Abstract
Plasticity in the spinal dorsal horn is thought to underlie the development of neuropathic pain. Calcineurin (protein phosphatase 3) plays an important role in plasticity in the brain. Here we examined whether chronic constriction injury (CCI) of the sciatic nerve modifies calcineurin expression in the spinal dorsal horn. Male rats were assigned to control (uninjured), sham-operated or CCI groups. CCI animals exhibited both a shift in weight bearing and a reduction in paw withdrawal latencies as signs of pain behavior. At 3 days (3D) the pain behavior was associated with a significant increase in calcineurin gene expression, enzyme activity and content of its Aα isoform in the ipsilateral spinal dorsal horn. In contrast, while the pain behavior persisted at 7 days (7D) calcineurin gene expression returned to control levels and activity and protein content decreased. A single intrathecal injection of MK-801 15 min before the ligation attenuated both signs of pain behavior in 3D but not 7D CCI animals. The same pre-treatment also prevented the CCI-associated increases in calcineurin in these animals. These data suggested an involvement of calcineurin in CCI-elicited neuropathic pain. The time-dependent divergent changes in calcineurin expression may underlie the different phases of neuropathic pain development.
Keywords: Central sensitization, Chronic constriction injury, Neuropathic Pain, Spinal Dorsal Horn, Phosphatase, Synaptic plasticity
It is now well-established that injury-elicited plasticity accompanies peripheral nerve injury and that this significant alteration in sensory processing in the spinal dorsal horn may ultimately contribute to the development of neuropathic pain (Ji and Strichartz, 2004; Latremoliere and Woolf, 2009; Sandkuhler, 2009). The development of neuropathic pain appears dependent upon some of the same mechanisms that give rise to activity-dependent synaptic plasticity in the brain (Citri and Malenka, 2008).
Synaptic plasticity is critically influenced by the actions of protein kinases and phosphatases at a synapse (Lee, 2006). More than a decade ago, Kandel and colleagues (1998) described how the interplay between protein kinase A (PKA) and calcineurin (protein phosphatase 3, previously protein phosphatase 2B) was essential in initiating and maintaining long-lasting enhancement of synaptic function in Aplysia, Drosophila, mice and rats. Activation of PKA by cyclic AMP, and the subsequent phosphorylation of target proteins, resulted in long-term memory storage. Activation of calcineurin led to the dephosphorylation of these target proteins to prevent the transition from short to long-term memory. Later studies in other brain areas confirmed the general role of calcineurin in negatively constraining the acquisition of spatial or aversive memory, or of long-lasting plasticity in ocular dominance, cocaine addiction, and vestibular compensation (Yang et al., 2005, Masumura et al., 2007, Baumgartel et al., 2008; Pulipparacharuvil et al., 2008; Wang et al., 2009).
Little is known about the role of calcineurin in the spinal dorsal horn. The phosphatase is highly localized in the superficial spinal dorsal horn with heavy staining in cell bodies and terminals in laminae I and II and only a few labeled neurons in laminae III and IV (Goto et al., 1990; Strack et al., 1996). The terminal staining was judged to be of dorsal horn origin because of the lack of immunoreactivity in sensory axons in the dorsal roots (Strack et al., 1996). In dorsal root ganglia (DRG), moderate staining was associated with DRG neuron cell bodies but not their processes in the ganglia. DRG neuron soma staining was diffuse and appeared to include the nucleus, in contrast to spinal cord neurons where the staining was granular and excluded the nucleus (Strack et al., 1996).
We previously reported that there was a significant decrease in calcineurin content in the spinal dorsal horn of animals exhibiting neuropathic pain 7 days after chronic constriction injury (CCI) of the sciatic nerve (Miletic et al., 2002). In the present study we extended our investigation by examining changes in calcineurin gene expression, enzyme activity, and content of its Aα isoform at two post-ligation periods, 3 days (3D) and 7 days (7D). These times represent the initial and established phases of neuropathic pain development. We chose to examine calcineurin Aα because this isoform is the most abundant in the spinal dorsal horn (Strack et al., 1996). We also investigated whether single intrathecal pre-treatment with MK-801, an NMDA receptor antagonist that blocks synaptic plasticity, would modify pain behavior and any CCI-associated changes in calcineurin expression.
Experimental Procedures
Animals and behavioral tests
Male Harlan-Sprague-Dawley rats (200-250g) were randomly assigned to control, sham-operated or CCI groups. All experiments were conducted in accordance with guidelines accepted by the International Association for the Study of Pain (Zimmerman, 1983). The animal protocol was approved by the Animal Care and Use Committee of the School of Medicine and Public Health at the University of Wisconsin-Madison.
A dual channel scale (Incapacitance Meter™, Stoelting, Chicago, IL), which separately measures the weight borne by each hind limb, was used for the weight-bearing test as detailed previously (Miletic and Miletic, 2008). Briefly, a 1s weighing period was used to average 20 measurements and obtain the weight borne by each limb separately. A weight distribution ratio for each animal was then calculated by dividing the injured leg weight over the uninjured leg weight. While control rats distribute their weight about equally, animals with a unilateral injury will shift their weight from an injured to an uninjured limb. This shift is taken as a measure of the level of discomfort in the injured limb. In other words, the less weight placed on an injured limb, the greater the pain.
Thermal hyperalgesia was assessed with the well-established hind paw withdrawal latency test using a plantar analgesia instrument (Stoelting, Chicago, IL). Animals were acclimated for 15-20 min. The ipsilateral, injured, paw was tested four times to obtain an average latency. Each test was separated by 5 min. Thermal withdrawal latencies were obtained both at baseline and 3 or 7 days after sciatic nerve exposure or ligation (second test latency). Animals that served as the corresponding control groups were tested at the same times. The weight-bearing test preceded the second latency test. Whenever possible, persons performing behavioral tests were blinded to treatment.
Anesthesia, sciatic ligation, intrathecal drug application, tissue collection
Animals were anesthetized with isoflurane. Body temperature was kept at 37°C with a homeothermic blanket system. Anesthesia was sufficiently deep to prevent arousal but light enough to permit spontaneous respiration. Adequate anesthesia was assessed by monitoring blink or ear reflexes, withdrawal to toe pinches, respiratory rate, and absence of spontaneous movements.
Loose ligation of the sciatic nerve (chronic constriction injury) was performed using the Bennett and Xie (1988) procedure. The sciatic nerve was exposed and loosely ligated with 4 simple interrupted 4-0 chromic gut sutures placed about 1mm apart. In sham-operated animals the sciatic nerve was exposed but not ligated. Control animals were anesthetized but were not subject to surgery.
In some experiments MK-801 (2μg/μl, Sigma-Aldrich, St. Louis, MO) or saline vehicle were injected intrathecally 15 min before sciatic exposure or ligation in a volume of 10μl as described previously (Miletic and Miletic, 2008).
For tissue collection animals were anesthetized with isoflurane and while still deeply anesthetized they were euthanized with an intracardiac injection of super-saturated potassium chloride (>350 mg/ml). A laminectomy rapidly (<2 min) exposed the lumbar spinal cord at L5, and the tissue was excised, cut into dorsal and ventral halves, and the dorsal half further divided into ipsilateral and contralateral quadrants. All tissues were stored at −80°C until use.
RT-PCR, enzyme activity assay and immunoblots
Calcineurin mRNA levels were determined by monitoring in real time the increase in fluorescence of SYBR-GREEN dye with the ABI Prism 7300 Sequence Detection System (Applied Biosystems, Foster City, CA) as detailed previously (Miyabe et al., 2006). Relative expression levels of calcineurin in each sample were determined using a standard curve of 3-fold serial dilutions. Average fold induction relative to control animals was determined after normalizing to the amount of 18S rRNA in each sample. A 2-fold or greater change was considered significant. Primer sequences are available upon request.
Calcineurin activity was obtained with a commercial kit (Enzo Life Sciences, Plymouth Meeting, PA) and expressed as nmol of phosphate released/min/mg of protein. Immunoblots were performed as described previously (Miletic et al., 2002). Calcineurin Aα (Millipore, Billerica, MA) was used at a dilution of 1:1000. Developed membranes were stripped and re-probed with beta III tubulin (1:1000; Promega, Madison, WI) as the loading control. Protein levels were estimated with the BioSpectrum 500 Image Analysis System (UVP, Upland, CA). The calcineurin Aα content within a gel was expressed over the beta III tubulin content, and then the content in sham-operated or CCI animals was normalized to those in control animals. The same normalization procedure was used for the enzyme activity assay.
Statistical analysis
ANOVA was used for the statistical data analysis. The main emphasis was on detecting differences in behavior, calcineurin message, activity or protein content between control, sham-operated and CCI animals, or vehicle and drug-treated animals. Significant effects were further analyzed with Scheffe’s post-hoc test. Statistical difference was inferred at p ≤ 0.05. The analysis was based on 6 animals in each group. All data are expressed as mean±SEM.
Results
Signs of pain behavior accompanied CCI
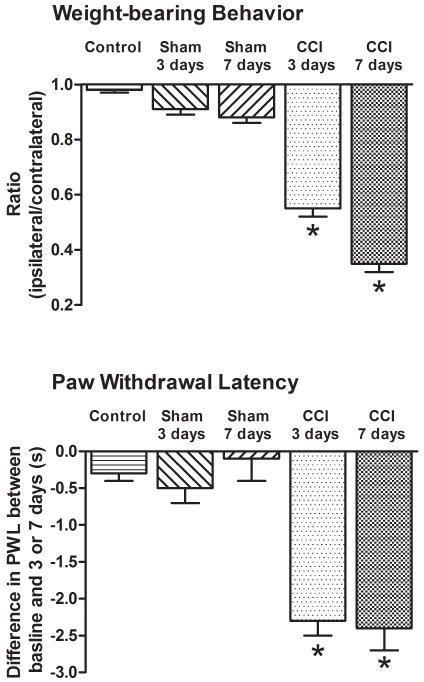
Both 3D and 7D CCI animals placed less weight on their injured, ipsilateral limb as their ipsilateral to contralateral ratio was reduced to 0.55±0.03 and 0.35±0.03, respectively (Fig. 1). In contrast, uninjured control (0.98±0.01), 3D sham (0.91±0.02) or 7D sham (0.88±0.02) animals did not show a similar shift in weight distribution suggestive of pain behavior. ANOVA indicated a significant difference among groups, F(4,25)=137.1, p<0.001. Scheffe’s post-hoc test confirmed that the difference was due to the weight-bearing ratio in CCI animals.
Figure 1. Pain behavior accompanied chronic constriction injury of the sciatic nerve.
Both 3D and 7D CCI animals exhibited a reduction in weight-bearing ratio (suggestive of pain behavior) and thermal hyperalgesia. On the other hand, neither control nor 3D or 7D sham-operated animals exhibited a shift in weight-bearing behavior or thermal hyperalgesia. *p<0.001.
In the same 3D and 7D CCI animals there was also a significant reduction in the post-ligation withdrawal latency (second test latency) of the injured, ipsilateral limb (5.9±0.2s and 5.4±0.2s) when compared to the pre-surgery baselines (8.2±0.2s and 7.8±0.2s, respectively). In contrast, there was no significant reduction in the second test withdrawal latency in control (8.0±0.3s vs. 8.3±), 3D sham (7.5±0.3s vs. 7.9±0.3s) or 7D sham (7.6±0.4s vs. 7.6±0.4s) animals. ANOVA confirmed no difference among groups at baseline, F (4,25)=0.8, p<1, but a significant difference later, F(4,25)=16.5, p<0.001. This difference was due to the second test latencies in the 3D and 7D CCI animals.
Time-dependent differential changes in calcineurin message, activity and Aα protein content were associated with CCI
After the last behavioral test, the animals were anesthetized, euthanized and their tissues collected for the biochemical assays.
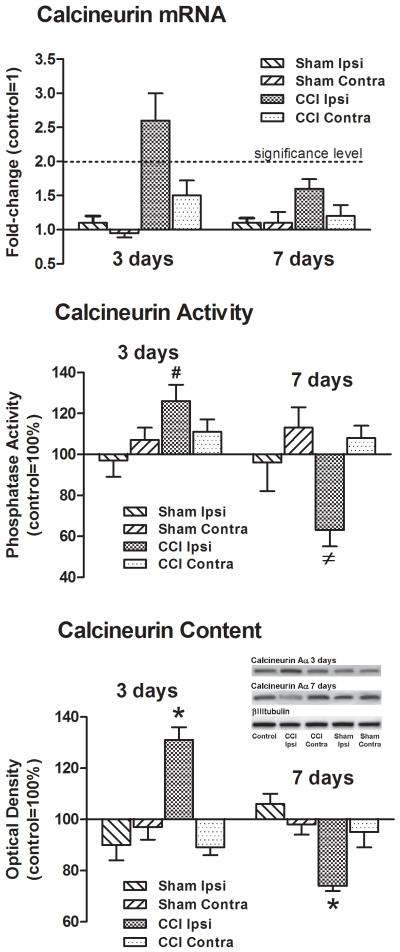
There was a significant increase in the calcineurin mRNA levels in the ipsilateral spinal dorsal horn of 3D CCI animals, 3.1±0.5-fold (Fig. 2). However, 7 days post-CCI the message levels returned to control values, 1.4±0.1-fold. Message levels in the contralateral dorsal horn of CCI animals, or either side of the spinal dorsal horn in 3D or 7D sham-operated animals were not different from controls.
Figure 2. Time-dependent changes in calcineurin message, activity and Aα protein content were associated with CCI.
There was a significant increase in calcineurin message in the ipsilateral, but not contralateral, spinal dorsal horn 3 days post-CCI. Calcineurin message returned to control levels at 7 days post-CCI. There were no changes in calcineurin message in 3D or 7D sham-operated animals. Similarly, both calcineurin activity and Aα protein content increased in the ipsilateral spinal dorsal horn of 3D CCI animals. However, 7 days post-ligation both decreased. Calcineurin activity or Aα protein content in the contralateral spinal dorsal horn of 3D or 7D CCI animals, or either side of the dorsal horn in 3D or 7D sham-operated animals was not different from controls. Beta III-tubulin was the loading control. #p<0.05, ≠p<0.005, *p<0.001.
A divergent change in enzyme activity was associated with CCI. Three days post-CCI there was significantly more activity in the ipsilateral spinal dorsal horn of CCI animals, 126±12%, F(4,25)=3.4, p<0.05 (Fig. 2). In contrast, there was significantly less activity in the ipsilateral spinal dorsal horn in 7D CCI animals, 60±8%, F(4,25)=5.6, p<0.005. The activity in the contralateral dorsal horn of 3D or 7D CCI animals (111±6% and (108±6%) or either side in 3D or 7D sham-operated animals (97±8% & 107±6% and 96±8% & 113±10%, respectively) was not different from controls.
A divergent change in the content of calcineurin Aα in the ipsilateral spinal dorsal horn was similarly associated with CCI. In 3D CCI animals there was significantly more calcineurin Aα, 131±5%, F(4,25)=15.5, p<0.001 (Fig. 2). In contrast, there was significantly less calcineurin Aα in 7D CCI animals, 74±2%, F(4,25)=11.7, p<0.001.
As with the enzyme activity, the content of the calcineurin Aα isoform in the contralateral dorsal horn of 3D or 7D CCI animals (95±5% and 98±4%) or either side in 3D or 7D sham-operated animals (91±6% & 97±5% and 106±4% & 98±4%, respectively) was not different from controls.
A single intrathecal injection of MK-801 attenuated pain behavior in 3D but not 7D animals
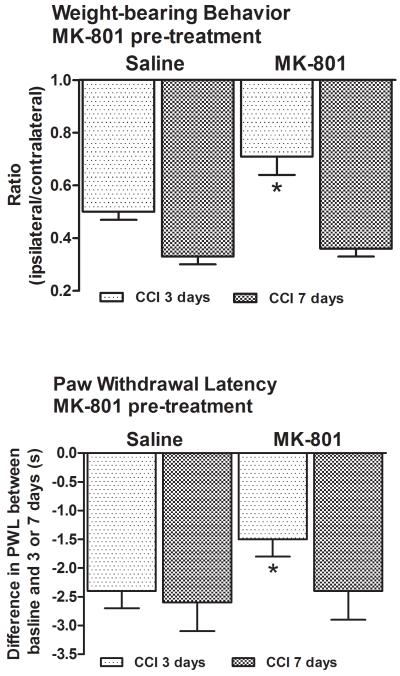
Single pre-treatment with the NMDA receptor antagonist MK-801 attenuated the shift in weight-bearing behavior in 3D CCI animals, 0.71±0.07, p<0.001 (Fig. 3). This was significantly different (p<0.001) from the behavior of vehicle-treated 3D animals (0.50±0.03) which was comparable to that seen previously (Fig. 1). In the same MK-801-treated 3D CCI animals thermal hyperalgesia was also attenuated (7.0±0.8s vs. 9.1±0.7s at baseline, Fig. 3). This was again significantly different (p<0.001) from the behavior of the vehicle-treated 3D CCI animals (5.0±0.3s vs. 9.3±0.2s at baseline).
Figure 3. Single intrathecal pre-treatment with MK-801 lessened signs of pain behavior in 3D but not 7D CCI animals.
MK-801 attenuated both pain behaviors 3 days post-ligation but had no effect 7 days post-ligation. *p<0.001.
On the other hand, a single intrathecal injection of MK-801 was ineffective in modifying the pain behavior of 7D CCI animals. These data suggested that the analgesic effect of MK-801 had dissipated by this time.
Control, 3D or 7D sham-operated animals treated with MK-801 or vehicle did not exhibit a shift in weight-bearing behavior or thermal hyperalgesia.
MK-801 pre-treatment prevented the ligation-associated increases in calcineurin message, activity and Aα protein content in 3D CCI animals
After the last behavioral test, the animals were anesthetized, euthanized and their tissues collected for the biochemical assays.
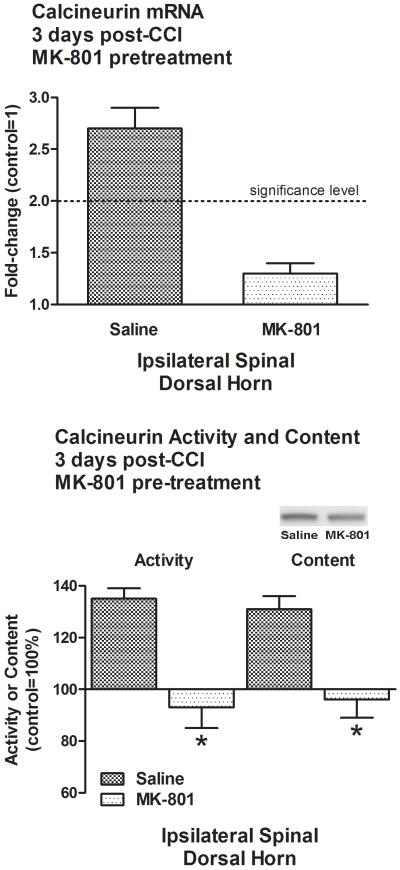
The injury-associated increase in calcineurin message in the ipsilateral spinal dorsal horn of 3D CCI animals was abolished by pre-treatment with MK-801 (1.3±0.1-fold, Fig. 4). The same pre-treatment also prevented increases in calcineurin activity (93±8%, p<0.001) and content of its Aα isoform (96±7%, p<0.001) in the ipsilateral spinal dorsal horn (Fig. 4).
Figure 4. Increases in calcineurin message, activity and Aα protein content were all prevented by MK-801 at 3 days post-CCI.
CCI-associated increases in calcineurin mRNA, activity and Aα protein content in the ipsilateral spinal dorsal horn of 3D CCI animals were abolished by MK-801 pre-treatment. *p<0.001.
In contrast, single pre-treatment with MK-801 or vehicle had no effect on calcineurin message, activity or Aα content in control, 3D or 7D sham-operated animals.
Discussion
In this study we examined the consequences CCI on calcineurin expression in the spinal dorsal horn. Our data established that 3D and 7D CCI animals exhibited both a shift in weight bearing behavior and thermal hyperalgesia. In 3D CCI animals this was associated with significantly more calcineurin message, activity and content of its Aα isoform in the ipsilateral dorsal horn. In 7D CCI animals the gene expression returned to control levels, and activity and protein content significantly decreased. Pre-treatment with MK-801 attenuated both signs of pain behavior 3 days but not 7 days post-CCI. The same pre-treatment prevented the injury-associated changes in message, activity and Aα content in the 3D CCI animals. To our knowledge, this is the first demonstration of nerve injury-associated and time-dependent divergent changes in calcineurin expression in the spinal dorsal horn.
These data are in agreement with our previous study (Miletic et al., 2002) in which we reported that the expression of thermal hyperalgesia 7 days post-CCI was accompanied by significant decreases in the content of both calcineurin Aα and Aβ isoforms in the spinal dorsal horn. Overall, our data provided further support for the notion of a close association between calcineurin expression and the development of neuropathic pain.
Our present results further suggested that the injury-associated increases in calcineurin expression in the spinal dorsal horn were mediated at least in part by NMDA receptors. There is a well-established association between injury-elicited plasticity in the spinal dorsal horn and the activation of NMDA receptors. However, the use of MK-801 as a pre-emptive analgesic for neuropathic pain has not proven beneficial in clinical trials of preemptive analgesia using randomized, controlled protocols (Taylor and Brennan, 2000). Our observation that MK-801 was effective 3 days post-CCI but not 7 days post-CCI suggested that the initial analgesic effect dissipated because of the disappearance of MK-801 through routine pharmacokinetics.
Calcineurin-inhibitor pain syndrome (CIPS) is a well-described complication associated with the use of the calcineurin inhibitors cyclosporine A and FK-506 (tacrolimus) in organ transplant patients (Smith, 2009). This clinical outcome suggests that there is an interdependent link between the development of pain and the inhibition of calcineurin activity. On the other hand, several publications reported that calcineurin inhibitors prevented the calcineurin-dependent activation of the transcription factor NFAT which, upon translocation to the nucleus, elicited the expression of many genes including those considered pro-nociceptive such as NGF, BDNF and COX-2 (Groth et al., 2004; 2007). It is possible that the phosphatase assumes a differential role based on its cellular location. Calcineurin activity in glial cells or in the cell bodies of primary afferent neurons may facilitate NFAT-dependent gene expression. In contrast, the loss of calcineurin activity within spinal dorsal horn neurons may allow for the development of injury-associated plasticity and thus by extension neuropathic pain.
It is also possible that the role of the phosphatase changes with the time after injury. Neuropathic pain, like synaptic plasticity, exhibits early, intermediate and late phases (Ji and Strichartz, 2004; Latremoliere and Woolf, 2009; Sandkuhler, 2009). Our data support a time-dependent differential role of calcineurin in CCI-elicited pain. The initial phase of pain may be associated with an increase in calcineurin expression in the spinal dorsal horn. This increase would promote NFAT-dependent up-regulation of genes that contribute to some of the initial signs of pain behavior. The later phase of pain may be associated with the loss of calcineurin function. This loss, and the ensuing lack of phosphatase dephosphorylating activity, would permit continual activation of numerous kinases whose contribution is necessary for the pain to be maintained.
Conclusions
The time-dependent divergent changes in calcineurin expression may differentially contribute to the development of neuropathic pain. Increases may promote the initial changes in injury-elicited plasticity that lead to the induction of neuropathic pain. The later loss of calcineurin may allow for neuropathic pain to be maintained because the lack of calcineurin dephosphorylating activity may permit the continual activation of numerous proteins known to contribute to long-lasting plasticity and thus by extension to fully developed neuropathic pain.
Research Highlights.
Calcineurin plays a central role in brain synaptic plasticity.
We investigated the role of calcineurin in nerve injury-associated plasticity in the dorsal horn.
Increases in calcineurin expression may be associated with neuropathic pain induction.
Decreases in calcineurin expression may be associated with neuropathic pain maintenance.
Acknowledgments
Supported in part by National Institutes of Health grants NS034870 and NS055042.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel T, Kandel E. Positive and negative regulatory mechanisms that mediate long-term memory storage. Brain Res Rev. 1998;26:360–378. doi: 10.1016/s0165-0173(97)00050-7. [DOI] [PubMed] [Google Scholar]
- Baumgartel K, Genoux D, Welzl H, Tweedie-Cullen RY, Koshibu K, Livingstone-Zatchej M, Mamie C, Mansuy IM. Control of the establishment of aversive memory by calcineurin and Zif268. Nature Neurosci. 2008;11:572–578. doi: 10.1038/nn.2113. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1997;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacol. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- Goto S, Hirano A, Pearson J. Calcineurin and synaptophysin in the human spinal cord of normal individuals and patients with familial dysautonomia. Acta Neuropathol. 1990;79:647–652. doi: 10.1007/BF00294243. [DOI] [PubMed] [Google Scholar]
- Groth RD, Dunbar RL, Mermelstein PG. Calcineurin regulation of neuronal plasticity. Biochem Biophys Res Comm. 2004;311:1159–1171. doi: 10.1016/j.bbrc.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Groth RD, Coicou LG, Mermelstein PG, Seybold VS. Neurotrophin activation of NFAT-dependent transcription contributes to the regulation of pro-nociceptive genes. J Neurochem. 2007;102:1162–1174. doi: 10.1111/j.1471-4159.2007.04632.x. [DOI] [PubMed] [Google Scholar]
- Ji RR, Strichartz G. Cell signaling and the genesis of neuropathic pain. Science’s STKE. 2004;252:re14. doi: 10.1126/stke.2522004re14. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK. Synaptic plasticity and phosphorylation. Pharmacol Therap. 2006;112:810–832. doi: 10.1016/j.pharmthera.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumura C, Horii A, Mitani K, Kitahara T, Uno A, Kubo T. Unilateral vestibular deafferentation-induced changes in calcium signaling-related molecules in the rat vestibular nuclear complex. Brain Res. 2007;1138:129–135. doi: 10.1016/j.brainres.2006.12.072. [DOI] [PubMed] [Google Scholar]
- Miletic G, Pankratz MT, Miletic V. Increases in the phosphorylation of cyclic AMP response element binding protein (CREB) and decreases in the content of calcineurin accompany neuropathic pain following chronic constriction injury in rats. Pain. 2002;99:493–500. doi: 10.1016/S0304-3959(02)00242-7. [DOI] [PubMed] [Google Scholar]
- Miletic G, Miletic V. Loose ligation of the sciatic nerve is associated with TrkB receptor-dependent decreases in KCC2 protein levels in the ipsilateral spinal dorsal horn. Pain. 2008;137:532–539. doi: 10.1016/j.pain.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyabe T, Miletic G, Miletic V. Loose ligation of the sciatic nerve in rats elicits transient up-regulation of Homer1a gene expression in the spinal dorsal horn. Neurosci Lett. 2006;398:296–299. doi: 10.1016/j.neulet.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, Greengard P, Nairn AC, Nestler EJ, Cowan CW. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Prog Brain Res. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- Smith HS. Calcineurin as a nociceptor modulator. Pain Physician. 2009;12:E309–E318. [PubMed] [Google Scholar]
- Strack S, Wadzinski BE, Ebner FF. Localization of the calcium/calmodulin-dependent protein phosphatase, calcineurin, in the hindbrain and spinal cord of the rat. J Comp Neurol. 1996;375:66–76. doi: 10.1002/(SICI)1096-9861(19961104)375:1<66::AID-CNE4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Taylor BK, Brennan TJ. Preemptive analgesia - moving beyond conventional strategies and confusing terminology. J Pain. 2000;1:77–84. [Google Scholar]
- Wang GP, Huang LQ, Wu HJ, Zhang L, You ZD, Zhao ZX. Calcineurin contributes to spatial memory impairment induced by rapid eye movement sleep deprivation. NeuroReport. 2009;20:1172–1176. doi: 10.1097/WNR.0b013e32832f0772. [DOI] [PubMed] [Google Scholar]
- Yang Y, Fischer QS, Zhang Y, Baumgartel K, Mansuy IM, Daw NW. Reversible blockade of experience-dependent plasticity by calcineurin in mouse visual cortex. Nature Neurosci. 2005;8:791–796. doi: 10.1038/nn1464. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]