Abstract
The activation of G-protein coupled receptors results in a cascade of events that include acute signaling, desensitization and internalization and it is thought that not all agonists affect each process to the same extent. The early steps in opioid receptor signaling, including desensitization, have been characterized electrophysiologically using brain slice preparations, while most previous studies of opioid receptor trafficking have been conducted in heterologous cell models. This study used transgenic mice that express an epitope-tagged (Flag) mu opioid receptor (FlagMOR) targeted to catecholamine neurons by regulatory elements from the tyrosine hydroxylase gene. Brain slices from there mice were used to study of tagged MOR receptors in neurons of the locus coeruleus (LC). Activation of the FlagMOR with [Met5]enkephalin (ME) produced a hyperpolarization that desensitized acutely to the same extent as native MOR in slices from wild type mice. A series of opioid agonists was then used to study desensitization and receptor trafficking in brain slices, which was monitored with a monoclonal antibody against the Flag epitope (M1) conjugated to Alexa594. Three patterns of receptor trafficking and desensitization were observed: 1. ME, etorphine and methadone resulted in both receptor desensitization and internalization; 2. Morphine and oxymorphone caused significant desensitization without evidence for internalization; 3. Oxycodone was ineffective in both processes. These results show that two distinct forms of signaling were differentially engaged depending on the agonist used to activate the receptor and support the hypothesis that ligand-specific regulation of opioid receptors occurs in neurons maintained in brain slices from adult animals.
Introduction
The activation of G-protein coupled receptors results in a cascade of processes and different agonists acting on a single receptor can result in varying signaling patterns (Urban et al., 2007). The mu-opioid receptor (MOR) is a Gi/o-linked receptor that activates a potassium conductance, inhibits voltage dependent calcium conductance and inhibits adenylyl cyclase (Williams et al., 2001). In the continued presence of agonist, the MOR desensitizes by a mechanism that is thought to be similar to that originally described for the β-adrenergic receptor (Krupnick and Benovic, 1998; Lefkowitz et al., 1998). The first step in receptor desensitization is thought to be a phosphorylation of the receptor by a G protein-coupled receptor kinase (GRK) followed by the binding to β-arrestin with high affinity. This process also transforms the receptor into a state where it can no longer couple to G proteins and is therefore physiologically inactive. Acute desensitization can take place within a few minutes and requires a saturating concentration of agonist (Connor et al., 2004; Gainetdinov et al., 2004; vonZastrow et al., 2003).
Like the β-adrenergic receptor, the MOR is trafficked into early endosomes followed by a multi-step process that recycles receptor back to the plasma membrane (Finn and Whistler, 2001; Tanowitz and vonZstrow, 2003). This trafficking pathway has been hypothesized to be a critical step in the recovery from desensitization where phosphatase activity is necessary for the reinsertion of receptors into the plasma membrane (vonZastrow et al., 2003; Ferguson et al., 1998; Luttrell and Lefkowitz 2002; Marie et al., 2006). The trafficking of MOR has been characterized in a variety of expression systems, although the ability to follow the internalization of receptors in neurons under more physiological conditions in real time has been limited.
Receptor activation, desensitization and internalization have different concentration dependence or require distinct agonist/receptor dwell times. In order to understand the complexity of signaling from a single receptor it is necessary to study two or more processes simultaneously or at least under identical conditions with a variety of agonists. This study measured acute signal transduction (membrane hyperpolarization), acute desensitization and receptor internalization resulting from the activation of an epitope-tagged MOR in a brain slice preparation.
Materials and Methods
FlagMOR-transgenic mice
The transgene was constructed by first ligating an 8.5 kb genomic fragment of the rat TH gene from plasmid pTH9000 (Min et al., 1994) containing 5’ regulatory sequences, the basal promoter, and 26 bp from the 5’ UTR in exon 1 of TH to a 0.7 kb cassette containing a heterologous intron 2 and splice donor/acceptor sites from the rabbit β-globin gene. The 2.2 kb Flag epitope-tagged MOR cDNA with a bovine growth hormone polyA sequence was obtained from excision of plasmid pcDNA3 SSF-MOR (Kim and vonZastrow, 2003) and ligated 3’ to the β-globin intron in a pBluescript SK+ plasmid vector. The Flag epitope (DYKDDDA) is immediately preceded by a modified influenza hemaglutinin signal peptide sequence that facilitates translocation of the modified receptor into the endoplasmic reticulum and production of functional protein. Co-translational cleavage of this sequence by signal peptidase is essential for subsequent binding of the M1 antibody to the free amino-terminal end of the Flag epitope. An 11.4 kb SalI-NotI transgene fragment was purified from the final construct and used for pronuclear microinjection into zygotes of B6D2 F2 mice by standard techniques. Two independent founders were identified by PCR genotyping of genomic DNA and backcrossed with C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) to obtain N1 hemizygous progeny for initial characterization. The one strain with readily detectable levels of FlagMOR by immunofluoresence in brain slices was further maintained by continued backcrossing with C57BL/6J mice. All data were collected from these hemizygous FlagMOR-Tg/+ mice ranging from generations N1 to N5. FlagMOR-Tg/+ mice were also crossed with MOR (Schuller et al., 1999) and β-arrestin2 knockout mice (Bohn et al., 2000) to generate mice that were hemizygous for the FlagMOR transgene and homozygous for either the MOR-KO (FlagMOR-Tg/+, MOR−/−) or the β-arrestin2-KO (FlagMOR-Tg/+, Arr−/−). All animal experiments were conducted in accordance with the National Institutes of Health guidelines and with approval from the Institutional Animal Care and Use Committee of the Oregon Health & Science University.
Fluorescent immunohistochemistry
Fixed brains from FlagMOR-Tg/+ mice perfused with 4% paraformaldehyde were prepared and sliced to 50 µm using a vibratome (Leica, Nussloch, Germany) as described previously (Ford et al., 2006). The primary antibody against the Flag epitope (M1, Sigma, St. Louis, MO) was chemically linked to Alexa 555 (monoclonal labeling kit, Molecular Probes, Eugene, OR) and used at a 1:500 dilution. Immunostaining was done using a free-floating method and tissue slices were incubated at 4°C for 18 hours. For tyrosine hydroxylase co-localization experiments, the tissues were permeabilized with 0.4% Triton X. The primary antibody against TH was a mouse-monoclonal antibody (1:5000, IncStar, Stillwater, MN) and the secondary antibody was Alexa 488-labeled goat-antimouse (1:1000, Molecular Probes, Eugene, OR). Images were collected from an Olympus microscope equipped with a confocal system, excitation/emission at 488 and 543 nm for Alexa 488 and Alexa 555, respectively.
Electrophysiolgy
Adult mice (4–10 weeks) were used for electrophysiology experiments. Animals were anesthesized with isoflurane, the brain was removed and sliced horizontally using a vibratome (Leica, Nussloch, Germany) in ice-cold artificial cerebro-spinal fluid (ACSF) containing the following (in mM): 126 NaCl, 2.5 KCl, 1.2 MgCl2, 1.2 NaH2PO4, 2.4 CaCl2, 21.4 NaHCO3 and 11 glucose. Slices (275 µm thickness) were incubated in warm (34°C) oxygenated ACSF containing (+)MK-801 (10 µM, Sigma-Aldrich, St. Louis, MO) for at least 30 min before use in experiments. Sharp glass electrodes (50–60 MΩ) filled with KCl (2 M) were used for intracellular recordings of membrane potentials. Experiments were performed at 35°C. Data was collected using Power Lab (Chart version 5.4, ADInstrument, Colorado Springs, CO) and acquired at 200Hz. Drugs were applied by perfusion at the rate of 1.5 ml/min.
Two-photon microscopy
Brain slices (200–220 µM) were prepared as those described for electrophysiological experiments. Slices were incubated in a solution containing M1 antibody (Sigma, St Louis MO) conjugated with Alexa 594 (Molecular Probes, Eugene, OR, 10 µg/ml, 45–60 min). In previous work the behavior of antibody-tagged receptors with unlabeled receptors was compared and there was no significant difference between the two conditions on the time scale of an hour (Tanowitz and vonZastrow, 2003; Whistler et al., 1999). The tissue was visualized with an upright microscope (Olympus, Center Valley, PA.) equipped with a custom-built two-photon apparatus. Data were acquired and collected using Scan Image Software (Pologruto et al., 2003). A z-series was collected at 1 µm intervals for 15 µm. Drugs were applied by perfusion. All experiments were done at 35°C. Drugs used were ME, oxycodone (Sigma), oxymorphone (Mallinkrodt, Hazelwood, MO), methadone, morphine, and etorphine (NIDA).
Quantification of receptor internalization
Analyses were done off-line with Image J (NIH) software. Images were selected with the Stacks-Reducing Plugin algorithm for quantification. The stack was Z-projected using Sum-Slices method. Five random ROIs were selected and averaged for background fluorescence. The average background fluorescence was then subtracted from the total fluorescent intensity of the whole frame. In control, data were obtained from slices before drug application. This fluorescent intensity was considered as total fluorescent receptors (C). After drug perfusion (15 min) followed by calcium free ACSF containing ethylene glycol-bis(β-aminoethylether) N,N,N’,N’-tetraacetic acid (0.5 mM EGTA, 10 min), a stack of images was collected and analyzed. This fluorescent intensity was termed internalized receptors (I). Percentage of internalization was calculated by (I/C) × 100 and averaged fluorescence from the drug free controls were subtracted.
Radioligand binding assay
Slices containing the locus coeruleus nuclei were prepared (~500 µm) and the area of the LC was dissected. The tissue was homogenized using a Dounce Tissue Grinder in ice-cold Tris HCl (50 mM, pH 7.4). Tissue from 6–10 animals was prepared for a binding assay using [3H]-diprenorphine (Amersham, Pittsburgh, PA) as described previously (Bunzow et al., 1995). Analyses were done using a one-site binding hyperbolic equation from Graph Pad Prism (San Diego, CA).
Results
FlagMOR-transgenic mice
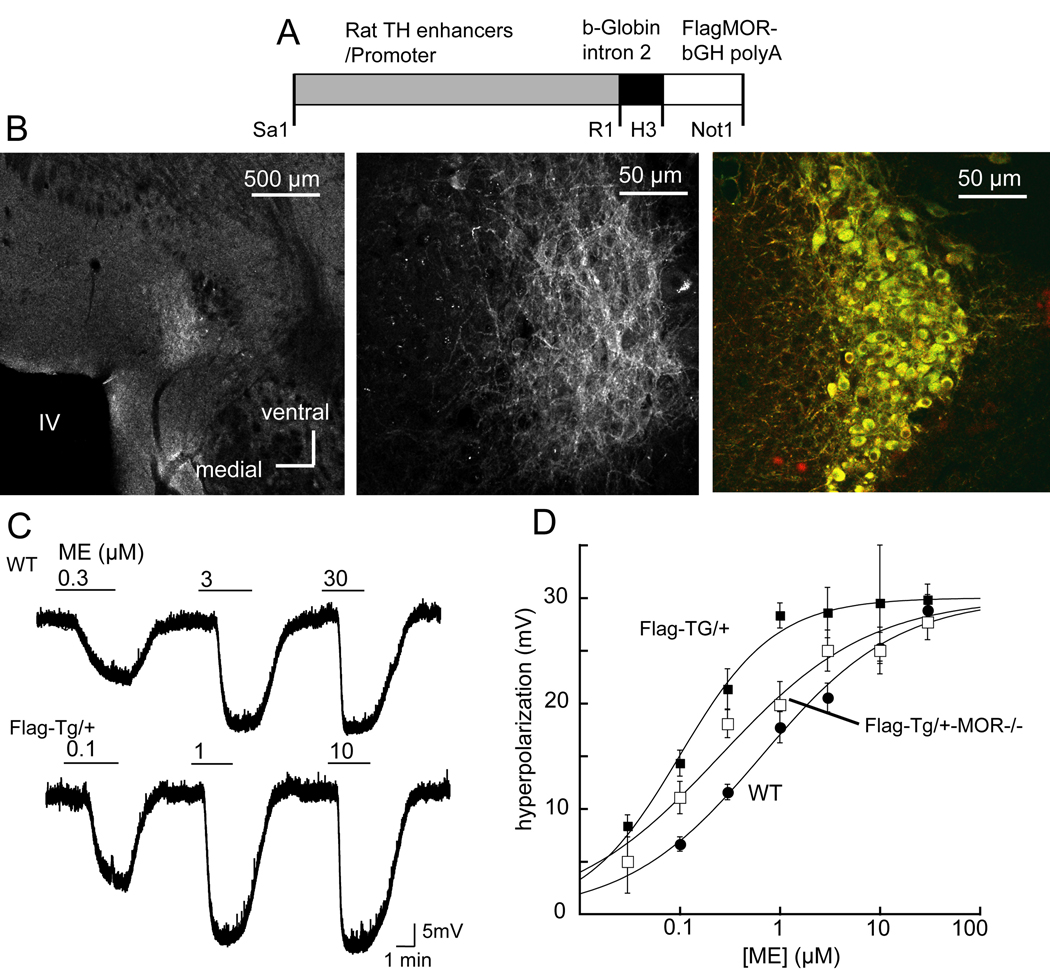
A transgenic mouse strain was generated that expresses a fusion protein consisting of the MOR with an amino-terminal Flag epitope targeted to catecholamine neurons by an 8.5 kb segment of genomic DNA from the rat tyrosine hydroxylase (TH) gene (Figure 1A). Staining of FlagMOR on the plasma membrane of locus coeruleus (LC) neurons was demonstrated using the M1-antibody conjugated with Alexa 555 and was limited to the area of LC and co-localized with TH immunoreactivity (Figure 1B). The FlagMOR was also detected in other catecholamine neurons in the olfactory bulb, arcuate nucleus, substantia nigra and ventral tegmental area (not shown). There was no obvious phenotypic difference between the transgenic mice and their wild type littermates. Hemizygous FlagMOR-Tg/+ mice grew and bred normally.
Figure 1.
Expression and signaling of FlagMOR in locus coeruleus (LC) neurons. A) Restriction map of the tyrosine hydroxylase (TH)-FlagMOR-Tg construct. B) Left, low power micrograph of a slice containing the LC. The light area is the LC stained using the M1 antibody conjugated with Alexa 555. Middle, higher power image showing fluorescent staining of processes and cell bodies. Right, FlagMOR was stained in red and TH was stained with a secondary antibody labeled with Alexa488. C. [Met]5enkephalin (ME) caused a concentration dependent hyperpolarization in slices from wild type and FlagMOR-Tg/+ mice. Cells from the FlagMOR-Tg/+ mice were more sensitive to ME. D. Concentration-response curves for ME in slices from wild type (solid circles, EC50=653 nM), FlagMOR-Tg/+ (filled squares, EC50=94 nM) and compound mutant FlagMOR-Tg/+, MOR−/− mice (open squares, EC50=151 nM).
Opioid receptor expression and function
Receptor number determined by a binding assay using [3H]diprenorphine indicated that LC neurons from the FlagMOR-Tg/+ mice expressed approximately 2-fold more MOR than wild type littermates (Bmax=732±220 fmol/mg protein, n=3 vs. 364±90 fmol/mg protein, n=4). The Kd from both groups of animals was the same (Kd=0.36±0.03 nM, n=3 for FlagMOR-Tg/+ and Kd=0.46±0.08 nM, n=4 for wild type, t-test P>0.05). This increased expression is similar to that reported recently for the delta opioid receptor (DOR) fused to EGFP (Scherrer et al., 2006). In homozygous DOR-EGFP knockin mice there was about a 2-fold increase in binding sites and a 3-fold increase in GTP-gamma-S binding (Scherrer et al., 2006).
Functional coupling of opioid receptors to a G protein-linked inwardly rectifying potassium channel (GIRK) was measured by intracellular recording of membrane potential in LC neurons (Figure 1C). The maximal hyperpolarization induced by [Met5]enkephalin (ME, 30 µM) was 30.1±1.3 mV and 28.8±1.1 mV in FlagMOR-Tg/+ and wild type mice (n=14 and 19 neurons, respectively, t-test P>0.05, Figure 1D). The membrane hyperpolarization induced by the alpha2-adrenoceptor agonist, UK14304 (3 µM) was also the same in transgenic and wild type mice (FlagMOR-Tg/+=23.9±1.6 mV, n=14, wild type=25.8±0.9 mV, n=24, t=test P>0.05) suggesting that the higher receptor numbers in FlagMOR-Tg/+ neurons did not interfere with activity of GIRK. Increased receptor expression in FlagMOR-Tg/+ mice did, however result in an increase in the potency of ME. There was a 7-fold leftward-shift in the EC50 in recordings made from slices taken from FlagMOR-Tg/+ mice compared to wild type (FlagMOR-Tg/+ EC50=94 nM; wild type EC50=658 nM; Figure 1D).
Hemizygous FlagMOR-Tg/+ mice were crossed with MOR knockout (MOR−/−) mice for two purposes. First, if opioids caused a hyperpolarization in neurons in the compound mutant mice, this would demonstrate that FlagMOR was functional in the absence of all endogenous MOR. Second, this genetic cross would decrease the total number of receptors on LC cells. The maximum hyperpolarization in neurons from compound mutant FlagMOR-Tg/+, MOR−/− mice was similar (29.7±1.4 mV, n = 15) to that observed in wild type and FlagMOR-Tg/+ mice. The EC50 for ME in slices from the FlagMOR-Tg/+, MOR−/− mice was 151 nM, 50% greater than that in the FlagMOR-Tg/+ mice (94 nM) and only 4-fold less than the EC50 of wild type animals (658 nM, Figure 1D).
FlagMOR internalization in brain slices
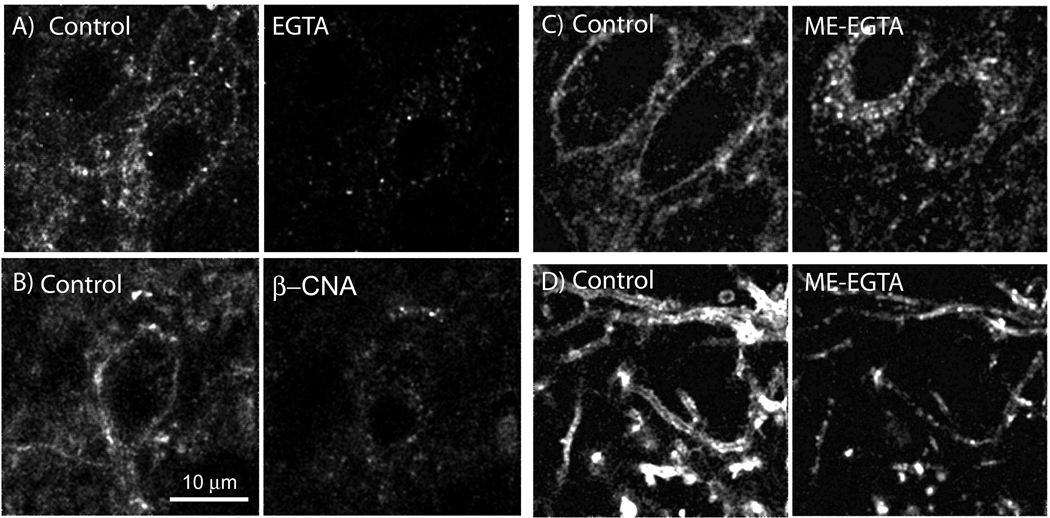
A useful feature of the detection system combining a Flag-epitope tagged receptor and M1 antibody is that the binding affinity between the two is decreased considerably upon the removal of calcium from the extracellular buffer. Slices were prepared identically to those used for electrophysiological experiments, incubated with the fluorescent-M1 antibody for 45 min and then placed in a tissue bath on the microscope for imaging. The fluorescent outlines of cell bodies were first visualized in each experiment (control, Figure 2A). When the superfusion solution was changed to one that was calcium free (EGTA 0.5 mM) for 10 min and the cells were imaged again, staining on the plasma membrane decreased to background levels (Figure 2A). This result confirmed that the fluorescence seen surrounding the cell body required calcium and that a 10 min wash with calcium free solution eliminated specific staining. In the next experiment an initial image was taken following staining and a second image was taken after treatment of the slice first with ME (30 µM, 15 min) followed by calcium free (EGTA) solution (10 min). The resulting image showed numerous fluorescent puncta remaining within the soma and processes (Figure 2C and D). To determine if the ME induced increase in intracellular puncta was receptor dependent, slices were treated with the irreversible opioid antagonist, β-chlornaltrexamine (β-CNA, 500 nM, 2 min) before the application of ME (30 µM, 15 min). The slice was then treated with calcium free (EGTA) solution and the result was a complete block of receptor internalization (Figure 2B, EGTA wash without ME treatment = 34.2±2.9 %, n=6; EGTA wash post β-CNA+ME – 38.7±4.3%, n=3). The results indicate that the appearance of intracellular fluorescent puncta within the cell was dependent on the activation of FlagMOR.
Figure 2.
Internalization of FlagMORs induced by ME is receptor dependent. A) Control (left) shows the initial staining pattern with an Alexa 594-conjugated anti-Flag antibody. Right, same slice after treatment with calcium-free (EGTA) solution. C&D) Internalization of FlagMOR induced by ME. Left, initial staining illustrating cell bodies (C) and dendrites (D). Right, staining following treatment of the slice with ME (30 µM, 15 min) followed by calcium-free (EGTA, 10 min) solution. B) Pretreatment with β-CNA (500 nM, 2 min) blocked internalization induced by ME. Left is control and the right side after ME (30 µM, 15 min).
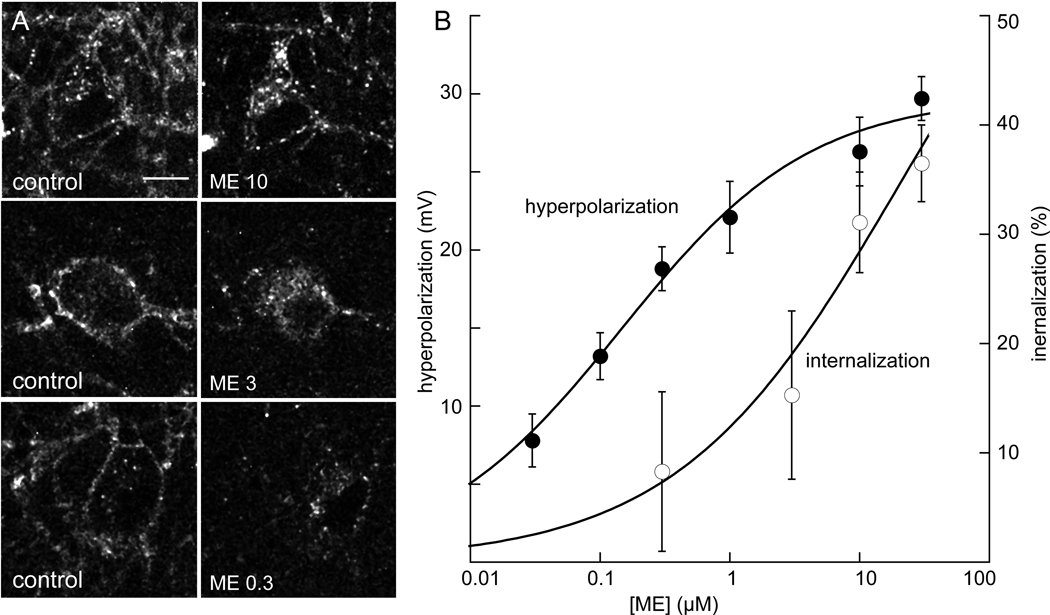
The concentration dependence of internalization induced by ME was determined in slices from FlagMOR-Tg/+, MOR−/− mice (Figure 4). Application of ME (300 nM) did not result in an accumulation of fluorescence that was different from control. At higher concentrations (3 and 10 µM) more intracellular fluorescence was observed and the concentration that was required to cause a half-maximal amount of internalization was about 3 µM, well above the EC50 (151 nM) for the hyperpolarization. Thus the concentrations of ME required to induce desensitization and internalization were significantly greater than that required to induce hyperpolarization (Harris and Williams, 1991) indicating that less receptor occupancy is required to mediate the activation of the potassium current.
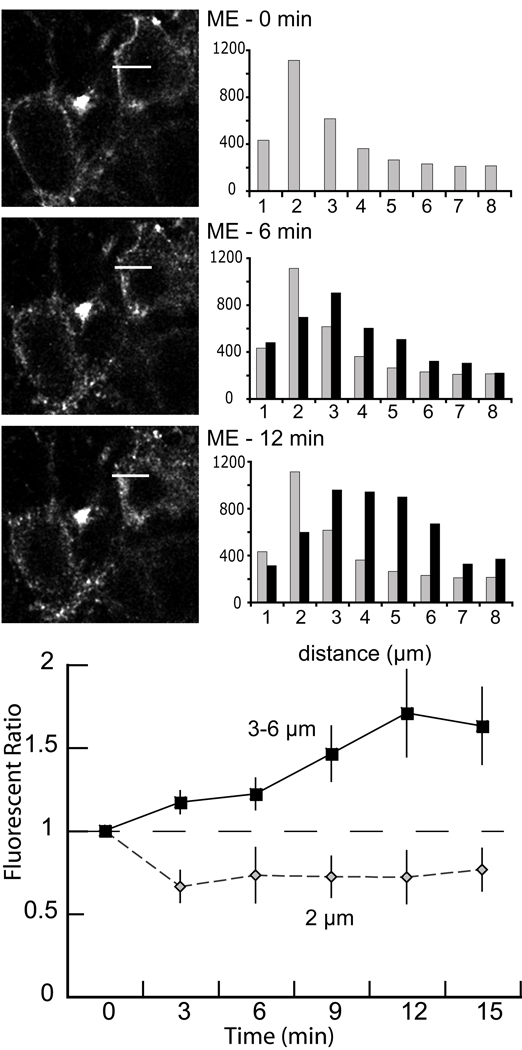
Figure 4.
The time course of receptor internalization. A) An illustration of a single experiment showing the time course of internalization with images collected at 6 min intervals (top before ME, middle 6 min after ME, bottom 12 min after ME). Each image is a single Z-scan (1 µm). The white bar in each image represented the area used to determine fluorescent intensity from the plot profile (0.5 × 8 µm, ImageJ). The fluorescence measured over a distance of 1 µm was added and plotted at the right for each image. Grey bars are the fluorescence measured before addition of ME. At 6 and 12 min the fluorescence moved away from the plasma membrane into the interior of the cell. B) Summary of the fluorescent ratio increasing in cytoplasm during 15 min perfusion of ME (30 µM, n=4). The fluorescence ratio was determined by dividing the counts measured after the addition of ME by the counts in the same area before addition of ME. Fluorescence decreased at the plasma membrane (2 µm, grey diamonds) and increased in the interior of the cell (3–6 µm, solid squares).
The time course of accumulation of intracellular fluorescence was examined by capturing images at 3 min intervals following the onset of superfusion with ME (30 µM, Figure 4, n=4). Within the first 3 min there was a detectable decrease in fluorescence at the plasma membrane and an increase in cytoplasmic fluorescent. Cytoplasmic puncta continued to increase over 12 min. This result demonstrated that detectable FlagMOR internalization was observed within 3 min, a period during which there is a significant amount of desensitization. With prolonged treatment, ME induced more cytoplasmic fluorescence in a perinuclear region.
Desensitization and recovery from desensitization
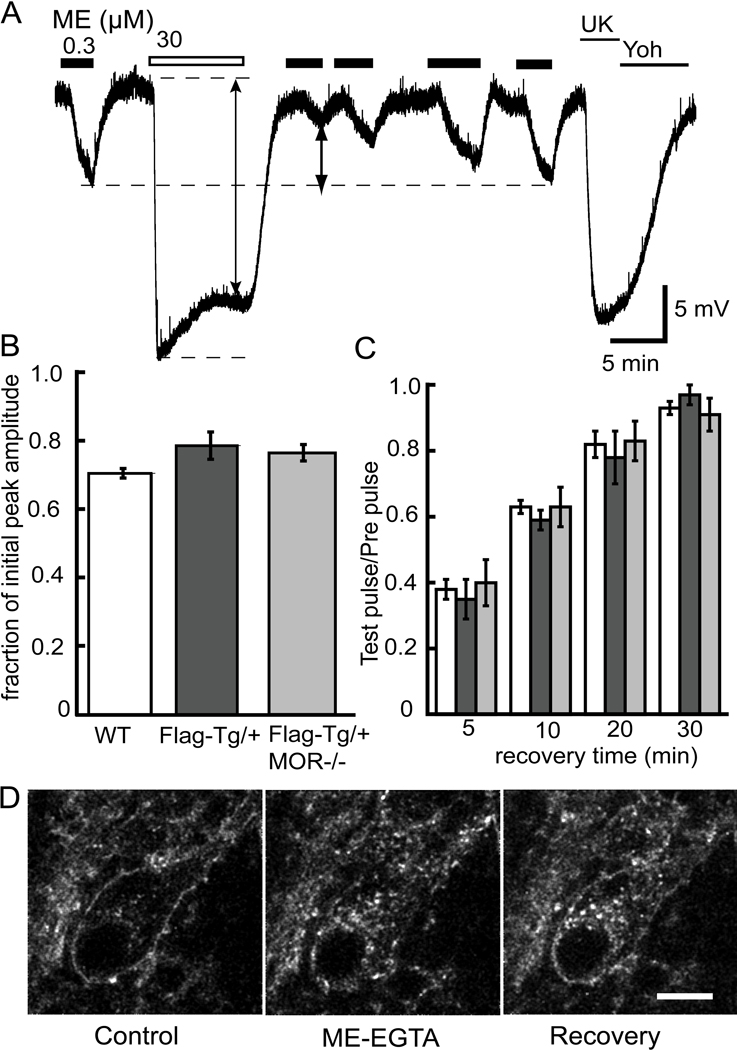
To determine if FlagMOR activity was regulated similarly to native receptors, desensitization was induced by a saturating concentration of ME (30 µM, 10 min). Prior to the application of the desensitizing concentration of ME, a pre-pulse of ME (EC50) was applied and the amplitude of the hyperpolarization was measured. Desensitization was measured in two ways. The first was to measure the decline in the peak hyperpolarization that occurred during the 10 min perfusion of ME (30 µM, Figure 5A). The amplitude of the hyperpolarization after 10 minutes decreased to 70%, 79% and 76% of the peak in wild type, FlagMOR-Tg/+, and FlagMOR-Tg/+, MOR−/− mice, respectively (Figure 5B, ANOVA P>0.05). Second, the amplitude of the hyperpolarization induced by an EC50 concentration of ME tested 5 min after washout of the desensitizing concentration of ME was decreased to 38±3% of the pre-pulse amplitude in wild type (ME 300 nM, n=9 neurons), 35±6% in FlagMOR-Tg/+ (ME 100 nM, n=5) and 40±7% in FlagMOR-Tg/+, MOR−/− (ME 100 nM, n=5, ANOVA P>0.05) mice.
Figure 5.
Recovery from desensitization and reinsertion of FlagMOR into plasma membrane. A) Voltage trace from a wild type mouse showing the hyperpolarization induced by ME (300 nM) before and after treatment of the slice with ME (30 µM) for 10 min. Following the washout of ME (30 µM) the hyperpolarization induced by ME (300 nM) was depressed and recovered completely after 30 min. At the end of the recording a saturating concentration of UK14304 (3 µM), an alpha-2-adrenoceptor agonist, and its blockade by the antagonist yohimbine (Yoh), was tested. B. Summary of the change in membrane potential during the 10-min treatment with ME (30 µM). The results indicate that the decline in the hyperpolarization was the same in slices from all three genotypes. C. Summary of the time course and extent of recovery from desensitization. There was no difference between genotypes. Given that the FlagMOR-Tg/+ and FlagMOR-Tg/+ MOR−/− were more sensitive to ME, the test concentration used to measure the recovery from desensitization was reduced to 100 nM. D) Return of receptors to the plasma membrane of cell bodies. Left panels are the initial staining. Middle taken after application of ME (30µM, 5 min) and EGTA solution (10 min). Right taken after a 45 min wash.
The time-course of recovery from desensitization was measured by repeated applications of ME (100 or 300 nM) over a 30 min period (Figure 5A and C). All three genotypes showed a similar extent and time course of recovery from desensitization (ANOVA P>0.05). Thus, FlagMORs were functional and regulated similarly to endogenous receptors.
Recycling of receptors was observed in experiments where the preparation was treated with ME (30 µM, 5 min), followed by a wash with calcium free EGTA buffer (10 min) and a 45-min wash with ACSF. The results show that fluorescent antibody-receptor complexes reappeared along the plasma membrane (Figure 5D). Thus the FlagMOR/fluorescent-M1 bound complex was capable of recycling in a time frame similar to the recovery from desensitization. Although only a fraction of the internalized receptors were able to traffic back to plasma membrane, the hyperpolarization induced by ME recovered almost completely. It is likely that the percentage of receptors that return to the plasma membrane is sufficient to result in a maximal hyperpolarization. The receptor-reserve reported in LC neurons suggests that only a small percentage of total receptors is necessary to elicit a maximal response (Christie et al., 1987).
Agonist-dependence of MOR desensitization and internalization
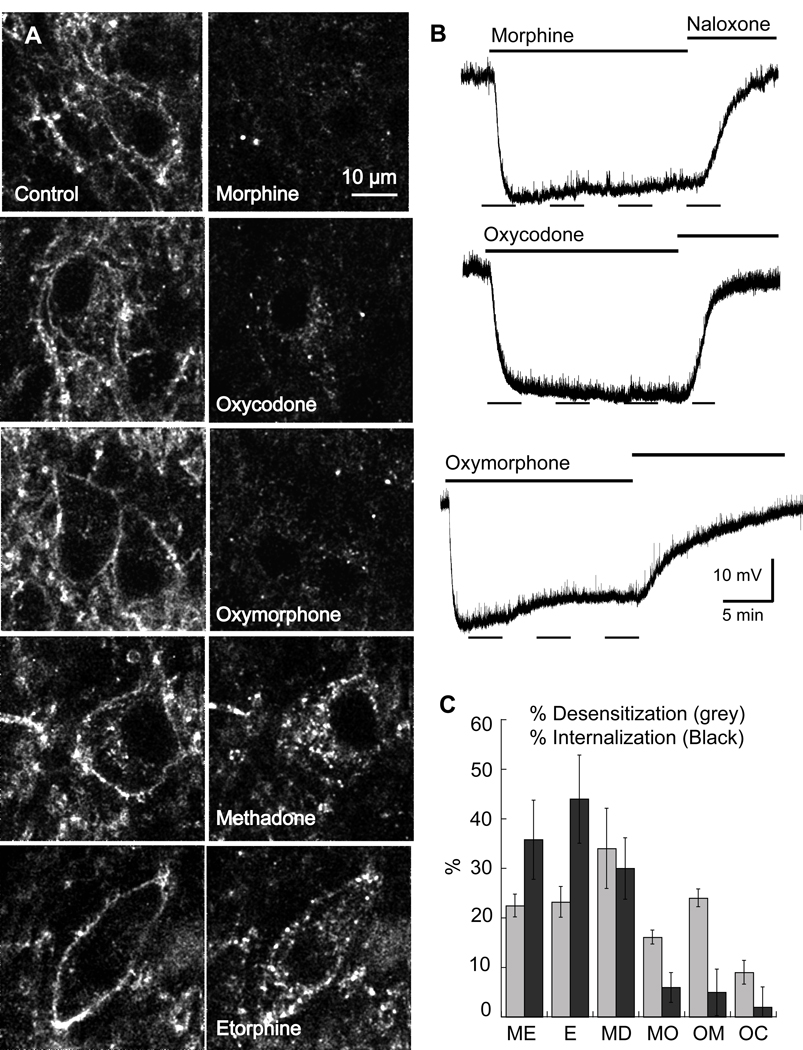
A series of opioid agonists was examined for the ability to induce desensitization and internalization in LC slices (Figure 6). As has been reported in other systems, ME, methadone and etorphine were all effective receptor-internalizing agents. The percent internalization induced by ME (30 µM) was 35±8% (n=6), etorphine (1 µM) 44±9% (n=6), and methadone (15 µM) 30±6% (n=6). The internalization induced by morphine (6±3%, n=6), oxymorphone (5±5%, n=6) and oxycodone (2±4%, n=6) was not different from background.
Figure 6.
Agonist-selective internalization. A) Images of the internalization. Left, control; right, after agonist (15 min) followed by calcium-free (EGTA) wash. Etorphine (E) and methadone (MD) caused dramatic internalization, while morphine (MO), oxymorphone (OM) and oxycodone (OC) caused very little. B) Example traces of the hyperpolarization induced by morphine, oxycodone and oxymorphone (15 µM each) all agonists that caused little or no internalization. C) Summary plot showing the decline in hyperpolarization (% desensitization, gray bars) and internalization (% internalization, black bars). The results show that different opioids produce different patterns of receptor regulation. [Met]5enkephalin, ME.
The ability of each opioid agonist to induce hyperpolarization and acute desensitization were also compared (Figure 6B and C). Each of the agonists produced a similar maximal hyperpolarization. Desensitization was measured as the decline in the amplitude of the hyperpolarization induced by each agonist during a 15 min application of a saturating concentration. The washout of all agonists except ME was very slow, so reversal of the hyperpolarization was obtained with the perfusion of naloxone (1 µM). Following return of the membrane potential to control the alpha-2-adrenoceptor agonist, UK14304 (3 µM) was perfused and the amplitude of the resulting hyperpolarization was measured. During a 15-minute application of ME (30 µM), etorphine (1 µM), morphine (15 µM) and oxymorphone (15 µM) the hyperpolarization declined by 22±4, 23±3, 16±1 and 24±2%, respectively (Figure 6C). Methadone (15 µM) caused the greatest decline in hyperpolarization (34±8%), however this was probably the result of a blockade of the potassium channel because the hyperpolarization induced by the alpha-2-adrenoceptor agonist, UK14304 was also reduced (Rodriquez-Martin et al., 2008). The amplitude of the hyperpolarization induced by UK14304 (3 µM) was 29.1±1.0 (n=20) following desensitization with all opioid agonists except methadone (17.8±1.5, n=5, p=0.002). Thus with the exception of methadone, the opioid induced desensitization was homologous. Oxycodone (15 µM) resulted in a decline of only 9±2%. The rank order for acute homologous desensitization was ME=etorphine=oxymorphone≥morphine>>oxycodone. Thus, oxymorphone and morphine caused desensitization without inducing internalization.
Internalization in β-Arrestin2-KO mice
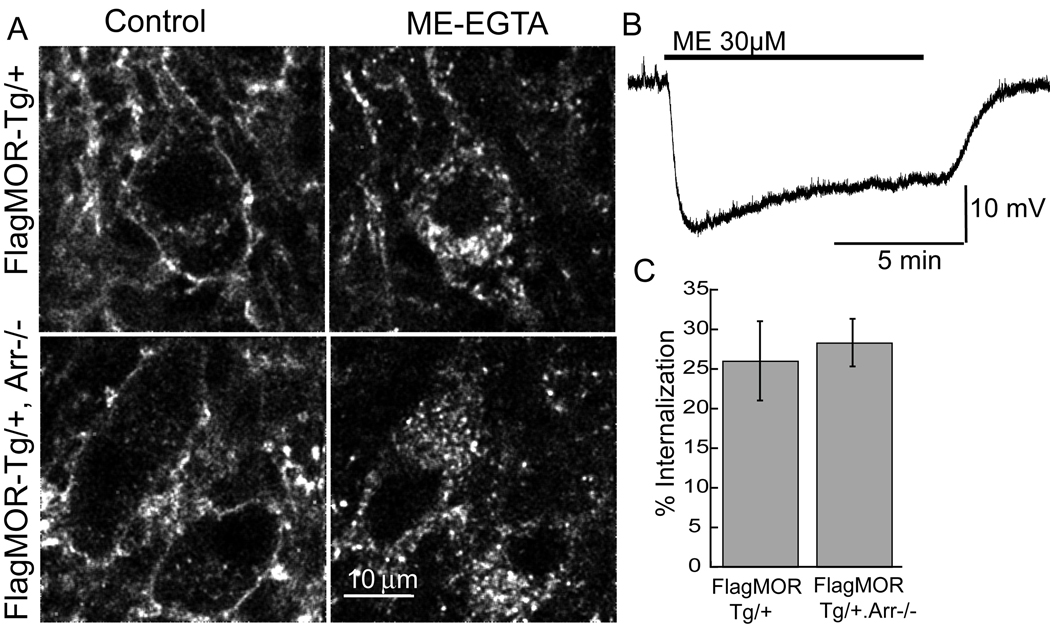
The elimination of β-arrestin might be expected to eliminate or at least decrease the amount of desensitization and internalization. Hemizygous FlagMOR-Tg/+ mice were crossed with β-arrestin2-KO (Arr−/−) mice to generate compound mutant FlagMOR-Tg/+, Arr−/− animals to determine the role of β-arrestin2 in desensitization and internalization. Intracellular recording of neurons in slices from Arr−/− animals showed that ME (30 µM) induced a hyperpolarization to 26.7±1.0 mV that declined to 76±3% of its initial amplitude after 10 min application (Figure 7B); values not significantly different from those observed in wild type mice (t-test; P>0.5). Morphine-induced desensitization in slices from the Arr−/− animals and was not different from that in slices from wild type animals (Arr−/− decreased to 84.6±2.6% of the peak, wild type 84.0±1.0) despite the fact that the maximum hyperpolarization was reduced (13.5±0.9 mV). Internalization induced by ME was examined in slices from FlagMOR-Tg/+, Arr−/− animals. Slices from these mice were treated with ME (30 µM, 15 min) and the percent internalization calculated. Internalization of FlagMOR did not differ between littermates lacking or expressing β-arrestin2 (FlagMOR-Tg/+, Arr−/−=28±3%, n=9 neurons; FlagMOR-Tg/+=26±5%, n=10, see Figure 7A and C). Thus elimination of β-arrestin2 had no effect on desensitization or internalization.
Figure 7.
[Met]5enkephalin (ME) induced MOR internalization occurs in absence of β-arrestin2. A. Image from FlagMOR-Tg/+ (top) and FlagMOR-Tg/+, Arr−/− LC slices in control (left) and following application of ME (30µM, 15 min) and calcium-free (EGTA) solution (right). Intracellular puncta were observed in both genotypes. B. Intracellular recording of the hyperpolarization produced by application of ME (30 µM). The response declines to approximately 76% of the initial amplitude during the 10 min application. C. Summary of the percent internalization caused by ME in FlagMOR-Tg/+, Arr−/− and their FlagMOR-Tg/+ littermates.
Discussion
In the present study, brain slices from transgenic mice were used to examine desensitization and trafficking of mu opioid receptors. Epitope-tagged (Flag) mu opioid receptors (FlagMOR) were targeted to locus coeruleus neurons using regulatory elements from the tyrosine hydroxylase gene. Using ME, the FlagMOR was activated and regulated in a similar way as the endogenous receptor. A series of opioid agonists was used to determine if different agonists activated distinct pathways. All agonists activated a potassium conductance that resulted in the same maximum hyperpolarization. The demonstration of agonist-selective regulation of desensitization and internalization indicates that the two processes are independent and are not necessarily serial events. Originally, morphine was described as the agonist that caused neither desensitization nor internalization (Alvarez et al., 2002), however recent results indicate that it can cause both desensitization (Borgland et al., 2003; Dang and Williams, 2005) and internalization (Haberstock-Debic et al., 2005). In contrast, the present results based on brain slices containing LC neurons show that morphine causes desensitization but no internalization. Oxymorphone was similar to morphine in that it caused robust desensitization in LC neurons from rats (Virk and Williams, 2008) and mice but was ineffective at evoking internalization of FlagMOR. The processes of internalization and desensitization have also been separated in experiments using cultured neurons treated with concanavalin A, where desensitization induced by ME and a dermorphin analog (dermorphin-Alexa 594) was induced in the absence of internalization (Arttamangkul et al., 2006). Thus it appears that although desensitization may be a precursor to internalization, internalization is not necessary for the expression of desensitization.
The agonists used in this study produced three distinct classes of effects. One group caused both desensitization and internalization and included ME, etorphine and methadone. Morphine and oxymorphone were distinct in that neither caused detectable internalization but both were capable of inducing desensitization. Oxycodone was the only agonist that neither desensitized nor internalized the receptor. In spite of the fact that oxycodone and oxymorphone differ only in a single methyl group, there was a distinct functional difference between the two compounds. Oxymorphone was more efficacious and much more effective at inducing desensitization than oxycodone (Virk and Williams, 2008). Given that oxymorphone is a natural metabolite of oxycodone, the receptor dependent processes of both oxycodone and oxymorphone must be considered in vivo.
The only way that desensitization could be compared using a series of agonists was by measuring the decline in the hyperpolarization during an extended application of a saturating concentration of each agonist. The slow washout of most agonists from brain slices prevented a more quantitative measurement of a decrease in sensitivity that was possible with ME (Figure 5A, 6B). The decline in peak hyperpolarization is not the most sensitive assay, as receptor reserve and the different efficacy of agonists result in a variable amount of desensitization. Given that near saturating concentrations of agonist are required for both desensitization and internalization, the decline in peak hyperpolarization would be blunted in experiments where highly efficacious agonists, such as ME or etorphine, were examined. Thus although a quantitative measure of desensitization is not possible, the rank order of the ability of various agonists to induce desensitization was reliably determined with this measure.
Morphine, oxymorphone and oxycodone are small opiate alkaloids having morphinan base structure while ME and methadone are linear and more flexible compounds. Considering the widely varying differences in chemical structure of opioid agonists, one may predict that different agonists could stabilize a range of receptor conformations and thus differentially affect the mechanisms leading to desensitization and internalization. Similar agonist dependent effects on receptor trafficking of β2-adrenoceptors (Swaminath et al., 2005) and dopamine D1 receptors (Ryman-Rasmussen et al., 2007) have been observed. Likewise, distinctive signaling induced by opioid agonists can vary depending on, perhaps subtle, differences in experimental conditions. Morphine is a good example in that it does not cause mu opioid receptor internalization in many cell types but did in cultured striatal neurons (Haberstock-Debic et al., 2005). It is also known that morphine can cause internalization in a system where G-protein receptor kinase (GRK) is over expressed (Zhang et al, 1998). Although phospho-MOR preferentially interacts with and has higher affinity to β-arrestin2 than β-arrestin1 (Bohn et al., 2000; Bohn et al, 2004; Oakley et al., 2000) the results with the β-arrestin2 KO mice suggest a possible interaction with β-arrestin1. It is clear that mice lacking β-arrestin2 have a dramatically altered response to some opioids. For example the tolerance to morphine is reduced, but treatment with etorphine resulted in tolerance that was the same as in wild-type animals (Bohn et al., 2004). These results are consistent with the idea that agonists can produce different patterns of receptor signaling in vivo (Stafford et al., 2001).
Although little work has been done at the cellular level with neurons from the β-arrestin2 knockout mice, the results in the present study are consistent with other studies that have reported that acute desensitization induced by opioid peptide agonists was not changed in either cultured dorsal root ganglion cells (Walwyn et al., 2007) or in LC neurons recorded in brain slices (Dang and Christie, personal communication). That desensitization and internalization of FlagMOR were both observed in brain slices from β-arrestin2 knockout mice suggests that mu opioid receptor signaling can occur by redundant pathways such that the control of receptor dependent signaling is ensured.
Conclusion
This study demonstrates the advantage of transgenic mice expressing an epitope-tagged receptor for the combined study of acute signaling through the activation of GIRK channels, desensitization and receptor trafficking. This approach allowed direct study of three acute receptor-dependent processes in a brain slice preparation. The role that these early signaling pathways have in the development of tolerance and dependence remains the subject of controversy and intense interest. Given that all opioid agonists result in tolerance under a variety of conditions, it is clear that no one process can completely account for the whole animal response to opioids. It is also clear that a single opioid agonist, such as morphine, has variable actions that are dependent on the cell under study and even the part of the cell under study. With the understanding of the mechanisms that underlie the early events following receptor activation it may be possible to develop a better understanding of the response of the whole animal to chronic opioid treatment.
Figure 3.
The concentration dependence of internalization induced by ME. A) Images were taken in control (left side) and after treatment of slices with different concentrations of ME for 15 min followed by a wash with calcium free solution (right side). B) Internalization required high concentrations of ME. The percent internalization caused by a 15-minute application of ME is plotted and compared with the concentration response to the hyperpolarization. All experiments were carried out using slices from FlagMOR-TG/+,MOR−/− animals.
Acknowledgments
The authors thank Mr. James Bunzow for making the FlagMOR-Tg construct, the OHSU Transgenic Mouse Models Shared Resource for pronuclear DNA microinjections, Dr. Shane Hentges for establishing the mouse colony and genotyping protocol, and Ms. Stephanie Gantz for the immunohistochemistry results. β-arrestin2 −/− animals were kindly provided by Dr Robert Lefkowitz.
Supported by JTW - DA08663 and NARSAD, SA - DA016627, NQ - NRSA DA023793.
Abbreviations
- FlagMOR
epitope-tagged (Flag) mu opioid receptor
- FlagMOR-Tg/+
hemizygous transgenic mice
- FlagMOR-Tg/+, MOR−/−
homozygous mu-opioid receptor knockout mice MOR-KO crossed with the transgenic mice
- FlagMOR-Tg/+, Arr−/−
homozygous β-arrestin2-KO mice crossed with the transgenic mice
- LC
locus coeruleus
- ME
[Met5]enkephalin
- GRK
G protein-coupled receptor kinase
- UK14304
5-bromo-6-[2-imidazolin-2-ylamino]quinoxaline
- EGTA
ethylene glycol-bis(β-aminoethylether) N,N,N’,N’-tetraacetic acid
- TH
tyrosine hydroxylase
- β-CNA
β-chlornaltrexamine
References
- Alvarez VA, Arttamangkul S, Dan V, Salem A, Whistler J, von Zastrow M, Grandy D, Williams JT. μ-opioid receptors: ligand dependent activation of potassium conductance, desensitization and internalization. J. Neurosci. 2002;22:5769–5776. doi: 10.1523/JNEUROSCI.22-13-05769.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arttamangkul S, Torrecilla M, Kobayashi K, Okano H, Williams JT. Separation of mu-opioid receptor desensitization and internalization: endogenous receptors in primary neuronal cultures. J Neurosci. 2006;26:4118–4125. doi: 10.1523/JNEUROSCI.0303-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408(6813):720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Dykstra LA, Lefkowitz RJ, Caron MG, Barak LS. Relative opioid efficacy is determined by the complements of the G protein-coupled receptor desensitization machinery. Mol Pharmacol. 2004;66:106–112. doi: 10.1124/mol.66.1.106. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Connor M, Osborne PB, Furness JB, Christie MJ. Opioid agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of mu-opioid receptors. J Biol Chem. 2003;278:18776–18784. doi: 10.1074/jbc.M300525200. [DOI] [PubMed] [Google Scholar]
- Bunzow JR, Zhang G, Bouvier C, Saez C, Ronnekleiv OK, Kelly MJ, Grandy DK. Characterization and distribution of a cloned rat mu-opioid receptor. J Neurochem. 1995;64:14–24. doi: 10.1046/j.1471-4159.1995.64010014.x. [DOI] [PubMed] [Google Scholar]
- Christie MJ, Williams JT, North RA. Cellular mechanisms of opioid tolerance: studies in single brain neurons. Mol Pharmacol. 1987;32:633–638. [PubMed] [Google Scholar]
- Connor M, Osborne PB, Christie MJ. Mu-opioid receptor desensitization: is morphine different? Br J Pharmacol. 2004;143:685–696. doi: 10.1038/sj.bjp.0705938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang VC, Williams JT. Morphine-induced mu-opioid receptor desensitization. Mol Pharmacol. 2005;68:1127–1132. doi: 10.1124/mol.105.013185. [DOI] [PubMed] [Google Scholar]
- Ferguson SS, Zhang J, Barak LS, Caron MG. Molecular mechanisms of G protein-coupled receptor desensitization and resensitization. Life Sci. 1998;62:1561–1565. doi: 10.1016/s0024-3205(98)00107-6. [DOI] [PubMed] [Google Scholar]
- Finn AK, Whistler JL. Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron. 2001;32:829–839. doi: 10.1016/s0896-6273(01)00517-7. [DOI] [PubMed] [Google Scholar]
- Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J. Neurosci. 2006;26:2788–2797. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- Haberstock-Debic H, Kim KA, Yu YJ, von Zastrow M. Morphine promotes rapid, arrestin-dependent endocytosis of mu-opioid receptors in striatal neurons. J Neurosci. 2005;25:7847–7857. doi: 10.1523/JNEUROSCI.5045-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Williams JT. Transient homologous mu-opioid receptor desensitization in rat locus coeruleus neurons. J Neurosci. 1991;11:2574–2581. doi: 10.1523/JNEUROSCI.11-08-02574.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KA, von Zastrow M. Neurotrophin-regulated sorting of opioid receptors in the biosynthetic pathway of neurosecretory cells. J. Neurosci. 2003;23:2075–2085. doi: 10.1523/JNEUROSCI.23-06-02075.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Pitcher J, Krueger K, Daaka Y. Mechanisms of beta-adrenergic receptor desensitization and resensitization. Adv Pharmacol. 1998;42:416–420. doi: 10.1016/s1054-3589(08)60777-2. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- Marie N, Aguila B, Allouche S. Tracking the opioid receptors on the way of desensitization. Cell Signal. 2006;18:1815–1833. doi: 10.1016/j.cellsig.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Min N, Joh TH, Kim KS, Peng C, Son JH. 5' upstream DNA sequence of the rat tyrosine hydroxylase gene directs high-level and tissue-specific expression to catecholaminergic neurons in the central nervous system of transgenic mice. Brain Res Mol Brain Res. 1994;27:281–289. doi: 10.1016/0169-328x(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- Pologruto TA, Sabatini BL, Svoboda K. ScanImage: flexible software for operating laser scanning microscopes. Biomed Eng Online. 2003;2:13. doi: 10.1186/1475-925X-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Martin I, Braksator E, Bailey CP, Goodchild S, Marrion NV, Kelly E, Henderson G. Methadone: does it really have low efficacy at mu-opioid receptors? Neuroreport. 2008;19:589–593. doi: 10.1097/WNR.0b013e3282f97b64. [DOI] [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, Griffith A, Oloff S, Vaidehi N, Brown JT, Goddard WA, 3rd, Mailman RB. Functional selectivity of dopamine D1 receptor agonists in regulating the fate of internalized receptors. Neuropharmacol. 2007;52:562–575. doi: 10.1016/j.neuropharm.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Laustriat D, Cao YQ, Basbaum AI, Dierich A, Vonesh JL, Gaveriaux-Ruff C, Kieffer BL. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci U S A. 2006;103:9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller AG, King MA, Zhang J, Bolan E, Pan YX, Morgan DJ, Chang A, Czick ME, Unterwald EM, Pasternak GW, Pintar JE. Retention of heroin and morphine-6 beta-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nat Neurosci. 1999;2:151–156. doi: 10.1038/5706. [DOI] [PubMed] [Google Scholar]
- Stafford K, Gomes B, Shen J, Yoburn BC. mu-Opioid receptor downregulation contributes to opioid tolerance in vivo. Pharm. Biochem. Behav. 2001;69:233–237. doi: 10.1016/s0091-3057(01)00525-1. [DOI] [PubMed] [Google Scholar]
- Swaminath G, Deupi X, Lee TW, Zhu W, Thian FS, Kobilka TS, Kobilka B. Probing the beta2 adrenoceptor binding site with catechol reveals differences in binding and activation by agonists and partial agonists. J Biol Chem. 2005;280:22165–22171. doi: 10.1074/jbc.M502352200. [DOI] [PubMed] [Google Scholar]
- Tanowitz M, von Zastrow M. A novel endocytic recycling signal that distinguishes the membrane trafficking of naturally occurring opioid receptors. J Biol Chem. 2003;278:45978–45986. doi: 10.1074/jbc.M304504200. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Virk MS, Williams JT. Agonist-specific regulation of μ-opioid receptor desensitization and recovery from desensitization. Mol Pharmacol. 2008;73:1301–1308. doi: 10.1124/mol.107.042952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zastrow M, Svingos A, Haberstock-Debic H, Evans C. Regulated endocytosis of opioid receptors: cellular mechanisms and proposed roles in physiological adaptation to opiate drugs. Curr Opin Neurobiol. 2003;13:348–353. doi: 10.1016/s0959-4388(03)00069-2. [DOI] [PubMed] [Google Scholar]
- Walwyn W, Evans CJ, Hales TG. β-arrestin2 and c-Src regulate the constitutive activity and recycling of μ opioid receptors in dorsal root ganglion neurons. J. Neurosci. 2007;27:5092–5104. doi: 10.1523/JNEUROSCI.1157-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler JL, Chuang HH, Chu P, Jan LY, von Zastrow M. Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron. 1999;23:737–746. doi: 10.1016/s0896-6273(01)80032-5. [DOI] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG. Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc Natl Acad Sci U S A. 1998;95:7157–7162. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]