Abstract
Objective
We sought to develop a computerized clinical decision support for clinicians treating patients with type 2 diabetes mellitus (T2DM).
Methods
We designed, developed, and tested a computer-assisted decision support (CADS) system using statistical analyses of self-monitoring of blood glucose data, laboratory data, medical and medication history, and individualized hemoglobin A1c goals. A rule-based expert system generated recommendations for changes in therapy and accompanying explanations.
Results
A clinical decision support system (CADS) was developed that considers 9 classes of medications and 69 regimens with combinations of up to 4 therapeutic agents. The preferred sequences of regimens can be customized. The program is integrated with a “comprehensive diabetes management system,” electronic medical record systems, and a method for uploading data from memory glucose meters via telephone without use of a computer or the Internet. The software provides a report to the clinician regarding the overall quality of glycemic control and identifies problems, e.g., hypoglycemia, hyperglycemia, glycemic variability, and insufficient data. The program can recommend continuation of current therapy, adjustment of dosages of current medications, or change of regimen and can provide explanations for its recommendations. If the user rejects the recommendations, the program will recommend alternative approaches. The CADS also provides access to Food and Drug Administration-approved prescribing information, guidelines from professional organizations, and selections from the general medical literature. The system has been extensively tested with real and synthetic data and is ready for evaluation in multicenter clinical trials.
Conclusion
A clinical decision support system to assist with the management of patients with T2DM was designed, developed, tested, and found to perform well.
Keywords: algorithm, artificial intelligence, clinical decision support, clinical diabetes, expert system, insulin, oral anti-diabetic agents, type 2 diabetes mellitus
Introduction
Software to adjust insulin dosages and regimens for patients with type 1 diabetes has been under development since 1985.1–6 Based in part on this experience,6 Mazze and colleagues7,8 developed “staged diabetes management” for type 2 diabetes mellitus (T2DM). However, to date, there has been no computer-assisted decision support (CADS) program for managing T2DM. Some have argued that the task is impossible and should not even be attempted on the basis that management of patients with T2DM is too complex for application of an “artificial intelligence” decision support system.
Computer-assisted decision support systems, which provide alerts, reminders, recommendations, and critiques of clinicians' decisions, have been demonstrated to improve quality of care in many but not all settings.9 Systems for selecting antibiotics,10,11 implementing cancer chemotherapy protocols,12 adjusting warfarin therapy,13,14 adjusting dosages in pediatric and geriatric settings, and warning of drug–drug interactions have been implemented and tested,15 generally resulting in improved compliance with guidelines and improved clinical outcomes. However, in three other applications (congestive heart failure, hypertension, or ischemic heart disease), clinical decision support systems did not appear to improve quality of care.16–18 In the face of the large and rapidly growing number of patients with T2DM worldwide, the failure of most patients to achieve the goals for glycemia and glycated hemoglobin (A1C)19–22 and a corresponding shortage of endocrinologists,23 such that the majority of patients with T2DM receive care from primary care providers (PCPs), including physicians, physician assistants, and nurse practitioners, a CADS system may potentially be of benefit to clinicians and their patients.
Developing a CADS system for diabetes poses many challenges. Since 1990, there has been a dramatic increase in the number of therapeutic options available in the United States, now reaching 13 classes of medications—sulfonylureas, biguanides, glinides, thiazolidinediones, dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) analogs, alpha-glucosidase inhibitors, a bile acid sequestrant, a dopamine agonist, an amylin analog, long-acting insulin analogs, intermediate acting insulin, and rapid-acting insulin analogs. There are multiple medications within most of these classes, multiple brand names for some drugs, and several commercially available combinations of two agents. The enormous number of possible combinations of therapeutic agents (more than 200) makes it difficult for clinicians to be familiar with the advantages and limitations of all available approaches. With 9 classes of agents (not including rapid-acting, short-acting, intermediate-acting, biphasic insulin and pramlintide) there are 9 forms of monotherapy, 36 potential types of dual therapy, 84 potential types of triple therapy, and 126 theoretically possible types of quadruple therapy, for a total of 256 regimens, including lifestyle modifications alone. The present version of the program handles 69 regimens, which is more than sufficient to cover the much smaller number of regimens (22 in addition to an unspecified number of regimens including insulin) considered by the American Association of Clinical Endocrinologists/American College of Endocrinology (AACE/ACE) algorithm24 or the 7 regimens offered by the American Diabetes Association/European Association for the Study of Diabetes algorithm.25 We do not advocate or recommend all of the possible combinatorial arrangements of therapy, but we include mention of many of them because some patients may be receiving an unusual, suboptimal, or inappropriate combination of therapies as prescribed by another clinician. Table A.9 in the Appendix provides a partial listing of inappropriate regimens; the CADS software screens for their presence and provides warnings as appropriate. New classes of medications are in clinical trials. There has also been a proliferation of treatment pathways or algorithms for treatment of patients with T2DM.8,24–28 Clinicians who closely monitor the literature may find it challenging to select a set of rules or treatment pathway for management of patients with T2DM. Several regimens are in widespread use by endocrinologists but have not yet been approved by the FDA (e.g., the combination of GLP-1 receptor agonists and insulin).29
Several additional challenges confront the clinician in managing patients with diabetes, including difficulty in assessing the quality of glycemic control, difficulty in obtaining adequate amounts of accurate self-monitoring of blood glucose (SMBG) data, insufficient time and resources for analysis and review of SMBG data, and limited accessibility of important clinical data. Clinicians often fail to identify current problems of glycemic control and fail to adjust therapy in a timely and effective manner.20,21,30,31 Thus, there appears to be the need for a clinical decision support system. We have developed a CADS system intended for use by PCPs. The system is based on the following inputs: (1) clinical information, including diagnoses (comorbidities), medication history (including medications that had been used previously and found to be ineffective or poorly tolerated), history of adverse events, and laboratory data; (2) SMBG data; (3) rules for titrating dosages of individual medications; (4) rules for adding or discontinuing medications; and (5) rules for individualizing goals for A1C and for glucose by time of day. The system provides the following outputs: (1) comprehensive analysis, display, and interpretation of the available SMBG data; (2) recommendations for possible changes in therapy with brief explanations of the rationale for those recommendations; (3) ability to recommend several options for therapy with explanations of their rationales; and (4) educational materials for health care professionals and patients, including prescribing information and access to the medical literature, including guidelines and algorithms from authoritative sources.
Methods
The present system was developed with the following design principles: safety, optimization of usability, flexibility, customizability, adaptability, inclusiveness, expansibility, and integration with existing electronic medical records, laboratory information systems, and other specialized electronic systems for diabetes case management. The CADS system has a number of design constraints in this prototype: (1) it does not consider use of an insulin pump [continuous subcutaneous insulin infusion (CSII)], gestational diabetes, type 1 diabetes, hospitalized patients, diabetic ketoacidosis, or hyperosmolar coma; (2) it does not include two classes of oral agents (colesevelam and bromocriptine) that are Food and Drug Administration (FDA) approved for management of T2DM; and (3) it does not currently include several injectable medications for preprandial use (regular human insulin, rapid-acting insulin analogs, biphasic insulins, pramlintide).
The CADS system is currently integrated with the comprehensive diabetes management program (CDMP),32 a multiplatform, Web-based system for diabetes case management currently used at several medical centers (Walter Reed Army Medical Center, Wilford Hall Medical Center, University of Hawaii-affiliated clinics, Boston Veterans Administration Hospital, the Indian Health Service, and Joslin Diabetes Center).
Patients can upload SMBG data even without direct access to a computer or the Internet. A MetriLink device (www.imetrikus.com) transmits data via telephone lines from the glucose meter to the iMetrikus server, which then transmits the data automatically via the Internet to the CDMP and CADS system. Alternatively, SMBG data can be uploaded via a computer using a password-protected patient portal, which also allows patients to review their data together with statistical and graphic analyses. The algorithms have been developed as table-driven logic to facilitate future changes. Similarly, the messages to the end users are table driven, making it easy to update those messages in accord with the prevailing philosophy and practices of medical centers or individual clinicians. The system has evolved through dozens of versions over a period of 5 years and has been tested extensively using synthetic and anonymized patient data. We have tested more than 1500 sets of input data to cover a wide range of parameters: target A1C and glucose target ranges; extent of SMBG data; patient characteristics (age), mixtures of hyperglycemia and hypoglycemia, and magnitude of glycemic variability; number of medications; types and combinations of medications; comorbid conditions (renal, hepatic, cardiac, gastrointestinal); adverse events presumably related to medications; drug–drug interactions; and failure to respond to previous medications. The logic and computer code has been modified iteratively in response to this testing. Several individuals have traced through the computer code to verify accuracy of implementation of the algorithms. Partially automated methods for testing of multiple cases have been set up and used extensively.
Results
Structure
The CADS system for diabetes management has four components. First, a “patient” component permits remote or clinic-based upload of glucose meter data into a database. The system provides options to provide patient-accessible graphic, statistical, and tabular summaries of the SMBG data, information regarding medications, and electronic communications between patient and clinical personnel. Second, a “health care professional” component enables the clinician to set goals for A1C and the target ranges for preprandial and postprandial glycemia for each patient or group of patients. It displays the current medications, medication history, allergies, contraindications, and comorbidities. It provides detailed statistical and graphic displays of the glucose data and identifies clinical problems such as hyperglycemia or hypoglycemia, excessive post-prandial excursions, or excessive glycemic variability within specified time periods (e.g., 07:00–09:00, before breakfast). The software then generates primary and alternative recommendations for patient management and provides options to accept or reject those recommendations. Third, an “administrator” component enables the medical leadership of each clinic or clinical practice to specify the medications to be included and revise the algorithms by adjusting types, doses, or combinations of medications. The algorithms utilize a prespecified sequence of regimens that reflects published algorithms24–28 as modified in accord with local preferences. Fourth, an “educational” component provides links to guidelines and algorithms for management of diabetes, FDA-approved prescribing information, and relevant literature.
Rules Underlying the Computer-Assisted Decision Support Program
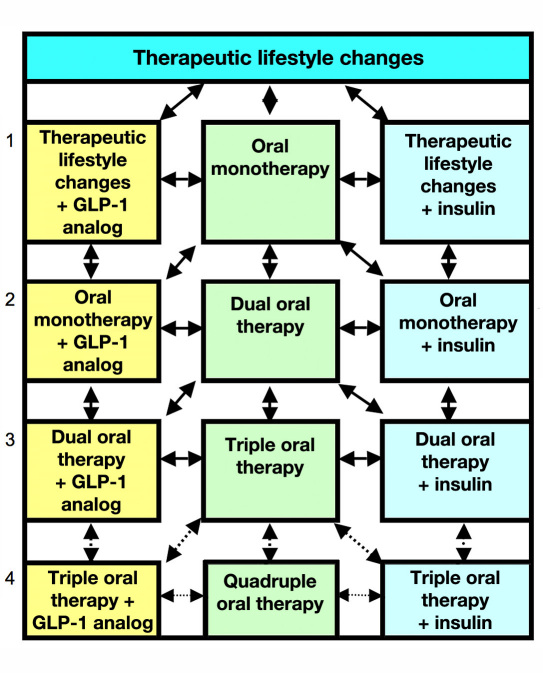
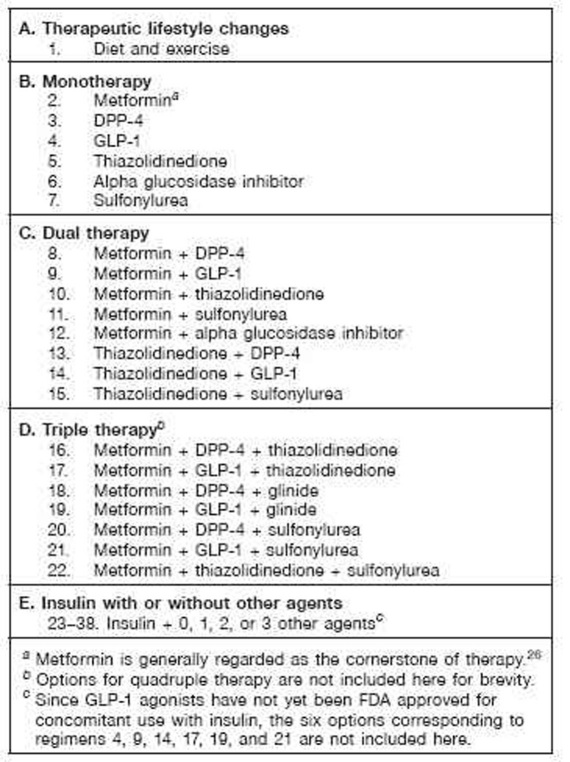
Figure 1 shows a simplified view of the sequence of regimens. Most commonly, an algorithm sequence progresses down the central column from one to two to three and possibly to four oral agents. The GLP-1 (left column) analogs can be used in lieu of one of the oral agents. Alternatively, the program can add insulin (right column), or when these regimens fail to achieve goal, may begin eliminating oral agents to reduce cost and complexity, relying progressively on insulin therapy. Figure 2 shows a schematic version of commonly used regimens and one of several options for sequence of use based in large part on the AACE/ACE algorithm.24 The clinician has the opportunity to accept or reject a recommendation; an alternative is then provided. Table 1 provides a summary of the logic used in this algorithm.
Figure 1.
Simplified overview of the algorithm. In addition to the transitions between adjacent therapies as shown by arrows, one can also transition from 0 to 2 or 0 to 3 medications or from 3 or 4 back to 0, 1, or 2. These additional transitions are not shown. The options for therapy involving four agents are generally avoided due to cost, complexity, issues of patient adherence, and difficulty in evaluating the efficacy of each of the agents.
Figure 2.
Sequence of treatment regimens. Each of these medication regimens can be combined with basal insulin, creating regimens 23–38. Many more combinations are potentially available. Combinations of insulin + GLP-1 agonists have not been approved by the FDA. Combinations of insulin + thiazolidinedione may lead to excessive weight gain, fluid retention, and occasionally (2% of cases) congestive heart failure and might increase the risk of ischemic heart disease. Combinations of a sulfonylurea or glinide with insulin therapy and other agents carry an increased risk of hypoglycemia. Agents that are expected to be effective for control of PPG (glinides, DPP.-4, GLP.-1, alpha glucosidase inhibitors) would ordinarily be used with basal insulin but not with prandial, premixed, or basal.bolus insulin therapy. Sitagliptin has been approved by the FDA for use with insulin; to date, saxagliptin has not been approved for use with insulin in the United States. Approved usage varies in other countries. Exenatide has been approved for monotherapy; to date, liraglutide has not been approved for monotherapy in the United States.
Table 1.
Simplified Overview of Algorithm
| Step | Procedure |
|---|---|
| 1. | Read data: patient identifier, target A1C, current medications, medication history, comorbidities, laboratory data, allergies, gender, contraindications, SMBG data. |
| 2. | Assess whether sufficient SMBG data are available and whether glycemic patterns are sufficiently stable with respect to longitudinal date in order to be able to make recommendations. |
| 3. | Assess current quality of glycemic control using multiple criteria (mean levels; preprandial and postprandial levels; postprandial excursions; variability; frequency, severity, and timing of hypoglycemia and hyperglycemia; and adequacy of SMBG monitoring). |
| 4. | Identify any new contraindications (e.g., allergy; cardiac, renal, hepatic disease; pregnancy; hospitalizations); if appropriate, discontinue one or more of the current medications. |
| 5. | Identify possible medication-related adverse events. If unacceptable and not expected to subside with temporary dosage adjustment, recommend discontinuation of that medication and replacement by another medication. |
| 6. | Assess adequacy of current therapy for all times of day: mean, standard deviation, percentage hyperglycemic, percentage hypoglycemic. |
| 7. | If patient has achieved goals, continue current regimen and dosages. |
| 8. | Assess problems related to hypoglycemia at each of eight specified time periods:a identify the medications that are primarily responsible for control of blood glucose during the time periods when hypoglycemia is occurring. Recommend dosage reduction for these medications. |
| 9. | Assess problems related to hyperglycemia at each of several (e.g., eight) specified time periods: identify the medications that are predominantly responsible for control of blood glucose during these time periods and recommend an increase in dosage of those medications if there are no problems with hypoglycemia during the time periods controlled by this same medication, provided that the dosage does not exceed the usual maximal effective dose. |
| 10. | Assess problems related to postprandial excursions at each of the three principal meals. Identify the medications primarily responsible for control of the postprandial excursions. Make recommendations regarding the meal content (protein, fat, carbohydrate), amount or glycemic index of carbohydrates, and timing of medications with respect to meals (e.g., GLP-1 agonists, DPP-4 inhibitors, glinides, alpha-glucosidase inhibitors). |
| 11. | Recommend advancement to the next regimen in the treatment pathway (algorithm). If the current medications have been titrated to their maximally effective doses and hyperglycemia or excessive postprandial excursions persist, then consider changing to the “next” regimen in the sequence, i.e., adding an additional agent with a different mechanism of action than the current medications. |
| 12. | Assess adequacy of SMBG monitoring. Recommend revised schedule for SMBG testing and provide explanation/rationale. |
| 13. | Assess complexity and cost of the current regimen; if excessively complex or costly, consider simplifying the regimen. |
| 14. | Periodically reassess treatment goals for A1C, mean glucose, and the preprandial glucose and PPG values. If the patient is unable to achieve goal or develops new comorbidities or other factors limiting treatment, adjust goals for glycemia to keep the risk of hypoglycemia within an acceptable level. |
| 15. | Provide explanations for recommendations, making it possible for the clinician to understand the logic being utilized by the program. |
| 16. | Allow the clinician to accept or reject (override) recommendations. |
| 17. | If the clinician rejects the initial set of recommendations, provide an alternative set of recommendations regarding therapy, together with explanations and rationale. |
| 18. | Provide access to educational materials for clinician and patient. |
| 19. | Generate instructions to be given to the patient. |
Time periods are also designated as “time buckets,” “time windows,” or “time segments,” e.g., “before lunch,” 11:00–12:30.
The logic underlying the CADS program can be shown in a series of tables (Appendix, Tables A.1–A.9). These include classes of medications (Table A.1); therapeutic regimens (69) based on combinations of 0 to 4 of these classes of medications [Table A.2; these regimens exclude combinations of classes of agents that are not approved by the FDA (e.g., GLP-1 agonists combined with insulin) and combinations of agents with similar mechanisms of action (e.g., sulfonylureas and glinides or DPP-4 inhibitors and GLP-1 agonists)]; an example of sequences of therapeutic regimens (Table A.3); an algorithm for reduction of dosage or discontinuation of a medication in response to significant problems with hypoglycemic episodes (Table A.4); an algorithm for increase of dosage of a medication in response to a significant problem with hyperglycemia (Table A.5); an algorithm for generating recommendations for change of treatment regimen with the addition of a new class of medication in response to failure to achieve goals for A1C, fasting plasma glucose (FPG) and/or postprandial glucose (PPG) (Table A.6);a simplified schematic representation of the pharmaco-dynamics of each class of medication that facilitates the identification of the medication(s) principally responsible for glycemic control at any specified time of day (Table A.7); suggested target levels for preprandial glucose and PPG values and recommended frequency of SMBG testing for specified target levels of A1C (Table A.8; as the target A1C level is lowered, the upper and lower limits for preprandial glucose and PPG values should also be adjusted); and inappropriate combinations of therapeutic agents (Table A.9).
Analysis of SMBG Data
We have developed several criteria to assess the following: (1) overall level of glycemic control; (2) hypoglycemia; (3) hyperglycemia; (4) variability; (5) patterns, e.g., post-prandial excursions, systematic changes during the day, rebound from hypoglycemia, and overtreatment of hyperglycemia; and (6) adequacy of monitoring (SMBG) as a function of the level of the desired intensity of control, type of regimen, and current A1C level. These criteria are table driven and can be modified (Appendix, Tables A.1–A.8). The following types of graphic displays can be generated:30,33–37 (1) glucose by date; (2) glucose by time of day; (3) glucose by day of the week; (4) glucose by time of day displayed separately for each day of the week; (5) pie charts and/or “stacked bar charts” to show the distribution of glucose values for any selected number of categories from “very very low” (e.g., <40 mg/dl) to “very very high” (e.g., >400 mg/dl) by date, time of day, or in relationship to meals or by day of the week;32 and (6) a “color-coded dot matrix” graphic display showing glucose by date and time of day in a new compact format.37
The program calculates the mean; standard deviation; standard error of the mean; minimum; 10th, 25th, 50th, 75th, and 90th percentiles; maximum; interquartile range; percentage of glucose observations above one or more specified thresholds for hyperglycemia; percentage of glucose values below one or more thresholds for hypoglycemia; and a measure of adequacy of monitoring. Results are presented for all data, and for eight separate times of day: before breakfast, after breakfast, before lunch, after lunch, before dinner, after dinner, bedtime, and overnight (03:00–04:00). Similar statistics are also reported for postprandial excursions when pairs of premeal and postmeal values are available for a given meal on the same day. Several scores for the quality of glycemic control are calculated, including overall control, hypoglycemia, hyperglycemia, postprandial excursions, variability, and adequacy of monitoring. The software then sets logical variables (“flags”) for these events or combinations of events, which can then be used to trigger the display of alerts and other messages to the clinician, e.g., “significant hypoglycemia is occurring in the ‘before dinner’ time period” or “insufficient number of glucose measurements.”
Interpretation of SMBG Data
The principal goal of the “interpretation” section of the program is to assess whether the current treatment should be modified. A preliminary analysis assesses the following:
Are the SMBG data current? Are there sufficient data to make recommendations?
What is the date of the most recent A1C value? Is it current? What is the A1C value?
Is the A1C value from the laboratory consistent with the A1C predicted on the basis of the SMBG data?38
Has the patient achieved goals for A1C value, average fasting glucose, and average PPG?28
Is the patient experiencing significant hypoglycemia or adverse events, or does the patient have any contraindications to any of the medications?
Is the patient female and of potential child-bearing age, and if so, are there any indications that she is pregnant or planning to become pregnant?
The program utilizes a very simplified schematic representation of pharmacodynamics to identify the types of medication and times of administration that are primarily responsible for control of glucose at any specified time of day using a rating on a scale of 1 to 10 (Table A.7). Since there are often diminishing returns in terms of efficacy for doses above 50% of the FDA-approved maximal doses for several oral anti-diabetic agents, the algorithm will not recommend increases above these levels. The program identifies glycemic “problems,” times of day when they occur, and the medications that are most likely to be primarily responsible. It first addresses problems involving hypo-glycemia, recommending that the clinician inquire whether there is an obvious or trivial explanation and then reducing the dosage of the responsible medication(s). After problems with hypoglycemia have been addressed, the program will generate recommendations regarding hyperglycemia, e.g., suggesting an increase in dosage for one of the current medications or suggesting the addition of another therapeutic agent. It may also suggest the substitution of one drug for another or the simultaneous addition of one or more medications and discontinuation of one or more of the current medications.
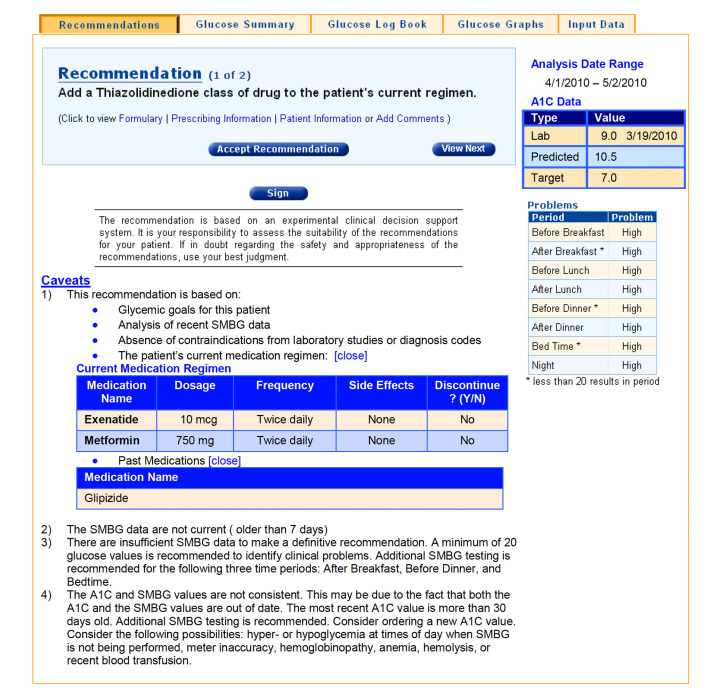
A typical output with a list of the major problems identified by analysis of the SMBG data and recommendations for modification of therapy is shown in Figure 3. All recommendations are accompanied by a caveat reminding clinicians to use their best judgment and, if in doubt, to seek further guidance from knowledgeable and authoritative sources before implementing recommendations generated by the program. The program can provide a brief explanation of the rationale underlying each recommendation. Users can accept or reject recommendations and record their own rationale.
Figure 3.
Representative output of the CADS program.
Discussion
We have successfully developed an operational CADS system. It interfaces with other systems to collect data from glucose meters via the MetriLink device and with the CDMP system, which provides the user interface. The CADS system interfaces with an electronic medical record to access additional clinical data (medication history, allergies, laboratory data, other diagnoses), although manual entry of medication and laboratory data is possible. Safety and appropriateness of the recommendations has been evaluated for a large range of combinations of patient data, regimens, target goals for A1C, extent of SMBG data, target ranges for hypoglycemia and hyperglycemia, and contraindications. The sequence of use and the pathway for addition of new drugs as shown in Figures 1 and 2, and Tables A.2 and A.3 illustrate a subset of possible pathways; CADS has flexibility that permits an “administrator” to specify nearly any conceivable pathway.
The logic underlying the CADS system has evolved considerably during its development since its initial presentation39,40 because of several factors: (1) additional approved agents for treatment (DPP-4 inhibitors, GLP-1 agonists) and indications (e.g., exenatide for monotherapy and the combination of sitagliptin and insulin), (2) new treatment algorithms,24–28 (3) the need for individualization of A1C goals in light of clinical trials indicating that the cardiovascular risks of intensive therapy are strongly dependent on duration of diabetes and the presence and severity of cardiovascular disease,41 (4) new measures of the overall quality of glycemic control and variability,42,43 (5) new methods for graphic display of SMBG data,33–37 and (6) the need for the ability to quickly and easily revise, test, and maintain the CADS system.
In the future, we plan to customize the systematic approach utilized here for application to special populations, e.g., the elderly, women who are pregnant or planning to become pregnant, women with gestational diabetes, T1DM patients, and T1DM or T2DM patients using CSII. Additional features need to be added to permit adjustment of prandial insulins when using either multiple daily injections or CSII, basal rates (CSII), and biphasic insulins.6 We have previously implemented computer algorithms for adjustment of several of these forms of insulin therapy6 based on the “Skyler algorithms.”44,45 Other planned generalizations and extensions of this system include adaptation to other health care systems, including those that rarely utilize SMBG for those patients with T2DM who are not receiving insulin, adaptation to languages other than English, use of mmol/liter rather than mg/dl, and the use of continuous glucose monitoring. Introduction of the CADS system places some potential burdens on both patient and clinician. The patient's glucose meter must be properly calibrated, accurate, and precise. The patient must adhere closely to the prescribed SMBG testing schedule and periodically submit the data to a central server. The clinician should be familiar with the algorithm, its principles, and the risk-benefit profile of each class of medications. Goals for glycemic control must be set appropriately and revised periodically as needed. The clinician would need to learn how to use the CADS/CDMP program and become familiar with routine use of the analysis, interpretation, and recommendations as provided by the program. Performance of the system will depend on the completeness and accuracy of input data.
Any program could potentially generate erroneous or suboptimal advice. The chief protection against this is the clinical judgment and experience of the clinician utilizing the program. However, there is always a possibility that the clinician will misread or misinterpret the output of the program. This risk is mitigated in part by redundancy within the program (e.g., severe or frequent hypoglycemic episodes should be apparent in the logbook displays, graphs, statistical tables, problem list, and recommendations). The user can override, ignore, or reject the recommendations of the CADS system and is instructed to immediately report any inappropriate or unsafe recommendation. The system is currently being implemented in a clinical research setting where its performance can be monitored carefully.
Acknowledgments
R. J. Kedziora of Estenda, Inc., and David Horne of Health-Sentry and LifeClinic, Inc., provided invaluable computer programming for the implementation of the CADS system. Susan Walker, R.N., Ph.D., and Lucia Novak, R.N., N.P., have conducted extensive testing of the program, examining more than 1500 real and simulated clinical cases in detail.
Abbreviations
- A1C
glycated hemoglobin
- CADS
computer-assisted decision support
- CDMP
comprehensive diabetes management program
- CSII
continuous subcutaneous insulin infusion
- DPP-4
dipeptidyl peptidase-4 inhibitor
- FDA
Food and Drug Administration
- FPG
fasting plasma glucose
- GLP-1
glucagon-like peptide-1 receptor agonist
- PCP
primary care provider
- PPG
postprandial glucose
- SMBG
self-monitoring of blood glucose
- T2DM
type 2 diabetes mellitus
References
- 1.Albisser AM. Six generations of the insulin dosage computer: a new clinical device for diabetes self-management through specialized centres. Horm Metab Res Suppl. 1990;24:140–144. [PubMed] [Google Scholar]
- 2.Chiarelli F, Tumini S, Morgese G, Albisser AM. Controlled study in diabetic children comparing insulin-dosage adjustment by manual and computer algorithms. Diabetes Care. 1990;13(10):1080–1084. doi: 10.2337/diacare.13.10.1080. [DOI] [PubMed] [Google Scholar]
- 3.Chanoch LH, Jovanovic L, Peterson CM. The evaluation of a pocket computer as an aid to insulin dose determination by patients. Diabetes Care. 1985;8(2):172–176. doi: 10.2337/diacare.8.2.172. [DOI] [PubMed] [Google Scholar]
- 4.Gross TM, Kayne D, King A, Rother C, Juth S. A bolus calculator is an effective means of controlling postprandial glycemia in patients on insulin pump therapy. Diabetes Technol Ther. 2003;5(3):365–369. doi: 10.1089/152091503765691848. [DOI] [PubMed] [Google Scholar]
- 5.Davidson PC, Steed RD, Bode BW. Glucommander: a computer-directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation. Diabetes Care. 2005;28(10):2418–2423. doi: 10.2337/diacare.28.10.2418. [DOI] [PubMed] [Google Scholar]
- 6.Pernick NL, Rodbard D. Personal computer programs to assist with self-monitoring of blood glucose and self-adjustment of insulin dosage. Diabetes Care. 1986;9(1):61–69. doi: 10.2337/diacare.9.1.61. [DOI] [PubMed] [Google Scholar]
- 7.Mazze RS, Etzwiler DD, Strock E, Peterson K, McClave CR, 2nd, Meszaros JF, Leigh C, Owens LW, Deeb LC, Peterson A. Staged diabetes management. Toward an integrated model of diabetes care. Diabetes Care. 1994;17(Suppl 1):56–66. [PubMed] [Google Scholar]
- 8.Mazze RS, Strock ES, Simonson GD, Bergenstal RM. New York: Wiley; 2007. Staged diabetes management: a systematic approach (revised second edition) http://media.wiley.com/product_data/excerpt/6X/04700612/047006126X.pdf. [Google Scholar]
- 9.Osheroff JA, Teich JM, Middleton B, Steen EB, Wright A, Detmer DE. A roadmap for national action on clinical decision support. J Am Med Inform Assoc. 2007;14(2):141–145. doi: 10.1197/jamia.M2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu VL, Buchanan BG, Shortliffe EH, Wraith SM, Davis R, Scott AC, Cohen SN. Evaluating the performance of a computer-based consultant. Comput Programs Biomed. 1979;9(1):95–102. doi: 10.1016/0010-468x(79)90022-9. [DOI] [PubMed] [Google Scholar]
- 11.Evans RS, Pestotnik SL, Classen DC, Clemmer TP, Weaver LK, Orme JF, Jr, Lloyd JF, Burke JP. A computer-assisted management program for antibiotics and other antiinfective agents. N Engl J Med. 1998;338(4):232–238. doi: 10.1056/NEJM199801223380406. [DOI] [PubMed] [Google Scholar]
- 12.Langlotz CP, Fagan LM, Tu SW, Sikic BI, Shortliffe EH. A therapy planning architecture that combines decision theory and artificial intelligence techniques. Comput Biomed Res. 1987;20(3):279–303. doi: 10.1016/0010-4809(87)90059-0. [DOI] [PubMed] [Google Scholar]
- 13.Rocha BH, Langford LH, Towner S. Computerized management of chronic anticoagulation: three years of experience. Stud Health Technol Inform. 2007;129(Pt 2):1032–1036. [PubMed] [Google Scholar]
- 14.Kelly JJ, Sweigard KW, Shields K, Schneider D, Eisenberg JM. Patient Safety Awards. Safety, effectiveness, and efficiency: a Web-based virtual anticoagulation clinic. Jt Comm J Qual Saf. 2003;29(12):646–651. doi: 10.1016/s1549-3741(03)29076-6. [DOI] [PubMed] [Google Scholar]
- 15.Schedlbauer A, Prasad V, Mulvaney C, Phansalkar S, Stanton W, Bates DW, Avery AJ. What evidence supports the use of computerized alerts and prompts to improve clinicians' prescribing behavior? J Am Med Inform Assoc. 2009;16(4):531–538. doi: 10.1197/jamia.M2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tierney WM, Overhage JM, Takesue BY, Harris LE, Murray MD, Vargo DL, McDonald CJ. Computerizing guidelines to improve care and patient outcomes: the example of heart failure. J Am Med Inform Assoc. 1995;2(5):316–322. doi: 10.1136/jamia.1995.96073834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tierney WM, Overhage JM, Murray MD, Harris LE, Zhou XH, Eckert GJ, Smith FE, Nienaber N, McDonald CJ, Wolinsky FD. Effects of computerized guidelines for managing heart disease in primary care. J Gen Intern Med. 2003;18(12):967–976. doi: 10.1111/j.1525-1497.2003.30635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tierney WM, Overhage JM, Murray MD, Harris LE, Zhou XH, Eckert GJ, Smith FE, Nienaber N, McDonald CJ, Wolinsky FD. Can computer-generated evidence-based care suggestions enhance evidence-based management of asthma and chronic obstructive pulmonary disease? A randomized, controlled trial. Health Serv Res. 2005;40(2):477–497. doi: 10.1111/j.1475-6773.2005.00368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291(3):335–342. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 20.Grant RW, Cagliero E, Dubey AK, Gildesgame C, Chueh HC, Barry MJ, Singer DE, Nathan DM, Meigs JB. Clinical inertia in the management of type 2 diabetes metabolic risk factors. Diabet Med. 2004;21(2):150–155. doi: 10.1111/j.1464-5491.2004.01095.x. [DOI] [PubMed] [Google Scholar]
- 21.Grant RW, Buse JB, Meigs JB, University HealthSystem Consortium (UHC) Diabetes Benchmarking Project Team Quality of diabetes care in U.S. academic medical centers: low rates of medical regimen change. Diabetes Care. 2005;28(2):337–442. doi: 10.2337/diacare.28.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodbard HW, Fox KM, Hardy E, Grandy S, SHIELD Study Group Disease control among adults with type 2 diabetes mellitus, hypertension and obesity. Abstract from the Annual Meeting of American Association of Clinical Endocrinologists; Boston, MA. 2010. [Google Scholar]
- 23.Rizza RA, Vigersky RA, Rodbard HW, Ladenson PW, Young WF, Jr, Surks MI, Kahn R, Hogan PF. A model to determine workforce needs for endocrinologists in the United States until 2020. Endocr Pract. 2003;9(3):210–219. doi: 10.4158/EP.9.3.210. [DOI] [PubMed] [Google Scholar]
- 24.Rodbard HW, Jellinger PS, Davidson JA, Einhorn D, Garber AJ, Grunberger G, Handelsman Y, Horton ES, Lebovitz H, Levy P, Moghissi ES, Schwartz SS. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009;15(6):540–559. doi: 10.4158/EP.15.6.540. [DOI] [PubMed] [Google Scholar]
- 25.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B, American Diabetes Association, European Association for Study of Diabetes Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woo V. Important differences: Canadian Diabetes Association 2008 clinical practice guidelines and the consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2009;52(3):552–555. doi: 10.1007/s00125-008-1236-0. [DOI] [PubMed] [Google Scholar]
- 27.Inzucchi SE. Diabetes facts and guidelines. Accessed August 28, 2010. http://medicine.yale.edu/intmed/endocrin/Images/695_21366_Takeda_Yale_KLL_tcm314-50135.PDF.
- 28.Rodbard HW, Blonde L, Braithwaite SS, Brett EM, Cobin RH, Handelsman Y, Hellman R, Jellinger PS, Jovanovic LG, Levy P, Mechanick JI, Zangeneh F, AACE Diabetes Mellitus Clinical Practice Guidelines Task Force American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(Suppl 1):1–68. doi: 10.4158/EP.13.S1.1. [DOI] [PubMed] [Google Scholar]
- 29.Buse JB, Bergenstal RM, Glass LC, Heilmann CR, Lewis MS, Kwan AY, Hoogwerf BJ, Rosenstock J. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2011;154(2):103–112. doi: 10.7326/0003-4819-154-2-201101180-00300. [DOI] [PubMed] [Google Scholar]
- 30.Rodbard D. Optimizing display, analysis, interpretation and utility of self-monitoring of blood glucose (SMBG) data for management of patients with diabetes. J Diabetes Sci Technol. 2007;1(1):62–71. doi: 10.1177/193229680700100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polonsky WH, Jelsovsky Z, Panzera S, Parkin CG, Wagner RS. Primary care physicians identify and act upon glycemic abnormalities found in structured, episodic blood glucose monitoring data from non-insulin-treated type 2 diabetes. Diabetes Technol Ther. 2009;11(5):283–291. doi: 10.1089/dia.2008.0087. [DOI] [PubMed] [Google Scholar]
- 32.Fonda SJ, Paulsen CA, Perkins J, Kedziora RJ, Rodbard D, Bursell SE. Usability test of an internet-based informatics tool for diabetes care providers: the comprehensive diabetes management program. Diabetes Technol Ther. 2008;10(1):16–24. doi: 10.1089/dia.2007.0252. [DOI] [PubMed] [Google Scholar]
- 33.Rodbard D. Display of glucose distributions by date, time of day, and day of week: new and improved methods. J Diabetes Sci Technol. 2009;3(6):1388–1394. doi: 10.1177/193229680900300619. Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodbard D. A semilogarithmic scale for glucose provides a balanced view of hyperglycemia and hypoglycemia. J Diabetes Sci Technol. 2009;3(6):1395–1401. doi: 10.1177/193229680900300620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazze RS, Lucido D, Langer O, Hartmann K, Rodbard D. Ambulatory glucose profile: representation of verified self-monitored blood glucose data. Diabetes Care. 1987;10(1):111–117. doi: 10.2337/diacare.10.1.111. [DOI] [PubMed] [Google Scholar]
- 36.Mazze RS, Strock E, Wesley D, Borgman S, Morgan B, Bergenstal R, Cuddihy R. Characterizing glucose exposure for individuals with normal glucose tolerance using continuous glucose monitoring and ambulatory glucose profile analysis. Diabetes Technol Ther. 2008;10(3):149–159. doi: 10.1089/dia.2007.0293. [DOI] [PubMed] [Google Scholar]
- 37.Rodbard D. New approaches to display of self-monitoring of blood glucose data. J Diabetes Sci Technol. 2009;3(5):1121–1127. doi: 10.1177/193229680900300515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, A1c-Derived Average Glucose Study Group Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31(8):1473–1478. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodbard D, Vigersky R. Development of algorithms for clinical decision support for primary care providers. Diabetes Technol Ther. 2005;7(2):409. [Google Scholar]
- 40.Vigersky RA, Galen RS, Horne D, Cavotta M, Rodbard D. Computer assisted decision support (CADS) for primary care of diabetes. Telemed E-Health. 2007;13:168. [Google Scholar]
- 41.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD, VADT Investigators Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 42.Rodbard D. Interpretation of continuous glucose monitoring data: glycemic variability and quality of glycemic control. Diabetes Technol Ther. 2009;11(Suppl 1):S55–S67. doi: 10.1089/dia.2008.0132. [DOI] [PubMed] [Google Scholar]
- 43.Rodbard D. New and improved methods to characterize glycemic variability using continuous glucose monitoring. Diabetes Technol Ther. 2009;11(9):551–565. doi: 10.1089/dia.2009.0015. [DOI] [PubMed] [Google Scholar]
- 44.Skyler JS, Skyler DL, Seigler DE, O'Sullivan MJ. Algorithms for adjustment of insulin dosage by patients who monitor blood glucose. Diabetes Care. 1981;4(2):311–318. doi: 10.2337/diacare.4.2.311. [DOI] [PubMed] [Google Scholar]
- 45.Schade DS, Santiago JV, Skyler JS, Rizza RA, Intensive insulin therapy . Amsterdam: Excerpta Medica; 1983. [Google Scholar]