Abstract
The opioid system modulates several physiological processes, including analgesia, the stress response, the immune response and neuroendocrine function1. Pharmacological and molecular cloning studies have identified three opioid-receptor types, δ, κ and µ, that mediate these diverse effects2,3. Little is known about the ability of the receptors to interact to form new functional structures, the simplest of which would be a dimer. Structural and biochemical studies show that other G-protein-coupled receptors (GPCRs) interact to form homodimers4,5. Moreover, two nonfunctional receptors heterodimerize to form a functional receptor, suggesting that dimerization is crucial for receptor function6–11. However, heterodimerization between two fully functional receptors has not been documented. Here we provide biochemical and pharmacological evidence for the heterodimerization of two fully functional opioid receptors, κ and δ. This results in a new receptor that exhibits ligand binding and functional properties that are distinct from those of either receptor. Furthermore, the κ–δ heterodimer synergistically binds highly selective agonists and potentiates signal transduction. Thus, heterodimerization of these GPCRs represents a novel mechanism that modulates their function.
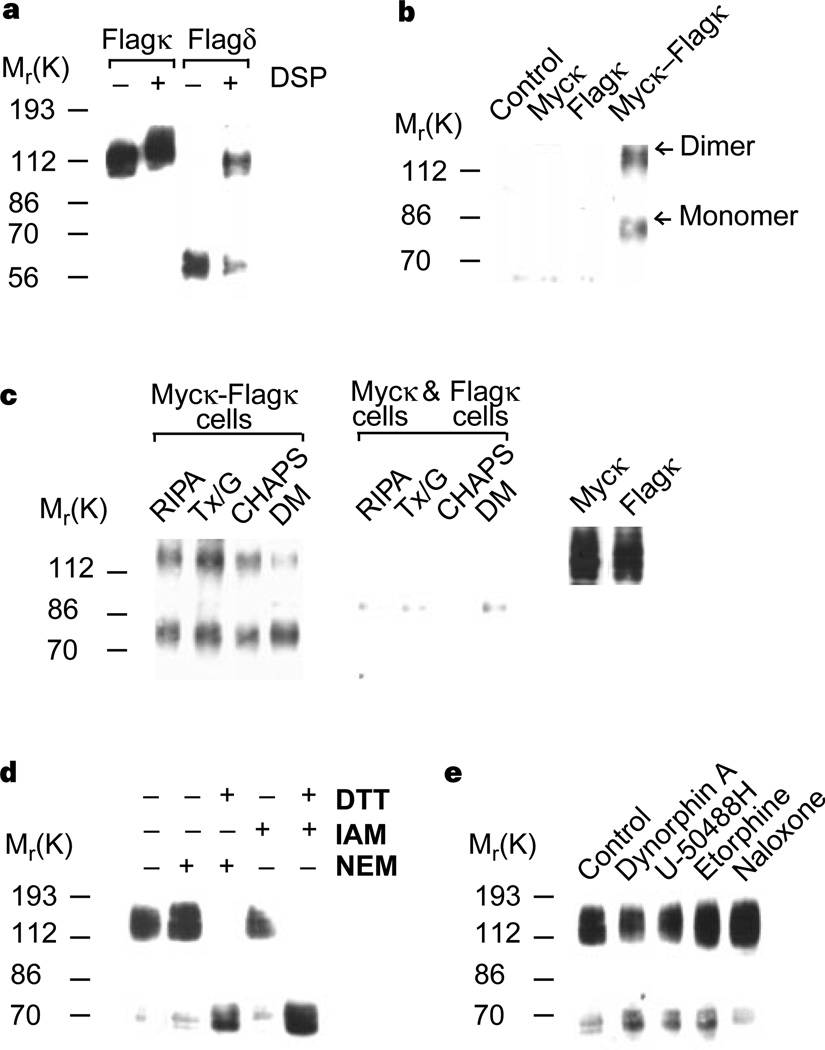
We have previously reported that δ-receptors exist as homodimers and that agonist treatment modulates the level of dimers12. Using western blotting, we examined lysates from cells expressing κ-receptors tagged with a Flag epitope to see if κ-receptors exist as dimers. We found that most κ-receptors exist as dimers of relative molecular mass (Mr) 130,000, regardless of the presence or absence of a crosslinker (Fig. 1a). The dimers are stable in 10% SDS buffer (not shown); this is unexpected, because δ-receptors show little or no dimeric forms in the absence of the crosslinker; crosslinking is required to stabilize δ-receptor dimers (Fig. 1a). The identity of this dimer was confirmed by immunoprecipitation experiments using differentially tagged receptors. We found that an antibody to the myc-tagged receptor can co-precipitate Flag-tagged receptors from cells expressing both myc-tagged and Flag-tagged receptors (Fig. 1b). The dimerization of κ-receptors is not induced by detergents or extraction conditions, as the receptors could be co-precipitated under a variety of conditions, but only from cells co-expressing myc-and Flag-tagged receptors (Fig. 1c), and not from a mixture of cells individually expressing the receptors (Fig. 1c). κ-dimers are destabilized in the presence of reducing agents (Fig. 1d), suggesting the involvement of disulphide bonds in receptor dimerization; recently, both the metabotropic glutamate receptor 5 and the calcium-sensing receptor have been shown to dimerize through disulphide bonds13,14. We found that agonist treatment does not induce monomerization of κ-receptor dimers (Fig. 1e), in contrast to δ-receptor dimers, which monomerize in the presence of agonists12. These results indicate that the properties of κ-receptor dimers and δ-receptor dimers may be different.
Figure 1.
Characteristics of κ-opioid-receptor homodimers. a, Immunoblotting of lysates from cells expressing Flag–κ receptors or Flag–δ receptors. b, c, Myc-tagged κ-receptors can be co-precipitated only from cells expressing both myc and Flag-tagged receptors (b) under a variety of extraction conditions and not from a mixture of cells individually expressing these receptors (c). Expression of myc- or Flag-tagged receptors was confirmed by immunoblotting with the appropriate antisera (right panel). d, e, Treatment of cells expressing κ-receptors with 1 mM DTT for 30 min followed by 5 mM iodacetamide (IAM) or N-ethylmaleimide (NEM) results in monomerization (d), whereas treatment with 100 nM agonists for 60 min does not (e). Immunoblotting experiments used anti-Flag antibodies; immunoprecipitation experiments used anti-myc antibodies.
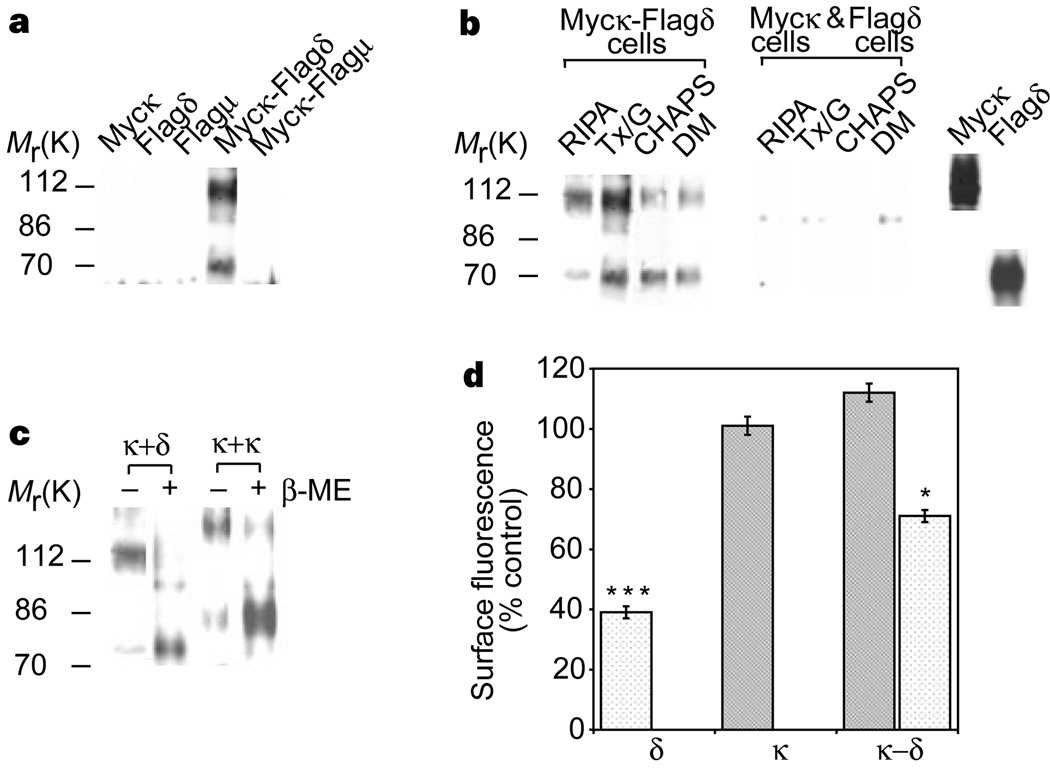
We examined the ability of κ-receptors to heterodimerize with δ-or µ-receptors by co-expressing myc-tagged κ-receptors with either Flag-tagged δ-receptors or Flag-tagged µ-receptors. Flag-tagged δ-receptors were detected in material immunoprecipitated using antibodies specific for myc-tagged κ-receptors (Fig. 2a). In contrast, Flag-tagged µ-receptors could not be detected under similar co-precipitation conditions (Fig. 2a). These results indicate that κ-receptors selectively dimerize with δ- but not with µ-opioid receptors. κ–δ heterodimers are stable in a variety of detergents and are not induced during solubilization/immunoprecipitation procedures (Fig. 2b). They are also destabilized by a reducing agent (Fig. 2c), suggesting a role for disulphide bonds inκ–δ heterodimerization.
Figure 2.
Characterization of κ–δ heterodimers. a, κ–δ heterodimers can be immunoprecipitated only from myc–κ- and Flag-δ-expressing cells and not from myc–κ- and Flag–µ-expressing cells. b, κ–δ heterodimers can be immunoprecipitated under a variety of extraction conditions and not from a mixture of cells individually expressing these receptors. c, Expression of myc- or Flag-tagged receptors in each cell line was confirmed by immunoblotting with the appropriate antisera (right panel). Treatment with β-mercaptoethanol 5% (β–ME) for 5 min results in the destabilization of dimers. d, Internalization of receptors in response to 1 µM etorphine for 60 min. Stippled bars, myc–δ; shaded bars, Flag–κ. Significant differences from untreated controls are indicated; *P < 0.05; ***P < 0.005 (n = 3). Immunoblotting experiments used anti-Flag antibodies; immunoprecipitation experiments used anti-myc antibodies.
We next examined the effect of heterodimerization on receptor trafficking using cells co-expressing κ- and δ-receptors. Etorphine is a potent, non-selective opioid agonist that binds both δ- and κ-receptors with high affinity. As was shown previously15–17, etorphine can induce robust internalization of δ- but not κ-receptors in cells individually expressing these receptors (Fig. 2d). In contrast, etorphine cannot induce substantial internalization of δ-receptors in cells expressing both κ- and δ-receptors; the internalization of δ-homodimers in these cells could account for the observed ~25–30% reduction in surface fluorescence (Fig. 2d). These results suggest a role for heterodimerization in altering the trafficking properties of these receptors.
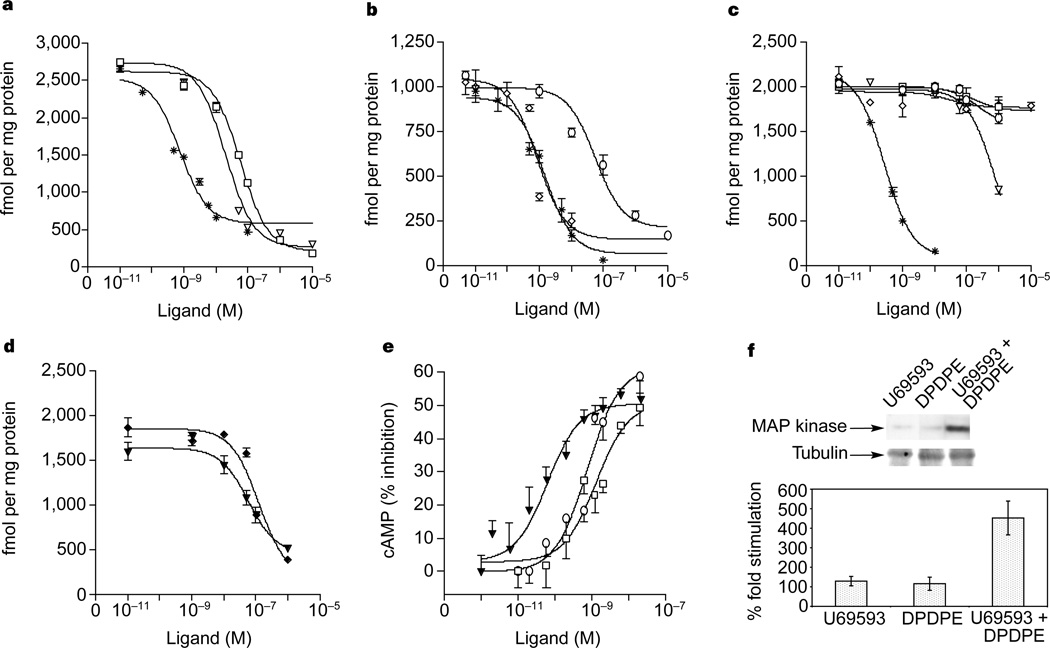
We compared the ligand-binding properties of κ–δ heterodimers with those of κ- or δ-receptors (Table 1), and examined the ability of highly selective agonists18,19 and antagonists20,21 to compete with 3H-diprenorphine (a non-selective opioid antagonist) in membranes from cells expressing either κ- or δ- or both κ- and δ-receptors. (Fig. 3a–c). We found that κ-receptors have high affinities for the κ-selective agonist (U69593) and antagonist (norbinaltorphimine). Similarly, δ-receptors have high affinities for the δ-selective agonist ([d-Pen2,d-Pen5]enkephalin; DPDPE) and antagonist (TIPPΨ; ref. 21). In contrast, κ–δ heterodimers show no significant affinity for either κ- or δ-selective agonists or antagonists (Fig. 3c; Table 1). However, the heterodimer shows a strong affinity for partially selective ligands (Table 1). The properties of these heterodimers are virtually identical to those of the previously reported κ-2-receptor subtype22. Although several studies have reported the presence of other subtypes of κ- (ref. 23) and δ- (refs 24, 25) opioid receptors, complementary DNAs corresponding to these subtypes have not been identified despite large-scale efforts by several laboratories. Recent work with δ-receptor knockout mice shows that both the δ1 and δ2 receptor subtypes are eliminated in these animals, indicating that the δ-receptor locus may encode both of these subtypes. It is possible that the heterodimerization of δ- or κ-receptors with other GPCRs could form a molecular basis for other receptor subtypes.
Table 1.
Ligand-binding properties of κ–δ heterodimer
| Ligand | Ki (nM) | ||
|---|---|---|---|
| κ | δ | κ–δ | |
| Agonists | |||
| U69593 | 14.4 ± 0.2 | >1,000 | >1,000 |
| DPDPE | >1,000 | 21.8 ± 2.1 | >1,000 |
| Dynorphin A | 1.3 ± 0.41 | 56.8 ± 0.3 | 5.6 ± 0.3 |
| EKC | 5.7 ± 0.79 | 105 ± 1.2 | 2.6 ± 0.2 |
| Etorphine | 1.5 ± 0.73 | 7.9 ± 0.03 | 7.0 ± 0.3 |
| Bremazocine | 0.4 ± 0.11 | 7.5 ± 0.03 | 1.2 ± 0.1 |
| Antagonists | |||
| Norbinaltorphimine | 4.9 ± 0.3 | 31.8 ± 1.2 | 126 ± 9.6 |
| TIPPΨ | >1,000 | 0.28 ± 0.6 | >1,000 |
| BNTX | 172 ± 19 | 5.2 ± 0.6 | 98 ± 19 |
| Naltrindole | 44.3 ± 1.1 | 1.0 ± 0.21 | 44 ± 1.1 |
| Naloxone | 14.9 ± 3.4 | 43.4 ± 0.3 | 7.5 ± 0.4 |
| Diprenorphine | 0.71 ± 0.27 | 1.43 ± 1.1 | 0.3 ± 0.1 |
| Combination of ligands | |||
| U69593 (+10 µM DPDPE) | 14.4 ± 0.4 | – | 9.2 ± 1.4 |
| DPDPE (+10 µM U69593) | – | 24.8 ± 1.6 | 20.0 ± 1.3 |
| Norbinaltorphimine (+10 µM TIPPΨ) | – | – | 0.02 ± 0.003 |
| U69593 (+10 µM TIPPΨ) | – | – | >1,000 |
Ligand affinities were determined by competition assays using 3H-diprenorphine as described. Mean ± s.e.m. (n = 3–4). BNTX, 7-benzylidenenaltrexone; EKC, ethylketocyclazocine;–, not done.
Figure 3.
Ligand binding and functional properties. a–c, Competition of 3H-diprenorphine binding by U69593 (square), norbinaltorphimine (triangle), diprenorphine (star), DPDPE (circle) and TIPPΨ (diamond) in membranes from cells expressing κ- (a), δ- (b) or κ- and δ- (c) receptors. d, Displacement of 3H-diprenorphine by U69593 in the presence of 10 µM DPDPE (triangle) or DPDPE in the presence of 10 µM U69593 (diamond). e, f, Decrease in intracellular cAMP (e) or increase in phospho-MAPK (f) by U69593 (square), DPDPE (circle) or U69593 + DPDPE (triangle). In e, the 50% inhibitory concentrations (nM) were: U69593, 1.3 ± 0.7; DPDPE, 0.9 ± 0.4; U69593 + DPDPE, 0.06 ± 0.03. Activation of homodimers in these cells could account for the effect seen by individual agonists. Error bars represent s.e.m. (n = 3–4).
We next examined whether the κ–δ heterodimer binds selective agonists synergistically. In the presence of a δ-selective agonist (DPDPE), a κ-agonist (U69593) binds the heterodimer with high affinity (Fig. 3d, Table 1). Similarly, in the presence of the κ-selective agonist (U69593), the δ-agonist (DPDPE) binds with high affinity (Fig. 3d, Table 1). Interestingly, whereas a combination of two selective antagonists also binds with high affinity, a combination of a selective agonist (U69593) and a selective antagonist (TIPPΨ) does not (Table 1). Also, synergistic binding is not observed in membranes from cells individually expressing κ- or δ-receptors (not shown). Taken together, these results imply that κ–δ heterodimerization results in a new binding site that is able to bind highly selective ligands synergistically.
We examined whether the synergistic binding of agonists leads to increased effector function. The activation of opioid receptors by agonists results in decreased levels of intracellular cyclic AMP and increased levels of phosphorylated mitogen-activated protein kinase (MAPK)26. We found that the potency of individual agonists in reducing intracellular cAMP levels is ~10–20-fold lower than that of the two combined (Fig. 3e). Similarly, we found a significant potentiation of MAPK phosphorylation by simultaneous treatment of cells with both agonists as compared to individual agonists (Fig. 3f). These results strongly suggest that the κ–δ heterodimer represents a functional receptor that is activated synergistically by selective ligands.
Our data provide biochemical and functional evidence for opioid-receptor heterodimerization. This is the first direct evidence for the heterodimerization of opioid-receptor types and for two fully functional GPCRs. The heterodimers have greatly reduced affinities for their selective ligands. Interestingly, selective agonists can cooperatively bind to heterodimers and induce synergistic functional responses. Heterodimerization could be a mechanism for activating the receptors on the co-release of selective endogenous peptides. Alternatively, κ–δ-opioid-receptor heterodimers could represent a hitherto uncharacterized receptor for a specific endogenous opioid peptide. The number of endogenous opioid peptides is far greater than the number of cloned opioid receptors27. Opioid-receptor subtypes resulting from heterodimerization of opioid receptors with other GPCRs could be targets for the action of these endogenous peptides. The physical interactions between GPCRs has enormous ramifications for our understanding of how their actions are regulated. Heterodimerization of opioid receptors also points to additional targets for the development of therapeutic drugs.
Methods
Generation of cell lines expressing opioid receptors
Cells stably expressing epitope-tagged rat κ- or mouse δ-receptors were generated as described elsewhere28. HEK293 or COS cells co-expressing myc-tagged κ- with Flag-tagged κ-, δ- or µ-receptors were generated by transfecting cells using calcium phosphate precipitation12 and collecting the cells 72 h later for transient expression. For stable expression, CHO cells were transfected with the Flag-tagged κ-receptor cDNA in a geneticin-selectable vector and myc-tagged δ-receptor cDNA in a hygomycin-selectable vector (pCEN4, Invitrogen) and selected with 500 µg ml−1 each of geneticin and hygromycin (Gibco). Surface expression was confirmed by flow cytometry using monoclonal anti-Flag (M1; Sigma) and polyclonal anti-myc (c-Myc A14; Santa Cruz) antibodies. Three clones expressing κ- plus δ-receptors at a ratio of approximately 1:1 were used for further studies.
Dimerization, immunoprecipitation and western blotting
Crosslinking with DSP (dithiobis-succinimydyl propionate; Pierce), SDS–PAGE and western blotting were carried out with lysates of whole cells or membranes essentially as described elsewhere12, except that cells were lysed in buffers containing the protease-inhibitor cocktail (10 µg ml−1 leupeptin, 10 κg ml−1 aprotinin, 10 mM EDTA, 1mM EGTA, 10 µg ml−1 bacitracin, 1 mM pepstatin A, 0.5mM phenylmethylsulphonyl fluoride and 1 mM E-64) and 100 mM iodoacetamide at 4 °C for 60 min (or 23 °C when lysing with SDS buffer). In most experiments we used Tx/G buffer (300 mM NaCl, 1% Triton X-100, 10% glycerol, 1.5mM MgCl2 and 1 mM CaCl2 in 50mM Tris-Cl, pH 7.4) for solubilization. Other solubilization buffers used were RIPA (1% NP-40, 0.5% deoxycholate, 0.5% SDS, 300mM NaCl), CHAPS (0.5% in 50mM NaPO4 buffer, pH 7.4), DM (0.5% dodecyl β-maltoside in 50 mM Tris-Cl, pH 7.4) and SDS (2% SDS in 50mM Tris-Cl, pH 6.8). For immunoprecipitation, 100–200 µg of proteins were incubated overnight at 4 °C with 1–2 µg of polyclonal anti-myc antibody. Immunocomplexes were isolated by incubation with 10% v/v of Protein A–Sepharose and analysed by western blotting using monoclonal anti-Flag antibody as described previously12. κ-receptors are differentially glycosylated in CHO and COS cells; this could account for differences in the size of monomers and dimers. Weak reducing conditions (10 mM dithiothreitol; DTT was added to the immunoprecipitate) were used to eliminate crossreactivity with nonspecific proteins; a portion of dimers are converted to monomers under these conditions.
Binding assays
For membrane preparation, HEK293 cells expressing single or combinations of receptors were washed with PBS buffer, collected with a rubber policeman in 5mM Tris-Cl buffer, pH 7.4, and incubated for 30 min at room temperature. Cells were disrupted by sonication and subjected to low-speed centrifugation to remove organelles and nuclei. The resulting supernatant was subjected to centrifugation at 50,000g for 10 min, and membranes were collected, washed three times, resuspended in 50mM Tris-Cl, pH 7.4, containing a protease-inhibitor cocktail and stored at −80 °C. Membranes were homogenized on thawing and 30–50 µg of membrane proteins were incubated with 0.5 nM or 5 nM 3H-diprenorphine (40 Ci mmol−1; NEN/Dupont) for 60 min at 37 °C in the absence or presence of 5–8 concentrations of unlabelled ligands; 1 µM unlabelled diprenorphine was used to obtain specific binding. 5 nM 3H-diprenorphine labels all receptors in κ–δ-expressing cells. Approximately 45% of the specific binding is not displaced by either 10 µM DPDPE or 10 µM U69593; this can be selectively labelled by 0.5 nM 3H-diprenorphine, so all studies characterizing the κ–δ heterodimer were carried out with 0.5 nM 3H-diprenorphine. Values for half-maximal inhibitory concentration (IC50) were determined from displacement curves using GraphPad Prism 2.0 and for inhibition constant (Ki) using the Cheng–Prusoff equation29.
Internalization assays
Ligand-induced internalization was carried out by flow cytometry as described elsewhere15, except that FITC-conjugated anti-mouse antibody was used to detect the monoclonal anti-Flag antibody (M1; Sigma) bound to Flag–κ receptors and phycoerythrin-conjugated anti-rabbit antibody was used to detect the polyclonal anti-myc antibody (c-Myc A14; Santa Cruz) bound to myc-tagged δ-receptors.
Functional assays
CHO cells co-expressing approximately 1:1 ratio of κ- and δ-receptors were treated with various doses of agonists (2 × DPDPE, 2 × U69593 or 1 × DPDPE + 1 × U69593) for 5 min at 37 °C. The intracellular cAMP level was measured by radioimmunoassay as described previously28. The level of phosphorylated MAPK was determined by western blotting30. Standardization was with tubulin measured in the same blots using anti-tubulin antibody (Sigma). NIH Image 1.61 software was used to densitize and quantify phospho-MAPK levels. The extent of MAPK phosphorylation in cells treated with 2 nM DPDPE or 2 nM U69593 or with 1 nM DPDPE + 1 nM U69593 is shown in Fig. 3f (upper panel). % fold stimulation refers to the agonist-induced increase in phospho-MAPK levels over untreated levels (taken as control, 100%).
Acknowledgements
We thank L. Fricker and S. Cvejic for critical reading of the manuscript and P. Schiller for the gift of TIPPΨ. This work is supported in part by grants from the NIH (NIDA and NINDS).
References
- 1.Herz A. Opioids. Vol. 1. Berlin: Springer; 1993. [Google Scholar]
- 2.Miotto K, Magendzo K, Evans CJ. In: The Pharmacology of Opioid Peptides. Tseng L, editor. Harwood, Singapore: 1995. pp. 57–71. [Google Scholar]
- 3.Kieffer BL. Recent advances in molecular recognition and signal transduction of active peptides: receptors for opioid peptides. Cell. Mol. Neurobiol. 1995;15:615–635. doi: 10.1007/BF02071128. [DOI] [PubMed] [Google Scholar]
- 4.Hebert TE, Bouvier M. Structural and functional aspects of G protein-coupled receptor oligomerization. Biochem. Cell Biol. 1998;76:1–11. doi: 10.1139/bcb-76-1-1. [DOI] [PubMed] [Google Scholar]
- 5.Gouldson PR, Snell CR, Bywater RP, Higgs C, Reynolds CA. Domain swapping in G-protein coupled receptor dimers. Protein Eng. 1998;11:1181–1193. doi: 10.1093/protein/11.12.1181. [DOI] [PubMed] [Google Scholar]
- 6.Maggio R, Vogel Z, Wess J. Co-expression studies with mutant muscarinic/adrenergic receptors provide evidence for intermolecular cross-talk between G-protein-linked receptors. Proc. Natl Acad. Sci. USA. 1993;90:3103–3107. doi: 10.1073/pnas.90.7.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monnot C, et al. Polar residues in the transmembrane domains of the type I angiotensin II receptor are required for binding and coupling: Reconstitution of the binding site by co-expression of two deficient mutants. J. Biol. Chem. 1996;271:1507–1513. doi: 10.1074/jbc.271.3.1507. [DOI] [PubMed] [Google Scholar]
- 8.Jones KA, et al. GABAB receptors function as a heteromeric assembly fo the subunits GABABR1 and GABABR2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 9.Kaupmann K, et al. GABAB-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- 10.White JH, et al. Heterodimerization is required for the formation of a functional GABAB receptor. Nature. 1998;396:679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- 11.Kuner R, et al. Role of heteromer formation in GABAB receptor function. Science. 1999;283:74–77. doi: 10.1126/science.283.5398.74. [DOI] [PubMed] [Google Scholar]
- 12.Cvejic S, Devi L. Dimerization of the delta opioid receptor; implications for a function in receptor internalization. J. Biol. Chem. 1997;272:26959–26964. doi: 10.1074/jbc.272.43.26959. [DOI] [PubMed] [Google Scholar]
- 13.Romano C, Yang W, O’Malley K. Metabotropic glutamate receptor 5 is a disulfide-linked dimer. J. Biol. Chem. 1996;271:28612–28616. doi: 10.1074/jbc.271.45.28612. [DOI] [PubMed] [Google Scholar]
- 14.Bai M, Trivedi S, Brown EM. Dimerization of the extracellular calcium-sensing receptor on the cell surface of CaR-transfected HEK-293 cells. J. Biol. Chem. 1998;273:23605–23610. doi: 10.1074/jbc.273.36.23605. [DOI] [PubMed] [Google Scholar]
- 15.Trapaidze N, Keith DE, Cvejic S, Evans CJ, Devi LA. Sequestration of the delta opioid receptor: Role of the C terminus in agonist-mediated internalization. J. Biol. Chem. 1996;271:29279–29285. doi: 10.1074/jbc.271.46.29279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keith DE, et al. Morphine activates opioid receptors without causing their rapid internalization. J. Biol. Chem. 1996;271:19021–19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- 17.Chu P, Murray S, Lissin D, von Zastrow M. Delta and kappa opioid receptors are differentially regulated by dynamin-dependent endocytosis when activated by the same alkaloid agonist. J. Biol. Chem. 1997;272:27124–27130. doi: 10.1074/jbc.272.43.27124. [DOI] [PubMed] [Google Scholar]
- 18.Lahti RA, Mickelson MM, McCall JM, Von Voigtlander P. [3H]U-69593, a highly selective ligand for the opioid κ receptor. Eur. J. Pharmacol. 1985;109:281–284. doi: 10.1016/0014-2999(85)90431-5. [DOI] [PubMed] [Google Scholar]
- 19.Roth G, et al. [D-Pen2,D-Pen5]enkephalin analogues with increased affinity and selectivity for δ opioid receptors. J. Med. Chem. 1990;258:299–303. doi: 10.1021/jm00163a041. [DOI] [PubMed] [Google Scholar]
- 20.Portoghese PS, Lipkowski AW, Takemori AE. Binaltrophimine and norbinaltrophimine, potent and selective κ-opioid receptor antagonists. Life Sci. 1987;40:1287–1292. doi: 10.1016/0024-3205(87)90585-6. [DOI] [PubMed] [Google Scholar]
- 21.Schiller P, et al. TIPPΨ: A highly potent and stable pseudopeptide δ opioid receptor antagonist with extraordinary δ selectivity. J. Med. Chem. 1993;36:3182–3187. doi: 10.1021/jm00073a020. [DOI] [PubMed] [Google Scholar]
- 22.Zukin RS, Echbali M, Olive D, Unterwald EM, Tempel A. Characterization and visualization of rat and guinea pig brain κ opioid receptors: Evidence for κ1 and κ2 opioid receptors. Proc. Natl Acad. Sci. USA. 1988:4061–4065. doi: 10.1073/pnas.85.11.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nock B. In: The Pharmacology of Opioid Peptides. Tseng L, editor. Harwood, Singapore: 1995. pp. 29–56. [Google Scholar]
- 24.Traynor JR, Elliott J. Delta opioid receptor subtypes and cross talk with mu receptors. Trends Pharmacol. Sci. 1993;14:84–86. doi: 10.1016/0165-6147(93)90068-u. [DOI] [PubMed] [Google Scholar]
- 25.Zaki PA, et al. Opioid receptor types and subtypes: the delta receptor as a model. Annu. Rev. Pharmacol. Toxicol. 1996;36:379–401. doi: 10.1146/annurev.pa.36.040196.002115. [DOI] [PubMed] [Google Scholar]
- 26.Jordan B, Devi LA. Molecular mechanisms of opiate receptor signal transduction. Br. J. Anaesth. 1998;81:12–19. doi: 10.1093/bja/81.1.12. [DOI] [PubMed] [Google Scholar]
- 27.Lord JAH, Waterfield AA, Hughes J, Kosterlitz HW. Endogenous opioid peptides:Multiple agonists and receptors. Nature. 1977;267:495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- 28.Cvejic S, Trapaidze N, Cyr C, Devi LA. Thr353, located within the COOH-terminal tail of the delta opiate receptor, is involved in receptor down-regulation. J. Biol. Chem. 1996;271:4073–4076. doi: 10.1074/jbc.271.8.4073. [DOI] [PubMed] [Google Scholar]
- 29.Cheng YC, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzyme reaction. Biochem. Pharmacol. 1973;22:3099–3102. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 30.Polakiewicz R, Schieferl SM, Dorner LF, Kansra V, Comb MJ. A mitogen-activated protein kinase pathway is required for mu-opioid receptor desensitization. J. Biol. Chem. 1998;273:12402–12406. doi: 10.1074/jbc.273.20.12402. [DOI] [PubMed] [Google Scholar]