Abstract
Closure of the middle ear is believed to be closely related to the evolutionary development of the mammalian jaw. However, few comprehensive descriptions are available on fetal development. We examined paraffin-embedded specimens of 20 mid-term human fetuses at 8–25 weeks of ovulation age (crown-rump length or CRL, 38–220 mm). After 9 weeks, the tympanic bone and the squamous part of the temporal bone, each of which was cranial or caudal to Meckel's cartilage, grew to close the lateral part of the tympanosquamosal fissure. At the same time, the cartilaginous tegmen tympani appeared independently of the petrous part of the temporal bone and resulted in the petrosquamosal fissure. Subsequently, the medial part of the tympanosquamosal fissure was closed by the descent of a cartilaginous inferior process of the tegmen tympani. When Meckel's cartilage changed into the sphenomandibular ligament and the anterior ligament of the malleus, the inferior process of the tegmen tympani interposed between the tympanic bone and the squamous part of the temporal bone, forming the petrotympanic fissure for the chorda tympani nerve and the discomalleolar ligament. Therefore, we hypothesize that, in accordance with the regression of Meckel's cartilage, the rapidly growing temporomandibular joint provided mechanical stress that accelerated the growth and descent of the inferior process of the tegmen tympani via the discomalleolar ligament. The usual diagram showing bony fissures around the tegmen tympani may overestimate the role of the tympanic bone in the fetal middle-ear closure.
Keywords: discomalleolar ligament, human fetus, middle ear, petrosquamosal fissure, petrotympanic fissure, tegmen tympani, tympanosquamosal fissure
Introduction
A closed middle ear is believed to have evolved in close relation to the development of the mammalian jaw (Allin, 1975), although in the long research history of this topic, the discussion has tended to concentrate on origins of the mammalian ear ossicles (Goodrich, 1930). The author of the present work also contributed to an understanding of the fetal origins of the human ear ossicles (Rodríguez-Vázquez, 2005, 2009; Rodríguez-Vázquez et al. 2006). However, no comprehensive description is available on the human fetal development of the temporal bone components (the tympanic bone, tegmen tympani, otic capsule, petrous part or squamous part) that cover or enclose the temporomandibular joint (TMJ) and the middle ear. By what steps did these bones close the middle ear?
Many key structures suggest a close relation between the middle-ear closure and TMJ development: (i) the tympanosquamosal fissure, which runs across the glenoid fossa of the temporal bone and which is subdivided by the tegmen tympani into the anterior and posterior fissures, i.e. the petrosquamosal fissure and the petrotympanic fissure (Paturet, 1951; Testut & Latarjet, 1975; Williams & Warwick, 1985; Rouvière & Delmas, 1987); (ii) the discomalleolar ligament, connecting the TMJ disk to the malleus (Kjellberg, 1904; Moffett, 1957; Coleman, 1970; Rodríguez-Vázquez et al. 1998); (iii) Meckel's cartilage, which also connects to the TMJ disk (Symons, 1952; Perry et al. 1985; Wong et al. 1985; Rodríguez-Vázquez et al. 1993; Mérida-Velasco et al. 1999); (iv) the os goniale, contributing to the formation of a part of the malleus (Rodríguez-Vázquez et al. 1992); and (v) the chorda tympani nerve.
Our study seeks to help in understanding the formation process of the petrotympanic and petrosquamosal fissures in humans and its relationship with the evolutionary processes in other mammals. Consequently, we investigated the fetal development of the middle-ear walls or of a separation between the middle ear and TMJ. We considered the tegmen tympani as the most critical structure because of its location surrounded by other key structures.
Materials and methods
The study was performed in accordance with the Declaration of Helsinki 1995 (as revised in Edinburgh 2000). We examined the paraffin-embedded histology of 25 mid-term human fetuses at 9–25 weeks of ovulational age (38–220 mm crown-rump length, CRL). The parameters used to determine gestational age were CRL, weight and cranial perimeter (O'Rahilly & Müller, 1996). The paraffin blocks studied contained whole parts of the head and neck. All specimens came from a large collection of the Embryology Institute of the University Complutense of Madrid. They originated from miscarriages and ectopic pregnancies from Department of Obstetrics of the University. Approval for the study was granted by the ethics committee of the University. After routine procedure for paraffin-embedded, frontal (five specimens), horizontal (five specimens) or sagittal (15 specimens) sections were prepared with a thickness of 10 μm to 1 mm, depending on specimen size. Sections were stained with hematoxylin-eosin or azocarmine.
Results
At 9–10 weeks, instead of a definite tympanosquamosal fissure, a large loose mass of mesenchymal tissue was found between the tympanic bone and the squamous part of the temporal bone (Fig. 1). The most bulky structure in this area was Meckel's cartilage, extending caudally from the middle ear. The widely open fissure divided Meckel's cartilage into two portions: the juxta-articular portion medial to the temporomandibular joint (TMJ) and the tympanic portion continuous with the malleus (Fig. 1C,D). The chorda tympani nerve ran in the medial side of Meckel's cartilage. Notably, in the cranial part of the fissure, cranial and lateral to Meckel's cartilage, the primitive discomalleolar ligament was identified as a distinct mesenchymal tract and it connected the TMJ disk to the tympanic portion of Meckel's cartilage (Fig. 1A,B). At this stage, the disk had formed earlier than and independently of the condyle head of the mandible. The os goniale was seen between Meckel's cartilage and the tympanic bone (Fig. 1A–C). The primitive maxillary or middle meningeal artery was seen near the chorda tympani nerve (Fig. 1). The tensor tympani muscle approached the malleus (Figs 1 and 2).
Fig. 1.
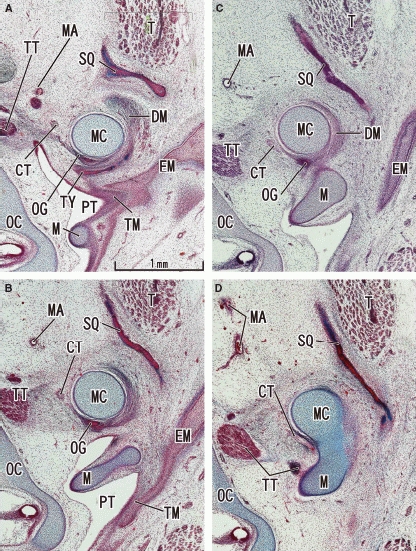
Earliest stage without the tegmen tympani: frontal sections of a 10-week human fetus. Panel A (or D) is the most ventral (or dorsal) level of the figure. A space between the squamous part of the temporal bone (SQ) and the tympanic bone (TY) corresponds to a future tympanosquamosal fissure which the discomalleolar ligament (DM) passed through. The chorda tympani nerve (CT) is medial to Meckel's cartilage (MC). The tegmen tympani has not yet appeared (cf. Fig. 2). All panels are prepared at the same magnification (scale bar in panel A). EM, external auditory meatus; M, malleus; MA, primitive maxillary artery including the middle meningeal artery; OC, otic capsule; OG, os goniale; PT, pharyngotympanic tube; T, temporalis muscle; TM, tympanic membrane; TT, tensor tympani muscle and its tendon.
Fig. 2.
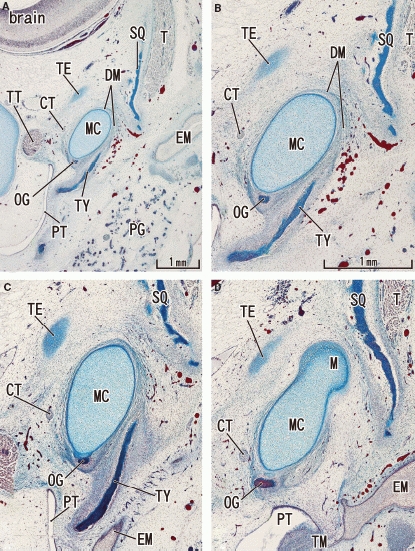
Tegmen tympani of the petrous part of the temporal bone appears in a 11-week human fetus. Frontal sections. Panel A (or D) most ventral (or dorsal) level of the figure. To show the topographical anatomy, panel A was prepared at lower magnification than panels B–D. The anlage of the tegmen tympani (TE) is a small oval cartilage located cranially and medially to the area of the tympanosquamosal fissure. Note the growth of the tympanic bone (TY) from the 10-week stage shown in Fig. 1. The squamous part (SQ) of the temporal bone narrows the tympanosquamosal fissure. Panels B–D are prepared at the same magnification (scale bar in panel B). CT, chorda tympani nerve; DM, discomalleolar ligament; EM, external auditory meatus; MC, Meckel's cartilage; OG, os goniale; PG, parotid gland; PT, pharyngotympanic tube; T, temporalis muscle; TM, tympanic membrane; TT, tensor tympani muscle and its tendon.
At 11 weeks, an anlage of the tegmen tympani was first identified as a small oval cartilage in the cranial and medial side of the wide tympanosquamosal fissure (Fig. 2). Depending on the growth of the tympanic bone and the squamous part of the temporal bone, the tympanosquamosal fissure became narrow in the lateral part. By contrast, although the tegmen tympani appeared, the tympanosquamosal fissure still opened widely in the medial part and allowed thick Meckel's cartilage to pass through (Fig. 2B,C). At this stage, the sphenoid bone did not reach the lateral site that included the future oval foramen (Fig. 3). Thus, no cartilages disturbed the chorda tympani nerve course. By contrast, the auriculotemporal nerve passed through a narrow slit between Meckel's cartilage and the condyle head of the mandible (Fig. 3C). The tensor veli palatini muscle was connected to the os goniale by a distinct fascia (Fig. 3B,C).
Fig. 3.
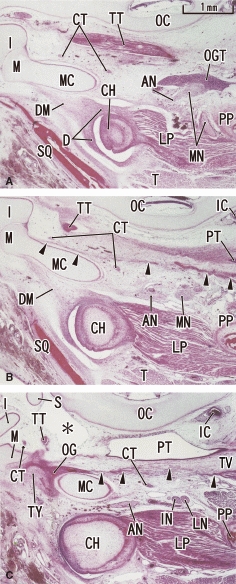
Horizontal sections of a 12-week human fetus showing the topographical anatomy of the medial side of the tympanosquamosal fissure. Panel A (or C) is the most superior (inferior) level of the figure. The discomalleolar ligament (DM) occupies the future tympanosquamosal fissure in panels A and B. The three panels show levels inferior to the tegmen tympani, but horizontal sections clearly display the topographical anatomy including the mandibular nerve (MN), otic ganglion (OGT) and pterygoid process (PP). A distinct fascia (arrowheads in panels B and C) connects the os goniale (OG) to the tensor veli palatine muscle (TV). Note the auriculotemporal nerve (AN; see also Fig. 6B) passing through a narrow slit between the condyle head (CH) and Meckel's cartilage (MC). Asterisk in panel C indicates a space made artificially during histological procedure. All panels were prepared at the same magnification (scale bar in panel A). IC, internal carotid artery; IN, inferior alveolar nerve; LN, lingual nerve. CT, chorda tympani nerve; D, temporomandibular joint disc; I, incus; LP, lateral pterygoid muscle; M, malleus; OC, otic capsule; PT, pharyngotympanic tube; S, stapes; SQ, squamous part of the temporal bone; T, temporalis muscle; TT, tensor tympani muscle and its tendon; TY, tympanic bone.
At 12 weeks, the tegmen tympani extended between the otic capsule and squamous part of the temporal bone. However, it did not reach the latter but a narrow slit remained, in contrast to the relatively wide tympanosquamosal fissure (Fig. 4). The tegmen tympani provided a horizontal roof over the middle ear, separating the malleus almost completely from the middle cranial fossa. Notably, through the tympanosquamosal fissure, a cranial part of the discomalleolar ligament from the TMJ disc reached the tegmen tympani (Fig. 4). At 13 weeks, although the tympanosquamosal fissure became narrow, depending on growth of the squamous part of the temporal bone and the tympanic bone, the medial part of the fissure was still open enough to allow thick Meckel's cartilage to pass through. Notably, the anterior end of the tegmen tympani, which received the discomalleolar ligament, enlarged and protruded inferiorly to form the cartilaginous inferior process (Fig. 5). The developing tympanic bone appeared to push the auriculotemporal nerve in an inferior direction (Fig. 5A).
Fig. 4.
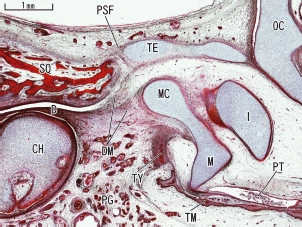
Growing tegmen tympani in a sagittal section of a 12-week human fetus. A sagittal section clearly displays the discomalleolar ligament (DM) connecting the disk (D) of the temporomandibular joint to Meckel's cartilage (MC). A part of the ligament connected with the most anterior part of the tegmen tympani (TE). The petrosquamosal fissure (PSF) was visible between the horizontally located tegmen tympani and the squamous part (SQ) of the temporal bone. CH, condyle head of the mandible; D, temporomandibular joint disc; I, incus; M, malleus; OC, otic capsule; PG, parotid gland; PT, pharyngotympanic tube; TM, tympanic membrane; TY, tympanic bone.
Fig. 5.
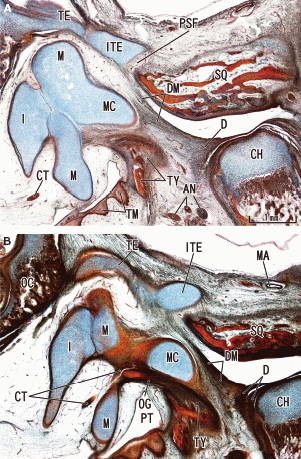
Inferior process of the tegmen tympani and the discomalleolar ligament in a 13-week human fetus. Sagittal sections. Panel A is lateral to panel B. Parts of the discomalleolar ligament (DM) extended anterocranially to attach to the developing inferior process of the tegmen tympani (ITE). These two panels are prepared at the same magnification. AN, auriculotemporal nerve; CH, condyle head of the mandible; CT, chorda tympani nerve; D, temporomandibular joint disc; I, incus; M, malleus; MA, primitive maxillary artery including the middle meningeal artery; MC, Meckel's cartilage; OC, otic capsule; OG, os goniale; PSF, petrosquamosal fissure; PT, pharyngotympanic tube; SQ, squamous part of the temporal bone; TE, tegmen tympani of the petrous part of the temporal bone; TM, tympanic membrane; TY, tympanic bone.
At 17 weeks, a reduction in thickness of Meckel's cartilage became evident (Fig. 6). The tegmen tympani finished ossification except for the inferior process attaching to the discomalleolar ligament (Fig. 6E,F). The os goniale changed into the anterior process of the malleus in the caudal side of Meckel's cartilage (Fig. 6A,B). The medial part of the tympanosquamosal fissure began to close by the growing inferior process of the tegmen tympani (Fig. 6E,F). The inferior process occupied a wedged position between the squamous part of the temporal bone and the tympanic bone. In the ventromedial side of the inferior process, the spine of the sphenoid bone and the definite maxillary artery were visible.
Fig. 6.
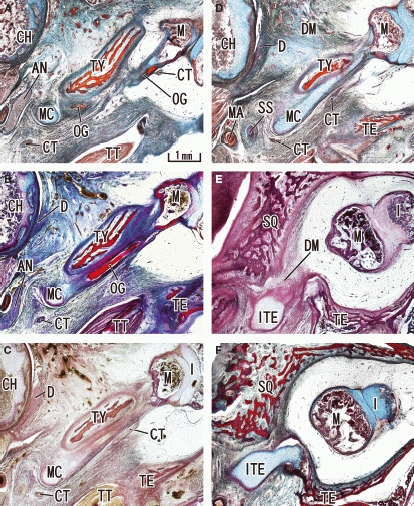
Regressing Meckel's cartilage and the growing tympanic bone in a 17-week human fetus. Transverse sections. Panel A (F) is the most caudal (cranial) level of the figure. Meckel's cartilage (MC) was small in size compared with the growing tympanic bone (TY) and other parts of the temporal bone. The os goniale (OG) is changing into the anterior process of the malleus, and the chorda tympani nerve (CT) accompanies the process (panels A–D). The auriculotemporal nerve (AN) passed through a narrow slit between the condyle head (CH) and Meckel's cartilage (MC) (panels A and B). The medial part of the tympanosquamosal fissure or space is closed by the inferior process of the tegmen tympani (ITE). The tegmen tympani (TE) has started ossification, in contrast to the cartilaginous inferior process. SS, spine of the sphenoid bone. D, temporomandibular joint disc; DM, discomalleolar ligament; I, incus; M, malleus; MA, primitive maxillary artery including the middle meningeal artery; SQ, squamous part of the temporal bone; TT, tensor tympani muscle and its tendon.
Until 25 weeks the tympanosquamosal fissure was closed in its lateral part because of the growth of the tympanic bone and the squamous part of the temporal bone (Fig. 7D). In the medial part, the inferior process of the tegmen tympani was visible as a wedge between the squamous part of the temporal bone and the tympanic bone. This descent of the inferior process formed the petrotympanic fissure (Fig. 7A–C). Meckel's cartilage disappeared in and around the middle ear and, instead, the remnant cartilage (i.e. the sphenomandibular ligament and the anterior ligament of the malleus) as well as the discomalleolar ligament occupied the narrow petrotympanic fissure (Fig. 7). The anterior process of the malleus was formed from the os goniale. The petrosquamosal fissure, which was occupied by dense connective tissue, and the inferior process of the tegmen tympani provided a roof for the two ligaments connecting to the middle ear (Fig. 7C,D). The semicircular canal was adjacent to the posterior wall of the middle ear, while the styloid process or Reichert's cartilage was embedded in the inferoposterior wall (Fig. 7A).
Fig. 7.
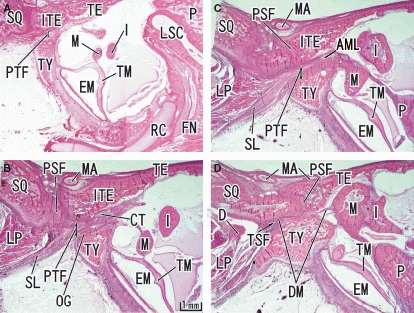
Closure of the tympanosquamosal fissure and the formation of the petrotympanic fissure seen in a 25-week human fetus. Sagittal sections. Panel A (D) is the most lateral (medial) level of the figure. The inferior process of the tegmen tympani (ITE) has descended to occupy a wedge position between the squamous part of the temporal bone (SQ) and the tympanic bone (TY). Subsequently, the petrotympanic fissure (PTF) is formed and the chorda tympani nerve (CT) and the sphenomandibular ligament (SL) passes through the fissure. AML, anterior ligament of the malleus; FN, facial nerve; LSC, lateral semicircular canal; RC, Reichert's cartilage; TSF, tympanosquamosal fissure. D, temporomandibular joint disc; DM, discomalleolar ligament; EM, external auditory meatus; I, incus; LP, lateral pterygoid muscle; M, malleus; MA, primitive maxillary artery including the middle meningeal artery; OG, os goniale; P, petrous part of the temporal bone; PSF, petrosquamosal fissure; TE, tegmen tympani of the petrous part of the temporal bone; TM, tympanic membrane.
Discussion
There are previous references showing fetal topographical relations among the TMJ disk, the discomalleolar ligament, the os goniale caudally to Meckel's cartilage, and the chorda tympani nerve medial to Meckel's cartilage (Harpman & Woollard, 1938; Symons, 1952; Moffett, 1957; Coleman, 1970; Smeele, 1988; Rodríguez-Vázquez et al. 1991, 1992, 1993). Unfortunately, the tympanosquamosal fissure was still widely open at the stages described. The present study is the first one available to reveal the comprehensive steps of middle-ear closure. An attempt is made to summarize the steps in Fig. 8.
Fig. 8.
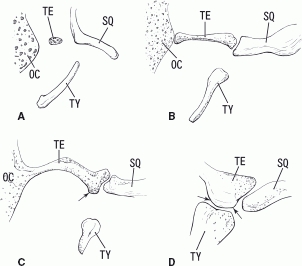
A diagram showing the growing tegmen tympani, especially its inferior process. Panels A, B, C and D correspond to 11 weeks (Fig. 2), 12 weeks (Fig. 4), 13 weeks (Fig. 5) and 25 weeks (Fig. 7) of gestation, respectively. The inferior process of the tegmen tympani (arrows) approaches the tympanic bone to form the final petrotympanic fissure. OC, otic capsule; SQ, squamous part of the temporal bone; TE, tegmen tympani of the petrous part of the temporal bone; TY, tympanic bone.
For the initial step, the squamous part of the temporal bone and the tympanic bone made the lateral part of the tympanosquamosal fissure. However, Meckel's cartilage was thick and opened the medial part of the fissure wide.
In the second step, the cartilaginous tegmen tympani appeared (Fig. 8A) and subsequently established a connection with the otic capsule (Fig. 8B,C). Thus, the petrosquamosal fissure appeared (Fig. 8B,C).
The third step is characterized by rapid growth of the inferior process of the tegmen tympani. The growth and descent of the cartilage narrowed the medial part of the tympanosquamosal fissure (Fig. 8C).
In the final step, Meckel's cartilage regressed to form the sphenomandibular ligament and the anterior ligament of the malleus, while the medial part of the tympanosquamosal fissure was wedged and closed by the descending inferior process of the tegmen tympani (Fig. 8D), and the narrow fissure contained the chorda tympani nerve and ligaments.
According to our observations, the petrosquamosal fissure formation was followed by the formation of the petrotympanic fissure. This consequence appears not to have been described previously. During the process, we noted a specific growth of the inferior process of the tegmen tympani: moreover, it maintained cartilage without ossification. Notably, throughout the steps, parts of the discomalleolar ligament connected the antero-inferior part of the tegmen tympani with the developing disk of the TMJ. We hypothesize that traction by the discomalleolar ligament avoids ossification and accelerates a descent of the inferior process (Fig. 9). The discomalleolar ligament was triangular, with its base towards the disk and with its apex on the malleus. This ligament came into the middle ear through the lateral part of the petrotympanic fissure, although the most lateral fibers of this ligament entered the medial part of the tympanosquamosal fissure (Rodríguez-Vázquez et al. 1998; Kim et al. 2004). The fetal discomalleolar ligament is most likely to transfer mechanical stress from the developing condyle because the latter development requires contraction of the lateral pterygoid muscle (Petrovic, 1972; Strutzmann & Petrovic, 1974; Perry et al. 1985; Sperber, 1989; Hinton, 1990; Takahashi, 1991; Ben-Ami et al. 1993; Takahashi et al. 1995).
Fig. 9.
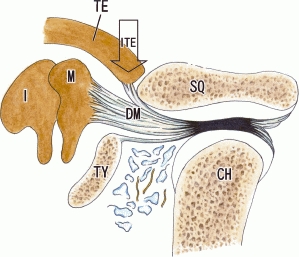
Hypothetical contribution of the discomalleolar ligament to the growth of the inferior process of the tegmen tympani. The discomalleolar ligament connected the disk of the temporomandibular joint to the malleus, and the inferior process of the tegmen tympani (ITE). The most lateral fibers of the discomalleolar ligament (DM) reached the malleus through the most medial area of the tympanosquamosal fissure. We hypothesize that the ligament plays a critical role in the descent and growth of the inferior process. CH, condyle head of the mandible; I, incus; M, malleus; SQ, squamous part of the temporal bone; TE, tegmen tympani of the petrous part of the temporal bone; TY, tympanic bone.
The developing TMJ disk also appears to exert mechanical stress on Meckel's cartilage and the os goniale to form the anterior process of the malleus or the sphenomandibular ligament, respectively. The growth of the pharynx may also contribute to the middle-ear closure because, as we reported previously (Rodríguez-Vázquez et al. 1991), the tensor veli palatine is connected with the os goniale by a distinct fascia. In steps of the middle-ear closure, we noted two nerves: the chorda tympani nerve and the auriculotemporal nerve. Notably, these nerves were located close together until 17 weeks. The auriculotemporal nerve was sandwiched between Meckel's cartilage and the condyle head. However, the chorda tympani nerve maintained its course in the petrotympanic fissure, whereas the auriculotemporal nerve course seemed to move in an inferior direction, depending both on regression of Meckel's cartilage and on the expansion of the tympanic bone.
In their famous scheme of temporal bone development, O'Rahilly & Müller (1996) did not explain closure mechanisms of the fissures. The tegmen tympani appears to be more critically important than the tympanic bone for the middle-ear closure. Taken together with the hypothetical traction role of the discomalleolar ligament, the middle-ear closure seems to occur in accordance with the development of the TMJ function.
The process described here is related to one of the central topics of the evolutionary biology in mammals, as it involves the transfer of accessory elements of the mandible (angular and articular) plus the quadrate, as strictly auditory bones, renamed tympanic, malleus, and incus (Allin, 1975). This transfer allowed the transition from a quadrato-articular reptilian-type joint (primary jaw articulation) to the mammalian squamoso-dental joint (Shute, 1956; Allin, 1975). In our opinion, the tympanosquamosal fissure allowed this articular transition in human development. It was known that the first reflex movements of the mouth opening were produced at 8–9 weeks of gestational age (Humphrey, 1968) and also that, at this developmental stage, the TMJ was not formed (Mérida-Velasco et al. 1999), but instead there was a joint between the dorsal end of Meckel's cartilage (which would give rise to the malleus) and the incus (Rodríguez-Vázquez et al. 1991). Therefore, the movements at this stage of development must involve this joint, which would be the primary jaw articulation (Mérida-Velasco et al. 1990). Wang et al. (2001) observed evidence of this transition in fossils of early Cretaceous mammals, identifying an ossified Meckel's cartilage which was related to the middle ear. These authors suggested that its function could be to move the mandible. This arrangement of Meckel's cartilage might be equivalent to that observed in human fetuses by Rodríguez-Vázquez et al. (1992, 1993).
Therefore, the closure of the middle ear would be the last phase of this joint transition, which divided the TMJ from the middle ear. This is why we hypothesize that when the TMJ started to perform its function, the discomalleolar ligament, through its attachment to the tegmen tympani, would end by closing the middle ear.
Regarding the variations of the petrotympanic fissure (Eckerdal, 1991; Schickinger et al. 1998; Koesling et al. 2005; Anagnostopoulou et al. 2008; Sato et al. 2008), and the chorda tympani nerve course (Fasel, 1986; Tóth et al. 2006), we consider them to be in direct relation to the degree of the descent of the inferior process of the tegmen tympani. Consequently, less descent would result in a wider petrotympanic fissure, easily allowing the passage of the discomalleolar ligament, sphenomandibular ligament, and its extension with the anterior ligament of the malleus by the same transmission force from the TMJ to the middle ear. This could explain the presence of hearing symptoms in the TMJ dysfunction syndrome (Schickinger et al. 1998; Kim et al. 2004; Anagnostopoulou et al. 2008; Sato et al. 2008; Sencimen et al. 2009).
References
- Allin EF. Evolution of the mammalian middle ear. J Morphol. 1975;147:403–438. doi: 10.1002/jmor.1051470404. [DOI] [PubMed] [Google Scholar]
- Anagnostopoulou S, Venieratos D, Antonopoulou M. Temporomandibular joint and correlated fissures: anatomical and clinical consideration. Cranio. 2008;26:88–95. doi: 10.1179/crn.2008.013. [DOI] [PubMed] [Google Scholar]
- Ben-Ami Y, Mark K, Franzen A, et al. Transformation of fetal secondary cartilage into embryonic bone in organ cultures of human mandibular condyles. Cell Tissue Res. 1993;271:317–322. doi: 10.1007/BF00318618. [DOI] [PubMed] [Google Scholar]
- Coleman RD. Temporomandibular joint: relation of the retrodiskal zone to Meckel's cartilage and lateral pterygoid muscle. J Dent Res. 1970;49:626–630. doi: 10.1177/00220345700490032701. [DOI] [PubMed] [Google Scholar]
- Eckerdal O. The petrotympanic fissure: a link connecting the tympanic cavity and the temporomandibular joint. Cranio. 1991;9:15–22. doi: 10.1080/08869634.1991.11678343. [DOI] [PubMed] [Google Scholar]
- Fasel J. The exit of the chorda tympani nerve through the external surface of the base of the skull. Acta Anat. 1986;126:205–207. [PubMed] [Google Scholar]
- Goodrich ES. Studies on the Structure and Development of Vertebrates. London: Macmillan; 1930. pp. 30–60. [Google Scholar]
- Harpman JA, Woollard HH. The tendon of the lateral pterygoid muscle. J Anat. 1938;73:112–115. [PMC free article] [PubMed] [Google Scholar]
- Hinton EJ. Myotomy of the lateral pterygoid muscle and condylar cartilage growth. Eur J Orthod. 1990;12:310–379. doi: 10.1093/ejo/12.4.370. [DOI] [PubMed] [Google Scholar]
- Humphrey T. The development of the mouth opening and related reflexes involving the oral area of human fetus. Ala J Med Sci. 1968;5:126–157. [PubMed] [Google Scholar]
- Kim HJ, Jung HS, Kaki HH, et al. The discomallear ligament and the anterior ligament of malleus: an anatomic study in human adults and fetuses. Surg Radiol Anat. 2004;26:39–45. doi: 10.1007/s00276-003-0170-6. [DOI] [PubMed] [Google Scholar]
- Kjellberg K. Beiträge zur Entwicklungsgeschichte des Kiefergelenks. Gegenbaurs Morphol Jahrb. 1904;32:159–184. [Google Scholar]
- Koesling S, Kunkel P, Schul T. Vascular anomalies, sutures and small canals of the temporal bone on axial CT. Eur J Radiol. 2005;54:335–343. doi: 10.1016/j.ejrad.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Mérida-Velasco JR, Rodríguez-Vázquez JF, Jiménez-Collado J. Meckelian articular complex. Eur Arch Biol. 1990;101:447–453. [Google Scholar]
- Mérida-Velasco JR, Rodríguez-Vázquez JF, Mérida-Velasco JA, Sánchez Montesinos I, et al. Development of the human temporomandibular joint. Anat Rec. 1999;255:20–33. doi: 10.1002/(SICI)1097-0185(19990501)255:1<20::AID-AR4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Moffett BC. The prenatal development of the human temporomandibular joint. Contrib Embryol. 1957;36:19–28. [Google Scholar]
- O'Rahilly R, Müller F. Human Embryology and Teratology. 2nd edn. New York: Wiley-Liss; 1996. pp. 433–448. [Google Scholar]
- Paturet G. Traité d'Anatomie Humaine. Vol. 1. Paris: Masson; 1951. pp. 530–535. [Google Scholar]
- Perry HT, Xu Y, Forbes DP. The embryology of the temporomandibular joint. Cranio. 1985;3:125–132. doi: 10.1080/08869634.1985.11678094. [DOI] [PubMed] [Google Scholar]
- Petrovic AG. Mechanisms and regulation of mandibular condylar growth. Acta Morphol Neerl-Scand. 1972;10:25–34. [PubMed] [Google Scholar]
- Rodríguez-Vázquez JF. Development of the stapes and associated structures in human embryos. J Anat. 2005;207:165–173. doi: 10.1111/j.1469-7580.2005.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Vázquez JF. Development of the stapedius muscle and pyramidal eminence in humans. J Anat. 2009;215:292–299. doi: 10.1111/j.1469-7580.2009.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Vázquez JF, Mérida-Velasco JR, Jiménez-Collado J. A study of the os goniale in man. Acta Anat. 1991;142:188–192. doi: 10.1159/000147188. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Vázquez JF, Mérida-Velasco JR, Jiménez-Collado J. Development of the human sphenomandibular ligament. Anat Rec. 1992;233:453–460. doi: 10.1002/ar.1092330312. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Vázquez JF, Mérida-Velasco JR, Jiménez-Collado J. Relationships between the temporomandibular joint and the middle ear in human fetuses. J Dent Res. 1993;72:62–66. doi: 10.1177/00220345930720010901. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Vázquez JF, Mérida-Velasco JR, Mérida-Velasco JA, et al. Anatomical considerations on the discomalleolar ligament. J Anat. 1998;192:617–621. doi: 10.1046/j.1469-7580.1998.19240617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Vázquez JF, Mérida-Velasco JR, Verdugo-López S, et al. Morphogenesis of the second pharyngeal arch cartilage (Reichert's cartilage) in human embryos. J Anat. 2006;208:179–189. doi: 10.1111/j.1469-7580.2006.00524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvière H, Delmas A. Anatomía Humana. Descriptiva, Topográfica y Funcional. Vol. 1. Barcelona: Masson; 1987. pp. 139–140. [Google Scholar]
- Sato I, Arai H, Asaumi R, et al. Classifications of tunnel-like structure of human petrotympanic fissure by cone beam CT. Surg Radiol Anat. 2008;30:323–326. doi: 10.1007/s00276-008-0327-4. [DOI] [PubMed] [Google Scholar]
- Schickinger B, Gstoettner W, Cerny C, et al. Variant petrotympanic fissure as possible cause of an otologic complication during TMJ arthroscopy. A case report. Int J Oral Maxillofac Surg. 1998;27:17–19. doi: 10.1016/s0901-5027(98)80089-1. [DOI] [PubMed] [Google Scholar]
- Sencimen M, Varol A, Baykal B, et al. Histological characteristics of ligaments between middle ear and temporomandibular joint. Eur J Dent. 2009;3:280–284. [PMC free article] [PubMed] [Google Scholar]
- Shute CCD. The evolution of the mammalian eardrum and tympanic cavity. J Anat. 1956;90:261–281. [PMC free article] [PubMed] [Google Scholar]
- Smeele LE. Ontogeny of relationship of human middle ear and temporomandibular (squamomandibular) joint. I. Morphology and ontogeny in man. Acta Anat. 1988;131:338–341. [PubMed] [Google Scholar]
- Sperber GH. Craniofacial Embryology. 4th edn. Cambridge: Wright; 1989. pp. 192–204. [Google Scholar]
- Strutzmann J, Petrovic AG. Effects of the resection of the external pterygoid muscle of the growth of the condylian cartilage in the young rat. Bull Assoc Anat. 1974;58:1107–1114. [Google Scholar]
- Symons NBB. The development of the human mandibular joint. J Anat. 1952;86:326–333. [PMC free article] [PubMed] [Google Scholar]
- Takahashi I. A histological study of the effect of the lateral pterygoid muscle activity on the growth of rat mandibular condylar cartilage. J Jpn Orthod Soc. 1991;50:368–382. [Google Scholar]
- Takahashi I, Mizoguchi I, Nakamura M, et al. Effects of lateral pterygoid muscle hyperactivity on differentiation of mandibular condyles in rats. Anat Rec. 1995;241:328–336. doi: 10.1002/ar.1092410306. [DOI] [PubMed] [Google Scholar]
- Testut L, Latarjet A. Tratado de Anatomía Humana. Vol. 1. Barcelona: Salvat Editores; 1975. pp. 528–531. [Google Scholar]
- Tóth M, Moser G, Patonay L, et al. Development of the anterior chordal canal. Ann Anat. 2006;188:7–11. doi: 10.1016/j.aanat.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hu Y, Meng J, et al. An ossified Meckel′s cartilage in two Cretaceous mammals and origin of the mammalian middle ear. Science. 2001;294:357–361. doi: 10.1126/science.1063830. [DOI] [PubMed] [Google Scholar]
- Williams PL, Warwick R. Gray Anatomía. Vol. 1. Barcelona: Salvat Editores; 1985. pp. 363–368. [Google Scholar]
- Wong GB, Weinberg S, Symington JM. Morphology of the developing articular disc of the human temporomandibular joint. J Oral Maxillofac Surg. 1985;43:565–569. doi: 10.1016/0278-2391(85)90121-1. [DOI] [PubMed] [Google Scholar]


