Opening and introduction
An important meeting titled “Defining the Objectives of the AIDS Vaccine Asian Network (AVAN)” was held 22–24 February 2009, in Beijing. This report describes the outcomes of this meeting. The meeting was jointly organised by the World Health Organization (WHO), the Joint United Nations Programme on HIV/AIDS (UNAIDS) and the Global HIV Vaccine Enterprise (GHVE) Regional Consultation on Expanding AIDS Vaccine Research and Development Capacity in Asia. Sixty-eight participants (Supplementary Digital Content 1) and observers gathered together from 16 countries including Australia, Cambodia, China, France, India, Indonesia, Japan, the Netherlands, the Philippines, the Russian Federation, Singapore, Sweden, Switzerland, Thailand, the United States of America and Viet Nam.
The meeting was highly relevant for the Asian region at a time when the HIV epidemic is still rampant despite concerted efforts by governments, communities, scientists and key stakeholders to stem its impact and advance. A great deal of work has been undertaken towards an AIDS vaccine over the past 15 years and the region has the essential assets to move the agenda forward.
Genesis of the 2009 Beijing meeting
In 1998, the first Asian meeting specifically devoted to AIDS vaccine research and development was convened by WHO-UNAIDS and the Japanese National Institute of Infectious Diseases (NIID) in Tokyo, Japan. In 2006, the regional strategy concept was revisited at the first WHO-UNAIDS regional consultation, Expanding Capacity and Accelerating AIDS Vaccine Development in Asia, hosted and co-organized by the University of Hokkaido, Sapporo, Japan. The 7 recommendations of the 2006 Sapporo meeting were to: First, create forums to facilitate interactions between funders and discuss funding opportunities, funding streams and funder capabilities. Second, facilitate linkages between research and development (R&D), innovation and discovery, in particular in emerging countries with strong innovation capacity, such as China, India and Thailand, and clinical trial capacity and production. Third, provide a forum for coordination of regional expertise, capacity building, and technical assistance. Fourth, promote advocacy and communication support for region-specific strategies. Fifth, contribute to and promote the implementation of the Scientific Strategic Plan of the GHVE. Sixth, prepare for future deployment of a vaccine by discussing regional approaches to ensure access, delivery capacity, demand estimates, epidemiology, vaccine characteristics, and strategies for delivery. Seventh, create a regional harmonization or regional authority for regulatory interactions, including regional licensure review and regional ethics committees with an initial ‘core’ working group.
To effectively implement and further facilitate the recommendation to develop an ongoing forum for coordination of regional expertise, capacity building and technical assistance, the establishment of AVAN was proposed.
In February 2009, the Chinese AIDS Vaccine Initiative (CAVI), GHVE and WHO-UNAIDS convened a follow-up meeting in Beijing, China to speed up implementation of recommendations of the Sapporo consultation.
Objectives of the 2009 Beijing meeting
The objectives of the 2009 Beijing meeting (beyond those established in Sapporo in 2006) were to:
define steps and milestones for implementation of recommendations of the 2006 Sapporo consultation;
constitute an AVAN Task Force, define its objectives, modus operandi, timeframes, and benchmarks;
review the current status of the HIV epidemic in Asia and the rationale for an AIDS vaccine;
review the progress in HIV vaccine R&D and implications of the latest findings, with emphasis on achievements, gaps and opportunities for Asia;
discuss challenges for engaging countries and communities in expanding pre-clinical and clinical trials, as well as increasing the regulatory and manufacturing capacity to accelerate the development of AIDS vaccines in Asia;
define the milestones for the development of a regional HIV vaccine strategy in alignment with the GHVE's Scientific Strategic Plan.
Expected outcomes of the 2009 Beijing meeting
The expected outcomes of this meeting were five-fold. First, provide updated data on the HIV epidemic and AIDS vaccine R&D landscape in Asia. Second, identify opportunities for regional collaboration and networking. Third, identify challenges and common strategies for building regional capacity in Asia. Fourth, to provide a roadmap for the constitution of the AVAN Task Force, its terms of reference, objectives, timeframe, and benchmarks. Fifth, the exchange of information and coordinated strategic plans between global, regional and country representatives.
Expected deliverables of the 2009 Beijing meeting
Three key expected deliverables of this meeting were formulated. First, a report suitable for publication will be prepared for the current meeting containing updated information on the state of the global and regional HIV epidemic and HIV vaccine R&D, summary of discussions and recommendations on addressing regional challenges and activities in support of advocacy for HIV vaccine R&D. Second, there is to be broad dissemination of the meeting outcomes and advocacy messages in support of HIV vaccine R&D in Asia at national and international levels. Third, recommendations to define a roadmap for the development of a regional HIV vaccine strategy, priorities and activities in alignment with the GHVE Scientific Strategic Plan will be developed by the AVAN Task Force.
HIV prevention technologies
HIV prevention technologies were reviewed during the meeting, with a particular emphasis on, how AIDS vaccines could be integrated once available. Effective HIV prevention interventions are available, but there is a need for increased efforts to strengthen management systems and services. New HIV prevention technologies, such as PrEP, have shown promising results in animal and human studies. Innovative approaches to studying new prevention technologies in high HIV prevalence settings and populations should be undertaken. Applied and clinical research on new prevention tools, including for population-based application of ART programmes, will need to be accelerated. Various new prevention tools should be envisaged as as working together to prevent HIV. In general, a better understanding of the shortcomings of primary prevention programmes will guide the introduction of new prevention technologies.
The NIAID/NIH strategy and current vaccine efforts
Simpler standard vaccine approaches, such as using inactivated or attenuated viruses, do not appear to be applicable to AIDS vaccine discovery (or to other chronic infections – tuberculosis, malaria, etc.). Therefore, the field needs to foster the development of new avenues towards vaccine discovery based on deeper understanding of immunological and pathogenic mechanisms. An improved understanding of antibody effector functions is needed. There is a need for a rational development of appropriate immunogens and better understanding of mucosal transmission, viral pathogenesis, manipulation of immune responses, including innate immunity, B-cell and T-cell regulation and function, proliferation, maturation, and phenotypes. New strategies for vaccine discovery include: reverse vaccinology, systems biology, exploitation of novel technologies such as genomic associations, siRNA, and in vitro ‘vaccination’. There is a need for innovation, the mining of nascent knowledge in related fields, and counter-intuitive thinking.
Priorities for future research
Priorities for future research that may lead to both new therapeutic and vaccine strategies include early events during the acute phase of infection, HIV reservoirs, interaction between viral factors and cellular partners, and co-infections. There is a need to shift from a ‘polarized’ to an ‘integrated’ vaccine research approach, considering both the innate, as well as the B- and T-cell components of immunity, including at the mucosal level. There needs to be a return to basic science to better understand early pathogenic mechanisms and basic immunology.
The Global HIV Vaccine Enterprise (GHVE)
GHVE is an alliance of independent organizations dedicated to accelerating the development of a preventive AIDS vaccine. Its creation was motivated by a widespread recognition of an urgent need to create a global approach to address the challenges of AIDS vaccine research and development that transcends individual funders, countries, researchers, and civil society. Central to GHVE's mission is promoting more efficient, faster ways for funders and researchers to share successes and failures and to avoid duplication of efforts. GHVE is a catalyst charged with bringing fresh thinking to the field and creating a collaborative and open approach to the research and development that is necessary to develop an AIDS vaccine. GHVE serves as a neutral convener, catalyst, and honest broker; mobilizes support for increased resources; targets significant new resources to priority areas; promotes collaboration and coordination; encourages efficient ways of information sharing; develops and maintains the GHVE Scientific Strategic Plan.
The AIDS Vaccine for Asia Network (AVAN)
The mission of AVAN is to “accelerate the development of an AIDS vaccine through expanding capacity for all aspects of AIDS vaccine research and development”. The creation of AVAN is timely as AVAN will promote the development of vaccines targeted towards the virus subtypes prevalent in the region. The group will be encouraged the participate in multinational clinical trials and therefore consider regional harmonization of regulatory policies and community engagement. The AVAN group's research strengths will contribute to diversifying scientific approaches and attract the very best minds to AIDS vaccine research. We envisage that AVAN will provide a unified and strong regional voice in global forums.
HIV epidemics in the Asia region
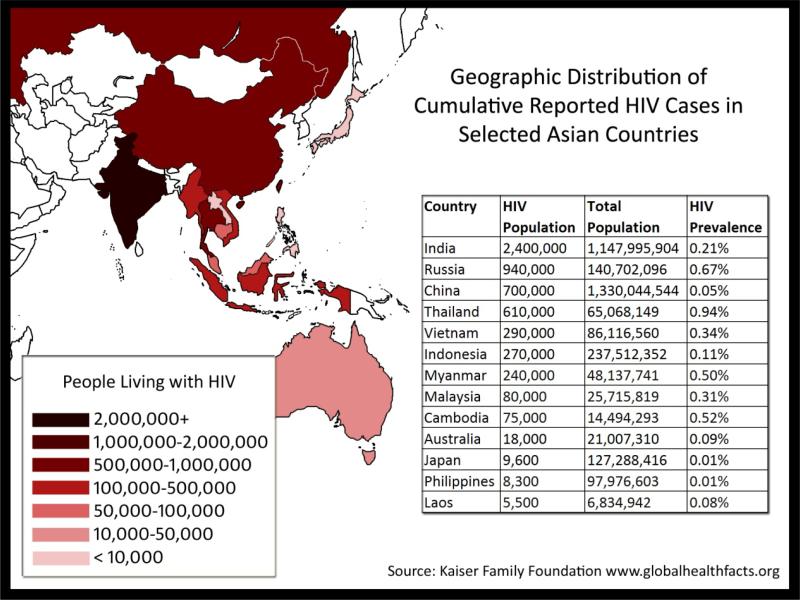
The total population of Asia is approximately four billion people (i.e. just over 60% of the world population). More than 500 million people are considered to be within at-risk populations for HIV infection, including youth, injecting drug users (IDUs), sex workers, men who have sex with men (MSM) and mobile populations. More than five million people have already been infected with HIV across the region, although the epidemic is highly variable across different countries, communities and populations [1].
Asian HIV epidemics are driven primarily by unsafe sex and injecting drug use and are therefore currently concentrated in groups with higher risk of HIV Infection. The epidemic is also slowly spreading to the general population (see Figure 1). Although some spectacular results have been achieved following the scaling up of available HIV prevention strategies in some countries and groups [1], the level of prevention, care and treatment coverage among most risk-associated groups remains dismally low. For example, significant increases in the number of new HIV infections are expected to occur across the region among MSM.
Figure 1. HIV-1 epidemic across Asia (2008 figures).
The estimated numbers of HIV-infected people across countries in Asia is shown, both as absolute numbers and as a proportion of the population.
Data from Kaiser Family Foundation
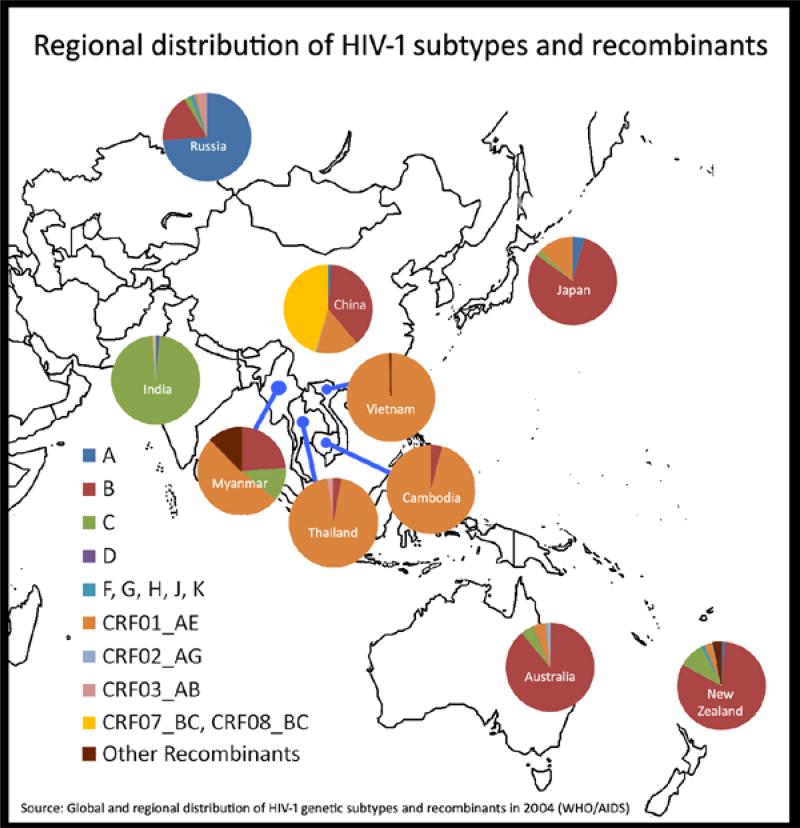
The geographic distribution of HIV subtypes and circulating recombinant forms (CRFs) is relatively homogeneous but varies by subcontinent: CRF_07B’/C and CRF01_AE in China [2]; subtype C in India [3]; and CRF01_AE in South-East Asia [4] (see Figure 2). Some aspects of the various epidemic can be explained by how founder viruses spread amongst defined risks groups. HIV strains within Asia countries will evolve differently to those in other populations owing to genetic differences such as HLA molecules. Unfortunately, HIV incidence data are relatively scarce and need to be bolstered for optimal planning of vaccine studies. The other main factors influencing AIDS vaccine development in the region include the distribution of multiple HIV subtypes and CRFs, the various modes of HIV transmission in the region, diverse host genetics, as well as the disparate social, cultural and political contexts in the region. How these interrelated factors will affect the research and development of AIDS vaccines across the region is unpredictable but they are significant considerations in the design of future studies. The complexity of the epidemic in the region poses substantial challenges but also affords tremendous opportunities for AIDS vaccine development.
Figure 2. Regional distribution of HIV-1 subtypes and recombinants.
The relative proportion of each subtype/recombinant is shown in the pie chart for each country.
Data from WHO/AIDS
Current efforts in AIDS vaccine research and development in Asia
Significant basic and clinical AIDS vaccine research and development efforts are already underway across the Asia region, with many notable achievements (see Tables 1 and 2). Several Asian countries now have national HIV-related vaccine plans and strategies. Further harmonization and consolidation of efforts will result in more productive associations and acceleration of vaccine research and development.
Table 1.
AIDS vaccine trials across Asia to date
| Country | Vaccine | Sponsor | Subtype | Phase/time | Reference |
|---|---|---|---|---|---|
| Thailand | V3 peptides | UBI | Multiclade | I/1994 | [5] |
| gp120 | Vaxgen, Chiron | E, B, B’/E | I/II,III/1995 | [6] | |
| gp160 | DoD | E | I/II | ||
| Canarypox + gp 160 vs 120 | US NIH/DoD | B/E | I/II/2003 | [7] | |
| Adenovirus type 5 | Merck | B | I/II/2003 | ||
| MVA | US NIH/DoD | A/E | I | [8] | |
| DNA + fowlpox | Australia | A/E | I/2004 | [9] | |
| Canarypox + gp 120 | US NIH/DoD | B/E | III/2006 | [10] | |
| China | V3peptides | UBI Co. | Multiclade | I/1993 | [5] |
| DNA + MVA | Baike Co. | CRF08_B/C | I /2005 | ||
| Tiantan vaccinia replicative | China CDC/EU | CRF 07_B/C | I /2006 | [11] | |
| DNA + Tiantan replicative | China CDC | CRF 07_B/C | I/2008 | [12] | |
| DNA + MVA | Baike Co. | CRF08_B/C | II/2009 | ||
| India | Adeno-associated virus | IAVI | C | I /2003 | [13] |
| MVA | IAVI | C | I/2005 | [14] | |
| AAV vs. DNA + MVA | IAVI | C | I /2009 | [15] | |
| Australia | DNA+ fowlpox | NIH | B | I/2004 | [16] |
| DNA + fowlpox | Australia | A/E | I | [9] | |
Table 2.
Examples of the current pipeline of pre-clinical AIDS vaccines being studied in Asia
| Vaccine vector | Immunity induced | Comment | Institution | References |
|---|---|---|---|---|
| Novel DNA vaccine vector | T and B cell immunity | Reduced DNA dose, enhanced immunogenicity | China CDC | [17] |
| Modified Tiantan vaccina | T-cell immunity, antibodies | Mucosal delivery with potentially less adverse effects compared to wild type Tiantan sp. | HK Univ., Tsinghua Univ., Chinese Academy of Medical Sciences, Peking Univ. Medical School, Chinese Academy of Sciences, China CDC | [18, 19] |
| CD40 ligand-expressing vaccines | Enhanced T- and B-cell immunity | Likely to improve durability of immunity. | University of Toronto | [20] |
| Envelope trimers | Neutralizing antibodies | Use of primary isolates may enhance antibody responses. | University of Melbourne | [21] |
| Peptides within particle-based vaccines | Durable T-cell immunity | Overlapping peptides induce broad T-cell immunity. Encapsulating peptides inside nanoparticles may permit durable immunity. | University of Melbourne | [22,23] |
| BCG vectors | T-cell and antibodies | Widely used vaccine vector with good safety record. | Osaka University | [24] |
| Influenza vectors | Mucosal T-cell immunity | Attenuated influenza strains with inserted HIV antigens could induce good pan-mucosal immunity. | University of Melbourne | [25] |
| Sendai virus vectors (alone or in prime-boost regimens) | Mucosal immunity | Attenuated parainfluenza virus vector could induce good pan-mucosal immunity. | The University of Tokyo/ DNAVEC Corporation/ China CDC | [26,27] |
Thailand
AIDS vaccine activities have a long and full history in Thailand, starting from the mid-1990's [28]. This reflects a strong political will to invest in AIDS vaccine research and development to stem the epidemic in Thailand. The experience in Thailand offers a good example of what can be achieved with international collaboration. Specifically, Thailand has conducted Phase I, II and III trials with the AIDSVax gp120 B’/E vaccine, as well as studies of other candidate vaccines (see Table 1) [6].
Important Phase II and, more recently, Phase III trials of the ALVAC (canarypox, vCP1521) and AIDSVAX gp120 B/E prime-boost regimens have also been conducted. The results of this trial (called ‘RV 144’), presented at the AIDS Vaccine Conference in 2009, showed a modest (31%) but significant (p = 0.04) reduction in new HIV infections; this result was from the modified intent to treat dataset. This landmark result is the first time an AIDS vaccine has exhibited protection against HIV acquisition, although there was some controversy about the borderline significance of the results. There were 74 new HIV infections among the 8,198 placebo recipients compared with 51 of the 8,197 participants who received the vaccine regimen [29]. Further research will now be required to understand the mechanisms of induction of immune protection and to further improve vaccine efficacy. In addition, the retention of the >16,000 volunteer base for this Phase III trial (the largest conducted to date) was 96%.
The Thailand experience in successfully conducting two Phase III efficacy trials has provided world-class expertise in collaborative teamwork, community engagement activities, good clinical practice and ethical compliance, sample repositories and good laboratory practices, volunteer retention strategies and large-scale data management approaches.
China
China's AIDS vaccine scientists have recently formed the Chinese AIDS Vaccine Initiative (CAVI) supported by the Government of China. The initiative includes projects aimed at cohort development, vaccine vector design, establishment of clinical trial units, a manufacturing facility using principles of good manufacturing practice (GMP), a primate centre, a humanized mouse centre and technical platforms encompassing both neutralizing antibody and T-cell expertise.
Chinese governmental research programmes and scientists have significantly invested in AIDS vaccine research. National AIDS vaccine research before 2007 consisted of Phase I trials with gp120 protein vaccines in 1993 and a DNA/MVA (modified vaccinia Ankara) trial in 2005–2006 (Table 1). Phase I trials on a DNA and Tiantan vaccinia vectors (replication-competent) are ongoing and proceeding towards phase II testing. Pre-clinical studies with DNA and modified Tiantan vaccinia as well as DNA, vaccinia and adenovirus vector approaches are also currently ongoing [11, 18, 19]. China's AIDS vaccine programme now has substantial capacity in primate centres, vaccine production capacity and clinical trial sites that will facilitate further research and development in the region.
India
AIDS vaccine trials have accelerated significantly in India in recent years. Activities include an adeno-associated virus vector Phase I trial, MVA vector vaccine trials [13,30] and a recent DNA/MVA Phase I prime-boost trial initiated in 2009 [16]. The International AIDS Vaccine Initiative (IAVI) is supporting Indian AIDS vaccine clinical trials and applied research on neutralizing antibody immunogens. Exploration of several novel concepts, including prime-boost regimens with CD40L adjuvants [20] as well as research towards development of improved Env-based immunogens, is also progressing. Improved awareness of AIDS vaccine issues, training of staff and development of trial sites have substantially improved with the conduct of AIDS vaccine research and clinical trials in India.
Australia
Australia has long-standing capacities in biomedical HIV research and clinical trial activities. A consortium termed the Australia-Thai HIV Vaccine Consortium conducted two recent trials, first in Sydney and then in Bangkok, of a DNA prime fowlpox virus boost vaccine using both B and CRF01_AE strains [16]. Australia was a clinical trial site of the adenovirus-based efficacy (STEP) trial that was not efficacious. Through collaborations within Australia, several new vaccine candidates are emerging. These include peptide-based vaccines, gp140 immunogens, recombinant influenza vectors and particle-based vaccine strategies [22,31]. In addition, improved and simpler assays to measure T-cell immunity and antibody-dependent cellular cytotoxicity (ADCC) are emerging [32,33]. These scientific approaches could be accelerated into expanded clinical trials in future collaborations across the Asia region.
Japan
Japan has developed a pipeline of promising AIDS vaccine candidates. These include recombinant Mycobacterium bovis/Bacillus Calmette-Guérin (BCG) vector-based regimens that are moving towards clinical trial development in collaboration with Thailand groups. In addition, novel Sendai virus vectors are being developed with the opportunity to deliver vaccines mucosally to induce mucosal immunity [24]. Sendai virus vectors combined with DNA vaccine candidates are moving towards clinical trials in collaboration with IAVI.
Vaccine development infrastructure and capacity building in Asia
Several international groups have provided high-level support for AIDS vaccine activities in the Asia region. Further expansion of this collaborative support will be critical in progressing future vaccine development efforts.
Armed Forces Research Institute of Medical Sciences-United States Military HIV Research Program (AFRIMS-USMHRP)
The Armed Forces Research Institute of Medical Sciences-United States Military HIV Research Program (AFRIMS-USMHRP), Bangkok, Thailand has made significant achievements in building capacity for highly specialized laboratories including in assessment of the HIV-specific humoral and cellular immune responses, including multi-parameter flow-cytometry; internationally accredited clinical laboratory facilities; specimen processing and archiving; human leukocyte antigen (HLA) typing capability, and high throughput genotyping assays. The Walter Reed Army Institute of Research, of the USMHRP, in collaboration with the Royal Thai Army, has sponsored and facilitated several clinical trials including the RV144 efficacy trial of an ALVAC prime and VaxGen envelope protein-boost regimen in Thailand.
Several areas have been identified as in urgent need of infrastructure and capacity building for cohort development and future vaccine trials. These include rapid testing and defining incident infections (including novel PCR techniques),central laboratories in-country to perform validated assays for vaccine trials, participation in external quality control programmes, capabilities to perform full-length single genome sequencing and analysis from plasma RNA, and development of novel assays to study host genetics and antibody dependent cellular cytotoxicity assays.
NIAID Division of Acquired Immunodeficiency Syndrome (DAIDS)
The NIAID Division of Acquired Immunodeficiency Syndrome (DAIDS) of the NIH supports basic, pre-clinical, and clinical research of candidate AIDS vaccines through its Vaccine Research Program (VRP). DAIDS has supported clinical and basic research across many countries throughout the region. There are several minimum requirements for trials for which DAIDS does not hold the Investigational New Drug (IND) Application. First, there needs to be adequate site capacity in terms of infrastructure and staffing. Second, political support for conducting AIDS vaccine trials must be present. Third, support from local institutions, communities and nongovernmental institutions must be present. Fourth, there must be investigator expertise and ability to coordinate large clinical research efforts and to work with high-risk populations. Finally, there must be adequate laboratory and pharmacy facilities and expertise.
International AIDS Vaccine Initiative (IAVI)
IAVI Collaborative Research Centres are located in eastern Africa (Kenya, Rwanda, Uganda) and southern Africa (South Africa, Zambia) and in India. IAVI has committed to supporting vaccine research and development, including clinical trial capacity building along with policy and preparedness activities in both India and China. These research centres are engaged in coordinating two long-term efforts: vaccine candidate R&D and developing trial capacity. This parallel development is essential for speed and efficacy.
IAVI observational clinical research studies have 4 broad aims. First, to develop capacity and infrastructure to conduct AIDS vaccine trials. Second, to understand the epidemiology and trial participants’ health issues. Third, to understand the nature of newly transmitted virus and immune mechanisms of HIV control. Fourth to engage the community in AIDS vaccine research and development.
These aims are achieved through building site- and country-specific capacity to support clinical research, working with vulnerable populations, building advisory mechanisms with leaders from multiple sectors and promoting coordination at multiple levels. Accreditation schemes are in place and most of the clinical research centres are now fully accredited. A particular emphasis is given to clinical research staff training in good clinical practice (GCP), good clinical laboratory practice (GCLP), laboratory techniques, gender sensitization, data management, communication, accounting and reporting procedures, advocacy and community mobilization, and team management. Since 2000, IAVI has completed 18 trials, enrolled more than 1000 volunteers, more than half of them in Africa and India.
HIV Vaccine Trials Network (HVTN)
The mission of the HIV Vaccine Trials Network (HVTN) is to enhance the discovery, and drive the development, of a safe and globally effective vaccine for the prevention of HIV through well-designed clinical research which objectively and ethically addresses the critical questions of the field. The HVTN has a number of research sites around the world and will be looking towards Asian sites in the coming years. The recent clinical research achievements by HVTN are illustrated by the initiation and follow-up of the STEP and Phambili Phase IIb trials, expanded laboratory programmes, and application of new analytical techniques. As a result of the outcome of the STEP trial in 2008, the priorities of the scientific agenda have shifted to account for new information and to meet new challenges. These priorities include: to better understand the outcome of data from STEP and Phambili trials, and to use these data to help define and evaluate conceptual improvements in T-cell-based vaccines, and to foster an iterative process between human and non-human primate studies that should allow the field to define such conceptual improvements. Several new clinical trials are planned.
To fill the gaps in our understanding, upcoming trials will address a series of questions. First, do mixtures of vaccines result in antigenic competition? Second, will priming and boosting with heterologous Adenovirus vector alter the magnitude, epitope breadth, and character of the immune responses? Third, does separating the site of vaccination increase the immunogenicity? Fourth, what are the behavioural, epidemiologic and biologic factors of HIV infection among injecting drug users in Xinjiang, China? Fifth, what are the virological factors associated with HIV transmission in the Phambili study? Finally, how can the HVTN assist in the development of a global systems biology approach to vaccinology?
Regional Emerging Diseases Intervention (REDI)
The Regional Emerging Diseases Intervention (REDI), is an intergovernmental organization jointly sponsored by Singapore and the USA, whose mission is to enhance the region's capability and capacity to effectively monitor, detect and respond to naturally occurring infectious diseases outbreaks or man-made health threats. It offers training and research focusing on avian influenza, dengue, Chikungunya, hand, foot and mouth disease (HFMD), and HIV. Many of REDI's activities related to disease outbreak have relevance to the planning and conduct of AIDS vaccine studies.
Other international initiatives
The Collaboration for AIDS Vaccine Discovery (CAVD), supported by the Bill and Melinda Gates Foundation, has an active network of HIV researchers across the world and will also be looking towards enhancing research on AIDS vaccines in Asia in the coming years. The EuroVacc network of AIDS vaccine researchers in Europe is evaluating HIV strains sourced from China with a view to further clinical trials in the region. WHO-UNAIDS and the Global HIV Vaccine Enterprise are actively engaged in policy development, clinical trial and research capacity strengthening, assisting the development of national AIDS vaccine plans and providing a global umbrella for AIDS vaccine development activities.
Preparing for Phase III vaccine trials in Thailand
Thirteen clinical trials, including two Phase III efficacy trials, have been conducted in Thailand since 1994. The various components involved include the regulatory authority, ethical review board, basic science research, clinical research, laboratory assessment (antibody responses – humoral, cellular), laboratory support and archiving, data management and analysis, community participation, and manufacturing capacity.
The National Regulatory Authority (NRA) benefited from a series of trainings and workshops to increase its capacity for licensing new vaccines and drugs with the help of WHO, the United States Food and Drug Administration (USFDA), NIH, and GHVE. Standard operating procedures are in place and a workshop has been held on inspection skills. Experts can be requested from international agencies. However, national staff are limited in number.
Institutional/ethical review boards have become very strong, especially in medical faculties during the past 2–3 years. Some are now accredited by the Forum of Ethical Review Committees in Asia and Western Pacific region (FERCAP). However, harmonization of ethics committees is crucial to minimize time for approval.
Full Phase I to Phase III clinical research teams are now established with more than 100 well-trained research nurses, technicians and research assistants, and enjoy the benefits of dedicated physical spaces and staff with full GCP training. These teams include AFRIMS, the Military HIV Research Programme, HIV-NAT/Thai Red Cross AIDS Research Centre; Chulalongkorn Hospital, Ministry of Public Health (MOPH), RIHES – Chiang Mai University – Faculty of Medicine; Siriraj Hospital – Mahidol University, Vaccine Trial Centre (VTC) – Faculty of Tropical Medicine – Mahidol University.
Laboratory capacity has been substantially strengthened over the past few years. Activities cover molecular epidemiology for monitoring circulating viruses in Thailand, HIV diagnosis, College of American Pathologists (CAP)-certification of laboratory safety, assessment of HIV-specific humoral and cellular immune responses, the Trial Registry and Repository Centre at MOPH and Bumrungrad Specimen Processing Laboratory. However, maintaining this capacity is questionable as a high level of commitment and financial support is needed.
Data management capacity is now self-sustaining at the Faculty of Tropical Medicine, Mahidol University, Bangkok. Volunteer relation and community engagement activities and community advisory boards (CAB) help in strengthening health system and heath-care delivery, although they may also lead to high expectations. The strengthening of manufacturing capacity remains difficult.
At the Vaccine and Cellular Immunology (VCI) laboratory, Chulalongkorn University and HIVNAT/Thai Red Cross AIDS Research Centre the initiation of a prime-boost Phase I trial with DNA and fowlpox vaccines has stimulated capacity strengthening, in particular at the laboratory level. The team now participates in HLA typing and epitope mapping for AIDS vaccine design under an NIH grant. Preclinical development of an Asian mosaic DNA vaccine is underway at VCI, Chulalongkorn Medical Research Centre, funded by Thai BIOTEC.
Many laboratories within Thailand and across Asia could potentially play a much greater role in basic vaccine R&D. There is interest from Thai funding agencies in funding committed vaccine development teams to take promising HIV vaccines from pre-clinical to early clinical studies.
Capacity and regulatory challenges in applying ‘good practice’ in AIDS vaccine research and clinical trials
Asia has abundant resources and a great capacity to apply good practice principles in clinical trials and manufacture of AIDS vaccine candidates. China and India have advanced biological industries with a production capacity of billions of doses of vaccine each year. Japan and the Republic of Korea also have an excellent capacity for good clinical, laboratory and manufacturing practice. Once an AIDS vaccine candidate advances beyond clinical trials, these countries have great potential to manufacture AIDS vaccine products that meet international standards. Thailand has accumulated significant capacity and experience in the clinical testing of novel AIDS vaccine candidates though conducting most of the efficacy trials to date. Furthermore, the recent significant increases in government investment into research and development for a preventive AIDS vaccine, particularly in China, are encouraging vaccine manufacturers to invest more in GMP. Taken as a whole, Asia has all the necessary resources and capacity to move an AIDS vaccine concept from laboratory research to field application.
However, transforming this capacity and potential into concerted activities and significant progress remains a great challenge facing AVAN investigators and supporters. Major hurdles are presented by the diversity of regulatory policies and review processes among different Asian countries, which hinder efforts to accelerate progress in clinical trials. The political will to support AIDS vaccine candidates among low-epidemic countries also falls short of expectations .
Current AIDS vaccine development in China, India, Japan, the Republic of Korea and Thailand is illustrative of the capacities and challenges of applying good practice in AIDS vaccine research and manufacturing.
China
In China, the National Institute for Control of Pharmaceutical and Biological Products (NICPBP) conducts technical assessments including: reviewing of the quality control (QC) methods and quality specifications proposed by vaccine manufacturers, laboratory testing of clinical lots of proposed vaccine candidates, lot release of vaccine batches for both licensed products and INDs intended to be used in clinical trials. The NICPBP makes recommendations to the State Food and Drug Administration (SFDA) on QC methods and specifications. SFDA, mainly through another branch (the Centre for Drug Evaluation), reviews and approves new vaccine clinical trial applications as well as the design of the clinical trials. Upon the close of each phase of clinical trials, data is reviewed by SDFA to decide whether or not these products should move on further. Guidelines from the NICPBP and SFDA outline regulatory requirements for clinical trials and development of preventive AIDS vaccines. These guidelines refer to comparable US FDA and WHO guidelines and ‘points to consider’ documents.
Procedures for registration and licensing begin with provincial or municipal review of proposals from manufacturers. SFDA and NICPBP undertake a parallel examination of the proposal and test materials, review the dossier and test data and consult with an Expert Committee for Drug Evaluation.
The primary responsibility for reviewing test results and QC, including specifications of the products and validation of the test methods, resides with NICPBP, which issues a QC report and comments to SFDA. Primary responsibility for reviewing proposed clinical trials, ascertaining the establishment of appropriate GCP, qualifications of staff, safety surveillance plans, and ethics committee approvals lie with SFDA.
China is facing the challenge of organizing AIDS vaccine efficacy trials in the coming years. International collaborations with both developed and developing countries, particularly Thailand, may represent an ideal solution to enhance the capacity to apply good practice to all phases of clinical trials, particularly efficacy trials. Another challenge facing China's AIDS vaccine development is the relatively complicated and lengthy procedures for clinical trial application and approval. Although SFDA has provided a fast approval process for AIDS vaccine candidates, further regulatory system evolution is needed to meet the demands of an expanding AIDS vaccine pipeline and increasing international collaborations.
Thailand
Prior to 2004, Thai Food and Drug Administration (FDA) regulations for new vaccines focused on the importation of investigational clinical trial material; the Thai FDA did not conduct clinical trial inspections or audits. In some cases, vaccines received conditional approval after examination of the proposed plan alone. Since 2004, GCP inspection has been required, as well as an IND-equivalent application requiring the local conduct of Phase I, II and III monitored clinical trials.
One good example is the clinical trial of the ALVA C-VaxGen vaccine combination in Thailand (RV-144). RV-144 is being conducted under a US IND as well as under the corresponding Thai FDA requirements. An end-of-Phase II process was required by USFDA, which included the development of a detailed package with information on all clinical data available from the many clinical trials previously conducted with the vaccines, as well as a meeting at which the sponsor and partners presented the plan and responded to questions by USFDA. Approval to proceed with Phase II was granted by USFDA, and official agreement was obtained from the Thai National Authority including approval for import of the vaccines, approval of quality assurance (QA)/QC reports, and approval of the safety evaluation plan.
Subsequent to the workshop, the results of the RV-144 trial have been announced and published showing a clear but modest efficacy of the vaccine [29]. This study has provided substantial encouragement to the field to identify the correlates of immunity and design, and to test improved vaccines. This trial sets a benchmark for future efficacy studies.
Thailand faces several challenges for future studies including: (a) developing legislative mechanisms to authorize and supervise clinical trials and to monitor and enforce GMP and GCP, (b) acquiring expertise to evaluate applications, including laboratory and clinical trials, market authorization and licensing, post-marketing surveillance, lot release, laboratory testing, GMP inspection and supervision of clinical trials. Thailand also faces challenges in training and implementing external experts or advisory boards for ethical and scientific review processes. In response to these challenges, Thailand has proposed a capacity-building strategy that includes implementing a clinical trial review process for ethical and scientific issues, implementing a stronger adverse events follow-up system, and providing advanced training in specific vaccine development technologies.
India
Review of vaccine applications in India is lengthy and involves three separate, independent institutions: the National Drug Authority, the National Food Authority, and the Biotechnology Review Authority. Vaccine trials also require reviews at the institutional ethics and scientific committees (National AIDS Research Institute (NARI) and Tuberculosis Research Centre (TRC), Drug Controller General of India (DCGI) office, Central Ethics Committee, Genetic Engineering Approval Committee, and the Health Minister's Screening Committee. In general, the review proceeds more expeditiously if USFDA has already approved the vaccine for clinical trials. Although India has developed guidelines for vaccine research and development including regulatory, GCP, and ethical guidelines, none are binding. In addition to this challenge, private sector involvement in AIDS vaccine research and development is limited because investment risks are not widely distributed and the regulatory review process is lengthy and complicated.
To address current challenges in the regulatory and manufacturing environments, India will need to develop a pipeline of robust candidates, enhance vaccine discovery, maintain good laboratory practice (GLP)-compliant labs with high throughput validated assays, and create repositories of relevant specimens and sequence databases of Indian isolates. In addition, there is a need for improved management of intellectual property and technology transfer to guarantee future access to products, as well as the development of human and material capacity to conduct Phase II and III trials. Finally, India needs a model similar to GHVE to harmonize and facilitate regulatory decision-making, perform risk benefit analyses, and to create an enabling environment for the sharing of scientific data and biological materials.
Japan and the Republic of Korea
Both Japan and the Republic of Korea have more than adequate capacity for manufacture and evaluation of vaccine products. However, despite the high-level of capacity in production and clinical trials for new vaccine candidates, an AIDS vaccine has never been a priority in these countries. Their relatively low HIV burden may explain their modest participation in global efforts on AIDS vaccine research and development. However, Asia could play a more significant role in the global AIDS vaccine field if greater commitments were to be made by Japan and the Republic of Korea. As a first step, enhancing the political will of their governments is of great importance.
Community considerations and needs for policy development – inclusion of vulnerable populations
By definition, HIV prevention research must follow the epidemic and thus populations at higher risk of HIV exposure are asked to volunteer for AIDS vaccine trials. The same factors that put individuals at higher risk of exposure to HIV in concentrated epidemics also make them vulnerable to cultural exclusion, social inequality, economic exploitation, and political oppression. Participating in an AIDS vaccine trial might increase vulnerability if it increases a participant's risk of exposure to stigmatization and discrimination because it highlights a study population's increased vulnerability to HIV exposure. A trial may also decrease vulnerability if it empowers the community or provides tangible assistance to participants, for example by improving the accessibility, affordability, and quality of appropriate health-care services in the community. AIDS vaccine researchers and advocates must work to decrease these vulnerabilities and increase the positive benefits of participating in a research trial.
A social and political analysis of a community can help researchers to better understand how to decrease vulnerabilities and help maximize benefits for volunteers. Findings from such analyses can be used to inform the design of research protocols that are sensitive to emerging information on incidental risks of social harm throughout the course of a trial. In some potential research populations, conditions affecting potential vulnerability or exploitation may be so severe that the risk outweighs the benefit of conducting the study in that population.
Early warning systems and quality assurance should be instituted in trials involving vulnerable populations. Research protocols might also include ongoing independent monitoring of a trial in relation to its impact on the vulnerabilities of communities participating in the study (additional to scientific and ethical review committees responsible for providing prior and continuing review of the trial).
Communities of people affected by research should play an active, informed role, working throughout trial conduct with site research staff and the principal investigator who is responsible for all aspects of a trial, including efforts to enhance community participation. This ‘participatory management’ benefits all parties; helps ensure smooth trial functioning; and builds community capacity to understand and inform the research process, raise concerns, and help find solutions to unexpected issues that may emerge once the trial is underway. AVAN should adopt genuine, transparent, meaningful participatory management principles by including community representatives on management structures of the network.
Although meaningful community engagement in clinical research is widely recognized as essential, it is also not without its challenges. These challenges can be magnified for AIDS vaccine researchers and trial sponsors who work in communities where individuals most at risk for HIV infection are also members of vulnerable populations. Special considerations and challenges exist for these communities and researchers and trial sponsors should take into account how others in the region have been successful in engaging with communities, communicating results and delivering important research benefits to the communities in which AIDS vaccine research takes place. Furthermore, AVAN can play a coordinating role to connect stakeholders to share lessons learnt as well as use its position to set the regional standard for good community engagement practices.
Unfavourable laws and prevailing stigma and discrimination make it difficult to reach vulnerable populations (e.g. MSM, sex workers, IDUs, migrants) that could participate in AIDS vaccine studies. To help overcome these challenges stakeholders must work to accelerate prevention efforts to stem HIV transmission, in particular among most at-risk populations and reduce HIV-associated stigma and create enabling environment for vulnerable communities to access health services and participate in HIV clinical research studies.
Community and vaccine preparedness activities also play a critical role in addressing some of the challenges described above. There is a need to build research literacy in trial site communities and beyond. Trial sites and sponsors in the region have formulated various activities recognizing the multiple and varying layers that make up each: health staff, trial staff, trial volunteers’ neighbours, volunteers’ families and of course the volunteers themselves.
Trial sites and sponsors in the region have had success implementing a range of community outreach activities with civil society, policy-makers, national and regional media and partners in the community. Local nongovernmental and community-based organizations, research organizations, media, religious leaders and doctors were consulted extensively, largely through consultative meetings, often held in high prevalence areas. Information was disseminated through these meetings, newsletters, info-kits and easy-to-understand brochures to explain the research. When meeting with policy-makers, research sponsors recognized the importance of building long-term partnerships through one-on-one consultations and regular briefings at the national and regional level. A similar approach was implemented when looking to build the capacity of local and national media to cover AIDS vaccine research. Sponsors held media briefings and one-on-one meetings with journalists and editors to help build understanding of vaccine science and the complex issues that are involved in clinical research.
Ongoing engagement is essential to help overcome the numerous challenges in retaining volunteers in any clinical trial, especially in a Phase III efficacy trial, which often takes several years. Strategies that have been implemented to improve retention include team-building, , extended clinic hours and a special tracking team.
Results from any biomedical prevention trial have an impact on AIDS vaccine research, the way it is communicated (how to maintain community and volunteer support in the face of failed products) or how future vaccine trials are designed (in the case of effective products that will need to be added to the standard of prevention for volunteers). No one working in HIV prevention research is operating in a silo, what happens in one trial, with one product, in one community, happens to the entire field.
The field must also work to sustain capacity (at clinical trial sites) and honestly communicate timelines. One of the biggest challenges in AIDS vaccine advocacy is managing expectations, as a balance must be struck between keeping up the momentum and support for research, while also preparing for what could, and likely will be, a long road. Timelines in vaccine development for any disease have often proved to be quite long and advocates need to manage these timelines carefully.
As identified, one of the critical priorities is to build research literacy, particularly around difficult scientific concepts (e.g. viral load reduction as a vaccine trial endpoint, which is different from what many commonly understand a vaccine to do: prevent infection). In a ‘post-STEP’ world in which AIDS vaccine trials are increasingly complex, earlier community engagement is essential. This early and more in-depth engagement should be guided by a new set of guidelines: the UNAIDS/AVAC Good participatory practice guidelines for biomedical HIV prevention trials (GPP). The GPP Guidelines are designed to provide systematic guidance on the roles and responsibilities of trial sponsors and trial implementers towards participants and their communities. The guidelines identify core principles, essential issues and minimum elements of how stakeholders should plan and evaluate community engagement in biomedical HIV prevention trials.
These and other guidelines can be especially useful in the Asian context to support community engagement and education work, especially in vulnerable populations. Given that Asia does not have a generalized epidemic, any larger-scale AIDS vaccine trials would need to take place in higher-risk cohorts, for this region most likely IDUs and MSM.
Thailand experience
There are many challenges related to community involvement in AIDS vaccine trials in Thailand, particularly in Phase III efficacy trials. A multilayered community approach should be adopted including health staff, trial staff, volunteers, their families and neighbours. Negative attitudes and misunderstanding related to AIDS vaccine trials include fears that the vaccine may cause HIV infection, or that the efficacy of the vaccine may be tested by deliberate exposure to the virus. Retention strategies are essential and should consider the interval between appointments, family and neighbourhood influences, the convenience of appointment dates/times, waiting times, and missed appointments due to mobility, among other issues. Strategies that must be put in place at the clinical service level include: improvement in service areas, team-building, provider-based services, volunteer relation activities, convenient-access clinics in Bangkok, and extended clinic hours. The communication plan for vaccine studies must include community engagement through a health forum, volunteer network, community network and a community advisory board. Additional challenges include how to communicate interim analysis data, the notion of co-primary end-point, and the viral-load effect. These were prominent issues during the release of the RV 144 trial results. Once an efficacious vaccine is identified, managing community expectations such as the likelihood for placebo recipients to be vaccinated and making a vaccine available at an affordable price remain important issues.
India experience
Much groundwork has been done on community participation in India during IAVI-sponsored AIDS vaccine trials. Key issues to be addressed include: stigma associated with HIV, mistrust of clinical trials, the important role of community and vaccine preparedness activities, the recruitment of volunteers in the complex Indian cultural context, the long approval process and bureaucracy, the need to develop trial site capacity and country scientific capacity, and the need for long-term commitment and support at all levels.
Engaging civil society as a partner and adviser to the AIDS vaccine programme was achieved though consulting and interacting extensively with nongovernmental and community-based organizations, women's organizations, policy-makers, doctors, research organizations, media, HIV positive people's groups and religious leaders, and through organizing consultative meetings in high-prevalence states.
Several advisory groups were constituted, including the National Advisory Board, National Consultation on Care and Treatment for AIDS Vaccine Trial Participants, Informed Consent Group, NGO Working Group, Gender Advisory Board, and Management Advisory Board for feasibility studies.
Formative research in at-risk communities (transgender and MSM) was conducted in 2007 to assess social structures, understand risk profiles, identify health-seeking patterns, and assess the quality of available services, potential barriers and opportunities for participating in AIDS vaccine research studies. A voluntary counselling and testing (VCT) workshop for MSM and transgender communities was held in 2007 reached several conclusions. First, the existing laws, stigma and discrimination make it difficult to reach vulnerable populations (MSM, sex workers, IDUs, migrants). Second, health systems are limited in capacity to deliver required services, in particular to vulnerable populations, hence the need for strengthening. Third, some populations are geographically scattered and there is a need to create links to close biomedical research and health infrastructure and services. Fourth, accelerating prevention efforts to stem HIV transmission, in particular among most at-risk populations, is a priority. Fifth, reducing HIV-associated stigma and creating an enabling environment for vulnerable communities to access health services and participate in HIV clinical research studies are key conditions for success.
Working group summaries and recommendations
During the Beijing meeting, participants were divided into two working groups (Supplementary Digital Content 2) to consider issues related to the creation of the AIDS Vaccine Asian Network (AVAN).
Working Group 1 (WG1): Defining a roadmap and the terms of reference for the AVAN Task Force
The objectives of Working Group 1 are to develop a roadmap and the terms of reference for the AVAN Task Force (e.g. objectives, modus operandi, timeframe, and milestones); to define the strategies, approaches, and milestones for the development of a regional Asian vaccine R&D strategy; and to make recommendations on how Asia can best contribute to the updating of the GHVE Scientific Strategic Plan.
Roadmap and terms of reference for the AVAN Task Force (TF)
The participants proposed the TF membership should consist of individuals with significant international scientific stature in vaccine R&D, a strong commitment to the vision of AVAN, experience in advocacy (both political and community), and an understanding of local and regional politics and funding mechanisms. Members will be nominated by consultation working group members, proposed by WHO and GHVE and the TF members will also chose alternate representatives. The duration of membership to the AVAN TF will be limited until the formal launch of AVAN. The AVAN TF membership will be finalized by March 2009 and the terms of reference for AVAN available by June 2009. AVAN will be presented at the AIDS Vaccine conference in Paris in October 2009.
The terms of reference of the TF include:
Developing a proposal for the structure, vision, mission, modus operandi, and the establishment of the AVAN Secretariat.
Identifying mechanisms to implement the Sapporo recommendations.
Determining needs for establishment of working groups on targeted issues, such as clinical trial capacity, molecular epidemiology, training etc.
Developing a proposal for the AVAN Strategic Plan.
Prioritizing objectives for AVAN.
Identifying initial targets for funding based on the Strategic Plan.
Identifying Asian scientists to attend AIDS Vaccine Conference 2009 and raising funds for attendance.
Regional vaccine R&D strategy – Objectives
Participants acknowledged the regional common challenges and strengths (though not just in one country) and therefore the need for a regional approach to AIDS vaccine R&D. AVAN could be a mechanism to foster scientific collaboration, educate governments, development agencies, and other donors. Participants identified regional priorities, including clinical trials capacity, young and early career investigators programmes, advocacy, need for developing regional cohorts, good laboratory practices, harmonization of assays and standard operating procedures, repository and molecular epidemiology, manufacturing capacity, industry participation, and product development programmes through public–private partnerships. (Note: Similar discussion took place in WG2).
Key objectives are to first create an inventory of existing scientific capacity, innovative ideas, and funding in the region. Second, develop a plan to harmonize regulatory and ethical requirements for AIDS vaccine trials. Third, to build trust and promote regional and international collaboration. Fourth, contribute to the GHVE scientific strategic plan and efforts by participating in scientific and communication working groups and proposing complementary topics not fully covered in the current plan.
Working Group 2 (WG2): Defining AVAN, identifying challenges and common strategies for building regional AIDS vaccine capacity in Asia
The objective of Working Group 2 (Supplementary Digital Content 2) was to help AVAN move towards the development of the common regional platforms in support of AIDS vaccine trials in Asia, in particular around regulatory, ethical and community considerations.
The AVAN mission, purpose and scope
Given the level of heterogeneity across different regions, nations, populations, and socio-cultural backgrounds, it was agreed that a lot could be gained by sharing the knowledge and experience of different partners. This would broaden advocacy opportunities for AIDS vaccine development and contribute to increasing research literacy across the region. It is important for the network to be aware of the heterogeneity in regional research capacity and infrastructure, as well as research experience, and therefore AVAN should take into account the needs of both more and less experienced members. It will also be important to take advantage of the existing local or regional mechanisms for networking in order to avoid confusion or duplication. It was noted that although there is a network addressing HIV treatment related issues (TREAT ASIA), there are no established networks in the region addressing HIV prevention.
The discussion of AVAN's purpose and scope was based on the recommendations from the Sapporo meeting, where the idea of creating AVAN was put forward. The group proposed a mission statement (Figure 3), which broadens the scope of the network by adding the element of ‘biomedical HIV prevention clinical trials’ in addition to AIDS vaccines. The following arguments were discussed in favour of such a change. First, HIV biomedical prevention trials are often conducted in populations with similar demographics and HIV epidemiological features in a region, country or population. Given the overlap, broadening the scope in terms of both capacity strengthening and avoiding competition for trial sites and populations seems rational. Second, current progress in development of HIV biomedical interventions, such as circumcision and PrEP, and negative results from recent AIDS vaccine trials, indicate that most likely first generation AIDS vaccines will need to be considered in the context of other preventive strategies. AIDS vaccine development therefore should not be conducted in isolation but rather should take into consideration the whole spectrum of novel preventive strategies and interventions. Third, given relatively small numbers of HIV clinical researchers in Asia, it is likely that many HIV biomedical researchers would already have been involved in research into a spectrum of HIV interventions. Fourth, the recent negative AIDS vaccine trial results (STEP trial) and the likelihood that it will be some time before there is success on the vaccine front, have moved other groups such as AVAC to consider broadening the scope of their focus to include other prevention modalities. It seems pragmatic to ensure that current investment in vaccine development and research infrastructure and vaccine trials be wisely managed so that centres and resources, such as equipment and personnel, do not remain idle and could be used for other prevention research and clinical trials to ensure sustaining this capacity.
Figure 3.
However, some concerns were raised regarding this broadening of scope. First, this could lead to a diffusion of effort and compromise the effectiveness of the network, undermining the initial purpose and spirit of bringing the network together in the first place. Second, strong advocacy on the vaccine front is needed along with the belief that an effective vaccine is the ‘end game’ or the ‘magic bullet’ in containing the epidemic; and therefore combining it with other strategies could risk diluting the highly challenging endeavour. Third, the slope for vaccine advocacy is steeper than that of advocating for other preventive interventions and therefore if the effort is diffused across biomedical prevention this may lead to an inadvertent slide towards lesser focus on vaccines. Fourth, should AVAN broaden its scope to include biomedical HIV preventive interventions, it would then also need to consider how to deal with the development of ‘therapeutic’ AIDS vaccines.
In conclusion, participants generally agreed that the scope could encompass both components, in that the primary thrust would still be AIDS vaccines but that biomedical preventive interventions should be part of the overall strategic considerations, which is reflected in the proposed mission statement (Figure 3).
Challenges, including regulatory, clinical trials and capacity building considerations
Several challenges exist in considering expanding HIV vaccine R&D capacity in Asia based on shared knowledge and experiences gained through conducting trials in the region. These include increasing community engagement, streamlining regulatory review processes, facilitating ethical review procedures, improving clinical trial acceptance, capacity and infrastructure, establishing accurate HIV epidemiological information, and harmonizing and validating procedures relevant to laboratory testing and clinical evaluation. The working group focussed on issues pertaining to increasing community engagement, streamlining regulatory review processes, as well as clinical research capacity.
Increasing community engagement
Engagement with the community is a critical aspect of conducting clinical trials. Sharing of experiences and solutions is instructive for network members. It is likely that many materials already developed could be useful and informative for groups that are only in the planning stages of community engagement. Examples of existing resources that could be made available through the network include community messages, and documents addressing important community procedures, such as CAB operating procedures. Also cited as potentially very useful were updated materials on key aspects of clinical trials, such as risk reduction guidance, and on issues related to post-vaccination seropositvity in AIDS vaccine trials. As mentioned above, AVAC/UNAIDS have developed Good participatory practice guidelines for biomedical HIV prevention trials that describes recommended practices for community engagement; this approach could also be considered and expanded through AVAN.
Streamlining regulatory review procedures: Relevant regulatory considerations
Most felt that it was likely that regional multi-centre trials would be required in testing future HIV biomedical interventions, especially vaccines. The opportunity to develop a network that could provide a common platform from which HIV-vaccine-specific regulatory issues could be discussed and addressed should be seized. There is tremendous diversity in the region in terms of innovation and biopharmaceutical industry as well as research capacity, accessibility and clarity of national regulatory review processes. Even when general policies are similar in principle among more established and experienced regulatory bodies, in particular when dealing with novel products, requirements often differ in specific details. Some requirements may seem unusual, unexpected or even unnecessarily rigorous when compared between different regulatory jurisdictions. There is a long-recognized and great need to harmonize these processes (Figure 4).
Figure 4.
Among the challenges discussed were the current situations in China, India and Japan. The China SFDA has specific requirements pertaining to experimental products manufactured outside of China if the trials are to be conducted in China. Foreign vaccine developers are required to transfer all QC processes to the designated China SFDA lab for validation and verification as well as provide access to all batch records. The current requirement, as it stands, complicates the regulatory pathway for collaborative efforts between Chinese and foreign partners. Some exceptions do exist to this rule, as in the case of drug development, where it is possible to conduct trials without meeting this requirement.
In India, despite the enormous numbers of clinical trials regularly conducted on many other products, where the regulatory review seems fairly rapid, the review approach taken with AIDS vaccines appears unusually lengthy and in some cases leads to significant delays.
In Japan, early phase clinical trials for specific products such as anything involving genetically modified organisms (GMO) also faces regulatory challenges. In addition, the need for an AIDS vaccine for the Japanese population is not recognized by the government and therefore the research community working on vaccines receives minimal support. However, lately, there has been some optimism that this stance may be changing to provide the opportunity for some Phase I trials to be conducted.
Some participants emphasized that initiating a dialogue with regulators early in the development process was critical for developing greater understanding of the regulatory requirements. Such a process could minimize unnecessary delays or costs. This dialogue would also provide an opportunity to identify regulatory issues which may require forging new mechanisms or procedures to facilitate appropriate national regulatory review. For example, in Tanzania, the process of developing a national HIV vaccine plan paved the way for an early dialogue with the regulatory authorities. This resulted in early discussions of gaps and issues in the regulatory review processes, which allowed for a smooth process in review and approval of a Phase I trial. Similarly in South Africa, conducting a clinical trial with an experimental GMO product prompted creation of specific review and oversight mechanisms.
It was highlighted that there have been several efforts initiated by WHO, national regulatory agencies and the pharmaceutical industry to engage regulators as a network to discuss regionally-relevant regulatory issues. This includes the Developing Country Vaccine Regulators Network (DCVRN), which can be used as a platform for engagement with regulators. In addition, for Association of Southeast Asian Nations (ASEAN) countries, the Pharmaceutical Product Working Group (PPWG) provides a forum that also discusses relevant regulatory issues from a regional perspective. Therefore, all agreed that AVAN could play a useful role in facilitating and informing such dialogue. It could provide a platform to keep key regulatory stakeholders informed about the science and progress in AIDS vaccine development, both from a general as well as from a product-specific perspective.
It was proposed that an essential preliminary step will be to do a quick survey of individuals and groups with experience in conducting trials in the region in order to identify regulatory issues of concern. This could be combined with a review of available regulatory guidance and documents. Such an analysis would not only identify the issues, but could also provide information on specificities around the issues and would allow a targeted and informed dialogue with national and regional regulators over AIDS vaccine development. It was also agreed that AVAN should make full use of current networks and forums to engage in this regulatory dialogue. One area to explore is how they can be engaged in order to ‘normalize’ some aspects of the HIV regulatory pathway. Another message that participants felt needed to be constantly voiced was that development and trials are not being conducted by ‘foreign developers’, who try to escape rigorous regulatory conditions in the country of origin and seek ‘easier conditions’ for testing a product. Figure 5 summarizes a proposed approach for AVAN when considering the challenges of streamlining regulatory review procedures.
Figure 5.
Clinical trial capacity considerations
Optimizing regional clinical research and trial infrastructure will be important and AVAN could play a role in facilitating several aspects of this process. Harmonization and standardization of clinical trial conduct through having template protocols for clinical trials would be useful. Training of study teams on principles of GCP and safety monitoring would be helpful. Harmonization of ethical review and approval processes could occur. Data management issues could also be assisted by AVAN. Developing clinical trial QA/QC methods between countries would help building validation capacity around clinical trial associated procedures. This would require consideration of country-specific requirements around material transfer agreements (MTAs).
A critical first step in undertaking any clinical trial would be to conduct an analysis of the normative landscape (formal, ethical, legal, regulatory, political and more informal such as social and cultural dimensions) to help inform the many aspects of clinical trial planning and conduct, such as knowledge on local standards of care and national policy around HIV. This will help AVAN members in dealing with issues such as not conducting AIDS vaccine trials in countries where there is a policy of mandatory testing for HIV or where there is no universal access to HIV prevention modalities.
Another fundamental element of clinical trial capacity is human resources. Development and training of investigators and study staff takes a long-term commitment and investment on many levels, and this is a critical consideration for sponsors of AIDS vaccine development.
Strategic areas of focus
The participants were convinced by the need for AVAN and proposed that some concrete activities could be considered along the lines of a strategic framework that were identified as priorities. Information exchange around best practices (GCP, GLP, GMP, SOP) and multi-disciplinary training is critical. Policy and advocacy work around national HIV vaccine plans and regulatory harmonization. Lastly, mobilizing resources to enhance all aspects of AIDS vaccine R&D will be crucial.
Preliminary activities
Several potential preliminary, start-up activities for AVAN were proposed.
Regional baseline HIV prevention landscape analysis
This analysis would be aimed at providing baseline information that would help develop potential activities for the network, such as conducting regional multi-centre clinical trials. These activities would include an epidemiological analysis of HIV demographics and mapping of current HIV prevention trial activities. Further, an inventory of policies relevant to clinical trial conduct such as existing regulatory review processes and guidelines for exchange of biological materials and data would be very helpful.
Epidemiological analysis of HIV demographics (e.g. UN Commission on AIDS in Asia 2008 report)
There is a need to collect and make accessible accurate, up-to-date, and comprehensive information on epidemiology of HIV in the region, in a manner relevant to the design and conduct of HIV prevention intervention trials. This is particularly important in view of the inherent challenges in developing and testing HIV preventive interventions in populations where the general incidence and prevalence are low, as well as in subgroups where HIV prevalence and incidence remains undetermined, such as in MSM. There is a need to expand molecular epidemiology studies in order to determine the distribution of HIV subtypes and the impact of these differences for vaccine trial design. Such information would help the network adopt appropriate strategies and develop approaches to vaccine development that are tailored to the needs of the region.
Mapping of current HIV prevention trial activity (e.g. Alliance for Microbicide Development and IAVI databases)
Knowledge about clinical trials and HIV prevention interventions, as well as baseline information that describes the level of community engagement, awareness, and activism could be helpful for researchers. This is essentially a knowledge management/database development type of activity. It was noted that UNAIDS had previously attempted to develop a research activity database to be used and managed by countries, which was apparently not systematically taken up. Therefore, some concern was expressed given that it could potentially be a rather large task and present challenges not only in establishing such an inventory or repository of information within the network but even more so in maintaining it. It was proposed that, as a starting strategy for this activity, existing databases could be used as sources of information to further populate such a database, as well as for focal points for member institutions or countries within the network.
Whatever effort that is taken to collect, document and communicate information for the network, the group agreed that a fundamental consideration will be determining the scope and audience. Some advocated that the network should start small and identify the scope of information that would be most useful for AVAN first and then expand to other stakeholders.
Mechanisms for implementing proposed activities
A method that has been useful in helping to provide a framework for the activities proposed above is the development of a national HIV vaccine development plan. Examples of how this has been used in Thailand and Tanzania were discussed. The process of developing such national HIV vaccine development plans also provides a platform for convening the key stakeholders at a local and national level and identifying key players who could play a critical role in the effort. National HIV vaccine plans brings together all the elements that need to be engaged with by the network, identifies areas that need strengthening and defines priorities and activities within those areas.
Such a plan also provides a platform through which the stakeholders in vaccine development could be proactive and anticipatory in terms of engaging with communities or approval bodies in the early conceptual stages of clinical development. The group recommended that the goals of AVAN should be facilitated in part by assisting and strengthening the development of national HIV vaccine development plans throughout countries in the region.
Conclusion
It was recommended that one of the key next steps was for the country participants to begin a dialogue with community stakeholders about the network; as well as with national or political stakeholders to gather support or buy-in, and feedback on the network.
In conducting such a dialogue, some clarity is required on what AVAN will be, whom it will represent and what it will do. It was proposed that some communication points about AVAN targeted at different audiences/stakeholders would be important to consider. A source for this could be the report from this meeting.
In terms of governance and setting up the network, it was concluded that it might be better to maintain an informal network approach in the beginning as national engagement on a formal level is likely to take much longer. Initiating a network that is more informal also facilitates a more flexible and adaptable approach to accommodate regional diversity, while forging on with already identified next steps and activities.
Supplementary Material
Acknowledgements
The Beijing meeting was sponsored by the World Health Organization, the National Institutes of Health Office of AIDS Research, the Global HIV Vaccine Enterprise (GHVE), and the China AIDS Vaccine Initiative (CAVI). The World Health Organization and the Joint United Nations Programme on HIV/AIDS (WHO-UNAIDS) and the Global HIV Vaccine Enterprise (GHVE) are extremely grateful to all participants who made this regional consultation a fruitful and successful meeting, in particular to the Ministry of Health of the People's Republic of China, to the Chinese Centre for Disease Control and Prevention for hosting this meeting. Thanks are also given to Michael Benenson, Jorge Flores, Deirdre Grant, Sonali Kochhar, Zarifah Reed, Candace Rosen and Yiming Shao who served as rapporteurs. The organizers also thank Jean Louis Excler for his assistance at the meeting and preparing the first draft of the meeting report. The authors also acknowledge the help of Tim France (iniscommunication.com) for technical editing of the paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1. www.unaids.org.
- 2.Lu L, Jia M, Ma Y, Yang L, Chen Z, Ho DD, Jiang Y, Zhang L. The changing face of HIV in China. Nature. 2008;455:609–611. doi: 10.1038/455609a. [DOI] [PubMed] [Google Scholar]
- 3.Deshpande A, Jauvin V, Pinson P, Jeannot AC, Fleury HJ. Phylogenetic analysis of HIV-1 reverse transcriptase sequences from 382 patients recruited in JJ Hospital of Mumbai, India, between 2002 and 2008. AIDS Res Hum Retroviruses. 2009;25:633–635. doi: 10.1089/aid.2008.0261. [DOI] [PubMed] [Google Scholar]
- 4.Wirachsilp P, Kantakamalakul W, Foongladda S, Chuenchitra T, Kohriangudom S, Athipanyasilp N, Tanprasertsuk S, Gasitrong M, Sutthent R. Surveillance of subtype and genetic variation of the circulating strains of HIV-1 in Thailand. Southeast Asian J Trop Med Public Health. 2007;38:814–827. [PubMed] [Google Scholar]
- 5.Lenihan F. China and Thailand to start trials of AIDS vaccines. BMJ. 1993;306:1564–1565. [PubMed] [Google Scholar]
- 6.Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, Hu D, Tappero JW, Choopanya K. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 7.Thongcharoen P, Suriyanon V, Paris RM, Khamboonruang C, de Souza MS, Ratto-Kim S, Karnasuta C, Polonis VR, Baglyos L, Habib RE, et al. A phase 1/2 comparative vaccine trial of the safety and immunogenicity of a CRF01_AE (subtype E) candidate vaccine: ALVAC-HIV (vCP1521) prime with oligomeric gp160 (92TH023/LAI-DID) or bivalent gp120 (CM235/SF2) boost. J Acquir Immune Defic Syndr. 2007;46:48–55. doi: 10.1097/QAI.0b013e3181354bd7. [DOI] [PubMed] [Google Scholar]
- 8.Earl PL, Cotter C, Moss B, VanCott T, Currier J, Eller LA, McCutchan F, Birx DL, Michael NL, Marovich MA, et al. Design and evaluation of multi-gene, multi-clade HIV-1 MVA vaccines. Vaccine. 2009;27:5885–5895. doi: 10.1016/j.vaccine.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Rose R, Chea S, Dale CJ, Reece J, Fernandez CS, Wilson KM, Thomson S, Ramshaw IA, Coupar BE, Boyle DB, et al. Subtype AE HIV-1 DNA and recombinant Fowlpoxvirus vaccines encoding five shared HIV-1 genes: safety and T cell immunogenicity in macaques. Vaccine. 2005;23:1949–1956. doi: 10.1016/j.vaccine.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, et al. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N Engl J Med. doi: 10.1056/NEJMoa0908492. in press. [DOI] [PubMed] [Google Scholar]
- 11.Shao Y, Li T, Shen X, Zhang J, Zhu H, Han L, Wang C, Lin W, Wang C, Fan H, et al. The Safety and Immunogenicity of the Replicative Tiantan Vaccinia HIV Vaccine in Phase I Clinical Trial.. Poster No. P13-18, AIDS Vaccine 2008, Cape Town, South Africa, Oct 13-16..2008. [Google Scholar]
- 12.Shao Y, Li T, Wolf H, Liu Y, Wang H, Zhu H, Lv W, Lin W, Chen J, Liang H, et al. The safety and immunogenicity of HIV-1 vaccines based on DNA and replication competent vaccinia vector in phase I clinical trial.. Poster No.P14-15LB, AIDS Vaccine 2009, Paris, Oct 19-22..2009. [Google Scholar]
- 13.Mehendale S, van Lunzen J, Clumeck N, Rockstroh J, Vets E, Johnson PR, Anklesaria P, Barin B, Boaz M, Kochhar S, et al. A phase 1 study to evaluate the safety and immunogenicity of a recombinant HIV type 1 subtype C adeno-associated virus vaccine. AIDS Res Hum Retroviruses. 2008;24:873–880. doi: 10.1089/aid.2007.0292. [DOI] [PubMed] [Google Scholar]
- 14.Berkley S. HIV vaccine trials in India. Nat Biotechnol. 2008;26:495. doi: 10.1038/nbt0508-495. author reply 496. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S, Aggarwal P, Vajpayee M, Pandey RM, Seth P. Development of a candidate DNA/MVA HIV-1 subtype C vaccine for India. Vaccine. 2006;24:2585–2593. doi: 10.1016/j.vaccine.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 16.Kelleher AD, Puls RL, Bebbington M, Boyle D, Ffrench R, Kent SJ, Kippax S, Purcell DF, Thomson S, Wand H, et al. A randomized, placebo-controlled phase I trial of DNA prime, recombinant fowlpox virus boost prophylactic vaccine for HIV-1. Aids. 2006;20:294–297. doi: 10.1097/01.aids.0000199819.40079.e9. [DOI] [PubMed] [Google Scholar]
- 17.Li D, Liu Y, Zhang Y, Xu J, Hong K, Sun M, Shao Y. Adjuvant effects of plasmid-generated hairpin RNA molecules on DNA vaccination. Vaccine. 2007;25:6992–7000. doi: 10.1016/j.vaccine.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 18.Huang X, Lu B, Yu W, Fang Q, Liu L, Zhuang K, Shen T, Wang H, Tian P, Zhang L, et al. A novel replication-competent vaccinia vector MVTT is superior to MVA for inducing high levels of neutralizing antibody via mucosal vaccination. PLoS One. 2009;4:e4180. doi: 10.1371/journal.pone.0004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai K, Liu Y, Liu M, Xu J, Huang W, Huang X, Liu L, Wan Y, Hao Y, Shao Y. Pathogenicity and immunogenicity of recombinant Tiantan Vaccinia Virus with deleted C12L and A53R genes. Vaccine. 2008;26:5062–5071. doi: 10.1016/j.vaccine.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Yu Q, Stone GW, Yue FY, Ngai N, Jones RB, Kornbluth RS, Ostrowski MA. CD40L expressed from the canarypox vector, ALVAC, can boost immunogenicity of HIV-1 canarypox vaccine in mice and enhance the in vitro expansion of viral specific CD8+ T cell memory responses from HIV-1-infected and HIV-1-uninfected individuals. Vaccine. 2008;26:4062–4072. doi: 10.1016/j.vaccine.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Center RJ, Wheatley AK, Campbell SM, Gaeguta AJ, Peut V, Alcantara S, Siebentritt C, Kent SJ, Purcell DF. Induction of HIV-1 subtype B and AE-specific neutralizing antibodies in mice and macaques with DNA prime and recombinant gp140 protein boost regimens. Vaccine. 2009;27:6605–6612. doi: 10.1016/j.vaccine.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 22.De Rose R, Fernandez CS, Smith MZ, Batten CJ, Alcantara S, Peut V, Rollman E, Loh L, Mason RD, Wilson CM, et al. Control of viremia following immunotherapy of SIV-infected macaques with peptide pulsed blood. Plos Pathogens. 2008;4:e1000055. doi: 10.1371/journal.ppat.1000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sexton A, Whitney P, Chong S, Zelikin A, Johnston A, De Rose R, Brooks A, Caruso F, Kent S. A protective vaccine delivery system for in vivo T cell stimulation using nanoengineered polymer hydrogel capsules. ACS Nano. doi: 10.1021/nn900715g. in press. [DOI] [PubMed] [Google Scholar]
- 24.Hiroi T, Goto H, Someya K, Yanagita M, Honda M, Yamanaka N, Kiyono H. HIV mucosal vaccine: nasal immunization with rBCG-V3J1 induces a long term V3J1 peptide-specific neutralizing immunity in Th1- and Th2-deficient conditions. J Immunol. 2001;167:5862–5867. doi: 10.4049/jimmunol.167.10.5862. [DOI] [PubMed] [Google Scholar]
- 25.Sexton A, De Rose R, Reece JC, Alcantara S, Loh L, Moffat JM, Laurie K, Hurt A, Doherty PC, Turner SJ, et al. Evaluation of recombinant influenza virus-simian immunodeficiency virus vaccines in macaques. J Virol. 2009;83:7619–7628. doi: 10.1128/JVI.00470-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawada M, Tsukamoto T, Yamamoto H, Iwamoto N, Kurihara K, Takeda A, Moriya C, Takeuchi H, Akari H, Matano T. Gag-specific cytotoxic T-lymphocyte-based control of primary simian immunodeficiency virus replication in a vaccine trial. J Virol. 2008;82:10199–10206. doi: 10.1128/JVI.01103-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu S, Feng X, Shu T, Matano T, Hasegawa M, Wang X, Ma H, Li H, Li Z, Zeng Y. Potent specific immune responses induced by prime-boost-boost strategies based on DNA, adenovirus, and Sendai virus vectors expressing gag gene of Chinese HIV-1 subtype B. Vaccine. 2008;26:6124–6131. doi: 10.1016/j.vaccine.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Pitisuttithum P. HIV vaccine research in Thailand: lessons learned. Expert Rev Vaccines. 2008;7:311–317. doi: 10.1586/14760584.7.3.311. [DOI] [PubMed] [Google Scholar]
- 29.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, et al. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 30.Ramanathan VDKM, Mahalingam J, Sathyamoorthy P, Narayanan PR, Solomon S, et al. A Phase 1 Study to Evaluate the Safety and Immunogenicity of a Recombinant HIV-1 Subtype C Modified Vaccinia Ankara Virus Vaccine Candidate in Indian volunteers. AIDS Res Human Retroviruses. doi: 10.1089/aid.2009.0096. in press. [DOI] [PubMed] [Google Scholar]
- 31.De Rose R, Zelikin A, Johnston APR, Sexton A, Chong SF, Cortez C, Mulholland W, Caruso F, Kent SJ. Binding, Internalisation and Antigen Presentation of Vaccine-Loaded Nanoengineered Capsules in Blood. Advanced Materials. 2008;20:4698–4703. [Google Scholar]
- 32.Stratov I, Chung A, Kent SJ. Robust NK cell-mediated human immunodeficiency virus (HIV)-specific antibody-dependent responses in HIV-infected subjects. J Virol. 2008;82:5450–5459. doi: 10.1128/JVI.01952-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaunders JJ, Munier ML, Seddiki N, Pett S, Ip S, Bailey M, Xu Y, Brown K, Dyer WB, Kim M, et al. High levels of human antigen-specific CD4+ T cells in peripheral blood revealed by stimulated coexpression of CD25 and CD134 (OX40). J Immunol. 2009;183:2827–2836. doi: 10.4049/jimmunol.0803548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.