Abstract
Despite some progress in reducing the rate of diabetic complications, the epidemic rise in incidence of diabetes mellitus ensures that there will be an increasing number of patients in the coming decades with complex health care management issues who will need efficient and effective care. The management of patients with diabetes is an ever-challenging endeavor attributable to several factors. These include, among others, (1) limited provider expertise, (2) decreasing time of a patient visit, (3) increasing complexity of drug management, (4) limited use of self-monitoring of blood glucose by patients and/or providers, (5) clinical inertia, and (6) nonadherence. Technology-driven innovative solutions, including those using virtual reality, are desperately needed to assist both patients and their providers in overcoming the exigencies of this protean disease.
Keywords: clinical inertia, medication adherence, primary care, telemedicine, type 2 diabetes mellitus
Introduction
This overview of management issues of type 2 diabetes mellitus (T2DM) is meant to provide a snapshot of the challenges confronting both the provider and patient to those with a limited knowledge of endocrinology and/or diabetology. From the outset, it is important to recognize that, to manage diabetes effectively and efficiently, a partnership between medical professionals and their patients (along with their caregivers) must be created. Each participant in this partnership has unique challenges, not the least of which is due to the limited amount of time and resources that can be devoted to this complex illness. Appropriate use of technology is likely to provide tools to help overcome some of these challenges.
Epidemiology
Diabetes prevalence is increasing at an alarming rate in the United States as well as worldwide. Depending on the criteria used to define diabetes [e.g., fasting blood glucose (BG) level, the BG level 2-hour postglucose, hemoglobin A1c (A1C), or some combination of these], the number of people with diabetes in the United States is between 20 and 27 million (with approximately 15% of those undiagnosed), which is a prevalence of 10–13% of our population.1 In China, there are 93 million adults with diabetes2 The number of persons with diabetes in the United States has approximately tripled since 1980. The burden of diabetes is not equally distributed among ethnic groups, age, and gender. For example, the prevalence of diabetes is 30% in Hispanic men aged 65–74 years, whereas it is only 10% in white men 45–64.3 The lifetime risk of developing diabetes is over 50% in Hispanic women compared with 32% in white women.4
Complications of Diabetes and Their Cost
The complications of diabetes are divided into those that are primarily microvascular (retinopathy, nephropathy, and neuropathy) or macrovascular (heart attacks, strokes, and peripheral vascular disease). This division is quite arbitrary since many of the complications are multi-factorial. Diabetes is the leading cause of blindness, nontraumatic amputations, and renal failure in adults and reduces life expectancy by 5–10 years, mainly due to the increase in cardiovascular events and cardiovascular deaths. Blindness is the result of one or more of the following ophthalmological disorders: retinopathy, premature cataract formation, and glaucoma. The retina is the most vascular region of the body because of the high oxygen demands required to convert light into electrical energy by the rods and cones. Chronic hyperglycemia causes microvascular damage to the retinal vessels through a variety of mechanisms too complex to discuss here. However, the end result is vascular permeability causing edema and/or hemorrhage into the retina or the vitreous humor. Up to 20% of newly diagnosed diabetes patients already have evidence of retinopathy, suggesting that their dysglycemia began years earlier.5 A yearly dilated eye examination by an eye care professional or a retinal photograph can detect early retinopathy and lead to preventative interventions such as laser photocoagulation treatment. Amputations are the result of both diabetic neuropathy and peripheral vascular disease. The neuropathy leads to loss of protective sensation in the feet, which become prone to callous formation, ulceration, and other injury. This, in turn, leads to infection of the skin (cellulitis) and/or bones of the foot (osteomyelitis) and gangrene. The peripheral disease associated with chronic hyperglycemia prevents adequate oxygenation of injured tissues and precludes wound healing. Renal failure or end-stage renal disease is preceded by years of declining renal function (nephropathy), which is mostly asymptomatic. The earliest manifestation of nephropathy is the presence of minute amounts of urinary protein (microalbumin), which is not detectable on routine urinalysis but can only be detected by specific testing. Early detection engenders the institution of measures that can prevent progression of the nephropathy, but this often is overlooked, because many providers are unaware of the lack of the sensitivity of the routine urinalysis in detecting microalbuminuria. Cardiovascular events (heart attacks and strokes) are arguably the most serious complications of diabetes, leading to a considerable morbidity and a shortened life expectancy. Detection of cardiovascular disease in asymptomatic patients is difficult, expensive, and often invasive (cardiac catheterization). Prevention of premature cardiovascular events involves complex interactive treatments with antihypertensives, lipid-lowering agents, and routine low-dose aspirin administration.
Given this information, it is not surprising that the direct ($116 billion) and indirect ($68 billion) costs of diabetes care have dramatically increased along with the epidemic increase in the number of those with diabetes since 2000.6 The cost of medical care per capita is approximately $10,000 per year compared with $2700 per year for those without diabetes. The vast majority of these costs are related to hospitalizations resulting from the chronic complications of diabetes, with only approximately 15–20% of the costs attributable to professional visits and pharmaceuticals.
Prevention of Diabetic Complications
The Diabetes Control and Complications Trial performed in patients with type 1 DM (T1DM) and the United Kingdom Prospective Diabetes Study along with the “Kumamoto” study done in patients with T2DM conclusively proved that improved glycemic control was important in reducing microvascular complications.7–9 Together, these studies showed that, for every 1% decrease in A1C—a measure of the average BG over the preceding 2–3 months—there is a 25% decrease in microvascular complications. Based on these studies, various organizations have developed guidelines for a target A1C. The American Diabetes Association (ADA) recommends that the goal for A1C should be below 7% (normal 4–6.1%),10 and the American Association of Clinical Endocrinologists recommends that it should be below 6.5%,11 corresponding to average BG values of 150 and 135 mg/dl, respectively (normal 70–126 mg/dl). Since 2000, the major professional organizations have recognized that there needs to be some individualization of goals for patients with diabetes based on factors such as age, life expectancy, and the presence of complications. The Veterans Health Administration/Department of Defense guidelines stratify goals from 7% to over 8%, according life expectancy and the presence/severity of diabetic complications.12 Furthermore, years of improved glycemic control appear to have a legacy effect and not only reduce the future rate of microvascular complications, but also decrease the incidence of macrovascular complications in both T1DM and T2DM.13,14 These studies and their implementation by diabetes care providers are likely to be an important reason that the complication rates have fallen. For example, in the years from 1995 to 2006, the rate of end-stage renal disease fell from 311 to 206 per 100,000 persons, visual impairment fell from 24% to 19%, and cardiovascular disease fell from 36.6% to 31.4% of patients with diabetes.3 Several studies have shown that improved glycemic control is cost-effective in both T1DM and T2DM despite the increase in cost of supplies, a greater number of clinic visits, and more pharmaceuticals used.15–20
Difficulties in Diabetes Management
Despite increased numbers of drugs for the treatment of diabetes and its co-morbidities of hypertension and hyperlipidemia and the increased accessibility, affordability, and accuracy of BG meters, glycemic control remains suboptimal in most patients. In fact, 42.3% of patients with DM have A1Cs over 7% according to 2004 National Health and Nutrition Examination Survey data, although there appears to be an improving trend.21 Moreover, only 12% of patients have achieved the combined goals of A1C below 7%, blood pressure less than 130/80 mm Hg, and low-density lipoprotein (LDL) cholesterol below 100 mg/dl.22
The burden of this treatment failure in T2DM is profound since it inevitably causes the development or progression of microvascular and macrovascular complications. The reasons why more patients do not reach appropriate goals for glycemic control are multiple and complex. They include, but are not limited to, (1) limited provider expertise, (2) limited time to evaluate a patient properly, (3) complexity of drug management, (4) lack of use of self-monitoring of blood glucose (SMBG) by patients and/or providers, (5) provider clinical inertia, and (6) patient nonadherence.
Limited Expertise
Patients with diabetes, with their comorbidities of hypertension and hyperlipidemia, are best monitored by highly skilled health care professionals who are equipped with the latest information to help ensure early detection of complications and appropriate treatment and to provide diabetes education to patients. Due to a dearth of endocrinologists and certified diabetes educators in both military and civilian health care settings,23 most diabetes care is administered by primary care providers (PCPs), including family practitioners, nurse generalists, nurse practitioners, and physician’s assistants. These health care professionals might not be equipped with the latest information and tools to provide state-of-the-art care to the vast majority of patients with diabetes.
Limited Time
Reviewing SMBG data is an important part of any medical visit of a patient with diabetes. Yet it is just one of many tasks that a typical PCP accomplishes in a day.24,25 This limits the amount of time they have to spend with patients—10 minutes is the norm. The potential utility of SMBG data is thus mitigated because reviewing the data is both time-consuming and complex. Data may be reviewed manually from a hand-written log, or the meter is downloaded to the provider’s computer. Manual review of the records precludes any statistical and graphical analysis of the data, reducing the likelihood that patterns and trends in the glucose will be recognized. Graphical/statistical display of downloaded data is more efficient but has its own set of barriers. There are dozens of meter manufacturers, and each manufacturer has its own proprietary software for data analysis and its own unique method for downloading data in the office or from the patient’s home. This requires a multiplicity of connecting cables and desktop programs for the provider’s computer. These considerations, along with the complex health issues that accompany even routine diabetes care, contribute to therapeutic inertia. Using technology for health care—alternately referred to as telemedicine, e-health, m-health, or i-health—has the potential for improving efficiency and efficacy, but that has yet to be convincingly documented. Although many studies have trumpeted the potential advantages of telemedicine, including Web-assisted or Web-based management of DM, most have addressed this using the Web for patient education, performance monitoring, risk stratification, and case management by nurses.26–28 Only a few studies have shown that using the Web and/or email improves glycemic control29,30 or can reduce the number of clinic visits,31 while others have not been able to show such an effect.32,33
Complexity of Drug Management
While lifestyle modification is the foundation of treatment for all patients with diabetes, over 85% of patients are managed pharmacologically.3 In addition, most patients with T2DM have several comorbid conditions, the most common of which are hypertension and hyperlipidemia. So the typical patient takes a minimum of five and up to nine medications in support of better diabetes outcomes—low-dose aspirin for prophylaxis against thrombosis, an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker for treatment of hypertension and/or prevention of nephropathy, a lipid-lowering agent like a statin for treatment of hypercholesterolemia and/or prevention of macrovascular disease, and two oral hypoglycemic agents. There are not only potential drug–drug interactions among these drug classes, but also among them and the other drugs patients take for other acute or chronic illnesses. It is also not surprising that medication adherence rates in these patients is low—another important factor complicating the ability to acheive glycemic control. While electronic medical records (EMRs) and computerized physician ordering entry (CPOE) systems can provide alerts to the most dangerous and/or well documented of these, there are undoubtedly many that occur that reduce efficacy and compliance as well as increase adverse effects. Currently, EMRs and CPOE systems are only available to the minority of PCPs who practice in large groups, clinics, or hospital-based practices. Incentives in the Health Care Reform Act for EMRs to be incorporated into all practices have the promise to improve this situation.
Lack of Use of Self-Monitoring of Blood Glucose
Self-monitoring of blood glucose is associated with improved glycemic control and adverse outcomes in both T1DM and in T2DM. The ADA recommends SMBG 3–4 times a day for patients with T1DM and at least once a day for pharmacologically treated T2DM. Each additional BG measurement results in a decrease A1C of 0.32%,34 and there is a lower rate of fatal and nonfatal cardiovascular events in those who monitor.35 In a survey of 44,181 pharmacologically treated diabetes patients in California, 60% of T1DM patients and 67% of T2DM patients performed SMBG less frequently than is recommended by the ADA.36 Despite this evidence showing the positive impact of SMBG, compliance with SMBG remains suboptimal, perhaps because no actionable advice is associated with its use. As discussed earlier, providers have little time to review the data.
Clinical Inertia
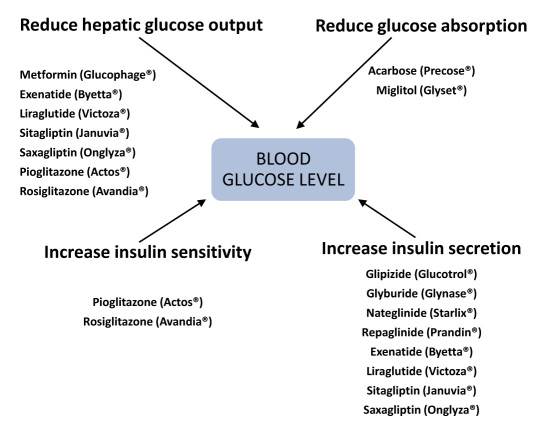
There is great inertia among those providers caring for patients with diabetes as demonstrated by the observation that switching to another agent or adding a second oral agent takes over 20 months in patients with inadequate glycemic control (A1C ≥8%).37 This results in the average patient accumulating nearly 5 A1C years of excess glycemic burden of >8.0% from diagnosis until starting insulin and approximately 10 A1C years of burden of >7.0%. One of the reasons for this inertia is that there are 10 classes of diabetes drugs (most of which have been introduced since 1995), with multiple drugs in each class. In fact, a total of 25 different agents can be used in monotherapy. Each drug in a class may affect one or more of the major pathophysiological abnormalities in T2DM: excessive glucose influx, increased hepatic glucose output, decreased insulin secretion, and decreased peripheral glucose uptake because of insulin resistance (Figure 1). There are 45 rational and U.S. Food and Drug Administration-permissible combinations that can be used in dual therapy and up to 120 combinations in triple therapy.
Figure 1.
Drug effects on the major factors controlling ambient BG.
Clinical inertia is a powerful force given the complexities of glycemic management, the uncertainty about what to do next, and the need to perform the required components of diabetes care, such as addressing hyper-tension, hyperlipidemia,10 and the often-heard statement in the patient in the exam room: “Oh, by the way, I’ve been having a problem with….”. This problem must be addressed and, assuming there are not life-threatening episodes of hypo- or hyperglycemia, it is easy to say, “Just try a little harder with your diet and exercise regimen, and we’ll address the sugars when you return in 3 months.” In addition to difficulties in reaching glycemic goals, providers have to manage the important comorbidities of diabetes such as hypertension and hyperlipidemia. They may not be familiar with the blood pressure and/or lipid goals in diabetes, or they may be reluctant to change and/or add antihypertensive medications as frequently as is necessary to reach established goals as was seen in a study of patients at clinics in the Veterans Health Administration.38
Patient Nonadherence
About one-third of patients with diabetes are nonadherent to their medications—a compliance rate that is lower than in many other medical conditions.39 Nonadherence negatively affects outcomes. For instance, Pladevall and colleagues40 found that nonadherent patients had an A1C 0.5% higher than those who were adherent to their metformin and a LDL that was 21 mg/dl higher than those who were adherent to a statin drug. Krapek and associates39 found 27% of patients who were prescribed at least one diabetic medication were nonadherent, which resulted in a 10% higher A1C. In a Hispanic population, there was a 36% prevalence of nonadherence with diabetes medication usage, which was associated with a 66% increase in diabetes-related deaths.41 Nonadherence is a complex phenomenon that may be, in part, the result of the polypharmacy often required to treat hyperglycemia and the frequent comorbid conditions of hypertension and hyperlipidemia. In some respects, this mirrors the provider’s difficulty in understanding his/her patient’s adherence rate when prescribing, renewing, and assessing these complex regimens.42 Reach 43 has suggested that adherence may be improved by using technology to provide rewards to the patient, e.g., less hypoglycemia using continuous glucose monitoring, and as a way to open up a conversation between the patient and the provider.
Summary
Effective diabetes management still remains an elusive goal for most providers and their patients. While the issues reviewed here represent important barriers to achieving that goal, there are many others not discussed that also have key impacts on a patient’s ultimate health. These include psychosocial issues (including depression and lack of motivation), access to and the cost of care (including medication and visit pay), ethnic disparities in care provision, and cultural divides in accepting advice from traditional health care professionals, among others. Thus it is unreasonable to expect that the next new “wonder drug” will be the silver bullet that cures this complex disease. Rather, such pharmacologic breakthroughs will be only one “tool” in the “ubiquitous health tool kit” that will be used by the partnership forged between patients and multidisciplinary health care teams. This partnership will need novel technology-driven methods for communication and motivation along with more traditional medical and educational approaches to improve long-term outcomes in patients with diabetes.
Abbreviations
- (CEG)
Clarke error grid
- (ICU)
intensive care unit
- (ISO)
International Organization for Standardization
- (IV)
intravenous
- (mid-IR)
mid-infrared
- (NICE-SUGAR)
Normoglycaemia in Intensive Care Evaluation and Survival Using Alogrithm Regulation
- (PVC)
polyvinyl chloride
- (SEE)
standard error of the estimate
- (TGC)
tight glycemic control
- (YSI)
Yellow Springs Instrument
Disclaimer
The opinions expressed in this article reflect the personal views of the author and not the official views of the United States Army or the Department of Defense.
References
- 1.Cowie CC, Rust KF, Byrd-Holt DD, Gregg EW, Ford ES, Geiss LS, Bainbridge KE, Fradkin JE. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988-2006. Diabetes Care. 2010;33(3):562–568. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang W, Lu J, Weng H, Jia W, Ji L, Xiao J, Shan Z, Liu J, Tian H, Ji Q, Zhu D, Ge J, Lin L, Chen L, Guo X, Zhao Z, Li Q, Zhou Z, Shan G, He J, China National Diabetes and Metabolic Disorders Study Group Prevalence of diabetes among men and women in China. N Eng J Med. 2010;362(12):1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Diabetes public health resource. http://www.cdc.gov/diabetes/
- 4.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290(14):1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 5.Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, 3rd, Klein R, American Diabetes Association Retinopathy in diabetes. Diabetes Care. 2004;27(Suppl 1):S84–S87. doi: 10.2337/diacare.27.2007.s84. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31(3):596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 7.Diabetes Control, Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 8.Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28(2):103–117. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 9.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 10.American Diabetes Association. Standards of medical care in diabetes-2010. Diabetes Care. 2010;33(Suppl 1):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodbard HW, Blonde L, Braithwaite SS, Brett EM, Cobin RH, Handelsman Y, Hellman R, Jellinger PS, Jovanovic LG, Levy P, Mechanick JI, Zangeneh F, AACE Diabetes Mellitus Clinical Practice Guidelines Task Force American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(Suppl 1):1–68. doi: 10.4158/EP.13.S1.1. [DOI] [PubMed] [Google Scholar]
- 12. United States Department of Veterans Affairs. Management of diabetes mellitus in primary care. http://www.healthquality.va.gov/Diabetes_Mellitus.asp.
- 13.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B, Diabetes Control Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 15.Menzin J, Langley-Hawthorne C, Friedman M, Boulange L, Cavanaugh R. Potential short-term economic benefits of improved glycemic control: a managed care perspective. Diabetes Care. 2001;24(1):51–55. doi: 10.2337/diacare.24.1.51. [DOI] [PubMed] [Google Scholar]
- 16.CDC Diabetes Cost-effectiveness Group. Cost-effectiveness of intensive glycemic control, intensified hypertension control, and serum cholesterol reduction for type 2 diabetes. JAMA. 2002;287(19):2542–2551. doi: 10.1001/jama.287.19.2542. [DOI] [PubMed] [Google Scholar]
- 17.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 18.Diabetes Control and Complications Trial Research Group. Lifetime benefits and costs of intensive therapy as practiced in the diabetes control and complications trial. JAMA. 1996;276(17):1409–1415. [PubMed] [Google Scholar]
- 19.Gilmer TP, O’Connor PJ, Manning WG, Rush WA. The cost to health plans of poor glycemic control. Diabetes Care. 1997;20(12):1847–1853. doi: 10.2337/diacare.20.12.1847. [DOI] [PubMed] [Google Scholar]
- 20.Herman WH, Eastman RC. The effects of treatment on the direct costs of diabetes. Diabetes Care. 1998;21(Suppl 3):C19–C24. doi: 10.2337/diacare.21.3.c19. [DOI] [PubMed] [Google Scholar]
- 21.Hoerger TJ, Segel JE, Gregg EW Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care. 2008;31(1):81–86. doi: 10.2337/dc07-1572. [DOI] [PubMed] [Google Scholar]
- 22.Cheung BM, Ong KL, Cherny SS, Sham PC, Tso AW, Lam KS. Diabetes prevalence and therapeutic target achievement in the United States, 1999 to 2006. Am J Med. 2009;122(5):443–453. doi: 10.1016/j.amjmed.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 23.Rizza RA, Vigersky RA, Rodbard HW, Ladenson PW, Young WF, Jr, Surks MI, Kahn R, Hogan PF. A model to determine the workforce needs for endocrinologists in the United States until 2020. J Clin Endocrinol Metab. 2003;88(5):1979–1987. doi: 10.1210/jc.2002-021288. [DOI] [PubMed] [Google Scholar]
- 24.Baron RJ. What’s keeping us so busy in primary care? A snapshot from one practice. N Eng J Med. 2010;362(17):1632–1636. doi: 10.1056/NEJMon0910793. [DOI] [PubMed] [Google Scholar]
- 25.Gottschalk A, Flocke SA. Time spent in face-to-face patient care and work outside the examination room. Ann Fam Med. 2005;3(6):488–493. doi: 10.1370/afm.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glasgow RE, Boles SM, McKay HG, Feil EG, Barrera M., Jr The D-Net diabetes self-management program: long-term implementation, outcomes, and generalization results. Prev Med. 2003;36(4):410–419. doi: 10.1016/s0091-7435(02)00056-7. [DOI] [PubMed] [Google Scholar]
- 27.Izquierdo RE, Knudson PE, Meyer S, Kearns J, Ploutz-Snyder R, Weinstock RS. A comparison of diabetes education administered through telemedicine versus in person. Diabetes Care. 2003;26(4):1002–1007. doi: 10.2337/diacare.26.4.1002. [DOI] [PubMed] [Google Scholar]
- 28.Peters AL, Davidson MB. Application of a diabetes managed care program. The feasibility of using nurses and a computer system to provide effective care. Diabetes Care. 1998;21(7):1037–1043. doi: 10.2337/diacare.21.7.1037. [DOI] [PubMed] [Google Scholar]
- 29.Alaoui A, Clement S, Khanafer N, Collmann J, Levine B, Mun SK. Diabetes home monitoring project. PACMEDTEK Symposium, Honolulu Hawaii. 1998 August. [Google Scholar]
- 30.Ober SK, Naito H, Low ME, Cutre KF, Crockett D, Skala MD, Asberry MT, Schumacher PO, Galen RS. A Web-based internet system of diabetes management. Diabetes. 2000;49(Suppl 1) Abstract 484. [Google Scholar]
- 31.Prendergast J, Bagdade J, Mayes P, Perrot M. Diabetes mellitus management with internet augmentation. Endocrine Society Annual Meeting; Toronto, Canada: 2000. Abstract 1823. [Google Scholar]
- 32.Vigersky RA, Anderson T, Filmore A, Thomas-Wharton D, Novak L, Heltzel IJ, Smith D, Kessler C, Clarkson J, Striegel A, Loughney M, Galen RS. The use of telemedicine in the management of poorly controlled patients with diabetes mellitus. Endocrine Society 85th Annual Meeting; 2003; Philadelphia, PA: [Google Scholar]
- 33.Grant RW, Wald JS, Schnipper JL, Gandhi TK, Poon EG, Orav EJ, Williams DH, Volk LA, Middleton B. Practice-linked online personal health records for type 2 diabetes mellitus: a randomized controlled trial. Arch Intern Med. 2008;168(16):1776–1782. doi: 10.1001/archinte.168.16.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schütt M, Kern W, Krause U, Busch P, Dapp A, Grziwotz R, Mayer I, Rosenbauer J, Wagner C, Zimmermann A, Kerner W, Holl RW, DPV Initiative Is the frequency of self-monitoring of blood glucose related to long-term metabolic control? Multicenter analysis including 24,500 patients from 191 centers in Germany and Austria. Exp Clin Endocrinol Diabetes. 2006;114(7):384–388. doi: 10.1055/s-2006-924152. [DOI] [PubMed] [Google Scholar]
- 35.Martin S, Schneider B, Heinemann L, Lodwig V, Kurth HJ, Kolb H, Scherbaum WA. Self-monitoring of blood glucose in type 2 diabetes and long-term outcome: an epidemiological cohort study. Diabetologia. 2006;49(2):271–278. doi: 10.1007/s00125-005-0083-5. [DOI] [PubMed] [Google Scholar]
- 36.Karter AJ, Ferrara A, Darbinian JA, Ackerson LM, Selby JV. Self-monitoring of blood glucose: language and financial barriers in a managed care population with diabetes. Diabetes Care. 2000;23(4):477–483. doi: 10.2337/diacare.23.4.477. [DOI] [PubMed] [Google Scholar]
- 37.Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27(7):1535–1540. doi: 10.2337/diacare.27.7.1535. [DOI] [PubMed] [Google Scholar]
- 38.Berlowitz DR, Ash AS, Hickey EC, Friedman RH, Glickman M, Kader B, Moskowitz MA. Inadequate management of blood pressure in a hypertensive population. N Eng J Med. 1998;339(27):1957–1963. doi: 10.1056/NEJM199812313392701. [DOI] [PubMed] [Google Scholar]
- 39.Krapek K, King K, Warren SS, George KG, Caputo DA, Mihelich K, Holst EM, Nichol MB, Shi SG, Livengood KB, Walden S, Lubowski TJ. Medication adherence and associated hemoglobin A1c in type 2 diabetes. Ann Pharmacother. 2004;38(9):1357–1362. doi: 10.1345/aph.1D612. [DOI] [PubMed] [Google Scholar]
- 40.Pladevall M, Williams LK, Potts LA, Divine G, Xi H, Lafata JE. Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care. 2004;27(12):2800–2805. doi: 10.2337/diacare.27.12.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuo YF, Raji MA, Markides KS, Ray LA, Espino DV, Goodwin JS. Inconsistent use of diabetes medications, diabetes complications, and mortality in older Mexican Americans over a 7-year period: data from the Hispanic established population for the epidemiologic study of the elderly. Diabetes Care. 2003;26(11):3054–3060. doi: 10.2337/diacare.26.11.3054. [DOI] [PubMed] [Google Scholar]
- 42.Heisler M, Hogan MM, Hofer TP, Schmittdiel JA, Pladevall M, Kerr EA. When more is not better: treatment intensification among hypertensive patients with poor medication adherence. Circulation. 2008;117(22):2884–2892. doi: 10.1161/CIRCULATIONAHA.107.724104. [DOI] [PubMed] [Google Scholar]
- 43.Reach G. Can technology improve adherence to long-term therapies? J Diabetes Sci Technol. 2009;3(3):492–499. doi: 10.1177/193229680900300313. [DOI] [PMC free article] [PubMed] [Google Scholar]