Abstract
Muscles are considered to play an important role in the ongoing daily loading of bone, especially in the masticatory apparatus. Currently, there are no measurements describing this role over longer periods of time. We made simultaneous and wireless in vivo recordings of habitual strains of the rabbit mandible and masseter muscle and digastric muscle activity up to ∼ 25 h. The extent to which habitually occurring bone strains were related to muscle-activity bursts in time and in amplitude is described. The data reveal the masseter muscle to load the mandible almost continuously throughout the day, either within cyclic activity bouts or with thousands of isolated muscle bursts. Mandibular strain events rarely took place without simultaneous masseter activity, whereas the digastric muscle only played a small role in loading the mandible. The average intensity of masseter-muscle activity bouts was strongly linked to the average amplitude of the concomitant bone-strain events. However, individual pairs of muscle bursts and strain events showed no relation in amplitude within cyclic loading bouts. Larger bone-strain events, presumably related to larger muscle-activity levels, had more constant principal-strain directions. Finally, muscle-to-bone force transmissions were detected to take place at frequencies up to 15 Hz. We conclude that in the ongoing habitual loading of the rabbit mandible, the masseter muscle plays an almost non-stop role. In addition, our results support the possibility that muscle activity is a source of low-amplitude, high-frequency bone loading.
Keywords: digastric muscle, electromyography, in vivo bone loading, in vivo bone strain, mandible, masseter muscle
Introduction
A relationship exists between skeletal muscle activity and the morphology and composition of bone (Shaw & Stock, 2009). Contractions of skeletal muscles are believed to have the most prominent and dynamic role in the habitual loading environment of bone tissue (Burr, 1997; Schoenau & Fricke, 2006). Profound changes in skeletal-muscle recruitment illustrate this muscle-bone relationship. For example, not only does the playing arm of tennis players have hypertrophied muscles, its humerus also has a higher bone mineral density (Kannus et al. 1994). In addition, the increase in playing-arm muscle size is correlated with the increase in several bone-strength-indicating parameters (Daly et al. 2004). Conversely, muscle paralysis has been shown to lead to cortical and trabecular bone mass decrease (Warner et al. 2006; Poliachik et al. 2010). The muscle-bone relationship might also exist on a more delicate level; the radius of the arm dominant in habitual everyday activities has a greater mass and volume than that of the non-dominant arm (MacIntyre et al. 1999). It is unclear, however, to what extent in time and in amplitude the daily activity of skeletal muscles is related to the daily loading of bone.
A suitable musculoskeletal system to study the muscle-bone relationship is the masticatory apparatus. As the mandible is not weight bearing and the gravitational forces working on it are small, the origin of the daily loads mandibular bone experiences can be assumed to lie mainly in muscle contractions and the resulting reaction forces. Several muscle groups insert on the mandible, but the masticatory muscles – due to their strength and functional relation to the jaw bones – may be considered the most important loaders. Paralysis of the masseter muscle, for example, results in both its own atrophy and in the subsequent atrophy, or growth retardation, of the mandible it inserts on (Matic et al. 2007; Kim et al. 2008).
The role of masticatory muscles in the mechanical loading of the mandible has been studied predominantly within the context of chewing behaviour using electromyography together with bone-load sensors (Weijs & de Jongh, 1977; Hylander et al. 1987; Teng & Herring, 1998). Extensive analyses have been performed on the relation of masticatory-muscle biopotentials to mandibular strain amplitudes within chewing cycles (Hylander & Johnson, 1989, 1993; Liu et al. 2004). Outside of these bouts of chewing behaviour the role of masticatory muscles in the everyday habitual loading of the mandible has been neglected mostly. Here, we hypothesise that the role of masticatory muscles in the habitual loading of the mandible might be near-continuous; expanding beyond the boundaries of mastication. Previous long-term measurements of habitual bone strain in the mandible revealed the existence of numerous isolated bone-strain events, occurring between bouts of more cyclic bone loading, which also contribute to the daily bone-strain history (de Jong et al. 2010b,c;).
Here we describe the habitual relationship between masticatory muscle activity and mandibular bone strain, without confining this to chewing behaviour. The hypothesis that daily bone strains are associated mainly with the activity of muscles – in terms of both time and amplitude – is tested. To this end, co-appearance of muscle activity and bone strain is analysed from long-term electromyograms of masseter and digastric muscle activity and simultaneously recorded mandibular bone strain. Also, amplitude distributions of habitual masseter and digastric activity bursts and bone-strain events are compared. The masseter muscles are the rabbit's main jaw closers, whereas the digastric muscles function as jaw openers (Schumacher & Rehmer, 1960; Weijs & Dantuma, 1981). We therefore expect a relation in time and in amplitude for masseter activity and mandibular strains. Habitual masseter-burst amplitudes and counts are compared with the co-appearing compressive principal-strain amplitudes and counts. Digastric activity is expected to elicit measurable bone strains, but without a relation in amplitude, as jaw opening will not result in reaction forces as large as during jaw closing.
Materials and methods
Laboratory animals
Ten adult (∼ 4 months old) male New Zealand white rabbits (Oryctolagus cuniculus) weighing 3.7 ± 0.2 kg were used for the experiments. Rabbits were used, as they are large enough to house both a bone-strain and an electromyography transmitter. The animals were kept in 73 × 73 × 46 cm3 cages, received food and water ad libitum, daily portions of hay, and a wooden block that served as an extra gnawing object. Lights were dimmed between 18:00 and 6:00 hours. The rabbits were allowed at least 2 weeks of acclimatisation before implantation of the transmitters. The experiments were approved by the Animal Ethics Committee of the Academic Medical Centre of the University of Amsterdam and executed in accordance with Dutch legislation.
Wireless electromyography (EMG) and bone-surface strain measurement
Masticatory-muscle biopotentials and bone-surface strain were recorded wirelessly using implantable transmitters (Langenbach et al. 2002; van Wessel et al. 2006; de Jong et al. 2010a). An overview of their main features follows below.
Two-channel TL11M2-F20-EET implants from Data Sciences International (DSI, St. Paul, MN, USA) recorded masseter and digastric muscle biopotentials with two indwelling electrodes per channel. Each electrode consisted of a silicone-tubed stainless steel double-helix wire with a diameter of 0.2 mm. The inter-electrode distance once placed in the sampled muscle was approximately 6 mm and the effective electrode length was 4 mm. The bipolar recordings were transmitted on a carrier frequency of 455 kHz to nearby DSI receivers (RMC-1). A DSI Data Exchange Matrix collected the data from the receivers and stored them onto a computer. The sample frequency was 250 Hz per channel. In five animals only masseter activity was recorded, in two others only digastric activity, and in three animals both masseter and digastric activity were recorded. All electrodes were placed unilaterally, at the left side. In the masseter muscle, electrodes were placed at the antero-ventral side of the superficial masseter, near the motor end plates (Widmer et al. 1997). In the digastric muscle, electrodes were placed in the middle of its muscle belly (Fig. 1). Variability in the electrode location was kept at a minimum.
Fig. 1.
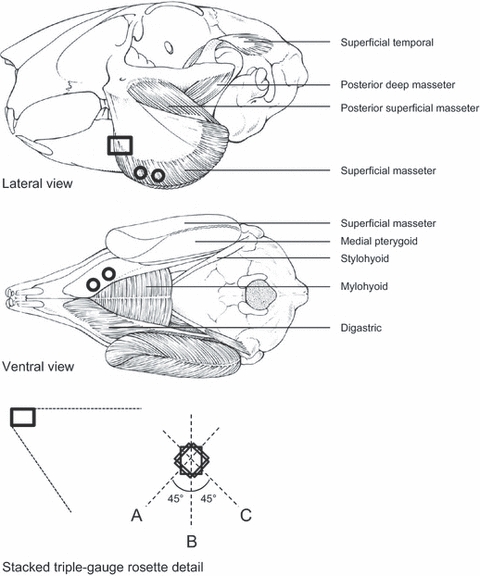
Schematic drawings of the rabbit skull with some skeletal muscles (top and middle drawings) and a detail depicting the orientations of the strain gauges (A,B,C) in the gauge rosette (bottom drawing). The circles in the top and middle drawings indicate the locations of the pairs of wire electrodes in the masseter and digastric muscles. The rectangle in the lateral view depicts the location of the strain-gauge rosette on the mandibular corpus, which in vivo is partly covered by the bulging masseter muscle.
A customised MicroStrain V-Link (Williston, VT, USA) connected to a stacked triple-gauge rosette (L2A-06-031WW-350; Vishay, Malvern, PA, USA) was used for the wireless bone-strain measurements. Strain measurements were transmitted on a carrier frequency of 2.4 GHz to a nearby MicroStrain USB Base Station, which stored the data on a desktop computer. Bone strain was sampled at a frequency of 617 Hz and measured in all 10 rabbits. The gauge rosette was positioned on the left lateral surface of the mandibular corpus, anteriorly of the masseter insertion and inferiorly of the molars. The orientations of the three gauges were rostroventral (A), vertical (B), and caudoventral (C), with an angle of 45° between gauges A and B and between gauges B and C (Fig. 1).
Surgical procedure and medications
The day before and the day after the aseptic surgical placement of the two implants, rabbits received 5.0 mg kg−1 enrofloxacine (Baytril, Bayer, Mijdrecht, the Netherlands) to suppress infections. Before surgery, rabbits were administered a subcutaneous dose of 0.03 mg kg−1 buprenorphine (Temgesic, Schering-Plough, Utrecht, the Netherlands), an analgesic. General anaesthesia was started with a subcutaneous dose of 15 mg kg−1 ketamine (Nimatek, EuroVet Animal Health, Bladel, the Netherlands) combined with 0.40 mg kg−1 dexmedetomidine (Dexdomitor, Orion Pharma, Espoo, Finland). The rabbit's eyes were covered with Oculentum Simplex (Pharmachemie B.V., Haarlem, the Netherlands) to prevent them from dehydrating. Anaesthesia was maintained through intratracheal dosage of 0.8–1.2% isoflurane in a 1 : 1 mixture of oxygen and air. Spirometry and oximetry monitored breathing frequency and oxygen saturation of the blood, respectively. Body temperature was kept at 37 °C using heating pads.
An incision was made in the rabbit's neck fold through which subcutaneous pockets were made to house the transmitters. The EMG electrodes and the wired strain-gauge rosette were led subcutaneously to a second incision in the mandibular region. Here, the electrodes were placed in either the masseter or the digastric, or in both muscles, using a longitudinally ground hypodermic needle as a slide (Nuijens et al. 1997). Electrodes were anchored at the muscle surface with a single suture. To attach the strain-gauge rosette, a mandibular bone-surface area of about 1 cm2 was cleared of the surrounding soft tissues and periosteum. The bone surface was cleaned with sterile swabs, degreased with an 80% ethanol solution, and dried to the air. The gauge rosette was glued to the bone with Histoacryl (B. Braun, Tuttlingen, Germany) and pressed firmly onto it for several minutes (Cochran, 1972).
Following surgery, the animal was given a subcutaneous dose of 2 mg kg−1 carprofen (Rimadyl, Pfizer Animal Health B.V., Capelle aan den IJssel, the Netherlands) to suppress inflammation and pain. After collection of the measurements, rabbits were sacrificed with an overdose of pentobarbital sodium (Euthesate, CEVA Santé Animale, Maassluis, the Netherlands).
Data analysis
Bone-strain recordings and electromyograms were analysed using the software program spike2 (version 5.21; Cambridge Electronic Design Limited, Cambridge, UK).
Masseter and digastric electromyograms were rectified and smoothed by calculating the moving root-mean-square value over Δt = 0.040 s. In each recording, the highest biopotential peak served as the 100% activity level of the sampled muscle, as rabbits cannot be ordered to give a maximum muscle contraction. All other peaks were expressed as a percentage of that biopotential. Distribution histograms were made of the EMG-peak amplitudes, or burst amplitudes, that crossed the 5% activity level of the recording. Histograms of muscle-burst amplitudes had a bin width of 2.5%.
Bone-strain recordings were rid of drift by subtracting the average of all data points from t − 3600 s to t + 3600 s from each data point at time t. The three drift-free strain-gauge recordings were used to calculate the compressive and tensile principal-strain signals. In the sections of the Results describing associations in time between masseter and digastric muscle bursts and mandibular strain, the tensile principal strain is used in the figures for clarity purposes (deviations from zero pointing upwards). Distribution histograms were made of the principal-strain amplitudes of all strain events of which the peak base lay below the threshold of 20 microstrain (με) and the peak maximum crossed the threshold of 30 με, safely above noise. The histograms had a bin width of 12.5 με.
For further analysis of the relation between masseter muscle activity and mandibular deformations, the compressive principal strain was used as compression at the site of measurement was expected to have a more clear relation to contractions of the masseter than tension. For six amplitude levels of compressive principal strain (10 ± 3 με, 35 ± 3 με, 75 ± 10 με, 150 ± 10 με, 300 ± 10 με, and 500 ± 10 με) accompanying masseter-burst amplitudes were collected. Amplitudes of compressive principal strain > 500 με were very rare and could not be included in the analysis. For each of the strain levels, 11 masseter bursts were collected by searching a strain event with the specific amplitude (e.g. −10 ± 3 με), writing down the accompanying muscle-burst amplitude, and doing so at 11 time points divided evenly over the length of the entire measurement per rabbit.
To evaluate whether the masseter muscle plays different roles in more intense (including feeding-behaviour-related) vs. less intense loading of the mandible, the average number of masseter-muscle bursts per h was compared to the average number of bone-strain events per h. This comparison was made for two amplitude domains which represented the more intense and less intense parts of loading. Chewing is a more intense cyclic masticatory behaviour eliciting compressive principal bone strains with amplitudes of 149–320 με on the working side of the mandible (Weijs & de Jongh, 1977). Using the masseter activity levels found with compressive principal strains of 150 ± 10 με, a division was made in the total count of masseter bursts above and below the mV level associated with this compressive principal-strain magnitude for each rabbit. The number of masseter bursts per strain event was assumed to be an indication of the role of the masseter in these two ranges of mandibular loading.
To quantify variation in the direction of the principal strains, the angle ϕ between the direction of the first principal strain and the orientation of gauge A of the strain-gauge rosette was calculated for 70 bone-strain events per rabbit. These strain events were collected from seven sites evenly divided over each mandibular bone-strain recording – including 10 successive strain events at each site. In this strain-event collection some care was taken to include larger strain events (i.e. compressive principal strain ≥ 150 με), which in practice meant ‘scrolling’ through the strain recordings away from a site to nearby large strain peaks. The direction of the first principal strain is oriented at an angle of 90° from the direction of the second principal strain. However, for eight rabbits, we simply used the angle ϕ (without adding or subtracting 90°) to plot against the second principal-strain amplitude in scatter distributions. The following formulas were used for the calculation of angle ϕ and the second principal strain:
in which εA, εB and εC were, respectively, the drift-removed strain values measured by the individual gauges A, B and C from the gauge rosette (Fig. 1), and ε2 is the second principal strain, i.e. the compressive principal strain.
The relation between digastric muscle activity and bone strain was too weak and therefore no such analyses were done for this muscle.
Statistics
The EMG amplitudes co-appearing with the various levels of compressive strain were tested for differences using two-tailed paired t-tests. Differences in mean EMG amplitude per compressive-strain level were considered significant when P ≤ 0.05.
Results
The rabbits recovered quickly from the placement of the two transmitters and started feeding and exploring as soon as 3 h after surgery. Simultaneous telemetric recordings of masticatory muscle activity and mandibular bone strain were started on the day of surgery. The average simultaneous recording length of muscle activity and bone strain was 16.6 ± 6.6 h (n = 10).
Throughout all recordings, longer and shorter bouts of activity of the masseter muscle coincided clearly with bouts of mandibular bone strain. The time between these bouts was filled with multitudes of isolated muscle bursts and co-appearing strain events (Fig. 2A). The co-appearance was especially pronounced for periods of higher muscle-activity levels and greater bone-strain amplitudes (> 150 με), which included chewing (WC de Jong, personal observation). On a smaller time scale the co-appearance of muscle-activity bouts and strain-event bouts, as well as of the isolated bursts and events, is illustrated even more clearly (Fig. 2B). Within bouts there was no correspondence between the individual muscle-burst amplitudes – in raw or root-mean-squared form – and the bone-strain event amplitudes (Fig. 2C). Occasionally, the level of an entire masseter-activity bout did not correspond at all with the amplitude of the accompanying strain-event series (Fig. 3). Between the bouts of more intense repetitive and rhythmic mandibular loading thousands of isolated strain events occurred, which were almost always accompanied by masseter muscle bursts (Fig. 4).
Fig. 2.
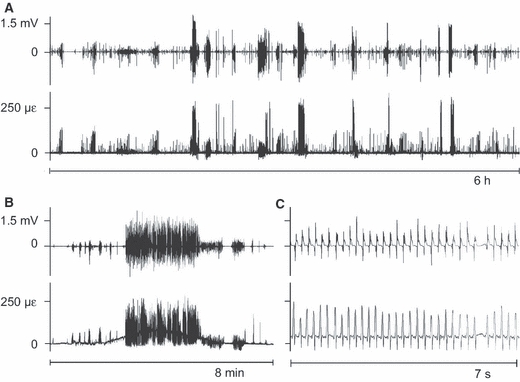
Representative stretches of unprocessed masseter electromyogram (EMG, upper graphs in each time window) and tensile principal bone strain (lower graphs). mV, millivolt, με, microstrain. Panels B and C have identical y-axes. Note the co-appearance throughout the recordings of masseter-activity bouts and strain-event episodes (A,B). This co-appearance persists to the level of individual muscle bursts and strain events (C). Negative values in the tensile principal strain graphs are an artefact of the drift-removal procedure which was applied before calculation of the principal strains.
Fig. 3.
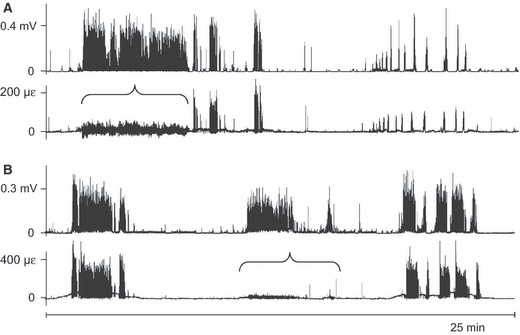
Two 25-min examples that include discordances in amplitude between co-appearing episodes of masseter activity and bone-strain events. The examples in A and B were taken from two different rabbits. In both A and B the upper graph is the electromyogram (processed with a root-mean-square function over Δt = 0.040 s) and the lower graph is the tensile principal bone strain. Horizontal braces indicate where strain amplitudes are lower compared to those co-appearing with muscle-activity bouts of the same burst activity level. Negative values in the tensile principal strain graphs are an artefact of the drift-removal procedure which was applied before calculation of the principal strains. Note that A and B have different y-axes.
Fig. 4.
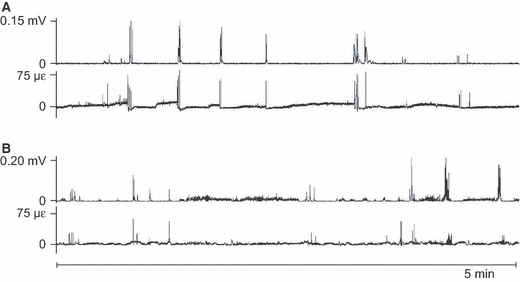
Representative 5-min stretches of root-mean-squared (Δt = 0.040 s) masseter electromyograms (upper graphs in A and B) and the simultaneously recorded mandibular tensile principal strain. Panels A and B were taken from different rabbits. Mandibular bone-strain events, although outside of cyclic loading bouts, are primarily the result of masseter activity.
The relation in time between activity of the digastric muscle and mandibular bone strain was not as pronounced as between the masseter muscle and mandibular bone strain. There were no clearly delineated bouts of digastric activity and bone strain (Fig. 5A,B). A one-on-one co-appearance of digastric bursts and strain events was present only occasionally (Fig. 5B). A relationship in the amplitudes of co-appearing digastric muscle bursts and bone-strain events was absent (Fig. 5C).
Fig. 5.
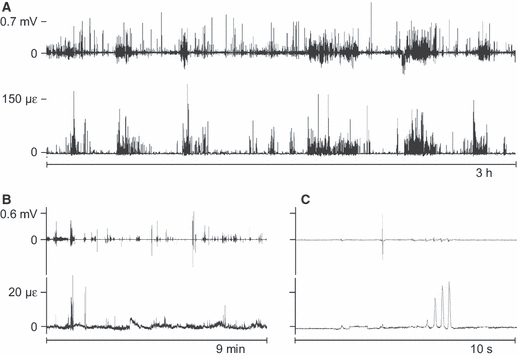
Representative stretches of unprocessed digastric electromyograms (EMG, upper graphs in each time window) and tensile principal bone strain (lower graphs). mV, millivolt, με, microstrain. Panels B and C have identical y-axes. On a larger time scale (A) some relation in time between digastric activity and mandibular bone strain can be detected. On smaller time scales (B,C), co-appearance of digastric bursts and strain events can also be seen, but there is no relation in amplitude.
In some instances, neither masseter nor digastric activity seemed to have a clear relation to the simultaneously recorded mandibular strain events (Fig. 6).
Fig. 6.
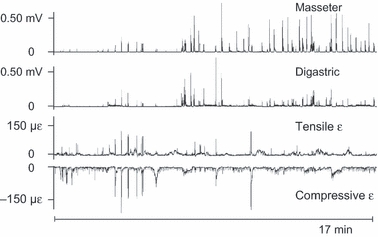
Two 17-min stretches of masseter and digastric electromyograms with the simultaneously recorded tensile and compressive principal strains. The EMGs are root-mean-squared (Δt = 0.040 s). Here, neither masseter nor digastric activity corresponds clearly with the bone-strain recording, although some co-appearance of muscle bursts and bone-strain events can be seen in the first 6 min.
Passages of co-appearing rhythmic masseter muscle bursts and strain events were manifold for the frequency of 5 Hz (Fig. 2C), well known as the frequency at which food is chewed and possibly a frequency used for all sorts of oral behaviours in the rabbit. Incidentally, masseter muscle bursts were found to coincide with the strain events they elicited up to frequencies of 15 Hz (Fig. 7). This illustrates that high-frequency muscular contractions may cause bone deformations with the same frequency.
Fig. 7.
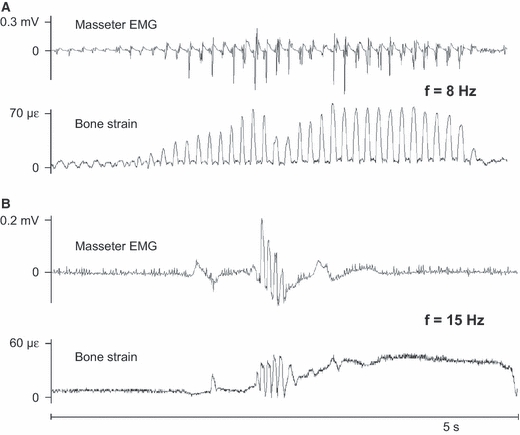
Two 5-s example recordings of high-frequency force transmission. Note that the amplitude scales differ between A and B. The examples were taken from two different rabbits. In both A and B the upper graphs are raw masseter electromyograms, used here because the root-mean-square function mostly removes high-frequency content, and the lower graphs are the tensile principal strain. In A the masseter muscle strains the mandible at a frequency of 8 Hz and in B the masseter muscle strains the mandible at a frequency of 15 Hz. mV, millivolt, με, microstrain.
In most rabbits, significantly increasing masseter-burst amplitudes were found with increasing compressive principal-strain amplitudes (Table 1). However, masseter bursts eliciting compressive principal strains of 500 με were not larger than those responsible for compressive principal strains of 300 με (Table 1). Above and below the masseter muscle activity level associated with the principal-strain amplitude of −150 με, the burst-number-to-strain-event ratio was the same (Table 2). On average, per hour, three times more masseter bursts were detected above the 5% activity level than strain events above the 30 με level. The interindividual variation was large, however, and in one rabbit 10 times more masseter bursts than strain events were registered, both above and below the −150 με level. Distribution histograms of the amplitudes of bursts of masseter and digastric activity and of bone-strain events displayed an exponential decrease in occurrence of greater amplitudes, but there was no clear resemblance between muscle-burst and bone-strain amplitude distributions (Fig. 8). Compared to the digastric muscle, masseter muscle activity contained more bursts for amplitudes above 20% of the peak voltage. Using the compressive principal bone strain, more strain events could be detected above the 30 με level, as evidenced by the larger area occupied by the event counts on the negative, i.e. compression amplitude half of the histogram compared to the positive, i.e. tension amplitude half.
Table 1.
Masseter burst amplitudes, from electromyograms (EMGs) root-mean-squared over Δt = 0.040 s, co-appearing with specific amplitudes of compressive principal bone strain. Masseter activity is expressed as a percentage of the peak burst voltage found in each recording. Shown are the means ± SD of 11 muscle bursts per bone-strain amplitude per rabbit. ‘–’ indicates that not enough strain events were found for that specific amplitude level. με, microstrain
| Compressive principal-strain amplitude level (με) | |||||||
|---|---|---|---|---|---|---|---|
| Individual | 10 | 35 | 75 | 150 | 300 | 500 | |
| Masseter muscle activity ± SD(% of peak voltage) | 1 | 5.5 ± 2.7 | 6.1 ± 1.1 | 16.9 ± 7.5* | 29.3 ± 6.8* | 41.0 ± 8.4* | 44.3 ± 10.1 |
| 2 | 3.8 ± 2.0 | 11.1 ± 5.8* | 19.4 ± 6.9* | 14.9 ± 3.8 | 33.4 ± 5.5* | 27.9 ± 6.2 | |
| 3 | 2.0 ± 1.6 | 6.5 ± 2.5* | 5.4 ± 3.3 | – | – | – | |
| 4 | 3.2 ± 1.5 | 9.4 ± 5.6* | 19.8 ± 11.0* | 40.9 ± 12.1* | 48.9 ± 14.0 | – | |
| 5 | 4.3 ± 1.3 | 14.2 ± 3.1* | 13.3 ± 4.6 | 18.2 ± 6.6* | 27.2 ± 7.5* | 32.3 ± 5.1 | |
| 6 | 7.1 ± 4.6 | 32.8 ± 10.1* | 54.5 ± 21.8* | 50.6 ± 9.6 | – | – | |
| 7 | 5.3 ± 3.7 | 12.8 ± 5.4* | 37.5 ± 14.8* | 75.0 ± 12.3* | – | – | |
| 8 | 5.3 ± 2.8 | 9.0 ± 3.5* | 15.6 ± 3.7* | 28.0 ± 6.5* | – | – | |
| Mean ± SD | 4.5 ± 1.6 | 12.7 ± 8.6 | 22.8 ± 15.7 | 36.7 ± 20.9 | 37.6 ± 9.4 | 34.8 ± 8.5 | |
Significantly larger than the EMG amplitudes from that rabbit accompanying the first lower bone compression level; P < 0.05.
Table 2.
Counts per hour of compressive strain events below and above the level of 150 με and masseter-muscle bursts below and above the mV level causing a compressive principal strain of 150 με. Only strain events with amplitudes > 30 με and muscle bursts above the 5% activity level are included in the counts (see Materials and methods section). In individual 3, no distinction could be made in the masseter bursts as there were not enough large strain events to measure the masseter burst level causing a compressive principal strain of 150 με (see Table 1). με, microstrain, mV, millivolt
| Individual | Events per hour ≤ 150 με | Bursts per hour ≤ mV150 με | Bursts/events | Events per hour > 150 με | Bursts per hour > mV150 με | Bursts/events |
|---|---|---|---|---|---|---|
| 1 | 1462 | 1440 | 1.0 | 213 | 277 | 1.3 |
| 2 | 981 | 496 | 0.5 | 130 | 257 | 2.0 |
| 3 | 159 | – | – | 4 | – | – |
| 4 | 512 | 757 | 1.5 | 103 | 207 | 2.0 |
| 5 | 586 | 1117 | 1.9 | 254 | 541 | 2.1 |
| 6 | 322 | 3094 | 9.6 | 8 | 81 | 10.1 |
| 7 | 769 | 4771 | 6.2 | 6 | 8 | 1.3 |
| 8 | 665 | 656 | 1.0 | 22 | 41 | 1.9 |
| Mean ± SD | 3.1 ± 3.5 | 3.0 ± 3.2 |
Fig. 8.
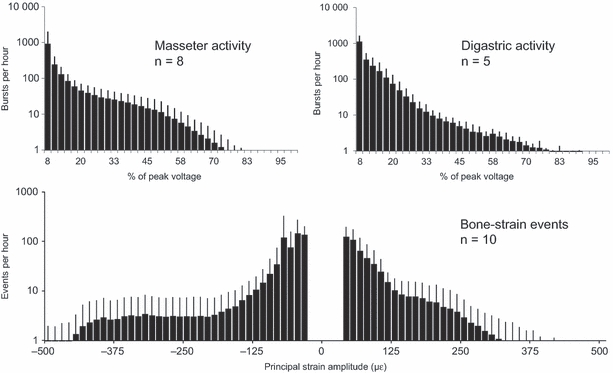
Average amplitude distributions of habitual masseter muscle bursts (n = 8), digastric muscle bursts (n = 5), and bone-strain events (n = 10). με, microstrain. Note that the y-axes have log scales. The thin bars indicate standard deviations. Per hour, there are more masseter bursts than digastric bursts above the 20% activity level. Negative strain amplitudes refer to compressive strain.
Greater amplitudes of compression were related to more constant values for the principal-strain orientation (Fig. 9). In most rabbits there was a tendency for angle ϕ to have values between 20 and 30° for second principal-strain amplitudes below −150 με. Therefore, the direction of the second principal strain was more horizontal than the orientation of gauge C when compression amplitudes exceeded the 150 με level.
Fig. 9.
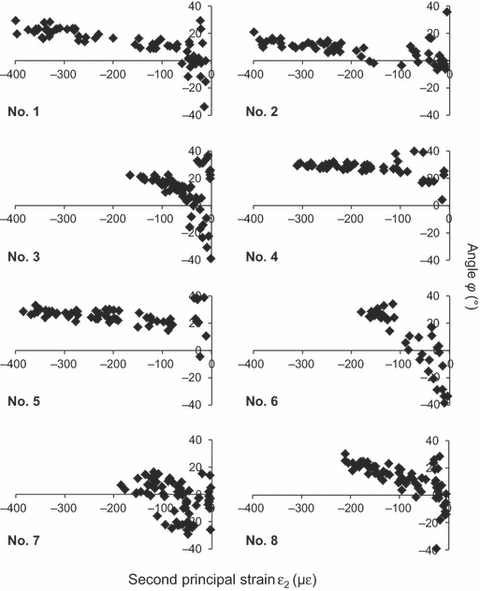
Scatter distributions of the angle ϕ plotted against the second principal-strain amplitude. Each dot indicates one bone-strain event. Positive angles are oriented counter-clockwise from the rostroventrally orientated gauge A, negative angles clockwise. The numbers in the lower left corners indicate the rabbit individuals. Note the tendency of ϕ to have more constant values with increasing amplitude of bone compression.
Discussion
Our measurements reveal masseter muscle contractions to be a primary contributor to the ongoing loading history of the rabbit mandible. The role of the masseter muscle was not confined to bouts of more intense cyclic loading, which include chewing, but also comprised thousands of isolated loading events with amplitudes mostly smaller than those of the bouts of cyclic loading. Masseter electromyograms greatly resembled mandibular bone-strain recordings in terms of co-appearing muscle bursts and strain events throughout entire recordings. However, the average number of masseter bursts per hour was greater than the average number of bone-strain events per hour (Table 2). One explanation is the exclusion from our analysis of strain events with principal amplitudes smaller than 30 με, although this does not explain the larger number of more intense masseter bursts compared to the number of strain events with principal amplitudes > 150 με. It is possible that not all registered masseter bursts resulted in a measurable mandibular bone strain.
Occasions where bone-strain events were not accompanied by a masseter activity burst were rare. Both larger and smaller bone-strain events featured co-appearing masseter bursts, almost without exception, suggesting equal roles of the masseter muscle in both high-intensity and low-intensity loading. Greater amplitudes of mandibular bone strain were related to both greater amplitudes of masseter activity and to a more constant orientation of the principal-strain directions. During large-amplitude loading events, the direction of the second principal strain in the surface of the mandibular corpus was aligned more horizontally. This could mean that the working line of the masseter muscle becomes more constant when the muscle exerts larger forces. This, however, is disputable as no other jaw-closing muscles were sampled and their contribution to larger strain events is unknown.
Other masticatory muscles than the masseter and the digastric will load the mandible, but no electromyograms were made of these muscles. Although the loads they exert on the mandible may cause strain events not accompanied by a registered masseter burst, the majority of their contractions are likely paralleled by the activity of the sampled masseter muscle. Especially during chewing, masticatory muscles primarily work in triplets: the masseter and medial pterygoid muscles of one side and the temporal muscle of the contralateral side (Langenbach & van Eijden, 2001). Although chewing is the main function of the masticatory apparatus, the daily loading history of the mandible includes much more than the short bouts of this behaviour. In all these oral behaviours the masseter muscle seems to play a main role, as indicated by the close fit between its activity and the mandibular bone strain throughout the day.
An amplitude relation between masseter activity and mandibular strain was present only on a coarse scale, evidenced by the visually matching amplitudes of complete bouts of muscle activity and bone strain and the increasing EMG amplitudes found for increasing compressive bone-strain levels. On a fine amplitude scale, amplitudes of individual masseter bursts were unrelated to those of their strain-event counterparts. This can be explained firstly by bone deformation being the result of several loads, of which masseter activity is only one. The activity of the other masticatory muscles, the facial muscles, the suprahyoid muscles, as well as additional reaction forces from incisors, molars and temporomandibular joints will load the mandible also. Second, concerning chewing bouts, the level of activation of each of the masticatory muscles and their exact activation pattern in time will vary from cycle to cycle, as the position and mechanical properties of the masticated food will change continuously (Morimoto et al. 1985). In Fig. 3, the discordance of masseter EMG amplitudes and strain amplitudes of complete activity bouts might simply be the result of a switch of working side and balancing side during mastication. Third, we sampled only the superficial part of the masseter muscle. As the rabbit masseter is extremely compartmentalised by aponeuroses (Schumacher & Rehmer, 1960; Weijs & Dantuma, 1981; Widmer et al. 1997), the magnitudes and directions of its contractions cannot always be captured by one or two EMG channels. Obviously, reaction forces on the mandible from outside of the rabbit – which would occur were the animal to, for example, scratch its head – might also have caused bone-strain events. Video registrations of the laboratory animal might have explained the behaviour behind some of those events. However, earlier attempts to film rabbit behaviours made it clear that many activities of the masticatory muscles were simply invisible from the outside of the animal.
Amplitudes of digastric muscle activity resembled very weakly or not at all those of mandibular bone strain. Digastric muscles are jaw openers and jaw opening generally does not need large muscle forces (Weijs & Muhl, 1987), nor does it bring about large reaction forces. The reaction forces that are present during opening arise from passive tensions in the soft tissues that cover the mandible and from the loads in the jaw joints. These loads are small and far away from the site of strain measurement and will therefore only elicit small bone strains near and underneath the gauge rosette. In contrast, the masseter is active mainly when the mandible cannot close any further, which results in larger bone-strain amplitudes. In addition, digastric muscles have small cross-sectional areas and lower weights compared to the masseter and pterygoid muscles (Weijs & Dantuma, 1981; Langenbach & Weijs, 1990) and, consequently, can exert only smaller maximal forces on the mandible. Situations in which the digastric muscles are more active than the masseter muscles, such as during grooming and limb licking (Yamada et al. 1993), could have given rise to instances of better resemblance between digastric activity and mandibular bone strain.
The data presented in this paper illustrate that the mechanical link between jaw-muscle activity and loading of the mandibular bone is strong. This strong mechanical link might explain the known functional relation between jaw-muscle activity and mandibular bone growth, modelling and maintenance. During growth in utero the presence of active masticatory muscles is known to be essential to a proper shaping of the mandible (Rot-Nikcevic et al. 2006). Impaired force output of masticatory muscles during post-natal growth results in a retarded mandibular bone growth as well (Kwon et al. 2007; Matic et al. 2007). In adulthood the functionality of the mechanical relation between masticatory muscle activity and mandibular bone morphology remains. Botulinum-toxin treatment of the masseter muscle in adult rats induces architectural changes in the mandible (Tsai et al. 2010). As injection of botulinum toxin type A into the masseter muscle is a procedure performed more and more, either for aesthetic (Kim et al. 2010; Wu, 2010) or medical purposes (Daelen et al. 1997; Tan & Jankovic, 2000), this might be of some consideration to the clinic.
Low-magnitude, high-frequency mechanical loads have been associated with anabolic effects on bone (Rubin et al. 2001; Midura et al. 2005; Goodship et al. 2009). Our data unveil that masticatory muscle bursts at 5% of peak activity are associated with small strains in the mandibular bone (Table 1). We found that masseter and digastric bursts at that activity level may take place at least 1000 times per hour (Fig. 8,Table 2), which is in accordance with earlier publications (van Wessel et al. 2005). Also, the frequency component of muscle activity is still very strong above 5 Hz and even 15 Hz force transmissions to bone were detected (Fig. 7). Therefore, the present study supports the possibility that part of all habitually occurring high-frequency strains might have their origin in muscle activity and that these strains quite possibly stimulate homeostatic bone turnover (Rubin et al. 1990; Turner et al. 1995; Fritton et al. 2000; Judex & Rubin, 2010).
This study demonstrates that wireless and simultaneous measurement of muscle activity and bone-surface strain in vivo is feasible for middle-sized and larger animals up to about 1 day. However, our methodology has limitations. Firstly, muscle activity and bone strain were recorded with two separate systems. Due to minute periods of signal dropout and variation in hardware clock rates, the two signals could not always be synchronised. Second, the synchronisation of the strain recordings and electromyograms was performed visually. Although a matching between a series of muscle-activity bursts and bone-strain events can be attained fairly easily, the exact relation in time of an individual pair of one muscle burst and the subsequent bone-strain event could not be studied objectively. Excitation-contraction lag times are known to be short for jaw-closing muscles, though, commonly featuring values from ∼ 13 ms to peak twitch tension in the cat masseter (Taylor et al. 1973), to ∼ 30 ms in the macaque masseter (Hylander & Johnson, 1993), to ∼ 43 ms in the pig temporalis (Teng & Herring, 1998). An implantable transmitter with sensors able to capture both muscle biopotentials and bone deformation would be more effective in quantifying the role of muscle activity in the daily habitual loading of bone.
To conclude, our results reveal that in the masticatory apparatus of the rabbit, jaw muscles play the main role in the ongoing habitual loading of the mandible. The mandible is loaded by jaw muscles not only during repetitive behaviours, such as chewing, but also almost continuously throughout the day outside of these cyclic bouts. Most bone-strain events can be attributed to masseter-muscle activity. Activity of the digastric muscle corresponds only weakly with the occurrence of mandibular strain events. A relation in masseter electromyogram amplitudes and bone-strain amplitudes exists on a coarse scale. Furthermore, muscle forces can dynamically strain the mandible up to frequencies of ∼ 15 Hz. The orientation of the principal strains become more constant with increasing strain amplitudes. This might indicate that the muscles loading the mandible during such high-intensity events have more constant working-line directions during larger force output.
Acknowledgments
We thank our colleagues at the Animal Research Institute Amsterdam (ARIA, Academic Medical Centre of the University of Amsterdam) for their kind and helpful assistance with the in vivo experiments, David Crick from Cambridge Electronic Design Limited for explaining specific features of spike2, Leo van Ruijven for providing customised software, and Vincent Everts for critical comments on the manuscript.
References
- Burr DB. Muscle strength, bone mass, and age-related bone loss. J Bone Miner Res. 1997;12:1547–1551. doi: 10.1359/jbmr.1997.12.10.1547. [DOI] [PubMed] [Google Scholar]
- Cochran GVB. Implantation of strain gages on bone in vivo. J Biomech. 1972;5:119–123. doi: 10.1016/0021-9290(72)90024-3. [DOI] [PubMed] [Google Scholar]
- Daelen B, Thorwirth V, Koch A. Treatment of recurrent dislocation of the temporomandibular joint with type A botulinum toxin. Int J Oral Maxillofac Surg. 1997;26:458–460. doi: 10.1016/s0901-5027(97)80014-8. [DOI] [PubMed] [Google Scholar]
- Daly RM, Saxon L, Turner CH, et al. The relationship between muscle size and bone geometry during growth and in response to exercise. Bone. 2004;34:281–287. doi: 10.1016/j.bone.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Fritton SP, McLeod KJ, Rubin CT. Quantifying the strain history of bone: spatial uniformity and self-similarity of low-magnitude strains. J Biomech. 2000;33:317–325. doi: 10.1016/s0021-9290(99)00210-9. [DOI] [PubMed] [Google Scholar]
- Goodship AE, Lawes TJ, Rubin CT. Low-magnitude high-frequency mechanical signals accelerate and augment endochondral bone repair: preliminary evidence of efficacy. J Orthop Res. 2009;27:922–930. doi: 10.1002/jor.20824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylander WL, Johnson KR. The relationship between masseter force and masseter electromyogram during mastication in the monkey Macaca fascicularis. Arch Oral Biol. 1989;34:713–722. doi: 10.1016/0003-9969(89)90078-2. [DOI] [PubMed] [Google Scholar]
- Hylander WL, Johnson KR. Modelling relative masseter force from surface electromyograms during mastication in non-human primates. Arch Oral Biol. 1993;38:233–240. doi: 10.1016/0003-9969(93)90033-i. [DOI] [PubMed] [Google Scholar]
- Hylander WL, Johnson KR, Crompton AW. Loading patterns and jaw movements during mastication in Macaca fascicularis: a bone-strain, electromyographic, and cineradiographic analysis. Am J Phys Anthropol. 1987;72:287–314. doi: 10.1002/ajpa.1330720304. [DOI] [PubMed] [Google Scholar]
- de Jong WC, Koolstra JH, van Ruijven LJ, et al. A fully implantable telemetry system for the long-term measurement of habitual bone strain. J Biomech. 2010a;43:587–591. doi: 10.1016/j.jbiomech.2009.09.036. [DOI] [PubMed] [Google Scholar]
- de Jong WC, Koolstra JH, Korfage JAM, et al. The daily habitual in vivo strain history of a non-weight-bearing bone. Bone. 2010b;46:196–202. doi: 10.1016/j.bone.2009.10.026. [DOI] [PubMed] [Google Scholar]
- de Jong WC, Korfage JAM, Langenbach GEJ. Variations in habitual bone strains in vivo: long bone versus mandible. J Struct Biol. 2010c;172:311–318. doi: 10.1016/j.jsb.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Judex S, Rubin CT. Is bone formation induced by high-frequency mechanical signals modulated by muscle activity? J Musculoskelet Neuronal Interact. 2010;10:3–11. [PMC free article] [PubMed] [Google Scholar]
- Kannus P, Haapasalo H, Sievänen H, et al. The site-specific effects of long-term unilateral activity on bone mineral density and content. Bone. 1994;15:279–284. doi: 10.1016/8756-3282(94)90289-5. [DOI] [PubMed] [Google Scholar]
- Kim JY, Kim ST, Cho SW, et al. Growth effects of botulinum toxin type A injected into masseter muscle on a developing rat mandible. Oral Dis. 2008;14:626–632. doi: 10.1111/j.1601-0825.2007.01435.x. [DOI] [PubMed] [Google Scholar]
- Kim NH, Park RH, Park JB. Botulinum toxin type A for the treatment of hypertrophy of the masseter muscle. Plast Reconstr Surg. 2010;125:1693–1705. doi: 10.1097/PRS.0b013e3181d0ad03. [DOI] [PubMed] [Google Scholar]
- Kwon TG, Park HS, Lee SH, et al. Influence of unilateral masseter muscle atrophy on craniofacial morphology in growing rabbits. J Oral Maxillofac Surg. 2007;65:1530–1537. doi: 10.1016/j.joms.2006.10.059. [DOI] [PubMed] [Google Scholar]
- Langenbach GEJ, van Eijden TMGJ. Mammalian feeding motor patterns. Am Zool. 2001;41:1338–1351. [Google Scholar]
- Langenbach GEJ, Weijs WA. Growth patterns of the rabbit masticatory muscles. J Dent Res. 1990;69:20–25. doi: 10.1177/00220345900690010201. [DOI] [PubMed] [Google Scholar]
- Langenbach GEJ, van Ruijven LJ, van Eijden TMGJ. A telemetry system to chronically record muscle activity in middle-sized animals. J Neurosci Methods. 2002;114:197–203. doi: 10.1016/s0165-0270(01)00528-3. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Green JR, Moore CA, et al. Time series analysis of jaw muscle contraction and tissue deformation during mastication in miniature pigs. J Oral Rehabil. 2004;31:7–17. doi: 10.1111/j.1365-2842.2004.01156.x. [DOI] [PubMed] [Google Scholar]
- MacIntyre NJ, Adachi JD, Webber CE. In vivo detection of structural differences between dominant and nondominant radii using peripheral quantitative computed tomography. J Clin Densitom. 1999;2:413–422. doi: 10.1016/s1094-6950(06)60407-1. [DOI] [PubMed] [Google Scholar]
- Matic DB, Yazdani A, Glenn Wells R, et al. The effects of masseter muscle paralysis on facial bone growth. J Surg Res. 2007;139:243–252. doi: 10.1016/j.jss.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Midura RJ, Dillman CJ, Grabiner MD. Low amplitude, high frequency strains imposed by electrically stimulated skeletal muscle retards the development of osteopenia in the tibiae of hindlimb suspended rats. Med Eng Phys. 2005;27:285–293. doi: 10.1016/j.medengphy.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Inoue T, Nakamura T, et al. Characteristics of rhythmic jaw movements of the rabbit. Arch Oral Biol. 1985;30:673–677. doi: 10.1016/0003-9969(85)90154-2. [DOI] [PubMed] [Google Scholar]
- Nuijens FW, Snelderwaard PC, Bout RG. An electromyographic technique for small animals. J Neurosci Methods. 1997;76:71–73. doi: 10.1016/s0165-0270(97)00081-2. [DOI] [PubMed] [Google Scholar]
- Poliachik SL, Bain SD, Threet D, et al. Transient muscle paralysis disrupts bone homeostasis by rapid degradation of bone morphology. Bone. 2010;46:18–23. doi: 10.1016/j.bone.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rot-Nikcevic I, Reddy T, Downing KJ, et al. Myf5−/−:MyoD−/− amyogenic fetuses reveal the importance of early contraction and static loading by striated muscle in mouse skeletogenesis. Dev Genes Evol. 2006;216:1–9. doi: 10.1007/s00427-005-0024-9. [DOI] [PubMed] [Google Scholar]
- Rubin CT, McLeod KJ, Bain SD. Functional strains and cortical bone adaptation: epigenetic assurance of skeletal integrity. J Biomech. 1990;23:43–54. doi: 10.1016/0021-9290(90)90040-a. [DOI] [PubMed] [Google Scholar]
- Rubin CT, Sommerfeldt DW, Judex S, et al. Inhibition of osteopenia by low magnitude, high-frequency mechanical stimuli. Drug Discov Today. 2001;6:848–858. doi: 10.1016/s1359-6446(01)01872-4. [DOI] [PubMed] [Google Scholar]
- Schoenau E, Fricke O. Interaction between muscle and bone. Horm Res. 2006;66:73–78. [Google Scholar]
- Schumacher GH, Rehmer H. Morphologische und funktionelle Untersuchungen an der Kaumuskulatur von Oryctolagus und Lepus. Gegenbaurs Morphol Jahrb. 1960;100:678–705. [Google Scholar]
- Shaw CN, Stock JT. Habitual throwing and swimming correspond with upper limb diaphyseal strength and shape in modern human athletes. Am J Phys Anthropol. 2009;140:160–172. doi: 10.1002/ajpa.21063. [DOI] [PubMed] [Google Scholar]
- Tan EK, Jankovic J. Treating severe bruxism with botulinum toxin. J Am Dent Assoc. 2000;131:211–216. doi: 10.14219/jada.archive.2000.0149. [DOI] [PubMed] [Google Scholar]
- Taylor A, Cody FWJ, Bosley MA. Histochemical and mechanical properties of the jaw muscles of the cat. Exp Neurol. 1973;38:99–109. doi: 10.1016/0014-4886(73)90011-3. [DOI] [PubMed] [Google Scholar]
- Teng S, Herring SW. Compressive loading on bone surfaces from muscular contraction: an in vivo study in the miniature pig, Sus scrofa. J Morphol. 1998;238:71–80. doi: 10.1002/(SICI)1097-4687(199810)238:1<71::AID-JMOR6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Tsai CY, Huang RY, Lee CM, et al. Morphologic and bony structural changes in the mandible after a unilateral injection of botulinum neurotoxin in adult rats. J Oral Maxillofac Surg. 2010;68:1081–1087. doi: 10.1016/j.joms.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Turner CH, Yoshikawa T, Forwood MR, et al. High frequency components of bone strain in dogs measured during various activities. J Biomech. 1995;28:39–44. doi: 10.1016/0021-9290(95)80005-0. [DOI] [PubMed] [Google Scholar]
- Warner SE, Sanford DA, Becker BA, et al. Botox induced muscle paralysis rapidly degrades bone. Bone. 2006;38:257–264. doi: 10.1016/j.bone.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijs WA, Dantuma R. Functional anatomy of the masticatory apparatus in the rabbit (Oryctolagus cuniculus L.) Neth J Zool. 1981;31:99–147. [Google Scholar]
- Weijs WA, de Jongh HJ. Strain in mandibular alveolar bone during mastication in the rabbit. Arch Oral Biol. 1977;22:667–675. doi: 10.1016/0003-9969(77)90096-6. [DOI] [PubMed] [Google Scholar]
- Weijs WA, Muhl ZF. The effects of digastric muscle tenotomy on jaw opening in the rabbit. Arch Oral Biol. 1987;32:347–353. doi: 10.1016/0003-9969(87)90090-2. [DOI] [PubMed] [Google Scholar]
- van Wessel T, Langenbach GEJ, van Ruijven LJ, et al. Daily number and lengths of activity bursts in rabbit jaw muscles. Eur J Neurosci. 2005;21:2209–2216. doi: 10.1111/j.1460-9568.2005.04054.x. [DOI] [PubMed] [Google Scholar]
- van Wessel T, Langenbach GEJ, Brugman P, et al. Daily activity of the rabbit jaw muscles during early postnatal development. Neuroscience. 2006;140:137–146. doi: 10.1016/j.neuroscience.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Widmer CG, Klugman D, English AW. Anatomical partitioning and nerve branching patterns in the adult rabbit masseter. Acta Anat (Basel) 1997;159:222–232. doi: 10.1159/000147988. [DOI] [PubMed] [Google Scholar]
- Wu WTL. Botox facial slimming/facial sculpting: the role of botulinum toxin-A in the treatment of hypertrophic masseteric muscle and parotid enlargement to narrow the lower facial width. Facial Plast Surg Clin North Am. 2010;18:133–140. doi: 10.1016/j.fsc.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Haraguchi N, Uchida K, et al. Jaw movements and EMG activities of limb-licking behaviour during grooming in rabbits. Physiol Behav. 1993;53:301–307. doi: 10.1016/0031-9384(93)90208-w. [DOI] [PubMed] [Google Scholar]


