Abstract
Candida krusei is a fungal pathogen of interest for the scientific community for its intrinsic resistance to fluconazole. Little is known about the interaction of this yeast with host immune cells. In this work, we have characterized the outcome of the interaction between C. krusei and murine macrophages. Once C. krusei was internalized, we observed different phenomena. In a macrophage-like cell line, C. krusei survived in a significant number of macrophages and induced filamentation and macrophage explosion. Phagocytosis of C. krusei led to actin polymerization around the yeast cells at the site of entry. Fluorescent specific staining with anti-Lamp1 and LysoTracker indicated that after fungal internalization, there was a phagolysosome maturation defect, a phenomenon that was more efficient when the macrophages phagocytosed killed yeast cells. Using cell line macrophages, we also observed macrophage fusion after cell division. When we used primary resident peritoneal macrophages in addition to macrophage explosion, we also observed a strong chemotaxis of uninfected macrophages to regions where C. krusei-infected macrophages were present. We also noticed yeast transfer phenomena between infected macrophages. Primary macrophages inhibited pseudohypha elongation more efficiently than the macrophage-like cell line, suggesting that C. krusei infection was better controlled by the former macrophages. Primary macrophages induced more tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) than the macrophage-like cell line. Our results demonstrate that C. krusei can exploit the macrophages for replication, although other different outcomes are also possible, indicating that the interaction of this pathogen with phagocytic cells is very complex and regulated by multiple factors.
INTRODUCTION
Fungal pathogens have arisen as a global threat to the health of immunocompromised patients. The management of these patients is complicated, due to their strong immunosuppression and to the fact that factors from both the pathogen and the host can contribute to the damage produced during infection (4).
Phagocytosis plays an important role in the host defense (see the review in reference 24). However, many pathogens have developed pathways to evade killing once they have been phagocytosed (see the review in reference 3). Moreover, some of these pathogens exploit the intracellular environment to replicate inside the macrophage and disseminate through the organism, the so-called Trojan horse model (5, 6, 21). This may offer a potential explanation for those pathogens which stay latent and spread within the host without triggering an immediate immune response. This mechanism has been shown for several fungal pathogens, in particular for Histoplasma capsulatum (7) and Cryptococcus neoformans (5). In addition, the activity of macrophages against some infections might determine the susceptibility of the host to that particular pathogen (28).
Candida krusei is a fungal pathogen that has been described in healthy hosts as an infrequent isolate of minor clinical significance inhabiting the mucosal surfaces (25). However, it is a pathogen of concern among immunocompromised patients, causing fungemia, endophthalmitis, arthritis, and endocarditis (12, 19). The widespread use of fluconazole to treat fungal infections has contributed to the appearance of C. krusei infections, since this species shows intrinsic resistance to this antifungal (22). Candida krusei can form pseudohyphae (11), a feature that might contribute to its virulence (26). Different studies have shown that C. krusei is less virulent than Candida albicans in terms of its adherence to epithelial cells and prosthetic devices, proteolytic potential, and production of phospholipases (10). Furthermore, C. krusei is different from other Candida spp. in its structural and metabolic features and exhibits different behavior patterns toward host defenses (25). Few studies have been conducted to determine the potential virulence of C. krusei in humans and laboratory animals. It is already known that C. krusei is less invasive than C. albicans, although it shows a heavy growth on the superficial epithelium (27). Besides this, C. krusei has been demonstrated to be more hydrophobic than other species of Candida, and this could play a critical role in the colonization of medical devices such as implants and catheters to which C. krusei has been shown to be more adherent (27). The ability to produce hydrolytic enzymes is considered a putative virulence factor of Candida spp. However, C. krusei does not produce these enzymes (25). These findings suggest that the virulence attributes of C. krusei could be more related to the host immune state than to specific fungal features.
In this work, we examined the intracellular behavior of this fungal pathogen once it has been phagocytosed by macrophages. We found that the interaction between macrophages and C. krusei is very complex and that multiple outcomes are possible, such as macrophage explosion, yeast expulsion, yeast transfer between macrophages, and macrophage fusion after division. Our findings indicate new aspects of C. krusei involved in the virulence of this organism.
MATERIALS AND METHODS
Yeast strains and growth conditions.
Candida krusei ATCC 6258 was cultured in liquid Sabouraud medium for 24 h at 30°C with moderate shaking (150 rpm) prior to experimental use.
Murine macrophage-like cells and culture conditions.
The adherent macrophage-like cell line RAW 264.7 was used for experimental work (23). This cell line is derived from the ascites of a tumor induced in a male mouse by intraperitoneal injection of Abelson murine leukemia virus (A-MuLV).
The cells were grown in feeding medium, which contained Dulbecco's modified Eagle's medium (Lonza, Verviers, Belgium) supplemented with 10% heat-inactivated fetal bovine serum (FBS; HyClone-Perbio), 10% NCTC medium (Sigma-Aldrich, Steinheim, Germany), and 1% nonessential amino acids (Sigma-Aldrich, Steinheim, Germany). The cells were regularly maintained at 37°C in a 5% CO2-enriched atmosphere.
Isolation of primary macrophages.
Primary macrophages were isolated from the peritoneal cavities of C57BL/6J mice (male, 8 to 12 weeks old) by washing the peritoneal cavities with phosphate-buffered saline (PBS), following the guidelines provided by the Committee for Bioethics and Animal Welfare from the Instituto de Salud Carlos III (approved protocol PA349). After centrifugation of the cell suspension, primary macrophages were counted using a hemocytometer. A suspension of 5 × 105 cells/ml in feeding medium was prepared, and 200 μl per well was added to 96-well tissue culture plates the day before the phagocytosis assays were performed.
Phagocytosis assays.
Phagocytosis assays were performed as described in reference 34. Briefly, RAW 264.7 macrophages were separated from tissue culture plates by continuing pipetting. The cells were centrifuged at 170 × g (1,000 rpm), and the pellet was suspended in 2 ml of fresh feeding medium. The cell density was estimated using a hemocytometer. A suspension of 2.5 ×105 cells/ml was prepared, and 200 μl per well was added (5 × 104 macrophages per well) to 96-well tissue culture plates (Costar, NY). For each condition, three wells were carried out in parallel. The plate was incubated overnight at 37°C in the presence of 5% CO2. Since these cells divide once during the night, it was estimated that 105 macrophage-like cells were present in each well at the moment they were exposed to the yeast cells. When primary macrophages were used, the cells obtained from the peritoneal cavity were directly placed on 96-well plates as described above and incubated overnight at 37°C in a 5% CO2-enriched atmosphere. Then, the medium was removed, and 200 μl of fresh feeding medium containing C. krusei at a cell density of 106 cells/ml or 5 × 105 cells/ml was added to each well. As a result, a 1:2 or 1:1 macrophage/yeast ratio was used in the macrophage-like cell line or the primary peritoneal macrophages, respectively. The phagocytosis was carried out for 2 h at 37°C in the presence of 5% CO2. Then, the wells were washed 5 times with feeding medium to remove the nonphagocytosed yeasts, and fresh feeding medium was added to the wells. Furthermore, phagocytosis assays were also performed using heat-killed C. krusei with both the macrophage-like cell line and primary macrophages.
Time-lapse recording and video processing.
The 96-well plate was placed under a Leica DMI 4000B microscope using a 20× lens objective in a 5% CO2 environment at 37°C. Pictures were taken every 3 min for around 18 h. The videos generated by the Leica software were exported as AVI documents and processed with ImageJ software (NIH) (http://rsb.info.nih.gov/ij). The final videos were generated by merging 8 frames per second, with each frame taken every 3 min, which means that a second from the video is equivalent to 24 min of the experiment.
Phagocytosis staining and quantification.
To quantify the phagocytosis, Giemsa staining was performed as described in reference 34. After phagocytosis, the medium was removed, and 100 μl of ice-cold methanol was added to each well. After 30 min of incubation at room temperature, methanol was removed, and the wells were washed 3 times with 200 μl of PBS. A 1:20 dilution of Giemsa stain (Merck) was added to each well for 30 min. Then, Giemsa staining solution was removed, and 100 μl of PBS was added to each well. Using a Leica DMI 3000B microscope, 5 pictures per well were taken to count the total number of macrophages and the number of macrophages with intracellular yeast. The phagocytosis percentage was calculated as (the number of infected macrophages/the number of total macrophages) × 100.
Intracellular/extracellular pseudohypha formation.
To test if the macrophage-like cell line or primary macrophages had different effects on pseudohypha formation, the following protocol was designed. Phagocytosis was carried out for 5 h as described above using a 1:1 macrophage/C. krusei cell ratio, and noninternalized yeast cells were isolated by gently washing the well twice with PBS. Five pictures per well were taken of these noninternalized cells to count the number of blastoconidia forming the pseudohyphae and to measure their length. To observe the yeasts internalized in the macrophages, the wells were washed again with PBS, and 100 μl of distilled water per well was added to produce the macrophage explosion. After 30 min of incubation at 37°C, we collected the medium from the wells and observed it under the microscope. Parallel samples of yeast cells incubated in feeding medium without macrophages were carried out as controls. The number of blastoconidia forming the pseudohyphae and their total length were calculated. The results were analyzed using GraphPad Prism 4.0 (GraphPad Software, Inc.) and PASW Statistics 17 software (SPSS SL).
Actin staining with fluorescent labeled phalloidin.
Macrophages were grown on poly-l-lysine-pretreated coverslips in cell culture plates (BD, France). The final macrophage density was 5 × 106 macrophages/ml. The C. krusei inoculum was prepared at 1.5 × 107 cells/ml and stained with 10 μg/ml calcofluor white (Sigma, St. Louis, MO) for 10 min at 37°C. Then, the inoculum was washed twice with sterile PBS and added to the coverslips. Phagocytosis, as described above and in the dark, was performed for 1 h, and then coverslips were washed three times to eliminate the nonphagocytosed yeasts. Cells were fixed with freshly prepared 4% p-formaldehyde for 10 min and washed with PBS. Coverslips were incubated with 0.1% Triton X-100 (Sigma, St. Louis, MO) for 5 min and washed again with PBS. Cells were incubated with 0.33 μM fluorescein isothiocyanate (FITC)-conjugated phalloidin solution (Invitrogen, Molecular Probes, Oregon) for 20 min and washed with PBS. Coverslips were place on slides with Fluoromount-G (SouthernBiotech), and pictures were taken with an SP5 confocal microscope (Leica Microsystems). Three-dimensional videos were generated from the confocal pictures using Voxx software (Indiana University), as previously described (18).
Immunofluorescence staining for lysosomal membrane protein LAMP1.
Candida krusei inoculum prepared at 1.5 × 107 cells/ml was stained with 10 μg/ml of calcofluor white (Sigma, St. Louis, MO), and phagocytosis using a 1:3 macrophage/C. krusei cell ratio was performed as described above. Cells were fixed with 4% p-formaldehyde for 10 min and washed with PBS. Coverslips were incubated with 0.1% Triton X-100 (Sigma, St. Louis, MO) for 5 min and washed again with PBS. Cells were incubated in 1% PBS-bovine serum albumin (BSA) for 15 min, and a 2.5-μg/ml solution of anti-Lamp1-biotin antibody (eBioscience) was added for 1 h. After incubation, coverslips were washed with PBS, and a 1 μg/ml solution of Streptavidin-Alexa 568 (Invitrogen, Molecular Probes, Oregon) was added for 30 min. Then, coverslips were washed again and place on slides with Fluoromount-G (SouthernBiotech). Finally, fluorescence was visualized using confocal microscopy, as described above.
Assessment of phagolysosome acidification.
Phagocytosis was performed at different times (30 min and 2 h), and nonphagocytosed yeast cells were removed by washing them with sterile PBS. LysoTracker red DND-99 (Invitrogen, Molecular Probes, Oregon) was added to the culture medium at a final concentration of 0.05 μM for 2 h at 37°C with 5% CO2. Parallel samples using heat-killed cells were carried out as positive controls. Coverslips were washed with PBS and mounted on slides with Fluoromount-G, and pictures were taken with a confocal microscope. To determine the acidification of the phagolysosomes, we examined the colocalization of LysoTracker red with C. krusei cells stained with calcofluor white (Sigma, St. Louis, MO). To establish statistical differences between live and heat-killed yeast cells, the experiments were performed in triplicate, and a t test was used to determine the P value.
Cytokine quantification produced by primary and RAW 264.7 macrophages in the presence of C. krusei.
Phagocytosis was carried out for 2 h as described above using a 2:1 macrophage/C. krusei cell ratio with both primary macrophages and RAW 264.7 macrophages. Supernatant was removed, fresh medium was added, and plates were incubated at 37°C with 5% CO2. Supernatants were collected after 4 h and an overnight incubation, and they were kept at −20°C after being centrifuged to discard residual macrophages and yeast cells. The experiment was performed in triplicate. The supernatants were analyzed following use of the cytometric bead array (CBA) mouse Th1/Th2/Th17 kit (BD Biosciences) using a FACSCalibur flow cytometer (BD Biosciences), and data were analyzed with FCAP Array software.
Statistics.
To assess statistical differences, the t test was performed using Microsoft Excel and GraphPad Prism 4.0 (GraphPad Software, Inc.). Differences were considered significant when P values were ≤0.05.
RESULTS
Interaction of C. krusei with macrophage-like cell line RAW 264.7.
We first performed phagocytosis assays as described in Materials and Methods using the macrophage-like cell line RAW 264.7. These macrophages had phagocytic activity in the presence of Candida krusei, and some macrophages were able to phagocytose not only blastoconidia but also pseudohyphae of a size similar to that of the macrophages (see Video S1 in the supplemental material). To quantify the phagocytosis, we stained the cells with Giemsa and found that around 10 to 20% of the macrophages had ingested C. krusei cells after 2 h without any opsonin added.
To study the intracellular behavior of C. krusei, we extensively washed the wells to remove the nonphagocytosed yeast cells after 2 h of phagocytosis. Then, the infected macrophages were observed using time-lapse microscopy. We observed that C. krusei survived inside the macrophages and induced pseudohypha formation (see Videos S1 and S2 in the supplemental material). This phenomenon occurred in 85% of the infected macrophages. The intracellular growth of the fungus yielded macrophage explosion (see Videos S1 and S2). During the overnight incubation, macrophages divided, but in the case of infected macrophages, we frequently observed that nascent macrophages fused after division, yielding a macrophage of a larger size (Fig. 1). This phenomenon was also observed when heat-killed yeast cells were used (result not shown). In some cases, C. krusei cells were not properly distributed among the two nascent macrophages, and prior to the fusion, pseudohypha sharing by both nascent macrophages was observed (Fig. 1; see also Video S1 in the supplemental material).
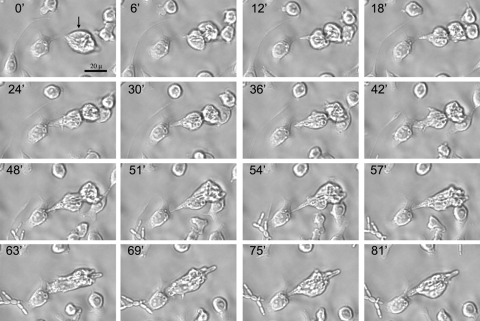
Fig. 1.
Division and fusion of macrophage-like RAW 264.7 cells. The sequence of images shows division of an infected macrophage (black arrow). After cell division, a fungal pseudohypha is trapped between both of the daughter macrophage cells. As a result, the macrophages fuse, and C. krusei continues intracellular proliferation. The scale bar shown in the first picture applies to all the panels. The time lapse between selected pictures is 3 or 6 min, as indicated in each panel.
Internalization of C. krusei by macrophage-like RAW 264.7 cells is associated with actin rearrangements.
Phagocytosis of C. krusei led to the polymerization of actin around the yeast cells at the site of entry. Using fluorescent phalloidin, we observed a bright ring around the ingested yeast cells (Fig. 2; see also Videos S3 and S4 in the supplemental material). In contrast, a diffuse fluorescence was observed when macrophages had no internalized C. krusei cells (Fig. 2).
Fig. 2.
Actin staining in RAW 264.7 macrophages infected with C. krusei. Actin staining of uninfected (top row) and infected (bottom row) macrophages using FITC-labeled phalloidin. C. krusei cells were stained with calcofluor white (in blue). Actin rearragements are pointed out with white arrows. DIC, differential interference contrast.
Candida krusei induces phagolysosome maturation defects in RAW 264.7 cells.
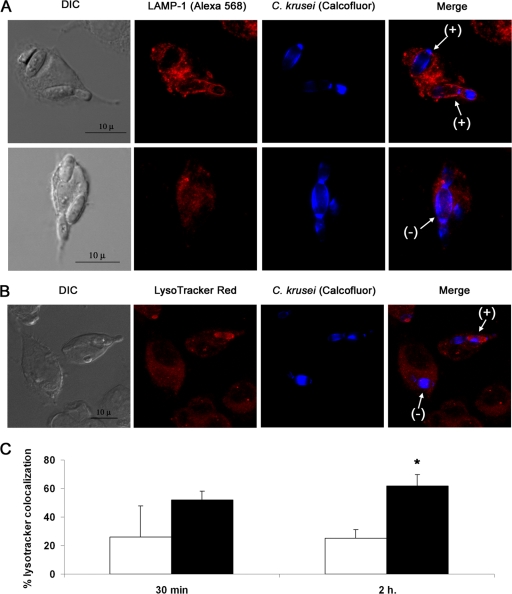
To investigate the mechanism by which C. krusei evaded killing after phagocytosis, we studied the phagolysosome maturation by using Lamp1 localization and analyzing the acidification of the phagolysosome using the LysoTracker red probe. In most of the macrophages, Lamp1 did not colocalize with the yeast cells, and colocalization was observed in only 20% of the macrophages (Fig. 3A shows representative images for these two situations). In agreement with this finding, we also found that acidification of the phagosomes in which C. krusei was observed was a rare phenomenon, and in the majority of the macrophages, there was not a colocalization between LysoTracker red and C. krusei cells. In Fig. 3B, we show an example of macrophages exhibiting the two patterns (colocalization or absence of colocalization). Since phagosome acidification is the final step of phagosome maturation, we carefully quantified the phenomenon at two different time points, 30 min (early time point) and 2 h (late time point). As shown in Fig. 3C, there was a low proportion of macrophages in which acidification of phagosomes containing C. krusei cells was observed, suggesting that C. krusei inhibits phagolysosome maturation. Previous findings in Candida albicans suggest that inhibition of phagolysosome maturation requires living cells (17), so we performed the same experiment but used heat-killed yeasts. As shown in Fig. 3C, phagosome acidification was observed in a higher proportion of macrophages, compared to the situation found with live yeast cells. These data indicate that C. krusei actively inhibits phagosome maturation and provide a mechanism for intracellular survival.
Fig. 3.
Phagosome maturation in RAW 264.7 macrophages infected with C. krusei. (A) Confocal microscopy of Lamp1 localization (red fluorescence) in macrophages with C. krusei cells (blue fluorescence) internalized after 1 h of phagocytosis. Arrows indicate positive (+; top row) and negative (−; bottom row) colocalization of Lamp1 with C. krusei. The scale bar shown in the first panel applies to the rest of the panels in the same row. (B) LysoTracker red localization in infected macrophages by confocal microscopy. C. krusei was stained with calcofluor white. Arrows indicate positive and negative colocalization of the dye with the yeast cells. The scale bar shown in the first panel applies to the rest of the panels in the same row. (C) The colocalization of C. krusei and the dye was measured by fluorescent microscopy after 30 min and 2 h of phagocytosis. The experiment was performed in triplicate, and the average values and standard deviations were plotted in the bar graphs. Black bars represent colocalization of heat-killed C. krusei and LysoTracker, while white bars represent colocalization of live yeast cells and the fluorescent dye. The asterisk denotes statistical difference (P < 0.05).
Interaction of C. krusei with primary peritoneal macrophages.
To confirm our results in a nonmodified phagocyte, we investigated the behavior of C. krusei in primary resident peritoneal murine macrophages obtained from the peritoneal cavities of C57BL/6J mice. These macrophages were less adherent than the RAW 264.7 cells. For this reason, removal of noninternalized yeasts produced a marked reduction in the number of macrophages adhered to the plate. To avoid this issue, we preferred to use a 1:1 macrophage/yeast cell ratio when working with primary macrophages rather than the 1:2 ratio used with RAW 264.7 cells, so elimination of noningested yeast did not require extensive washes of the well. The phagocytosis percentage was also around 20%, and similar to the situation found with the macrophage-like cell line, we observed pseudohypha formation and macrophage explosion after internalization of the yeast cells, confirming that C. krusei can survive inside phagocytic cells. However, this phenomenon occurred less frequently than that in the RAW 264.7 cells, and it was found in around 50% of the infected primary macrophages.
In addition to intracellular survival, we also observed other different phenomena. Primary resident macrophages exhibited a strong chemotactic effect, in which macrophages migrated to regions where other infected macrophages or C. krusei cells were present (see Video S5 in the supplemental material). This phenomenon was found to be more noticeable when infected macrophages exploded and C. krusei cells were released and was significantly decreased when using heat-killed yeast cells (result not shown).
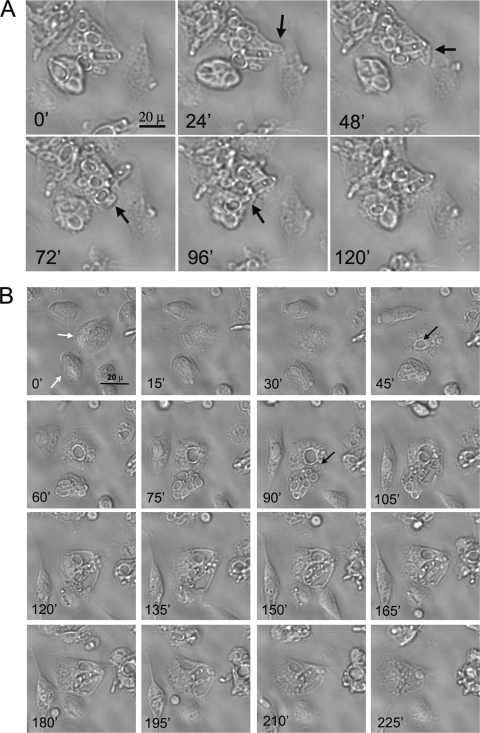
We also observed that infected macrophages extruded C. krusei cells, a phenomenon that did not have an effect on the integrity of the macrophage (Fig. 4A; see also Video S6 in the supplemental material). In some cases, these yeasts that were extruded from macrophages were engulfed by surrounding macrophages (see Video S5). More striking, we occasionally found that an infected macrophage was able to transfer intracellular yeast cells to another macrophage (Fig. 4B; see also Video S7). In these cases, there was a partial fusion of the cell membranes of the donor and recipient macrophages. In addition, previous to the transfer, the recipient macrophage formed an intracellular compartment. After the membrane fusion, the yeasts were transferred to this last compartment in the recipient macrophage (Fig. 4B; see also Video S7). Although C. krusei survived in a significant number of infected primary macrophages, we also observed that these macrophages were able to “destroy” the yeast cells. In these cases, intracellular yeast cells lost their visual integrity, which was accompanied by the appearance of small vesicles in the macrophage (see Video S8).
Fig. 4.
Extrusion of blastoconidia and yeast transfer between infected primary macrophages. (A) Expulsion of yeast cells from primary macrophages. Black arrows indicate yeast cells expulsed by the macrophage. The time lapse between pictures is 18 min. The scale bar shown in the first picture applies to the rest of the pictures. (B) Yeast transfer between primary macrophages. Two infected macrophages (white arrows) fuse, and yeast blastoconidia are transferred from the lower macrophage to the upper one. Prior to the transfer, an intracellular compartment appears in the recipient macrophage (black arrows in panels corresponding to 45 and 90 min), compartments in which the yeast cells will be located after the transfer. The scale bar shown in the first picture applies to the rest of the pictures. The time lapse between selected pictures is 15 min.
Pseudohypha formation during phagocytosis.
When phagocytosis was performed with the macrophage-like cell line and primary macrophages, we observed differences in the outcome of the interaction, which suggested that primary macrophages had a stronger antifungal effect. In consequence, we investigated if these two types of macrophages had different effects on pseudohypha formation. Candida krusei induced pseudohypha elongation in feeding medium, which was registered by the appearance of new blastoconidia (4 to 5, compared to 2 at time zero) (Fig. 5A). When the yeast cells were incubated in the presence of the macrophage-like cell line RAW 264.7, the yeast produced pseudohyphae as the control, independently of whether they were found inside or outside macrophages. In contrast, when C. krusei cells were incubated with primary resident macrophages, we noticed that the pseudohyphae were composed of a lower number of blastoconidia (Fig. 5B and C). This difference was observed in both intracellular and extracellular yeast cells. These results were also confirmed by measuring the total length of the pseudohyphae (Fig. 5B and C).
Fig. 5.
Pseudohypha formation after phagocytosis. (A) Images of C. krusei at time zero and after 5 h of incubation in feeding medium during the phagocytosis assay. (B) Graphic showing a distribution of the number of blastoconidia forming the pseudohypha and its length under all the experimental conditions. (C) Table showing the mean number of blastoconidia forming the pseudohypha and the length of the pseudohypha. The P value resulting from the Student t analysis considering the value obtained for the control yeast cells incubated for 5 h in feeding medium as a reference value for all the comparisons is shown. Statistical differences of these comparisons are highlighted in boldface.
Pattern of cytokine production after C. krusei phagocytosis.
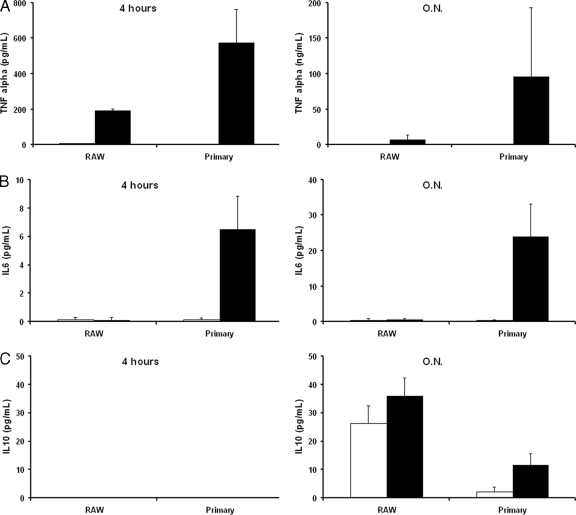
Our previous findings indicate that the interaction between C. krusei and phagocytic cells is different, depending on whether macrophage-like cells or primary macrophages were used. Since macrophage activation is an important step to determine the antimicrobial activity of the phagocytic cells, we investigated if the macrophage-like cell line RAW 264.7 and primary macrophages produced different cytokines after challenge with C. krusei. Primary macrophages produced higher levels of the proinflammatory cytokine tumor necrosis factor alpha (TNF-α) both at 4 h and after overnight incubation. As a consequence, primary macrophages accumulated interleukin-6 (IL-6), a cytokine not detected in the supernatants of the infected macrophage-like cell line. Moreover, IL-10, an anti-inflammatory cytokine, was produced at higher levels by RAW 264.7 macrophages (Fig. 6).
Fig. 6.
Cytokine production by RAW 264.7 and primary macrophages. RAW 264.7 and primary macrophages were exposed to C. krusei, and the cytokines produced were measured as described in Materials and Methods after 4 h and overnight incubation (O.N.). The experiment was performed in triplicate, and the average values and standard deviations are plotted in the bar graphs. (A) TNF-α; (B) IL-6; (C) IL-10. Black bars, macrophages exposed to C. krusei; white bars, control macrophages not infected with yeasts.
DISCUSSION
The capacity for intracellular pathogenesis is an important feature because the outcome of interaction of macrophages with fungal pathogens might determine the susceptibility of the host to the infection (28, 32). In this work, we demonstrate that the emerging fungal pathogen Candida krusei can survive and exploit the intracellular macrophage environment for its replication, which may influence the dissemination through the organism. The mechanisms involved in intracellular pathogenesis are diverse. Mycobacterium tuberculosis inhibits phagosome-lysosome fusion (20), and this mechanism allows a latent infection in the host that can last years. Among fungi, the best-characterized intracellular pathogen is Histoplasma capsulatum, which impairs phagosome-lysosome fusion (7, 29). Another well-known example is Cryptococcus neoformans. This encapsulated yeast has developed different mechanisms to evade killing by the macrophages, including formation of leaky phagolysosomes (30) and induction of capsule enlargement, which confers protection to free radicals (33). In the case of C. krusei, we have observed that there is a defect in the maturation of the phagolysosome by using different markers of this process, such as Lamp1 and LysoTracker. This finding is in agreement with the related species Candida albicans, for which a reduction of phagosome maturation and acidification defects have been described (8, 17). Similar findings have been found in the survival of Candida albicans inside epithelial cells (35).
One striking aspect of our work is the number of possible outcomes for the interaction between this fungus and the macrophages. For example, in macrophage-like cell lines, we observed macrophage fusion after cell division. Although we did not observe this phenomenon in primary macrophages because they do not divide, we observed a strong chemotactic effect of the macrophages on the areas where C. krusei was present. These findings suggest that the host immune system has developed mechanisms to control the intracellular parasitism by producing a more effective cellular response. This response was decreased when macrophages were exposed to heat-killed C. krusei, which could mean that there is a specific response against the pathogen by macrophages and not only because of the presence of foreign particles.
In addition, we observed other phenomena, especially when we used primary macrophages rather than macrophage-like cell lines. For example, we observed how macrophages extruded yeast cells. At the moment, we do not know if this phenomenon is produced by the macrophage to eliminate intracellular yeast that cannot be destroyed or if it is a mechanism developed by the pathogen to divide in new niches. We also observed yeast transfer between macrophages, which might represent a new strategy for the pathogen to infect adjacent cells without exiting the intracellular space and, thus, avoid exposure of the microbe to antimicrobial compounds in the extracellular space, such as specific antibody and complement. However, it could also reflect a mechanism developed by the immune system in which macrophages overloaded with yeast cells transfer part of the fungal burden to uninfected or less-infected macrophages. Some of these phenomena (macrophage fusion, yeast extrusion, and transfer between macrophages) have been described in other fungal pathogens (1, 2, 13, 15, 16), which suggests that these processes represent a universal strategy developed by the host cells to control the infection independently of the pathogen engulfed.
It is noteworthy that differences in the outcome of the interaction were found depending on whether cell lines or primary macrophages were used. In general, primary macrophages demonstrated a higher capacity to control the fungal intracellular pathogenesis. Primary macrophages produced higher levels of proinflammatory cytokines, indicating that they were strongly activated in the presence of C. krusei cells. In particular, primary macrophages strongly induced the production of TNF-α, a well-known proinflammatory cytokine produced by macrophages and one that induces phagocytosis and antimicrobial responses in these cells. In contrast, the induction of anti-inflammatory cytokines, such as IL-10, was stronger in the macrophage-like cell line. The different cytokine production patterns observed between the two types of macrophages suggest that the different behaviors observed might be related to the activation state of the phagocytes. In addition, our findings highlight that macrophage-like cell lines might have partially lost their antimicrobial activity, which raises a note of warning in the interpretation of data exclusively obtained with these cell types. Our work also demonstrates that even when using the same type of macrophages, the population is not homogenous, and multiple phenomena are observed in the same experiment, which suggests that the interaction depends on individual traits, such as the activation state of each macrophage. In this context, it has been shown that the cell cycle and postmitotic events might influence the outcome of the interaction (13, 14), which highlights a feature that could partially explain the different behavior of each macrophage in the well. In addition, the specific production of cytokines by a certain macrophage could influence the behavior of the surrounding macrophages. In this sense, it has been shown that the intracellular parasitism of Cryptococcus neoformans can be modulated by the addition of different cytokines, and in particular, induction of Th1 and Th17 responses in the macrophage produces a reduction in the capacity of intracellular replication of this fungus (31).
In conclusion, our results demonstrate that C. krusei can evade killing by macrophages. This finding had not been previously described and highlights a new mechanism by which this pathogen can survive and disseminate in the host. We also describe other processes developed by the macrophage (such as macrophage fusion, chemotaxis, or yeast cell transfer between phagocytic cells) that might reflect mechanisms developed by the immune system to control the infection. We argue that the balance between these processes is important to determine the development of the infection, and for this reason, we believe that our results could have profound implications on the understanding of C. krusei pathogenesis.
Supplementary Material
ACKNOWLEDGMENTS
R.G.-R. is supported by a FPI fellowship (reference BES-2009-015913) from the Spanish Ministry of Science and Innovation. O.Z. is funded by grants SAF-2008-03761 and PCI2006-a7-0606 from the Spanish Ministry of Science and Innovation.
We thank David Martin from Leica Microsystems for technical assistance with the time-lapse microscopy and Enrique Viguera for providing the animals used in this study.
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
Published ahead of print on 21 March 2011.
REFERENCES
- 1. Alvarez M., Casadevall A. 2007. Cell-to-cell spread and massive vacuole formation after Cryptococcus neoformans infection of murine macrophages. BMC Immunol. 8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alvarez M., Casadevall A. 2006. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr. Biol. 16:2161–2165 [DOI] [PubMed] [Google Scholar]
- 3. Bliska J. B., Casadevall A. 2009. Intracellular pathogenic bacteria and fungi—a case of convergent evolution? Nat. Rev. Microbiol. 7:165–171 [DOI] [PubMed] [Google Scholar]
- 4. Casadevall A., Pirofski L. A. 2003. The damage-response framework of microbial pathogenesis. Nat. Rev. Microbiol. 1:17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Charlier C., et al. 2009. Evidence for a role of monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect. Immun. 77:120–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drevets D. A., et al. 2004. The Ly-6Chigh monocyte subpopulation transports Listeria monocytogenes into the brain during systemic infection of mice. J. Immunol. 172:4418–4424 [DOI] [PubMed] [Google Scholar]
- 7. Eissenberg L. G., Goldman W. E., Schlesinger P. H. 1993. Histoplasma capsulatum modulates the acidification of phagolysosomes. J. Exp. Med. 177:1605–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernandez-Arenas E., et al. 2009. Candida albicans actively modulates intracellular membrane trafficking in mouse macrophage phagosomes. Cell. Microbiol. 11:560–589 [DOI] [PubMed] [Google Scholar]
- 9.Reference deleted.
- 10. Horn D. L., et al. 2009. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin. Infect. Dis. 48:1695–1703 [DOI] [PubMed] [Google Scholar]
- 11. Kurtzman C. P., Fell J. W. (ed.). 1997. The yeasts: a taxonomical study. Elsevier, Amsterdam, Netherlands [Google Scholar]
- 12. Lewis R. E. 2009. Overview of the changing epidemiology of candidemia. Curr. Med. Res. Opin. 25:1732–1740 [DOI] [PubMed] [Google Scholar]
- 13. Luo Y., Alvarez M., Xia L., Casadevall A. 2008. The outcome of phagocytic cell division with infectious cargo depends on single phagosome formation. PLoS One 3:e3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luo Y., Tucker S. C., Casadevall A. 2005. Fc- and complement-receptor activation stimulates cell cycle progression of macrophage cells from G1 to S. J. Immunol. 174:7226–7233 [DOI] [PubMed] [Google Scholar]
- 15. Ma H., Croudace J. E., Lammas D. A., May R. C. 2007. Direct cell-to-cell spread of a pathogenic yeast. BMC Immunol. 8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ma H., Croudace J. E., Lammas D. A., May R. C. 2006. Expulsion of live pathogenic yeast by macrophages. Curr. Biol. 16:2156–2160 [DOI] [PubMed] [Google Scholar]
- 17. Marcil A., et al. 2008. Analysis of PRA1 and its relationship to Candida albicans-macrophage interactions. Infect. Immun. 76:4345–4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maxson M. E., Dadachova E., Casadevall A., Zaragoza O. 2007. Radial mass density, charge, and epitope distribution in the Cryptococcus neoformans capsule. Eukaryot. Cell 6:95–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Merz W. G., Karp J. E., Schron D., Saral R. 1986. Increased incidence of fungemia caused by Candida krusei. J. Clin. Microbiol. 24:581–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nguyen L., Pieters J. 2005. The Trojan horse: survival tactics of pathogenic mycobacteria in macrophages. Trends Cell Biol. 15:269–276 [DOI] [PubMed] [Google Scholar]
- 21. Peluso R., Haase A., Stowring L., Edwards M., Ventura P. 1985. A Trojan horse mechanism for the spread of visna virus in monocytes. Virology 147:231–236 [DOI] [PubMed] [Google Scholar]
- 22. Pfaller M. A., et al. 2008. Candida krusei, a multidrug-resistant opportunistic fungal pathogen: geographic and temporal trends from the ARTEMIS DISK Antifungal Surveillance Program, 2001 to 2005. J. Clin. Microbiol. 46:515–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raschke W. C., Baird S., Ralph P., Nakoinz I. 1978. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell 15:261–267 [DOI] [PubMed] [Google Scholar]
- 24. Russel D. G., Gordon S. 2009. Phagocyte-pathogen interaction: macrophages and the host response to infection, 1st ed. ASM Press, Washington, DC [Google Scholar]
- 25. Samaranayake Y. H., Samaranayake L. P. 1994. Candida krusei: biology, epidemiology, pathogenicity and clinical manifestations of an emerging pathogen. J. Med. Microbiol. 41:295–310 [DOI] [PubMed] [Google Scholar]
- 26. Samaranayake Y. H., Wu P. C., Samaranayake L. P., Ho P. L. 1998. The relative pathogenicity of Candida krusei and C. albicans in the rat oral mucosa. J. Med. Microbiol. 47:1047–1057 [DOI] [PubMed] [Google Scholar]
- 27. Samaranayake Y. H., Wu P. C., Samaranayake L. P., So M., Yuen K. Y. 1994. Adhesion and colonisation of Candida krusei on host surfaces. J. Med. Microbiol. 41:250–258 [DOI] [PubMed] [Google Scholar]
- 28. Shao X., et al. 2005. An innate immune system cell is a major determinant of species-related susceptibility differences to fungal pneumonia. J. Immunol. 175:3244–3251 [DOI] [PubMed] [Google Scholar]
- 29. Strasser J. E., et al. 1999. Regulation of the macrophage vacuolar ATPase and phagosome-lysosome fusion by Histoplasma capsulatum. J. Immunol. 162:6148–6154 [PubMed] [Google Scholar]
- 30. Tucker S. C., Casadevall A. 2002. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc. Natl. Acad. Sci. U. S. A. 99:3165–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Voelz K., Lammas D. A., May R. C. 2009. Cytokine signalling regulates the outcome of intracellular macrophage parasitism by Cryptococcus neoformans. Infect. Immun. 77:3450–3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zaragoza O., Alvarez M., Telzak A., Rivera J., Casadevall A. 2007. The relative susceptibility of mouse strains to pulmonary Cryptococcus neoformans infection is associated with pleiotropic differences in the immune response. Infect. Immun. 75:2729–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zaragoza O., et al. 2008. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell. Microbiol. 10:2043–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zaragoza O., Taborda C. P., Casadevall A. 2003. The efficacy of complement-mediated phagocytosis of Cryptococcus neoformans is dependent on the location of C3 in the polysaccharide capsule and involves both direct and indirect C3-mediated interactions. Eur. J. Immunol. 33:1957–1967 [DOI] [PubMed] [Google Scholar]
- 35. Zhao X. R., Villar C. C. 2011. Trafficking of Candida albicans through oral epithelial endocytic compartments. Med. Mycol. 49:212–217 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.