Abstract
Background
One of the most serious complications after major orthopedic surgery is deep wound or periprosthetic joint infection. Various risk factors for infection after hip and knee replacement surgery have been reported, including patients' comorbidities and surgical technique factors. We investigated whether hyperglycemia and diabetes mellitus (DM) are associated with infection that requires surgical intervention after total hip and knee arthroplasty.
Methods
We reviewed our computerized database for elective primary total hip and knee arthroplasty from 2000 to 2008. Demographic information, past medical history of patients, perioperative biochemistry, and postoperative complications were reviewed.
Patients were divided into two groups: infected group (101 patients who had surgical intervention for infection at our institution within 2 years after primary surgery) and noninfected group (1847 patients with no intervention with a minimum of one year follow-up. The data were analyzed using t, chi-squared, and Fisher's exact tests.
Results
There were significantly more diabetes patients in the infected group compared with the noninfected group (22% versus 9%, p < .001). Infected patients had significantly higher perioperative blood glucose (BG) values: preoperative BG (112 ± 36 versus 105 ± 31 mg/dl, p = .043) and postoperative day (POD) 1 BG (154 ± 37 versus 138 ± 31 mg/dl, p < .001). Postoperative morning hyperglycemia (BG >200 mg/dl) increased the risk for the infection more than two-fold. Non-DM patients were three times more likely to develop the infection if their morning BG was >140 mg/dl on POD 1, p = .001. Male gender, higher body mass index, knee arthroplasty, longer operative time and hospital stay, higher comorbidity index, history of myocardial infarction, congestive heart failure, and renal insufficiency were also associated with the infection.
Conclusions
Diabetes mellitus and morning postoperative hyperglycemia were predictors for postoperative infection following total joint arthroplasty. Even patients without a diagnosis of DM who developed postoperative hyperglycemia had a significantly increased risk for the infection.
Keywords: blood glucose, diabetes mellitus, hip and knee arthroplasty, infection, orthopedic surgery
Introduction
Total hip or knee replacement surgery are major operations frequently performed in the United States. Over 500,000 hip and knee arthroplasties are performed each year, and by year 2030, the number is projected to increase to over 4 million.1 One of the most serious complications after total hip and knee replacement surgery is deep wound or periprosthetic joint infection (PJI), which increases morbidity and mortality, doubling rehospitalization rates and increasing health care costs more than three times.2
It has been estimated that more than 8% of patients with diabetes mellitus (DM) undergo primary or revision total hip or knee arthroplasty in the United States.3 The American Diabetes Association reported on their Web site (www.diabetes.org) that, in 2007, the prevalence of DM in the United States was 23.6 million (7.8% of the population), with 1.6 million new cases of DM each year. Patients with DM have increased risk for postoperative complications. Diabetes mellitus may increase risk for postoperative infection after laminectomy,4 cardiac surgery,5 and noncardiac surgery.6 Although DM was associated with infection after total joint arthroplasty,7–10 some studies did not find this correlation.11–13 Actual peri-operative blood glucose (BG) levels were not measured in these studies.
One study found that a majority of non-DM patients develop hyperglycemia during the first 2 days after major limb surgery, but high glucose could not be correlated with infection following arthroplasty because there was no postoperative infections in their cohort up to a year following the surgery.14 In contrast, perioperative hyperglycemia increased the rate of infection after cardiac surgery,15 general surgery,16 colorectal surgery,17 spinal surgery,18 and mastectomy19 but did not increase the risk for infections after esophagectomy.20 Whether postoperative hyperglycemia increases the risk for PJI after hip and knee arthroplasty is not established.
Hyperglycemia may result from medications, stress, impaired glucose tolerance, or uncontrolled DM. Stress hyperglycemia is common in surgical patients. It is usually a manifestation of counter-regulatory hormonal responses and proinflammatory mediators. Acute hyper-glycemia may impair the ability of the host to combat infection.21 Thus hyperglycemia may have an impact on infection after total joint arthroplasty independent of DM status.
In this retrospective study, we investigated the effects of perioperative hyperglycemia and DM on the incidence of infection that requires surgical intervention after total hip and knee arthroplasty.
Methods
After obtaining the institutional review board approval, we queried our institutional computerized database from January 2000 to February 2008. The database prospectively records all hip and knee surgeries performed at our institution. In this study, we included patients who underwent primary total hip or knee arthroplasty, developed PJI, and subsequently had surgical intervention for the infection within 2 years of the primary procedure at our institution (infected group). Periprosthetic joint infection was defined as a deep infection below fascia with involvement of muscle and/or bone. Because diagnosis of PJI after hip and knee arthroplasty still presents significant clinical challenge, we included in the study patients who had surgical interventions that confirmed the clinical diagnosis of PJI.
As a control group, we identified patients who underwent primary hip or knee arthroplasty during the same time period, did not undergo surgical treatment for infection at our institution postoperatively, and had follow-up data for a minimum of 1 year postoperatively (noninfected group).
Demographic information, past medical history of patients, perioperative biochemistry, and postoperative complications were reviewed. All perioperative BG levels were recorded. Preoperative BG values were random BG values (patients were not instructed to fast) taken at the preoperative visit. For postoperative BG values, we used morning fasting BG. Since our hospital database records the time of measurement of BG but does not record whether glucose values were fasting or not, we used only BG values taken before 1:00 pm on the day of surgery (patients were fasting overnight prior to surgery and did not eat before dinnertime after the surgery). For subsequent post-operative days (PODs), we used BG values closest to 6:00 am but not later than 8:00 am (when breakfast is served in the hospital). During the period of the study, preoperative glycemic control was left to the patients' primary care physician and endocrinologist. If preoperative random glucose was ≥200 mg/dl, a letter was sent to the physician to address glycemic control. There was no hospital policy regarding BG preoperative glycemic control in patients with BG ≥200 mg/dl on the morning of surgery. Postoperatively, patients were treated if BG was ≥180 mg/dl. Persistent hyperglycemia called for an endocrinology consult and insulin infusion. The severity of patients' comorbidities was calculated using Charlson comorbidity index modified by Deyo et al.22 and the American Society of Anesthesiologists physical status (ASAPS).
All patients received standardized perioperative management for infection prophylaxis throughout the study period. Intravenous antibiotic (cefazolin or vancomycin in allergic patients) was given within 1 h of the incision and for 24 h postoperatively. The surgery was performed in operating rooms with vertical laminar flow and use of helmet aspirator suits and double gloves. Skin was prepared with alcohol and iodine lavage. Uncemented and cemented prostheses were used, respectively, for hip and knee arthroplasties, without postoperative drains. Regional anesthesia, spinal, and/or epidural, targeting a mean arterial pressure of 60 mm Hg, was used unless contraindicated. Over 90% of hip and knee surgeries were performed under spinal/epidural anesthesia at our institution. Thromboembolic prophylaxis was with intraoperative intravenous heparin 1000 IU and postoperative warfarin titrated to international normalized ratio (INR) 1.5 to 2.0.
Statistical Analysis
Data analysis was performed using t-tests for all continuous variables and chi-squared or Fisher's exact tests for all categorical variables. Blood glucose values were analyzed as continuous and categorical variables. Categories were (1) BG ≤100 mg/dl, (2) BG >100 mg/dl to BG ≤200 mg/dl, and (3) BG >200 mg/dl. A one-way analysis of variance was used to assess the association between perioperative BG levels and time to infection in the infected cohort. Further cross-sectional analysis was performed to assess the combinatorial effects of DM and hyperglycemia on likelihood for infection. For this analysis, hyperglycemia was defined as BG >140 mg/dl on POD 1; p < .05 was considered statistically significant.
Results
A total of 101 patients were included in the infected group and 1847 patients in the noninfected group. Surgical interventions included resection of arthroplasty components and insertion of an antibiotic spacer (40 patients), deep (i.e., with arthrotomy) irrigation and debridement (I&D) with or without polyethylene exchange (56 patients), superficial I&D (2 patients), or one-stage revision arthro-plasty for infection (3 patients). Operative notes were reviewed to confirm that the infection was the cause for the reoperation. In 5 cases in which this diagnosis was not clear, medical records were reviewed further to confirm infection. Four of these 5 patients had positive intraoperative cultures, and the final patient had a superficial I&D for wound dehiscence with a subsequent resection and antibiotic spacer placement less than 1 year following the I&D. Only one patient developed infection in both hips performed 3 years apart, each infection developed within 2 years of surgery. We counted this patient as two separate episodes of infection. The interventions for the infected joint were performed on average of 0.4 ± 0.5 years after the primary surgery (range 0–1.9 years).
There was no difference between infected and noninfected patients in age and ethnicity as well as preoperative hemoglobin and white blood cell count (Tables 1 and 2). Infected patients tended to be male and had a significantly higher body mass index (BMI), longer operative time, longer hospital stay, were undergoing knee (versus hip) arthroplasty, and had bilateral (versus unilateral) surgery (Tables 1 and 2). Both comorbidity indices, the ASAPS score, and the Charlson comorbidity index were significantly associated with infection as well as history of myocardial infarction (MI), congestive heart failure, and renal disease (Tables 1 and 2). Patients who became infected were more likely to have had homologous blood transfusions and were less likely to have had autologous blood transfusions (Table 2). Preoperative laboratory values associated with infection included higher creatinine and blood urea nitrogen (BUN), higher INR, and lower platelet count. Significant post-operative labs included higher creatinine and BUN, higher INR, and higher white blood cell count (Tables 1 and 2).
Table 1.
Patients' Data, Continuous Variablesa
| Variable | Infected (n = 101) | Noninfected (n = 1847) | p |
|---|---|---|---|
| Age (years) | 64.7 ± 11.6 | 62.9 ± 11.8 | 0.143 |
| BMI (kg/m2) | 32 ± 9 | 30 ± 7 | 0.003b |
| Hospital stay (days) | 6.5 ± 8.3 | 4.1 ± 1.4 | <0.001b |
| Operative time (minutes) | 135.2 ± 69.6 | 108.8 ± 35.5 | <0.001b |
| Preoperative labs | |||
| Creatinine (mg/dl) | 1.0 ± 0.3 | 0.9 ± 0.3 | <0.001b |
| Hemoglobin (g/dl) | 13.4 ± 1.7 | 13.3 ± 1.3 | 0.666 |
| INR | 1.10 ± 0.26 | 1.02 ± 0.20 | 0.002b |
| Platelet count (1000 cells/μl) | 242.5 ± 81.1 | 259.5 ± 63.7 | 0.012b |
| BUN (mg/dl) | 20.3 ± 7.2 | 17.6 ± 6.1 | <0.001b |
| Serum WBC (1000 cells/μl) | 7.5 ± 2.2 | 7.3 ± 2.0 | 0.305 |
| Postoperative Labs | |||
| Creatinine (mg/dl) | 1.1 ± 0.4 | 0.9 ± 0.3 | <0.001b |
| Hemoglobin (g/dl) | 10.2 ± 1.2 | 10.4 ± 1.2 | 0.094 |
| INR | 1.42 ± 0.25 | 1.36 ± 0.21 | 0.006b |
| Platelet count (1000 cells/μl) | 207.3 ± 74.3 | 208.3 ± 56.0 | 0.878 |
| BUN (mg/dl) | 16.8 ± 9.5 | 12.3 ± 5.2 | <0.001b |
| Serum WBC (1000 cells/μl) | 9.8 ± 2.8 | 9.1 ± 2.4 | 0.006b |
Data are mean ± standard deviation. WBC, white blood cell count.
p < .05.
Table 2.
Patients' Data, Categorical Variables
| Variable | Infected (n = 101) | Noninfected (n = 1847) | p | ||
|---|---|---|---|---|---|
| (N) | Percentages (%) | (N) | Percentages (%) | ||
| Gender (male/female) | 56/45 | 55/45 | 682/1165 | 37/63 | <0.001a |
| Ethnicity (white/black/other) | 83/14/3b | 83/14/3 | 1288/164/15b | 88/11/1 | 0.129 |
| Joint (hip/knee) | 31/70 | 31/69 | 1084/763 | 59/41 | <0.001a |
| Laterality (unilateral/bilateral) | 75/24b | 76/24 | 1591/256 | 86/14 | 0.007a |
| ASAPS score (1–2/3–4) | 28/53b | 35/65 | 734/470b | 61/39 | <0.001a |
| Charlson index (≤3/>3) | 74/27 | 73/27 | 1618/229 | 88/12 | <0.001a |
| Prior MI | 9 | 9 | 64/1783 | 3 | 0.011a |
| Congestive heart failure | 7 | 7 | 15/1832 | 1 | <0.001a |
| Lung disease | 12 | 12 | 177/1670 | 10 | 0.488 |
| Connective tissue disease | 4 | 4 | 67/1780 | 4 | 0.784 |
| Renal disease | 7 | 7 | 11/1836 | 1 | <0.001a |
| Homologous transfusions | 44b | 45 | 319/1526b | 17 | <0.001a |
| Autologous transfusions | 54b | 56 | 1347/497b | 73 | <0.001a |
| DM | 22 | 22 | 162/1685 | 9 | <0.001a |
p < .05.
Values do not add up to the total number of patients (some data were missing).
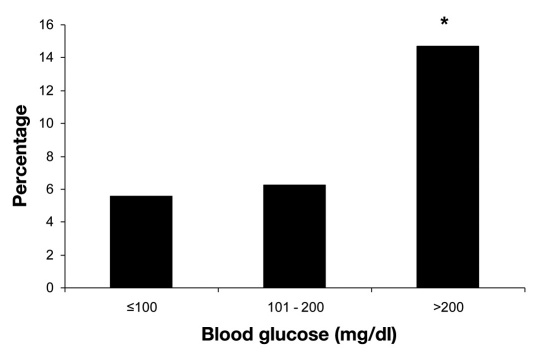
Analysis of BG as continuous values showed that patients in the infected group were more likely to have had higher perioperative BG values. Patients with PJI had significantly higher preoperative BG (112 ± 36 versus 105 ± 31 mg/dl, p = .043) and POD 1 BG (154 ± 37 versus 138 ± 31 mg/dl, p < .001). Categorical analysis of perioperative glucose values showed only significant differences between groups on POD 1, but taken all together, patients with BG >200 mg/dl have had a greater than two-fold increased rate of the infection (Table 3). The infection rate of PJI and fasting BG levels during total hospitalization was also statistically significant, p < .001 (Figure 1). Patients in the infected group were also more likely to have a prior diagnosis of DM, p < .001 (Table 2).
Table 3.
Categorical Perioperative Fasting Glucose Values
| Glucose value | Infected | Noninfected | p | ||||
|---|---|---|---|---|---|---|---|
| ≤100 mg/dl | 101–200 mg/dl | >200 mg/dl | ≤100 mg/dl | 101–200 mg/dl | >200 mg/dl | ||
| Day of surgery | 0 | 5 | 0 | 12 | 26 | 0 | 0.300 |
| POD 1 | 1 | 53 | 6 | 41 | 880 | 37 | 0.049a |
| POD 2 | 1 | 7 | 1 | 9 | 99 | 7 | 0.777 |
| POD 3 | 17 | 36 | 2 | 232 | 412 | 8 | 0.296 |
| POD 4 | 3 | 10 | 1 | 79 | 151 | 6 | 0.428 |
| All (%)b | 22 (15) | 111 (78) | 10 (7) | 373 (18) | 1668 (79) | 58 (3) | <0.001a |
p < .05.
Value in parentheses is the percentage of patients in each BG category in infected and noninfected groups.
Figure 1.
The infection rate of PJIs and fasting BG levels during total hospitalization. The asterisk represents p < .001 for >200 versus ≤100 mg/dl and >200 versus 101–200 mg/dl. p < .001 for trend.
In cross-sectional analysis, we compared the likelihood of developing PJI in patients with low and high BG values on POD 1 and with or without DM. In non-DM patients, high BG on POD 1 was significantly associated with an increased risk for the postoperative infection, p < .001 (Table 4). There was no association between an increased risk for infection and high BG on POD 1 among DM patients. When we compared infection rates for DM patients with a cutoff of 180 mg/dl, patients with high BG had a 22% (8/37) infection rate compared to 9% (5/54) in patients with low BG but did not reach statistical significance (p = .23), possibly due to a low number of patients.
Table 4.
Cross-sectional Analysis Results with Postoperative Day 1 Glucose Values
| BG | Infected/noninfected | p | |
|---|---|---|---|
| N (%) | |||
| DM | ≤140 mg/dl | 3/22 (14%) | 1 |
| >140 mg/dl | 10/69 (14%) | ||
| No DM | ≤140 mg/dl | 20/582 (3%) | <0.001a |
| >140 mg/dl | 27/285 (9%) |
p < .05.
Discussion
Our retrospective study showed that perioperative fasting BG increased, by more than two-fold, the risk of infection requiring surgical intervention after total hip and knee arthroplasty. Even patients without a diagnosis of DM were three times more likely to develop the infection if their fasting BG on POD 1 was >140 mg/dl. The study also confirmed known risk factors for the infection: increased BMI, higher ASAPS score and Charlson index, history of MI, congestive heart failure or renal disease, longer duration of the surgery and longer hospital stay, higher INR, and homologous transfusions.
Several studies have evaluated risk factors for infection after hip and knee replacement surgery. Diabetes mellitus as a risk factor for infection is controversial. Some studies have found an association between DM and infection,7–10 but some studies have not.11–13 The controversy might exist because the diagnosis of DM was used from the discharge summaries or because the studies were not powered enough to show the difference. More importantly, peri-operative BG levels were not measured in these studies. As our study showed, postoperative hyperglycemia may have more influence on the prediction on the infection in non-DM patients than in patients diagnosed with DM (Table 4). Hence perioperative BG levels may be more important than diagnosis of DM in prediction of the PJI after hip and knee arthroplasty. Indeed, in general and vascular surgery patients, postoperative hyperglycemia increased the risk for postoperative infection independent of preoperative BG levels and DM status.6
Although our cohort of infected cases is among the largest in which the risks for the postoperative infection after total hip and knee arthroplasty were evaluated, it might yet be underpowered to show the difference in BG level on every POD. But when all fasting BG levels were taken together, hyperglycemia was significantly associated with the infection, p < .001 (Table 4). We used BG levels on POD 1 for cross sectional analysis since these values were the most complete in our data set. Due to the small number of DM patients with the infection, we divided BG into only two categories. Although there was a trend for higher rate of infection in DM patients if BG was >180 mg/dl, it did not reach statistical significance, probably because of the small number of patients. Conversely, in non-DM patients, the infection rate was statistically significant if BG was >140 mg/dl, p < .001 (Table 4). Similar to our study, fasting BG values at 6:00 am on POD 1 and POD 2 <200 mg/dl are used for measuring quality control for surgical site infection prevention after cardiac surgery in the Surgical Care Improvement Project, the Joint Commission on Accreditation of Healthcare Organizations performance measure set, and the National Quality Forum standards.
A limitation of our study is its retrospective nature. All retrospective studies depend on the quality and availability of the data and the accuracy and completeness of the medical records. Some patients may be lost in follow-up, and BG values might be missing from the database. We used a 2-year follow-up period to increase the power of the study, but we did not have data on glycemic control during this period nor preoperative hemoglobin A1c values. Non-DM patients who developed hyperglycemia during the perioperative period may not have increased BG later in the recovery period. How many patients eventually develop DM if they had perioperative hyperglycemia is unknown. We did not perform a multivariate analysis because of the low number of patients with PJI and BG values. Also, the number of BG values was too small to calculate BG variability, which might be important since the variability increases oxidative stress and triggers coagulation and inflammation cascades.23 Finally, although we limited our uninfected cohort to patients with at least one year of known follow-up at our institution, it is possible that we may have missed some patients who were operated on at another institution.
The strength of our study is that it was performed at a high-volume orthopedic center that has strict perioperative protocols; as a result, patients received uniform infection precautions, antibiotic prophylaxis, and thromboprophylaxis. Additionally, by including only patients who developed PJI that required operative interventions, we defined our cohort to the group of clinically important infections that are known to increase morbidity, mortality, and health care costs. Patients with severe orthopedic postoperative infections have substantially greater physical limitations and significant reductions in their health-related quality of life.2 Finally, all BG values used in the study were tested in the central laboratory. Point of care measure-ments were not used for the analysis.
The mechanism of how hyperglycemia might cause postoperative infections has been described elsewhere.21 A review article reported that the most obvious findings related to hyperglycemia included reduced neutrophil activity (e.g., chemotaxis, formation of reactive oxygen species, phagocytosis of bacteria) and concluded that acute, short-term hyperglycemia affects all major components of innate immunity and impairs the ability of the host to combat infection.21
Our study suggests that the goal of perioperative glycemic control after orthopedic surgery should be <200 mg/dl on morning BG values to reduce incidence of PJI that requires surgical intervention. Controlling BG post-operatively might be a simple way to reduce the rate of infection after major orthopedic surgery. A prospective randomized controlled study is needed to determine whether tight control of preoperative BG levels leads to a decreased incidence of PJI in this clinical setting.
Conclusion
Our study showed that perioperative morning hyperglycemia >200 mg/dl increased by more than two times the risk of infection that requires surgical intervention after elective total hip and knee arthroplasty. Even patients without diagnosis of DM had a three-fold increased risk for the infection if fasting BG on POD 1 was >140 mg/dl.
Abbreviations
- ASAPS
American Society of Anesthesiologists physical status
- BG
blood glucose
- BMI
body mass index
- BUN
blood urea nitrogen
- DM
diabetes mellitus
- I&D
irrigation and debridement
- INR
international standardized ratio
- MI
myocardial infarction
- PJI
periprosthetic joint infection
- POD
postoperative day
References
- 1.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 2.Whitehouse JD, Friedman ND, Kirkland KB, Richardson WJ, Sexton DJ. The impact of surgical–site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol. 2002;23(4):183–189. doi: 10.1086/502033. [DOI] [PubMed] [Google Scholar]
- 3.Bolognesi MP, Marchant MH, Jr, Viens NA, Cook C, Pietrobon R, Vail TP. The impact of diabetes on perioperative patient outcomes after total hip and total knee arthroplasty in the United States. J Arthroplasty. 2008;23(6 Suppl 1):92–98. doi: 10.1016/j.arth.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Friedman ND, Sexton DJ, Connelly SM, Kaye KS. Risk factors for surgical site infection complicating laminectomy. Infect Control Hosp Epidemiol. 2007;28(9):1060–1065. doi: 10.1086/519864. [DOI] [PubMed] [Google Scholar]
- 5.Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67(2):352–362. doi: 10.1016/s0003-4975(99)00014-4. [DOI] [PubMed] [Google Scholar]
- 6.Ramos M, Khalpey Z, Lipsitz S, Steinberg J, Panizales MT, Zinner M, Rogers SO. Relationship of perioperative hyperglycemia and post-operative infections in patients who undergo general and vascular surgery. Ann Surg. 2008;248(4):585–591. doi: 10.1097/SLA.0b013e31818990d1. [DOI] [PubMed] [Google Scholar]
- 7.Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 Suppl):84–88. doi: 10.1016/j.arth.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Serna F, Mont MA, Krackow KA, Hungerford DS. Total knee arthroplasty in diabetic patients. Comparison to a matched control group. J Arthroplasty. 1994;9(4):375–379. doi: 10.1016/0883-5403(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 9.England SP, Stern SH, Insall JN, Windsor RE. Total knee arthroplasty in diabetes mellitus. Clin Orthop Relat Res. 1990;(260):130–134. [PubMed] [Google Scholar]
- 10.Marchant MH, Jr, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621–1629. doi: 10.2106/JBJS.H.00116. [DOI] [PubMed] [Google Scholar]
- 11.Meding JB, Reddleman K, Keating ME, Klay A, Ritter MA, Faris PM, Berend ME. Total knee replacement in patients with diabetes mellitus. Clin Orthop Relat Res. 2003;(416):208–216. doi: 10.1097/01.blo.0000093002.90435.56. [DOI] [PubMed] [Google Scholar]
- 12.Chiu FY, Lin CF, Chen CM, Lo WH, Chaung TY. Cefuroxime-impregnated cement at primary total knee arthroplasty in diabetes mellitus. A prospective, randomised study. J Bone Joint Surg Br. 2001;83(5):691–695. doi: 10.1302/0301-620x.83b5.11737. [DOI] [PubMed] [Google Scholar]
- 13.Papagelopoulos PJ, Idusuyi OB, Wallrichs SL, Morrey BF. Long term outcome and survivorship analysis of primary total knee arthroplasty in patients with diabetes mellitus. Clin Orthop Relat Res. 1996;(330):124–132. doi: 10.1097/00003086-199609000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Pili-Floury S, Mitifiot F, Penfornis A, Boichut N, Tripart MH, Christophe JL, Garbuio P, Samain E. Glycaemic dysregulation in nondiabetic patients after major lower limb prosthetic surgery. Diabetes Metab. 2009;35(1):43–48. doi: 10.1016/j.diabet.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi GY, Murad MH, Flynn DN, Erwin PJ, Cavalcante AB, Bay Nielsen H, Capes SE, Thorlund K, Montori VM, Devereaux PJ. Effect of perioperative insulin infusion on surgical morbidity and mortality: systematic review and meta-analysis of randomized trials.7. Mayo Clin Proc. 2008;83(4):418–430. doi: 10.4065/83.4.418. [DOI] [PubMed] [Google Scholar]
- 16.Ata A, Lee J, Bestle SL, Desemone J, Stain SC. Postoperative hyperglycemia and surgical site infection in general surgery patients. Arch Surg. 2010;145(9):858–864. doi: 10.1001/archsurg.2010.179. [DOI] [PubMed] [Google Scholar]
- 17.McConnell YJ, Johnson PM, Porter GA. Surgical site infections following colorectal surgery in patients with diabetes: association with postoperative hyperglycemia. J Gastrointest Surg. 2009;13(3):508–515. doi: 10.1007/s11605-008-0734-1. [DOI] [PubMed] [Google Scholar]
- 18.Olsen MA, Nepple JJ, Riew KD, Lenke LG, Bridwell KH, Mayfield J, Fraser VJ. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg Am. 2008;90(1):62–69. doi: 10.2106/JBJS.F.01515. [DOI] [PubMed] [Google Scholar]
- 19.Vilar-Compte D, Alvarez de Iturbe I, Martín-Onraet A, Pérez-Amador M, Sánchez-Hernández C, Volkow P. Hyperglycemia as a risk factor for surgical site infections in patients undergoing mastectomy. Am J Infect Control. 2008;36(3):192–198. doi: 10.1016/j.ajic.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Vriesendorp TM, DeVries JH, Hulscher JB, Holleman F, van Lanschot JJ, Hoekstra JB. Early postoperative hyperglycaemia is not a risk factor for infectious complications and prolonged in-hospital stay in patients undergoing oesophagectomy: a retrospective analysis of a prospective trial. Crit Care. 2004;8(6):R437–R442. doi: 10.1186/cc2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turina M, Fry DE, Polk HC., Jr Acute hyperglycemia and the innate immune system: clinical, cellular, and molecular aspects. Crit Care Med. 2005;33(7):1624–1633. doi: 10.1097/01.ccm.0000170106.61978.d8. [DOI] [PubMed] [Google Scholar]
- 22.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 23.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]