Abstract
Ovariectomized (OVX) sheep are now considered to be useful models for a variety of metabolic bone disorders. The specific aim of this study was to determine the effects of ovariectomy on the structural parameters and material density of the subchondral bone of the ovine tibial plateau as measured by microcomputed tomography (MicroCT). Twenty-three sheep were examined in this study; 10 of the sheep underwent ovariectomy (OVX), and the remainder (n = 13) were kept as controls (CON). These animals were then sacrificed at 12 months post-operatively. Three-dimensional analyses were performed of osteochondral samples (15 mm deep) which were obtained from the medial tibial plateau using MicroCT. Bone volume fraction of the subchondral trabecular bone was reduced in the ovariectomized sheep as compared to control animals (0.439 vs. 0.483, P = 0.038). Trabeculae were also significantly thinner in the OVX group (0.220 vs. 0.252 mm, P = 0.010), with reduced connectivity density (7.947 vs. 11.524 mm−3, P = 0.014). There was a trend towards lower numbers of individual trabeculae present in the OVX group as compared to controls, but this did not reach significance (2.817 vs. 3.288 mm−1, P = 0.1). There was also increased trabecular separation in the OVX group, which again fell short of significance (0.426 vs. 0.387 mm, P = 0.251). There was no difference in hydroxyapatite concentration (HA) between the two groups (929 vs. 932 mgHA cm−3, P = 0.687). In conclusion, significant alterations of the trabecular architecture under the tibial plateau were observed following 12 months of oestrogen-deficiency in this ovine model. Despite these marked morphological and structural density differences, the material densities were equal in the two groups.
Keywords: microcomputed tomography, osteoarthritis, osteoporosis, subchondral bone, tibial plateau
Introduction
The skeleton is in a constant state of flux, adapting its form in response to alterations in dynamic load (Wolff, 1870) and also repairing or replacing bone damaged at an ultrastructural level (Burr, 2002). Ovariectomized (OVX) sheep are now considered to be useful models for a variety of metabolic bone disorders, including bone mineral density loss, osteoporosis and alterations in trabecular bone architecture associated with oestrogen deficiency (Thorndike & Turner, 1998; Johnson et al. 2002; Newton et al. 2004; Brennan et al. 2009; Kennedy et al. 2009a). A number of musculoskeletal similarities exist between the human and ovine skeletons (Osterhoff et al. 2011) and the ovine bone remodelling cycle is comparable to that of humans, being of approximately 3 months’ duration (Lee et al. 2002). The alterations typically observed post-ovariectomy include a reduction in bone volume fraction (BV/TV), a reduction in the number of trabeculae, a reduction in the thickness of the individual trabeculae and an increased distance between them (Turner et al. 1995; Jiang et al. 2005). The effects of interventions such as ovariectomy on cortical bone are less extensively documented. Cortical porosity does increase in the diaphysis of the ovine metatarsal 1 year post ovariectomy (2.07 vs. 1.04%) (Kennedy et al. 2009b).
It has been documented that there can be a large degree of variability within the skeleton regarding trabecular, and cortical, structure depending on the bone sampled (Mitton et al. 1998; Rubin et al. 2002; Cornish et al. 2006), and even between specific sites within the same bone (Cornish et al. 2006; Kennedy et al. 2009a). Previous studies using an ovariectomized ovine model to investigate osteoporosis within our own unit have confirmed that bone turnover is significantly elevated at 12 months post-ovariectomy (Kennedy et al. 2009b), with a trend towards reduction in bone volume fraction and trabecular thickness at a number of sites (Brennan, 2008; Kennedy et al. 2008). This current study is now using this same ovine model to examine the trabecular bone structure in yet another site – the tibial subchondral bone, as some authors have hypothesised that alterations in the microstructure of this region may play a role in the development of osteoarthritis (Karvonen et al. 1998; Kawcak et al. 2001; Burr, 2004).
The use of the ovine model to investigate osteoarthritis is not new. The ovine stifle joint may be considered to be a 1 : 3 scale model of the human knee joint, with only minor morphological exceptions (Osterhoff et al. 2011). Most ovine studies in the past have typically induced osteoarthritis in this joint by means of an experimental injury; monoarticular studies may do this by means of causing an injury to an articular structure, such as meniscectomy, in order to destabilise the joint, or by placing the joint under abnormal mechanical loading (Pritzker, 1994; Little et al. 1997; Hwa et al. 2001; Parker et al. 2003; Little & Smith, 2008). The use of ovariectomy alone as a model of osteoarthritis is a relatively recent addition to the literature, however. Ovariectomy has been shown to have a detrimental effect on the structural, material and biomechanical properties of ovine articular cartilage (Turner et al. 1997; Cake et al. 2005). Further studies have shown that these effects are ameliorated by post-operative oestrogen replacement therapy (Turner et al. 1997; Parker et al. 2003; Sniekers et al. 2008).
Given the expanding literature regarding osteoarthritic changes within the cartilage of the stifle joint following ovariectomy, we feel that investigation and quantification of alterations in the subchondral bone structure following this procedure, independent of any additional pathophysiological changes, is of importance to establish the natural history of bone turnover and microstructural changes in this model. Post-ovariectomy subchondral bone changes have been documented in very few studies to date (Waarsing et al. 2004; Little & Smith, 2008), and not as yet in an ovine model. Specifically, this study aims to provide a comparison of the subchondral bone in control and ovariectomized sheep with regard to a number of structural parameters, at 1 year post-ovariectomy.
Materials and methods
Animals and study design
Twenty-three skeletally mature ewes were included in this study; approval was obtained from the ethics committee in the School of Veterinary Science in University College Dublin and an animal licence, number B100/2443, was granted by the Department of Health under the Cruelty to Animals Act, 1876. The precise age of the animals was not known but the range was between 5 and 9 years. Animals were randomly allocated to ovariectomized or control groups; ovariectomy was performed on 10 of the sheep (OVX), and the remainder (n = 13) were kept as controls (CON). The sheep were kept at pasture for 12 months and then sacrificed. Bones were immediately harvested and stored at −20 °C.
Specimen preparation
Removal of the plateau from the intact ovine tibia was initially performed using a Struers Minitom Diamond Saw. The intact tibia was placed in the specimen holder of the Diamond Saw, and the proximal 1.5–2 cm of the tibia removed. Following this, further cuts were made using the Diamond Saw to remove a 7 × 5 mm osteochondral specimen (ca. 15 mm deep) from the anterior aspect of the medial plateau (Fig. 1).
Fig. 1.
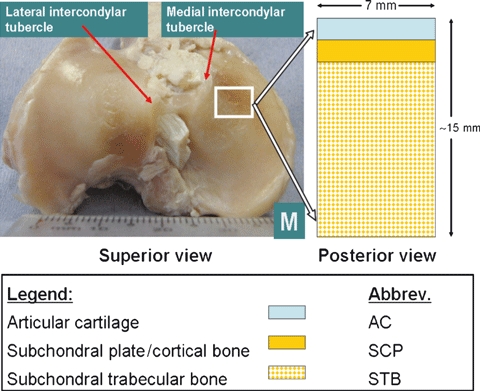
Osteochondral specimen from Ovine Stifle joint.
Microcomputed tomography (MicroCT)
Three-dimensional analyses were performed of these specimens using desktop microcomputed tomography (μCT40; Scanco Medical, Basserdorf, Switzerland). Specimens were placed in a 20.5-mm diameter polyetherimide specimen holder, and secured in position using synthetic foam. Care was taken to ensure that all specimens were orientated identically: specimens were placed with the anterior surface facing inferiorly; the subchondral plate of the specimen faced into the MicroCT; rotation of the specimen was eliminated. The scan was performed at 8 μm resolution, with a beam intensity of 70 kVp (I = 114 μA) and a scan integration time of 230 ms (Bouxsein et al. 2010). The MicroCT was routinely calibrated with a phantom containing hydroxyapatite (HA) densities of 0, 100, 200, 400 and 800 mgHA cm−3, so that HA concentration of the specimens could be ascertained. The MicroCT initially performed a ‘scout view’ of the specimen. Reference lines were then placed 4.16 mm (416 slices) apart; the resultant scan time for each specimen was ca. 36 min.
For analysis of the subchondral trabecular bone, a volume of interest (VOI) was defined within the sample. A 7 × 5-mm area (700 × 500 voxels) was taken from each complete 2D slice, beginning 1.5 mm below the nadir of the chondral surface of each individual slice (Fig. 2). A global thresholding system was used; specifically, a threshold value of 210 was applied, with a sigma of 0.8 and a support value of 1. These were then reconstructed into a 3-dimensional image by the scanco software, with automated analysis of standard morphological parameters and hydroxyapatite concentration. The thickness of the subchondral plate was measured on 10 random 2-dimensional sections of each specimen; the mathematical mean of these measurements was then adjudged to be the subchondral thickness of the specimen.
Fig. 2.
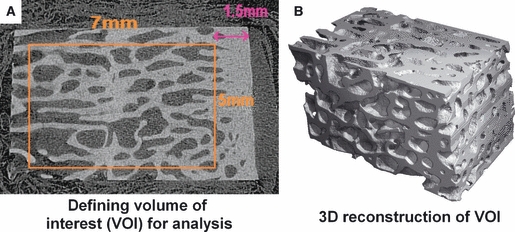
Definition and reconstruction of the Volume of Interest within the Osteochondral specimen for analysis on MicroCT.
Statistical analysis
sigmastat version 3.00 (SPSS Inc.) was used for statistical analysis. All datasets were initially analysed for normality and equal variance. A t-test was performed if the appropriate criteria were met (P > 0.05 and P = 0.10, respectively); if either criterion was not met, a Mann–Whitney U-test was performed instead. Differences were considered significant for values of P < 0.05. With regard to power analysis, the majority of analyses reached the desired power of 0.800. For trabecular analysis, the only failure was the analysis of hydroxyapatite concentration – Power of performed test with alpha = 0.050: 0.050; the power of the analysis of subchondral plate thickness was also low, however, at 0.300 (desired power with alpha = 0.050 is 0.800).
Results
On examination of the subchondral bone microstructure, quantifiable differences were found between the two experimental groups. Bone volume fraction of the subchondral trabecular bone was assessed as the fraction of bone volume per total volume (BV/TV); this was lower in ovariectomized animals than in the control group, and this difference reached statistical significance (0.439 vs. 0.483, P = 0.038, Mann–Whitney U-test; Fig. 3).
Fig. 3.
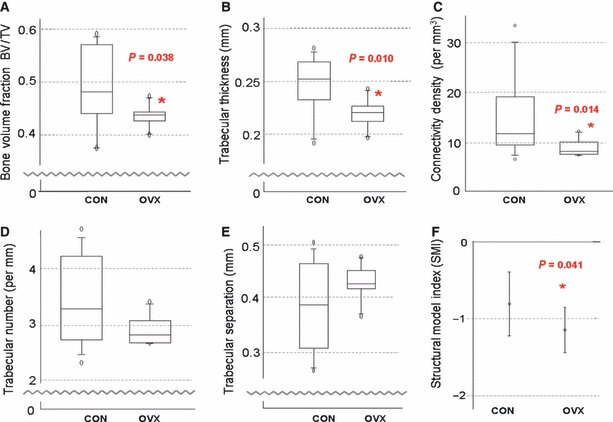
Analysis of Microstructural Properties. (A) Bone Volume fraction. (B) Trabecular thickness. (C) Connectivity Density. (D) Trabecular number. (E) Trabecular separation. (F) Structural Model Index.
Trabecular thickness was also observed to be reduced in the ovariectomized group, as shown in Fig. 3B (0.220 vs. 0.252 mm, P = 0.010, Mann–Whitney U-test); this difference was statistically significant. Connectivity density (a measure of the number of connections between trabeculae present per mm3) was also significantly reduced in the ovariectomized group (7.947 vs. 11.524 mm−3, P = 0.014, Mann–Whitney U-test).
When the number of individual trabeculae present per mm was examined, there was a trend towards a lower number being present in the ovariectomized group than in the control group (2.817 vs. 3.288 mm−1, P = 0.1, Mann–Whitney U-test; Fig. 3C); however, this failed to reach significance. The median distance between the individual trabeculae (trabecular separation or Tb.Sp.) was observed to be greater in the ovariectomized group, although this again failed to reach significance (0.426 vs. 0.387 mm, P = 0.251, Mann–Whitney U-test).
Given all of the above structural differences between the two study groups, it is perhaps unsurprising that there was also a significant difference in the structural model index (SMI) (−1.159 vs. −0.822, P = 0.041, t-test; 95% confidence interval for difference of means: 0.0160–0.658; Fig. 3F). While SMI is often described as a value from 0 to 3 (an ideal plate and cylinder, respectively), a negative SMI may be seen in dense bone, and indicates that there are more concave than convex surfaces present (Issever et al. 2003). As such, it is sensitive to relative volume changes; dilatation of an enclosed space will cause SMI to become increasingly negative.
Finally, when the hydroxyapatite concentration of the trabeculae was ascertained (this being used as an indirect measurement of the material density of the bone) it was revealed that there was no variation in this parameter between the two groups, despite the aforementioned marked morphological and structural density differences observed (929 vs. 932 mgHA cm−3, P = 0.687, t-test; 95% confidence interval for difference of means: −1.315 to 16.846).
The subchondral plate was observed to be thinner in OVX sheep than in controls, but this result fell just short of significance (1.326 vs. 0.992, P = 0.08, t-test; 95% confidence interval for difference of means: −0.0432 to 0.712). As previously stated, however, the power of this test was extremely low. The hydroxyapatite concentration of the subchondral plate revealed no variation between the two groups (994.8 vs. 996.3, P = 0.868, t-test).
Discussion
Ovariectomy is now being employed by some centres as an animal model for investigation of osteoarthritis, in addition to its more established role as a model for osteoporosis (Thorndike & Turner, 1998; Johnson et al. 2002; Newton et al. 2004; Little & Smith, 2008; Brennan et al. 2009; Kennedy et al. 2009a; Osterhoff et al. 2011). This procedure has been shown to have a detrimental effect on the structural, material and biomechanical properties of ovine articular cartilage (Turner et al. 1997; Cake et al. 2005). However, the effects of ovariectomy in isolation on the subchondral bone structure have not been documented extensively to date. Age-related alterations in the structure and material properties of the subchondral trabecular bone in the human proximal tibia have been investigated within a small number of studies, although for analysis purposes subjects were divided purely into groups based upon age, which included both genders (Ding et al. 1997, 2002). Bone volume fraction and trabecular thickness were found to decrease significantly after the age of 60, with the trabeculae shifting from a plate-like to a rod-like microstructure (Ding et al. 2002).
One of the handful of studies to examine subchondral trabecular changes post-ovariectomy is a longitudinal study in rat tibiae, performed using in vivo MicroCT scanning (Waarsing et al. 2004). When examining the epiphyseal bone, there was a 25% reduction in the trabecular bone volume of the ovariectomized rats at 4 weeks; this increased to 30% at 10 weeks. While this was predominantly due to initial thinning of the trabeculae in the first 4 weeks (130–116 μm), a gradual increase in the thickness of the few remaining trabeculae from week 4 to week 14 was observed (116–122 μm). In contrast, the sham-operated rats showed no changes in bone volume over the course of the study, and the trabeculae showed a mild thickening (116 mm to 121 μm).
Our data were consistent with these findings; we also demonstrated a significant reduction in the trabecular bone volume fraction in ovariectomized animals as compared to the control group (0.439 vs. 0.483, P = 0.038). It is well established that oestrogen withdrawal by ovariectomy results in substantial increases in basic multicellular unit (BMU) activation, which is responsible for bone remodelling, within 12 months (Newman et al. 1995; Kennedy et al. 2009b), particularly so in epiphyseal regions, where considerable volumes of bone marrow are present. The subchondral bone region is one of relatively high density (reflected in the negative SMI data) (Issever et al. 2003), thus this region has a considerable trabecular surface area on which newly activated BMUs could act. It follows intuitively that increased bone remodelling in a region densely packed with trabeculae would result in reduced bone volume fraction, without necessitating a corresponding reduction in trabecular number.
Indeed, this hypothesis is further supported by our finding of a significant reduction in trabecular thickness within our ovariectomized sheep as compared to controls, with an increase in the distance between individual trabeculae (Tb.Sp.). These data compare favourably with findings from other established models of osteoarthritis examining these parameters. The intra-articular collagenase injection osteoarthritis model in high-bone mass (C3H/HeJ) mice also shows thinning of subchondral trabeculae and increased trabecular spacing after 4 weeks (Botter et al. 2008). A canine anterior cruciate ligament transaction model (ACLT) has shown similar findings, with trabeculae being significantly thinner in the operated, osteoarthritic knee (as compared to contralateral) for up to 54 months post ACLT (Dedrick et al. 1993). However, these findings do vary somewhat. A guinea pig model (hindlimb) also recorded initial thinning of the trabeculae, but this was then followed by subsequent trabecular thickening, but these animals were not skeletally mature (Layton et al. 1988). It should be noted, however, that the local effects of osteoarthritis induced by ovariectomy and those induced by alteration of the mechanical loading of the joint (i.e. by meniscectomy) may be quite different (Pajamaki et al. 2008).
Despite the marked morphological and structural density differences observed, the material density of the trabecular bone (i.e. hydroxyapatite concentration) was equal at 1 year in both the control and the ovariectomized animals. Therefore, despite the increased turnover and morphological changes occurring, the mineralization was unaltered, suggesting that while osteoclastic activity was ongoing to reduce the bone volume fraction, very little new young undermineralized tissue is being deposited in compensation.
As stated, this study was performed to examine the natural history of structural alterations within the tibial subchondral trabecular bone post-ovariectomy, in view of the increased use of this procedure as a model for the development of osteoarthritis within the stifle joint. Some authors now question whether the changes in the underlying subchondral bone may actually precede the damage seen within the articular cartilage (Radin & Rose, 1986; Karvonen et al. 1998; Kawcak et al. 2001; Burr, 2004). Certainly, once osteoarthritis is established, whatever the initiating cause, there is absolute consensus that subchondral alterations do occur, which will then have a considerable effect on the stresses within the overlying cartilage (Karvonen et al. 1998; Burr, 2004).
This study had a number of limitations. First, the study population was non-uniform; for reasons of availability, mixed breeds of sheep were used in this experiment. Different breeds may have different natural metabolic states and also different metabolic responses to ovariectomy, which could affect the structural parameters investigated. Additionally, while all animals were skeletally mature (with an age range of between 5 and 9 years) the precise age of each sheep was undocumented and unobtainable. Also, while initial power analysis and sample size calculations were performed in advance of purchase, these were determined based on the use of these animals as a model of osteoporosis, not osteoarthritis. However, on statistical analysis, the vast majority of tests reached the desired power of 0.800, the only exception being analysis of trabecular hydroxyapatite concentration as previously described. Of note, while seasonal variations in ovine bone turnover due occur, due to the longer oestrous cycles present in these animals during the summer months as compared to the rest of the year, all animals were sacrificed on the same (November) day, so any effects from this physiological variable should be minimised (Kennedy et al. 2009b).
In conclusion, this study shows significant alterations within the subchondral trabecular architecture at 1 year post-ovariectomy, with reduced bone volume fraction, thinning of individual trabeculae and an increase in trabecular separation. In light of the fact that the ovariectomized ewe has been proven to be a valid model for osteoarthritis (Turner et al. 1997; Cake et al. 2005), the role that these microstructural alterations may play in the pathophysiology of osteoarthritis warrants further consideration.
Acknowledgments
The authors thank Peter O'Reilly for technical assistance with MicroCT. They also thank Brian Cloak and the staff at the Lyons Research Facility, UCD, for animal care and handling. This project was funded by the Higher Education Authority in Ireland under the PRTL Cycle III and by a Research Committee grant from the Royal College of Surgeons in Ireland.
Conflict of interest statement
None of the authors has any conflict of interests to report.
References
- Botter SM, Van Osch GJ, Waarsing JH, et al. Cartilage damage pattern in relation to subchondral plate thickness in a collagenase-induced model of osteoarthritis. Osteoarthritis Cartilage. 2008;16:506–514. doi: 10.1016/j.joca.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Bouxsein ML, Boyd SK, Christiansen BA, et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- Brennan O. Bone Quality and Its Relationship with Bone Fragility and Osteoporosis. Dublin: Department of Anatomy; 2008. Royal College of Surgeons in Ireland. [Google Scholar]
- Brennan O, Kennedy OD, Lee TC, et al. Biomechanical properties across trabeculae from the proximal femur of normal and ovariectomised sheep. J Biomech. 2009;42:498–503. doi: 10.1016/j.jbiomech.2008.11.032. [DOI] [PubMed] [Google Scholar]
- Burr DB. Targeted and nontargeted remodeling. Bone. 2002;30:2–4. doi: 10.1016/s8756-3282(01)00619-6. [DOI] [PubMed] [Google Scholar]
- Burr DB. Anatomy and physiology of the mineralized tissues: role in the pathogenesis of osteoarthrosis. Osteoarthritis Cartilage. 2004;12(Suppl A):S20–S30. doi: 10.1016/j.joca.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Cake MA, Appleyard RC, Read RA, et al. Ovariectomy alters the structural and biomechanical properties of ovine femoro-tibial articular cartilage and increases cartilage iNOS. Osteoarthritis Cartilage. 2005;13:1066–1075. doi: 10.1016/j.joca.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Cornish RJ, Wilson DF, Logan RM, et al. Trabecular structure of the condyle of the jaw joint in young and mature sheep: a comparative histomorphometric reference. Arch Oral Biol. 2006;51:29–36. doi: 10.1016/j.archoralbio.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Dedrick DK, Goldstein SA, Brandt KD, et al. A longitudinal study of subchondral plate and trabecular bone in cruciate-deficient dogs with osteoarthritis followed up for 54 months. Arthritis Rheum. 1993;36:1460–1467. doi: 10.1002/art.1780361019. [DOI] [PubMed] [Google Scholar]
- Ding M, Dalstra M, Danielsen CC, et al. Age variations in the properties of human tibial trabecular bone. J Bone Joint Surg Br. 1997;79:995–1002. doi: 10.1302/0301-620x.79b6.7538. [DOI] [PubMed] [Google Scholar]
- Ding M, Odgaard A, Linde F, et al. Age-related variations in the microstructure of human tibial cancellous bone. J Orthop Res. 2002;20:615–621. doi: 10.1016/S0736-0266(01)00132-2. [DOI] [PubMed] [Google Scholar]
- Hwa SY, Burkhardt D, Little C, et al. The effects of orally administered diacerein on cartilage and subchondral bone in an ovine model of osteoarthritis. J Rheumatol. 2001;28:825–834. [PubMed] [Google Scholar]
- Issever AS, Burghardt A, Patel V, et al. A micro-computed tomography study of the trabecular bone structure in the femoral head. J Musculoskelet Neuronal Interact. 2003;3:176–184. [PubMed] [Google Scholar]
- Jiang Y, Zhao J, Geusens P, et al. Femoral neck trabecular microstructure in ovariectomized ewes treated with calcitonin: MRI microscopic evaluation. J Bone Miner Res. 2005;20:125–130. doi: 10.1359/JBMR.041008. [DOI] [PubMed] [Google Scholar]
- Johnson RB, Gilbert JA, Cooper RC, et al. Effect of estrogen deficiency on skeletal and alveolar bone density in sheep. J Periodontol. 2002;73:383–391. doi: 10.1902/jop.2002.73.4.383. [DOI] [PubMed] [Google Scholar]
- Karvonen RL, Miller PR, Nelson DA, et al. Periarticular osteoporosis in osteoarthritis of the knee. J Rheumatol. 1998;25:2187–2194. [PubMed] [Google Scholar]
- Kawcak CE, Mcilwraith CW, Norrdin RW, et al. The role of subchondral bone in joint disease: a review. Equine Vet J. 2001;33:120–126. doi: 10.1111/j.2042-3306.2001.tb00589.x. [DOI] [PubMed] [Google Scholar]
- Kennedy OD, Brennan O, Mahony NJ, et al. Effects of high bone turnover on the biomechanical properties of the L3 vertebra in an ovine model of early stage osteoporosis. Spine. 2008;33:2518–2523. doi: 10.1097/BRS.0b013e318186b292. [DOI] [PubMed] [Google Scholar]
- Kennedy OD, Brennan O, Rackard SM, et al. Variation of trabecular microarchitectural parameters in cranial, caudal and mid-vertebral regions of the ovine L3 vertebra. J Anat. 2009a;214:729–735. doi: 10.1111/j.1469-7580.2009.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy OD, Brennan O, Rackard SM, et al. Effects of ovariectomy on bone turnover, porosity, and biomechanical properties in ovine compact bone 12 months postsurgery. J Orthop Res. 2009b;27:303–309. doi: 10.1002/jor.20750. [DOI] [PubMed] [Google Scholar]
- Layton MW, Goldstein SA, Goulet RW, et al. Examination of subchondral bone architecture in experimental osteoarthritis by microscopic computed axial tomography. Arthritis Rheum. 1988;31:1400–1405. doi: 10.1002/art.1780311109. [DOI] [PubMed] [Google Scholar]
- Lee TC, Staines A, Taylor D. Bone adaptation to load: microdamage as a stimulus for bone remodelling. J Anat. 2002;201:437–446. doi: 10.1046/j.1469-7580.2002.00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little C, Smith M. Animal models of osteoarthritis. Curr Rheumatol Rev. 2008;4:175–182. [Google Scholar]
- Little C, Smith S, Ghosh P, et al. Histomorphological and immunohistochemical evaluation of joint changes in a model of osteoarthritis induced by lateral meniscectomy in sheep. J Rheumatol. 1997;24:2199–2209. [PubMed] [Google Scholar]
- Mitton D, Cendre E, Roux JP, et al. Mechanical properties of ewe vertebral cancellous bone compared with histomorphometry and high-resolution computed tomography parameters. Bone. 1998;22:651–658. doi: 10.1016/s8756-3282(98)00036-2. [DOI] [PubMed] [Google Scholar]
- Newman E, Turner AS, Wark JD. The potential of sheep for the study of osteopenia: current status and comparison with other animal models. Bone. 1995;16:277S–284S. doi: 10.1016/8756-3282(95)00026-a. [DOI] [PubMed] [Google Scholar]
- Newton BI, Cooper RC, Gilbert JA, et al. The ovariectomized sheep as a model for human bone loss. J Comp Pathol. 2004;130:323–326. doi: 10.1016/j.jcpa.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Osterhoff G, Loffler S, Steinke H, et al. Comparative anatomical measurements of osseous structures in the ovine and human knee. Knee. 2011;18:98–103. doi: 10.1016/j.knee.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Pajamaki I, Sievanen H, Kannus P, et al. Skeletal effects of estrogen and mechanical loading are structurally distinct. Bone. 2008;43:748–757. doi: 10.1016/j.bone.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Parker D, Hwa S, Sambrook P, et al. Estrogen replacement therapy mitigates the loss of joint cartilage proteoglycans and bone mineral density induced by ovariectomy and osteoarthritis. APLAR J Rheumtol. 2003;6:116–127. [Google Scholar]
- Pritzker KP. Animal models for osteoarthritis: processes, problems and prospects. Ann Rheum Dis. 1994;53:406–420. doi: 10.1136/ard.53.6.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res. 1986;213:34–40. [PubMed] [Google Scholar]
- Rubin C, Turner AS, Muller R, et al. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J Bone Miner Res. 2002;17:349–357. doi: 10.1359/jbmr.2002.17.2.349. [DOI] [PubMed] [Google Scholar]
- Sniekers YH, Weinans H, Bierma-Zeinstra SM, et al. Animal models for osteoarthritis: the effect of ovariectomy and estrogen treatment – a systematic approach. Osteoarthritis Cartilage. 2008;16:533–541. doi: 10.1016/j.joca.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Thorndike EA, Turner AS. In search of an animal model for postmenopausal diseases. Front Biosci. 1998;3:c17–c26. doi: 10.2741/a260. [DOI] [PubMed] [Google Scholar]
- Turner AS, Alvis M, Myers W, et al. Changes in bone mineral density and bone-specific alkaline phosphatase in ovariectomized ewes. Bone. 1995;17:395S–402S. doi: 10.1016/8756-3282(95)00317-7. [DOI] [PubMed] [Google Scholar]
- Turner AS, Athanasiou KA, Zhu CF, et al. Biochemical effects of estrogen on articular cartilage in ovariectomized sheep. Osteoarthritis Cartilage. 1997;5:63–69. doi: 10.1016/s1063-4584(97)80032-5. [DOI] [PubMed] [Google Scholar]
- Waarsing JH, Day JS, Van Der Linden JC, et al. Detecting and tracking local changes in the tibiae of individual rats: a novel method to analyse longitudinal in vivo micro-CT data. Bone. 2004;34:163–169. doi: 10.1016/j.bone.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Wolff J. Uber die innere Architectur der Knochen und ihre Bedeutung fur die Frange vom Knochenwachsthum. Virchow's Arch. 1870;50:389–450. [Google Scholar]


