Abstract
Human gingival epithelial cells (GEC) produce peptides, such as β-defensins and the cathelicidin LL-37, that are both antimicrobial and that modulate the innate immune response. In myeloid and airway epithelial cells, the active form of vitamin D3 [1,25(OH)2D3] increases the expression and antibacterial activity of LL-37. To examine the activity of vitamin D on the innate immune defense of the gingival epithelium, cultured epithelial cells were treated with either 10−8 M 1,25(OH)2D3 or ethanol for up to 24 h. A time-dependent induction of LL-37 mRNA up to 13-fold at 24 h in both standard monolayer and three-dimensional cultures was observed. Induction of the vitamin D receptor and the 1-α-hydroxylase genes was also observed. The hydroxylase was functional, as LL-37 induction was observed in response to stimulation by 25(OH)D3. Through microarray analysis of other innate immune genes, CD14 expression increased 4-fold, and triggering receptor expressed on myeloid cells-1 (TREM-1) was upregulated 16-fold after 24 h of treatment with 1,25(OH)2D3. TREM-1 is a pivotal amplifier of the innate immune response in macrophages, leading to increased production by inflammatory response genes. Activation of TREM-1 on the GEC led to an increase in interleukin-8 (IL-8) mRNA levels. Incubation of three-dimensional cultures with 1,25(OH)2D3 led to an increase in antibacterial activity against the periodontal pathogen Aggregatibacter actinomycetemcomitans when the bacteria were added to the apical surface. This study is the first to demonstrate the effect of vitamin D on antibacterial defense of oral epithelial cells, suggesting that vitamin D3 could be utilized to enhance the innate immune defense in the oral cavity.
INTRODUCTION
The initial defense against the microbial pathogens associated with periodontal disease includes the regulated expression of a number of host defense peptides, such as β-defensins and cathelicidins (reviewed in reference 14). The only human cathelicidin, LL-37, is a multifunctional peptide, with antimicrobial activity against both Gram-positive and Gram-negative bacteria, as well as some viruses (reviewed in reference 58). In addition, it exhibits chemotactic properties and plays a role in dendritic cell maturation, identifying it as an important mediator in the innate and adaptive immune systems (reviewed in reference 6). LL-37 gene expression can be induced by live bacteria (36) or by bacterial products such as lipopolysaccharide (LPS) (30, 39), and the active LL-37 peptide is processed from the inactive human CAP-18 (hCAP-18) precursor by proteolysis. Lack of LL-37 is associated with two human disorders, morbus Kostmann and Papillon-Lefèvre syndrome, in which there is severe periodontal disease associated with colonization by the periodontal pathogen Aggregatibacter actinomycetemcomitans (8, 11, 41). LL-37 gene expression can be induced by live bacteria (36) or by bacterial products such as LPS (30, 39), making the peptide part of the innate immune defense of the gingival epithelium.
Activation of an innate immune response such as this typically proceeds through binding to pattern recognition receptors such as Toll-like receptors (TLRs). In addition to these, receptors in the triggering receptor expressed on myeloid cells (TREM) family also regulate the innate immune response. Initially identified on myeloid cells, activation of TREM-1 and -2 regulate the innate immune response at a finer level (reviewed in references 21 and 28). The natural ligand of TREM-1 is still unknown, but activation by a specific cross-linking antibody can lead to proinflammatory cytokine secretion, and it can act synergistically with TLRs to modulate the inflammatory response (3, 4, 18, 40, 42). Partial inhibition of TREM-1 increases survival of mice in an experimental model of sepsis. However, a more complete inhibition increases mortality due to reduced neutrophil function (22).
LL-37 expression can also be induced by the active form of vitamin D3 [1,25(OH)2D3] (23, 35, 51, 56). In humans, active vitamin D is produced from circulating, inactive vitamin D [25(OH)D] by 1-α-hydroxylase (Cyp27B1) (2, 33). Cyp27B1 was originally found in the proximal tubules of the kidneys but since then has been identified in a variety of tissues. Epithelial, breast, prostate, and immune system cells (monocytes, macrophages, and dendritic cells) all produce the vitamin D-activating 1-α-hydroxylase (2, 33).
In the vitamin D3 pathway, active vitamin D3 binds the nuclear vitamin D receptor (VDR), which then acts either as a homodimer or heterodimer with members of the retinoid X receptor (RXR) family as a transcription factor for the many genes, including the LL-37 gene, that contain vitamin D response elements (VDRE) (2, 33, 52). When the genes are stimulated directly with 1,25(OH)2D3, increased expression is seen (52). In addition to its role in calcium homeostasis, vitamin D3 has been associated with varied regulatory effects on cell proliferation and differentiation, especially in the immune system (2, 33), and has an established antiproliferative role in breast cancer (13).
In this study, we investigated the effect of the active form of vitamin D3 [1,25(OH)2D3] on the innate immune response of cultured GEC with respect to expression of LL-37, innate immune mediators, and antibacterial activity against a periodontal pathogen, A. actinomycetemcomitans.
MATERIALS AND METHODS
Cell culture.
The human oral keratinocyte cell line OKF6/TERT (obtained with material transfer agreement from the laboratory of James Rhinewald, Harvard University) was grown in keratinocyte serum-free medium (KSFM) supplemented with l-glutamine and penicillin-streptomycin-fungizone (Sigma-Aldrich). Calcium chloride was added to 0.03 M, and bovine pituitary extract and epithelial growth factor supplied with the medium were added per the manufacturer's instructions. Three-dimensional (3D) Transwell cultures were grown as previously described (15, 16). Primary cultures of normal human gingival epithelial cells were grown as previously described (31, 55).
RNA isolation and reverse transcription-PCR.
Total RNA was isolated using the RNeasy minikit (Qiagen, Valencia, CA) and was reversed transcribed with SuperScript II reverse transcriptase with an oligo(dT) primer (Invitrogen, Carlsbad, CA). Control reactions without reverse transcriptase were carried out to demonstrate no nonspecific amplification.
Quantification of mRNA levels was carried out relative to the housekeeping gene encoding β2-microglobulin (β2M) using the MyiQ iCycler (Bio-Rad Laboratories, Hercules, CA) as previously described (56). The quantitative real-time (QRT) PCR primers were the following (forward and reverse, respectively): for TREM-1, 5′-TGGTCTTCTCTGTCCTGTTTG-3′ and 5′-ACTCCCTGCCTTTTACCTC-3′; LL-37, 5′-GTCACCAGAGGATTGTGACTTCAA-3′ and 5′-TTGAGGGTCACTGTCCCCATA3′; Cyp27B1, 5′-AACCCTGAACAACGTAGTCTGCGA-3′ and 5′-ATGGTCAACAGCGTGGACACAAA-3′; VDR, 5′-CTTCAGGCGAAGCATGAAGC-3′ and 5′-CCTTCATCATGCCGATGTCC-3′; pro-platelet basic protein (PPBP), 5′-TGCTGAACTCCGCTGCATGTGT-3′ and 5′-TCCCATCCTTCAGTGTGGCTATCA-3′; β2M, 5′-CTCCGTGGCCTTAGCTGTG-3′ and 5′-TTTGGAGTACGCTGGATAGCCT-3′. Quantitative real-time PCR analysis was performed in the MyiQ iCycler (Bio-Rad) with SYBR green core mix (Applied Biosystems) and equal amounts of cDNA. PCR conditions were 95°C for 14 min, 60 cycles at 95°C for 1 min and 60°C for 1 min, and then 55°C for 15 min. The results were quantified based on the 2−ΔΔCT value compared to that of the housekeeping gene.
PCR products were verified by subcloning using the pTOPO-TA vector (Invitrogen) and automated sequence analysis.
Array analysis.
Triplicate cultures of OKF6/TERT cells were seeded in 25-cm2 tissue culture flasks and, when near confluence, treated with either ethanol or 10−8 M 1,25(OH)2D3 for 24 h and lysed, and the RNA samples were extracted as described above. Pooled RNA samples were used for array analysis in the RT2 Profiler PCR array for human innate and adaptive immune responses (SABiosciences) per the manufacturer's instructions. The array analysis was carried out on the MyiQ iCycler (Bio-Rad).
Immunoblotting.
Whole-cell lysates were prepared in a radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 1 mM phenylmethylsulfonyl fluoride [PMSF]) plus protease inhibitors (Roche). The lysates were subjected to SDS-PAGE on a 10-to-20%-gradient Tricine Ready Gel (Bio-Rad), transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad), and blocked. Primary antibodies to LL-37 were anti-LL-37 (H-40) (1:350) (Santa Cruz Biotechnology) and AL37-7 mouse polyclonal serum (1:250) (a kind gift of Toshi Kawai, Forsyth Institute). Secondary antibodies were goat anti-rabbit IgG–horseradish peroxidase (HRP) or goat anti-mouse IgG–HRP (Pierce/Thermo Fisher), and visualization was done using chemiluminescence (Pierce/Thermo Fisher).
Immunofluorescence.
OKF6/TERT cells were seeded on sterile coverslips and grown for 1 to 2 days to confluence. The cells were treated with either ethanol or 10−8 M 1,25(OH)2D3 for 24 h and fixed in 1.0% paraformaldehyde in phosphate-buffered saline (PBS). After PBS washes, the cells were blocked for 10 min at 25°C in 10% goat serum (Invitrogen), washed in PBS, and incubated overnight at 4°C with anti-TREM-1 (FL-234) (1:200) (Santa Cruz Biotechnology) or with rabbit serum at the same concentration as an isotype control (Santa Cruz Biotechnology). After PBS washes, incubation with a secondary antibody (Alexa Fluor 488 goat anti-rabbit IgG; Invitrogen) for 1.5 h at room temperature, and further PBS washes, the coverslips were mounted on slides using Vectashield mounting medium. The cells were visualized using an Olympus D70 fluorescent microscope and software.
Bacterial culture.
A. actinomycetemcomitans (strain CU1000) was streaked from a frozen culture onto Trypticase soy agar with 0.6% yeast supplemented with 0.75% dextrose, 0.4% sodium bicarbonate, bacitracin (75 mg/liter), and vancomycin (5 mg/liter) and was incubated at 37°C in 10% CO2. A single colony was used to inoculate 30 ml of growth medium (Trypticase soy broth with 0.6% yeast supplemented with 0.75% dextrose, 0.4% sodium bicarbonate, bacitracin [75 mg/liter], and vancomycin [5 mg/liter]) in a T75 tissue culture flask and incubated at 37°C in 10% CO2. Nonaggregated bacteria were harvested by scraping them into 1 ml PBS, followed by separation into a suspension of single cells by vortexing the mixture and allowing the aggregates to settle.
Bacterial killing assay.
Three-dimensional cultures of OKF6/TERT cells were assayed for antibacterial activity against A. actinomycetemcomitans by direct application of 103 CFU to the apical surface as previously described (15). Viable colonies were quantified by washing the surface with PBS, plating the colonies on A. actinomycetemcomitans growth medium (AAGM)-agar plates, and incubating the plates overnight at 37°C.
RESULTS
LL-37 induction in gingival epithelial cells in response to 1,25(OH)2D3.
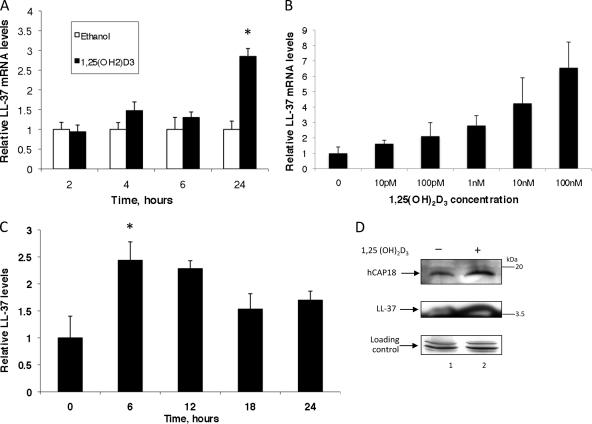
We previously demonstrated that treatment of airway epithelial cells with 1,25(OH)2D3 leads to an increase in LL-37 mRNA and protein levels, as well as antibacterial activity (9). To determine if gingival epithelial cells respond similarly, the oral keratinocyte cell line OKF6/TERT was grown as a monolayer and treated with either 1,25(OH)2D3 or vehicle control (ethanol) for increasing times. Quantitative RT-PCR (Fig. 1 A) demonstrates a time-dependent upregulation of hCAP-18/LL-37 mRNA, with an increase of 3-fold at 24 h, and a dose-dependent induction (Fig. 1B). To confirm that this expression is not confined to the immortalized cell line, we also observed a similar pattern of induction in a primary culture of gingival epithelial cells (Fig. 1C), albeit with different kinetics of induction, which may reflect the variability of the cultures.
Fig. 1.
Induction of LL-37 in gingival epithelial cells in response to 1,25(OH)2D3. OKF6/TERT (A and B) or primary gingival epithelial cells (C) were cultured in the presence of 10−8 M 1,25(OH)2D3 or ethanol (0.1%) for increasing times (A and C) or for 24 h in the presence of increasing concentrations of 1,25(OH)2D3 (B). Total mRNA was isolated, and LL-37 mRNA levels were quantified by QRT-PCR. Results are mRNA levels of 1,25(OH)2D3-stimulated cultures compared to those of ethanol-treated cultures at each time point, normalized to the level for β2M (n = 3; bars indicate mean ± standard deviation). (D) LL-37 protein levels were visualized by Western blot analysis of whole-cell lysates of OKF6/TERT cells cultured in an air-liquid interface (ALI) and stimulated with 1,25(OH)2D3 for 18 h. Tubulin was visualized as a loading control. The increase in LL-37 mRNA levels in panel A is significant at 24 h as quantified by t test (P < 0.001). The dose response in panel B is significant as measured by analysis of variance (ANOVA) (P < 0.0001).
To determine if this upregulation extended to the protein level, whole-cell extracts of OKF6/TERT cells, either mock or 1,25(OH)2D3 treated for 24 h, were prepared and immunoblotted (Fig. 1C). Western blot analysis demonstrates induction of both the precursor hCAP-18 and the mature LL-37 peptide (Fig. 1D).
Expression of the vitamin D pathway in GEC.
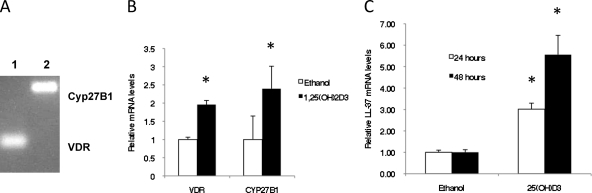
While the typical pathway for the generation of the active 1,25(OH)2D3 in the body requires two enzymes primarily found in the liver and kidney, there is some evidence of expression of these enzymes in other tissues. To examine whether the enzymes that could activate vitamin D are expressed in GEC, we performed RT-PCR on mRNA from OKF6/TERT cells treated with ethanol or 1,25(OH)2D3 using primers for Cyp27B1 (1-α-hydroxylase) and the vitamin D receptor (VDR). The results in Fig. 2 A indicate that the genes encoding these enzymes are expressed in this cell line. Incubation of OKF6/TERT cells with 1,25(OH)2D3 leads to an increase in mRNA levels of both VDR and Cyp27B1 (Fig. 2B). To determine whether the expression led to active 1-α-hydroxylase enzymes, we incubated the cells with inactive 25(OH)D3, as well as 1,25(OH)2D3, and quantified the levels of LL-37 mRNA. The results in Fig. 2C demonstrate that the inactive form of vitamin D can be activated in gingival epithelial cells and leads to an increase in antimicrobial peptide gene expression.
Fig. 2.
Expression of the vitamin D pathway in GEC. (A) Expression of vitamin D-related genes, i.e., those encoding VDR (lane 1) and Cyp27B1 (1-α-hydroxylase) (lane 2), in unstimulated cultures was observed by gel electrophoresis of RT-PCR products. (B) Cultures of OKF6/TERT cells were incubated for 2 h with 10−8 M 1,25(OH)2D3 or ethanol. Total mRNA was isolated, and relative levels of VDR and Cyp27B1 were quantified by QRT-PCR as described in the text. Results are means ± standard deviations for triplicate cultures, compared to ethanol-treated cultures, normalized to the result with β2M. The increase in VDR mRNA levels is significant (*, P = 0.0001). The increase in Cyp27B1 mRNA levels is significant (**, P = 0.03). (C) Functionality of the hydroxylase is shown by incubation of OKF6/TERT cells with either ethanol or 10−8 M 25(OH)D3 for 24 h and quantification of LL-37 mRNA levels as described above. The increases in LL-37 mRNA levels with 25(OH)D3 are significant at 24 h (*, P = 0.0003) and 48 h (**, P = 0.00005) as measured by Student's t test.
Induction of innate immune genes in GEC.
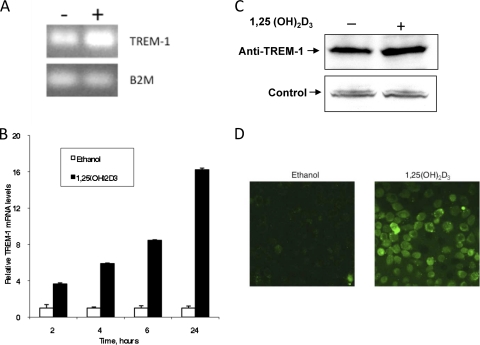
To determine whether other innate immune genes are regulated by vitamin D, we stimulated OKF6/TERT cells with 10−8 M 1,25(OH)2D3 or ethanol for 24 h. Total mRNA was isolated, transcribed into cDNA, and subjected to PCR microarray analysis using RT2 Profiler human innate and adaptive immune response PCR array (SABiosciences). The results shown in Table 1 identify four immunity-related genes stimulated by 1,25(OH)2D3, in addition to the LL-37 gene. The gene exhibiting the highest induction in these cells was the TREM-1 gene. To confirm and quantify this effect, we stimulated OKF6/TERT cells with 1,25(OH)2D3 and quantified the expression of TREM-1 by QRT-PCR. The results shown in Fig. 3 A demonstrate that TREM-1 is expressed at low levels in control cultures and induced by 1,25(OH)2D3. Quantitative RT-PCR shows that TREM-1 is induced by 1,25(OH)2D3 in a time-dependent manner, with a maximum induction at 24 h (Fig. 3B). Western blot analysis of whole-cell lysates of 3D cultures stimulated with 1,25(OH)2D3 on the basolateral surface confirms that protein levels rise in response to 1,25(OH)2D3 as well (Fig. 3C). Vitamin D treatment increases the surface expression of TREM-1, as observed by immunofluorescence analysis of OKF6/TERT cells stimulated with 1,25(OH)2D3 for 24 h (Fig. 3D).
Table 1.
Genes induced by 1,25(OH)2D3 in OKF6/TERT cells
Inhibitory IL-1 decoy receptor.
Pro-platelet basic protein.
Fig. 3.
Induction of TREM-1 in GEC. OKF6/TERT cells were grown in 3D cultures for increasing times in the presence of either ethanol or 10−8 M 1,25(OH)2D3. (A and B) Total mRNA was isolated, and TREM-1 mRNA levels were observed by gel electrophoresis of RT-PCR products of 24-h-stimulated cultures (A) and quantified by QRT-PCR of triplicate cultures at each time point as described in the text (B). (C) Western blot analysis of whole-cell lysates using anti-TREM-1 antibody. (D) Immunofluorescence of nonpermeabilized OKF6/TERT cultures grown on coverslips and treated with anti-TREM-1 antibody, visualized with an Alexa Fluor-coupled secondary antibody. Magnification, ×400. Time-dependent induction of TREM-1 was significant as measured by ANOVA (P < 0.0001).
A lower level of induction was also observed for three other genes in the panel, those coding for CD14, interleukin-1 decoy receptor (IL-1R2), and pro-platelet basic protein (PPBP, also known as CXCL7). Quantitative PCR analysis of these genes determined that IL-1R2 was expressed at very low levels. A band was observed for PPBP, but no significant induction was demonstrated by quantitative PCR (data not shown). A maximal 4.4-fold induction of CD14 mRNA levels was observed at 24 h after treatment with 1,25(OH)2D3 (P = 0.002).
Effect of vitamin D treatment on innate immunity and antibacterial activity of gingival cells.
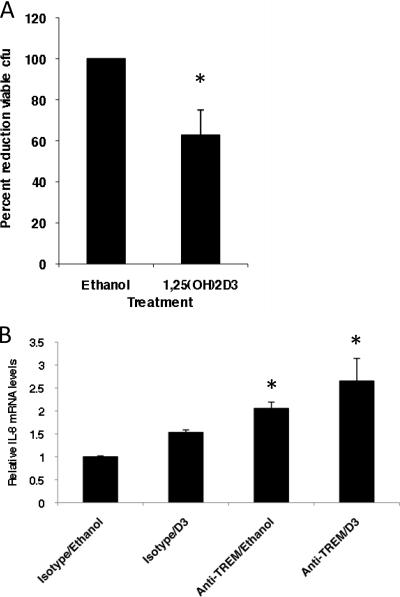
To examine the effect of vitamin D treatment on the host defense function of gingival cells, we quantified the effect of vitamin D-mediated induction in GEC on antibacterial activity using a 3D air-liquid interface culture system (15). OKF6/TERT cells were grown on a collagen layer which included 3T3 cells as a feeder layer and were treated with ethanol or 1,25(OH)2D3 added to the basolateral medium for 24 h. Both LL-37 and TREM-1 were induced in this type of culture by 1,25(OH)2D3, similar to the results with submerged cultures (data not shown). This culture system allows us to better quantify the effects on the production of antibacterial activity by adding bacteria to the apical surface of the cultures and measuring their viability after stimulation. Cultures stimulated with 1,25(OH)2D3 for 24 h exhibit a significant increase in antibacterial activity on the apical surface as measured by the ability to kill added cultures of A. actinomycetemcomitans (Fig. 4 A). Since it was observed that bacterial stimulation acted synergistically with the activation of TREM-1 to induce an innate immune response in macrophages (29), we stimulated the 3D cultures with 1,25(OH)2D3 for 24 h to induce TREM-1, followed by treatment with A. actinomycetemcomitans and either anti-TREM-1 or an isotype antibody. The anti-TREM antibody cross-links the receptor to act as a ligand and results in the stimulation of an NF-κB-regulated innate immune response (40). We measured the induction in IL-8 mRNA levels to quantify this effect. The results in Fig. 4B demonstrate that activation of the innate immune response by bacterial stimulation, in concert with vitamin D stimulation, leads to a functional activation of TREM-1. We did not observe any induction of IL-8 with cultures not treated with bacteria (data not shown).
Fig. 4.
Antibacterial host defense response of gingival epithelial cells. Three-dimensional cultures of OKF6/TERT cells were grown as described in the text. (A) Antibacterial activity was measured on the surface of OKF6/TERT cells cultured in ALI, stimulated for 24 h with either ethanol or 10−8 M 1,25(OH)2D3 (D3). Bacteria were incubated on the surface of the 3D cultures for 3 h and plated to quantify viable colonies. Results are shown as the mean reduction in viable colonies compared to that of the control result (± standard deviation) for triplicate cultures. The reduction in colonies is significant by a paired t test (n = 4 independent experiments of triplicate cultures; P = 0.04). (B) Cultures were treated with either ethanol or 10−8 M 1,25(OH)2D3 for 24 h, followed by the addition of 500 CFU live bacteria to the surface for 1 h. Anti-TREM-1 antibody or an isotype control was added to the surface for a further 3 h, and total mRNA was isolated as described above. Levels of IL-8 mRNA were quantified by QRT-PCR and are shown relative to that of the isotype/ethanol control, normalized to β2-microglobulin. Induction of IL-8 mRNA is significant in anti-TREM-1/ethanol-treated cultures, compared with that of the control (*, P = 0.001), and in anti-TREM-1/vitamin D-treated cultures (**, P = 0.02) by t test.
DISCUSSION
Antimicrobial defense of the gingival epithelium involves the recognition of microbes by cell surface receptors such as TLRs, which leads to the induction of host defense genes, such as those encoding defensins, cathelicidins, and cytokines. The expression of these response genes can be regulated by a variety of factors, including bacterial products and proinflammatory cytokines. However, a therapeutic induction to bolster the natural antibacterial defense of the gingival epithelium would preferably be carried out by mediators such as vitamin D, which have few detrimental effects. Thus, our observation that the active form of vitamin D, 1,25(OH)2D3, can induce the expression of not only an antimicrobial peptide such as LL-37 but also the innate immune regulator TREM-1 suggests that this pathway can be useful for the treatment or prevention of infectious diseases in the oral cavity. Furthermore, the endogenous expression of 1-α-hydroxylase in the gingival epithelial cells means that systemic vitamin D treatment, which would increase serum levels of 25(OH)D3, may be sufficient to activate the innate immune response in these cells.
In the oral cavity, LL-37 was initially identified in infiltrating neutrophils (9) but was subsequently observed in salivary glands as well, in both human (53) and murine (38) oral tissues. Furthermore, the LL-37 gene was inducible in gingival epithelial cells by bacteria, including A. actinomycetemcomitans (26). LL-37 has broad-spectrum activity against Gram-positive and Gram-negative microorganisms, including those associated with periodontal disease (27). A rare disorder, morbus Kostmann, is associated with the complete absence of LL-37 and is characterized by, among other symptoms, chronic periodontitis and overgrowth with A. actinomycetemcomitans (8, 41). Individuals with another genetic disorder, Papillon-Lefèvre syndrome, demonstrate a deficiency in LL-37 and exhibit severe periodontitis. This may be due to a deficiency in serine proteinases that process hCAP-18 to the mature, active LL-37 peptide (11). Together, these studies suggest a role for LL-37 in the natural defense against colonization by periodontal pathogens such as A. actinomycetemcomitans.
While initially isolated as an antimicrobial peptide, LL-37 has been discovered to exhibit numerous other activities. As its expression in the epithelium is induced in response to infection and inflammation (17), it was proposed to play other roles in inflammation besides antimicrobial. Indeed, LL-37 demonstrates chemotactic activity for neutrophils, monocytes, and some T cells (54) and induces IL-8 secretion from epithelial cell lines (49). Furthermore, LL-37 affects dendritic cell (DC) maturation and DC-mediated T-cell polarization and can act synergistically with the DC maturation cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF) to activate signal transduction pathways in monocytes (45). Together, these multiple activities of LL-37 (as reviewed in reference 7) suggest that it plays an important, multifunctional role in host defense; thus, induction with vitamin D could bolster this defense.
The induction by vitamin D of other aspects of the innate immune response can also increase the defense against periodontal disease. TREM-1 has been described as a fine-tuning regulator of the inflammatory response, which works in concert with TLR stimulation (reviewed in reference 21). While its expression and function have traditionally been described as limited to cells of the myeloid lineage, TREM-1 has also been identified in gastric (44) and airway (43) epithelial cells. Inflammatory processes do not appear to induce TREM-1 expression, in contrast to bacteria and bacterial products (3, 5). Partial inhibition of TREM-1 can protect against problems associated with polymicrobial colonization (59), while activation of the receptor (via the cross-linking antibody) leads to increased host defense against bacterial colonization (29). Surprisingly, LL-37 has recently been observed to be involved in the regulation of TREM-1 in neutrophils (1), suggesting that the vitamin D-regulated genes encoding both of these proteins are involved in the maintenance of the innate immune defense. Furthermore, the bacterially mediated induction of LL-37 and interactions of LL-37, bacterial LPS (37), and TREM-1 activation (1) suggest a complex host defense mechanism in these cells that can be modulated or bolstered by vitamin D.
Induction of CD14 by vitamin D has already been demonstrated in epithelial cells (24) and is similarly regulated in oral epithelial cells. Since functional TLR4 has been observed in GEC (20), the observation of CD14 in these cells and its induction by vitamin D supports its role in amplification of the innate immune defense. Another surprising gene we observed to be expressed in these cells was the gene encoding PPBP (also known as CXCL7). While PPBP is generally considered a platelet-derived chemokine (reviewed in reference 50), there is some evidence of antibacterial activity (34) similar to that of other chemokines. Examination of the promoter region of the PPBP gene did identify a putative VDRE; however, our experiments observed only a statistically insignificant (2.9-fold, P = 0.08) increase in mRNA levels in stimulated cells. Regulation of the PPBP gene by vitamin D may require other factors, or it may occur in less time than the shortest time (2 h) we examined. We did not observe expression of this gene in airway epithelium stimulated with 10−8 M 1,25(OH)2D3 (data not shown), suggesting that if the PPBP gene plays a role in epithelial cell defense, it may be unique to gingival cells.
As vitamin D and calcium deficiencies can lead to increased inflammation (19), it is reasonable to hypothesize that there is an association with periodontal disease. Indeed, numerous studies have demonstrated an association between certain restriction fragment length polymorphisms (RFLPs) in the VDR gene and different forms of periodontitis. Specifically, in Japanese (47), Chinese (48), and Brazilian (10) populations, the presence of a specific restriction fragment length polymorphism is associated with chronic periodontitis. Other studies have demonstrated a similar association with generalized aggressive periodontitis (32) and with early onset periodontitis (25, 46), although it is likely that this association requires the presence of other markers (57). The specific RFLPs that have been studied are in the coding region of the VDR and are found at the start site (where the absence of a FokI site leads to a three-residue-shorter protein, which may affect its transcriptional activity) in intron 8 (a BsmI polymorphism) and in exon 9 (a TaqI polymorphism). A recent meta-analysis of these polymorphisms has demonstrated a strong association (12) with chronic periodontitis. The association between the polymorphic allele frequencies and periodontal disease in the studies cited above suggest that the regulation of the vitamin D response may affect the susceptibility to periodontal disease. While this has usually been ascribed to the role of vitamin D in bone loss, it is also possible that vitamin D-mediated gene regulation of the innate immune response may be associated with the initial defense against colonization by periodontal pathogens.
In summary, as periodontal disease is associated with the adherence and colonization of pathogenic bacteria at the gingival epithelium, followed by the inflammation that occurs in response to this colonization and invasion, prevention of colonization with direct antimicrobial activity, as well as an enhancement of the natural innate immune response, may have a profound effect. While development of new antibiotics can temporarily address the colonization, the increase in antibiotic-resistant organisms makes this approach less effective. Since bacteria do not develop resistance to antimicrobial peptides such as LL-37, the potential of these peptides as therapies is great. However, their development as exogenous antibiotics has been hampered by a variety of factors, including difficulty with their large-scale production. The better possibilities of therapy with antimicrobial peptides lie with agents that can induce or enhance their endogenous production in the tissues.
ACKNOWLEDGMENTS
We thank Sylvia Christakos and Puneet Dhawan for their helpful advice.
This work was funded by U.S. Public Health Service grant R21DE18781 to G.D.
Footnotes
Published ahead of print on 21 March 2011.
REFERENCES
- 1. Amatngalim G. D., Nijnik A., Hiemstra P. S., Hancock R. E. 2010. Cathelicidin peptide LL-37 modulates TREM-1 expression and inflammatory responses to microbial compounds. Inflammation doi:10.1007/s10753-010-9248-6 [DOI] [PubMed] [Google Scholar]
- 2. Bikle D. D. 2010. Vitamin D: newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol. Metab. 21:375–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bleharski J. R., et al. 2003. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J. Immunol. 170:3812–3818 [DOI] [PubMed] [Google Scholar]
- 4. Bouchon A., Dietrich J., Colonna M. 2000. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 164:4991–4995 [DOI] [PubMed] [Google Scholar]
- 5. Bouchon A., Facchetti F., Weigand M. A., Colonna M. 2001. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 410:1103–1107 [DOI] [PubMed] [Google Scholar]
- 6. Bowdish D. M., Davidson D. J., Hancock R. E. 2006. Immunomodulatory properties of defensins and cathelicidins. Curr. Top. Microbiol. Immunol. 306:27–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bowdish D. M., et al. 2005. Impact of LL-37 on anti-infective immunity. J. Leukoc. Biol. 77:451–459 [DOI] [PubMed] [Google Scholar]
- 8. Carlsson G., et al. 2006. Periodontal disease in patients from the original Kostmann family with severe congenital neutropenia. J. Periodontol. 77:744–751 [DOI] [PubMed] [Google Scholar]
- 9. Dale B. A., et al. 2001. Localized antimicrobial peptide expression in human gingiva. J. Periodontal Res. 36:285–294 [DOI] [PubMed] [Google Scholar]
- 10. de Brito R. B., Jr., Scarel-Caminaga R. M., Trevilatto P. C., de Souza A. P., Barros S. P. 2004. Polymorphisms in the vitamin D receptor gene are associated with periodontal disease. J. Periodontol. 75:1090–1095 [DOI] [PubMed] [Google Scholar]
- 11. de Haar S. F., Hiemstra P. S., van Steenbergen M. T. J. M., Everts V., Beertsen W. 2006. Role of polymorphonuclear leukocyte-derived serine proteinases in defense against Actinobacillus actinomycetemcomitans. Infect. Immun. 74:5284–5291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deng H., et al. 2010. BsmI, TaqI, ApaI, and FokI polymorphisms in the vitamin D receptor gene and periodontitis: a meta-analysis of 15 studies including 1,338 cases and 1,302 controls. J. Clin. Periodontol. doi:10.1111/j.1600-051X.2010.01685.x [DOI] [PubMed] [Google Scholar]
- 13. Dhawan P., Wieder R., Christakos S. 2009. CCAAT enhancer-binding protein alpha is a molecular target of 1,25-dihydroxyvitamin D3 in MCF-7 breast cancer cells. J. Biol. Chem. 284:3086–3095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diamond G., Beckloff N., Ryan L. K. 2008. Host defense peptides in the oral cavity and the lung: similarities and differences. J. Dent. Res. 87:915–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diamond G., Yim S., Rigo I., McMahon L. 2010. Measuring antimicrobial peptide activity on epithelial surfaces in cell culture. Methods Mol. Biol. 618:371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dongari-Bagtzoglou A., Kashleva H. 2006. Development of a highly reproducible three-dimensional organotypic model of the oral mucosa. Nat. Protoc. 1:2012–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dorschner R. A., et al. 2001. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J. Investig. Dermatol. 117:91–97 [DOI] [PubMed] [Google Scholar]
- 18. Dower K., Ellis D. K., Saraf K., Jelinsky S. A., Lin L. L. 2008. Innate immune responses to TREM-1 activation: overlap, divergence, and positive and negative cross-talk with bacterial lipopolysaccharide. J. Immunol. 180:3520–3534 [DOI] [PubMed] [Google Scholar]
- 19. Ebert R., Schutze N., Adamski J., Jakob F. 2006. Vitamin D signaling is modulated on multiple levels in health and disease. Mol. Cell Endocrinol. 248:149–159 [DOI] [PubMed] [Google Scholar]
- 20. Eskan M. A., et al. 2008. TLR4 and S1P receptors cooperate to enhance inflammatory cytokine production in human gingival epithelial cells. Eur. J. Immunol. 38:1138–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ford J. W., McVicar D. W. 2009. TREM and TREM-like receptors in inflammation and disease. Curr. Opin. Immunol. 21:38–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gibot S., et al. 2007. TREM-1 promotes survival during septic shock in mice. Eur. J. Immunol. 37:456–466 [DOI] [PubMed] [Google Scholar]
- 23. Gombart A. F., Borregaard N., Koeffler H. P. 2005. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 19:1067–1077 [DOI] [PubMed] [Google Scholar]
- 24. Hansdottir S., et al. 2008. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J. Immunol. 181:7090–7099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hennig B. J., Parkhill J. M., Chapple I. L., Heasman P. A., Taylor J. J. 1999. Association of a vitamin D receptor gene polymorphism with localized early-onset periodontal diseases. J. Periodontol. 70:1032–1038 [DOI] [PubMed] [Google Scholar]
- 26. Hosokawa I., et al. 2006. Innate immune peptide LL-37 displays distinct expression pattern from beta-defensins in inflamed gingival tissue. Clin. Exp. Immunol. 146:218–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ji S., et al. 2007. Susceptibility of various oral bacteria to antimicrobial peptides and to phagocytosis by neutrophils. J. Periodontal Res. 42:410–419 [DOI] [PubMed] [Google Scholar]
- 28. Klesney-Tait J., Turnbull I. R., Colonna M. 2006. The TREM receptor family and signal integration. Nat. Immunol. 7:1266–1273 [DOI] [PubMed] [Google Scholar]
- 29. Lagler H., et al. 2009. TREM-1 activation alters the dynamics of pulmonary IRAK-M expression in vivo and improves host defense during pneumococcal pneumonia. J. Immunol. 183:2027–2036 [DOI] [PubMed] [Google Scholar]
- 30. Larrick J. W., et al. 1995. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect. Immun. 63:1291–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Laube D., et al. 2008. Differential regulation of innate immune response genes in gingival epithelial cells stimulated with Aggregatibacter actinomycetemcomitans. J. Periodontal Res. 43:116–123 [DOI] [PubMed] [Google Scholar]
- 32. Li S., et al. 2008. Association of vitamin D receptor gene polymorphisms in Chinese patients with generalized aggressive periodontitis. J. Periodontal Res. 43:360–363 [DOI] [PubMed] [Google Scholar]
- 33. Lin R., White J. H. 2004. The pleiotropic actions of vitamin D. Bioessays 26:21–28 [DOI] [PubMed] [Google Scholar]
- 34. Linge H. M., et al. 2008. The human CXC chemokine granulocyte chemotactic protein 2 (GCP-2)/CXCL6 possesses membrane-disrupting properties and is antibacterial. Antimicrob. Agents Chemother. 52:2599–2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu P. T., et al. 2006. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311:1770–1773 [DOI] [PubMed] [Google Scholar]
- 36. Midorikawa K., et al. 2003. Staphylococcus aureus susceptibility to innate antimicrobial peptides, β-defensins and CAP18, expressed by human keratinocytes. Infect. Immun. 71:3730–3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mookherjee N., et al. 2006. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J. Immunol. 176:2455–2464 [DOI] [PubMed] [Google Scholar]
- 38. Murakami M., Ohtake T., Dorschner R. A., Gallo R. L. 2002. Cathelicidin antimicrobial peptides are expressed in salivary glands and saliva. J. Dent. Res. 81:845–850 [DOI] [PubMed] [Google Scholar]
- 39. Nell M. J., Tjabringa G. S., Vonk M. J., Hiemstra P. S., Grote J. J. 2004. Bacterial products increase expression of the human cathelicidin hCAP-18/LL-37 in cultured human sinus epithelial cells. FEMS Immunol. Med. Microbiol. 42:225–231 [DOI] [PubMed] [Google Scholar]
- 40. Netea M. G., et al. 2006. Triggering receptor expressed on myeloid cells-1 (TREM-1) amplifies the signals induced by the NACHT-LRR (NLR) pattern recognition receptors. J. Leukoc. Biol. 80:1454–1461 [DOI] [PubMed] [Google Scholar]
- 41. Putsep K., Carlsson G., Boman H. G., Andersson M. 2002. Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet 360:1144–1149 [DOI] [PubMed] [Google Scholar]
- 42. Radsak M. P., Salih H. R., Rammensee H. G., Schild H. 2004. Triggering receptor expressed on myeloid cells-1 in neutrophil inflammatory responses: differential regulation of activation and survival. J. Immunol. 172:4956–4963 [DOI] [PubMed] [Google Scholar]
- 43. Rigo I. Induction of triggering receptor expressed on myeloid cells (TREM-1) in airway epithelial cells by 1,25(OH)2 vitamin D3. Innate Immun., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schmausser B., et al. 2008. Triggering receptor expressed on myeloid cells-1 (TREM-1) expression on gastric epithelium: implication for a role of TREM-1 in Helicobacter pylori infection. Clin. Exp. Immunol. 152:88–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Scott M. G., Davidson D. J., Gold M. R., Bowdish D., Hancock R. E. 2002. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 169:3883–3891 [DOI] [PubMed] [Google Scholar]
- 46. Sun J. L., et al. 2002. Relationship between vitamin D receptor gene polymorphism and periodontitis. J. Periodontal Res. 37:263–267 [DOI] [PubMed] [Google Scholar]
- 47. Tachi Y., et al. 2003. Vitamin D receptor gene polymorphism is associated with chronic periodontitis. Life Sci. 73:3313–3321 [DOI] [PubMed] [Google Scholar]
- 48. Tachi Y., et al. 2001. Association of vitamin D receptor gene polymorphism with periodontal diseases in Japanese and Chinese. Nucleic Acids Res. Suppl. 2001:111–112 [DOI] [PubMed] [Google Scholar]
- 49. Tjabringa G. S., et al. 2003. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J. Immunol. 171:6690–6696 [DOI] [PubMed] [Google Scholar]
- 50. von Hundelshausen P., Petersen F., Brandt E. 2007. Platelet-derived chemokines in vascular biology. Thromb Haemost. 97:704–713 [DOI] [PubMed] [Google Scholar]
- 51. Wang T. T., et al. 2004. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 173:2909–2912 [DOI] [PubMed] [Google Scholar]
- 52. Wang T. T., et al. 2005. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol. Endocrinol. 19:2685–2695 [DOI] [PubMed] [Google Scholar]
- 53. Woo J. S., et al. 2003. Expression of cathelicidin in human salivary glands. Arch. Otolaryngol. Head Neck Surg. 129:211–214 [DOI] [PubMed] [Google Scholar]
- 54. Yang D., et al. 2000. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 192:1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yao L., et al. 2010. Porphyromonas gingivalis infection sequesters pro-apoptotic Bad through Akt in primary gingival epithelial cells. Mol. Oral Microbiol. 25:89–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yim S., Dhawan P., Ragunath C., Christakos S., Diamond G. 2007. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3). J. Cyst. Fibros. 6:403–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yoshihara A., et al. 2001. Analysis of vitamin D and Fcgamma receptor polymorphisms in Japanese patients with generalized early-onset periodontitis. J. Dent. Res. 80:2051–2054 [DOI] [PubMed] [Google Scholar]
- 58. Zanetti M. 2004. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 75:39–48 [DOI] [PubMed] [Google Scholar]
- 59. Zeng H., Ornatowska M., Joo M. S., Sadikot R. T. 2007. TREM-1 expression in macrophages is regulated at transcriptional level by NF- kappaB and PU.1. Eur. J. Immunol. 37:2300–2308 [DOI] [PubMed] [Google Scholar]