Abstract
The tetraspanins are a superfamily of transmembrane proteins with diverse functions and can form extended microdomains within the plasma membrane in conjunction with partner proteins, which probably includes receptors for bacterial adhesins. Neisseria meningitidis, the causative agent of meningococcal disease, attaches to host nasopharyngeal epithelial cells via type IV pili and opacity (Opa) proteins. We examined the role of tetraspanin function in Neisseria meningitidis adherence to epithelial cells. Tetraspanins CD9, CD63, and CD151 were expressed by HEC-1-B and DETROIT 562 cells. Coincubation of cells with antibodies against all three tetraspanin molecules used individually or in combination, with recombinant tetraspanin extracellular domains (EC2), or with small interfering RNAs (siRNAs) significantly reduced adherence of Neisseria meningitidis. In contrast, recombinant CD81, a different tetraspanin, had no effect on meningococcal adherence. Antitetraspanin antibodies reduced the adherence to epithelial cells of Neisseria meningitidis strain derivatives expressing Opa and pili significantly more than isogenic strains lacking these determinants. Adherence to epithelial cells of strains of Staphylococcus aureus, Neisseria lactamica, Escherichia coli, and Streptococcus pneumoniae was also reduced by pretreatment of cells with tetraspanin antibodies and recombinant proteins. These data suggest that tetraspanins are required for optimal function of epithelial adhesion platforms containing specific receptors for Neisseria meningitidis and potentially for multiple species of bacteria.
INTRODUCTION
The tetraspanins are a family of mammalian transmembrane proteins comprising 33 members. All share similar structural motifs with four transmembrane domains (TM1 to -4), a small extracellular loop (EC1), and a large extracellular loop (EC2) (18). The tetraspanins can homo- and heterodimerize while also associating with partner proteins, including CD46 (17) and members of the immunoglobulin superfamily (7), to form tetraspanin-enriched microdomains (TEMs) (18). These associations allow the tetraspanins to facilitate many functions through several signaling pathways, including the binding and processing of pathogens. Some viruses have been shown to utilize the tetraspanins for host cell entry, including HIV-1 with CD63 (39) and hepatitis C virus (HCV) with CD81 (25). In contrast, the contribution of tetraspanins to bacterial cell attachment and entry is not well defined.
Neisseria meningitidis, the cause of meningococcal disease, has well-described antigenic and phase-variable adhesins involved in attachment to the epithelial barrier (23). Type IV pili and the opacity proteins (Opa) require host cell membrane proteins for the facilitation of attachment and invasion of host cells. Pilus-mediated attachment may require CD46 on epithelial cells (14, 32). This initial adhesion process places the meningococcus in proximity to the host cell for a secondary stage of attachment with different adhesins such as the opacity proteins Opa and Opc. Neisseria meningitidis possesses three to four phase-variable opa genes (28, 30), which encode proteins with distinguishable characteristics due to variable regions within their three surface-exposed loops (19). Opa proteins are able to bind both the carcinoembryonic antigen-related cell adhesion molecule (CEACAM) receptors and heparan sulfate proteoglycans (HSPGs) found on human epithelial cells (6, 35). CEACAMs are members of the immunoglobulin superfamily consisting of 12 differentially expressed proteins, where CEACAM1 is the most widely distributed protein upon host cells.
A small number of studies have suggested a possible role for tetraspanins in bacterial attachment to cells. Uropathogenic Escherichia coli (UPEC) interacts with the uroplakins through the use of the FimH adhesin (37), while Listeria monocytogenes requires CD81 for entry into epithelial cells (31). A relationship with meningococcal adherence is possible, because tetraspanins associate with members of the immunoglobulin superfamily (16), while CD9 and CD151 associate indirectly with CD46, through integrins (17). Furthermore, intracellular CD63 is depleted after the action of meningococcal IgA protease within lysosomes (2).
Here, we investigate the role of the tetraspanins in meningococcal colonization of epithelial cells and further study their involvement with multiple bacterial species. We demonstrate that interference with tetraspanin function modifies meningococcal adherence to epithelial cells, likely via an effect on specific receptors, and demonstrate a wider involvement of tetraspanins in the adherence of multiple diverse bacterial species.
MATERIALS AND METHODS
Strains and bacterial growth conditions.
The N. meningitidis derivatives tested and their relevant characteristics are shown in Table 1. Strains of Neisseria lactamica (NL1009), Streptococcus pneumoniae (D39), Escherichia coli (ATCC 25922; kindly provided by Mark Thomas, Sheffield Medical School), and Staphylococcus aureus (NCTC 6571) were utilized in this study. Solid cultures were grown on Columbia horse blood or chocolate agar (Oxoid, Ltd., Cambridge, United Kingdom) aerobically at 37°C overnight in a humidified atmosphere. Liquid cultures were grown in Mueller-Hinton broth (MHB), brain heart infusion (BHI), or tryptone soya broth (TSB; Oxoid) microaerobically at 37°C in a humidified atmosphere with constant agitation. Freshly grown aerobic plates were used to inoculate all liquid cultures.
Table 1.
N. meningitidis strains and adhesin variants utilized in this study
Cell culture.
Maintenance of DETROIT 562 (ATCC CCL-138; American Type Culture Collection, Manassas, VA), a human pharynx carcinoma cell line, required Eagle's modified essential medium (EMEM; Lonza Group, Ltd., Basel, Switzerland); additional supplements of 2 mM glutamine, 1% nonessential amino acids, 1 mM pyruvate (Lonza), and 0.1% lactalbumin hydrolysate (LH; Sigma-Aldrich Company, Ltd., Gillingham, United Kingdom); and 10% (vol/vol) heat-inactivated fetal calf serum (HI-FCS). The human endometrial adenocarcinoma epithelial cell line HEC-1-B (ATCC HTB-113) was maintained in EMEM (Lonza) supplemented with 10% HI-FCS. Cell lines were grown at 37°C in a humidified atmosphere with 5% (vol/vol) CO2 and passaged with trypsin-EDTA when confluent.
Antibodies and recombinant GST fusion proteins.
Monoclonal antibodies or Fab fragments directed against CD9 (602.29 and 602.29 Fab) (1), CD63 (H5C6 and H5C6 Fab) (3), CD151 (14A2) (9), and the IgG isotype control (JC1) (24) were purified from hybridoma supernates generated in house with protein G Sepharose (Amersham-Pharmacia, United Kingdom). Anti-CD166 monoclonal antibodies (MAbs) (B-6; MCA1926F) and an anti-CEACAM MAb (D14HD11) were purchased from Santa Cruz Biotechnology, United States, AbD Serotec, United Kingdom, and Abcam, United Kingdom, respectively. The mouse antimeningococcal monoclonal antibody 2-1-P15 (02/310; National Institute for Biological Standards and Control [NIBSC]) was utilized for staining procedures. Recombinant glutathione S-transferase (GST) fusion proteins were assembled from CD9, CD63, and CD151 tetraspanin EC2 extracellular domains fused with GST (12).
Cell surface tetraspanin expression analysis.
Tetraspanin expression in epithelial cell lines was measured by flow cytometry. Adherent cells were grown to approximately 1 × 106, detached by trypsin-EDTA treatment, and transferred to tubes. Cells were fixed with 1% paraformaldehyde, centrifuged, and treated with relevant antibodies (602.29, 20 μg ml−1; H5C6, 20 μg ml−1; 14A2, 32.5 μg ml−1; and MCA1926F, 1 μg ml−1) followed by a goat anti-mouse fluorescein isothiocyanate (FITC)-conjugated antibody if required (Sigma Aldrich, United Kingdom), both at 4°C for 60 min. Labeled cells were analyzed with an LSR II cytometer (Becton Dickinson, Oxford, United Kingdom), and the results were analyzed with BD FACSDiva software (Becton Dickinson).
Effect of tetraspanin antibodies on bacterial adherence.
Inhibition of meningococcal adherence by antitetraspanin antibodies was demonstrated by using coverslips seeded with approximately 1.5 × 105 epithelial cells and blocked by immersion in 5% bovine serum albumin (BSA). Cells were washed with phosphate-buffered saline (PBS) and treated with antitetraspanin and control antibodies (602.29, 602.29 Fab, H5C6, H5C6 Fab, JC1, and B-6 at 20 μg ml−1 and 14A2 at 32.5 μg ml−1) for 30 min at 37°C. Combination treatment mixed anti-CD9, -CD63, and -CD151 antibodies (602.29 and H5C6 at 5.73 μg ml−1 and 14A2 at 8.6 μg ml−1). After washing to remove excess antibody, cells were incubated with bacteria for 60 min at a multiplicity of infection (MOI) of 300, except for N. meningitidis ¢13- and ¢2-infected cells at an MOI of 30 and S. aureus at an MOI of 1. Cells were washed and fixed with 2% paraformaldehyde.
Effect of GST-tetraspanin EC2s on bacterial adherence.
Using the adherence assay described above, recombinant GST fusion proteins were added to epithelial cells (CD9-EC2, CD63-EC2, and CD81-EC2 at 20 μg ml−1 and CD151-EC2 at 32.5 μg ml−1). Combination protein treatment mixed CD9, CD63, and CD151 recombinant proteins (at concentrations the same as those of the antibodies). A control treatment of free GST (20 μg ml−1) was used.
Effect of tetraspanin modulation by siRNA on bacterial attachment.
Small interfering RNA (siRNA) transfection was conducted following the manufacturer's instructions (Thermo Scientific Dharmacon). HEC-1-B cells seeded at 7.5 × 104 were incubated for 48 h with either medium alone or a variety of siRNAs (siGENOME nontargeting siRNA 1, human GAPDH [glyceraldehyde-3-phosphate dehydrogenase] control, CD9, CD63, and CD151 at 40 nM) (Thermo Scientific, United States). Transfection was performed with DharmaFECT 1 (Thermo Scientific). After incubation, transfection efficiency was tested by flow cytometry, and adherence assays were performed.
Immunofluorescence microscopy.
Fixed coverslips were washed and treated with (i) antimeningococcal antibody or antitetraspanin antibody followed by goat anti-mouse FITC-conjugated antibody to visualize all external meningococci or tetraspanins and (ii) stained with DAPI (4′,6-diamidino-2-phenylindole) to visualize DNA and nuclei. Antibodies were incubated at room temperature and diluted in PBS. Vectashield mounting medium with DAPI (Vector Labs, Burlingame, CA) was used to mount coverslips, allowing examination on a Leica DMRB fluorescent microscope. A total of 100 cells were counted, and the numbers of bacteria associated, either bound or internalized, were noted. Tetraspanins were visualized on a bright-field and fluorescence Leica DMRB upright microscope.
Statistical analysis.
All data were analyzed for normality by skewness using GraphPad Prism 5.01 (GraphPad Software, Inc., La Jolla, CA). Specific statistical considerations and the tests used are described separately for each subsection. All analyses used GraphPad Prism 5 for Windows version 5.01. Data are given as means ± standard deviations (SD). Significance was established at P ≤ 0.05.
RESULTS
Tetraspanins are variably expressed on epithelial cells.
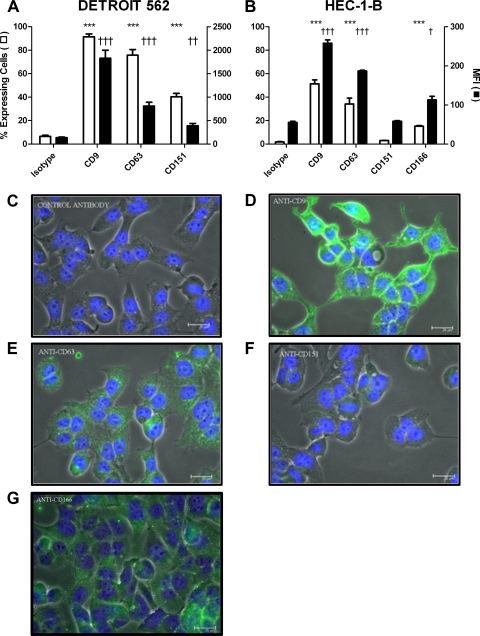
Expression of tetraspanins by epithelial cells was examined by flow cytometry and immunofluorescence (Fig. 1) and normalized against nonspecific isotype control antibody JC1. CD9 was richly expressed on both species of epithelial cell, while CD63 and CD151 were expressed but at much lower levels of intensity. As expected, the nontetraspanin epithelial cell molecule CD166 was expressed strongly. CD9 was most intense at intercellular junctions, while CD63 and CD151 exhibited punctate expression patterns.
Fig. 1.
Expression of tetraspanins by epithelial cells. (A) DETROIT 562 cells. n = 6. (B) HEC-1-B cells. n = 3. Data were collected by flow cytometry. Values are means ± SD. *, significance in the percentage of expressing cells compared to control cells; †, significance in the median fluorescence intensity (MFI) compared to control cells. ***/†††, P ≤ 0.001, and ††, P ≤ 0.01, by one-way analysis of variance (ANOVA) with Tukey's multiple-comparison test. (C to G) Visualization of protein distribution on HEC-1-B cells by fluorescence microscopy in JC1 (C), CD9 (D), CD63 (E), CD151 (F), and CD166 (G). Scale bar, 25 μm.
Adherence of N. meningitidis is reduced after treatment of epithelial cells with antitetraspanin antibodies and Fab fragments.
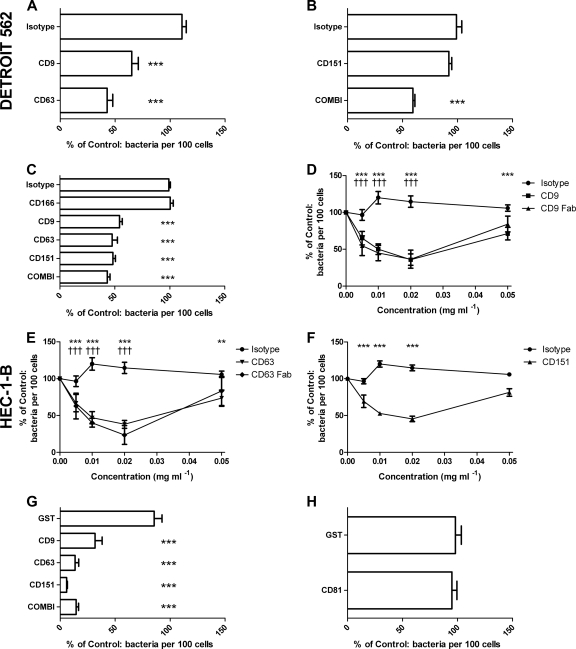
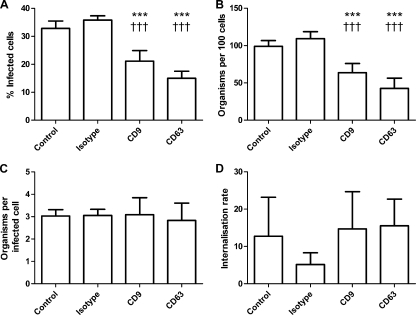
Treatment of epithelial cells with antitetraspanin MAbs significantly reduced meningococcal adherence. In DETROIT 562 cells, pretreatment with anti-CD9 (34.97% ± 14.56%) or anti-CD63 (57.28% ± 12.16%) antibodies significantly reduced bacterial adherence to epithelial cells (Fig. 2A). Anti-CD151 MAbs had no significant effect on meningococcal adherence (Fig. 2B). A combination of antitetraspanin MAb treatments also significantly reduced bacterial adherence (40.5% ± 3.96%) (Fig. 2B). Treatment of HEC-1-B cells with antitetraspanin MAbs also caused significant inhibition of bacterial adhesion (CD9, 45.74% ± 5.75%; CD63, 52.34% ± 11.39%; CD151, 51.84% ± 6.1%; a combination of all three antibodies, 56.99% ± 6.47%) (Fig. 2C). As expected, anti-CD166 MAbs had no significant effect on meningococcal adherence, supporting a specific role for the tetraspanin MAbs (Fig. 2C). Treatment with various concentrations of Fab fragments demonstrated a typical dose response with significant reductions in meningococcal adherence (Fig. 2D to F). Untransformed data revealed that single tetraspanin MAb treatments significantly reduced meningococcal adherence (Fig. 3). However, in all experiments with tetraspanin antibodies, a subset of cells were unaffected by tetraspanin treatment; 15 to 20% of treated cells were colonized by numbers of bacteria similar to those found on control cells (Fig. 3C), which may be the result of variation in tetraspanin expression during the cell cycle. Despite the reduction in adherence, there was no significant effect on bacterial internalization of bound bacteria (Fig. 3D).
Fig. 2.
Pretreatment of tetraspanins with antibodies, Fab fragments, or recombinant tetraspanin peptides reduces N. meningitidis adherence to epithelial cells. DETROIT 562 (A and B) or HEC-1-B (C to H) cells were immersed in medium alone, the isotype control (JC1), or anti-CD166 (C) or were treated with antitetraspanin treatments 602.29 and 602.29 Fab (anti-CD9), H5C6 and H5C6 Fab (anti-CD63), 14A2 (anti-CD151), or a combination (COMBI) containing all three antibodies for 30 min at 37°C (A to F). Cells were treated with single recombinant GST-EC2 fusion proteins, CD9, CD63, CD81, CD151, a combination containing all three proteins, or a GST control (G and H). Samples with treatment were calculated as a percentage of samples with medium alone, which was set at 100%. n ≥ 5. Values are means ± SD. *, significance from cells treated with medium alone; †, significance from anti-CD166 treatment; **, P ≤ 0.01, and ***/†††, P ≤ 0.001, by one-way ANOVA with Tukey's multiple-comparison test.
Fig. 3.
Untransformed data showing blockade of tetraspanins with antibodies reduces N. meningitidis adherence to DETROIT 562 epithelial cells. This figure reflects the transformed data demonstrated in Fig. 2A. The method was performed and the results were collected as described previously (Fig. 2). Graphs show adherence, by measurement of cells binding bacteria, total number of bacteria binding 100 cells, and the average number of bacteria bound to a positive cell (A to C) and internalization (D). n = 6. Values are means ± SD. *, statistical significance against infected control cells; †, statistical significance against isotype control-treated cells; ***, P ≤ 0.001, and †††, P ≤ 0.001, by one-way ANOVA with Tukey's multiple-comparison test.
Recombinant GST-EC2 tetraspanin fusion proteins inhibit meningococcal adherence to epithelial cells.
Treatment of HEC-1-B cells with recombinant GST-EC2 fusion proteins significantly reduced meningococcal adherence, particularly that of CD63 (86.57% ± 7.55%) and CD151 (94.16% ± 1.59%) (Fig. 2G). CD81 EC2 proteins demonstrated no significant reduction in meningococcal association (Fig. 2H). Combination treatment, consisting of CD9, CD63, and CD151 EC2 proteins at the same dose as single recombinant protein treatments, significantly reduced meningococcal adherence (85.54% ± 4.85%) (Fig. 2G). At the doses used, recombinant protein treatments reduced meningococcal adherence by approximately 4-fold more than antitetraspanin MAb (Fig. 2). We found no evidence of direct bacterial binding to recombinant tetraspanins using a solid-phase assay (see Fig. S1 in the supplemental material).
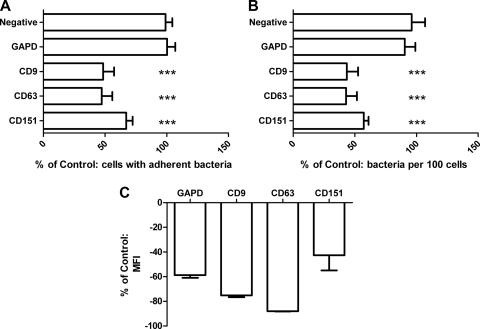
Tetraspanin modulation by siRNAs reduced meningococcal adherence to epithelial cells.
Reduced expression of tetraspanins following siRNA treatment decreased meningococcal adherence to epithelial cells (Fig. 4). siRNA treatments produced large reductions in GAPDH (−58.82% ± 2.05%) and the tetraspanins (CD9, −75.19% ± 1.50%; CD63; −87.99% ± 0.25%; CD151, −42.61% ± 12.38%) (Fig. 4C). Pretreatment of cells with a variety of siRNAs significantly reduced meningococcal adherence to epithelial cells; in contrast, reduction of the positive control (GAPDH) had no effect on meningococcal adherence (Fig. 4A and B).
Fig. 4.
Modulation of tetraspanins by siRNA reduces meningococcal adherence to HEC-1-B epithelial cells. HEC-1-B cells were incubated in either medium alone or a variety of siRNAs (nontargeting siRNAs, glyceraldehyde-3-phosphate dehydrogenase [GAPD], CD9, CD63, and CD151 at 40 nM) for 48 h. (A and B) Cells were infected for 60 min with MC58, and adherence was measured by fluorescence microscopy. Samples with treatment were calculated as a percentage of samples with medium alone, which was set at 100%. n = 6. Values are means ± SD. ***, P ≤ 0.001 by one-way ANOVA with Tukey's multiple-comparison test. (C) Relative expression of the respective proteins on treated cells was measured by flow cytometry. n = 3. Values are means ± SD.
Differential binding of Neisseria adhesin variants suggests CEACAM and CD46 require tetraspanins for meningococal adherence.
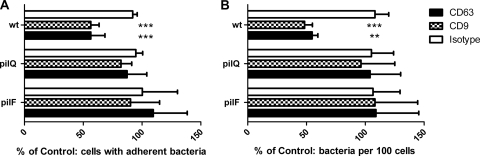
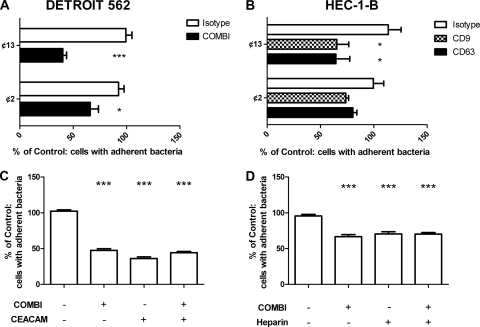
In contrast to wild-type bacteria, no significant reduction in adherence of pilQ and pilF mutants to tetraspanin MAb-treated HEC-1-B cells was observed (Fig. 5). Attachment of wild-type piliated bacteria to untreated HEC-1-B cells was approximately 6-fold greater than that of the Pil− mutants. A comparison of the adherence of acapsulate Opa+ and Opa− variants to HEC-1-B and DETROIT 562 cell lines is shown in Fig. 6. The percentages of HEC-1-B and DETROIT 562 epithelial cells associated with acapsulate bacteria after antitetraspanin MAb treatment were significantly reduced compared to that in the cells treated with medium alone; this was not observed with the Opa− variant on HEC-1-B cells (Fig. 6B). Tetraspanin treatment significantly reduced Opa− variant association with the DETROIT 562 cells, although this was reduced from that of the parent strain. In these experiments, the CD151 antibody was not tested.
Fig. 5.
Involvement of type IV pili in tetraspanin-mediated adherence to HEC-1-B epithelial cells. Strains that lack pilQ and pilF are unable to express type IV pili. Cells treated with no antibody, the isotype control, or a combination (COMBI) of antitetraspanins were infected with bacteria separately (MOI of 300). Adhesion was measured by microscopy. Samples with antibody were calculated as a percentage of the samples with medium alone, which was set at 100%. wt, wild type. (A) Change in the number of infected cells; (B) change in the number of organisms per 100 cells. n = 6. Values are means ± SD. **, P ≤ 0.01, and ***, P ≤ 0.001, by one-way ANOVA with Tukey's multiple-comparison test.
Fig. 6.
Opa receptor involvement in tetraspanin-mediated adherence to epithelial cells. ¢13 and ¢2, derivatives of the parent strain MC58, do not express capsule, and the latter also lacks Opa proteins. DETROIT 562 (A) and HEC-1-B (B) cells were treated with medium alone, the isotype control, and a combination (COMBI) of antitetraspanins or singular antitetraspanins. Cells were infected with the MC58 derivatives for 60 min (MOI of 30). DETROIT 562 (C) and HEC-1-B (D) cells were treated with medium alone, the isotype control, the antitetraspanin combination, anti-CEACAM, or a combination of the two. Cells were infected with MC58 for 60 min (MOI of 300). Adhesion was measured by fluorescent microscopy. Samples with antibody were calculated as a percentage of samples with medium alone, which was set at 100%. n = 6. Values are means ± SD. *, P ≤ 0.05, and ***, P ≤ 0.001, by one-way ANOVA with Tukey's multiple-comparison test.
CEACAM and HSPG blockade demonstrate analogous effects to tetraspanin blockade.
Blockade of CEACAM and HSPG demonstrated significant reductions in meningococcal association analogous to tetraspanin blockade (Fig. 6C and D). Pretreatment with a combination antitetraspanin MAb treatment (58.32% ± 5.64%) or an anti-CEACAM MAb treatment (64.28% ± 10.01%) significantly reduced meningococcal association with epithelial cells. Combination of these treatments also significantly reduced meningococcal association, but no additive effect was demonstrated (Fig. 6C). Pretreatment of HEC-1-B cells with heparin (50.49% ± 6.18%), antitetraspanin MAb, or a combination of the two demonstrated similar effects (Fig. 6D).
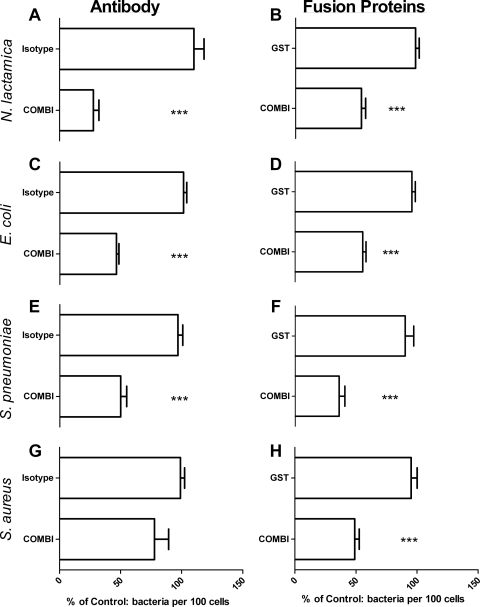
Tetraspanins influence epithelial cell adherence of multiple bacterial species.
Blockade of the tetraspanins with a combination of antitetraspanin MAbs significantly reduced the association of several species (Fig. 7), particularly N. lactamica (72.3% ± 10.29%) (Fig. 7A), S. pneumoniae (50.07% ± 11.83%) (Fig. 7E), and E. coli (53.52% ± 4.83%) (Fig. 7C). The effect on S. aureus adhesion was not significant (Fig. 7G). However, HEC-1-B cells treated with a combination of recombinant GST-EC2 fusion proteins demonstrated significantly reduced bacterial adherence in all strains tested compared to cells treated with medium alone (∼40 to 60%) (Fig. 7B, D, F, and H).
Fig. 7.
Blockade of tetraspanins affects many bacterial adhesion cascades. Cells were treated with antibody (A, C, E, and G) or with recombinant GST:EC2 tetraspanin protein (B, D, F, and H). Cells were infected with N. lactamica (A and B), E. coli (C and D), S. pneumoniae (E and F), or S. aureus (G and H). Coverslips were stained with DAPI, and the results were collected by fluorescence microscopy. Treated samples were calculated as a percentage of the samples with medium alone, which was set at 100%. n = 6. Values are means ± SD. ***, P ≤ 0.001 by one-way ANOVA with Tukey's multiple-comparison test.
DISCUSSION
We have demonstrated that tetraspanins mediate adherence of multiple bacterial species to human epithelial cells, likely due to facilitation of specific receptor-adhesin engagement. By coating plates with recombinant tetraspanin peptides, we found that tetraspanins were not acting as direct receptors for bacterial adherence (see Fig. S1 in the supplemental material), suggesting an indirect effect of tetraspanins on adhesin-receptor interactions. The epithelial cell lines exhibited highly variable cell surface expression levels of tetraspanin proteins (Fig. 1). These findings reflect current knowledge of tetraspanin distribution; CD9 is mostly found on the cell surface, while its recycling is minimal, whereas CD63 has a high rate of internalization, being predominantly associated with late endosomal compartments (26), with lower levels of the protein displayed on the cell surface. CD151 is similar to CD63 and has a high rate of internalization (38), with approximately 50% of the protein presented on the cell surface.
Previous reports have suggested that uropathogenic Escherichia coli (UPEC) and Listeria monocytogenes use members of the tetraspanin superfamily, the uroplakins and CD81, respectively, for adherence to cells (11, 31, 33, 40). In the present study, we have further analyzed several bacterial species to determine if they are affected by tetraspanin blockade. Both antitetraspanin MAbs and GST-EC2 fusion proteins caused a general reduction of bacterial adherence (Fig. 6). These data suggest that the tetraspanins have a general involvement in bacterial adherence, perhaps because of their property of association with partner proteins. We found that GST-EC2 fusion protein treatments were more potent than antibody treatment, suggesting that the GST-EC2 fusion proteins produce a more global, nonspecific disruption of tetraspanin function compared to the MAbs.
Antitetraspanin Fab fragments are able to reduce meningococcal adherence in an analogous manner to whole antibody (Fig. 2), demonstrating the effects described here are not due to the cross-linking of receptors. We further observe that interference of CD166, a strongly expressed epithelial cell marker, is unable to reduce meningococcal adherence refuting a possible effect by steric hindrance. Tetraspanin modulation by siRNA exhibited comparable reductions in meningococcal adherence, demonstrating further evidence that this phenomenon is tetraspanin specific. The mechanism of action of GST-EC2 fusion proteins is unclear, but it is likely the soluble EC2 domains intercalate with endogenous TEMs and alter TEM function, interfering with tetraspanin associations with other proteins resulting in disruption of the TEMs (4). In nature, it is likely that the tetraspanins form an “adhesion platform” containing the required receptors for meningococcal adherence, as has previously been suggested with CD9 and CD81 during HIV infection (10). Redundancy of the tetraspanins is typical within these microdomains as complex interactions within the TEM can allow proximal tetraspanins to interact with the inhibited tetraspanin “partner” proteins and mediate their functions. However, treatment of cells with a combination of MAbs did not result in an additive effect on meningococcal adherence, suggesting that either tetraspanin adhesion platforms do not demonstrate tetraspanin redundancy or further tetraspanin blockade during combination treatment is required.
The pharyngeal cell line DETROIT 562 and the endometrial cell line HEC-1-B both support high levels of bacterial adherence and are commonly used in bacterial infection studies. We have demonstrated that type IV pili and Opa variants are relatively less affected by tetraspanin blockade, suggesting that both type IV pilus and opacity protein receptors are associated with the tetraspanins. The putative pilus receptor, CD46, and the most common Opa receptor, CEACAM, are well characterized and are excellent candidates for tetraspanin partner proteins. Previous reports indicated that CD46 associates with CD9 as well as several integrins (17), and several members of the immunoglobulin superfamily have been reported to associate with the tetraspanins (5, 27), including heparin-binding epidermal growth factor (HB-EGF) as a receptor for diphtheria toxin (13) and tetraspanin interactions with B-CAM and EpCAM (15); however, there are currently no reports demonstrating CEACAM association with the tetraspanins. HEC-1-B cells do not express CEACAM (29), yet Opa variant adherence is less affected by tetraspanin blockade than that of wild-type bacteria. This piece of perplexing data would suggest a secondary Opa receptor is associated with the tetraspanins: perhaps the heparan sulfate proteoglycans (HSPGs) may be a component of the tetraspanin adhesion platform.
In conclusion, we have shown that blockade of tetraspanins CD9, CD63, and CD151, either with antibody or with recombinant peptides, inhibits adherence of Neisseria meningitidis. The effect of the tetraspanins was significantly reduced in isogenic strains of N. meningitidis lacking the adhesins Opa and pilin, suggesting that the tetraspanins are involved in optimal organization of receptors for specific meningococcal adhesins. Furthermore, we show that the tetraspanins are generally involved in the attachment of multiple species of bacteria to the surface of epithelial cells, demonstrating a wider role in bacterial adherence. Previous reports have also suggested tetraspanin involvement in pathogenesis, whether viral or bacterial (20, 40). However, our study suggests a larger involvement of the tetraspanins not just as receptors but as facilitators of adhesion platforms, allowing bacteria to rapidly associate with cells. These novel findings will prove useful in dissection of a multitude of microbial adhesion cascades and perhaps initiate clinical tetraspanin treatments to reduce colonization of the epithelial barrier, the first step in bacterial pathogenesis.
Supplementary Material
ACKNOWLEDGMENTS
L.G. was supported by the United Kingdom Health Protection Agency. We thank Christoph Tang, Mumtaz Virji, and Mark Thomas for the contribution of bacterial strains to this work.
We are grateful to the estate of the late Peter Milne for a bequest which made this work possible.
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
Published ahead of print on 4 April 2011.
REFERENCES
- 1. Andrews P. W., Knowles B. B., Goodfellow P. N. 1981. A human cell-surface antigen defined by a monoclonal antibody and controlled by a gene on chromosome 12. Somatic Cell Genet. 7:435–443 [DOI] [PubMed] [Google Scholar]
- 2. Ayala P., Lin L., Hopper S., Fukuda M., So M. 1998. Infection of epithelial cells by pathogenic neisseriae reduces the levels of multiple lysosomal constituents. Infect. Immun. 66:5001–5007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Azorsa D. O., Hyman J. A., Hildreth J. E. 1991. CD63/Pltgp40: a platelet activation antigen identical to the stage-specific, melanoma-associated antigen ME491. Blood 78:280–284 [PubMed] [Google Scholar]
- 4. Barreiro O., et al. 2008. Endothelial adhesion receptors are recruited to adherent leukocytes by inclusion in preformed tetraspanin nanoplatforms. J. Cell Biol. 183:527–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Charrin S., et al. 2003. EWI-2 is a new component of the tetraspanin web in hepatocytes and lymphoid cells. Biochem. J. 373:409–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Vries F. P., Cole R., Dankert J., Frosch M., van Putten J. P. 1998. Neisseria meningitidis producing the Opc adhesin binds epithelial cell proteoglycan receptors. Mol. Microbiol. 27:1203–1212 [DOI] [PubMed] [Google Scholar]
- 7. Ellerman D. A., Ha C., Primakoff P., Myles D. G., Dveksler G. S. 2003. Direct binding of the ligand PSG17 to CD9 requires a CD9 site essential for sperm-egg fusion. Mol. Biol. Cell 14:5098–5103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Exley R. M., et al. 2009. Identification of meningococcal genes necessary for colonization of human upper airway tissue. Infect. Immun. 77:45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fitter S., Tetaz T. J., Berndt M. C., Ashman L. K. 1995. Molecular cloning of cDNA encoding a novel platelet-endothelial cell tetra-span antigen, PETA-3. Blood 86:1348–1355 [PubMed] [Google Scholar]
- 10. Gordon-Alonso M., et al. 2006. Tetraspanins CD9 and CD81 modulate HIV-1-induced membrane fusion. J. Immunol. 177:5129–5137 [DOI] [PubMed] [Google Scholar]
- 11. Hassuna N., Monk P. N., Moseley G. W., Partridge L. J. 2009. Strategies for targeting tetraspanin proteins: potential therapeutic applications in microbial infections. BioDrugs 23:341–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higginbottom A., et al. 2003. Structural requirements for the inhibitory action of the CD9 large extracellular domain in sperm/oocyte binding and fusion. Biochem. Biophys. Res. Commun. 311:208–214 [DOI] [PubMed] [Google Scholar]
- 13. Iwamoto R., et al. 1994. Heparin-binding EGF-like growth factor, which acts as the diphtheria toxin receptor, forms a complex with membrane protein DRAP27/CD9, which up-regulates functional receptors and diphtheria toxin sensitivity. EMBO J. 13:2322–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kallstrom H., Liszewski M. K., Atkinson J. P., Jonsson A. B. 1997. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol. Microbiol. 25:639–647 [DOI] [PubMed] [Google Scholar]
- 15. Le Naour F., et al. 2006. Profiling of the tetraspanin web of human colon cancer cells. Mol. Cell. Proteomics 5:845–857 [DOI] [PubMed] [Google Scholar]
- 16. Levy S., Todd S. C., Maecker H. T. 1998. CD81 (TAPA-1): a molecule involved in signal transduction and cell adhesion in the immune system. Annu. Rev. Immunol. 16:89–109 [DOI] [PubMed] [Google Scholar]
- 17. Lozahic S., et al. 2000. CD46 (membrane cofactor protein) associates with multiple beta1 integrins and tetraspans. Eur. J. Immunol. 30:900–907 [DOI] [PubMed] [Google Scholar]
- 18. Maecker H. T., Todd S. C., Levy S. 1997. The tetraspanin superfamily: molecular facilitators. FASEB J. 11:428–442 [PubMed] [Google Scholar]
- 19. Malorny B., Morelli G., Kusecek B., Kolberg J., Achtman M. 1998. Sequence diversity, predicted two-dimensional protein structure, and epitope mapping of neisserial Opa proteins. J. Bacteriol. 180:1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martin F., et al. 2005. Tetraspanins in viral infections: a fundamental role in viral biology? J. Virol. 79:10839–10851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McGuinness B. T., et al. 1991. Point mutation in meningococcal por A gene associated with increased endemic disease. Lancet 337:514–517 [DOI] [PubMed] [Google Scholar]
- 22. McNeil G., Virji M. 1997. Phenotypic variants of meningococci and their potential in phagocytic interactions: the influence of opacity proteins, pili, PilC and surface sialic acids. Microb. Pathog. 22:295–304 [DOI] [PubMed] [Google Scholar]
- 23. Merz A. J., So M. 2000. Interactions of pathogenic neisseriae with epithelial cell membranes. Annu. Rev. Cell Dev. Biol. 16:423–457 [DOI] [PubMed] [Google Scholar]
- 24. Muranova T. A., et al. 2004. Crystallization of a carbamatase catalytic antibody Fab fragment and its complex with a transition-state analogue. Acta Crystallogr. D Biol. Crystallogr. 60:172–174 [DOI] [PubMed] [Google Scholar]
- 25. Pileri P., et al. 1998. Binding of hepatitis C virus to CD81. Science 282:938–941 [DOI] [PubMed] [Google Scholar]
- 26. Pols M. S., Klumperman J. 2009. Trafficking and function of the tetraspanin CD63. Exp. Cell Res. 315:1584–1592 [DOI] [PubMed] [Google Scholar]
- 27. Sala-Valdes M., et al. 2006. EWI-2 and EWI-F link the tetraspanin web to the actin cytoskeleton through their direct association with ezrin-radixin-moesin proteins. J. Biol. Chem. 281:19665–19675 [DOI] [PubMed] [Google Scholar]
- 28. Stern A., Brown M., Nickel P., Meyer T. F. 1986. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell 47:61–71 [DOI] [PubMed] [Google Scholar]
- 29. Swanson K. V., et al. 2001. CEACAM is not necessary for Neisseria gonorrhoeae to adhere to and invade female genital epithelial cells. Cell. Microbiol. 3:681–691 [DOI] [PubMed] [Google Scholar]
- 30. Tettelin H., et al. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809–1815 [DOI] [PubMed] [Google Scholar]
- 31. Tham T. N., et al. 2010. Tetraspanin CD81 is required for Listeria monocytogenes invasion. Infect. Immun. 78:204–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tobiason D. M., Seifert H. S. 2001. Inverse relationship between pilus-mediated gonococcal adherence and surface expression of the pilus receptor, CD46. Microbiology 147:2333–2340 [DOI] [PubMed] [Google Scholar]
- 33. van Spriel A. B., Figdor C. G. 2010. The role of tetraspanins in the pathogenesis of infectious diseases. Microbes Infect. 12:106–112 [DOI] [PubMed] [Google Scholar]
- 34. Virji M., et al. 1991. The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells. Mol. Microbiol. 5:1831–1841 [DOI] [PubMed] [Google Scholar]
- 35. Virji M., Makepeace K., Ferguson D. J., Watt S. M. 1996. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Mol. Microbiol. 22:941–950 [DOI] [PubMed] [Google Scholar]
- 36. Virji M., et al. 1995. Opc- and pilus-dependent interactions of meningococci with human endothelial cells: molecular mechanisms and modulation by surface polysaccharides. Mol. Microbiol. 18:741–754 [DOI] [PubMed] [Google Scholar]
- 37. Xie B., et al. 2006. Distinct glycan structures of uroplakins Ia and Ib: structural basis for the selective binding of FimH adhesin to uroplakin Ia. J. Biol. Chem. 281:14644–14653 [DOI] [PubMed] [Google Scholar]
- 38. Yang X., et al. 2002. Palmitoylation of tetraspanin proteins: modulation of CD151 lateral interactions, subcellular distribution, and integrin-dependent cell morphology. Mol. Biol. Cell 13:767–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yoshida T., et al. 2008. A CD63 mutant inhibits T-cell tropic human immunodeficiency virus type 1 entry by disrupting CXCR4 trafficking to the plasma membrane. Traffic 9:540–558 [DOI] [PubMed] [Google Scholar]
- 40. Zhou G., et al. 2001. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: evidence from in vitro FimH binding. J. Cell Sci. 114:4095–4103 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.