Abstract
The relationship between intensity of inflammatory stimulation and production of α 2-macroglobulin (α2M) and α 1-acid glycoprotein (AAG) in rats was investigated. Sprague-Dawley rats were injected with turpentine oil at doses of 0.05, 0.2 or 0.4 mL/rat. Serum levels of α2M, interleukin (IL)-6 and cytokine-induced neutrophil chemoattractant-1 (CINC-1) were measured by enzyme-linked immunosorbent assay, and AAG was measured by single radial immunodiffusion. Peak serum levels of α2M and AAG in rats injected at 0.05 mL/rat were significantly lower than those at 0.2 or 0.4 mL/rat. However, no significant differences were observed for peak serum levels of these acute-phase proteins between 0.2 and 0.4 mL/rat. Furthermore, peak serum levels of IL-6 and CINC-1 in rats injected at 0.05 mL/rat were significantly lower than those at 0.2 or 0.4 mL/rat. Thus, the production of these acute-phase proteins has upper limits, even under increased strength of inflammatory stimulation in rats injected with turpentine oil.
Keywords: α2M, AAG, IL-6, CINC-1, rats
Acute-phase protein levels increase during acute inflammation, e.g. bacterial infection, trauma and surgical treatment.1–3 C-reactive protein is a typical acute-phase protein in humans and dogs,4–7 while α 2-macroglobulin (α2M) is a typical acute-phase protein in rats.8–10 α2M levels increase in rats subjected to acute inflammation by inoculation with microorganisms, injection of turpentine oil or after surgical treatment.9 α 1-acid glycoprotein (AAG) contributes to protein binding with anionic drug substances, and also increases following inflammatory stimulation.11,12
Peak levels of α2M differ after injection of turpentine oil, inoculation with microorganisms or surgical treatment.8,9 Furthermore, peak levels of AAG also differ after injection of turpentine oil, inoculation with microorganisms and gastric bleeding by injection of indomethacin.12 Peak levels of these acute-phase proteins are thus presumed to differ with the magnitude of inflammatory stimulation in rats. α2M and AAG are considered to be typical inflammatory markers in rats. However, there has been little basic research on these proteins, and little is known about the differences in production based on the magnitude of stimulation. Thus, degree of inflammation cannot be estimated based on production of α2M or AAG.
In the present study, the changes in serum levels of α2M and AAG in rats subjected to various dosages of turpentine oil to induce acute inflammation were investigated in an effort to clarify whether the production of acute-phase proteins varies with the magnitude of inflammatory stimulation.
Materials and methods
Animals
Eighteen nine-week-old Sprague-Dawley rats were purchased from Charles River, Inc (Yokohama, Japan), and were used in the present study. Rats were kept in isolators at a temperature of 23 ± 2°C, and a relative humidity of 55 ± 10%, on a 12/12 h dark/light cycle (06:00–18:00) with air exchanged 12 times or more per hour. Rats were fed MF (Oriental Yeast Co, Ltd, Tokyo, Japan), and were allowed free access to water.
All experiments were approved by the Institutional Review Board of Azabu University and conducted in accordance with the Institute's Animal Experimentation Guidelines (Japanese Association for Laboratory Animal Science, JALAS, 1987).
Animal experiment design
Rats were divided into three groups (n = 6 for each group). Turpentine oil (Wako Pure Chemical Industries, Ltd, Osaka, Japan) was injected intramuscularly into rats at 0.05, 0.2 or 0.4 mL/rat. Acute-phase protein levels are known to increase after intramuscular injection of turpentine oil,8,9,13–15 which has been used to induce acute inflammation in several studies, primarily because turpentine oil is able to induce constant acute inflammation, as compared with infection by microorganisms or other chemical compounds.8,9 Turpentine oil was thus used to induce acute inflammation in this study. Blood (0.3 mL) was collected before injection and at 6, 12, 24, 48, 72 and 96 h after administration. Blood was collected from the venae cervicalis superficialis using a syringe under mild anaesthesia with pentobarbital (Kyoritsu Seiyaku Corporation, Tokyo, Japan). Pentobarbital was intravenously administered at small doses (4 mg/kg) in order to ensure only short-term effects. Sera were obtained by centrifugation at 1600 g for 15 min, and were stored at −80°C until analysis.
Measurement of α2M and AAG
Serum levels of α2M were measured by enzyme-linked immunosorbent assay (ELISA) according to the procedure of Honjo et al. 16 Serum levels of AAG were measured by single radial immunodiffusion using a commercial kit (Institute for Metabolic Ecosystem Co, Ltd, Miyagi, Japan).
Measurement of interleukin-6 and cytokine-induced neutrophil chemoattractant-1
Serum levels of interleukin-6 (IL-6) and cytokine-induced neutrophil chemoattractant (CINC-1) were measured by ELISA using commercial kits. Commercial ELISA kits were purchased from BioSource International, Inc (Camarillo, CA, USA) for IL-6 and from Panapharm Laboratories Co, Ltd (Kumamoto, Japan) for CINC-1.
Statistics
All values are expressed as means ± SD (n = 6). Analysis of significance between variables was performed using unpaired Student's t-test. P values of <0.05 were considered to be statistically significant.
Results
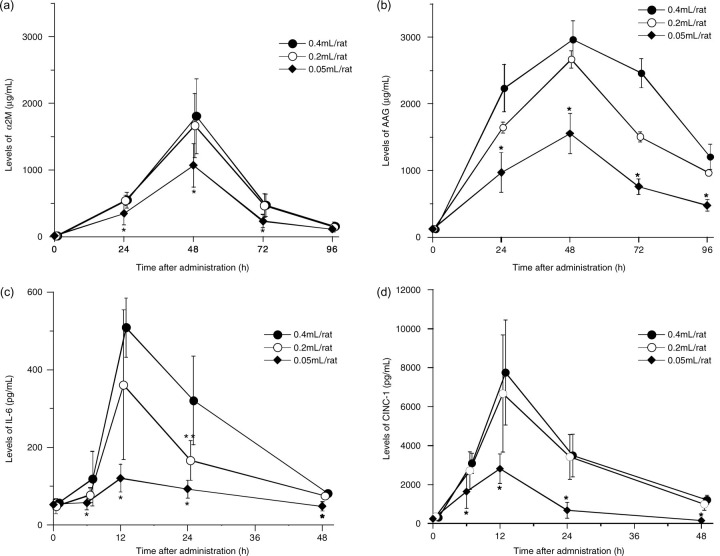
Changes in serum levels of α2M, AAG, IL-6 and CINC-1 are shown in Figure 1. Peak levels of α2M were observed at 48 h after injection of 0.05, 0.2 or 0.4 mL/rat. Peak α2M levels were 1071.2 ± 324.4 µg/mL in the 0.05 mL/rat injection group, 1667.4 ± 478.7 µg/mL in the 0.2 mL/rat injection group and 1808.0 ± 563.5 µg/mL in the 0.4 mL/rat injection group. Peak levels in the 0.05 mL/rat injection group were significantly lower than those in the 0.2 and 0.4 mL/rat injection groups. However, no significant differences were observed between the 0.2 and 0.4 mL/rat injection groups.
Figure 1.
Changes in serum levels of acute-phase proteins and cytokines in rats injected with turpentine oil at 0.05, 0.2 or 0.4 mL/rat. (a) α 2-macroglobulin (α2M), (b) α 1-acid glycoprotein (AAG), (c) interleukin-6 (IL-6) and (d) cytokine-induced neutrophil chemoattractant-1 (CINC-1). Data are expressed as means ± SD (n = 6). *P < 0.05, significantly different versus 0.2 or 0.4 mL/rat injection group, as analysed by Student's t-test. **P < 0.05, significantly different versus 0.4 mL/rat injection group, as analysed by Student's t-test
Serum levels of AAG also peaked at 48 h after injection in all groups. Peaks for AAG were 1554.8 ± 302.8 µg/mL in the 0.05 mL/rat injection group, 2667.6 ± 451.1 µg/mL in the 0.2 mL/rat injection group and 2962.8 ± 285.0 µg/mL in the 0.4 mL/rat injection group. Peak levels of AAG in the 0.05 mL/rat injection group were significantly lower than those in the 0.2 and 0.4 mL/rat injection groups.
Peak serum levels of IL-6 and CINC-1 were observed at 12 h after injection in all injection groups. Peak levels of IL-6 were 120.5 ± 36.0 pg/mL in the 0.05 mL/rat injection group, 361.0 ± 192.9 pg/mL in the 0.2 mL/rat injection group and 508.6 ± 76.2 pg/mL in the 0.4 mL/rat injection group. Peak levels in the 0.05 mL/rat injection group were significantly lower than in the 0.2 and 0.4 mL/rat injection groups. No significant differences were observed between the 0.2 and the 0.4 mL/rat injection groups, except at 24 h after injection.
Peaks for CINC-1 were 2812.3 ± 755.8 pg/mL in the 0.05 mL/rat injection group, 6734.8 ± 2898.2 pg/mL in the 0.2 mL/rat injection group and 7449.8 ± 2698.5 pg/mL in the 0.4 mL/rat injection group. Peaks levels in the 0.05 mL/rat injection group were significantly lower than those in the 0.2 and 0.4 mL/rat injection groups. No significant differences were observed between the 0.2 and 0.4 mL/rat injection groups.
Discussion
Serum levels of the typical acute-phase proteins α2M and AAG in rats after inflammatory stimulation with various doses of turpentine oil were examined in an effort to clarify the differences in production during various degrees of inflammatory stimulation. Serum levels of α2M and AAG in rats injected with 0.05 mL/rat were significantly lower than those injected with 0.2 and 0.4 mL/rat; however, no significant differences were observed between the 0.2 and 0.4 mL/rat injection groups. These results suggest that production of α2M and AAG increases in proportion with turpentine oil dose. Production of α2M and AAG was similar above 0.2 mL/rat; however, limited production was observed. Thus, it was necessary to carefully evaluate the degree of inflammation based on the levels of these acute-phase proteins.
IL-6 is known to regulate the synthesis of acute-phase proteins,17–22 while IL-8 regulates the production of acute-phase proteins in humans.23,24 However, IL-8 has not been observed in rats, and CINC-1 is considered to be the counterpart of human IL-8.25–28 On the other hand, serum levels of seven cytokines (IL-1, IL-2, IL-4, IL-6, CINC-1, IL-10 and interferon-γ) in rats injected with turpentine oil have been investigated, and only serum levels of IL-6 and CINC-1 were elevated prior to increases in α2M.9 In contrast, levels of the cytokines IL-1, IL-2, IL-4, IL-10 and interferon-γ did not change.9 IL-6 and CINC-1 also increased prior to AAG in rats injected with turpentine oil.12 Furthermore, the changes in serum levels of α2M and AAG after repeated injection of turpentine oil at doses of 0.05 and 0.4 mL/rat showed a similar pattern as with single stimulation, and peak serum levels in rats injected with 0.05 and 0.4 mL/rat were largely comparable. This phenomenon was thought to be caused by differences in the production of IL-6 and CINC-1 in the two groups.29 Thus, IL-6 and CINC-1 apparently contribute to the production of α2M and AAG in rats.16 In the present study, levels of IL-6 and CINC-1 in rats injected with 0.05 mL were also significantly lower than those injected with 0.2 and 0.4 mL/rat. The levels of both cytokines did not differ significantly between the 0.2 and the 0.4 mL/rat injection groups. The four parameters evaluated in the present study showed similar patterns. The significant differences in production of α2M and AAG independent of the turpentine oil dosage are considered to be attributable to significant differences in the production of IL-6 and CINC-1. These results thus suggest that IL-6 and CINC-1 regulate the production of α2M and AAG.
In summary, the production of α2M and AAG has an upper limit, irrespective of the magnitude of stimulation. Production of α2M and AAG showed similar trends to those of IL-6 and CINC-1; thus, the kinetics of these cytokines influenced the production of α2M and AAG.
ACKNOWLEDGEMENTS
This research was partially supported by the Promotion and Mutual Aid Corporation for Private Schools of Japan, Grant-in-Aid for Matching Fund Subsidy for Private Universities.
REFERENCES
- 1. Capsi D, Baltz ML, Snel F, et al. Isolation and characterization of C-reactive protein from the dog. Immunology 1984;53: 307–13 [PMC free article] [PubMed] [Google Scholar]
- 2. Ceron JJ, Eckersall PD, Martinez-Subiela S. Acute phase in dogs and cats: current knowledge and future perspectives. Vet Clin Pathol 2005;34:85–99 [DOI] [PubMed] [Google Scholar]
- 3. Eckersall PD, Harvey MJA, Ferguson JM, Renton JP, Nickson DA, Boyd JS. Acute phase proteins in canine pregnancy (Canis familiaris). J Reprod Fertil 1993;47:159–64 [PubMed] [Google Scholar]
- 4. Capsi D, Snel FW, Batt RM, et al. C-reactive protein in dogs. Am J Vet Res 1987;48:919–21 [PubMed] [Google Scholar]
- 5. Karadag F, Kirdar S, Karul AB, Ceylan E. The value of C-reactive protein as a marker of systemic inflammation in stable chronic obstructive pulmonary disease. Eur J Intern Med 2008;19:104–8 [DOI] [PubMed] [Google Scholar]
- 6. Morris SG, Gomez D, Prasad KR. C-reactive protein in liver cancer surgery. Eur J Surg Oncol 2008;34:727–9 [DOI] [PubMed] [Google Scholar]
- 7. Volanakis JE. Human C-reactive protein: expression, structure, and function. Mol Immunol 2001;38:189–97 [DOI] [PubMed] [Google Scholar]
- 8. Jinbo T, Motoki M, Yamamoto S. Variation of serum α2-macroglobulin concentration in healthy rats and rats inoculated with Staphylococcus aureus or subjected to surgery. Comp Med 2001;51:332–5 [PubMed] [Google Scholar]
- 9. Jinbo T, Sakamoto T, Yamamoto S. Serum α2-macroglobulin and cytokine measurements in an acute inflammation model in rats. Lab Anim 2002;36:153–7 [DOI] [PubMed] [Google Scholar]
- 10. Westrhenen R, Westra WM, Born J, et al. Alpha-2-macroglobulin and albumin are useful serum proteins to detect subclinical peritonitis in the rat. Perit Dial Int 2006;26:101–7 [PubMed] [Google Scholar]
- 11. Fournier T, Medjoubi NN, Porquet D. Alpha-1-acid glycoprotein. Biochem Biophys Acta 2000;1482:157–71 [DOI] [PubMed] [Google Scholar]
- 12. Honjo T, Kuribayashi T, Seita T, et al. Variations in α 1-acid glycoprotein (α1 AG) and inflammatory cytokines (IL-6, CINC-1) in healthy rats following acute inflammation – α1-acid glycoprotein and cytokines in rats. Vet Biochem 2006;43:9–15 [Google Scholar]
- 13. Kusahio K, Shimizu H, Kimura F, et al. Effect of excessive acute-phase response on liver regeneration after partial hepatectomy in rats. Hepatogastroenterology 2009;56:824–8 [PubMed] [Google Scholar]
- 14. Sheikh N, Dudas J, Ramadori G. Changes of gene expression of iron regulatory proteins during turpentine oil-induced acute-phase response in the rat. Lab Invest 2007;87:713–25 [DOI] [PubMed] [Google Scholar]
- 15. Viatte L, Grone HJ, Hentza MW, Glay B. In vivo role(s) of the iron regulatory proteins (IRP) 1 and 2 in aseptic local inflammation. J Mol Med 2009;87:913–21 [DOI] [PubMed] [Google Scholar]
- 16. Honjo T, Kuribayashi T, Mokonuma Y, Yamaga A, Yamazaki S, Yamamoto S. The effects of interleukin-6 and cytokine-induced neutrophil chemoattractant-1 on α2-macroglobulin production in rat. Exp Anim 2010;59:589–94 [DOI] [PubMed] [Google Scholar]
- 17. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448–54 [DOI] [PubMed] [Google Scholar]
- 18. Heinrich PC, Castell JV, Andus T. Interleukin-6 and acute phase response. Biochem J 1990;265:621–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fattori BE, Cappelletti M, Costa P, et al. Defective inflammatory response in interleukin 6-deficient mice. J Exp Med 1994;180:1243–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bourantas KL, Dalekos GN, Makis A, Chaidos A, Tsiara S, Mavridis A. Acute phase proteins and interleukins in steady state sickle cell disease. Eur J Haematol 1998;61:49–54 [DOI] [PubMed] [Google Scholar]
- 21. Castell JV, Gomez-Lechon MJ, Martina D, Hirano T, Kishimoto T, Heinrich PC. Recombinant human interleukin-6 (IL-6/BSF-2/HSF) regulates the synthesis of acute phase proteins in human hepatocytes. FEBS 1988;232:347–50 [DOI] [PubMed] [Google Scholar]
- 22. Yoshida T, Kaneshi T, Shimabukuro T, Sunagawa M, Ohta T. Serum C-reactive protein and its relation to cardiovascular risk factors and adipocytokines in Japanese children. J Clin Endocrinol Metab 2006;91:2133–7 [DOI] [PubMed] [Google Scholar]
- 23. Yamashita K, Fujinaga T, Miyamoto T. Canine acute phase response relationship between serum cytokine activity and acute phase protein in dogs. J Vet Med Sci 1994;56:487–92 [DOI] [PubMed] [Google Scholar]
- 24. Wigmore JS, Fearon K, Maingay J, Lai P, Ross J. Interleukin-8 can mediate acute-phase protein production by isolated human hepatocytes. Am J Physiol Endocrinol Metab 1997;273:720–6 [DOI] [PubMed] [Google Scholar]
- 25. Watanabe K, Koizumi F, Kurashige Y, Tsurufuji S, Nakagawa H. Rat CINC, a member of the interleukin-8 family, is a neutrophil-specific chemoattractant in vivo . Exp Mol Pathol 1991;55:30–7 [DOI] [PubMed] [Google Scholar]
- 26. Watanabe K, Konishi K, Fujioka M, Kinoshita S, Nakagawa H. The neutrophil chemoattractant produced by the kidney epithelioid cell line NRK-52E is a protein related to the KC/gro protein. J Biol Chem 1989;25:19559–63 [PubMed] [Google Scholar]
- 27. Watanabe K, Suematsu M, Iida M, et al. Effect of rat CINC/gro, a member of the intereukin-8 family, on leukocytes in microcirculation of rat mesentery. Exp Mol Pathol 1992;56:60–9 [DOI] [PubMed] [Google Scholar]
- 28. Iida M, Watanabe K, Tsurufuji M, Takahashi K, Iizuka Y, Turufuji S. Levels of neutrophil chemotactic factor CINC/gro, a member of interleukin-8 family, associated with lipopolysaccharaide-induced inflammation in rats. Infect Immnun 1992;60:1268–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Honjo T, Kuribayashi T, Matsumoto M, Yamazaki S, Yamamoto S. Kinetics of α 2-macrogobulin and α 1-acid glycoprotein in rats subjected to repeated acute inflammatory stimulation. Lab Anim 2010;44:150–4 [DOI] [PubMed] [Google Scholar]