Abstract
The mechanisms that govern the initial interaction between Paracoccidioides brasiliensis, a primary dimorphic fungal pathogen, and cells of the innate immunity need to be clarified. Our previous studies showed that Toll-like receptor 2 (TLR2) and TLR4 regulate the initial interaction of fungal cells with macrophages and the pattern of adaptive immunity that further develops. The aim of the present investigation was to assess the role of MyD88, an adaptor molecule used by TLRs to activate genes of the inflammatory response in pulmonary paracoccidioidomycosis. Studies were performed with normal and MyD88−/− C57BL/6 mice intratracheally infected with P. brasiliensis yeast cells. MyD88−/− macrophages displayed impaired interaction with fungal yeast cells and produced low levels of IL-12, MCP-1, and nitric oxide, thus allowing increased fungal growth. Compared with wild-type (WT) mice, MyD88−/− mice developed a more severe infection of the lungs and had marked dissemination of fungal cells to the liver and spleen. MyD88−/− mice presented low levels of Th1, Th2, and Th17 cytokines, suppressed lymphoproliferation, and impaired influx of inflammatory cells to the lungs, and this group of cells comprised lower numbers of neutrophils, activated macrophages, and T cells. Nonorganized, coalescent granulomas, which contained high numbers of fungal cells, characterized the severe lesions of MyD88−/− mice; the lesions replaced extensive areas of several organs. Therefore, MyD88−/− mice were unable to control fungal growth and showed a significantly decreased survival time. In conclusion, our findings demonstrate that MyD88 signaling is important in the activation of fungicidal mechanisms and the induction of protective innate and adaptive immune responses against P. brasiliensis.
INTRODUCTION
Toll-like receptors (TLRs), one of the most important groups of innate immune receptors, have been shown to participate in the recognition of several pathogens (9, 19, 34). These receptors have been characterized as a family of evolutionarily conserved type I transmembrane proteins displaying leucine-rich extracellular domains. The cytoplasmic portions of TLRs are homologous to the intracellular signaling domain of the interleukin 1 (IL-1) receptor, and they are known as TIR domains. Intracellular pathways are activated by TLRs through the homophilic interactions of their TIR domains with those of adaptor proteins, the best characterized of which is myeloid differentiation factor 88 (MyD88). This adaptor protein is used by all TLRs except TLR3. MyD88 signaling leads to the activation of protein kinases and the expression of transcription factors that trigger the expression of genes involved in the inflammatory response (19, 27). In addition, the IL-1R family of cytokine receptors, including IL-18R and IL-33R, contains intracellular TIR domains and, like the majority of TLRs, uses MyD88 as an adaptor molecule to activate cells and induce the expression of specific genes such as the IL-1β, IL-6, and tumor necrosis factor alpha (TNF-α) genes (1, 11, 26).
IL-1β, IL-18, and IL-33 are members of the IL-1 family of inflammatory cytokines and are involved in the differentiation of the Th17, Th1, and Th2 immune responses, respectively (11, 26). Interestingly, MyD88-dependent TLR signaling has been implicated in the transcription of pro-IL-1β and pro-IL-18, the inactive precursors of IL-1β and IL-18, which are then cleaved to their active forms by different host- or pathogen-derived proteases (1, 26). Thus, deficient MyD88 signaling can affect multiple pathways of cell activation and cytokine production and can influence the development of innate and adaptive immune responses against pathogens.
Several studies have demonstrated the importance of MyD88 signaling in the immunoprotection against fungal infections caused by Aspergillus fumigatus, Candida albicans, and Cryptococcus neoformans (2, 5, 12). This adaptor molecule was shown to regulate the fungicidal mechanisms, the production of cytokines, and the activity of effector and Treg cells and to affect the efficiency of the immunological mechanisms induced by these pathogens.
Paracoccidioidomycosis (PCM) is a systemic granulomatous disease caused by the dimorphic fungus Paracoccidioides brasiliensis, which constitutes the most prevalent deep mycosis in Latin America (16). The molecular mechanisms controlling the initial steps of P. brasiliensis and phagocyte interactions are not well understood. It is known, however, that unstimulated macrophages are permissive to P. brasiliensis growth while cytokine-activated macrophages are able to control P. brasiliensis proliferation (6, 8).
In previous work, our group demonstrated the role of TLR2 in pulmonary PCM. TLR2 deficiency leads to increased Th17 immunity associated with diminished expansion of Treg cells and increased lung pathology due to unrestrained inflammatory reactions (23). In addition, a more severe P. brasiliensis infection associated with increased production of Th17 cytokines, enhanced proinflammatory immunity, and impaired expansion of regulatory T cells was shown to be regulated by TLR4 expression (22). Moreover, TLR2, TLR4, and dectin-1 were suggested to be involved in the recognition and internalization of P. brasiliensis by human monocytes and neutrophils, indicating an important role for these pathogen receptors in the immune response against the fungus (3). Another group, however, reported that MyD88 is not essential for an effective defense against a systemic P. brasiliensis infection (17).
In the present work, we verified that the absence of MyD88 signaling by peritoneal macrophages resulted in decreased fungicidal ability associated with diminished synthesis of IL-12 and nitric oxide (NO). Accordingly, after pulmonary infection, MyD88−/− mice presented a severe infection, decreased levels of pulmonary Th1, Th2, and Th17 cytokines, impaired lymphoproliferative response, diminished expansion of Treg cells, and high mortality rates associated with extensive fungal lesions that affected several organs. Altogether, our findings demonstrate that the MyD88 adaptor molecule plays an important role not only in the fungicidal mechanisms of innate immune cells but also in the induction of the effector and regulatory cells of the adaptive immune response against this fungal pathogen.
MATERIALS AND METHODS
Fungus.
P. brasiliensis Pb18, a highly virulent isolate, was used throughout this investigation (18). Pb18 yeast cells were maintained by weekly subcultivation in semisolid culture medium. Washed yeast cells were adjusted to 20 × 106 cells/ml (in vivo infection) and 4 × 104 cells/ml (in vitro infection) based on hemocytometer counts. Viability was determined with Janus Green B vital dye (Merck) and was always higher than 85%.
Mice and i.t. infection.
MyD88−/− mice on a C57BL/6 background were kindly provided by S. Akira (Osaka University, Japan). C57BL/6 control (wild-type [WT]) mice were obtained from our Isogenic Breeding Unit (Departmento de Imunologia, Instituto de Ciências Biomédicas, Universidade de São Paulo, São Paulo, Brazil) and used at 8 to 12 weeks of age. Mice were anesthetized and subjected to intratracheal (i.t.) P. brasiliensis infection as previously described (10). Briefly, after intraperitoneal anesthesia, the animals were i.t. infected with 106 P. brasiliensis yeast cells, contained in 50 μl of phosphate-buffered saline (PBS). Mice were studied at 48 h and 8 weeks postinfection. The experiments were approved by the Ethics Committee on Animal Experiments of our institution.
PI labeling of P. brasiliensis yeast cells.
P. brasiliensis yeast cells were washed in PBS and heat killed at 60°C for 1 h. Before the labeling, the yeast suspension was sonicated using 3 cycles of 10 s each (21% amplitude) with Sonics (Vibra Cell VCX 750; Sonics & Materials) to eliminate aggregates. The yeast cells were washed, adjusted to 1 × 106 cells/ml in PBS, and then incubated with propidium iodide (PI; 100 μg/ml; Sigma) for 30 min at 37°C. The yeast suspension was then washed three times with PBS and stored at 4°C.
Phagocytic and fungicidal assays.
Thioglycolate-induced peritoneal macrophages were isolated by adherence (2 h at 37°C in 5% CO2) to plastic-bottom tissue culture plates (1 × 106 cells/well in 24-well plates for fungicidal assays). Macrophages were washed to remove nonadherent cells and cultivated overnight with fresh complete medium (Dulbecco's modified Eagle's medium [DMEM]; Sigma) containing 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin in the presence or absence of recombinant gamma interferon (IFN-γ; 20 ng/ml in culture medium; BD Pharmingen). For phagocytic assays, macrophage cultures were infected with P. brasiliensis yeast cells labeled with propidium iodide (PI) at a macrophage/yeast ratio of 1:1. The cells were cocultivated for 2 h at 37°C in 5% CO2 to allow fungal adhesion and ingestion. Supernatants were removed and cells washed with PBS to remove any noningested or nonadhered yeast cells. Cells were then harvested and the macrophages were then labeled with anti-CD11b fluorescein isothiocyanate (FITC; eBioscience) for 20 min at 4°C. As P. brasiliensis yeast cells have high levels of variability in size and granularity (different sizes, numbers of buds, and numbers of nuclei), the granulocyte gates as defined by size (forward scatter [FSC]) and granularity (side scatter [SSC]) to determine the macrophage population were not used. The total cells present in the samples were analyzed by flow cytometry (FACSCalibur; BD Pharmingen). For fungicidal assays, IFN-γ-primed and unprimed macrophages were infected with P. brasiliensis yeast cells at a macrophage/yeast ratio of 25:1 and cocultivated for 2 h. The monolayers were then washed to remove nonadherent cells and incubated for an additional 48-h period. Plates were centrifuged (400 × g, 10 min, 4°C) and supernatants obtained and stored at −70°C and further analyzed for the presence of nitrite and cytokines. The wells were washed with distilled water to lyse macrophages and suspensions collected in individual tubes. One hundred microliters of cell homogenates was assayed for the presence of viable yeast cells. All assays were done with five wells per condition in more than three independent experiments.
CFU assays, mortality rates, and histological analysis for determining severity of infections.
The numbers of viable microorganisms in cell cultures and infected organs (lungs, liver, and spleen) from experimental and control mice were determined by counting the number of CFU. Animals from each group were sacrificed, and the enumeration of viable organisms was done as previously described (31). The numbers (log10) of viable P. brasiliensis per gram of tissue (in vivo) or per ml of cell homogenate (in vitro) are expressed as the means ± standard errors. Mortality studies were done with groups of 9 or 10 mice inoculated i.t. with 1 × 106 yeast cells or PBS. Deaths were registered daily, and experiments were repeated twice. For histological examinations, the left lung of the infected mouse was removed, fixed in 10% formalin, and embedded in paraffin. Five-micrometer sections were stained by hematoxylin-eosin (H&E) for an analysis of the lesions and silver stained (Grocott stain) for fungal evaluation. Pathological changes were analyzed based on the size, morphology, and cell composition of granulomatous lesions, the presence of fungi, and the intensity of the inflammatory infiltrates. Morphometrical analysis was performed using a Nikon DXM 1200c digital camera (magnification of ×100) and Nikon NIS Elements AR 2.30 software. The area of lesions was measured (in μm2) using 10 microscopic fields per slide for 6 animals per group. Results were expressed as the means ± standard errors for the total area of lesions for each animal.
Measurement of cytokines and NO.
Supernatants from lung homogenates or cell cultures were separated and stored at −70°C. Cytokines (IL-1β, IL-33, transforming growth factor β [TGF-β], IL-4, IL-5, IL-23, IL-17, IL-12, IL-10, IL-6, TNF-α, and IFN-γ) levels were measured by a capture enzyme-linked immunosorbent assay (ELISA) with antibody pairs purchased from eBioscience or BD Pharmingen. The ELISA procedure was performed according to the manufacturer's protocol, and absorbances were measured with a Versa Max microplate reader (Molecular Devices). NO production was quantified by the accumulation of nitrite in the supernatants from in vitro and in vivo protocols by a standard Griess reaction (13). All determinations were performed in duplicate and expressed in μM NO.
Quantitative analysis of IL-18 mRNA expression.
RNA was extracted from infected lungs using Trizol reagent (Invitrogen), and cDNA was synthesized from 2 μg RNA using a high-capacity RNA-to-cDNA kit (Applied Biosystems) according to the manufacturer's instructions. IL-18 mRNA expression was quantified relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression using assay-on-demand primers and probes, TaqMan universal master mix, and an ABI Prism 7000 apparatus (Applied Biosystems).
Assessment of leukocyte subpopulations in lung inflammatory exudates.
After 48 h and 8 weeks of infection, lungs from each mouse were digested enzymatically for 30 min with collagenase (1 mg/ml) and DNase (30 μg/ml) in culture medium (Sigma). Lung cell suspensions were centrifuged in the presence of 20% Percoll (Sigma) to separate leukocytes from cell debris. Total lung leukocyte numbers were assessed in the presence of trypan blue using a hemocytometer; viability was >85%. For differential leukocyte counts, samples of lung cell suspensions were cytospun (Shandon Cytospin) onto glass slides and stained by the Diff-Quik blood stain (Baxter Scientific). A total of 200 to 400 cells were counted from each sample. For flow cytofluorometry, lung leukocytes were resuspended at 106 cells/ml in staining buffer (PBS-0.1% NaN3-1% fetal calf serum). Fc receptors were blocked by unlabeled anti-CD16/32 antibodies (BD Biosciences) and cells stained for 20 min at 4°C. Phycoerythrin (PE)-labeled anti-CD40, CD86, CD69, and dectin-1, fluorescein isothiocyanate (FITC)-labeled anti-IAK, CD80, TLR-2, TLR-4, and CD4, and Alexa Fluor 488 anti-CD25, PE-Cy7 anti-CD4, and peridinin chlorophyll protein (PerCP) complex-Cy5.5 anti-CD11b and anti-CD8 monoclonal antibodies (MAbs; BD Biosciences) were used. Cells were fixed with 1% paraformaldehyde (Sigma) and stored in the dark at 4°C until they were analyzed in a flow cytometer. The acquisition and analysis gates were restricted to the lymphocytes or macrophages. Treg cells were characterized by intracellular staining for Foxp3, using a Treg staining kit from BD Biosciences. Surface staining of CD25+ and intracellular FoxP3 (PE) expression were back-gated on the CD4+ T cell population. For intracellular cytokine (IL-4, IFN-γ, and IL-17) staining, cells were stimulated for 6 h in complete medium in the presence of 50 ng/ml phorbol 12-myristate 13-acetate, 500 ng/ml ionomycin (both from Sigma-Aldrich), and monensin (3 mM; eBioscience). After surface staining for CD4 (Pacific Blue anti-CD4) and CD8 (Alexa Fluor 488 anti-CD8), cells were fixed, permeabilized, and stained by PerCP-Cy5.5 anti-IFN-γ, PE-Cy7 anti-IL-4, and PE anti-IL-17 antibodies (eBioscience, San Diego, CA). The cell surface expression of leukocyte markers as well as intracellular expression of FoxP3, IL-4, IFN-γ, and IL-17 in lung-infiltrating leukocytes (LIL) were analyzed with a FACSCanto flow cytometer (BD Pharmingen) using the FlowJo software program (Tree Star, Inc., Ashland, OR).
Lymphoproliferation assay.
Cells were assayed for proliferation using an in vitro fluorescence-based assay. Briefly, 1 × 106 cells from spleens were stained with 1 μl (5 mM) of carboxyfluorescein diacetate-succinimidyl ester (CFSE; Molecular Probes) in PBS and 5% fetal calf serum for 15 min at room temperature. Stained cells were cultured for 3 days in the presence of anti-CD3 antibodies (0.3 μg/ml), anti-CD28 monoclonal antibodies (2.5 μg/ml) (BD Biosciences), or P. brasiliensis soluble antigen (100 μg/ml). A minimum of 100,000 events were acquired with a FACSCalibur flow cytometer using Cell-Quest software (BD Pharmingen). The proliferation index (p) was calculated as the mean fluorescence intensity (MFI) of unstimulated cultures/MFI of stimulated cultures.
Statistical analysis.
Data were analyzed by Student's t test or two-way analysis of variance depending on the number of experimental groups. Differences between survival times were determined with the log rank test using GraphPad Prism 5 for Windows (GraphPad Software). P values under 0.05 were considered significant.
RESULTS
MyD88 deletion leads to a less pronounced response of macrophages to P. brasiliensis infection.
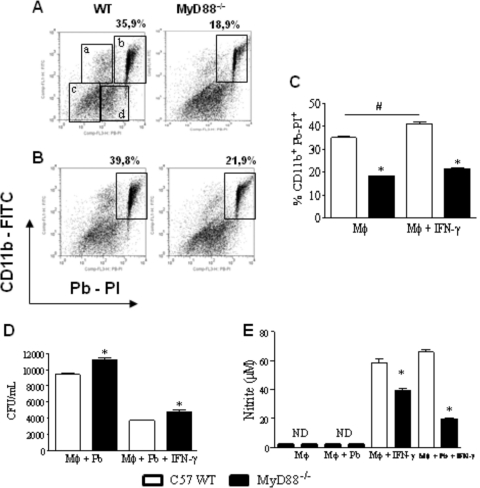
Macrophage cultures (1 × 106/well) performed in 24-well plates were preactivated or not with IFN-γ (20 ng/ml) and infected with 1 × 106 heat-killed PI-labeled yeast cells/well (1:1 fungus/macrophage ratio). After 2 h of incubation, supernatants were aspirated, the monolayer was gently washed with PBS, and the cells were harvested. To determine the number of ingested/adhered Pb yeast cells, macrophages were incubated with FITC-labeled anti-CD11b and analyzed by flow cytometry. Compared with the level for WT macrophages, a lower number of yeast cells were associated (ingested/adhered) with MyD88−/− macrophages (Fig. 1A and C). The same was observed with IFN-γ-primed macrophages (Fig. 1B and C).
Fig. 1.
Macrophages from MyD88−/− mice have a decreased ability to interact with P. brasiliensis yeast cells. For phagocytic assays, unprimed (A, C) and IFN-γ-primed (20 ng/ml, overnight) (B, C) peritoneal macrophages from MyD88−/− and WT C57BL/6 mice were infected with 1 × 106/well heat-killed PI-labeled yeast cells (1:1 fungus/macrophage ratio). After 2 h, the supernatants were aspirated, the cells washed, and harvested macrophages labeled with FITC anti-CD11b antibodies and analyzed by flow cytometry. Four different cell populations were identified (regions a to d). (a) Single positive FITC-labeled cells (CD11b+ cells that do not phagocytose or adhere to Pb-PI); (b) double-positive cells (PI-Pb-FITC macrophages); (c) dead cells; and (d) nonphagocytosed PI-Pb. For fungicidal assays, IFN-γ-primed and unprimed peritoneal macrophages were infected with P. brasiliensis yeast cells at a macrophage/yeast ratio of 25:1. After 48 h of cocultivation, the supernatants were obtained to characterize NO and cytokine production; monolayers were lysed and assayed for the presence of viable yeast cells by a CFU assay (D). Supernatants were used to determine the levels of nitrites using the Griess reagent (E). Data are means ± standard errors of the means (SEM) of results from quintuplicate samples from one experiment representative of three independent determinations. # and *, P < 0.05.
Macrophages were cultivated with P. brasiliensis yeast cells for an additional 48-h period. Supernatants were removed and assayed for the presence of nitric oxide and cytokines and cell homogenates plated for CFU determinations. As shown in Fig. 1D, the absence of MyD88 signaling led to increased recovery of viable yeast cells from untreated and IFN-γ-primed macrophages (20 ng/ml). In addition, higher levels of NO were produced by macrophages from WT mice than by those from MyD88−/− mice (Fig. 1E).
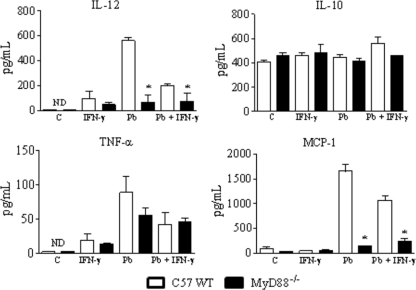
When cytokines in macrophage supernatants were measured, no differences in IL-10 and TNF-α were observed, but IL-12 was produced in decreased concentrations by MyD88−/− macrophages. The same occurred with the MCP-1 chemokine (Fig. 2).
Fig. 2.
Macrophages from MyD88−/− mice secrete diminished levels of IL-12 and MCP-1. IFN-γ-treated (20 ng/ml) or untreated macrophages of MyD88−/− and WT mice were challenged with viable P. brasiliensis yeast cells (1:25 fungus/macrophage ratio) and cultivated for 48 h at 37°C in 5% CO2. Supernatants were then obtained and used for cytokine measurements using ELISA. Data are means ± SEM of results from triplicate samples from one experiment representative of 3 independent determinations. *, P < 0.05.
Absence of MyD88 signaling increases mortality rates associated with increased fungal loads and tissue pathology.
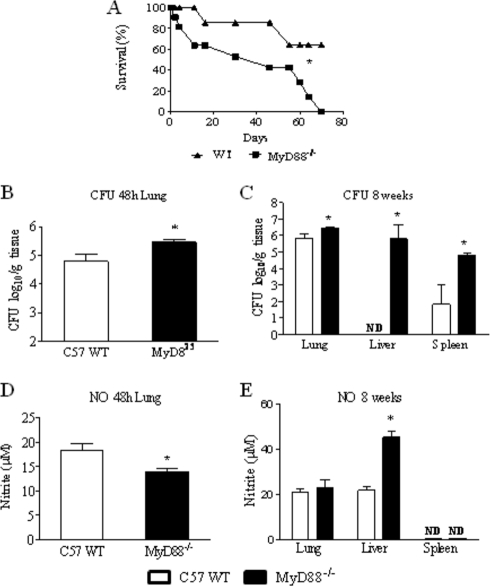
To assess the influence of MyD88 deficiency in the disease outcome, the mortality of P. brasiliensis-infected MyD88−/− and WT (n = 9 or 10) mice was registered daily after infection with 1 × 106 P. brasiliensis yeast cells. As shown in Fig. 3 A, at the 70th day of infection, all (10) MyD88−/− mice were dead. In the same period, 6 out of 9 WT mice were still alive.
Fig. 3.
Absence of MyD88 signaling increases mortality rates and tissue fungal burdens. (A) Survival times of MyD88−/− (n = 9) and WT (n = 10) mice after i.t. infection with 1 × 106 P. brasiliensis yeast cells was determined in a period of 70 days. The results are representative of two independent experiments. *, P < 0.05. (B and C) Recovery of fungal loads (CFU) from organs of MyD88−/− and WT mice. The bars represent mean ± SEM log10 numbers of CFU obtained from groups of 6 to 8 mice at 48 h (B) and 8 weeks (C) after infection. Levels of NO (μM) present in tissue homogenates obtained 48 h (D) and 8 weeks (E) after fungal infection. The results are representative of three experiments with equivalent results. *, P < 0.05.
After infection of groups (n = 6 to 8) of MyD88−/− and WT mice i.t. with 1 × 106 P. brasiliensis yeast cells, the severity of infection was assessed at early (48-h) and late (8-week) periods of the disease. As soon as 48 h after infection, MyD88−/− mice presented increased fungal loads in the lungs (Fig. 3B). At a later time (week 8), increased fungal burdens were recovered from lungs, livers, and spleens of MyD88−/− mice. The most striking differences in fungal loads were seen in the livers. No P. brasiliensis growth was detected in WT mice, whereas almost 1 million yeast cells were recovered from MyD88−/− mice (Fig. 3C). The augmented fungal burden of MyD88−/− mice observed in the lungs at 48 h of infection was accompanied by low levels of pulmonary NO (Fig. 3D). At week 8, however, elevated levels of NO were detected in the liver homogenates of MyD88−/− mice, which presented marked fungal loads (Fig. 3E). No measurable levels of NO were detected in the spleens of both mouse strains.
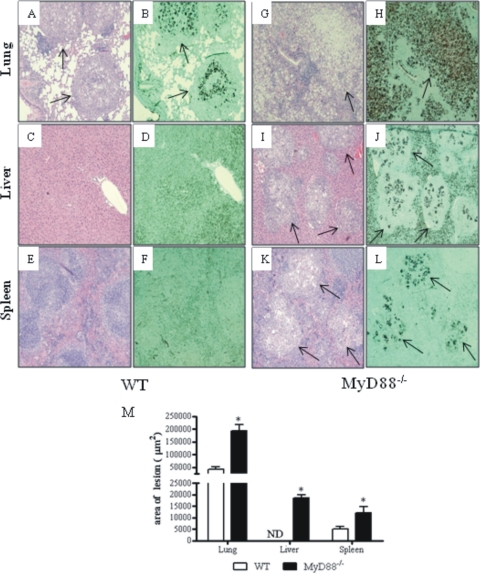
To better characterize the severity of P. brasiliensis infection, histopathological examination of lungs, livers, and spleens was done at week 8 of infection. As can be seen in Fig. 4, a more severe infection with intense destruction of tissue was observed in MyD88−/− mice. Pulmonary lesions in MyD88−/− mice replaced the largest part of normal tissue and were composed of confluent necrotic lesions of various sizes containing many budding yeast cells, surrounded by a small number of inflammatory mononuclear cells (Fig. 4G and H). The lesions in the lungs of WT mice (Fig. 4A) occupied a smaller area and were composed of sometimes confluent, organized granulomas of smaller sizes. Besides, the reduced numbers of fungal cells were surrounded by intense inflammatory reactions (Fig. 4A and B). The livers and spleens of WT mice presented a normal morphology (Fig. 4C to F), in contrast to those MyD88−/− mice which contained wide necrotic lesions with an elevated number of yeast cells accompanied by surrounding inflammatory exudates (Fig. 4I to L). The total areas of lesions in histological sections were quantified and are shown in panel M of Fig. 4. At week 8, the areas of lesions of MyD−/− mice were significantly larger than those of WT mice. Thus, the higher level of fungal growth observed in the organs of MyD88−/− mice was concomitant with increased tissue pathology.
Fig. 4.
Photomicrographs of lesions of WT (A to F) and MyD88−/− (G to L) mice at week 8 of infection with 1 × 106 P. brasiliensis yeast cells. Compared with those of MyD88−/− mice (G), the pulmonary lesions of WT mice (A) were smaller (arrows) and composed of organized granulomas containing lower numbers of yeast cells (B). The pulmonary lesions of MyD88−/− mice were composed of confluent, necrotic, unorganized granulomas of various sizes (G) containing an elevated number of fungal cells (arrow in H) and replaced almost all the normal tissue (G, H). The livers (C, D) and spleens (E, F) of WT mice presented a normal morphology; in contrast, the livers (I, J) and spleens (K, L) of MyD88−/− mice presented extensive necrotic lesions (arrows in I and K) containing an elevated number of yeast cells (arrows in panels J and L) surrounded by mononuclear inflammatory exudates. H&E (A, C, E, G, I, K)- and Groccot (B, D, F, H, J, L)-stained lesions (magnification, ×100). *, P < 0.05. (M) Total area of lesions in the lungs, livers and spleen of mice (n = 6) at week 8 after infection. *, P < 0.05.
MyD88-deficiency results in reduced levels of pro- and anti-inflammatory cytokines.
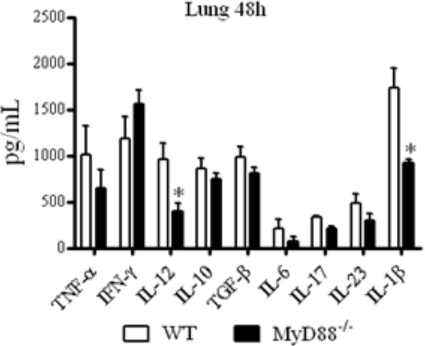
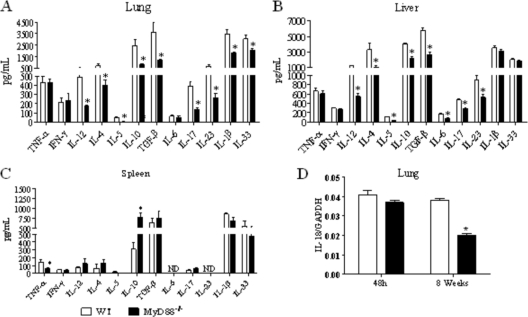
Forty-eight hours after infection, at the innate phase of immunity, reduced levels of IL-12 and IL-1β were detected in the lungs of MyD88−/− mice (Fig. 5). At week 8, reduced concentrations of IL-12, IL-4, IL-5, IL-10, TGF-β, IL-17, IL-23, IL-1β, and IL-33 were detected in the lungs of MyD88−/− mice (Fig. 6 A). Besides these cytokines, MyD88-deficient mice presented decreased levels of hepatic IL-6 (Fig. 6B). When splenic cytokines were measured, only reduced levels of TNF-α concomitant with elevated levels of IL-10 were detected in MyD88−/− mice (Fig. 6C). The message for IL-18 was also detected in the lungs, and impaired expression was detected in the lungs at week 8 but not at the early postinfection period (48 h) of infection (Fig. 6D).
Fig. 5.
Early after infection, lung homogenates of MyD88−/− mice presented decreased levels of IL-12 and IL-1β. MyD88−/− and WT mice were infected with 1 × 106 yeast cells of P. brasiliensis, and 48 h later, lungs were collected and disrupted in 5.0 ml of PBS, and supernatants were analyzed for cytokine content by capture ELISA. The bars depict means ± SEM of cytokine levels (6 to 8 animals per group). The results are representative of two independent experiments. *, P < 0.05.
Fig. 6.
At week 8 after infection, organs from MyD88−/− mice presented decreased levels of Th1, Th2, and Th17 cytokines. Lung (A), liver (B), and spleen (C) homogenates of MyD88−/− mice presented decreased levels of cytokines. At week 8 after i.t. infection with 1 × 106 yeast cells of P. brasiliensis, organs from MyD88−/− and WT mice were collected and disrupted in 5.0 ml of PBS and supernatants analyzed for cytokine content by capture ELISA. The bars depict means ± SEM of cytokine levels (6 to 8 animals per group). The results are representative of three independent experiments. *, P < 0.05. (D) Quantitative PCR analysis of IL-18 expression in the lungs of P. brasiliensis-infected WT and MyD88−/− mice. Total lung RNA was obtained, reverse transcribed, and cDNA amplified. Real-time PCR was performed using TaqMan universal master mix. Amplified products were normalized to the amount of GAPDH products. Data represent the means ± SEM of results from two independent experiments.
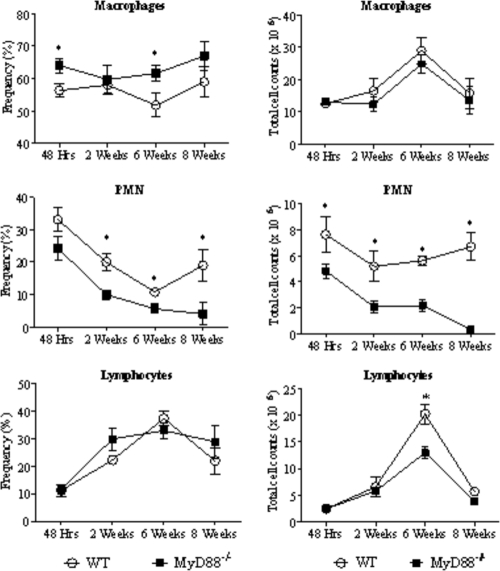
MyD88−/− mice exhibit decreased recruitment of PMN leukocytes to the lungs.
To better characterize the inflammatory reaction at the site of infection, leukocyte recruitment to the lung tissues of P. brasiliensis-infected MyD88−/− and WT mice was studied at several postinfection periods. As can be seen in Fig. 7, lower frequencies (left panels) and numbers (right panels) of polymorphonuclear (PMN) cells were observed in the lungs of MyD88−/− mice than in their normal controls. No important differences were noted in the total counts of lymphocytes and macrophages, except the significantly elevated numbers of lymphocytes in the lungs of WT mice at week 6 after infection (Fig. 7).
Fig. 7.
MyD88 deficiency determines a decreased recruitment of PMN cells to the lungs. Frequency (left side panels) and absolute number (right panels) of leukocyte subsets (macrophages, PMN neutrophils, and lymphocytes) in the lung-infiltrating leukocytes (LIL) from MyD88−/− and WT mice inoculated i.t. with 1 million P. brasiliensis yeast cells. At different postinfection periods (48 h, 2, 6, and 8 weeks), lungs of both mouse strains (n = 6 to 8) were excised, washed in PBS, minced, and digested enzymatically. Lung cell suspensions were obtained and cytospun onto glass slides. Cells were stained by the Diff-Quik bloodstain. Data are expressed as means ± SEM. *, P < 0.05.
Absence of MyD88 signaling determines decreased numbers of activated mononuclear phagocytes and T cells.
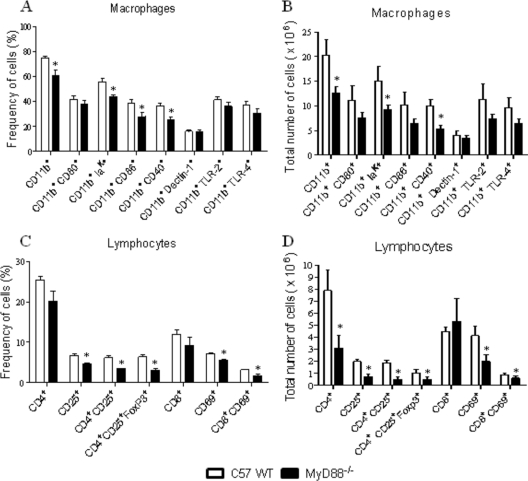
We have further analyzed the phenotype and activation status of lung infiltrating leukocytes at week 8 of P. brasiliensis infection (Fig. 8). To determine the activation profile of pulmonary mononuclear phagocytes (macrophages and dendritic cells), the expression of CD11b, major histocompatibility complex (MHC) class II (IAK), CD80, CD86, CD40, dectin-1, TLR2, and TLR4 molecules was assessed by flow cytometry. As can be seen in Fig. 8A and B, besides diminished frequencies and numbers of CD11b+ cells, MyD88−/− mice presented decreased presence of cells expressing IAK and CD40 markers. No differences in CD80, CD86, dectin-1, TLR2, and TLR4 expression by CD11b+ phagocytes were detected. When lymphocytes were characterized, a significantly reduced recruitment of CD4+ CD25+ and CD8+ CD69+ T cells to the lungs of MyD88−/− mice was detected (Fig. 8C and D). The presence of CD4+ CD25+ FoxP3+ T cells was characterized by flow cytometry in the CD4+ subpopulation of lung-infiltrating lymphocytes. Surface staining of CD25+ and intracellular FoxP3+ expression were back-gated on the CD4+ T cell population. As can be seen in Fig. 8C and D, diminished frequencies and numbers of CD4+ CD25+ FoxP3+ T cells were observed in the lung inflammatory lymphocytes of MyD88−/− mice. Thus, in pulmonary PCM, MyD88 signaling appears to affect the induction of effector and regulatory T cells.
Fig. 8.
Decreased numbers of activated macrophages, T lymphocytes, and regulatory T cells were detected in the lungs of MyD88−/− mice at week 8 of infection. Characterization of leukocyte subsets by flow cytometry in the lung-infiltrating leukocytes (LIL) from MyD88−/− and WT mice inoculated i.t. with 1 × 106 P. brasiliensis yeast cells. At week 8 after infection, lungs of both mouse strains (n = 6 to 8) were excised and digested enzymatically. Cell suspensions were obtained and stained as described in Materials and Methods. The stained cells were analyzed immediately with FACSCanto equipment gating on macrophages or lymphocytes, as judged from forward and side light scatters. Twenty thousand cells were counted, and the data are expressed as percentage and absolute number of positive cells. For characterization of Treg cells (CD4+ CD25+ FoxP3+), surface staining of CD25+ and intracellular FoxP3 expression were back-gated on the CD4+ T cell population. Data are expressed as means ± SEM and are representative of two independent experiments. *, P < 0.05.
MyD88−/− mice present an impaired lymphoproliferative response.
To characterize the proliferative activity of lymphocytes, spleen cells were obtained from WT and MyD88−/− mice at week 8 of infection. CFSE-labeled lymphocytes were in vitro stimulated with P. brasiliensis antigen and anti-CD3 and anti-CD28 MAbs and cultivated for 72 h. As presented in Table 1, with all stimuli used, the lymphoproliferative response of MyD88−/− splenocytes was lower than that of WT mice. This experiment indicates that MyD88−/− mice mount a deficient T cell response as revealed by the diminished migration of T cells to the site of infection and the impaired lymphoproliferative activity detected.
Table 1.
Proliferation indexes of spleen lymphocytes obtained from P. brasiliensis-infected MyD88−/− and WT mice at week 6 of infectiona
| Treatment |
p |
|
|---|---|---|
| MyD88−/− | WTb | |
| Lymphocytes-AgPb | 0.86 | 1.87 |
| Lymphocytes-anti-CD28 | 0.69 | 1.31 |
| Lymphocytes-anti-CD28-AgPb | 0.87 | 4.62 |
Spleen cell suspensions were labeled with CFSE and cultured for 3 days in the presence of anti-CD3 (0.3 μg/ml) and anti-CD28 (2.5 μg/ml) antibodies and P. brasiliensis soluble antigen (100 μg/ml). The intensity of CFSE was assessed by flow cytometry.
The proliferation index (p) was calculated as the mean fluorescence intensity (MFI) of unstimulated cultures/MFI of stimulated cultures. Data are representative of two independent experiments.
MyD88−/− mice present decreased numbers of Th17 cells in the lungs.
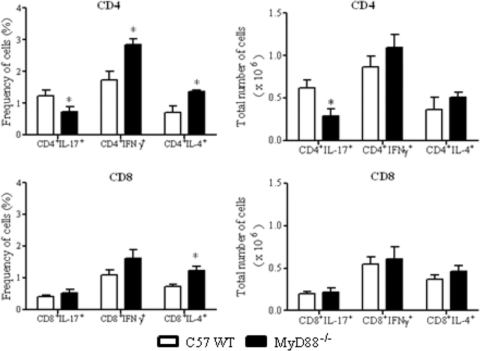
To better clarify the importance of MyD88 signaling in the polarization of T cell responses, the phenotypes of IL-17, IFN-γ, and IL-4-producing cells were defined in the inflammatory infiltrates of lungs at week 8 postinfection. These cytokines were assessed by intracellular staining in NK, Tγδ, CD4+, and CD8+ T cells. As shown in Fig. 9, significantly diminished frequencies and numbers of CD4+ IL-17+ T cells were detected in the lungs of MyD88−/− mice. Despite the increased percentages of CD4+ IFN-γ+, CD4+ IL-4+, and CD8+IL-4+ cells, no significant increases in the total numbers of these cells were detected in MyD88−/− mice. NK and Tγδ cells were negative for all intracellular cytokines assayed. These findings indicate that absence of MyD88 signaling induces a marked impairment of Th17 immunity of P. brasiliensis-infected hosts.
Fig. 9.
Decreased numbers of CD4+IL-17+ cells were detected in the lungs of MyD88−/− mice. Groups (n = 6 or 7) of WT and MyD88−/− mice were infected with 1 × 106 P. brasiliensis yeast cells. The presence of IL-17+, IFN-γ+, and IL-4+ CD4+ and CD8+ T cells in the lung-infiltrating leukocytes was assessed by intracellular cytokine staining by flow cytometry at week 8 after infection. Lung cells were restimulated in vitro with phorbol myristate acetate (PMA)-ionomycin for 6 h and subjected to intracellular staining for IL-17, IL-4, and IFN-γ. The lymphocyte population was gated by the forward/side scatters. The results are from one experiment and are representative of two independent experiments. *, P < 0.05.
DISCUSSION
The ability to interact with distinct conserved molecules of pathogens makes TLRs key elements of innate immunity (27). Due to the broad activities of MyD88, infections in MyD88−/− mice generally result in reduced survival, increased pathogen load, and diminished secretion of IL-12 (14, 24, 30, 35). Similar results were demonstrated in this study in a pulmonary model of infection caused by P. brasiliensis. In this report, we first verified that MyD88−/− macrophages have a decreased ability to interact with P. brasiliensis yeast cells, and this behavior was also observed when macrophages were primed with IFN-γ. The low phagocytic and fungicidal abilities of and the impaired NO and IL-12 production by these cells demonstrated that MyD88 signaling is important for macrophage activation and, consequently, for the control of P. brasiliensis growth. In addition, low IL-12 and MCP-1 production may negatively influence CD4+ T cell activation and the migration of mononuclear cells to the site of infection, which have been observed in infected MyD88−/− mice. Although MyD88−/− macrophages were able to produce NO, the levels were lower than those produced by WT cells. In murine PCM and other fungal infections, the production of NO, an important fungicidal mediator (25), correlates with fungal loads and the synthesis of proinflammatory mediators, including cytokines, chemokines, and leukotrienes. Enhanced NO production and fungicidal function were detected in WT and MyD88−/− macrophages activated by IFN-γ. Previous studies have indicated that IFN-γ R1 signaling is partially dependent on MyD88 (27, 32). Thus, it is possible that this low-level but evident NO secretion was induced by MyD88-independent IFN-γ R1 signaling or other inducible nitric oxide synthase (iNOS)-activating mediators induced by MyD88-independent pathogen recognition receptors (PRRs). Although no differences in TLR2, TLR4, or dectin-1 expression were detected in lung inflammatory mononuclear phagocytes, other pathogen receptors, such as C-type lectin receptors (e.g., mannose receptors [MRs] or dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin [DC-SIGN]), may be involved in the macrophage response in MyD88−/− mice.
The increased fungal burden observed in the early infection period (48 h) was consistent with data obtained using infected macrophages in vitro. A more severe infection associated with decreased IL-12 and IL-1β production was observed in MyD88−/− mice. These proinflammatory cytokines mediate phagocyte activation in the innate phase of immunity but are also involved in the induction of Th1 and Th17 adaptive immune responses, respectively (26). A more severe infection of the lungs, liver, and spleen was also detected at a later postinfection time point (8 weeks). Despite the high levels of NO produced, the dissemination and growth of fungal cells in the livers of MyD88−/− mice were extensive, suggesting that the activation of iNOS was induced by MyD88-independent mechanisms triggered by the elevated fungal burden.
Lower levels of Th1 (IL-12 and IL-18), Th2 (IL-33, IL-4, IL-5, and IL-10), and Th17 (TGF-β, IL-1β, IL-6, IL-23, and IL-17) cytokines were found in the lungs and/or livers of MyD88−/− mice after 8 weeks of infection. Moreover, the impaired cell migration to inflammatory sites and suppressed polyclonal and antigen-specific lymphoproliferative responses observed in MyD88−/− splenocytes suggest that absence of MyD88 signaling profoundly affects Th1, Th2, and Th17 immune responses in pulmonary PCM.
In addition to defective MyD88-dependent TLR signaling, deficient production of cytokines from the IL-1 family (IL-1β, IL-18, and IL-33) and cell signaling by their receptors (IL-1R, IL-18R, and IL-33R) appear to be involved in the deficient immunity observed in MyD88−/− mice. Indeed, IL-1β, IL-18, and IL-33 are involved in the differentiation of Th17, Th1, and Th2 cells of adaptive responses, respectively (11, 26). Interestingly, MyD88-dependent TLR signaling has been implicated in the transcription of pro-IL-1β and pro-IL-18 into their active forms by caspase 1 or other inflammatory or pathogen-derived proteases (1, 11, 26). Thus, the deficient cytokine production and impaired cell activation (likely due to defective IL-1R family signaling) observed here appear to have contributed to the suppressed T cell immunity developed by P. brasiliensis-infected MyD88−/− mice.
The reduced synthesis of IL-1β, TGF-β, and IL-23 was associated with defective Th17 responses in MyD88−/− mice. This deficiency was confirmed by the evaluation of intracellular cytokines, which demonstrated a lower number of CD4+IL-17+ T cells in the lungs of MyD88−/− mice. Interestingly, in murine candidiasis, dectin-1, dectin-2, and MRs, which are not involved in TIR domain-mediated signaling, were shown to be involved in fungal recognition and the induction of Th17 immunity (28, 29, 36). However, in pulmonary PCM, the two MyD88-dependent receptors, TLR2 and TLR4, have been shown to be antagonistically involved in Th17 development: TLR2 inhibited Th17 immunity but expanded Treg cell numbers, whereas TLR4 induced Th17 cells and inhibited Treg development (9, 22, 23).
In several models of infection, the control of neutrophil influx to the inflammatory sites is mediated by MyD88 (7, 37). Furthermore, Th17 immunity is generally associated with enhanced synthesis of CXC chemokines and the induction of neutrophils chemotaxis to inflammatory sites (20, 21, 38). The increased influx of neutrophils into the lungs of P. brasiliensis-infected WT mice paralleled the increase in production of Th17 cytokines. This finding is consistent with our previous report showing that Th17 polarization in pulmonary PCM was associated with PMN-rich inflammatory reactions. These cells appear to efficiently control fungal loads; however, they may also mediate deleterious effects due to their ability to cause tissue damage (23).
In addition to the reduced numbers of activated CD4+ and CD8+ T cells, MyD88 signaling affected the numbers and activation state of inflammatory myeloid cells. Reduced numbers of CD11b+ mononuclear phagocytes expressing MHC class II (IAK) and CD40 molecules were found in the lungs of MyD88−/− mice after 8 weeks of infection. In addition, a reduced influx of CD4+ CD25+ FoxP3+ Treg cells was also observed. This result is consistent with the work by Sutmuller et al. (33), who previously showed that Treg cell proliferation is dependent on MyD88 signaling. Recently, the plasticity of Th/Treg cells has been demonstrated by studies showing that under certain in vivo or in vitro conditions, they can convert to other Th cell phenotypes. Thus, FoxP3+ Treg cells (natural and induced Tregs) can acquire a Th17 phenotype in the presence of IL-6; however, these cells express transcription factors of both T cell subpopulations (39, 40). Our studies demonstrated that WT mice develop a high number of CD4+IL-17+ T cells in addition to elevated numbers of FoxP3+ Treg cells. We have not assessed the concomitant presence of FoxP3 and RORγt in the IL-17-positive cells; however, this interconversion cannot be ruled out in our model.
The absence of MyD88 signaling resulted in increased mortality rates in infected mice. Interestingly, this phenomenon was not observed in TLR4- or TLR2-deficient mice (22, 23). Increased mortality was associated with uncontrolled fungal growth, impaired T cell immunity, and the absence of organized granulomatous lesions. We previously reported that impaired T cell immunity and excessive fungal growth in the livers of WT C57BL/6 mice were linked with the increased mortality observed in the late phase of infection (15). Thus, it is tempting to hypothesize that the marked growth of yeast cells in the livers of MyD88-deficient mice contributes to the augmented mortality that we observed. Working with A. fumigatus, Bretz et al. (5) also showed that MyD88 signaling is essential for controlling pulmonary fungal burden and organizing inflammatory reactions. A recent study, however, showed equivalent fungal growth and cytokine production in MyD88−/− and normal mice infected with P. brasiliensis, suggesting that MyD88 signaling is not essential for an effective defense against the fungus (17). This discrepancy may be due to the fact that those experiments were performed with a different strain and, perhaps more importantly, using a different infection route. It should be noted, however, that our previous studies (22, 23) showed that TLR2 and TLR4, which signal using MyD88 as an adapter molecule, influence the severity of PCM. Taken together, our results suggest that MyD88-dependent signaling pathways downstream from TLRs and IL-1Rs contribute to host defense against pulmonary paracoccidioidomycosis.
As demonstrated with another dimorphic fungal pathogen (4, 12), P. brasiliensis yeast cells appear to use MyD88-mediated signaling to mount protective Th responses that include prevalent Th17 expansion. This response pattern results in PMN-rich inflammatory reactions, which are able to restrain fungal growth and dissemination into other organs and tissues. Using a MyD88-influenced pathway of cell activation (12), WT mice developed FoxP3+ Treg cells that were able to control adaptive immunity and excessive inflammation. This balanced immunity mediated by Th1/Th2/Th17 cells appeared to control fungal growth without significant tissue damage, which led to an extended survival time in WT mice. In contrast, the absence of MyD88 signaling appears to profoundly suppress the development of adaptive immunity, as shown by decreased levels of Th1/Th2 and Th17 cytokines, suppressed lymphoproliferative activity, and diminished activation and migration of mononuclear phagocytes and T cells (CD4+ and CD8+) to the site of infection. This defective innate immunity and impaired adaptive immunity, including deficient Treg expansion, resulted in uncontrolled fungal growth, which contributed significantly to the tissue pathology observed in MyD88−/− mice. In conclusion, the absence of MyD88 signaling in pulmonary PCM results in profound deleterious effects due to the combined deficiency of innate and adaptive immunity, which results in severe tissue pathology and precocious host mortality.
ACKNOWLEDGMENTS
We are grateful to Tania A. Costa for her invaluable technical assistance and S. Akira and Ricardo Gazzinelli for generously providing the MyD88−/− breeders used in this study.
This work was supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Pesquisas (CNPq).
Footnotes
Published ahead of print on 21 March 2011.
REFERENCES
- 1. Adachi O., et al. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143–150 [DOI] [PubMed] [Google Scholar]
- 2. Biondo C., et al. 2005. MyD88 and TLR2, but not TLR4, are required for host defense against Cryptococcus neoformans. Eur. J. Immunol. 35:870–878 [DOI] [PubMed] [Google Scholar]
- 3. Bonfim C. V., Mamoni R. L., Blotta M. H. 2009. TLR-2, TLR-4 and dectin-1 expression in human monocytes and neutrophils stimulated by Paracoccidioides brasiliensis. Med. Mycol. 47:722–733 [DOI] [PubMed] [Google Scholar]
- 4. Bonifazi P., et al. 2009. Balancing inflammation and tolerance in vivo through dendritic cells by the commensal Candida albicans. Mucosal Immunol. 2:362–374 [DOI] [PubMed] [Google Scholar]
- 5. Bretz C., et al. 2008. MyD88 signaling contributes to early pulmonary responses to Aspergillus fumigatus. Infect. Immun. 76:952–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brummer E. 1994. Interaction of Paracoccidioides brasiliensis with host defense cells, p. 213–223 In Franco M., Lacaz C. S., Restrepo A., Del Negro G. (ed.), Paracaccidioidomycosis. CRC Press, Boca Raton, FL [Google Scholar]
- 7. Cai S., Batra S., Shen L., Wakamatsu N., Jeyaseelan S. 2009. Both TRIF- and MyD88-dependent signaling contribute to host defense against pulmonary Klebsiella infection. J. Immunol. 183:6629–6638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Calich V. L., et al. 2008. Innate immunity to Paracoccidioides brasiliensis infection. Mycopathologia 165:223–236 [DOI] [PubMed] [Google Scholar]
- 9. Calich V. L. G., et al. 2008. Toll-like receptors and fungal infections: the role of TLR2, TLR4 and MyD88 in paracoccidioidomycosis. FEMS Immunol. Med. Microbiol. 53:1–7 [DOI] [PubMed] [Google Scholar]
- 10. Cano L. E., Singer-Vermes L. M., Vaz C. A. C., Russo M., Calich V. L. G. 1995. Pulmonary paracoccidioidomycosis in resistant and susceptible mice: relationship among progression of infection, bronchoalveolar cell activation, cellular immune response, and specific isotype patterns. Infect. Immun. 63:1777–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Casanova J. L., Abel L., Quintana-Murci L. 2011. Human TLRs and IL-1Rs in host defense: natural insights from evolutionary, epidemiological, and clinical genetics. Annu. Rev. Immunol. 29:447–491 [DOI] [PubMed] [Google Scholar]
- 12. De Luca A., et al. 2007. Functional yet balanced reactivity to Candida albicans requires TRIF, MyD88, and IDO-dependent inhibition of Rorc. J. Immunol. 179:5999–6008 [DOI] [PubMed] [Google Scholar]
- 13. Ding A. H., Nathan C. F., Stuehr D. J. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. J. Immunol. 141:2407–2412 [PubMed] [Google Scholar]
- 14. Edelson B. T., Unanue E. R. 2002. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J. Immunol. 169:3869–3875 [DOI] [PubMed] [Google Scholar]
- 15. Felonato M., et al. 2010. CD28 exerts protective and detrimental effects in a pulmonary model of paracoccidioidomycosis. Infect. Immun. 78:4922–4935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Franco M. 1987. Host-parasite relationships in paracoccidioidomycosis. J. Med. Vet. Mycol. 25:5–18 [DOI] [PubMed] [Google Scholar]
- 17. González A., Yáñez A., Gozalbo D., Gil M. L. 2008. MyD88 is dispensable for resistance to Paracoccidioides brasiliensis in a murine model of blood-borne disseminated infection. FEMS Immunol. Med. Microbiol. 54:365–374 [DOI] [PubMed] [Google Scholar]
- 18. Kashino S. S., et al. 2000. Resistance to Paracoccidioides brasiliensis infection is linked to a preferential Th1 immune response, whereas susceptibility is associated with absence of IFN-gamma production. J. Interferon Cytokine Res. 20:89–97 [DOI] [PubMed] [Google Scholar]
- 19. Kawai T., Akira S. 2007. TLR signaling. Semin. Immunol. 19:24–32 [DOI] [PubMed] [Google Scholar]
- 20. Ley K., Smith E., Stark M. A. 2006. IL-17A-producing neutrophil-regulatory T lymphocytes. Immunol. Res. 34:229–242 [DOI] [PubMed] [Google Scholar]
- 21. Liang S. C., et al. 2007. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J. Immunol. 179:7791–7799 [DOI] [PubMed] [Google Scholar]
- 22. Loures F. V., Pina A., Felonato M., Araújo E. F., Calich V. L. G. 2010. TLR4 signaling leads to a more severe fungal infection associated with enhanced proinflammatory immunity and impaired expansion of regulatory T cells. Infect. Immun. 78:1078–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loures F. V., Pina A., Felonato M., Calich V. L. G. 2009. TLR2 is a negative regulator of Th17 cells and tissue pathology in a pulmonary model of fungal infection. J. Immunol. 183:1279–1290 [DOI] [PubMed] [Google Scholar]
- 24. Muraille E., et al. 2003. Genetically resistant mice lacking MyD88-adapter protein display a high susceptibility to Leishmania major infection associated with a polarized Th2 response. J. Immunol. 170:4237–4241 [DOI] [PubMed] [Google Scholar]
- 25. Nascimento F. R., Calich V. L., Rodríguez D., Russo M. 2002. Dual role for nitric oxide in paracoccidioidomycosis: essential for resistance, but overproduction associated with susceptibility. J. Immunol. 168:4593–4600 [DOI] [PubMed] [Google Scholar]
- 26. Netea M. G., et al. 2010. IL-1beta processing in host defense: beyond the inflammasomes. PLoS Pathog. 6:e1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O'Neill L. A. J., Bowie A. G. 2007. The family of five: TIR-domain-containing adaptors in Toll-like receptor signaling. Nat. Rev. Immunol. 7:353–364 [DOI] [PubMed] [Google Scholar]
- 28. Reid D. M., Gow N. A., Brown G. D. 2009. Pattern recognition: recent insights from Dectin-1. Curr. Opin. Immunol. 21:30–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saijo S., et al. 2010. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32:681–691 [DOI] [PubMed] [Google Scholar]
- 30. Scanga C. A., et al. 2002. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 168:5997–6001 [DOI] [PubMed] [Google Scholar]
- 31. Singer-Vermes L. M., Ciavaglia M. C., Kashino S. S., Burguer E., Calich V. L. G. 1992. The source of the growth-promoting factor(s) affects the plating efficiency of Paracoccidioides brasiliensis. J. Med. Vet. Mycol. 30:261–264 [DOI] [PubMed] [Google Scholar]
- 32. Sun D., Ding A. 2006. MyD88-mediated stabilization of interferon-gamma-induced cytokine and chemokine mRNA. Nat. Immunol. 7:375–381 [DOI] [PubMed] [Google Scholar]
- 33. Sutmuller R. P., et al. 2006. Toll-like receptor 2 controls expansion and function of regulatory T cells. J. Clin. Invest. 116:485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takeda K., Kaisho T., Akira S. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335–376 [DOI] [PubMed] [Google Scholar]
- 35. Takeuchi O., Hoshino K., Akira S. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392–5396 [DOI] [PubMed] [Google Scholar]
- 36. van de Veerdonk F. L., et al. 2009. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe 5:329–340 [DOI] [PubMed] [Google Scholar]
- 37. Wiersinga W. J., Wieland C. W., Roelofs J. J., van der Poll T. 2008. MyD88 dependent signaling contributes to protective host defense against Burkholderia pseudomallei. PLoS One 3:e3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu Q., et al. 2007. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 9:78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu L., Kitani A., Fuss I., Strober W. 2007. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J. Immunol. 178:6725–6729 [DOI] [PubMed] [Google Scholar]
- 40. Yang X. O., et al. 2008. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity 29:44–56 [DOI] [PMC free article] [PubMed] [Google Scholar]