Abstract
Pathogenic rickettsiae are the causative agents of Rocky Mountain spotted fever, typhus, and other human diseases with high mortality and an important impact on society. Although survivors of rickettsial infections are considered immune to disease, the molecular basis of this immunity or the identification of protective antigens that enable vaccine development was hitherto not known. By exploring the molecular pathogenesis of Rickettsia conorii, the agent of Mediterranean spotted fever, we report here that the autotransporter protein, rickettsial outer membrane protein B (rOmpB), constitutes a protective antigen for this group of pathogens. A recombinant, purified rOmpB passenger domain fragment comprised of amino acids 36 to 1334 is sufficient to elicit humoral immune responses that protect animals against lethal disease. Protective immunity requires folded antigen and production of antibodies that recognize conformational epitopes on the rickettsial surface. Monoclonal antibodies (MAbs) 5C7.27 and 5C7.31, which specifically recognize a conformation present in the folded, intact rOmpB passenger domain, are sufficient to confer immunity in vivo. Analyses in vitro indicate this protection involves a mechanism of complement-mediated killing in mammalian blood, a means of rickettsial clearance that has not been previously described. Considering the evolutionary conservation of rOmpB and its crucial contribution to bacterial invasion of host cells, we propose that rOmpB antibody-mediated killing confers immunity to rickettsial infection.
INTRODUCTION
Spotted fever group (SFG) rickettsiae, such as Rickettsia conorii and R. rickettsii, causative agents of Mediterranean spotted fever (MSF) and Rocky Mountain spotted fever (RMSF), respectively, are obligate intracellular Gram-negative bacteria. Naturally transmitted to human hosts by tick bite inoculation, they spread through the circulatory system and proliferate in vascular endothelial cells (42) throughout the body, including the lungs, brain, liver, heart, kidney, and skin. Localized replication of rickettsiae at the inoculation site and subsequent tissue damage often give rise to a necrotic lesion, or eschar. Further damage to the vascular endothelium and infiltration of perivascular mononuclear cells cause increased fluid leakage into the interstitial space, often resulting in a dermal rash. These symptoms facilitate proper diagnosis of the infectious agent. However, the disease often manifests itself as nondescript fever and flu-like symptoms, leading to misdiagnosis. The widespread endothelial infection of rickettsiae induces microvascular leakage that can lead to severe clinical manifestations, including noncardiogenic pulmonary edema, interstitial pneumonia, acute renal failure, hemorrhagic rash, peripheral edema, and hypovolemia. The mortality rate for untreated RMSF is estimated to be as high as 23% (47). The severity of the disease, existence of antibiotic-resistant strains (51), and documented risk for aerosol transmission (28, 37, 41) have led to classification of rickettsial species as category B and C priority pathogens by the U.S. Centers for Disease Control and Prevention (CDC).
Recovery from rickettsiosis confers robust immunity and protection from reinfection (5, 21, 22), suggesting that protective antigen(s) must exist. Formalin-fixed rickettsial preparations have been shown to reduce mortality rates but have failed to prevent disease onset (10, 15, 16, 29). Sera from rabbits or mice that survived R. rickettsii or R. conorii infections are sufficient to confer passive immunity in guinea pig, mouse, and rhesus macaque animal models (5, 19, 44). Western blot analyses of these sera detect two abundant and high-molecular-weight antigens from SFG rickettsial lysates predicted to be rickettsial outer membrane proteins A (rOmpA; 155 kDa) and B (rOmpB; 120 kDa) (19, 55). Thus, rOmpA and rOmpB have been proposed to be protective antigens; however, direct evidence is still lacking.
Monoclonal antibodies (MAbs) have been generated following sublethal infection of mice with R. rickettsii or R. conorii strains. MAbs generated against R. rickettsii have been shown to protect Swiss-Webster mice from lethal R. rickettsii challenge (2, 4, 32, 33) and to prevent fever development in guinea pigs (32). MAbs generated against R. conorii afford complete protection against lethal R. conorii challenge in a mouse model of endothelial disease (19). The molecular identities of protective antigens recognized by therapeutic MAbs have not been elucidated but are speculated to encompass rOmpA and rOmpB (4, 19, 33). Efforts to test this conjecture have included the use of sonicated E. coli extracts enriched with overproduced recombinant rOmpA (34, 35, 46), as well as rompA baculovirus-transfected Sf9 insect cell extracts (43) for the active vaccination of mice and guinea pigs. Both approaches have proven successful in achieving full protection against SFG rickettsial challenges. However, immunization of mice with purified rOmpA showed incomplete (14 to 29%) and variable protection (11, 13). Modest levels of protection have also been observed upon immunization of mice with purified denatured peptides of rOmpB; in particular, 29 to 43% protection could be achieved by immunizing animals with unfolded fragments within the rOmpB passenger domain (amino acids 451 to 1308), but not the β-peptide (amino acids 1335 to 1704) (13).
rOmpB (Sca5) is a major rickettsial surface antigen (24, 52) that belongs to a family of proteins in Gram-negative bacteria called autotransporters, many of which function as virulence factors. Autotransporters have modular structures composed of an N-terminal signal peptide for translocation across the plasma membrane, followed by the so-called passenger domain that carries the functional attributes of the protein and a C-terminal β-barrel-rich translocation domain, or β-peptide, that serves as a pore for passage of the passenger domain across the outer membrane (27). rOmpB is initially translated as a 168-kDa protein and later proteolytically processed into a 32-kDa β-peptide and a 120- to 130-kDa extracellular and outer membrane-associated passenger domain (25). We have previously shown that production of rOmpB in a heterologous Escherichia coli system is sufficient to mediate attachment to and invasion of nonphagocytic mammalian cells (9, 45). Bacterial attachment is competitively inhibited by addition of the purified, recombinant passenger domain (amino acids 36 to 1334), implying the functional significance of this portion of the protein (9).
In this study, we use purified recombinant rOmpB antigens encompassing various lengths of the passenger domain to evaluate the protective molecular attributes of rOmpB in the lethal R. conorii infection of endothelium-targeted rickettsiosis using C3H/HeN mice (49). We show that the integrity of the folded passenger domain must be maintained to afford protection. By using a series of monoclonal rOmpB antibodies generated with this protective antigen, we identify one MAb that can protect animals from lethal infection. This protection was shown in vitro to be associated with complement-mediated killing of R. conorii in murine blood, a previously undefined mechanism of rickettsial clearance. Our findings demonstrate that immunization with rOmpB is sufficient to protect mice against lethal R. conorii infection and that this protection requires immune recognition of specific conformational epitopes.
MATERIALS AND METHODS
Cell lines and bacterial strains.
Vero and HeLa cells were cultured under standard conditions as described previously (9). E. coli BL21(DE3) or TOP10 was grown in LB Miller broth at 37°C, supplemented with carbenicillin (50 μg/ml) or kanamycin sulfate (50 μg/ml) where appropriate. R. conorii Malish 7 was propagated and isolated from Vero cell cultures as described previously (1). R. conorii was further purified from Vero cells by needle lysis and centrifugation over a 20% sucrose cushion (16,000 × g; 4°C; 30 min) and stored at −80°C in SPG buffer (218 mM sucrose, 3.8 mM KH2PO4, 7.2 mM K2HPO4, 4.9 mM l-glutamate, pH 7.2). The 50% lethal dose (LD50) of Vero cell-isolated R. conorii in male C3H/HeN mice was determined to be 4 × 106 PFU.
Quantification of R. conorii infectious titers.
Enumeration of viable R. conorii cells was determined by limiting dilution and infection of Vero or HeLa cells. Ninety-six-well plates containing cells at 90% confluence were aspirated of medium and infected with 10-fold serial dilutions of R. conorii in ice-cold brain heart infusion (BHI) at 8 to 16 replicate wells per dilution in a 10-μl volume. Bacterial contact was induced by centrifugation at 300 × g for 5 min and incubation at room temperature for an additional 10 min. Two hundred microliters of complete Dulbecco's modified Eagle's medium (DMEM) was added to each well and incubated for 4 to 6 days at 34°C and 5% CO2, whereupon the plates were fixed with 4% PFA (4% paraformaldehyde, 4% sucrose, phosphate-buffered saline [PBS], pH 7.4), permeabilized with 0.1% Triton X-100, and processed for total R. conorii immunofluorescence using anti-RCPFA (1 μg/ml) and secondary detection with goat anti-rabbit Alexa Fluor 488 (Invitrogen). R. conorii-positive wells were counted, and the infectious titer, expressed as PFU/ml, was calculated by the method of Reed and Muench (39).
Plasmid construction.
rompB gene fragments were amplified by PCR from a chromosomal preparation of R. conorii Malish 7 containing the rompB gene (GenBank accession no. AAL03623.1). Construction of pYC7, pYC9, and pYC11 are described elsewhere (9, 40). rompB-containing plasmids were generated by directional cloning into the specified vector using the primers and restriction enzymes indicated in Table S1 in the supplemental material. pYC55 was generated by site-directed mutagenesis of pET-22b (Novagen) to remove the pelB sequence and modify the frame of the BamHI restriction site. Mutagenesis was done using the specified primers (see Table S1 in the supplemental material) according to a modified protocol described previously (50). Plasmids pYC82 and pEC3, containing R. conorii sca15264-5479 (GenBank accession no. AAL02557.1) and murine actin, respectively, were constructed using the TOPO TA cloning kit (Invitrogen). R. conorii sca15264-5479 and murine actin were PCR amplified from purified R. conorii or the C3H/HeN mouse chromosome, respectively, using the primers listed in Table S1 in the supplemental material, and cloned into pCR2.1.
Sera and antibodies.
Anti-R. conorii mouse sera (numbers 1 to 10) were generated in mice infected with a sublethal dose of Vero cell-purified R. conorii (7.63 × 105 PFU) by intravenous (i.v.) retro-orbital injection and reinfected on day 12 with 7.9 × 106 PFU R. conorii. Twenty-two days after the initial infection, mouse blood was drawn by cardiac puncture and serum was recovered.
Rabbit polyclonal antibodies were generated as described previously (31). Anti-rOmpBFL serum was generated from immunization with rOmpB36-1655 excised from SDS-PAGE of an E. coli outer membrane preparation of rOmpB (9) and quantified by densitometry in ImageJ, followed by subsequent boosts with rOmpB36-1655 (full length) purified under denaturing conditions. Anti-rOmpB35-1334 rabbit serum was generated by immunization with His6-SUMO-rOmpB35-1334 purified under native conditions. Anti-RCPFA was generated by immunization with purified R. conorii (1 × 108 PFU), fixed with 4% PFA, and resuspended in PBS.
Anti-RC71 (9) and anti-RC72 are rabbit anti-R. conorii sera/antibodies generously provided by Patricia Crocquet-Valdes and David H. Walker (UTMB, Galveston, TX).
rOmpB MAbs were generated at the Frank W. Fitch Monoclonal Facility (University of Chicago) as described previously (38), with the differences noted. Briefly, three female BALB/c mice (designated NEP, LEP, and REP) were immunized and boosted intraperitoneally (i.p.) with rOmpB36-1334 purified under nondenaturing conditions. Following the initial immunization scheme (38), the LEP mouse, which showed the strongest immune reactivity to antigen, was selected for subsequent hybridoma generation. Splenocytes were harvested and fused with the mouse myeloma cell line SP2/mIL-6. Hybridomas were screened by enzyme-linked immunosorbent assay (ELISA) and flow cytometry, and antigen-specific clones were subcloned to produce MAb-secreting hybridomas arising from single cells. The rOmpB MAbs generated include 5B4.2, 5C7.27, 5C7.31, and 6B6.6 of the IgG2a isotype and 4B1.15 and 4H2.20 of the IgG2b isotype. The rOmpB MAb titers against glutathione S-transferase (GST)-rOmpB36-1334 were determined to be 10 ng/ml. Isotype-matched control antibodies 7B8.35 (IgG2a) and 12CA5 (IgG2b) were purified and supplied by the Frank W. Fitch Monoclonal Antibody Facility. The hybridoma for Staphyloccocus aureus anti-alpha-hemolysin 7B8.38 was generously provided by Julie Bubeck-Wardenburg (University of Chicago).
Mapping rOmpB antigen recognition.
One-milliliter cultures of E. coli TOP10 transformed with plasmids pYC42-pYC53 and pYC83-pYC86 were induced with 0.1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) at 30°C for 2 h to express GST-rOmpB peptide fusions (A to L). Induced bacteria were normalized to 1 ml at an optical density at 600 nm (OD600) of 0.5, and proteins were extracted using B-PER lysis buffer (Pierce). B-PER-insoluble protein fractions were solubilized in SDS-PAGE sample buffer and resolved on 10% SDS-PAGE gels for immunoblot analyses with polyclonal anti-GST (Z-5) antibody (Santa Cruz Biotechnologies), anti-rOmpBFL rabbit sera (1:20,000), anti-rOmpB35-1334 rabbit sera (1:30,000), anti-rOmpB36-1334 mouse sera (NEP, LEP, and REP; 1:5,000), anti-RCPFA rabbit sera (1:20,000), anti-RC71 rabbit sera (1:1,000), anti-RC72 rabbit polyclonal antibody (1:1,000), anti-R. conorii mouse sera (1:500), and rOmpB monoclonal 5B4.2 or 6B6.6 antibodies (0.01 μg/ml). Reactivity of antibodies against rOmpB was detected using the appropriate rabbit anti-mouse or goat anti-rabbit horseradish peroxidase (HRP) conjugates (1:5,000; Sigma).
Protein expression and purification.
Overnight bacterial cultures were diluted 1:20 into 1 liter of fresh medium and grown at 37°C to mid-exponential phase (OD600 = 0.5 to 0.6) and induced under the specified conditions. GST-tagged (expressed from pYC11, pYC13, pYC14, pYC15, pYC16, and pYC17) and His-tagged (pYC69) rOmpB fusions were expressed and purified under native conditions from E. coli BL21(DE3) (Stratagene). Cultures were cooled on ice for 30 min and then induced using 0.1 M IPTG at 25°C overnight. Bacterial suspensions were lysed by passage through the French pressure cell twice (1,500 lb/in2), and rOmpB fusions were purified on the appropriate 5 ml GSTrap-FF or HisTrap-FF column (GE Healthcare) using an Äkta FPLC (GE Healthcare). Fractions containing fusion proteins were pooled and dialyzed into 10% glycerol-Tris-buffered saline (TBS) (50 mM Tris, pH 8, 150 mM NaCl) and then snap-frozen in liquid nitrogen and stored at −80°C.
Purified GST-rOmpB36-1334 (pYC11) and His6-SUMO-rOmpB35-1334 (pYC69) used for immunizations were further processed to remove the affinity tag. The GST tag was cleaved using thrombin (GE Healthcare) as described by the manufacturer and then allowed to flow over a GSTrap-FF column and a benzamidine column (GE Healthcare) to remove the GST and thrombin, respectively. Untagged native rOmpB36-1334 in PBS was stored at −80°C for use in immunizations for rOmpB MAb production. His6-SUMO-rOmpB35-1334 in TBS containing 0.2% NP-40 and 1 mM dithiothreitol (DTT) was cleaved at 4°C overnight with gentle rocking with SUMO protease 1 (1 U/100 μg protein; Life Sensors). SUMO protease (His6 tagged) and liberated His6-SUMO tag were separated from the untagged rOmpB35-1334 (rOmpBN) by affinity purification over Ni-nitrilotriacetic acid (Ni-NTA) agarose beads (Qiagen). The flowthrough containing rOmpBN was buffer exchanged into PBS and stored at −80°C until it was used for mouse immunization studies.
His6-rOmpB peptides (amino acids 36 to 204 [A; pYC62], 353 to 499 [D; pYC57], 950 to 1098 [J; pYC66], 1148 to 1334 [L; pYC68]), and His6-rOmpB36-1334 (rOmpBD; pYC7) were expressed in E. coli BL21(DE3) and purified under denaturing conditions. Cultures were induced at 37°C with 0.5 mM IPTG for 4 h. Bacteria were lysed as described above, and His6-rOmpB fusions were purified by Ni-NTA affinity chromatography as described previously (40). Eluate fractions positive for rOmpB were pooled, buffer exchanged, concentrated using Amicon Ultra (Millipore) into 1 M urea-PBS, and stored at −80°C until they were used for immunizations. Protein concentrations were quantified by bicinchoninic acid assay (Pierce).
ELISA.
Antibody titers and antigen recognition were determined by ELISA. Ninety-six-well MaxiSorp plates (Nunc) were coated with 1 μg of protein in bicarbonate buffer (0.1 M Na2CO3, 0.1 M NaHCO3, pH 9.6) overnight at 4°C. Antibodies or sera prepared in 1% bovine serum albumin (BSA)-PBS at the indicated concentrations were incubated in triplicate wells and subsequently developed with the appropriate goat anti-rabbit or rabbit anti-mouse HRP-conjugated (1:10,000) secondary antibodies and a TMB substrate kit (Pierce). Colorimetric differences were analyzed with a spectrophotometer at 450 nm.
Mouse immunizations and challenges.
For active immunizations, groups (n = 13) of 5- to 7-week-old male C3H/HeN mice (Harlan Sprague Dawley) were immunized by intramuscular injection in the hind leg with 0.1-ml aliquots of 50 μg of recombinant rOmpBN, rOmpBD, or rOmpB peptide A, D, J, or L in PBS emulsified 1:1 with colonization factor antigen (CFA). Booster immunizations with proteins emulsified in incomplete Freund adjuvant (IFA) were done 21 and 42 days after initial immunization. Blood sampling occurred on day 56 to determine serum antibody titers by ELISA against the immunization antigen (titer ± standard error of the mean [SEM]): PBS, 52 ± 22; rOmpBD, 20,899 ± 4,850; rOmpBN, 45,155 ± 11,264; A, 18,077 ± 4,289; D, 40,354 ± 7,388; J, 4,431 ± 1,451; and L, 3,804 ± 980. Mice were challenged on day 63 by i.v. retro-orbital injection with 4 LD50 R. conorii diluted in 0.1 ml SPG buffer.
MAbs were screened for protective properties against R. conorii by adoptive transfer. Groups of mice (n = 13) were injected i.p. with 200 μg of purified MAb 1 h prior to being challenged by i.v. retro-orbital injection with 3.5 LD50 R. conorii.
Infected mice were monitored twice daily for signs of disease and daily for weight loss. Animals that exhibited symptoms of severe disease that were consistent with not recovering from the infection were removed from the study and scored as succumbing to the infection. These symptoms included ruffled fur, hunched posture, shallow respiration, immobility when touched, and weight loss of at least 15% of the initial body weight. At the time of death of the control mice, three predesignated mice from each experimental group were sacrificed, and their spleens, kidneys, livers, hearts, and lungs were extracted to determine the R. conorii load. Survival and weight loss curves were determined for at least 10 mice per group. The experiments were repeated at least twice with similar results. Animal experiments were performed in accordance with institutional guidelines and protocols approved by the Institutional Biosafety Committee and the Institutional Animal Care and Use Committee at the University of Chicago.
PCR quantification of R. conorii levels.
R. conorii loads in mouse organs were quantified by TaqMan quantitative PCR (qPCR). Sections of spleen, kidney, liver, heart, and lung were harvested from mice and stored at −80°C until they were processed. Total chromosomes from digested tissue homogenates were purified as described previously (53), eluted in 50 μl of H2O, and normalized to 50 μg/ml. TaqMan qPCRs were run in multiplex, with primer sets used to construct pEC3 (sca1) and pYC82 (actin) and fluorescent probes (Integrated DNA Technologies) annealing to sca1 and actin (see Table S1 in the supplemental material) in each reaction well and analyzed on the 7300 Real-Time PCR System (Applied Biosystem). Standard curves were generated using pEC3 and pYC82. PCRs were run as follows: 95°C for 6 min, followed by 50 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 20 s. VIC and FAM (6-carboxyfluorescein) fluorescence emissions were recorded for each reaction at the 72°C stage. R. conorii presence in organs was expressed as the average number of R. conorii sca1 genes per murine actin gene copy.
Flow cytometry.
R. conorii organisms were fixed for 20 min in 4% PFA and subsequently washed in cold PBS. Fixed R. conorii organisms were incubated with mouse sera, MAbs, or mouse monoclonal F(ab′)2 at the specified concentration, counterstained with anti-RCPFA polyclonal antibody (1 μg/ml), and then labeled with both goat anti-rabbit IgG Alexa Fluor 488- and goat anti-mouse IgG Alexa Fluor 546-conjugated secondary antibodies (Molecular Probes). Bacteria were analyzed with a BD LSR-II flow cytometer using fluorescein isothiocyanate (FITC) and phycoerythrin (PE) parameters and FloJo software. The analyses of rOmpB detection relative to naive mouse sera or isotype-matched MAbs or F(ab′)2 (PE) were predicated on positive detection of rabbit anti-RCPFA (FITC) fluorescence.
F(ab′)2 inhibition assays.
Monoclonal antibody F(ab′)2 fragments were generated and purified using the F(ab′)2 Preparation Kit (Thermo Scientific). Vero cells were seeded into 96-well plates at 90% confluence (104 cells/well) 24 h prior to infection. R. conorii diluted to 4 × 106 PFU/ml in DMEM were preadsorbed for 15 min at 25°C with monoclonal F(ab′)2 at 10 or 50 μg/ml or with PBS only. R. conorii mixtures (50 μl) were added to each well of Vero cells (multiplicity of infection, 10). Bacterial contact with host cells was initiated by centrifugation at 300 × g for 5 min, and then the plates were incubated at 34°C and 5% CO2 for 30 min. Following infection, the cells were washed 5 times with PBS, fixed with 4% PFA at 25°C for 20 min, and then processed for differential immunofluorescence staining. Extracellular R. conorii organisms were labeled using IgG-purified anti-RCPFA (1 μg/ml) followed by Alexa Fluor 546-conjugated goat anti-rabbit IgG (1:1,000) prior to permeabilization of the mammalian cells with 0.1% Triton X-100. Total R. conorii organisms were stained with anti-RCPFA, followed by Alex Fluor 488-conjugated goat anti-rabbit IgG. Cellular nuclei were labeled with 4′,6-diamidino-2-phenylindole (DAPI) (1:10,000). Images of the infections were digitally captured on a Nikon Eclipse TE2000-u microscope coupled to a charge-coupled device (CCD) camera using ×200 magnification to reveal total associated R. conorii, extracellular R. conorii, and host cell nuclei in each field. Bacteria and host nuclei were manually enumerated in Image J. R. conorii association was calculated as a ratio of total R. conorii per cellular nucleus, while invasion was determined as intracellular R. conorii organisms as a percentage of the total associated organisms.
R. conorii survival in mouse blood and plasma.
Whole blood was collected by cardiac puncture of C3H/HeN male mice and mixed with 10 μg/ml lepirudin (Bayer) to prevent coagulation. Aliquots (225 μl) of the pooled blood were supplemented with 50 μg/ml of the indicated MAb (IgG2a, 5B4.2 and 5C7.31; IgG2b, 4B1.15) or an equivalent volume of PBS and inoculated with 25 μl of 107 PFU/ml of R. conorii in SPG. The PBS samples were put on ice for the duration of the experiment (initial inoculum). The infected samples containing MAbs were incubated at 37°C with rotation for 60 min, at which time all blood aliquots were incubated on ice for 15 min in a final concentration of 1% saponin (in PBS) to lyse eukaryotic cells. The lysed blood was centrifuged at 16,000 × g for 10 min to pellet R. conorii, and the supernatants were removed. The pellets were resuspended in 1 ml ice-cold BHI medium, and R. conorii titers were determined by limiting dilution on Vero cells. R. conorii survival was calculated as the percentage of the average initial inoculum recovered at 60 min.
Plasma was generated by centrifugation of lepirudin-treated blood at 10,000 × g for 3 min for removal of blood cells. Heat inactivation of the plasma was done at 56°C for 30 min, followed by incubation on ice for 5 min. Plasma samples were supplemented with MAb/PBS and infected as described above. Following the 60-min time point, all samples were incubated on ice and brought to 1-ml volume with ice-cold BHI, and viable R. conorii titers and survival were determined as described above.
C3 deposition on R. conorii.
R. conorii organisms were incubated with PBS containing 10% human (CompTech) or C3H/HeN mouse serum supplemented with 0.008, 0.04, 0.2, 1.0, or 5.0 μg/ml 5C7.31 or IgG2a at 37°C for 20 min with rotation. Cells were immediately placed on ice, washed twice in PBS, and fixed with 4% PFA, and surface-bound C3 was detected with FITC-conjugated goat F(ab′)2 anti-human C3 (Protos Immunoresearch). R. conorii was additionally labeled with rabbit anti-RCPFA followed by a goat-anti rabbit Alexa Fluor 546 conjugate. The binding of C3 antibody to R. conorii was measured by flow cytometry (as described above) on the anti-RCPFA (PE)-positive population.
Statistical analyses.
Statistics were computed in GraphPad Prism. In analyses of rickettsial levels in mouse organs in active and passive immunization experiments, significance was determined using the Kriskal-Wallis test, followed by Dunn's posttest for multiple pairwise comparisons of experimental groups to the control group. In studies pertaining to the active immunizations, rOmpB-immunized mouse groups (rOmpBD, rOmpBN, A, D, J, and L) were compared to PBS group values. In the MAb studies, the 5B4.2, 5C7.31, and 6B6.6 groups were compared to the IgG2a control samples, while 4B1.15 and 4H2.20 were analyzed against the IgG2b control samples. All statistically significant comparisons, where the P value was <0.05, were reported.
RESULTS
Mice infected with R. conorii produce antibodies against rOmpB.
Previous experiments suggested that rOmpB is a highly immunogenic rickettsial antigen with potential protective attributes (2, 19, 24, 33, 52). We first examined whether immune sera from R. conorii-infected mice specifically recognized GST-rOmpB36-1334 protein carrying the recombinant passenger domain of rOmpB (amino acids 36 to 1334) fused to GST (see Fig. S1A in the supplemental material). This recombinant rOmpB fusion remains soluble under native conditions and competitively inhibits R. conorii association with host Vero cells (9) (see Fig. S1B in the supplemental material). Using an ELISA, we confirmed that each of the 10 hyperimmune sera contained antibodies directed against GST-rOmpB36-1334 (see Fig. S1C in the supplemental material). The IgG antibody recognition is specific for rOmpB and not the GST tag. Sera from uninfected mice served as negative controls.
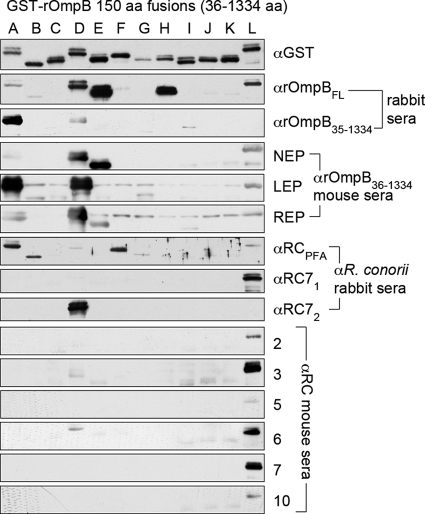
In order to identify antigenic regions of rOmpB, we cloned and expressed in E. coli 12 overlapping 150-amino-acid rOmpB peptides, fused to GST, that span the rOmpB passenger domain (constructs A to L; see Fig. S1A in the supplemental material). All GST fusions, A through L, in E. coli lysates were resolved by SDS-PAGE and transferred onto a blot for identification of immune-reactive species (Fig. 1). The presence of antibodies to each of these rOmpB peptides was examined through the use of various serum samples that have previously been demonstrated to exhibit reactivity to the recombinant rOmpB passenger domain. The most apparent result of these queries is that, regardless of the method for inducing anti-rOmpB seroreactivity, the L fragment was almost universally recognized while the B, C, F, G, H, I, J, and K fragments were rarely or not recognized. Additionally, fragments A and D were frequently detected.
Fig. 1.
Epitope mapping of R. conorii rOmpB by Western blotting. Twelve overlapping 150-amino-acid (aa) peptides (abbreviated as peptides A through L) spanning the rOmpB passenger domain (amino acids 36 to 1334) were resolved by SDS-PAGE in order to query for the presence of specific reactive antibodies contained within serum samples known to possess anti-rOmpB seroreactivity. The presence of all 12 peptides was demonstrated through probing with anti-GST antibody. In all, 14 serum samples were used for Western blotting. The serum samples were derived from recombinant-protein vaccination or infection with R. conorii. Vaccination-derived serum samples include those from rabbits and mice immunized with rOmpB peptides, including full-length rOmpB (rOmpBFL) or the amino acid 36 to 1334 peptide encompassing the rOmpB passenger domain (rOmpB36-1334). Additionally, sera from rabbits infected with live (RC71 and RC72) or immunized with paraformaldehyde-fixed (RCPFA) R. conorii and mice infected with R. conorii were used (numbers 2, 3, 5, 6, 7, and 10).
Protective efficacy of rOmpB antigens.
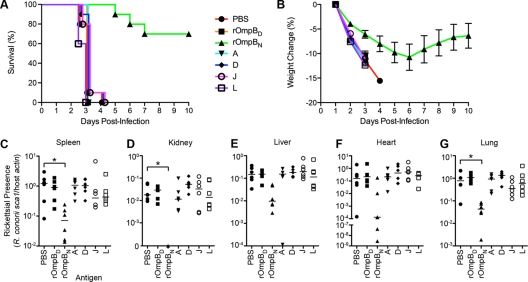
We wondered whether fragments of rOmpB exhibiting greater antigenicity, as shown in Fig. 1, might also confer protective immunity against infection by R. conorii. To test this hypothesis, we selected three rOmpB peptides, A, D, and L, recognized by several sera in our experiment, as well as J, which did not elicit any immune reaction (Fig. 1). All four peptides were expressed as a His6-tagged fusion, purified from E. coli under denaturing conditions (see Fig. S1D in the supplemental material), and utilized to immunize mice. To address the protective contributions of immune responses generated against conformational versus linear epitopes, we also immunized mice with the full-length rOmpB passenger domain purified under native (rOmpBN) or denaturing (rOmpBD) conditions (see Fig. S1E in the supplemental material). The immunized mice were then challenged with 4 LD50 of R. conorii and monitored for survival (Fig. 2 A) and weight change (Fig. 2B), the latter of which served as a correlate for morbidity. All animals, with the exception of those immunized with native rOmpB35-1334 (rOmpBN), exhibited severe morbidity by day 4 postinfection and were euthanized. The majority of the rOmpBN-immunized mice exhibited delayed signs of morbidity and reduced mortality (70% survival at day 10). By day 7 postchallenge, these mice displayed signs of recovery, such as weight gain, reduced ruffling, and greater activity, and by day 10, the surviving rOmpBN-immunized mice no longer exhibited signs of distress.
Fig. 2.
Active immunization of mice with native rOmpB36-1334 protects against lethal challenges with R. conorii. (A) Survival curve of rOmpB-immunized mice following lethal challenge with R. conorii. Cohorts of 10 C3H/HeN mice immunized with PBS, denatured (D) or native (N) rOmpB36-1334, or the rOmpB 150-amino-acid peptides (A, D, J, and L) were challenged with 4 LD50 R. conorii and monitored for survival over the course of 10 days. Animals that exhibited symptoms of severe disease that were consistent with not recovering from the infection were removed from the study and scored as succumbing to the infection. Shown is a representative survival curve of two experiments. (B) Daily percent weight change of the actively immunized mice (from panel A) after lethal infection with R. conorii. The data points represent the mean percent weight changes of up to 10 mice compared to the initial weight on day 1. The standard deviation is indicated by error bars. (C to G) Rickettsial presence in mouse organs. At the time of death of the PBS-immunized mice, predesignated mice from each of the rOmpB-immunized groups were sacrificed, and their spleens (C), kidneys (D), livers (E), hearts (F), and lungs (G) were harvested to assess the rickettsial load. R. conorii levels were quantified by TaqMan qPCR from total purified DNA of organ homogenates and are presented as the ratio of R. conorii sca1 to murine actin genomic copies. The data are combined from two independent experiments with a total of six mice, with the median of each group is denoted by the horizontal line. Kriskal-Wallis analysis with Dunn's posttest was used for group comparisons, as detailed in Materials and Methods. *, P < 0.05.
At the time of death corresponding to the mock-immunized mice, predesignated mice from each group were sacrificed and necropsied for their spleens, kidneys, livers, hearts, and lungs to enumerate the rickettsial burden. As shown in Fig. 2C to G, the levels of R. conorii present in organs from mice immunized with the denatured peptides A, D, J, and L and rOmpBD were similar to those observed in the mock-treated mice. In contrast, mice immunized with rOmpBN consistently harbored lower R. conorii levels in spleen, kidney, and lungs; rickettsial levels were also reduced in the liver and heart, though these differences were not statistically significant. These results demonstrate that immunization with folded rOmpB passenger domain (amino acids 35 to 1334) but not linear, denatured peptides generates protective immunity against lethal R. conorii infections.
Binding of rOmpB antibodies to R. conorii.
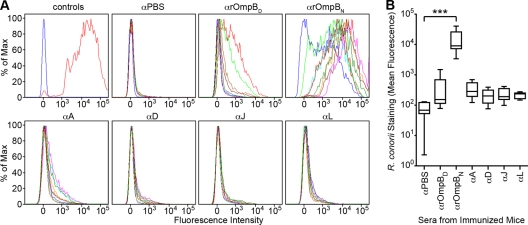
rOmpB is displayed on the surface of R. conorii and promotes adhesion to host cells (9). We wondered whether animals immunized for the active challenge (Fig. 2) developed antibodies capable of binding rOmpB on the surface of R. conorii. To test this, PFA-fixed R. conorii organisms were incubated with serum or mock, rOmpBD, rOmpBN, A, D, J, or L immunized and then incubated with fluorophore-conjugated secondary antibodies for analysis by flow cytometry. As shown in Fig. 3 A, surface staining of R. conorii was observed with the sera of rOmpBN-immunized mice only. Comparison of the mean fluorescence intensity of R. conorii detection by rOmpBN sera compared to sera isolated from PBS- and denatured rOmpB protein-immunized mice shows a 2-fold (logarithmic)-increased difference in staining (Fig. 3B). This result indicates that both protective immunity and antibody recognition of conformational epitopes on the surface of R. conorii are optimally achieved by using the intact and functional passenger domain of rOmpB. Neither of the smaller, unfolded fragments of rOmpB nor the unfolded passenger domain was sufficient to fulfill these molecular activities.
Fig. 3.
Surface recognition of R. conorii by IgG in a subset of rOmpB-immunized animals. (A) Flow cytometric analyses of R. conorii recognition by immunized mouse sera. Sera from PBS- and rOmpB-immunized C3H/HeN mice were analyzed by flow cytometry for IgG antibodies that could recognize rOmpB epitopes on the R. conorii surface. Sera collected from actively immunized mice were diluted 1/1,000 and used to stain PFA-fixed R. conorii. Mouse antibody recognition of R. conorii was detected using a secondary goat anti-mouse IgG Alexa Fluor 546 conjugate. Controls (top left) show the fluorescence intensity shift between naïve mouse serum-labeled (blue) and anti-R. conorii mouse serum-labeled (red) R. conorii. Sera from eight PBS- or nine rOmpB-immunized mice per group were analyzed. The fluorescence spectrum of each mouse is displayed in a different color. At least 8,000 R. conorii organisms were measured per spectrum. (B) Box-and-whisker plots of the combined mean fluorescence from the flow cytometric analyses of the serum staining of R. conorii done in panel A. The box encompasses the 25 to 75% quartile, with the inner line representing the median and the whiskers denoting the minimum and maximum mean fluorescence values for each of the sera from the seven groups of immunized mice. One-way analysis of variance (ANOVA) with Dunnett's test was used for group comparisons. ***, P < 0.001.
Protective efficacy of rOmpB monoclonal antibodies in passive immunization studies.
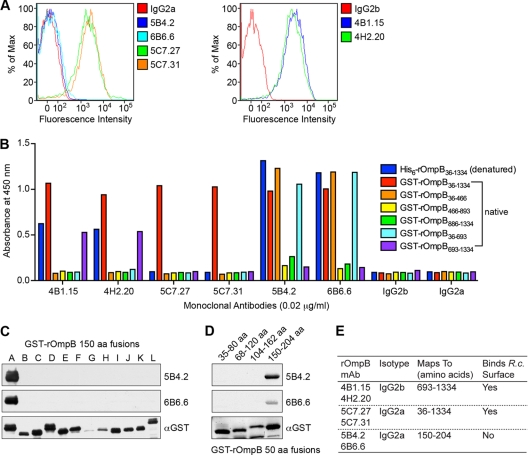
To delineate the types of antibodies produced by rOmpB immunization that potentially contribute to the observed protection (Fig. 2A), we generated MAbs against recombinant rOmpB36-1334 purified under nondenaturing conditions. Six mouse MAbs were examined, and their abilities to bind surface-exposed rOmpB were established by flow cytometry. Four MAbs (4B1.15, 4H2.20, 5C7.27, and 5C7.31) detected rOmpB on the R. conorii surface, whereas two MAbs (5B4.2 and 6B6.6) and the isotype-matched antibody controls (IgG2a and IgG2b) did not (Fig. 4 A). These antibodies were further characterized by ELISA using the passenger domain (amino acids 36 to 1334) purified under denaturing conditions, as well as GST-rOmpB fusions purified under native conditions and carrying either the intact passenger domain (amino acids 36 to 1334), halves of the passenger domain (amino acids 36 to 693 or 693 to 1334), or thirds (amino acids 36 to 466, 466 to 893, or 886 to 1334), as depicted in Fig. S1A in the supplemental material. The data suggest that MAbs 5C7.27 and 5C7.31 recognize the native, full passenger domain exclusively (Fig. 4B). In contrast, MAbs 4B1.15, 4H2.20, 5B4.2, and 6B6.6 recognize both the native and denatured passenger domain, as well as smaller fragments of rOmpB. Specifically, MAbs 4B1.15 and 4H2.20 recognize a conformational epitope present in the second half of the passenger domain (amino acids 693 to 1334) but absent in the smaller passenger domain thirds that encompass the region (Fig. 4B). MAbs 5B4.2 and 6B6.6 recognize a nonnative epitope located within the first third of the passenger domain (Fig. 4B) contained within amino acids 36 to 204 of the passenger domain (Fig. 4C). A more refined mapping experiment revealed that MAbs 5B4.2 and 6B6.6 bind a linear epitope located between amino acids 150 and 204 (Fig. 4D). Neither MAb 4B1.15, 4H2.20, 5C7.27, nor 5C7.31 detected rOmpB by denaturing Western blot analyses (data not shown). A summary of the antibody characteristics is depicted in Fig. 4E.
Fig. 4.
Characterization of monoclonal antibodies generated against rOmpB36-1334 purified under native conditions. (A) Flow cytometric analyses of R. conorii surface recognition using six anti-rOmpB MAbs relative to isotype-matched antibody controls. PFA-fixed R. conorii organisms were incubated with MAbs at 0.01 μg/ml, followed by secondary detection using a goat-anti-mouse Alexa Fluor 546 IgG conjugate. At least 10,000 R. conorii organisms were analyzed per spectrum. (B) The anti-rOmpB MAbs were mapped by ELISA for recognition of denatured (blue) and native (red) forms of rOmpB36-1334 and various truncations of the rOmpB passenger domain purified under native conditions. MAbs were used at 0.01 μg/ml. (C) 5B4.2 and 6B6.6 anti-rOmpB monoclonal antibodies were mapped by Western immunoblot analyses against E. coli extracts expressing GST-tagged 150-amino-acid peptides spanning amino acids 36 to 1334 of rOmpB. The anti-GST blot shows the expression of each of the rOmpB peptide fusions. (D) 5B4.2 and 6B6.6 anti-rOmpB MAbs were mapped by Western immunoblot analyses against E. coli extracts expressing GST fusions of 50 amino acids overlapping peptides of rOmpB spanning 36 to 204 amino acids. The anti-GST blot shows expression of the GST-rOmpB peptides. (E) Summarized characteristics of each of the anti-rOmpB MAbs. R.c., R. conorii.
Comparison of these MAbs in a passive-immunization study revealed that only the 5C7 group of MAbs protect mice in a lethal R. conorii challenge (Fig. 5A). Mice were passively immunized with each rOmpB MAb or isotype-matched control MAbs (IgG2a or IgG2b) and then challenged intravenously with 3.5 LD50 of R. conorii. The animals were monitored several times daily for survival (Fig. 5A), and weights were recorded daily over a 10-day period (Fig. 5B). At the time of death of the passively immunized, isotype-matched control mice, predesignated animals from each rOmpB MAb-immunized group (5B4.2, 5C7.27, 5C7.31, 6B6.6, 4B1.15, and 4H2.20) were sacrificed, and their spleens, livers, kidneys, hearts, and lungs were extracted to quantify the degree of bacterial colonization. Animals that received MAbs 4B1.15, 4H2.20, 5B4.2, and 6B6.6 succumbed to infection at rates similar to those of control animals (Fig. 5A and B) and harbored levels of R. conorii in organ tissues similar to those in the isotype-matched control groups (Fig. 5C to G). In contrast, mice immunized with MAb 5C7.31 exhibited reduced morbidity and mortality and significantly lower R. conorii levels in all examined organs. Similar results were obtained for MAb 5C7.27 (data not shown). These results indicate that the ability of a MAb to bind surface-exposed rOmpB, as with 4B1.15 and 4H2.20 (Fig. 4A), does not necessarily correlate with protection. MAb 5C7.31 not only binds surface-exposed rOmpB (Fig. 4A), but also interacts exclusively with the folded, intact passenger domain (Fig. 4B), suggesting that these two parameters may serve as correlates of protection. Taken together, the results demonstrate that only a subset of rOmpB conformation-specific antibodies that bind the rickettsial surface can sufficiently protect mice from fatal rickettsial infections.
Fig. 5.
A subset of conformation-specific rOmpB monoclonal antibodies protect passively immunized mice in a lethal R. conorii infection. (A) Survival curve of passively immunized mice following lethal challenge with R. conorii. Cohorts of 10 C3H/HeN mice immunized with MAbs generated against rOmpB (5B4.2, 5C7.31, 6B6.6, 4B1.15, and 4H2.20) or isotype-matched controls (IgG2a and IgG2b) were challenged with 3.5 LD50 R. conorii, and their survival was monitored over the course of 10 days. Shown is a representative survival curve of two experiments. (B) Percent daily weight change of the passively immunized mice (from panel A) after lethal infection with R. conorii. The data points represent the mean percent weight changes of up to 10 mice relative to the weights recorded on day 1 postinfection. The standard deviation is indicated by error bars. (C to G) Rickettsial presence in mouse organs. At the time of death of the isotype-matched IgG2a and IgG2b passively immunized mice, predesignated mice from each of the other groups were sacrificed, and their spleens (C), kidneys (D), livers (E), hearts (F), and lungs (G) were harvested to determine the rickettsial load. R. conorii levels were quantified by TaqMan qPCR from total chromosomal extracts of organ homogenates and are presented as the ratio of R. conorii sca1 to murine actin gene copies. The data are combined from two independent experiments. Each data point represents the rickettsial levels from one mouse, with the median of each group denoted. The Kriskal-Wallis test with Dunn's posttest was used for group comparisons to the isotype controls as detailed in Materials and Methods. *, P < 0.05; **, P < 0.01.
MAb 5C7.31 reduces R. conorii survival in blood.
We next investigated the mechanism of the 5C7.31 MAb-mediated protection. Antibodies can protect by three mechanisms: neutralization of antigen function, opsonophagocytosis, and/or complement-mediated killing. We previously showed that rOmpB functions in bacterial adherence and invasion of host cells (9) and that R. conorii association with host Vero cells can be competitively inhibited by addition of purified rOmpB passenger domain (see Fig. S1B in the supplemental material). Given the contribution of rOmpB to these rickettsial infection processes, we first examined whether the protection afforded by 5C7.31 could be attributed to impeding R. conorii interactions with host cells. F(ab′)2 fragments of isotype-matched MAbs and representative rOmpB MAbs (Fig. 4E) were generated and shown to retain rOmpB binding activity comparable to that of intact MAbs (data not shown). Next, monoclonal rOmpB F(ab′)2 fragments were tested for their ability to prevent R. conorii association with and invasion of Vero cells. Neither preadsorbtion of R. conorii with 5C7.31 F(ab′)2 fragments (otherwise protective as a MAb) or with 5B4.2 or 4B1.15 F(ab′)2 fragments (otherwise capable of binding surface-exposed rOmpB) prevented rickettsial association or invasion of mammalian cells relative to R. conorii treated with isotype-matched IgG2a/IgG2b F(ab′)2 or PBS (Fig. 6A and B). These results suggest that binding of 5C7.31 MAb does not interfere with R. conorii adherence to or entry into host cells.
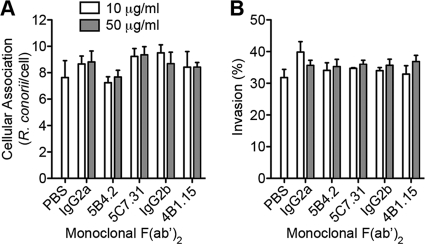
Fig. 6.
Anti-rOmpB monoclonal F(ab′)2 does not inhibit R. conorii cellular association or invasion. (A and B) Effects of monoclonal F(ab′)2 fragments on R. conorii association with and invasion of host Vero cells. Monolayers of Vero cells were infected for 30 min with R. conorii preadsorbed for 15 min with 10 (white) or 50 (gray) μg/ml purified F(ab′)2 monoclonal fragments. Unassociated bacteria were washed from monolayers, and samples were processed for immunofluorescence to assess total bacterial association (A) and percent invasion (B) of the total associated bacteria. The data shown are the representative means ± SEM from one of three experiments, where the infections were assayed in quadruplicate, and at least 50 eukaryotic cells were counted per replicate. Two-way ANOVA revealed no differences in adherence and invasion frequencies of R. conorii when preadsorbed with the monoclonal rOmpB or isotype-matched F(ab′)2 fragments at increasing concentrations.
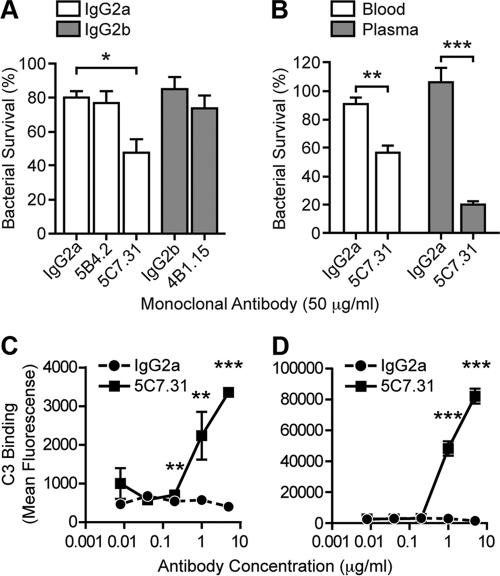
To examine the role of 5C7.31 in opsonophagocytic and complement-mediated killing, we next asked whether MAb 5C7.31 affected R. conorii survival in whole blood collected from C3H/HeN mice. R. conorii organisms were inoculated into blood supplemented with 5C7.31, the nonprotective rOmpB MAbs 5B4.2 and 4B1.15, or isotype-matched MAbs and were assessed for survival following 60 min of incubation (see Materials and Methods). As shown in Fig. 7A, neither 5B4.2 nor 4B1.15 reduced R. conorii survival in blood compared to controls. In contrast, incubation with MAb 5C7.31 led to a significant reduction of R. conorii (32% compared with the IgG2a isotype-matched MAb control) (Fig. 7A).
Fig. 7.
Anti-rOmpB 5C7.31 MAb reduces R. conorii viability in mouse blood through a complement-dependent mechanism. (A) R. conorii survival in mouse blood. Fresh blood drawn from C3H/HeN mice was infected with 106 PFU/ml R. conorii. Blood samples were supplemented with rOmpB (5B4.2, 5C7.31, and 4B1.15) or isotype-matched control (IgG2a and IgG2b) MAbs at the specified concentrations and incubated with rotation at 37°C. After 60 min, samples were cooled on ice and blood cells were lysed with saponin. Viable R. conorii organisms in each sample were enumerated by limiting dilution on monolayers of Vero cells. Bacterial survival was calculated as the percentage of viable R. conorii organisms recovered from the blood at 60 min relative to that at 0 min prior to the addition of MAb. The data are the combined mean survival ± SEM from three independent experiments performed with triplicate samples. (B) R. conorii survival in freshly drawn mouse blood or plasma supplemented with anti-rOmpB 5C7.31 or the IgG2a isotype-matched MAb, similarly to that described above. Plasma was generated by removal of cells from blood by centrifugation at 10,000 × g for 3 min. The data are the combined mean survival ± SEM from two independent assays done in quadruplicate. (C and D) R. conorii was incubated with PBS-10% mouse (C) or human (D) serum supplemented with 0.008, 0.04, 0.2, 1.0, or 5.0 μg/ml 5C7.31 or IgG2a at 37°C for 20 min. The cells were washed twice in PBS and fixed with 4% PFA, and surface-bound C3 was detected with FITC-conjugated goat F(ab′)2 anti-human C3. The binding of C3 antibody to R. conorii was measured by flow cytometry. The data are combined from three independent analyses conducted in triplicate (twice) and duplicate. The error bars represent the SEM. A total of 10,000 R. conorii organisms were analyzed per replicate. In panel A, one-way ANOVA with Bonferroni's test was used for group comparisons within isotype-matched groups. Panels B, C, and D were analyzed by two-tailed Student's t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To determine whether MAb 5C7.31-dependent killing was mediated in a cell-dependent manner, the bacterial survival experiment was repeated in either plasma or blood (Fig. 7B). As before, R. conorii killing was increased when MAb 5C7.31 was added to blood, but this killing effect was further enhanced when plasma was used instead of blood (Fig. 7B). These data suggest that in the absence of professional phagocytic cells, R. conorii must be killed by complement, a heat-labile component of plasma. Indeed, heat inactivation of the plasma completely eliminated the rickettsicidal effect of MAb 5C7.31 on R. conorii (data not shown), indicating that the 5C7.31 MAb mediates killing of R. conorii in blood by complement.
C3 is the most abundant complement component in serum and is common to both the classical and alternative complement pathways (18). Cleavage of C3, and its resultant activation, leads to the release of the cleavage product C3a and the deposition of C3b on the bacterial surface, the latter of which gives rise to subsequent amplification of the complement cascade (17). To verify the involvement of complement in MAb 5C7.31-mediated R. conorii killing, we examined deposition of C3 components on the bacterial surface. R. conorii was incubated in C3H/HeN mouse or human serum with increasing concentrations of 5C7.31 or the IgG2a isotype-matched control and then analyzed by flow cytometry for C3 binding. As depicted in Fig. 7C, C3 was deposited on R. conorii in mouse sera containing MAb 5C7.31 compared to the control, specifically at the higher antibody concentrations tested (0.2, 1, and 5 μg/ml). Interestingly, increased C3 binding was also observed in human sera containing 5C7.31 (Fig. 7D), suggesting that this MAb also fixes human complement. In contrast, there was no significant difference in C3 association with R. conorii in mouse or human serum containing the IgG2a isotype-matched MAb versus no MAb (data not shown), indicating that C3 deposition is not enhanced by the presence of irrelevant antibodies. Collectively, these results suggest that protection afforded by passive immunization with MAb 5C7.31 is facilitated through complement killing of bacteria in blood.
DISCUSSION
Protective immunity is achieved upon neutralization of critical virulence factors that represent protective antigens. If a host mounts the proper immune response against a protective antigen, it will be protected against reinfection by the cognate pathogen. This is true for rickettsial diseases, as humans surviving infections elicit long-term protective immunity (21, 22). This suggests the existence of a discrete rickettsial antigen(s) that is involved in generating protective immune responses. The molecular identification of such antigens is complicated by the fact that rickettsiae are intracellular pathogens that cannot be grown in the absence of a host cell and because, until recently, directed genetic mutagenesis of the organism was not possible (14). Thus, the discovery of rickettsial protective antigens has relied heavily on the analysis of hyperimmune reactive species in convalescent hosts. Such approaches suggested that recognition of conformational epitopes of high-molecular-weight rickettsial antigens rOmpA and rOmpB is crucial for protection against SFG rickettsiosis (3, 19, 33).
We have previously shown that rOmpB is important for the molecular pathogenesis of R. conorii (9), and we now show its value as a protective antigen by immunization of animals with the recombinant rOmpB passenger domain purified under native conditions. Immunization with linear peptides of rOmpB or the denatured passenger domain did not afford protection. The corresponding antibodies against these linear epitopes also lack the capacity to bind to the surfaces of intact R. conorii organisms. We find that antibody detection of rOmpB on the surface of R. conorii is uniquely dependent on the recognition of conformational epitopes by polyclonal and monoclonal antibodies elicited through immunization with soluble protein purified under native conditions. By comparing several MAbs, we additionally demonstrate that this property alone is insufficient to abrogate disease. Indeed, protective rOmpB MAbs (5C7.27 and 5C7.31) not only bind the rickettsial surface specifically, they also recognize an epitope of rOmpB exclusively displayed by the folded, full-length passenger domain. Our studies suggest that optimal induction of rOmpB-mediated humoral immunity will be predicated on the production and enrichment of such conformation-specific protective antibodies.
Anti-rOmpB 5C7.31 MAb promotes killing of R. conorii when supplemented in naïve murine plasma lacking phagocytic cells but containing active complement. These results are consistent with the idea that anti-rickettsial antibodies have the capacity to fix complement (8, 32). Antibody-mediated clearance of rickettsiae has previously been attributed to Fc receptor-mediated phagocytosis (6, 7, 19, 20, 23, 30, 54), though the role of complement in this process is poorly understood (23). It has been proposed that typhus group (TG) species R. prowazekii and R. typhi may be altogether resistant to complement-mediated killing using human or mouse convalescent-phase sera (7, 54). The effect of complement as a possible host defense mechanism against rickettsial infection has not been extensively explored. The results of our findings show that R. conorii is susceptible to complement-mediated killing specifically in the presence of the rOmpB MAb, 5C7.31, and that this correlates with increased C3 deposition on the bacterial surface. Together, these observations indicate that in the absence of a particular antibody, R. conorii may otherwise be inherently resistant to the effects of complement. Thus, to our knowledge, this is the first molecular evidence illustrating R. conorii susceptibility to complement-mediated killing by specific conformational rOmpB antibodies. Examination of the in vivo contribution of complement to anti-rickettsial immunity is under way.
What is the potential therapeutic value of rOmpBN conformational antibodies, such as MAb 5C7.31? The intravenous inoculation of R. conorii into the bloodstream of mice immediately predisposes the rickettsiae to antibody opsonization and subsequent complement-mediated eradication. During the tick's blood meal in the natural transmission of SFG rickettsiae, it is thought that some bacteria may be inoculated into the vasculature of mammalian hosts. Therefore, it may be possible that proper rOmpB immunization may perturb arthropod-transmitted rickettsial infections, perhaps by reducing the initial viable dose and infectivity of the rickettsiae and enabling more efficient clearance of the infection.
In addition, the intracellular lifestyle of rickettsiae has commonly led to the belief that circulating antibodies are ineffective in clearing an established infection. However, two previous studies provide insight into the therapeutic use of anti-rickettsial antibodies for the treatment of infections early after the onset of disease symptoms. A study of RMSF pathogenesis using guinea pigs and rhesus monkeys revealed that animals treated with R. rickettsii rabbit hyperimmune sera after the establishment of disease symptoms ultimately resolved the infection and recovered fully (44). A more recent study using a murine model of infection demonstrated that supplementing anti-R. conorii monoclonal antibodies in previously infected C3H/HeN SCID mice prolonged survival and reduced the bacterial load in organs (19). While the nature of antibody accessibility to rickettsiae during the course of infection is unclear, these findings demonstrate that protective anti-rickettsial antibodies administered after the onset of SFG disease symptoms can reduce the severity of, and potentially resolve, the disease. We thus hypothesize that anti-rickettsial monoclonal antibodies, such as rOmpB 5C7.31, could be used as an alternative therapy to antibiotic treatment of rickettsial disease, where the efficacy of antibodies would be unaffected by antibiotic-resistant strains of rickettsiae. In light of the historical and now potential use of rickettsial pathogens as bioweapons (12, 26, 48) and the engineering of antibiotic-resistant strains (51), investigation into new therapies for the treatment and prevention of SFG and TG rickettsial diseases is of the utmost importance.
Supplementary Material
ACKNOWLEDGMENTS
We thank P. Crocquet-Valdes and D. H. Walker (UTMB, Galveston, TX) for providing the RC7 rabbit antibodies, C. D. Lima (Memorial Sloan-Kettering Cancer Center, New York, NY) for the pET28-smt3 plasmid, and J. Bubeck-Wardenburg for the 7B8.35 (IgG2a) monoclonal antibody. We are indebted to C. McShan at the Frank W. Fitch Monoclonal Facility (University of Chicago) for production of MAbs; L. Quenee and N. Ciletti for guidance with the animal experiments; A. Cheng, V. Thammavongsa, H. Kim, and O. Schneewind for help with experimental designs; D. Missiakas for careful reading and editing of the manuscript; and members of the Martinez laboratory for discussion.
Y.G.-Y.C. was supported by Genetics and Regulation Training Grant T32 GM007197 at the University of Chicago. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID), Infectious Diseases Branch (AI 72606; J.J.M.). We acknowledge membership within the Region V Great Lakes Regional Center of Excellence (GLRCE) in Biodefense and Emerging Infectious Diseases Consortium and technical support from the GLRCE Animal Research and Immunology Core (GLRCE) (NIAID Award U54-AI-057153). Statistical assistance was provided by the Biostat Clinic at the University of Chicago (supported by Grant number UL1RR024999 from the National Center For Research Resources).
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
Published ahead of print on 28 March 2011.
REFERENCES
- 1. Ammerman N. C., Beier-Sexton M., Azad A. F. 2008. Laboratory maintenance of Rickettsia rickettsii. Curr. Protoc. Microbiol. 3:3A.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anacker R. L., List R. H., Mann R. E., Hayes S. F., Thomas L. A. 1985. Characterization of monoclonal antibodies protecting mice against Rickettsia rickettsii. J. Infect. Dis. 151:1052–1060 [DOI] [PubMed] [Google Scholar]
- 3. Anacker R. L., Mann R. E., Gonzales C. 1987. Reactivity of monoclonal antibodies to Rickettsia rickettsii with spotted fever and typhus group rickettsiae. J. Clin. Microbiol. 25:167–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anacker R. L., McDonald G. A., List R. H., Mann R. E. 1987. Neutralizing activity of monoclonal antibodies to heat-sensitive and heat-resistant epitopes of Rickettsia rickettsii surface proteins. Infect. Immun. 55:825–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anigstein L., Bader M. N., Young G. 1943. Protective effect of separate inoculation of spotted fever virus and immune serum by intradermal route. Science 98:285–286 [DOI] [PubMed] [Google Scholar]
- 6. Beaman L., Wisseman C. L., Jr 1976. Mechanisms of immunity in typhus infections. V. Demonstration of Rickettsia mooseri-specific antibodies in convalescent mouse and human serum cytophilic for mouse peritoneal macrophages. Infect. Immun. 14:1065–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beaman L., Wisseman C. L., Jr 1976. Mechanisms of immunity in typhus infections. VI. Differential opsonizing and neutralizing action of human typhus rickettsia-specific cytophilic antibodies in cultures of human macrophages. Infect. Immun. 14:1071–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bengtson I. A., Topping N. H. 1942. Complement-fixation in rickettsial diseases. Am. J. Public Health Nations Health 32:48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan Y. G., Cardwell M. M., Hermanas T. M., Uchiyama T., Martinez J. J. 2009. Rickettsial outer-membrane protein B (rOmpB) mediates bacterial invasion through Ku70 in an actin, c-Cbl, clathrin and caveolin 2-dependent manner. Cell Microbiol. 11:629–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clements M. L., et al. 1983. Reactogenicity, immunogenicity, and efficacy of a chick embryo cell-derived vaccine for Rocky Mountain spotted fever. J. Infect. Dis. 148:922–930 [DOI] [PubMed] [Google Scholar]
- 11. Crocquet-Valdes P. A., et al. 2001. Immunization with a portion of rickettsial outer membrane protein A stimulates protective immunity against spotted fever rickettsiosis. Vaccine 20:979–988 [DOI] [PubMed] [Google Scholar]
- 12. Croddy E. A., Wirtz J. J., Larsen J. A. 2005. Typhus (Rickettsia Prowazekii), p. 293.In Croddy E. A., Wirtz J. J. (ed.), Weapons of mass destruction: an encyclopedia of worldwide policy, technology, and history, vol. 2 ABC-CLIO, Inc., Santa Barbara, CA [Google Scholar]
- 13. Díaz-Montero C. M., Feng H. M., Crocquet-Valdes P. A., Walker D. H. 2001. Identification of protective components of two major outer membrane proteins of spotted fever group Rickettsiae. Am. J. Trop. Med. Hyg. 65:371–378 [DOI] [PubMed] [Google Scholar]
- 14. Driskell L. O., et al. 2009. Directed mutagenesis of the Rickettsia prowazekii pld gene encoding phospholipase D. Infect. Immun. 77:3244–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dumler J. S., Wisseman C. L., Jr., Fiset P., Clements M. L. 1992. Cell-mediated immune responses of adults to vaccination, challenge with Rickettsia rickettsii, or both. Am. J. Trop. Med. Hyg. 46:105–115 [DOI] [PubMed] [Google Scholar]
- 16. DuPont H. L., et al. 1973. Rocky Mountain spotted fever: a comparative study of the active immunity induced by inactivated and viable pathogenic Rickettsia rickettsii.. J. Infect. Dis. 128:340–344 [DOI] [PubMed] [Google Scholar]
- 17. Dyer J. K., Bourque J. A., Steeves J. D. 2005. The role of complement in immunological demyelination of the mammalian spinal cord. Spinal Cord 43:417–425 [DOI] [PubMed] [Google Scholar]
- 18. Erdei A., Fust G., Gergely J. 1991. The role of C3 in the immune response. Immunol. Today 12:332–337 [DOI] [PubMed] [Google Scholar]
- 19. Feng H. M., Whitworth T., Olano J. P., Popov V. L., Walker D. H. 2004. Fc-dependent polyclonal antibodies and antibodies to outer membrane proteins A and B, but not to lipopolysaccharide, protect SCID mice against fatal Rickettsia conorii infection. Infect. Immun. 72:2222–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feng H. M., Whitworth T., Popov V., Walker D. H. 2004. Effect of antibody on the rickettsia-host cell interaction. Infect. Immun. 72:3524–3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fox J. P., Everritt M. G., Robinson T. A., Conwell D. P. 1954. Immunization of man against epidemic typhus by infection with avirulent Rickettsia prowazekii (strain E); observations as to post-vaccination reactions, the relation of serologic response to size and route of infecting dose, and the resistance to challenge with virulent typhus strains. Am. J. Hyg. (London) 59:74–88 [PubMed] [Google Scholar]
- 22. Fox J. P., Jordan M. E., Gelfand H. M. 1957. Immunization of man against epidemic typhus by infection with avirulent Rickettsia prowazekii strain E. IV. Persistence of immunity and a note as to differing complement-fixation antigen requirements in post-infection and post-vaccination sera. J. Immunol. 79:348–354 [PubMed] [Google Scholar]
- 23. Gambrill M. R., Wisseman C. L., Jr 1973. Mechanisms of immunity in typhus infections. 3. Influence of human immune serum and complement on the fate of Rickettsia mooseri within the human macrophages. Infect. Immun. 8:631–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gilmore R. D., Jr., Joste N., McDonald G. A. 1989. Cloning, expression and sequence analysis of the gene encoding the 120 kD surface-exposed protein of Rickettsia rickettsii. Mol. Microbiol. 3:1579–1586 [DOI] [PubMed] [Google Scholar]
- 25. Hackstadt T., Messer R., Cieplak W., Peacock M. G. 1992. Evidence for proteolytic cleavage of the 120-kilodalton outer membrane protein of rickettsiae: identification of an avirulent mutant deficient in processing. Infect. Immun. 60:159–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harris S. 1992. Japanese biological warfare research on humans: a case study of microbiology and ethics. Ann. N. Y. Acad. Sci. 666:21–52 [DOI] [PubMed] [Google Scholar]
- 27. Jacob-Dubuisson F., Fernandez R., Coutte L. 2004. Protein secretion through autotransporter and two-partner pathways. Biochim. Biophys. Acta 1694:235–257 [DOI] [PubMed] [Google Scholar]
- 28. Kenyon R. H., Kishimoto R. A., Hall W. C. 1979. Exposure of guinea pigs to Rickettsia rickettsii by aerosol, nasal, conjunctival, gastric, and subcutaneous routes and protection afforded by an experimental vaccine. Infect. Immun. 25:580–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kenyon R. H., Sammons L. S., Pedersen C. E., Jr 1975. Comparison of three Rocky Mountain spotted fever vaccines. J. Clin. Microbiol. 2:300–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keysary A., McCaul T. F., Winkler H. H. 1989. Roles of the Fc receptor and respiratory burst in killing of Rickettsia prowazekii by macrophagelike cell lines. Infect. Immun. 57:2390–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim H. K., et al. 2010. IsdA and IsdB antibodies protect mice against Staphylococcus aureus abscess formation and lethal challenge. Vaccine 28:6382–6392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lange J. V., Walker D. H. 1984. Production and characterization of monoclonal antibodies to Rickettsia rickettsii. Infect. Immun. 46:289–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li H., Lenz B., Walker D. H. 1988. Protective monoclonal antibodies recognize heat-labile epitopes on surface proteins of spotted fever group rickettsiae. Infect. Immun. 56:2587–2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McDonald G. A., Anacker R. L., Garjian K. 1987. Cloned gene of Rickettsia rickettsii surface antigen: candidate vaccine for Rocky Mountain spotted fever. Science 235:83–85 [DOI] [PubMed] [Google Scholar]
- 35. McDonald G. A., Anacker R. L., Mann R. E., Milch L. J. 1988. Protection of guinea pigs from experimental Rocky Mountain spotted fever with a cloned antigen of Rickettsia rickettsii. J. Infect. Dis. 158:228–231 [DOI] [PubMed] [Google Scholar]
- 36. Reference deleted.
- 37. Oster C. N., et al. 1977. Laboratory-acquired Rocky Mountain spotted fever. The hazard of aerosol transmission. N. Engl. J. Med. 297:859–863 [DOI] [PubMed] [Google Scholar]
- 38. Quenee L. E., et al. Amino acid residues 196-225 of LcrV represent a plague protective epitope. Vaccine 28:1870–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reed L. J., Muench H. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493–497 [Google Scholar]
- 40. Riley S. P., et al. 2010. The Rickettsia conorii autotransporter protein Sca1 promotes adherence to nonphagocytic mammalian cells. Infect. Immun. 78:1895–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saslaw S., Carlisle H. N. 1966. Aerosol infection of monkeys with Rickettsia rickettsii. Bacteriol. Rev. 30:636–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Silverman D. J., Bond S. B. 1984. Infection of human vascular endothelial cells by Rickettsia rickettsii. J. Infect. Dis. 149:201–206 [DOI] [PubMed] [Google Scholar]
- 43. Sumner J. W., Sims K. G., Jones D. C., Anderson B. E. 1995. Protection of guinea-pigs from experimental Rocky Mountain spotted fever by immunization with baculovirus-expressed Rickettsia rickettsii rOmpA protein. Vaccine 13:29–35 [DOI] [PubMed] [Google Scholar]
- 44. Topping N. H. 1940. Rocky mountain spotted fever; treatment of infected laboratory animals with immune rabbit serum. U.S. Government Printing Office, Washington, DC [Google Scholar]
- 45. Uchiyama T., Kawano H., Kusuhara Y. 2006. The major outer membrane protein rOmpB of spotted fever group rickettsiae functions in the rickettsial adherence to and invasion of Vero cells. Microbes Infect. 8:801–809 [DOI] [PubMed] [Google Scholar]
- 46. Vishwanath S., McDonald G. A., Watkins N. G. 1990. A recombinant Rickettsia conorii vaccine protects guinea pigs from experimental boutonneuse fever and Rocky Mountain spotted fever. Infect. Immun. 58:646–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Walker D. H. 1989. Rocky Mountain spotted fever: a disease in need of microbiological concern. Clin. Microbiol. Rev. 2:227–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Walker D. H. 2009. The realities of biodefense vaccines against Rickettsia. Vaccine 27(Suppl. 4):D52–D55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Walker D. H., Popov V. L., Wen J., Feng H. M. 1994. Rickettsia conorii infection of C3H/HeN mice. A model of endothelial-target rickettsiosis. Lab. Invest. 70:358–368 [PubMed] [Google Scholar]
- 50. Wang W., Malcolm B. A. 1999. Two-stage PCR protocol allowing introduction of multiple mutations, deletions and insertions using QuikChange Site-Directed Mutagenesis. Biotechniques 26:680–682 [DOI] [PubMed] [Google Scholar]
- 51. Weiss E., Dressler H. R. 1962. Increased resistance to chloramphenicol in Rickettsia prowazekii with a note on failure to demonstrate genetic interaction among [sic]. J. Bacteriol. 83:409–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Williams J. C., Walker D. H., Peacock M. G., Stewart S. T. 1986. Humoral immune response to Rocky Mountain spotted fever in experimentally infected guinea pigs: immunoprecipitation of lactoperoxidase 125I-labeled proteins and detection of soluble antigens of Rickettsia rickettsii. Infect. Immun. 52:120–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wilson T., Carson J. 2001. Rapid, high-throughput extraction of bacterial genomic DNA from selective-enrichment culture media. Lett. Appl. Microbiol. 32:326–330 [DOI] [PubMed] [Google Scholar]
- 54. Wisseman C. L., Jr., Waddell A. D., Walsh W. T. 1974. Mechanisms of immunity in typhus infections. IV. Failure of chicken embryo cells in culture to restrict growth of antibody-sensitized Rickettsia prowazekii. Infect. Immun. 9:571–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xu W., Raoult D. 1997. Distribution of immunogenic epitopes on the two major immunodominant proteins (rOmpA and rOmpB) of Rickettsia conorii among the other rickettsiae of the spotted fever group. Clin. Diagn. Lab. Immunol. 4:753–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.