Neuropeptide Y | Energy homeostasis, proliferation of preadipocytes | (149) |
| Hepcidin | Proinflammatory | (150) |
| IL-8 | Proinflammatory, chemotaxic | (151) |
| IL-18 | Proinflammatory | (152) |
| Thrombospondin-1 | Proinflammatory | (153) |
| Serum amyloid A | Proinflammatory, lipolytic | (154) |
| Chemerin | Impairs glucose tolerance | (155) |
Excessive TG accumulation within adipocytes, presumably linked to adipose tissue overload as a result of positive energy balance, leads to adipocyte hypertrophy and a dysregulation of adipokine secretory patterns. This has been primarily attributed to an imbalance between secretions of pro- compared to antiinflammatory adipokines. Thus, obesity is associated with a chronic low-grade inflammation in the adipose tissue (18, 19). Although adipocytes are a source of proinflammatory cytokines in obesity (15, 20), cells of the stromal vascular fraction such as preadipocytes (21), macrophages, and adipose stem cells can produce even higher levels of these cytokines (22). Major cell types that play key roles in the inflammatory response during onset of obesity are illustrated in Figure 1.
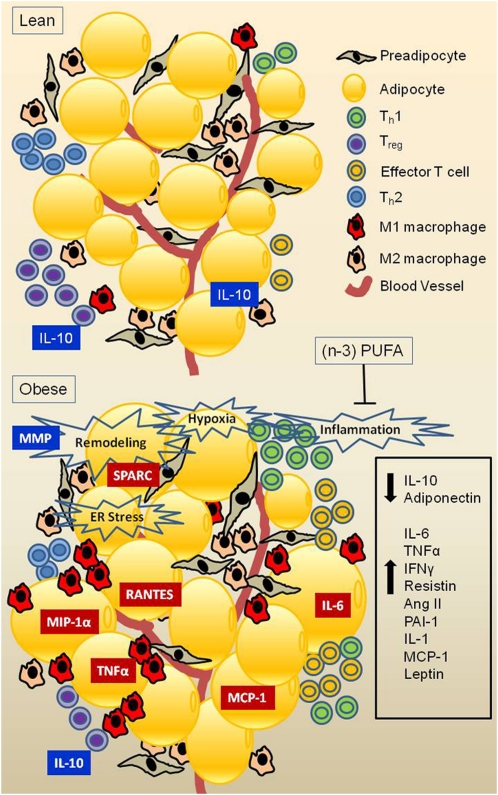
Figure 1.
Obesity-associated immune cell infiltration of adipose tissue. Lean individuals exhibit higher ratios of M2:M1 macrophage, Th1:Th2 T cell, and regulatory:effector T cell. Excessive TG accumulation leads to adipose tissue remodeling, relative hypoxia, and ER stress, which trigger production of chemokines and changes in the above cell ratios, culminating in increased production of proinflammatory adipokines and reduced production of antiinflammatory adipokines.
Although the exact trigger for the onset of adipose tissue inflammation is hitherto unknown, several possible mechanisms have been suggested and are discussed below. In a state of positive energy balance, adipose tissue expands to accommodate the storage of excess TG. Adipose tissue remodeling via degradation of the ECM and adipogenesis are 2 key processes in this expansion. Matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases play important roles in ECM degradation and adipose tissue remodeling (23, 24). Defective adipose tissue expansion as a result of dysregulation of any of the above factors could lead to adipocyte injury, death, and inflammation (25). For example, factors that promote adipose tissue fibrosis, such as secreted protein acidic and rich in cysteine, are associated with obesity and adipose tissue inflammation (25).
Rodent studies show that increasing adipose tissue mass without a similar magnitude increase in supporting vasculature could lead to tissue hypoxia, triggering the expression of hypoxia-inducible factor-1 and inflammatory genes (26). Similarly, oxygen partial pressure in subcutaneous adipose tissue negatively correlates with adiposity in humans (27). Thus, hypoxia could be a trigger for adipose tissue inflammation. Both animal and human studies support the role of adipose tissue endoplasmic reticulum (ER) stress as another key elicitor for subsequent inflammation in obesity (28–31).
Although there is evidence that adipose tissue expansion per se is an important initiator of the inflammatory processes during the development of obesity, other lines of evidence suggest that type of dietary fat is also an important factor in triggering this process. For example, the NF-κB pathway in the visceral adipose tissue is activated 2 h after consumption of a meal rich in SFA in rodents (32). Other studies have shown similar chronic effects of fatty acids in triggering inflammation in the adipose tissue (33–35). Given that these fatty acids are ligands for Toll-like receptors (TLR) 2 and 4, and because TLR2 and 4 are expressed in human adipocytes (36), it is likely that the effect of SFA on adipose tissue inflammation is mediated via these receptors. Indeed, obesity-induced adipose tissue inflammation is attenuated in mice with a mutation in TLR4 (37). Furthermore, TLR2 knockout mice are also protected from high-fat-diet–induced insulin resistance and β-cell dysfunction (38). Other receptors of the innate immune system such as Nod-like receptors are also implicated in obesity and high-saturated fat–associated adipose inflammation (39).
Conversely, there are studies showing that adipose tissue inflammation can be reduced without changing adipose mass (40, 41). This is also consistent with our recent findings that EPA reverses high-fat–induced metabolic disorders and adipose inflammation (42). In this study, EPA supplementation reversed high-saturated fat diet-induced insulin resistance and hepatic steatosis and increased adipose tissue MCP-1 and plasminogen activator inhibitor (PAI)-1 levels in C57BL/6J mice. Moreover, high-fat feeding–induced adipose tissue inflammation is not completely resolved following energy restriction (43). Taken together, the current research suggests a role of dietary fat in the onset of adipose tissue inflammation.
Adipose tissue inflammation in obesity is characterized by macrophage infiltration (18, 44) (Fig. 1). Adipose tissue macrophages (ATM) are classified into 2 main types. M1 or classically activated macrophages are stimulated by IFNγ and LPS and produce proinflammatory cytokines such as TNFα, IL-6, and IL-1 and reactive oxygen species such as NO (Fig. 1). M2 or alternatively activated macrophages are activated by IL-4 and IL-13 and express antiinflammatory factors IL-10, TGFβ, IL-1 receptor antagonist-a, IL-4, and arginase (45, 46). Phenotypically, murine ATM express the F4/80 antigen. The murine M1 ATM highly express cluster of differentiation (CD) 11c, and the M2 express macrophage galactose N-acetyl-galactosamine specific lectin (MGL) 1 (47). In contrast, human ATM express CD14, whereas CD11c is only poorly expressed. Human ATM also express CD206, CD209, and CD163 (47).
Obesity induces an M2 to M1 shift in ATM populations, characterized by a reduction in antiinflammatory IL-10 and arginase production and an increase in proinflammatory TNFα production (48) (Fig. 1). This increase in M1 ATM could be due to either a phenotypic switch from M2 to M1 or to additional recruitment of M1 macrophages from blood vessels. Lipotoxicity of macrophages seems to play a major role in the phenotypic switch of M2 to M1 (49). Detailed mechanisms of the M2 to M1 switch have previously been reviewed and summarized by Olefsky et al. (16). Briefly, TLR4 ligands such as SFA activate NF-κB and activator protein 1 transcription factors, leading to increased production of proinflammatory cytokines such as TNFα, IL-6, and IL-1, giving rise to the M1 phenotype. In the lean adipose tissue, this is prevented by repression of TLR4-responsive genes by nuclear receptor corepressor complexes. PPARγ along with IL-4 and IL-13 prevent the signal-dependent turnover of nuclear receptor corepressor and thus help maintain the M2 phenotype. However, it is worth noting that the M1:M2 polarization may be a more complex concept, because recent evidence has shown that in humans, even M2 macrophages are capable of producing excessive amounts of proinflammatory cytokines in obesity (50).
Evidence for M1 recruitment originated from studies showing increased MGL1− C-C motif chemokine receptor (Ccr)+ macrophages recruited around necrotic adipocytes in high-fat-diet–fed mice, whereas the MGL+ ATM levels remained unchanged (51). Adipose tissue from obese animals expresses high levels of chemokines such as MCP-1, macrophage inflammatory protein-1α, and RANTES (Regulated Upon Activation, Normal T-Cell Expressed and Secreted); chemokine receptors such as Ccr2 and Ccr5 (18); and adhesion molecules such as P-selectin glycoprotein ligand (PSGL)-1); (52). The expression of these chemokines, chemokine receptors, and adhesion molecules play a major role in the recruitment of macrophages to adipose tissue in obesity. Indeed, mice overexpressing MCP-1 in the adipose tissue have higher macrophage infiltration, whereas MCP-1 knockout mice are protected from high-fat-diet–induced ATM infiltration (53). In agreement with these findings, Ccr2-deficient mice exhibit low ATM numbers (54), whereas PSGL-1 knockout mice are protected from high-fat-diet–induced adipose inflammation (52). In contrast, however, macrophage inflammatory protein-1α–deficient mice are not protected against high-fat-diet–induced macrophage infiltration into adipose tissue (55). Thus, although MCP-1 appears to be a key mediator of the initiation of adipose tissue inflammation in obesity, the exact mechanisms remain to be elucidated. It is likely that adipocyte hypertrophy, a hallmark of inflamed adipose tissue, is critical in the pathogenesis of these metabolic disorders. This is further supported by clinically relevant findings that adipocyte size positively correlates with MCP-1 expression in humans (56, 57).
Recent evidence also points toward involvement of T cells in obesity-associated adipose tissue inflammation (58). Nishimura et al. (59) showed that CD8 (+) effector T cells infiltrate the adipose tissue in high-fat–fed mice, with a concurrent reduction in CD4 (+) helper (Th) and regulatory T cells. Moreover, these changes occur before the adipose tissue infiltration with macrophages. The adipose tissue infiltration of macrophages is prevented by genetic depletion of CD8 (+) T cells. Feuerer et al. (60) showed that the number of Treg cells in the white adipose tissue of obese mice is significantly lower than in lean ones. Winer et al. (61) showed that obese mice have a higher Th1:Th2 ratio promoting IFNγ secretion from adipose tissue (Fig. 1). Taken together, this suggests that T cells are early modulators of adipose tissue inflammation in obesity. The cytokine profile of these T cells could play an important role in determining the M1/M2 phenotype of ATM.
Molecular mechanisms of insulin resistance
Insulin resistance is defined as an inadequate response by insulin-sensitive tissues (liver, skeletal muscle, and adipose tissue) to normal circulating levels of insulin (62). At physiological levels, insulin inhibits hepatic glucose production, promotes skeletal muscle glucose uptake, and inhibits lipolysis. Insulin resistance leads to impairments in insulin-mediated suppression of hepatic glucose production, skeletal muscle glucose disposal, and inhibition of lipolysis, leading to relative hyperglycemia and increased plasma levels of FFA (16). In response to the relative hyperglycemia, there is a compensatory response by the pancreatic β-cells, which secrete more insulin. This hypersecretion of insulin in turn increases skeletal muscle glucose uptake and inhibits hepatic glucose production to maintain normoglycemia. Thus, insulin-resistant individuals maintain normoglycemia through overproduction and secretion of higher insulin levels (63). Long-term insulin resistance and hypersecretion of insulin eventually leads to pancreatic β-cell failure. These events result initially in prediabetes and glucose intolerance and later progress to hyperglycemia and type 2 diabetes (63).
Insulin exerts its physiological actions on insulin-sensitive tissues via activation of a cascade of intracellular signaling events, all of which have been previously reviewed (64, 65). Briefly, insulin binds to the insulin receptor, triggering its autophosphorylation as well as tyrosine phosphorylation of downstream substrates, including the insulin receptor substrates (IRS). The latter is a critical step in eliciting IRS binding to Src-homology-2 domain of the regulatory subunit of phosphatidylinositol 3-kinase (PI3K). This binding subsequently activates the catalytic subunit of PI3K, which in turn catalyzes the formation of lipid second messenger PIP3. Binding of this lipid moiety to proteins with pleckstrin-homology domains leads to their activation. Additionally, activation of 3-phosphoinositide–dependent protein kinase 1, leads to activation of Akt/protein kinase B. Akt/protein kinase B is a serine/threonine kinase that targets several downstream proteins. Moreover, phosphorylation of Rab small GTPases and inactivation of AS160 by Akt initiates cytoskeletal reorganization and results in translocation of glucose transporter-4 into the cell membrane and facilitates glucose entry into cells.
Akt also phosphorylates and deactivates glycogen synthase kinase 3, which leads to activation of glycogen synthase and subsequent glycogen synthesis. Akt also regulates transcription of several genes involved in gluconeogenesis and lipogenesis via control of winged helix or forkhead box O class of transcription factors. A well-studied example is inhibition by Akt of the forkhead box O-mediated activation of hepatic gluconeogenic genes in the liver (64). Thus, the net effect of these signaling and activation cascades is increased glucose entry into cells as well as increased flux of glucose into intra-cellular metabolic pathways of skeletal muscle and adipose tissue and reduced hepatic gluconeogenesis.
Downregulation of insulin receptor protein level, as seen in obesity, can result in insulin resistance (64). Defective insulin signaling at various levels of the above cascade is also known to be associated with insulin resistance. A reduction in IRS protein levels is also associated with insulin resistance. Hyperinsulinemia itself can reduce IRS protein via transcriptional regulation (65). Suppressor of cytokine signaling (SOCS)-3 blocks the interaction between insulin receptor and IRS and contributes to insulin resistance (66). Serine phosphorylation of IRS by FFA, cytokines (67), and activation of NF-κB–mediated inflammatory pathways (68) is also known to induce insulin resistance. SOCS1 and 3 are also known to induce degradation of IRS (69). Further downstream, higher expression of the regulatory subunit of PI3K is also associated with insulin resistance (70).
Proposed mechanisms for obesity-induced insulin resistance
Obesity induces insulin resistance in skeletal muscle, liver, and adipose tissue (16). Several models have been put forward to explain mechanisms of obesity-induced insulin resistance. The chronic low-grade inflammation occurring in adipose tissue is considered to be a major factor in the pathogenesis of obesity-induced insulin resistance. There are several lines of evidence to support this model. First, adipose-specific overexpression of proinflammatory cytokines such as MCP-1 or Agt induces whole-body insulin resistance (53, 71). Second, neutralization or knockdown of inflammatory mediators such as TNFα, MCP-1, Ccr-2, and PSGL-1 protects rodents from high-fat-diet–induced insulin resistance (52–54, 72). Finally, overexpression of antiinflammatory adipokines such as adiponectin protects rodents from high-fat-diet–induced insulin resistance (73).
Increased proinflammatory cytokines can induce insulin resistance by several mechanisms. As outlined earlier, proinflammatory cytokines can induce SOCS3 expression, which in turn can inhibit insulin signaling by inhibiting IRS action (66). Proinflammatory cytokines also activate numerous intracellular serine kinases such as jun N-terminal kinase (JNK) and inhibitor of κB kinase. These serine kinases can also inhibit insulin signaling at various levels (68). Indeed, JNK1 or inhibitor of κB kinase-β knockout mice and mice with adipose-specific JNK inactivation are protected from insulin resistance (74–76). Finally, increased circulating FFA levels due to adipose tissue insulin resistance can in turn inhibit insulin signaling via serine phosphorylation of IRS (67) and lead to insulin resistance in skeletal muscle and liver.
Although the imbalance of pro- and antiinflammatory adipokines can induce insulin resistance via paracrine effects, the endocrine effects of these adipokines are especially important in the development of insulin resistance in skeletal muscle and liver (72). For example, circulating levels of adiponectin, an adipokine exclusively secreted by the adipose tissue, is positively correlated with insulin sensitivity in both humans and rodents (77). Moreover, individuals with high-plasma adiponectin levels have a lower risk of developing type 2 diabetes (78). Finally, abdominal adiposity correlates with plasma C-reactive protein levels, indicating that systemic markers of inflammation are also increased with obesity (79). Whereas adipose tissue inflammation in obesity plays a key role in the development of insulin resistance, adipose inflammation in the absence of obesity does not seem to induce insulin resistance (80). Thus, it is important to use mouse models with at least some degree of obesity when studying the contribution of individual inflammatory mediators to insulin resistance.
Increased lipid deposition in skeletal muscle and liver is also considered to be a factor linked to the pathogenesis of insulin resistance (62). Indeed, obese, insulin-sensitive individuals have lower skeletal muscle and liver lipids than obese, insulin-resistant individuals (81). This ectopic lipid deposition is attributed to the inability of the adipose tissue (mainly subcutaneous) to store excess energy due to reduced differentiation/remodeling capacity. This is also characterized by increased visceral fat mass. Although the exact mechanism of these defects in adipogenesis/remodeling is not known, proinflammatory cytokines such as TNFα are implicated because of their known inhibitory effects on adipogenesis (82), in a PPARγ-dependent manner. Conversely, PPARγ agonists such as thiazolidinediones are known to increase both adipogenesis and insulin sensitivity. Increased lipid accumulation in the liver and skeletal muscle is associated with increased fatty acid flux, which leads to excessive accumulation of fatty acid intermediates such as ceramide (62). These lipid intermediates activate intracellular serine kinases that can lead to inhibition of insulin signaling. Ceramide can also directly inhibit Akt (83). Indeed, pharmacological inhibition of ceramide synthesis protects rodents from obesity-associated insulin resistance (84).
Long-chain (n-3) PUFA for improvement of insulin resistance and metabolic derangements in obesity
(n-3) and (n-6) PUFA are the 2 main classes of essential fatty acids. Linoleic acid (LA) is the parent long-chain (n-6) PUFA, which can be converted into arachidonic acid (AA) (Fig. 2). α-Linolenic acid (ALA) is the parent (n-3) fatty acid, which can be converted to EPA and DHA. The latter fatty acids are found primarily in foods of marine origin such as oily fish. The ratio of (n-6):(n-3) PUFA in the Western diet ranges from about 10:1 to 20:1 (85), whereas in countries with a relatively higher fish consumption such as Japan, this ratio is 4:1 (86). Dietary intake of these fatty acids affects the proportion of AA:EPA ratio in phospholipids, which affects cardiovascular disease risk (87). The (n-3) index is a measure of erythrocyte EPA+DHA:total fatty acid ratio, which has been proposed to be used as a cardiovascular disease risk factor. Individuals with a low (n-3) index have a higher risk of cardiac events (88)
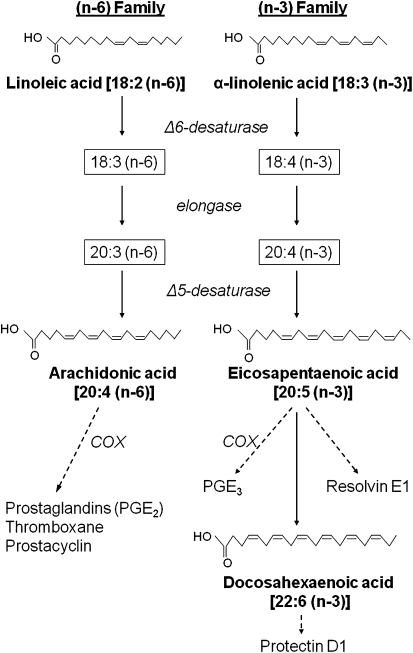
Figure 2.
Metabolism of (n-3) and (n-6) fatty acids. LA and ALA are the parent (n-6) and (n-3) long-chain PUFA. LA is converted to AA and ALA is converted to EPA and DHA. AA-derived eicosanoids are proinflammatory, whereas EPA-derived ones are less so. EPA and DHA metabolites such as resolvins and protectins have important roles in the resolution of inflammation. COX, cyclooxygenase.
The TG-lowering and cardioprotective actions of EPA and DHA are well established. Additionally, rodent studies show that EPA and DHA also prevent and reverse insulin resistance associated with high-fat (42) or high-sucrose feeding (9). Because insulin resistance associated with these dietary conditions is due to defects in adipose tissue, skeletal muscle, and hepatic function (discussed above), it is particularly important to understand how EPA and DHA modulate the functions of these organs.
Effects of EPA and DHA on adipose tissue function
EPA and DHA reduce adiposity in humans (89) especially when combined with energy restriction (5). These fatty acids also prevent the development of high-fat-diet–induced adiposity and adipocyte hypertrophy in rodents (42, 90). There are 2 possible mechanisms for these antiobesity effects of EPA and DHA. First, these fatty acids are known to increase fatty acid oxidation in liver, adipose tissue (91), and small intestine (92) in rodents in vivo and adipocytes (93) and myotubules (94) in vitro. Fish oil also increases fatty acid oxidation in humans with a reduction in respiratory quotient (89). Second, they are known to inhibit hepatic lipogenesis (Fig. 3). Both these processes shift the balance of fatty acid metabolism toward oxidation rather than storage. EPA and DHA activate AMP-activated protein kinase (AMPK) in adipose tissue and cultured adipocytes, which could be a mechanism for their effect on fatty acid oxidation (95, 96). Further, these PUFA are also known to induce mitochondrial biogenesis (3) (Fig. 4).
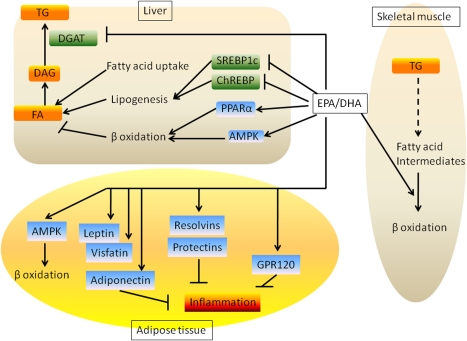
Figure 3.
Integrated effects of EPA and DHA on liver, skeletal muscle, and adipose tissue metabolism. EPA and DHA promote hepatic fatty acid oxidation and suppress lipogenesis. This leads to reduced accumulation of TG in the liver. These fatty acids also increase adipose tissue fatty acid oxidation and increase secretion of adiponectin, leptin, and visfatin. EPA and DHA also alleviate adipose tissue inflammation via GPR120 and resolvins/protectins. In the skeletal muscle, EPA and DHA promote fatty acid oxidation, thereby preventing accumulation of fatty acid intermediates. All these mechanisms account for the EPA- and DHA-mediated improvement in insulin sensitivity.
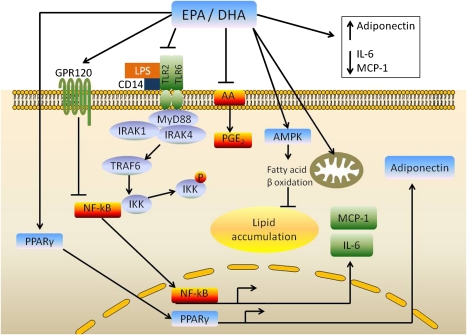
Figure 4.
Mechanisms by which EPA and DHA modulate adipose tissue function. LPS binds to TLR2/TLR6 complex via CD14. The binding of LPS to TLR2 stimulates IL-6 gene transcription by activation and recruitment of myeloid differentiation factor 88 (MyD88) and IL-1 receptor-associated kinase (IRAK)-4. The latter phosphorylates IRAK-1, followed by activation of TNF receptor-associated factor (TRAF)-6 and NF-κB. The latter translocates into the nucleus and activates IL-6 gene transcription. EPA and DHA inhibit LPS-induced IL-6 gene expression in adipose stem cells via regulation of IRAK1 and NF-κB pathways. EPA and DHA also inhibit the NF-κB pathway via GPR120. These fatty acids also activate PPARγ, leading to increased production of adiponectin, in addition to their known antiinflammatory effects mediated by antagonism of the (n-6) fatty acid arachidonic acid (AA). Finally, EPA and DHA activate AMPK and mitochondrial biogenesis, both of which increase fatty acid oxidation and lower lipid accumulation in adipocytes.
Although it is possible that improvements of systemic insulin resistance due to EPA and DHA are secondary to reduction in adipose mass, this could also be due to direct actions of these fatty acids in improving adipose tissue function. Indeed, some studies have shown EPA- and DHA-mediated insulin sensitivity is preserved even in the presence of increased adipose mass (97). EPA and DHA modulate adipokine secretion from adipose tissue (Figs. 3 and 4). They increase plasma adiponectin levels in obese humans (98, 99) and rodents (100), which could be a potential mechanism by which EPA and DHA improve insulin sensitivity. This effect of EPA and DHA on adiponectin is PPARγ-dependent, because adiponectin is not elevated in response to fish oil in mice lacking PPARγ (101). They also induce leptin and visfatin secretion and reduce the expression of several proinflammatory cytokines from the adipose tissue, including TNFα, IL-6, MCP-1, and PAI-1 (42, 102–104). Current evidence suggests that these antiinflammatory actions of EPA and DHA play a major role in their insulin-sensitizing effects.
ATM infiltration and phenotypic switch are causally linked to insulin resistance in obesity (discussed previously in this review). EPA and DHA prevent high-fat-diet–induced ATM infiltration in mice (105). Production of proinflammatory cytokines by macrophages is dependent on activation of the NF-κB and JNK pathways. EPA and DHA bind to G protein-coupled receptor (GPR) 120, and inhibit NF-κB and JNK, attenuating this response (106) (Fig. 4). The importance of this receptor, which is present in both adipocytes and macrophages, is highlighted by the finding that the EPA-mediated improvement in insulin sensitivity is absent in mice lacking GPR120. More specifically, TGF-β activated kinase (TAK)-1 is necessary for the activation of NF-κB and JNK, which in turn is dependent upon association of TAK-1 with TAK-1 binding protein-1. EPA or DHA binding to GPR120 leads to its internalization along with β-arrestin2. This complex associates with TAK-1 binding protein-1 and prevents the activation of TAK-1 binding protein-1, thereby preventing activation of NF-κB and JNK. Another GPR, GPR40, is also activated by long-chain PUFA (107).
Recent evidence has highlighted the role of EPA and DHA in resolving inflammation, through mechanisms involving EPA-derived resolvin E1 and DHA-derived protectin D1 (108). In high-fat–fed mice, protectin D1 is lacking in the adipose tissue and skeletal muscle. Moreover, transgenic restoration of (n-3) PUFA and protectin D1 prevents the high-fat-diet–induced insulin resistance, highlighting the important role of this DHA derivative (109).
AA-derived eicosanoids such as PGE2 and thromboxane A2 are proinflammatory, whereas EPA-derived ones such as PGE3 are less inflammatory. Because higher EPA and DHA intakes result in higher incorporation into membrane phospholipids at the expense of AA, it is possible that EPA and DHA supplementation reduces production of AA-derived eicosanoids. This can also contribute to antiinflammatory effects of these fatty acids. Indeed, we previously showed that both murine and human adipocytes secrete prostaglandins (110) and EPA significantly reduced adipose PGE2 secretion and antagonized AA-induced PGE2 secretion from cultured adipocytes (111) (Fig. 4).
Adipose stem cells are a major source of proinflammatory cytokines in adipose tissue (22). Recently, we tested whether EPA and/or stearidonic acid [18:4 (n-3), EPA precursor] inhibit LPS-mediated inflammation in mouse adipose stem cells (112). Our results demonstrated that both stearidonic acid and EPA significantly reduced LPS-induced IL-6 secretion and IL-6 mRNA expression via TLR2 and NF-κB–mediated pathways (Fig. 4).
EPA and DHA and hepatic insulin sensitivity
The effect of (n-3) PUFA, mainly EPA, on lowering plasma TG is well established. This effect is at least in part due to their ability to inhibit hepatic enzyme diacylglycerol acyltransferase (113), which catalyzes the final reaction of TG synthesis. In addition to this TG-lowering effect, EPA and DHA also prevent the development of hepatic steatosis associated with high-saturated fat feeding in rodents (114).
Lipid accumulation in the liver depends on nonesterified fatty acid delivery to the liver, de novo lipogenesis, and the rate of fatty acid oxidation (Fig. 3). In obesity, there is a net increase in fatty acid availability, promoting lipid deposition in the liver. Moreover, lipogenic gene transcription factors such as sterol regulatory element-binding protein-1c, are expressed at a higher level in obesity (115). This leads to increased expression of hepatic lipogenic genes such as fatty acid synthase and stearoyl-CoA desaturase 1 (116). Further, obesity is also associated with suppression of PPARα (115) leading to reduced fatty acid oxidation (116). All these processes are linked to the development of hepatic steatosis. Excessive lipid accumulation in the liver leads to hepatic insulin resistance and blunting of insulin-mediated suppression of hepatic glucose production.
EPA reduces lipogenesis and increases fatty acid oxidation (117), preventing lipid accumulation in the liver, leading to improvements in hepatic insulin resistance (Fig. 3). Moreover, EPA reduces lipogenesis via inhibition of lipogenic transcription factors such as sterol regulatory element-binding protein-1c, nuclear factor-Y (118), and carbohydrate-responsive element-binding protein (119). EPA stimulates fatty acid oxidation via activation of PPARα (116, 120–122) and AMPK (123). PPARα is required for EPA’s beneficial effects on hepatic insulin sensitivity, as evidenced by a lack of EPA effect in restoring hepatic insulin sensitivity in PPARα null mice fed a high-fat diet (124). Interestingly, these mice continue to exhibit low-plasma TG levels, concomitant with diacylglycerol accumulation in the liver, suggesting that EPA exerts a PPARα-independent effect on hepatic diacylglycerol acyltransferase. AMPKα2 is another signaling enzyme, coordinately regulated with PPARα during fat oxidation. As expected, and in line with the PPARα null mice phenotype, AMPKα2 null mice do not exhibit EPA’s beneficial effects on improvement in hepatic insulin sensitivity (125).
EPA and DHA and skeletal muscle metabolism
TG accumulation in skeletal muscle fibers has been linked to insulin resistance (discussed previously). Proposed mediators include increased fatty acid availability and impaired fatty acid oxidation in the skeletal muscle. The latter is also associated with accumulation of fatty acid intermediates such as diacylglycerol and ceramides. Exposure of myotubules to EPA enhances glucose uptake (126), indicating increased insulin sensitivity. EPA also protects from the development of high-fat–induced skeletal muscle insulin resistance in vivo (127), with improvements in muscle glycogen synthesis (100). Interestingly, some studies have shown that EPA increases TG accumulation, along with increases in fatty acid β oxidation (Fig. 3) and improvements in skeletal muscle insulin sensitivity both in vitro (94) and in vivo (128).
Because EPA and DHA also reduce skeletal muscle ceramide content (100), it is possible that their effect on maintaining skeletal muscle insulin sensitivity is related to their ability to normalize fatty acid oxidation with lower accumulation of fatty acid intermediates. SFA induce skeletal muscle insulin resistance via activation of the NF-κB pathway (129). Because EPA and DHA inhibit this pathway in other tissues, it will be interesting to determine whether EPA and DHA inhibit this pathway in the skeletal muscle and subsequently prevent the SFA-mediated insulin resistance.
Although EPA and DHA consistently improve insulin resistance in rodent models of obesity, this is not the case for humans with type 2 diabetes. In a previous review of clinical studies on the use of (n-3) PUFA for glycemic control of individuals with type 2 diabetes, possible causes for this lack of effect were suggested to be due to inadequate dose and lack of control of the background diet (12). It is also possible that EPA and DHA delay the progression of metabolic syndrome and/or prediabetes to type 2 diabetes. Further clinical studies testing this hypothesis are warranted.
Conclusions
In summary, obesity and increased adiposity are associated with a chronic, low-grade inflammation in the adipose tissue. Current evidence suggests that adipose tissue hypoxia, immune cell chemotaxis to adipose tissue followed by subsequent activation, ER stress, and SFA-mediated activation of innate immune receptors play a role in the trigger of this inflammatory process. ATM and T lymphocytes are the 2 key immune cell types that orchestrate these processes. Adipose tissue inflammation and associated hepatic steatosis is causally linked to the development of skeletal muscle and systemic insulin resistance.
EPA and DHA prevent excessive adiposity and insulin resistance in rodents. Mechanistically, this is related to the ability of these fatty acids to increase hepatic, skeletal muscle, and adipose tissue fatty acid oxidation and their ability to reduce lipogenesis. EPA and DHA also have important antiinflammatory properties that modulate adipose tissue inflammation via GPR120-mediated suppression of macrophage proinflammatory cytokine secretion, resolvin, and protectin-mediated resolution of inflammation. Through modulation of adipokine secretion, these fatty acids also favor insulin sensitivity. Further studies in obese humans are warranted to study whether these fatty acids can prevent and reverse the progression of metabolic syndrome to type 2 diabetes.
Acknowledgments
N.S.K. and N.M.-M. analyzed data and wrote the paper; K.J.C. reviewed and edited the paper; N.M.-M. had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by a National Institute of Food and Agriculture-National Research Initiative award (2005-35200-15224), an AHA Southeast Affiliate Predoctoral Fellowship, the University of Tennessee (UT) Obesity Research Center, UT AgResearch, and UT Extension.
Author disclosures: N. S. Kalupahana, K. J. Claycombe, and N. Moustaid-Moussa, no conflicts of interest.
Abbreviations used: AA, arachidonic acid; ALA, α-linolenic acid; AMPK, AMP-activated protein kinase; ATM, adipose tissue macrophage; Ccr, C-C motif chemokine receptor; CD, cluster of differentiation; ECM, extracellular matrix; ER, endoplasmic reticulum; GPR, G protein–coupled receptor; IRS, insulin receptor substrate; JNK, jun N-terminal kinase; LA, linoleic acid; M1, classically activated macrophage; M2, alternatively activated macrophage; MCP, monocyte chemotactic protein; MGL, macrophage galactose N-acetyl-galactosamine specific lectin; PAI, plasminogen activator inhibitor; PI3K, phosphatidylinositol 3-kinase; PSGL, P-selectin glycoprotein ligand; SOCS, suppressor of cytokine signaling; TAK, TGF-β activated kinase; Th, helper T cell; TLR, Toll-like receptor.
Literature Cited
- 1.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5 [DOI] [PubMed] [Google Scholar]
- 2.Chapkin RS, Kim W, Lupton JR, McMurray DN. Dietary docosahexaenoic and eicosapentaenoic acid: emerging mediators of inflammation. Prostaglandins Leukot Essent Fatty Acids. 2009;81:187–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flachs P, Rossmeisl M, Bryhn M, Kopecky J. Cellular and molecular effects of n-3 polyunsaturated fatty acids on adipose tissue biology and metabolism. Clin Sci (Lond). 2009;116:1–16 [DOI] [PubMed] [Google Scholar]
- 4.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–57 [DOI] [PubMed] [Google Scholar]
- 5.Mori TA, Bao DQ, Burke V, Puddey IB, Watts GF, Beilin LJ. Dietary fish as a major component of a weight-loss diet: effect on serum lipids, glucose, and insulin metabolism in overweight hypertensive subjects. Am J Clin Nutr. 1999;70:817–25 [DOI] [PubMed] [Google Scholar]
- 6.Ruzickova J, Rossmeisl M, Prazak T, Flachs P, Sponarova J, Veck M, Tvrzicka E, Bryhn M, Kopecky J. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids. 2004;39:1177–85 [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Periz A, Horrillo R, Ferre N, Gronert K, Dong B, Moran-Salvador E, Titos E, Martinez-Clemente M, Lopez-Parra M, et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23:1946–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Storlien LH, Kraegen EW, Chisholm DJ, Ford GL, Bruce DG, Pascoe WS. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science. 1987;237:885–8 [DOI] [PubMed] [Google Scholar]
- 9.Ghafoorunissa Ibrahim A, Rajkumar L, Acharya V. Dietary (n-3) long chain polyunsaturated fatty acids prevent sucrose-induced insulin resistance in rats. J Nutr. 2005;135:2634–8 [DOI] [PubMed] [Google Scholar]
- 10.Kabir M, Skurnik G, Naour N, Pechtner V, Meugnier E, Rome S, Quignard-Boulange A, Vidal H, Slama G, et al. Treatment for 2 mo with n 3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr. 2007;86:1670–9 [DOI] [PubMed] [Google Scholar]
- 11.Rivellese AA, Maffettone A, Iovine C, Di Marino L, Annuzzi G, Mancini M, Riccardi G. Long-term effects of fish oil on insulin resistance and plasma lipoproteins in NIDDM patients with hypertriglyceridemia. Diabetes Care. 1996;19:1207–13 [DOI] [PubMed] [Google Scholar]
- 12.Hendrich S. (n-3) Fatty acids: clinical trials in people with type 2 diabetes. Advances in Nutrition. 2010; 1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nettleton JA, Katz R. n-3 Long-chain polyunsaturated fatty acids in type 2 diabetes: a review. J Am Diet Assoc. 2005;105:428–40 [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–8 [DOI] [PubMed] [Google Scholar]
- 15.Kim S, Moustaid-Moussa N. Secretory, endocrine and autocrine/paracrine function of the adipocyte. J Nutr. 2000;130:S3110–5 [DOI] [PubMed] [Google Scholar]
- 16.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46 [DOI] [PubMed] [Google Scholar]
- 17.Alexaki VI, Notas G, Pelekanou V, Kampa M, Valkanou M, Theodoropoulos P, Stathopoulos EN, Tsapis A, Castanas E. Adipocytes as immune cells: differential expression of TWEAK, BAFF, and APRIL and their receptors (Fn14, BAFF-R, TACI, and BCMA) at different stages of normal and pathological adipose tissue development. J Immunol. 2009;183:5948–56 [DOI] [PubMed] [Google Scholar]
- 18.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P, Mariman E, Renes J, Keijer J. The secretory function of adipocytes in the physiology of white adipose tissue. J Cell Physiol. 2008;216:3–13 [DOI] [PubMed] [Google Scholar]
- 21.Harkins JM, Moustaid-Moussa N, Chung YJ, Penner KM, Pestka JJ, North CM, Claycombe KJ. Expression of interleukin-6 is greater in preadipocytes than in adipocytes of 3T3–L1 cells and C57BL/6J and ob/ob mice. J Nutr. 2004;134:2673–7 [DOI] [PubMed] [Google Scholar]
- 22.Zhou HR, Kim EK, Kim H, Claycombe KJ. Obesity-associated mouse adipose stem cell secretion of monocyte chemotactic protein-1. Am J Physiol Endocrinol Metab. 2007;293:E1153–8 [DOI] [PubMed] [Google Scholar]
- 23.Chavey C, Mari B, Monthouel MN, Bonnafous S, Anglard P, Van Obberghen E, Tartare-Deckert S. Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J Biol Chem. 2003;278:11888–96 [DOI] [PubMed] [Google Scholar]
- 24.Maquoi E, Munaut C, Colige A, Collen D, Lijnen HR. Modulation of adipose tissue expression of murine matrix metalloproteinases and their tissue inhibitors with obesity. Diabetes. 2002;51:1093–101 [DOI] [PubMed] [Google Scholar]
- 25.Kos K, Wilding JP. SPARC: a key player in the pathologies associated with obesity and diabetes. Nat Rev Endocrinol. 2010;6:225–35 [DOI] [PubMed] [Google Scholar]
- 26.Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293:E1118–28 [DOI] [PubMed] [Google Scholar]
- 27.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boden G, Duan X, Homko C, Molina EJ, Song W, Perez O, Cheung P, Merali S. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 2008;57:2438–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA, Ozawa K, Ogawa S, Hori M, et al. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem. 2005;280:847–51 [DOI] [PubMed] [Google Scholar]
- 30.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61 [DOI] [PubMed] [Google Scholar]
- 31.Shen X, Zhang K, Kaufman RJ. The unfolded protein response: a stress signaling pathway of the endoplasmic reticulum. J Chem Neuroanat. 2004;28:79–92 [DOI] [PubMed] [Google Scholar]
- 32.Magne J, Mariotti F, Fischer R, Mathe V, Tome D, Huneau JF. Early postprandial low-grade inflammation after high-fat meal in healthy rats: possible involvement of visceral adipose tissue. J Nutr Biochem. 2010;21:550–5 [DOI] [PubMed] [Google Scholar]
- 33.Kennedy A, Martinez K, Chuang CC, LaPoint K, McIntosh M. Saturated fatty acid-mediated inflammation and insulin resistance in adipose tissue: mechanisms of action and implications. J Nutr. 2009;139:1–4 [DOI] [PubMed] [Google Scholar]
- 34.LaRosa PC, Miner J, Xia Y, Zhou Y, Kachman S, Fromm ME. Trans-10, cis-12 conjugated linoleic acid causes inflammation and delipidation of white adipose tissue in mice: a microarray and histological analysis. Physiol Genomics. 2006;27:282–94 [DOI] [PubMed] [Google Scholar]
- 35.Poirier H, Shapiro JS, Kim RJ, Lazar MA. Nutritional supplementation with trans-10, cis-12-conjugated linoleic acid induces inflammation of white adipose tissue. Diabetes. 2006;55:1634–41 [DOI] [PubMed] [Google Scholar]
- 36.Bes-Houtmann S, Roche R, Hoareau L, Gonthier MP, Festy F, Caillens H, Gasque P, Lefebvre d'Hellencourt C, Cesari M. Presence of functional TLR2 and TLR4 on human adipocytes. Histochem Cell Biol. 2007;127:131–7 [DOI] [PubMed] [Google Scholar]
- 37.Suganami T, Mieda T, Itoh M, Shimoda Y, Kamei Y, Ogawa Y. Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochem Biophys Res Commun. 2007;354:45–9 [DOI] [PubMed] [Google Scholar]
- 38.Ehses JA, Meier DT, Wueest S, Rytka J, Boller S, Wielinga PY, Schraenen A, Lemaire K, Debray S, et al. Toll-like receptor 2-deficient mice are protected from insulin resistance and beta cell dysfunction induced by a high-fat diet. Diabetologia. 2010;53:1795–806 [DOI] [PubMed] [Google Scholar]
- 39.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abe M, Matsuda M, Kobayashi H, Miyata Y, Nakayama Y, Komuro R, Fukuhara A, Shimomura I. Effects of statins on adipose tissue inflammation: their inhibitory effect on MyD88-independent IRF3/IFN-beta pathway in macrophages. Arterioscler Thromb Vasc Biol. 2008;28:871–7 [DOI] [PubMed] [Google Scholar]
- 41.DeFuria J, Bennett G, Strissel KJ, Perfield JW II, Milbury PE, Greenberg AS, Obin MS. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. J Nutr. 2009;139:1510–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalupahana NS, Claycombe K, Newman SJ, Stewart T, Siriwardhana N, Matthan N, Lichtenstein AH, Moustaid-Moussa N. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J Nutr. 2010;140:1915–22 [DOI] [PubMed] [Google Scholar]
- 43.Kalupahana NS, Voy BH, Saxton AM, Moustaid-Moussa N. Energy-restricted high-fat diets only partially improve markers of systemic and adipose tissue inflammation. Obesity (Silver Spring). 2011;19:245–54 [DOI] [PubMed] [Google Scholar]
- 44.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35 [DOI] [PubMed] [Google Scholar]
- 46.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86 [DOI] [PubMed] [Google Scholar]
- 47.Zeyda M, Stulnig TM. Adipose tissue macrophages. Immunol Lett. 2007;112:61–7 [DOI] [PubMed] [Google Scholar]
- 48.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prieur X, Mok CY, Velagapudi VR, Nunez V, Fuentes L, Montaner D, Ishikawa K, Camacho A, Barbarroja N, et al. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 2011;60:797–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Gyori G, Zlabinger GJ, Stulnig TM. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond). 2007;31:1420–8 [DOI] [PubMed] [Google Scholar]
- 51.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato C, Shikata K, Hirota D, Sasaki M, Nishishita S, Miyamoto S, Kodera R, Ogawa D, Tone A, et al. P-selectin glycoprotein ligand-1 deficiency is protective against obesity-related insulin resistance. Diabetes. 2011;60:189–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Surmi BK, Webb CD, Ristau AC, Hasty AH. Absence of macrophage inflammatory protein-1{alpha} does not impact macrophage accumulation in adipose tissue of diet-induced obese mice. Am J Physiol Endocrinol Metab. 2010;299:E437–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–33 [DOI] [PubMed] [Google Scholar]
- 57.Eiras S, Teijeira-Fernandez E, Salgado-Somoza A, Couso E, Garcia-Caballero T, Sierra J, Juanatey JR. Relationship between epicardial adipose tissue adipocyte size and MCP-1 expression. Cytokine. 2010;51:207–12 [DOI] [PubMed] [Google Scholar]
- 58.Lumeng CN, Maillard I, Saltiel AR. T-ing up inflammation in fat. Nat Med. 2009;15:846–7 [DOI] [PubMed] [Google Scholar]
- 59.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–20 [DOI] [PubMed] [Google Scholar]
- 60.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333–46 [DOI] [PubMed] [Google Scholar]
- 64.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96 [DOI] [PubMed] [Google Scholar]
- 65.Cheng Z, Tseng Y, White MF. Insulin signaling meets mitochondria in metabolism. Trends Endocrinol Metab. 2010;21:589–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, Hilton DJ, Hotamisligil GS, Van Obberghen E. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J Biol Chem. 2001;276:47944–9 [DOI] [PubMed] [Google Scholar]
- 67.Zick Y. Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Sci STKE. 2005;2005:pe4. [DOI] [PubMed] [Google Scholar]
- 68.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem. 2002;277:42394–8 [DOI] [PubMed] [Google Scholar]
- 70.Ueki K, Fruman DA, Yballe CM, Fasshauer M, Klein J, Asano T, Cantley LC, Kahn CR. Positive and negative roles of p85 alpha and p85 beta regulatory subunits of phosphoinositide 3-kinase in insulin signaling. J Biol Chem. 2003;278:48453–66 [DOI] [PubMed] [Google Scholar]
- 71.Kalupahana NS, Voy BH, Fletcher S, Stewart T, Kim JH, Quignard-Boulange A, Wasserman D, Moustaid-Moussa N. Mechanisms linking overproduction of angiotensinogen by adipose tissue to inflammation, glucose intolerance and insulin resistance. Obesity. 2009;17(suppl 2):S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91 [DOI] [PubMed] [Google Scholar]
- 73.Luo N, Liu J, Chung BH, Yang Q, Klein RL, Garvey WT, Fu Y. Macrophage adiponectin expression improves insulin sensitivity and protects against inflammation and atherosclerosis. Diabetes. 2010;59:791–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–6 [DOI] [PubMed] [Google Scholar]
- 75.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–8 [DOI] [PubMed] [Google Scholar]
- 76.Zhang X, Xu A, Chung SK, Cresser JH, Sweeney G, Wong RL, Lin A, Lam KS. Selective inactivation of c-Jun NH2-terminal kinase in adipose tissue protects against diet-induced obesity and improves insulin sensitivity in both liver and skeletal muscle in mice. Diabetes. 2011;60:486–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6 [DOI] [PubMed] [Google Scholar]
- 78.Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, Knowler WC, Krakoff J. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–8 [DOI] [PubMed] [Google Scholar]
- 79.Brooks GC, Blaha MJ, Blumenthal RS. Relation of C-reactive protein to abdominal adiposity. Am J Cardiol. 2010;106:56–61 [DOI] [PubMed] [Google Scholar]
- 80.Tang T, Zhang J, Yin J, Staszkiewicz J, Gawronska-Kozak B, Jung DY, Ko HJ, Ong H, Kim JK, et al. Uncoupling of inflammation and insulin resistance by NF-kappaB in transgenic mice through elevated energy expenditure. J Biol Chem. 2010;285:4637–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–16 [DOI] [PubMed] [Google Scholar]
- 82.Gustafson B, Gogg S, Hedjazifar S, Jenndahl L, Hammarstedt A, Smith U. Inflammation and impaired adipogenesis in hypertrophic obesity in man. Am J Physiol Endocrinol Metab. Epub 2009 Jul 21 [DOI] [PubMed] [Google Scholar]
- 83.Stratford S, DeWald DB, Summers SA. Ceramide dissociates 3′-phosphoinositide production from pleckstrin homology domain translocation. Biochem J. 2001;354:359–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schmitz-Peiffer C. Targeting ceramide synthesis to reverse insulin resistance. Diabetes. 2010;59:2351–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Simopoulos AP. Evolutionary aspects of diet: the omega-6/omega-3 ratio and the brain. Mol Neurobiol. Epub 2011 Jan 29 [DOI] [PubMed] [Google Scholar]
- 86.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood). 2008;233:674–88 [DOI] [PubMed] [Google Scholar]
- 87.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93:950–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aarsetoey H, Aarsetoey R, Lindner T, Staines H, Harris WS, Nilsen DW. Low levels of the omega-3 index are associated with sudden cardiac arrest and remain stable in survivors in the subacute phase. Lipids. 2011;46:151–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Couet C, Delarue J, Ritz P, Antoine JM, Lamisse F. Effect of dietary fish oil on body fat mass and basal fat oxidation in healthy adults. Int J Obes Relat Metab Disord. 1997;21:637–43 [DOI] [PubMed] [Google Scholar]
- 90.Flachs P, Mohamed-Ali V, Horakova O, Rossmeisl M, Hosseinzadeh-Attar MJ, Hensler M, Ruzickova J, Kopecky J. Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet. Diabetologia. 2006;49:394–7 [DOI] [PubMed] [Google Scholar]
- 91.Flachs P, Horakova O, Brauner P, Rossmeisl M, Pecina P, Franssen-van Hal N, Ruzickova J, Sponarova J, Drahota Z, et al. Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce beta-oxidation in white fat. Diabetologia. 2005;48:2365–75 [DOI] [PubMed] [Google Scholar]
- 92.van Schothorst EM, Flachs P, Franssen-van Hal NL, Kuda O, Bunschoten A, Molthoff J, Vink C, Hooiveld GJ, Kopecky J, et al. Induction of lipid oxidation by polyunsaturated fatty acids of marine origin in small intestine of mice fed a high-fat diet. BMC Genomics. 2009;10:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guo W, Xie W, Lei T, Hamilton JA. Eicosapentaenoic acid, but not oleic acid, stimulates beta-oxidation in adipocytes. Lipids. 2005;40:815–21 [DOI] [PubMed] [Google Scholar]
- 94.Wensaas AJ, Rustan AC, Just M, Berge RK, Drevon CA, Gaster M. Fatty acid incubation of myotubes from humans with type 2 diabetes leads to enhanced release of beta-oxidation products because of impaired fatty acid oxidation: effects of tetradecylthioacetic acid and eicosapentaenoic acid. Diabetes. 2009;58:527–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Figueras M, Olivan M, Busquets S, Lopez-Soriano FJ, Argiles JM. Effects of eicosapentaenoic Acid (EPA) treatment on insulin sensitivity in an animal model of diabetes: improvement of the inflammatory status. Obesity (Silver Spring). 2011;19:362–9 [DOI] [PubMed] [Google Scholar]
- 96.Lorente-Cebrian S, Bustos M, Marti A, Martinez JA, Moreno-Aliaga MJ. Eicosapentaenoic acid stimulates AMP-activated protein kinase and increases visfatin secretion in cultured murine adipocytes. Clin Sci (Lond). 2009;117:243–9 [DOI] [PubMed] [Google Scholar]
- 97.Ide T. Interaction of fish oil and conjugated linoleic acid in affecting hepatic activity of lipogenic enzymes and gene expression in liver and adipose tissue. Diabetes. 2005;54:412–23 [DOI] [PubMed] [Google Scholar]
- 98.Krebs JD, Browning LM, McLean NK, Rothwell JL, Mishra GD, Moore CS, Jebb SA. Additive benefits of long-chain n-3 polyunsaturated fatty acids and weight-loss in the management of cardiovascular disease risk in overweight hyperinsulinaemic women. Int J Obes (Lond). 2006;30:1535–44 [DOI] [PubMed] [Google Scholar]
- 99.Itoh M, Suganami T, Satoh N, Tanimoto-Koyama K, Yuan X, Tanaka M, Kawano H, Yano T, Aoe S, et al. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler Thromb Vasc Biol. 2007;27:1918–25 [DOI] [PubMed] [Google Scholar]
- 100.Kuda O, Jelenik T, Jilkova Z, Flachs P, Rossmeisl M, Hensler M, Kazdova L, Ogston N, Baranowski M, et al. n-3 fatty acids and rosiglitazone improve insulin sensitivity through additive stimulatory effects on muscle glycogen synthesis in mice fed a high-fat diet. Diabetologia. 2009;52:941–51 [DOI] [PubMed] [Google Scholar]
- 101.Neschen S, Morino K, Rossbacher JC, Pongratz RL, Cline GW, Sono S, Gillum M, Shulman GI. Fish oil regulates adiponectin secretion by a peroxisome proliferator-activated receptor-gamma-dependent mechanism in mice. Diabetes. 2006;55:924–8 [DOI] [PubMed] [Google Scholar]
- 102.Perez-Echarri N, Perez-Matute P, Marcos-Gomez B, Baena MJ, Marti A, Martinez JA, Moreno-Aliaga MJ. Differential inflammatory status in rats susceptible or resistant to diet-induced obesity: effects of EPA ethyl ester treatment. Eur J Nutr. 2008;47:380–6 [DOI] [PubMed] [Google Scholar]
- 103.Perez-Echarri N, Perez-Matute P, Marcos-Gomez B, Martinez JA, Moreno-Aliaga MJ. Effects of eicosapentaenoic acid ethyl ester on visfatin and apelin in lean and overweight (cafeteria diet-fed) rats. Br J Nutr. 2009;101:1059–67 [DOI] [PubMed] [Google Scholar]
- 104.Puglisi MJ, Hasty AH, Saraswathi V. The role of adipose tissue in mediating the beneficial effects of dietary fish oil. J Nutr Biochem. 2011;22:101–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Todoric J, Loffler M, Huber J, Bilban M, Reimers M, Kadl A, Zeyda M, Waldhausl W, Stulnig TM. Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n-3 polyunsaturated fatty acids. Diabetologia. 2006;49:2109–19 [DOI] [PubMed] [Google Scholar]
- 106.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–6 [DOI] [PubMed] [Google Scholar]
- 108.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.White PJ, Arita M, Taguchi R, Kang JX, Marette A. Transgenic restoration of long-chain n-3 fatty acids in insulin target tissues improves resolution capacity and alleviates obesity-linked inflammation and insulin resistance in high-fat-fed mice. Diabetes. 2010;59:3066–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim S, Whelan J, Claycombe K, Reath DB, Moustaid-Moussa N. Angiotensin II increases leptin secretion by 3T3–L1 and human adipocytes via a prostaglandin-independent mechanism. J Nutr. 2002;132:1135–40 [DOI] [PubMed] [Google Scholar]
- 111.Wortman P, Miyazaki Y, Kalupahana NS, Kim S, Hansen-Petrik M, Saxton AM, Claycombe KJ, Voy BH, Whelan J, et al. n3 and n6 Polyunsaturated fatty acids differentially modulate prostaglandin E secretion but not markers of lipogenesis in adipocytes. Nutr Metab (Lond). 2009;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hsueh HW, Zhou Z, Whelan J, Allen KGD, Moustaid-Moussa N, Kim H, Claycombe KJ. Stearidonic and eicosapentaenoic acids inhibit interleukin-6 expression in ob/ob mouse adipose stem cells via toll-like receptor-2 mediated pathway1. J Nutr. Epub 2011 May 11 [DOI] [PubMed] [Google Scholar]
- 113.Rustan AC, Nossen JO, Christiansen EN, Drevon CA. Eicosapentaenoic acid reduces hepatic synthesis and secretion of triacylglycerol by decreasing the activity of acyl-coenzyme A:1,2-diacylglycerol acyltransferase. J Lipid Res. 1988;29:1417–26 [PubMed] [Google Scholar]
- 114.Saraswathi V, Gao L, Morrow JD, Chait A, Niswender KD, Hasty AH. Fish oil increases cholesterol storage in white adipose tissue with concomitant decreases in inflammation, hepatic steatosis, and atherosclerosis in mice. J Nutr. 2007;137:1776–82 [DOI] [PubMed] [Google Scholar]
- 115.Pettinelli P, Del Pozo T, Araya J, Rodrigo R, Araya AV, Smok G, Csendes A, Gutierrez L, Rojas J, et al. Enhancement in liver SREBP-1c/PPAR-alpha ratio and steatosis in obese patients: correlations with insulin resistance and n-3 long-chain polyunsaturated fatty acid depletion. Biochim Biophys Acta. 2009;1792:1080–6 [DOI] [PubMed] [Google Scholar]
- 116.Sekiya M, Yahagi N, Matsuzaka T, Najima Y, Nakakuki M, Nagai R, Ishibashi S, Osuga J, Yamada N, et al. Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology. 2003;38:1529–39 [DOI] [PubMed] [Google Scholar]
- 117.Madsen L, Rustan AC, Vaagenes H, Berge K, Dyroy E, Berge RK. Eicosapentaenoic and docosahexaenoic acid affect mitochondrial and peroxisomal fatty acid oxidation in relation to substrate preference. Lipids. 1999;34:951–63 [DOI] [PubMed] [Google Scholar]
- 118.Teran-Garcia M, Adamson AW, Yu G, Rufo C, Suchankova G, Dreesen TD, Tekle M, Clarke SD, Gettys TW. Polyunsaturated fatty acid suppression of fatty acid synthase (FASN): evidence for dietary modulation of NF-Y binding to the Fasn promoter by SREBP-1c. Biochem J. 2007;402:591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dentin R, Benhamed F, Pegorier JP, Foufelle F, Viollet B, Vaulont S, Girard J, Postic C. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation. J Clin Invest. 2005;115:2843–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hein GJ, Bernasconi AM, Montanaro MA, Pellon-Maison M, Finarelli G, Chicco A, Lombardo YB, Brenner RR. Nuclear receptors and hepatic lipidogenic enzyme response to a dyslipidemic sucrose-rich diet and its reversal by fish oil n-3 polyunsaturated fatty acids. Am J Physiol Endocrinol Metab. 2010;298:E429–39 [DOI] [PubMed] [Google Scholar]
- 121.Svegliati-Baroni G, Candelaresi C, Saccomanno S, Ferretti G, Bachetti T, Marzioni M, De Minicis S, Nobili L, Salzano R, et al. A model of insulin resistance and nonalcoholic steatohepatitis in rats: role of peroxisome proliferator-activated receptor-alpha and n-3 polyunsaturated fatty acid treatment on liver injury. Am J Pathol. 2006;169:846–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Neschen S, Moore I, Regittnig W, Yu CL, Wang Y, Pypaert M, Petersen KF, Shulman GI. Contrasting effects of fish oil and safflower oil on hepatic peroxisomal and tissue lipid content. Am J Physiol Endocrinol Metab. 2002;282:E395–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Suchankova G, Tekle M, Saha AK, Ruderman NB, Clarke SD, Gettys TW. Dietary polyunsaturated fatty acids enhance hepatic AMP-activated protein kinase activity in rats. Biochem Biophys Res Commun. 2005;326:851–8 [DOI] [PubMed] [Google Scholar]
- 124.Neschen S, Morino K, Dong J, Wang-Fischer Y, Cline GW, Romanelli AJ, Rossbacher JC, Moore IK, Regittnig W, et al. n-3 Fatty acids preserve insulin sensitivity in vivo in a peroxisome proliferator-activated receptor-alpha-dependent manner. Diabetes. 2007;56:1034–41 [DOI] [PubMed] [Google Scholar]
- 125.Jelenik T, Rossmeisl M, Kuda O, Jilkova ZM, Medrikova D, Kus V, Hensler M, Janovska P, Miksik I, et al. AMP-activated protein kinase alpha2 subunit is required for the preservation of hepatic insulin sensitivity by n-3 polyunsaturated fatty acids. Diabetes. 2010;59:2737–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aas V, Rokling-Andersen MH, Kase ET, Thoresen GH, Rustan AC. Eicosapentaenoic acid (20:5 n-3) increases fatty acid and glucose uptake in cultured human skeletal muscle cells. J Lipid Res. 2006;47:366–74 [DOI] [PubMed] [Google Scholar]
- 127.Storlien LH, Jenkins AB, Chisholm DJ, Pascoe WS, Khouri S, Kraegen EW. Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes. 1991;40:280–9 [DOI] [PubMed] [Google Scholar]
- 128.Kusunoki M, Tsutsumi K, Hara T, Ogawa H, Nakamura T, Miyata T, Sakakibara F, Fukuzawa Y, Suga T, et al. Ethyl icosapentate (omega-3 fatty acid) causes accumulation of lipids in skeletal muscle but suppresses insulin resistance in OLETF rats. Otsuka Long-Evans Tokushima Fatty. Metabolism. 2003;52:30–4 [DOI] [PubMed] [Google Scholar]
- 129.Sinha S, Perdomo G, Brown NF, O'Doherty RM. Fatty acid-induced insulin resistance in L6 myotubes is prevented by inhibition of activation and nuclear localization of nuclear factor kappa B. J Biol Chem. 2004;279:41294–301 [DOI] [PubMed] [Google Scholar]
- 130.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70 [DOI] [PubMed] [Google Scholar]
- 131.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–703 [DOI] [PubMed] [Google Scholar]
- 132.McTernan CL, McTernan PG, Harte AL, Levick PL, Barnett AH, Kumar S. Resistin, central obesity, and type 2 diabetes. Lancet. 2002;359:46–7 [DOI] [PubMed] [Google Scholar]
- 133.Jones BH, Standridge MK, Taylor JW, Moustaid N. Angiotensinogen gene expression in adipose tissue: analysis of obese models and hormonal and nutritional control. Am J Physiol. 1997;273:R236–42 [DOI] [PubMed] [Google Scholar]
- 134.Massiera F, Bloch-Faure M, Ceiler D, Murakami K, Fukamizu A, Gasc JM, Quignard-Boulange A, Negrel R, Ailhaud G, et al. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 2001;15:2727–9 [DOI] [PubMed] [Google Scholar]
- 135.Yvan-Charvet L, Quignard-Boulange A. Role of adipose tissue renin-angiotensin system in metabolic and inflammatory diseases associated with obesity. Kidney Int. 2011;79:162–8 [DOI] [PubMed] [Google Scholar]
- 136.Takahashi K, Mizuarai S, Araki H, Mashiko S, Ishihara A, Kanatani A, Itadani H, Kotani H. Adiposity elevates plasma MCP-1 levels leading to the increased CD11b-positive monocytes in mice. J Biol Chem. 2003;278:46654–60 [DOI] [PubMed] [Google Scholar]
- 137.Christiansen T, Richelsen B, Bruun JM. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int J Obes (Lond). 2005;29:146–50 [DOI] [PubMed] [Google Scholar]
- 138.Morange PE, Alessi MC, Verdier M, Casanova D, Magalon G, Juhan-Vague I. PAI-1 produced ex vivo by human adipose tissue is relevant to PAI-1 blood level. Arterioscler Thromb Vasc Biol. 1999;19:1361–5 [DOI] [PubMed] [Google Scholar]
- 139.Mohamed-Ali V, Flower L, Sethi J, Hotamisligil G, Gray R, Humphries SE, York DA, Pinkney J. beta-Adrenergic regulation of IL-6 release from adipose tissue: in vivo and in vitro studies. J Clin Endocrinol Metab. 2001;86:5864–9 [DOI] [PubMed] [Google Scholar]
- 140.Lira FS, Rosa JC, Yamashita AS, Koyama CH, Batista ML, Jr, Seelaender M. Endurance training induces depot-specific changes in IL-10/TNF-alpha ratio in rat adipose tissue. Cytokine. 2009;45:80–5 [DOI] [PubMed] [Google Scholar]
- 141.Frydelund-Larsen L, Akerstrom T, Nielsen S, Keller P, Keller C, Pedersen BK. Visfatin mRNA expression in human subcutaneous adipose tissue is regulated by exercise. Am J Physiol Endocrinol Metab. 2007;292:E24–31 [DOI] [PubMed] [Google Scholar]
- 142.Castan-Laurell I, Vitkova M, Daviaud D, Dray C, Kovacikova M, Kovacova Z, Hejnova J, Stich V, Valet P. Effect of hypocaloric diet-induced weight loss in obese women on plasma apelin and adipose tissue expression of apelin and APJ. Eur J Endocrinol. 2008;158:905–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kloting N, Graham TE, Berndt J, Kralisch S, Kovacs P, Wason CJ, Fasshauer M, Schon MR, Stumvoll M, et al. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab. 2007;6:79–87 [DOI] [PubMed] [Google Scholar]
- 144.Gealekman O, Burkart A, Chouinard M, Nicoloro SM, Straubhaar J, Corvera S. Enhanced angiogenesis in obesity and in response to PPARgamma activators through adipocyte VEGF and ANGPTL4 production. Am J Physiol Endocrinol Metab. 2008;295:E1056–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Peeraully MR, Jenkins JR, Trayhurn P. NGF gene expression and secretion in white adipose tissue: regulation in 3T3–L1 adipocytes by hormones and inflammatory cytokines. Am J Physiol Endocrinol Metab. 2004;287:E331–9 [DOI] [PubMed] [Google Scholar]
- 146.Juge-Aubry CE, Somm E, Chicheportiche R, Burger D, Pernin A, Cuenod-Pittet B, Quinodoz P, Giusti V, Dayer JM, et al. Regulatory effects of interleukin (IL)-1, interferon-beta, and IL-4 on the production of IL-1 receptor antagonist by human adipose tissue. J Clin Endocrinol Metab. 2004;89:2652–8 [DOI] [PubMed] [Google Scholar]
- 147.Kloting N, Berndt J, Kralisch S, Kovacs P, Fasshauer M, Schon MR, Stumvoll M, Bluher M. Vaspin gene expression in human adipose tissue: association with obesity and type 2 diabetes. Biochem Biophys Res Commun. 2006;339:430–6 [DOI] [PubMed] [Google Scholar]
- 148.Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, Hansen BC, Shuldiner AR, Fried SK, McLenithan JC, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290:E1253–61 [DOI] [PubMed] [Google Scholar]
- 149.Kos K, Harte AL, James S, Snead DR, O'Hare JP, McTernan PG, Kumar S. Secretion of neuropeptide Y in human adipose tissue and its role in maintenance of adipose tissue mass. Am J Physiol Endocrinol Metab. 2007;293:E1335–40 [DOI] [PubMed] [Google Scholar]
- 150.Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, Iannelli A, Staccini-Myx A, Casanova D, et al. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology. 2006;131:788–96 [DOI] [PubMed] [Google Scholar]
- 151.Bruun JM, Pedersen SB, Richelsen B. Regulation of interleukin 8 production and gene expression in human adipose tissue in vitro. J Clin Endocrinol Metab. 2001;86:1267–73 [DOI] [PubMed] [Google Scholar]
- 152.Wood IS, Wang B, Jenkins JR, Trayhurn P. The pro-inflammatory cytokine IL-18 is expressed in human adipose tissue and strongly upregulated by TNFalpha in human adipocytes. Biochem Biophys Res Commun. 2005;337:422–9 [DOI] [PubMed] [Google Scholar]
- 153.Varma V, Yao-Borengasser A, Bodles AM, Rasouli N, Phanavanh B, Nolen GT, Kern EM, Nagarajan R, Spencer HJ III, et al. Thrombospondin-1 is an adipokine associated with obesity, adipose inflammation, and insulin resistance. Diabetes. 2008;57:432–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang RZ, Lee MJ, Hu H, Pollin TI, Ryan AS, Nicklas BJ, Snitker S, Horenstein RB, Hull K, et al. Acute-phase serum amyloid A: an inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS Med. 2006;3:e287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Becker M, Rabe K, Lebherz C, Zugwurst J, Goke B, Parhofer KG, Lehrke M, Broedl UC. Expression of human chemerin induces insulin resistance in the skeletal muscle but does not affect weight, lipid levels, and atherosclerosis in LDL receptor knockout mice on high-fat diet. Diabetes. 2010;59:2898–903 [DOI] [PMC free article] [PubMed] [Google Scholar]