Abstract
Francisella tularensis, the causative agent of tularemia, survives and proliferates within macrophages of the infected host as part of its pathogenic strategy, through an intracellular life cycle that includes phagosomal escape and extensive proliferation within the macrophage cytosol. Various in vitro models of Francisella-macrophage interactions have been developed, using either opsonic or nonopsonic phagocytosis, and have generated discrepant results on the timing and extent of Francisella phagosomal escape. Here we have investigated whether either complement or antibody opsonization of the virulent prototypical type A strain Francisella tularensis subsp. tularensis Schu S4 affects its intracellular cycle within primary murine bone marrow-derived macrophages. Opsonization of Schu S4 with either human serum or purified IgG enhanced phagocytosis but restricted phagosomal escape and intracellular proliferation. Opsonization of Schu S4 with either fresh serum or purified antibodies redirected bacteria from the mannose receptor (MR) to the complement receptor CR3, the scavenger receptor A (SRA), and the Fcγ receptor (FcγR), respectively. CR3-mediated uptake delayed maturation of the early Francisella-containing phagosome (FCP) and restricted phagosomal escape, while FcγR-dependent phagocytosis was associated with superoxide production in the early FCP and restricted phagosomal escape and intracellular growth in an NADPH oxidase-dependent manner. Taken together, these results demonstrate that opsonophagocytic receptors alter the intracellular fate of Francisella by delivering bacteria through phagocytic pathways that restrict phagosomal escape and intracellular proliferation.
INTRODUCTION
Professional phagocytes such as macrophages express a variety of phagocytic receptors that recognize invading microorganisms either through pathogen-associated molecular patterns (PAMPs) or via opsonin deposition and initiate phagocytosis and destruction of the ingested particle for antigen presentation and subsequent immune responses (43). Depending on the receptors engaged, phagocytosis triggers various signaling cascades that lead to differential maturation of phagosomes, antigen presentation, and proinflammatory responses (43). In the case of opsonophagocytosis, Fcγ receptor (FcγR)-mediated uptake typically promotes efficient killing of microbes through the induction of an oxidative burst and is proinflammatory, while complement receptor-mediated phagocytosis is associated with neither an oxidative burst nor a proinflammatory response (43). In light of these functional differences, pathogenic microbes with intracellular survival capabilities may take advantage of less microbicidal phagocytic pathways in order to avoid destruction and promote their survival.
Francisella tularensis is a highly infectious Gram-negative bacterium that causes tularemia, a zoonotic disease that affects a variety of small mammals and is transmissible via arthropod vectors (17, 32). Human tularemia can be contracted through arthropod bites, direct contact with infected tissues, ingestion, or inhalation of aerosolized bacteria (31). In the latter case, the pneumonic form can lead to up to 25% mortality if untreated (31). Among the three subspecies of F. tularensis, Francisella tularensis subsp. tularensis (type A) and Francisella tularensis subsp. holarctica (type B) account for most cases of human tularemia, whereas Francisella tularensis subsp. mediasiatica is considered nonpathogenic. Additionally, the closely related species Francisella novicida is nonpathogenic to humans and yet remains highly virulent in rodents, making it a widely used surrogate model for tularemia. A major virulence attribute of F. tularensis is its ability to survive and proliferate within phagocytes of the infected host, of which macrophages are an important target (20). Using a variety of Francisella-phagocyte interaction models, several laboratories have identified the phagocytic receptors engaged during Francisella uptake. The mannose receptor (MR) (2, 40), the scavenger receptor A (SRA) (33), the complement receptor CR3 (CD11b/CD18) (2, 4, 9, 40), FcγRs (2, 33), and surface-exposed nucleolin (3) are involved in phagocytosis of either opsonized or unopsonized Francisella strains. Once ingested, Francisella resides within a phagosome, the Francisella-containing phagosome (FCP), that initially interacts with early and late compartments of the endocytic pathway (6, 8, 11, 37), prior to phagosomal escape and bacterial release into the cytosol (6, 8, 11, 18, 37, 44). Bacteria then undergo extensive replication within the macrophage cytosol that culminates in apoptotic and pyroptotic cell death and bacterial release (23, 24, 27, 39). While these stages of the Francisella intracellular cycle are commonly accepted, some discrepancies exist about the kinetics of phagosomal escape. Ultrastructural studies have revealed bacteria in intact phagosomes until 2 to 4 h postinfection (p.i.) (11, 18, 26, 28, 37, 41), arguing for a late phagosomal disruption process, and yet other studies based on immunofluorescence microscopy phagosomal integrity assays have reported completion of phagosomal escape within 1 h p.i. (6–8, 36, 38, 44). Although one could invoke technical differences in the models used and differential sensitivity of the methodologies employed in these studies to explain such discrepancies, a consistent difference between the studies reporting slow and those reporting rapid phagosomal escape was the use of opsonins for phagocytic uptake of Francisella. While most studies using unopsonized bacteria have reported rapid phagosomal disruption (6–8, 36, 38, 44), opsonization of Francisella with fresh serum can be associated with the targeting of bacteria to different phagocytic receptors (2, 4, 9, 33, 40) and with a belated phagosomal escape (11, 28, 41).
Because of this correlation between opsonization, the phagocytic receptors engaged, and slower kinetics of phagosomal escape, we postulated that the mode of Francisella uptake by macrophages influences its intracellular fate. Here we have examined this hypothesis by comparing the behavior of the highly virulent strain F. tularensis subsp. tularensis Schu S4 in primary murine macrophages under opsonic and nonopsonic conditions. We show that targeting bacteria to opsonophagocytic pathways is deleterious to its intracellular fate, since it restricts phagosomal escape and intracellular proliferation, either by altering phagosomal maturation or through the activation of specific microbicidal mechanisms. These findings argue for the host capacity to control Francisella proliferation in tissues where humoral responses are functional.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The prototypic type A virulent strain, F. tularensis subsp. tularensis Schu S4, was obtained from Rick Lyons (University of New Mexico, Albuquerque, NM). Green fluorescent protein (GFP)-expressing Schu S4 was described previously (8). F. tularensis subsp. tularensis Schu S4 was grown on modified Mueller-Hinton (mMH) plates for 3 days at 37°C under 7% CO2. Immediately prior to infection of murine bone marrow-derived macrophages (BMMs), a few colonies from a freshly streaked mMH plate were resuspended in mMH broth and optical density at 600 nm (OD600) was measured to estimate bacterial numbers. All manipulations of F. tularensis strain Schu S4 were performed in a biosafety level 3 facility according to standard operating procedures approved by the Rocky Mountain Laboratories Institutional Biosafety Committee.
Macrophage culture and infection.
Bone marrow cells were isolated from femurs of 6- to 10-week-old, female mice and differentiated into macrophages for 5 days at 37°C and 7% CO2, in 1-g/liter-glucose Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum (FBS; Invitrogen), 10% L929-conditioned medium, and 2 mM l-glutamine in non-tissue-culture-treated petri dishes. After 5 days, loosely adherent BMMs were washed with phosphate-buffered saline (PBS), harvested by incubation in chilled cation-free PBS supplemented with 1 g/liter d-glucose on ice for 10 min, resuspended in complete medium, and replated in 24-well cell culture-treated plates at a density of 1 × 105 macrophages/well. BMMs were further incubated at 37°C under a 7% CO2 atmosphere for 48 h, being replenished with complete medium 24 h before infection.
C57BL/6J mice, scavenger receptor A-knockout mice (SRA-KO; strain name B6.Cg-Msr1tm1Csk/J), and complement receptor CR3-knockout mice (CD11b-KO; strain name B6.129S4-Itgamtm1Myd/J) were obtained from The Jackson Laboratory (Bar Harbor, ME). FcRγ-knockout mice (FcRγ-KO; strain name B6.129P2-Fcer1gtm1Rav N12) were purchased from Taconic Farms, Inc. (Hudson, NY). Mannose receptor-knockout (MR-KO) mice (25) were a gift from Michel Nussenzweig (Rockefeller University, New York, NY) and kindly provided by Alan Harmsen (Montana State University, Bozeman, MT). BMMs from KO mice were analyzed for lack of receptor surface expression by flow cytometry, using rat anti-mouse CD11b, CD204, and CD206 antibodies (AbD Serotec, Raleigh, NC) and rat anti-mouse CD16/CD32 antibodies (eBioscience, San Diego, CA), to detect surface expression of CR3, SRA, MR, and FcγRIII/II, respectively.
For either complement or IgG opsonization, approximately 108 bacteria were incubated prior to infection in DMEM supplemented with 10% heat-inactivated FBS in the presence of either 10% human serum or 0.27 μg/ml mouse monoclonal anti-F. tularensis lipopolysaccharide (LPS) IgG antibody (US Biologicals, Swampscott, MA), respectively, for 30 min at 37°C, concentrations that did not induce agglutination of bacteria. Human serum was obtained from a healthy donor with no history of tularemia, in accordance with a protocol approved by the Institutional Review Board for Human Subjects, National Institute of Allergy and Infectious Diseases. Blood was drawn and incubated for 30 min at 37°C and then centrifuged for 8 min, 2,000 × g, at room temperature. The serum phase was harvested and snap-frozen and then stored at −80°C in single-use aliquots. C3 deposition through serum opsonization and IgG coating were verified by immunostaining opsonized bacteria with goat anti-human complement C3 antibody (Sigma-Aldrich, St. Louis, MO) followed by either Alexa Fluor 488-conjugated donkey anti-goat antibodies or Alexa Fluor 488-conjugated goat anti-mouse antibodies, respectively (data not shown). For infections, opsonized bacteria were directly diluted in complete medium and 0.5 ml was added to chilled BMMs at an appropriate multiplicity of infection (MOI). In phagocytosis experiments, BMMs were infected with both unopsonized and opsonized bacteria at an MOI of 5. In phagosomal escape and replication experiments, an MOI of 50 was used for unopsonized bacteria to normalize phagocytic levels for all conditions. For complement opsonization, 10% fresh human serum was also added to the infection medium. Bacteria were centrifuged onto macrophages at 400 × g for 10 min at 4°C, and infected BMMs were incubated for 20 min at 37°C under a 7% CO2 atmosphere including an initial, rapid warm-up in a 37°C water bath to synchronize bacterial uptake. Infected BMMs were then washed 5 times with DMEM to remove extracellular bacteria and incubated for 40 min in complete medium and then for an additional 60 min in complete medium containing 100 μg/ml gentamicin to kill extracellular bacteria. Thereafter, infected BMMs were incubated in gentamicin-free medium until processing.
Determination of bacterial CFU and intracellular growth.
BMMs (1 × 105/well) were infected as described above, washed 3 times with sterile PBS, and then lysed with 1 ml of sterile deionized water for 3 min at room temperature, followed by repeated pipetting to complete lysis. Serial dilutions of the lysates were rapidly plated onto mMH plates, and plates were incubated for 3 days at 37°C under 7% CO2 before enumeration of CFU. The number of viable intracellular bacteria per well was determined in triplicate for each condition, and at least 3 independent experiments were performed. Replication index was calculated as fold change in CFU between 1 and 12 h p.i.
Immunofluorescence microscopy.
Macrophages grown on 12-mm glass coverslips in 24-well plates were infected for the appropriate time, washed 3 times with PBS, fixed with 3% paraformaldehyde (pH 7.4) at 37°C for 20 min, washed 3 times with PBS, and then incubated for 10 min in 50 mM NH4Cl in PBS in order to quench free aldehyde groups. Samples were blocked and permeabilized in blocking buffer (10% horse serum, 0.1% saponin in PBS) for 30 min at room temperature. Cells were labeled by incubating inverted coverslips onto drops of primary antibodies diluted in blocking buffer for 45 min at room temperature. Primary antibodies used were mouse anti-F. tularensis LPS (US Biologicals, Swampscott, MA), goat polyclonal anti-EEA-1 (N-19; Santa Cruz Biotechnology, Santa Cruz, CA), and rat anti-mouse LAMP-1 (clone 1D4B, developed by J. T. August and obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA). Bound antibodies were detected by incubation with 1:500 dilutions in blocking buffer of Alexa Fluor 488–donkey anti-mouse, Alexa Fluor 586–donkey anti-goat, and Alexa Fluor 568–donkey anti-rat antibodies for 45 min at room temperature. Cells were washed twice with 0.1% saponin in PBS, once in PBS, and once in H2O and then mounted in Mowiol 4-88 mounting medium (Calbiochem, Gibbstown, NJ). For FCP acidification measurements, infected cells were incubated for 2 min at 37°C under a 7% CO2 atmosphere with 0.5 μM Lysotracker Red DND-99 (Molecular Probes); fixed with 3% paraformaldehyde (PFA), pH 7.4, at 37°C for 20 min; washed 3 times with PBS and once in H2O; and then mounted in Mowiol 4-88 mounting medium and analyzed immediately. Samples were observed on a Carl Zeiss Axio Imager epifluorescence microscope equipped with a Plan-Apochromat 63×/1.4 objective for quantitative analysis or on a Carl Zeiss LSM 710 confocal laser scanning microscope for image acquisition. Confocal images of 1,024 by 1,024 pixels were acquired and assembled using Adobe Photoshop CS.
Phagosomal integrity assay.
To quantify Francisella escape from its initial phagosome, phagosomal integrity assays were performed as described previously (8). Briefly, live infected BMMs were first permeabilized with digitonin to allow for cytosolic delivery of Alexa Fluor 488-conjugated anti-Francisella antibodies, which labeled bacteria located in the cytosol or within a compromised phagosome. Cells were then fixed, and all bacteria were labeled after saponin permeabilization using Alexa Fluor 568-conjugated anti-Francisella antibodies, yielding samples with differentially labeled cytosolic and vacuolar bacteria (6, 8). Samples were observed on a Carl Zeiss Axio Imager epifluorescence microscope equipped with a Plan-Apochromat 63×/1.4 objective for quantitative analysis.
Measurements of ROS.
Reactive oxygen species (ROS) production by infected BMMs was measured using a luminol-based chemiluminescence method, as described previously (12). BMMs were seeded at a density of 6 × 104 cells/well in 96-well plates and infected at an MOI of either 200 (unopsonized control) or 80 (serum or IgG opsonization). After 5 min of incubation in a 37°C water bath, the macrophage medium was replaced with 100 μl of Krebs-Ringer (KRG) buffer (120 mM NaCl, 5 mM KCl, 1.7 mM KH2PO4, 8.3 mM Na2HPO4, 10 mM glucose, 1 mM CaCl2, and 1.5 mM MgCl2, pH 7.3) containing 100 μM luminol (Sigma) and 12 U/ml horseradish peroxidase (HRP; Sigma), and chemiluminescence was immediately measured at 25°C over 60 min at 1-min intervals using an Infinite M1000 plate reader (Tecan US Inc., Durham, NC). As a positive control of ROS production, BMMs were treated with 1 μg/ml Escherichia coli Ultra Pure LPS K-12 (InvivoGen, San Diego, CA) for 24 h prior to measurements, and phorbol-12-myristate-13-acetate (PMA; Sigma) in KRG buffer was added at a final concentration of 1 μM immediately before measurement. Killed IgG-opsonized Schu S4 bacteria were prepared by incubating approximately 2 × 108 bacteria in 3% PFA in PBS for 15 min at room temperature. PFA-fixed bacteria were retrieved by centrifugation at 12,000 × g for 2 min and resuspended in DMEM supplemented with 10% heat-inactivated FBS prior to IgG opsonization. All assays were run in triplicate.
RESULTS
Opsonization alters Francisella phagosomal escape and cytosolic proliferation.
In order to compare the intracellular fates of unopsonized and opsonized virulent Francisella, BMMs were infected with either unopsonized, serum-opsonized, or IgG-opsonized F. tularensis subsp. tularensis strain Schu S4 at the same multiplicity of infection (MOI = 5) and phagocytosis was evaluated at 1 h postinfection (p.i.). In agreement with previous reports in other Francisella-phagocyte infection models (2, 33, 40), both serum and IgG opsonization increased phagocytosis of Schu S4 by at least 1 order of magnitude (Fig. 1 A). In agreement, immunofluorescence microscopy analysis of infected BMMs revealed larger numbers of intracellular bacteria under both serum and IgG opsonization conditions (Fig. 1D), indicating that both complement and antibody opsonization enhanced Francisella uptake by BMMs. Intracellular bacteria were mostly individual, with some in small clusters of 2 or 3 bacteria upon serum opsonization (Fig. 1D). To determine whether opsonization affects Francisella intracellular trafficking, we first examined phagosomal escape of opsonized bacteria over 4 h p.i., a time frame that precedes replication of cytosolic bacteria (4 to 16 h p.i.) (44) and excludes overestimation of the cytosolic bacterial population. Complement-opsonized Schu S4 showed a slower and incomplete phagosomal escape compared to control, unopsonized Schu S4, which reached the macrophage cytosol within 1 to 2 h p.i. (87% ± 4.8% of cytosolic bacteria at 2 h p.i.; Fig. 1B), since only 57% ± 6.2% of bacteria were detected in the cytosol by 4 h p.i. (Fig. 1B). Similarly, IgG opsonization of Schu S4 impaired phagosomal escape, with a maximum of 63% ± 14% of cytosolic bacteria at 4 h p.i. Hence, opsonization of Francisella negatively impacts phagosomal escape. To confirm these results, we examined which intracellular compartments opsonized bacteria inhabit. While most unopsonized or IgG-opsonized Schu S4 bacteria did not colocalize with the late endosomal marker LAMP-1 at 1 h p.i., consistent with being mostly cytosolic, the majority of complement-opsonized Schu S4 bacteria were located within LAMP-1-positive compartments, in agreement with impaired phagosomal escape and a vacuolar location (Fig. 1D). By 8 h p.i., replicating unopsonized Schu S4 bacteria were cytosolic, while opsonized bacteria were both cytosolic and vacuolar. A large fraction of serum-opsonized bacteria were located within endosomal vacuoles, and some cytosolic bacteria showed patterns of replication (Fig. 1D). Taken together, these results indicate that serum opsonization and, to a lesser extent, IgG opsonization restrict phagosomal escape and generate a heterogenous population of both vacuolar and cytosolic intracellular bacteria. To extend these findings, we investigated whether cytosolic proliferation of Francisella was affected by opsonization. To this end, we measured the replication index of unopsonized and opsonized Schu S4, as the fold change in recoverable intracellular bacteria between 1 and 12 h p.i., a time frame that encompasses the Schu S4 cytosolic replication phase in BMMs (44). To ensure that the increased levels of bacterial uptake upon opsonization did not affect these experiments, MOIs were adjusted to normalize entry levels. While unopsonized Schu S4 replicated 404- ± 152-fold, within this time frame, serum- and IgG-opsonized bacteria achieved only 28- ± 7.9- and 22- ± 5.1-fold replication, respectively (Fig. 1C), indicating that Francisella opsonization dramatically reduces its ability to proliferate within the macrophage cytosol.
Fig. 1.
Opsonization of Schu S4 affects its intracellular cycle. (A) Phagocytosis of unopsonized (control), serum-opsonized, and IgG-opsonized Schu S4 by C57BL/6 BMMs. BMMs were infected for 20 min with unopsonized bacteria or bacteria opsonized with either fresh human serum (serum) or a purified anti-Francisella LPS antibody (IgG) and washed extensively, and intracellular CFU were enumerated at 1 h p.i., as described in Materials and Methods. Values are means ± standard deviations of 10 independent experiments performed in triplicate. (B) Phagosomal escape of unopsonized and either serum- or IgG-opsonized Schu S4 in C57BL/6 BMMs. BMMs were infected with either unopsonized or serum-opsonized or IgG-opsonized bacteria as described in Materials and Methods, and phagosomal integrity assays were performed at 0.5, 1, 2, and 4 h p.i. Values are means ± standard deviations of three independent experiments. (C) Intracellular proliferation of unopsonized and opsonized Schu S4. Intracellular viable bacteria from BMMs infected with either unopsonized, serum-opsonized, or IgG-opsonized Schu S4 were enumerated at 1 and 12 h p.i., and replication indexes were calculated as fold changes in CFU between 12 and 1 h p.i. Values are means ± standard deviations of three independent experiments. (D) Representative confocal fluorescence micrographs of uptake (1 h p.i.) and replication (8 h p.i.) of either unopsonized (top panels) or serum-opsonized (middle panels) or IgG-opsonized (bottom panels) Schu S4 by C57BL/6 BMMs. Bacteria appear in green, and LAMP-1 appears in red. Insets indicate areas that are magnified and shown as single channels and overlays. For serum-opsonized Schu S4 at 8 h p.i., insets a and b represent vacuolar and cytosolic bacteria, respectively. Arrows indicate LAMP-1-positive, vacuolar bacteria. Bars, 10 and 2 (insets) μm. Asterisks denote statistically significant differences in either uptake (A) or replication (C) compared to C57BL/6 BMMs, according to a one-way analysis of variance followed by a Dunnett posttest (P < 0.05).
Opsonization directs Francisella to different phagocytic receptors.
Opsonin deposition on bacterial surfaces targets oncoming organisms to dedicated opsonic receptors on macrophages, thereby altering the phagocytic pathways engaged for bacterial uptake and the macrophage responses to bacterial ingestion (43). To investigate the possibility that the altered intracellular fate of opsonized Francisella is determined by the phagocytic pathways activated, we first identified which phagocytic receptors are engaged by unopsonized and opsonized Schu S4 by use of BMMs generated from a series of knockout mice for specific phagocytic receptors. Since uptake of various Francisella strains by phagocytes has reportedly involved the mannose receptor (MR), the scavenger receptor A (SRA), the complement receptor CR3, and Fcγ receptors depending on the opsonization conditions (2, 4, 9, 33, 40), we tested phagocytic uptake of unopsonized, serum-opsonized, and IgG-opsonized Schu S4 in C57BL/6, MR-KO, SRA-KO, CD11b-KO, and FcRγ-KO BMMs, the latter of which lack expression of FcγRI, FcγRIII, and FcεRI receptors due to the targeted deletion of the FcR common γ subunit (42). Phagocytosis of unopsonized Schu S4 was reduced by about 50% in both MR-KO and SRA-KO BMMs, compared to C57BL/6 BMMs, although statistical significance was achieved only for MR-KO BMMs (Fig. 2 A). In contrast, phagocytosis of serum-opsonized Schu S4 was significantly reduced by about 75% in SRA-KO and CD11b-KO BMMs (Fig. 2B), in agreement with previous findings (2, 9, 33, 40), but neither in MR-KO nor in FcγR-KO BMMs (Fig. 2B). This indicates that phagocytosis of serum-opsonized bacteria was driven mostly by complement deposition and not by potentially present serum antibodies. Phagocytosis of IgG-opsonized Schu S4 was essentially driven via FcγR, since it was reduced by more than 90% in FcRγ-KO BMMs and not affected in other receptor-deficient BMMs (Fig. 2C). Hence, unopsonized Francisella bacteria are phagocytosed in part via the MR (and perhaps marginally via the SRA), while phagocytosis of serum-opsonized bacteria engages both CR3 and the SRA and that of IgG-opsonized bacteria mostly involves FcγRs. Altogether, these results demonstrate that opsonization of Francisella redirects its phagocytic uptake through different receptors.
Fig. 2.
Opsonization targets Francisella to different phagocytic receptors. BMMs from either C57BL/6, MR-KO, SRA-KO, CD11b-KO, or FcRγ-KO mice were infected with either unopsonized (A), serum-opsonized (B), or IgG-opsonized (C) Schu S4, as described in Materials and Methods, and intracellular CFU were enumerated at 1 h p.i. Results are expressed as percentages of phagocytic uptake in control C57BL/6 BMMs and are the means ± standard deviations of three to six independent experiments. Asterisks denote statistically significant differences in phagocytic uptake compared to control BMMs, according to a one-way analysis of variance followed by a Dunnett posttest (P < 0.05).
Opsonization of Francisella alters FCP early trafficking events.
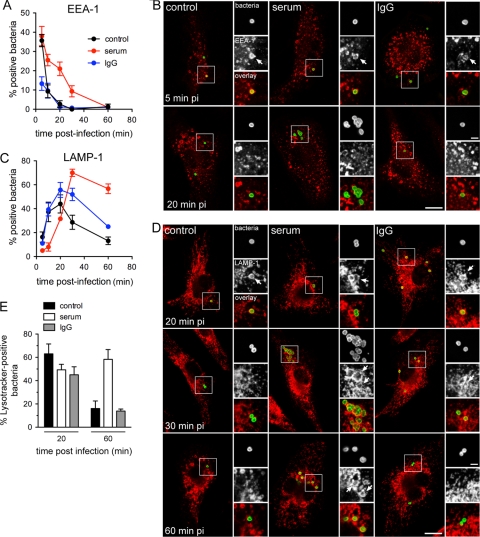
Since the efficiency of Francisella phagosomal escape depends upon trafficking events and maturation of the early FCP (8, 36), we sought to determine whether opsonization affects FCP maturation prior to phagosomal escape. For this purpose, we examined trafficking of early FCPs containing either unopsonized or serum- or IgG-opsonized Schu S4 along the endocytic compartment using immunofluorescence confocal microscopy. A rapid and transient labeling of FCPs containing either control, serum-opsonized, or IgG-opsonized Schu S4 with the early endosomal protein EEA-1 was detected (Fig. 3 A and B), peaking under all conditions at 5 min p.i., indicating that all FCPs transiently interacted with early endosomes following phagocytic uptake. These interactions were either less pronounced or more rapid for IgG-opsonized bacteria, since a maximum of 13% ± 6.1% of EEA-1-positive FCPs was detected at 5 min p.i. (Fig. 3A and B). Interestingly, FCPs containing serum-opsonized bacteria retained EEA-1 for a significantly longer period than did FCPs containing either control or IgG-opsonized bacteria, indicating a slower FCP maturation process upon serum opsonization (Fig. 3A and B). This was confirmed when examining subsequent LAMP-1 acquisition by FCPs, which was significantly delayed on FCPs containing serum-opsonized bacteria during the first 30 min p.i. compared to either control or IgG opsonization conditions (Fig. 3C and D). While FCPs containing either control or IgG-opsonized bacteria rapidly acquired LAMP-1 on their membranes and excluded it past 20 min p.i., consistent with phagosomal disruption (6, 8) (Fig. 3C and D), serum opsonization delayed LAMP-1 acquisition by FCPs, the majority of which subsequently retained this endosomal protein (Fig. 3C and D). To extend this data, we examined whether FCPs containing either unopsonized or opsonized Schu S4 become acidic, since FCPs are transiently acidified during early maturation (8, 36). Using Lysotracker Red DND-99, the majority of unopsonized bacteria were located within an acidified compartment at 20 min but not at 60 min p.i., consistent with phagosomal disruption (8) (Fig. 3E). Opsonization with either serum or IgGs did not significantly alter the percentage of Lysotracker-positive bacteria at 20 min p.i., indicating that all FCPs experienced some levels of acidification. By 60 min p.i., most IgG-opsonized bacteria were located in a nonacidic compartment, while the majority of serum-opsonized bacteria remained within an acidified compartment at 60 min p.i. (Fig. 3E), consistent with their colocalization with LAMP-1 (Fig. 3C). Taken together, these results indicate that, compared to either nonopsonic or IgG opsonization conditions, serum opsonization of Francisella promotes a slower maturation of the FCPs, which does not culminate in the loss of endosomal membranes normally associated with phagosomal disruption.
Fig. 3.
Serum opsonization of Schu S4 alters FCP early trafficking. BMMs were infected with either unopsonized (control), serum-opsonized (serum), or IgG-opsonized (IgG) Schu S4. At various times postinfection, samples were fixed and processed for immunolabeling of bacteria and either EEA-1 or LAMP-1. (A) Quantification of colocalization of intracellular Schu S4 with EEA-1. Data are means ± standard deviations of three independent experiments. (B) Representative confocal micrographs of BMMs that were infected with either unopsonized (control), serum-opsonized (serum), or IgG-opsonized (IgG) Schu S4 for either 5 or 20 min. Bacteria (appearing in green) are within EEA-1-positive FCPs (appearing in red) at 5 min p.i. Arrows in insets indicate bacteria within EEA-1-positive FCPs. Bars, 10 or 2 (inset) μm. (C) Quantification of colocalization of intracellular Schu S4 with LAMP-1. Data are means ± standard deviations of three independent experiments. (D) Representative confocal micrographs of BMMs that were infected with either unopsonized (control), serum-opsonized (serum), or IgG-opsonized (IgG) Schu S4 for either 20, 30, or 60 min. Arrows in insets indicate bacteria (appearing in green) within LAMP-1-positive FCPs (appearing in red). Bars, 10 or 2 (inset) μm. (E) Quantification of colocalization of intracellular Schu S4 with the acidic probe Lysotracker DND-99. BMMs were infected with either unopsonized (control), serum-opsonized (serum), or IgG-opsonized (IgG) GFP-expressing Schu S4; loaded with Lysotracker DND-99, as described in Materials and Methods; and analyzed at 20 and 60 min p.i. Values are means ± standard deviations of three independent experiments.
Because of the obvious differences in FCP maturation observed between unopsonized and opsonized bacteria, we examined in more detail how opsonization affects the kinetics of phagosomal escape of Francisella. We focused our analysis on the first 90 min p.i., which are sufficient to ensure complete escape into the cytosol of nonopsonic bacteria (8). While the percentage of cytosolic unopsonized bacteria progressively increased from 22% ± 14% at 20 min p.i. to 86% ± 5.6% at 90 min p.i. (Fig. 4 A), the fraction of cytosolic IgG-opsonized bacteria increased steadily over 60 min but did not exceed 63% ± 7.4% (Fig. 4C), consistent with some retained colocalization with LAMP-1 at 1 h p.i. (Fig. 3C) and incomplete phagosomal escape by 4 h p.i. (Fig. 1B). Phagosomal disruption by serum-opsonized bacteria was even more impaired since it did not occur until after 30 min p.i., indicating a significant delay in phagosomal disruption, and did not involve more than 53.5% + 9.0% of bacteria (Fig. 4B). Hence, while IgG opsonization restricts phagosomal escape to a fraction of intracellular bacteria, serum opsonization induces a delayed and incomplete phagosomal escape of Francisella that correlates with a slow maturation of the early FCP.
Fig. 4.
CR3 and FcγRs restrict phagosomal escape of opsonized Schu S4. (A) BMMs from C57BL/6, SRA-KO, and MR-KO mice were infected with unopsonized Schu S4 and processed for phagosomal integrity assays at 20, 30, 45, 60, and 90 min p.i. (B) BMMs from C57BL/6, CD11b-KO, and SRA-KO mice were infected with serum-opsonized Schu S4 and processed for phagosomal integrity assays at 20, 30, 45, 60, and 90 min p.i. (C) BMMs from C57BL/6, FcRγ-KO, and gp91Phox-KO mice were infected with IgG-opsonized Schu S4 and processed for phagosomal integrity assays at 20, 30, 45, 60, and 90 min p.i. At least 100 bacteria were analyzed per condition. Values are expressed as the percentages of cytosolic bacteria and are the means ± standard deviations of three to four independent experiments. Asterisks denote statistically significant differences in phagosomal escape compared to C57BL/6 BMMs, according to a one-way analysis of variance followed by a Dunnett posttest (P < 0.05). In panels B and C, asterisks colors indicate the receptor knockout that yields statistically significant differences from the C57BL/6J control BMMs.
Phagocytosis via the complement receptor CR3 and Fcγ receptors restricts phagosomal escape.
Having established which phagocytic receptors contribute to the uptake of unopsonized and opsonized Francisella (Fig. 2), and given how opsonization affects FCP maturation and the extent of phagosomal escape (Fig. 3 and 4), we examined the effect of specific receptor knockouts on the kinetics of phagosomal escape of opsonized and unopsonized bacteria to assess the permissivity of the various phagocytic pathways engaged. Phagosomal escape of unopsonized Schu S4 in either MR-KO or SRA-KO BMMs was similar to that observed in control C57BL/6 BMMs (Fig. 4A), indicating that uptake via these nonopsonic receptors does not influence the efficiency of phagosomal disruption. In contrast, the impaired phagosomal escape of serum-opsonized bacteria observed in C57BL/6 BMMs did not occur in CD11b-KO BMMs, since 59% ± 4.7% of serum-opsonized bacteria were cytosolic at 30 min p.i. in CD11b-KO BMMs, compared to 14.7% ± 6.7% in wild-type BMMs (Fig. 4B). This indicates that uptake via the CR3 is restrictive to phagosomal escape of Francisella. This restriction was specific for this receptor, since phagosomal escape of serum-opsonized bacteria in SRA-KO BMMs was not significantly different from that in C57BL/6 BMMs (Fig. 4B). Yet, phagosomal escape remained incomplete in both CD11b-KO and SRA-KO BMMs (Fig. 4B), indicating that serum opsonization also affects completion of phagosomal escape regardless of the phagocytic pathway engaged. Phagosomal escape of IgG-opsonized bacteria in FcRγ-KO BMMs proceeded more efficiently than that in C57BL/6 BMMs and reached levels comparable to those of unopsonized bacteria in wild-type BMMs by 60 and 90 min p.i. (Fig. 4A and C), indicating that FcγR-mediated uptake also restricts phagosomal escape. Altogether, these results demonstrate that both the complement receptor CR3 and Fcγ receptors are restrictive to Francisella phagosomal escape but seem to affect the efficiency and completion of this process differently.
Phagocytosis via Fcγ receptors restricts cytosolic proliferation of Francisella.
Since opsonic and nonopsonic receptors influence the extent of phagosomal escape of Francisella, which is a prerequisite to intracellular replication, we examined how restrictive and permissive receptors involved in Schu S4 uptake affect intracellular proliferation. In these experiments, MOIs were adjusted to ensure comparable uptake levels for all opsonization conditions and BMMs tested (data not shown). Compared to C57BL/6 BMMs, cytosolic proliferation of unopsonized bacteria was significantly decreased by about 50% in MR-KO BMMs but not in SRA-KO or the nonrelevant CD11b-KO BMMs (Fig. 5 A). This suggests that MR, which is involved in phagocytosis of unopsonized Schu S4, promotes intracellular proliferation of Francisella. The absence of either SRA, CR3, or MR did not restore intracellular proliferation of serum-opsonized Schu S4 (Fig. 5B), arguing that, although SRA and CR3 are involved in uptake of serum-opsonized Francisella, the specific phagocytic pathways that their engagement triggers do not play a role in the restricted intracellular proliferation observed for these bacteria. In contrast, knockout of Fcγ receptors increased intracellular proliferation of IgG-opsonized bacteria (Fig. 5C), while that of either SRA or CD11b did not (Fig. 5C), indicating that FcγR-mediated uptake restricts intracellular proliferation of Francisella.
Fig. 5.
Contribution of phagocytic receptors to intracellular proliferation of Schu S4. BMMs from either C57BL/6 (A to C), MR-KO (A and B), SRA-KO (A to C), CD11b-KO (A to C), or FcRγ-KO (C) mice were infected with either unopsonized (control) (A), serum-opsonized (serum) (B), or IgG-opsonized (IgG) (C) Schu S4, and intracellular CFU were enumerated at 1 and 12 h p.i. Replication index was calculated as fold change in intracellular CFU between 1 and 12 h p.i. Values are means ± standard deviations of three to four independent experiments. Asterisks denote statistically significant differences in replication compared to C57BL/6 BMMs, according to a one-way analysis of variance followed by a Dunnett posttest (P < 0.05).
NADPH oxidase activity restricts phagosomal escape and intracellular proliferation of IgG-opsonized Francisella.
A well-established macrophage response induced by FcγR-mediated phagocytosis is the oxidative burst, an enhanced production of reactive oxygen species within newly formed phagosomes by the recruitment and activation of the NADPH oxidase complex (43). To test whether the incomplete phagosomal escape and limited intracellular proliferation of IgG-opsonized Schu S4 resulted from enhanced NADPH activity on newly formed FCPs, we first examined whether phagocytosis of either unopsonized or opsonized Schu S4 triggers ROS production. Compared to untreated controls that showed background levels of superoxide production, BMMs from C57BL/6 and FcRγ-KO mice generated high levels of ROS in response to Escherichia coli LPS and PMA treatments (Fig. 6 A), indicating that these cells are capable of an oxidative burst. ROS production was dependent upon the NADPH oxidase, since it was absent in LPS/PMA-activated gp91Phox-KO BMMs (Fig. 6A). When fed PFA-killed, IgG-opsonized Schu S4 for 5 min, C57BL/6 BMMs produced detectable ROS levels, unlike FcRγ-KO BMMs and gp91Phox-KO BMMs (Fig. 6B), demonstrating that phagocytosis of killed, IgG-opsonized Francisella triggers a rapid, FcγR-mediated, NADPH oxidase-dependent oxidative burst. Infection of C57BL/6 BMMs for 5 min with live unopsonized or opsonized Schu S4 triggered ROS production above background levels only upon IgG opsonization (Fig. 6C), similar to killed bacteria, although in a more transient manner (compare Fig. 6B and C). ROS production upon phagocytosis of IgG-opsonized Schu S4 was abolished in FcRγ-KO BMMs and gp91Phox-KO BMMs (Fig. 6D and E), indicating that it is FcγR mediated and NADPH oxidase dependent. Taken together, these results demonstrate that FcγR-mediated phagocytosis of IgG-opsonized Francisella triggers a transient NADPH oxidase-dependent ROS production within the FCP.
Fig. 6.
FcγR-mediated uptake of IgG-opsonized Schu S4 restricts intracellular proliferation through NADPH oxidase-dependent ROS production. (A) ROS production by either C57BL/6, FcRγ-KO, or gp91Phox-KO BMMs that were left untreated or were pretreated with E. coli LPS for 24 h and stimulated with PMA (LPS+PMA). Cells were placed in luminol-containing KRG buffer, and luminescence (cps) was measured over a 60-min period. Data are means ± standard deviations from triplicate samples of a representative experiment out of three. (B) ROS production by either C57BL/6, FcRγ-KO, or gp91Phox-KO BMMs after 5 min of phagocytosis of PFA-killed IgG-opsonized Schu S4. Data are means ± standard deviations from triplicate samples of a representative experiment out of three. (C to E) ROS production by either C57BL/6 (C), FcRγ-KO (D), or gp91Phox-KO (E) BMMs after 5 min of infection with either unopsonized (control), serum-opsonized (serum), or IgG-opsonized (IgG) live Schu S4. Data are means ± standard deviations from triplicate samples of a representative experiment out of three. (F) BMMs from gp91Phox-KO mice were infected with either unopsonized (control), serum-opsonized (serum), or IgG-opsonized (IgG) Schu S4, and intracellular CFU were enumerated at 1 and 12 h p.i. Replication index was calculated as fold change in intracellular CFU between 1 and 12 h p.i. Values are means ± standard deviations of three independent experiments.
Since FcγR-mediated uptake restricts phagosomal escape (Fig. 4C), we examined this intracellular stage in gp91Phox-KO BMMs. Knockout of the gp91Phox subunit of the NADPH oxidase was sufficient to restore phagosomal escape of IgG-opsonized Schu S4 to levels observed in FcRγ-KO BMMs (Fig. 4C) that were comparable to those of unopsonized bacteria in C57BL/6 BMMs (Fig. 4A). This demonstrates that the incomplete phagosomal escape experienced by bacteria phagocytosed through FcγRs is due to NADPH oxidase activity. Furthermore, cytosolic proliferation of IgG-opsonized Schu S4 was restored in gp91Phox-KO BMMs (Fig. 6F) to levels observed in FcRγ-KO BMMs (Fig. 5C), arguing for an NADPH oxidase-dependent restriction of intracellular proliferation of IgG-opsonized Francisella. Importantly, the effect of NADPH oxidase inactivation on Schu S4 intracellular proliferation was specific to IgG opsonization, since cytosolic replication of serum-opsonized Schu S4 was not restored in gp91Phox-KO BMMs (Fig. 5B and 6F).
DISCUSSION
Several phagocytic receptors involved in the uptake of F. tularensis and F. novicida by macrophages and monocytes have been recently identified and include both opsonic and nonopsonic receptors depending on the infection model used (2, 11, 33, 40). Yet, whether engagement through these various receptors is either beneficial or detrimental to Francisella intracellular fate has been largely ignored, in particular in the context of opsonophagocytosis that may trigger enhanced microbicidal responses from the phagocytes (43). Based upon discrepancies in the intracellular behavior of Francisella in various phagocyte infection models (2, 8, 11, 18, 28, 33, 37, 40), here we have investigated whether targeting the virulent F. tularensis type A strain Schu S4 to different phagocytic pathways alters its intracellular fate. We show that engagement of opsonic receptors triggers phagocytic pathways that are less permissive to Francisella phagosomal escape and intracellular proliferation, since targeting of bacteria either to the complement receptor CR3 via serum opsonization or to FcγRs via IgG opsonization affects the speed and extent of phagosomal escape and limits intracellular replication in the cytosol.
Our findings of the restrictive role of CR3-mediated uptake in vacuolar escape and proliferation of Francisella are reminiscent of the demonstrated role of CR3 in killing Listeria monocytogenes (15, 16). Complement-opsonized Listeria bacteria are also defective for phagosomal escape (15, 16), arguing for a restrictive role of CR3 toward bacteria with a cytosolic lifestyle. Inversely, complement receptors do not seem to be deleterious to vacuolar pathogens, since complement-mediated uptake of Salmonella enterica serovar Typhimurium promotes intracellular replication in RAW 264.7 cells (14), and uptake of Mycobacterium tuberculosis via various complement receptors does not impair intramacrophage survival and growth (45). While complement receptors differentially affect the intracellular fate of bacterial pathogens, FcγR-mediated uptake consistently alters the trafficking of pathogens, regardless of their intracellular lifestyle. For example, FcγRs target Toxoplasma gondii (30), Streptococcus pneumoniae (19), Mycobacterium tuberculosis (1), and Legionella pneumophila (21) to the lysosomal compartment. IgG opsonization led to retention of ∼30% of Francisella bacteria in endosomal compartments and restriction of cytosolic replication. This illustrates that FcγR-mediated uptake can influence the intracellular fate of a parasite not only when it is enclosed within a vacuole but also when it is free in the cytosol.
Our findings that opsonization impairs phagosomal escape are consistent with previous reports of the differential kinetics of phagosomal escape observed between infection models using either unopsonized or serum-opsonized Francisella (6, 8, 11, 18, 26, 37, 38, 41) and likely account for the apparent discrepancies of the literature. An additional controversy in Francisella intracellular trafficking that may depend upon the mode of uptake is whether the early FCP becomes acidified or not. While studies using unopsonized F. tularensis and F. novicida have detected transient FCP acidification prior to phagosomal disruption (8, 36), others using serum-opsonized F. tularensis have not (10, 11), suggesting that differential uptake mechanisms may alter the maturation process of the FCP. Under our experimental conditions, we detected some acidification of FCPs containing either unopsonized or opsonized bacteria, but our qualitative measurements based on Lysotracker DND-99 fluorescence may not accurately reflect functionally significant variations in intraphagosomal pH. We could not quantify the intraphagosomal pH of FCPs using ratiometric imaging of pH-sensitive and pH-insensitive fluorescent probes (13), as these failed to accumulate in detectable amounts in FCPs within the short time frame of analysis. It therefore remains possible that FCPs generated following uptake of serum-opsonized Francisella do not acidify to levels observed with unopsonized bacteria, a hypothesis that is consistent with (i) the requirement of acidification for efficient phagosomal disruption (8, 36) and (ii) the impaired phagosomal escape upon CR3-mediated uptake (this study).
The intracellular fate of serum-opsonized Francisella remains to be clarified. FCPs containing serum-opsonized bacteria undergo a slower maturation process that correlates with delayed and incomplete phagosomal escape. While CR3-mediated uptake accounts for a delayed phagosomal escape, neither the inactivation of CR3 nor that of the SRA restores phagosomal escape or efficient intracellular proliferation. This suggests the occurrence of additional receptor-independent restrictive processes that need to be characterized. At the cellular level, serum opsonization generated a heterogenous population of both vacuolar and cytosolic bacteria that persist over time (Fig. 1D). The ratio of both populations slowly evolved in favor of cytosolic bacteria, the percentage of which increased from 51% ± 7.9% to 68% ± 6.4% between 1 and 8 h p.i. (Fig. 1B and data not shown), presumably due to some replication of cytosolic bacteria. Yet, whether vacuolar bacteria replicated or, inversely, were killed remains to be determined. Although persistent, the fate of this vacuolar population cannot quantitatively account for the overall limited proliferation of serum-opsonized bacteria, indicating that even the cytosolic population of serum-opsonized bacteria does not replicate as efficiently as unopsonized bacteria do. It is therefore possible that complement opsonization triggers yet-to-be-identified receptor-independent responses within the FCP and/or the cytosol that counteract proliferation of opsonized cytosolic Francisella.
Antibody opsonization of Schu S4 targeted bacteria almost exclusively to FcγRs and impaired phagosomal escape and intracellular proliferation, indicating that FcγR-mediated phagocytosis of Francisella restricts bacterial proliferation. We identified the effector of this restriction as the phagosomal NADPH oxidase and showed that production of reactive oxygen species (ROS) occurs within FCPs containing IgG-opsonized bacteria rapidly after uptake and compromises their ability to undergo complete escape and proliferate intracellularly. Comparatively, inactivation of the NADPH oxidase restored neither phagosomal escape of serum-opsonized Schu S4 (data not shown) nor their intracellular proliferation (Fig. 5D), consistent with the lack of an oxidative response upon CR3-mediated phagocytosis (43). Although apparently contradictory, our results are in agreement with the recent demonstration that virulent and attenuated F. tularensis subspecies inhibit NADPH oxidase assembly and activity in neutrophils (28, 29), since antibody opsonization with a subagglutinating concentration of immune serum still triggered a moderate respiratory burst before the onset of Francisella-mediated inhibition of NADPH oxidase (29). We therefore propose that a moderate FcγR-dependent oxidative burst occurs in BMMs upon phagocytosis of antibody-opsonized Francisella, a transient response that is, however, not sufficient for bacterial killing but harmful enough to affect phagosomal escape and bacterial replication. IgG-opsonized Francisella, although mostly cytosolic (70% ± 6.4% at 1 h p.i., Fig. 1B), displayed restricted intracellular replication compared to unopsonized bacteria, a phenomenon that we traced to ROS production in the early FCP. It nonetheless remains to be determined whether ROS production is the only FcγR-dependent process that restricts intracellular proliferation of Francisella. Indeed, FcγR-mediated signaling downstream of phagocytosis is sufficient for lysosomal targeting and intracellular growth restriction of L. pneumophila and the Mycobacterium bovis BCG strain (21), suggesting that FcγR-dependent signaling may affect the ability of Francisella to grow in the macrophage cytosol.
The mannose receptor, which is engaged by unopsonized subspecies of F. tularensis and by F. novicida in both murine and human phagocytes (this study and references 2 and 40), promotes efficient phagosomal escape and intracellular proliferation of Schu S4 in murine BMMs, arguing that MR-mediated phagocytosis is a permissive route for Francisella infection of macrophages. Similarly, MR-mediated uptake of Mycobacterium tuberculosis via its lipoarabinomannan promotes inhibition of phagosome-lysosome fusion (22), further arguing that the MR is a permissive receptor for intracellular pathogens. This is contrary to the findings of Rodriguez et al. showing that interleukin-4 (IL-4)-mediated control of F. tularensis intramacrophage growth is MR dependent and retains bacteria in an acidified compartment (34), suggesting that the MR is restrictive to Francisella phagosomal escape and intracellular growth. However, this study did not specifically examine phagosomal escape, nor did it rule out that IL-4-induced changes other than a potential enhanced MR-mediated uptake are responsible for restricted growth of Francisella. Additional receptors likely contribute to the uptake of unopsonized Francisella and trigger phagocytic pathways that may be less permissive for intracellular growth than is MR-mediated uptake, since replication of unopsonized bacteria in MR-KO BMMs was reduced (Fig. 5A) while phagosomal escape was not significantly affected (Fig. 4A). The SRA may also contribute to uptake of unopsonized bacteria, but this remains unclear, as its absence reproducibly, yet nonsignificantly, decreased uptake of unopsonized Schu S4 (Fig. 2A) and slowed down phagosomal escape (Fig. 4A). The SRA is also engaged upon serum opsonization of Schu S4 (this study), consistent with results obtained using the attenuated F. holarctica strain LVS (33), and yet does not play a significant role in restricting phagosomal escape and intracellular proliferation of complement-opsonized bacteria. It may, however, represent a more permissive route of entry for serum-opsonized bacteria than the CR3 receptor, since a rapid phagosomal escape of serum-opsonized Schu S4 was restored in CD11b-KO but not in SRA-KO BMMs. Nonetheless, SRA-mediated phagocytic pathways remain restrictive to intracellular proliferation of serum-opsonized bacteria, arguing again that receptor-independent processes limit replication of serum-opsonized Francisella.
Although some of the underlying mechanisms remain to be characterized, our results clearly illustrate how the mode of entry of a pathogen influences its intracellular outcome. Opsonization restricts phagosomal escape and proliferation of Francisella. This phenomenon may play a significant role in vivo in the context of tissues where humoral immunity-based mechanisms are in place to recognize invading bacteria. While Francisella resists serum complement-mediated killing (35), possibly due to the lack of deposition of the C5b-C9 membrane attack complex (5), deposition of C3 fragments targets bacteria to complement receptors on phagocytes (2, 5, 11, 40), which likely restricts the ability of Francisella to proliferate intracellularly. Similarly, opsonization by circulating antibodies in immune hosts may contribute to restricting the bacterium's intracellular proliferation. In addition to the apoptotic and pyroptotic cell death processes induced in Francisella-infected cells that control bacterial proliferation in tissues (27), our results highlight the likely existence of humoral immunity-based mechanisms that control F. tularensis intracellular proliferation during the infection process.
ACKNOWLEDGMENTS
We are grateful to Michel Nussenzweig and Alan Harmsen for providing mannose receptor-KO mice, to staff at the Rocky Mountain Lab Veterinary Branch for mouse breeding, to Kevin Braughton for human serum collection, to Rebecca Anderson for assistance with flow cytometry, to Joel Swanson for helpful suggestions, and to Audrey Chong and Leigh Knodler for critical reading of the manuscript.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print on 21 March 2011.
REFERENCES
- 1. Armstrong J. A., Hart P. D. 1975. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J. Exp. Med. 142:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balagopal A., et al. 2006. Characterization of the receptor-ligand pathways important for entry and survival of Francisella tularensis in human macrophages. Infect. Immun. 74:5114–5125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barel M., et al. 2008. A novel receptor-ligand pathway for entry of Francisella tularensis in monocyte-like THP-1 cells: interaction between surface nucleolin and bacterial elongation factor Tu. BMC Microbiol. 8:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ben Nasr A., et al. 2006. Critical role for serum opsonins and complement receptors CR3 (CD11b/CD18) and CR4 (CD11c/CD18) in phagocytosis of Francisella tularensis by human dendritic cells (DC): uptake of Francisella leads to activation of immature DC and intracellular survival of the bacteria. J. Leukoc. Biol. 80:774–786 [DOI] [PubMed] [Google Scholar]
- 5. Ben Nasr A., Klimpel G. R. 2008. Subversion of complement activation at the bacterial surface promotes serum resistance and opsonophagocytosis of Francisella tularensis. J. Leukoc. Biol. 84:77–85 [DOI] [PubMed] [Google Scholar]
- 6. Checroun C., Wehrly T. D., Fischer E. R., Hayes S. F., Celli J. 2006. Autophagy-mediated reentry of Francisella tularensis into the endocytic compartment after cytoplasmic replication. Proc. Natl. Acad. Sci. U. S. A. 103:14578–14583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Child R., Wehrly T. D., Rockx-Brouwer D., Dorward D. W., Celli J. 2010. Acid phosphatases do not contribute to the pathogenesis of type A Francisella tularensis. Infect. Immun. 78:59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chong A., et al. 2008. The early phagosomal stage of Francisella tularensis determines optimal phagosomal escape and Francisella pathogenicity island protein expression. Infect. Immun. 76:5488–5499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clemens D. L., Lee B. Y., Horwitz M. A. 2005. Francisella tularensis enters macrophages via a novel process involving pseudopod loops. Infect. Immun. 73:5892–5902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clemens D. L., Lee B. Y., Horwitz M. A. 2009. Francisella tularensis phagosomal escape does not require acidification of the phagosome. Infect. Immun. 77:1757–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clemens D. L., Lee B. Y., Horwitz M. A. 2004. Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infect. Immun. 72:3204–3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dahlgren C., Karlsson A. 1999. Respiratory burst in human neutrophils. J. Immunol. Methods 232:3–14 [DOI] [PubMed] [Google Scholar]
- 13. Drecktrah D., Knodler L. A., Howe D., Steele-Mortimer O. 2007. Salmonella trafficking is defined by continuous dynamic interactions with the endolysosomal system. Traffic (Copenhagen, Denmark) 8:212–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Drecktrah D., Knodler L. A., Ireland R., Steele-Mortimer O. 2006. The mechanism of Salmonella entry determines the vacuolar environment and intracellular gene expression. Traffic (Copenhagen, Denmark) 7:39–51 [DOI] [PubMed] [Google Scholar]
- 15. Drevets D. A., Canono B. P., Campbell P. A. 1992. Listericidal and nonlistericidal mouse macrophages differ in complement receptor type 3-mediated phagocytosis of L. monocytogenes and in preventing escape of the bacteria into the cytoplasm. J. Leukoc. Biol. 52:70–79 [DOI] [PubMed] [Google Scholar]
- 16. Drevets D. A., Leenen P. J., Campbell P. A. 1993. Complement receptor type 3 (CD11b/CD18) involvement is essential for killing of Listeria monocytogenes by mouse macrophages. J. Immunol. 151:5431–5439 [PubMed] [Google Scholar]
- 17. Farlow J., et al. 2005. Francisella tularensis in the United States. Emerg. Infect. Dis. 11:1835–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Golovliov I., Baranov V., Krocova Z., Kovarova H., Sjostedt A. 2003. An attenuated strain of the facultative intracellular bacterium Francisella tularensis can escape the phagosome of monocytic cells. Infect. Immun. 71:5940–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gordon S. B., Irving G. R., Lawson R. A., Lee M. E., Read R. C. 2000. Intracellular trafficking and killing of Streptococcus pneumoniae by human alveolar macrophages are influenced by opsonins. Infect. Immun. 68:2286–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hall J. D., et al. 2008. Infected-host-cell repertoire and cellular response in the lung following inhalation of Francisella tularensis Schu S4, LVS, or U112. Infect. Immun. 76:5843–5852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Joller N., et al. 2010. Antibodies protect against intracellular bacteria by Fc receptor-mediated lysosomal targeting. Proc. Natl. Acad. Sci. U. S. A. 107:20441–20446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kang P. B., et al. 2005. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J. Exp. Med. 202:987–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lai X. H., Golovliov I., Sjostedt A. 2001. Francisella tularensis induces cytopathogenicity and apoptosis in murine macrophages via a mechanism that requires intracellular bacterial multiplication. Infect. Immun. 69:4691–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lai X. H., Sjostedt A. 2003. Delineation of the molecular mechanisms of Francisella tularensis-induced apoptosis in murine macrophages. Infect. Immun. 71:4642–4646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee S. J., et al. 2002. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science 295:1898–1901 [DOI] [PubMed] [Google Scholar]
- 26. Lindgren H., Stenman L., Tarnvik A., Sjostedt A. 2005. The contribution of reactive nitrogen and oxygen species to the killing of Francisella tularensis LVS by murine macrophages. Microbes Infect. 7:467–475 [DOI] [PubMed] [Google Scholar]
- 27. Mariathasan S., Weiss D. S., Dixit V. M., Monack D. M. 2005. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J. Exp. Med. 202:1043–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McCaffrey R. L., Allen L. A. 2006. Francisella tularensis LVS evades killing by human neutrophils via inhibition of the respiratory burst and phagosome escape. J. Leukoc. Biol. 80:1224–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McCaffrey R. L., et al. 2010. Multiple mechanisms of NADPH oxidase inhibition by type A and type B Francisella tularensis. J. Leukoc. Biol. 88:791–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mordue D. G., Sibley L. D. 1997. Intracellular fate of vacuoles containing Toxoplasma gondii is determined at the time of formation and depends on the mechanism of entry. J. Immunol. 159:4452–4459 [PubMed] [Google Scholar]
- 31. Oyston P. C., Sjostedt A., Titball R. W. 2004. Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat. Rev. Microbiol. 2:967–978 [DOI] [PubMed] [Google Scholar]
- 32. Petersen J. M., Mead P. S., Schriefer M. E. 2009. Francisella tularensis: an arthropod-borne pathogen. Vet. Res. 40:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pierini L. M. 2006. Uptake of serum-opsonized Francisella tularensis by macrophages can be mediated by class A scavenger receptors. Cell. Microbiol. 8:1361–1370 [DOI] [PubMed] [Google Scholar]
- 34. Rodriguez A. R., et al. 2011. Mast cell/IL-4 control of Francisella tularensis replication and host cell death is associated with increased ATP production and phagosomal acidification. Mucosal Immunol. 4:217–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sandstrom G., Lofgren S., Tarnvik A. 1988. A capsule-deficient mutant of Francisella tularensis LVS exhibits enhanced sensitivity to killing by serum but diminished sensitivity to killing by polymorphonuclear leukocytes. Infect. Immun. 56:1194–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Santic M., Asare R., Skrobonja I., Jones S., Abu Kwaik Y. 2008. Acquisition of the vacuolar ATPase proton pump and phagosome acidification are essential for escape of Francisella tularensis into the macrophage cytosol. Infect. Immun. 76:2671–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Santic M., Molmeret M., Abu Kwaik Y. 2005. Modulation of biogenesis of the Francisella tularensis subsp. novicida-containing phagosome in quiescent human macrophages and its maturation into a phagolysosome upon activation by IFN-gamma. Cell. Microbiol. 7:957–967 [DOI] [PubMed] [Google Scholar]
- 38. Santic M., et al. 2007. A Francisella tularensis pathogenicity island protein essential for bacterial proliferation within the host cell cytosol. Cell. Microbiol. 9:2391–2403 [DOI] [PubMed] [Google Scholar]
- 39. Santic M., Pavokovic G., Jones S., Asare R., Kwaik Y. A. 2010. Regulation of apoptosis and anti-apoptosis signalling by Francisella tularensis. Microbes Infect. 12:126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schulert G. S., Allen L. A. 2006. Differential infection of mononuclear phagocytes by Francisella tularensis: role of the macrophage mannose receptor. J. Leukoc. Biol. 80:563–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schulert G. S., et al. 2009. Francisella tularensis genes required for inhibition of the neutrophil respiratory burst and intramacrophage growth identified by random transposon mutagenesis of strain LVS. Infect. Immun. 77:1324–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Takai T., Li M., Sylvestre D., Clynes R., Ravetch J. V. 1994. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell 76:519–529 [DOI] [PubMed] [Google Scholar]
- 43. Underhill D. M., Ozinsky A. 2002. Phagocytosis of microbes: complexity in action. Annu. Rev. Immunol. 20:825–852 [DOI] [PubMed] [Google Scholar]
- 44. Wehrly T. D., et al. 2009. Intracellular biology and virulence determinants of Francisella tularensis revealed by transcriptional profiling inside macrophages. Cell. Microbiol. 11:1128–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zimmerli S., Edwards S., Ernst J. D. 1996. Selective receptor blockade during phagocytosis does not alter the survival and growth of Mycobacterium tuberculosis in human macrophages. Am. J. Respir. Cell Mol. Biol. 15:760–770 [DOI] [PubMed] [Google Scholar]