Abstract
Cell polarity is critical for cell migration and requires localized signal transduction in subcellular domains. Recent evidence demonstrates that activation of ERK1/2 (extracellular-signal-regulated kinase 1/2) in focal adhesions is essential for cell migration. GIT1 (G-protein-coupled receptor kinase-interacting protein 1) has been shown to bind paxillin and regulate focal-adhesion disassembly. We have previously reported that GIT1 binds to MEK1 [MAPK (mitogen-activated protein kinase)/ERK kinase 1] and acts as a scaffold to enhance ERK1/2 activation in response to EGF (epidermal growth factor). In the present study we show that GIT1 associates with ERK1/2 in focal adhesions and this association increases after EGF stimulation. The CC (coiled-coil) domain of ERK1/2 is required for association with GIT1, translocation to focal adhesions, and cell spreading and migration. Immunofluorescent staining showed that, after EGF stimulation, GIT1 co-localized with pERK1/2 (phosphorylated ERK1/2) in focal adhesions. The binding of GIT1 and ERK1/2 was functionally important, since transfecting an ERK2 mutant lacking the CC domain [ERK2(del CC)] significantly decreased pERK1/2 translocation to focal adhesions, cell spreading and migration induced by EGF. In summary, the CC domain of ERK1/2 is necessary for binding to GIT1, for ERK1/2 activation in focal adhesions, and for cell spreading and migration.
Keywords: extracellular-signal-regulated kinase 1/2 (ERK1/2), focal adhesion, G-protein-coupled receptor kinase-interacting protein 1 (GIT1)
1. Introduction
Cell migration is critical at many stages of embryonic development, and is essential for wound healing and tumour cell invasion (Shattil and Ginsberg, 1997). Cell migration is regulated by coordinated changes in the actin cytoskeleton and cell-adhesion molecules. Activation of ERK1/2 (extracellular-signal-regulated kinase 1/2) is a key step in the cascade responsible for cell migration in response to many growth factors and hormones (Sakagami et al., 1995). ERK1/2 are present in several subcellular compartments, including the cytoplasm, plasma membrane, focal adhesions and nucleus (Kyriakis and Avruch, 1996; Shattil and Ginsberg, 1997). ERK1/2 localization has important functional consequences, and several scaffold proteins have been shown to regulate subcellular localization of ERK1/2. For example, KSR (kinase suppressor of Ras) transports MEK1 [MAPK (mitogen-activated protein kinase)/ERK kinase 1] from the cytoplasm to the plasma membrane and facilitates activation of ERK1/2 in response to membrane receptor stimulation (Xing et al., 1997; Muller et al., 2001). MP1 (MEK partner 1) is an ERK1/2 scaffold that is specifically targeted to endosomes by the adaptor protein P14 (Schaeffer et al., 1998). A previous report has demonstrated that Sef acts as a MEK–ERK1/2 scaffold localized to the Golgi apparatus (Torii et al., 2004). We previously reported that GIT1 (G-protein-coupled receptor kinase-interacting protein 1) associates with ERK1/2 and acts as a scaffold to enhance ERK1/2 activation at focal adhesions in response to EGF (epidermal growth factor) (Yin et al., 2004, 2005). The presence of active ERK1/2 in focal adhesions is essential for cell migration and is independent of de novo gene transcription (Kyriakis and Auruch, 1996). Critical signals required for migration that are regulated by ERK1/2 include activation of the intracellular protease calpain, which promotes cell detachment (Shattil and Ginsberg, 1997), and stimulation of MLCK [MLC (myosin light chain) kinase] and MLC phosphorylation (Glading et al., 2001; Webb et al., 2004).
The structures of ERK1/2 are similar, with a central kinase domain flanked by short N-and C-terminal domains. The N-terminal domain of ERK2 was shown to be important in regulating MEK1 association, substrate targeting and localization (Eblen et al., 2001). A portion of the C-terminal domain was shown to be required for ERK dimerization and nuclear translocation (Xu et al., 2001). Two groups independently identified a common docking motif, found in all known ERK family members, that mediates interactions with MEKs, MAPK phosphatase 3 and substrate MNK1 (MAPK-interacting kinase) (Tanoue et al., 2000; Zhang et al., 2003). We showed previously that GIT1 was a scaffold for MEK1 and ERK1/2 which mediated localization to focal adhesions (Yin et al., 2004, 2005). Furthermore, we showed that among the three putative CC (coiled-coil) domains in GIT1, only the CC2 domain (amino acids 426–474) (Yin et al., 2004) was required for interaction with ERK1/2 in focal adhesions. However, the domains of ERK1/2 required for binding to GIT1 remain poorly defined. In the present study we show that a CC domain present in ERK2 comprising amino acids 322–343 mediated binding to GIT1, and is required for localization to focal adhesions and subsequent regulation of cell migration.
2. Materials and methods
2.1. Cell culture
HeLa cells were cultured in DMEM (Dulbecco’s modified Eagle’s medium) supplemented with 10% (v/v) FBS (fetal bovine serum), 100 units/ml penicillin and 100 mg/ml streptomycin at 37°C in 5% CO2.
2.2. DNA expression plasmids and reagents
Xpress–GIT1, GST (glutathione transferase)–GIT1, HA (haemag-glutinin)–MEK1 and HA–ERK2 constructs were prepared as described previously and were sequenced to verify appropriate construction (Eblen et al., 2001; Xu et al., 2001; Yin et al., 2004, 2005). FLAG-tagged ERK2 was a gift from Dr Melanie Cobb (The University of Texas Southwestern Medical Center at Dallas, Dallas, TX, U.S.A.). FLAG-tagged ERK2(del 10–25) was a gift from Dr Michael J. Weber (University of Virginia, Charlottesville, VA, U.S.A.). For other constructs, PCR products of ERK2 (amino acids 1–343) were ligated into BamHI and XhoI restriction enzyme sites of pCMV-Tg2B. The truncated ERK2 construct [ERK2(del CC), lacking the CC domain present in amino acids 322–343] was ligated into pCMV-Tg2B using the Quikchange® site-directed mutagenesis kit (Stratagene). An anti-GIT1 monoclonal antibody and anti-GST antibody were purchased from BD Transduction Laboratories. Antibodies specific for FLAG-M2 were from Sigma. The anti-ERK1/2 and anti-pERK1/2 (phosphorylated ERK1/2) antibodies were from Promega. The anti-ERK1/2 monoclonal antibodies for immunofluoresence were purchased from Zymed.
2.3. Cell fractionation
HeLa cells, transfected with the indicated cDNAs, from 100 mm dishes were collected and homogenized in TEEND buffer [25 mM Tris/HCl, 5 mM EGTA, 2 mM EDTA, 100 mM NaF, 5 mM DTT (dithiothreitol) and 0.2 mM PMSF] with protease inhibitors. Cell fractions were prepared as described previously (Yin et al., 2004). The protein concentrations of each fraction were determined using BCA (bicinchoninic acid) reagent (Pierce).
2.4. Immunoprecipitation and immunoblotting assays
HeLa cells were co-transfected with Xpress–GIT1(WT) and FLAG– ERK2 using Lipofectamine™ 2000. After transfection for 24 h, cells were serum-starved for 6 h. Cell fractions were prepared as described above. Cytoskeletal fractions were immunoprecipitated with 2 µg of anti-Xpress antibody. They were then separated and probed with anti-FLAG and anti-Xpress antibodies and then with an HRP (horseradish peroxidase)-conjugated secondary antibody (Amersham Biosciences). Western blotting bands were visualized using the ECL (enhanced chemiluminescence) technique.
2.5. Immunofluoresence
HeLa cells were starved with serum-free DMEM for 6 h and then stimulated with EGF. Cells were fixed with 4% formaldahyde for 10 min, washed three times with PBS, permeabilized with 0.05% Triton X-100 for 5 min, and blocked with 10% normal goat serum for 1 h. Cells were incubated with anti-pERK1/2 or anti-GIT1 antibodies diluted in PBS, followed by Alexa Fluor® 546 goat anti-mouse or rabbit IgG (H+L) for red fluorescence or by Alexa Fluor® 488 goat anti-mouse or rabbit (H+L) for green fluorescence (Molecular Probes) in PBS at a final concentration of 1.5–2 µg/ml each.
2.6. Spreading and chamber assays
For the spreading assay, HeLa cells were transfected with different constructs for 24 h, serum-starved for 6 h and then suspended in DMEM containing trypsin inhibitor (0.5 µg/ml). Cells were washed with serum-free DMEM three times and incubated at 37°C and 5% CO2 for 30 min and allowed to adhere to 10 µg/ml fibronectin for the times indicated. The cell areas were calculated by ImageJ software. Statistical analysis was performed using a Student’s t test. A Boyden chamber assay was performed as described previously (Yin et al., 2004). Scanning density analysis was performed with NIH image software and statistical analysis was performed using the Student’s t test.
3. Results
3.1. ERK2 interaction with GIT1 is increased after EGF stimulation in HeLa cells
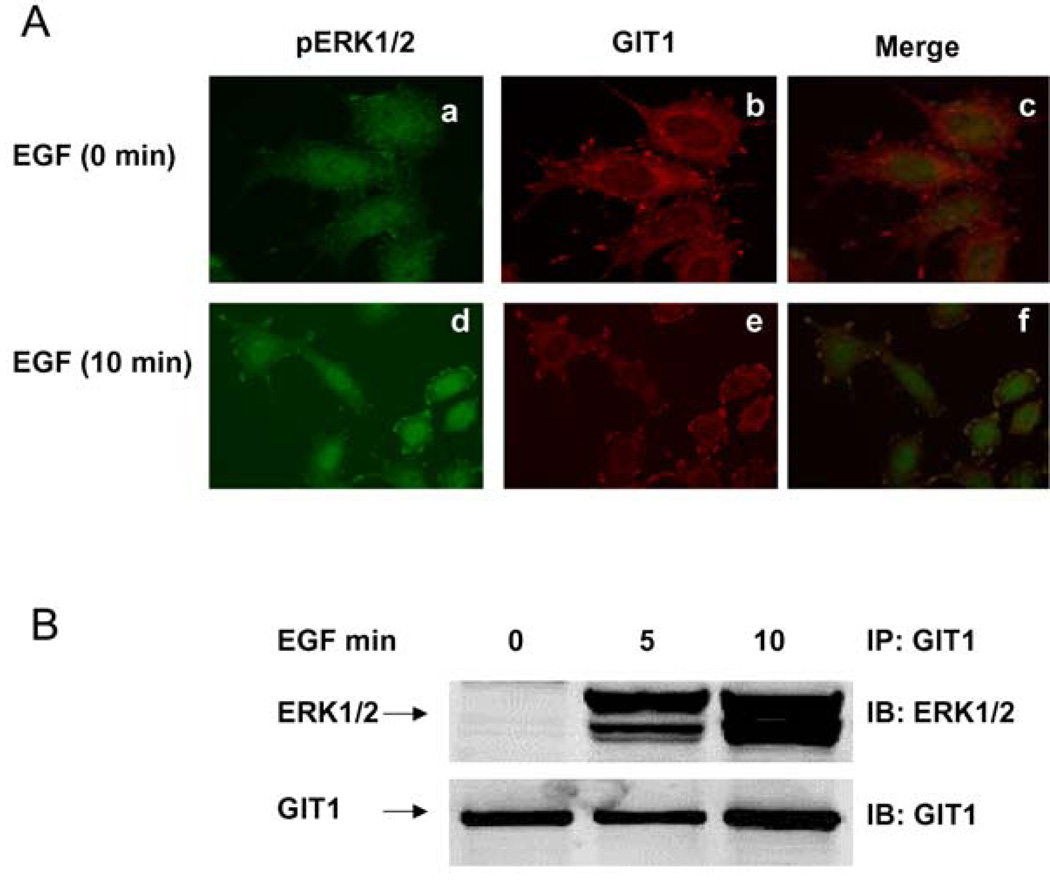
We have previously demonstrated that ERK1/2 translocates to focal adhesions after EGF stimulation (Yin et al., 2004, 2005). The CC2 domain of GIT1 is required for interaction with ERK2 (Yin et al., 2004), but the domains of ERK1/2 required for GIT1 interaction have not been identified. To demonstrate that HeLa cells are a useful model, we stimulated cells with EGF for 10 min and performed immunofluorescence for GIT1 and pERK1/2 (as a marker for activated ERK1/2). Under basal conditions pERK1/2 was diffusely located with no concentration at the plasma membrane (Figure 1A, a). In contrast, GIT1 was located in the perinuclear region and in discrete membrane-associated structures typical of focal adhesions (Figure 1A, b). There was essentially no co-localization when the images were merged (Figure 1A, c). EGF stimulation caused dramatic translocation of pERK1/2 to the plasma membrane (Figure 1A, d) in a pattern that strongly resembled the appearance of GIT1 (Figure 1A, e). When the images were merged, there was significant co-localization of pERK1/2 and GIT1, especially in structures typical of focal adhesions. To provide further evidence for EGF-mediated association between GIT1 and ERK2 we performed a cell-fractionation experiment (Figure 1B). In brief, HeLa cells were serum-starved for 6 h and stimulated with EGF for 5 and 10 min. Cell lysates were prepared and the cytoskeletal fractions were analysed. GIT1 was immunoprecipitated, and associated ERK1/2 was detected by Western blot analysis. There was a very large increase in the GIT1–ERK1/2 interaction after EGF stimulation (Figure 1B). These results are consistent with previous reports that ERK1/2 co-localizes with paxillin and that pERK1/2 localizes to focal adhesions in fibroblasts (Fincham et al., 2000).
Figure 1. GIT1 associates with pERK1/2 in HeLa cells.
(A) HeLa cells were serum-starved for 6 h and stimulated with saline (a–c) or 10 ng/ml EGF (d–f) for 10 min. Cells were fixed with 4% formaldehyde and stained with an anti-pERK1/2 antibody (a and d) or an anti-GIT1 antibody (b and e). Panels c and fare the merged images. (B) HeLa cells were serum-starved for 6 h and stimulated with EGF (10 ng/ml) for the times indicated. Cytoskeleton fractions were immunoprecipitated with an anti-GIT1 antibody and probed with an anti-ERK1/2 antibody (top panel). To confirm equal protein loading, the blot was reprobed with the anti-GIT1 antibody (bottom panel). These results were reproducibly obtained in three independent experiments. IB, immunoblot; IP, immunoprecipitation.
3.2. The CC domain of ERK2 is required for association with GIT1
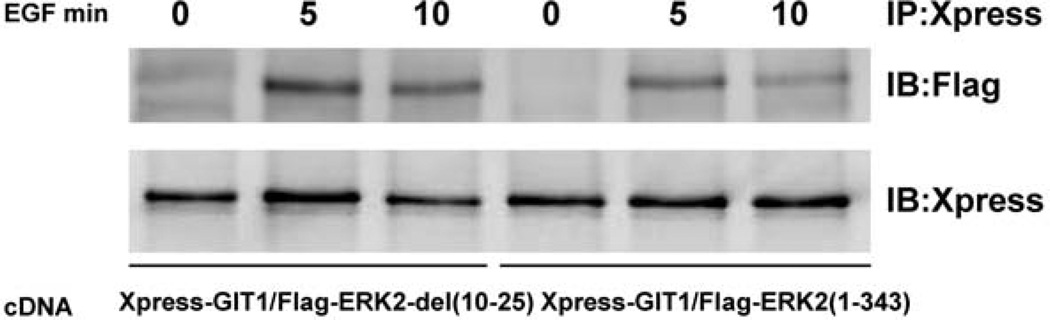
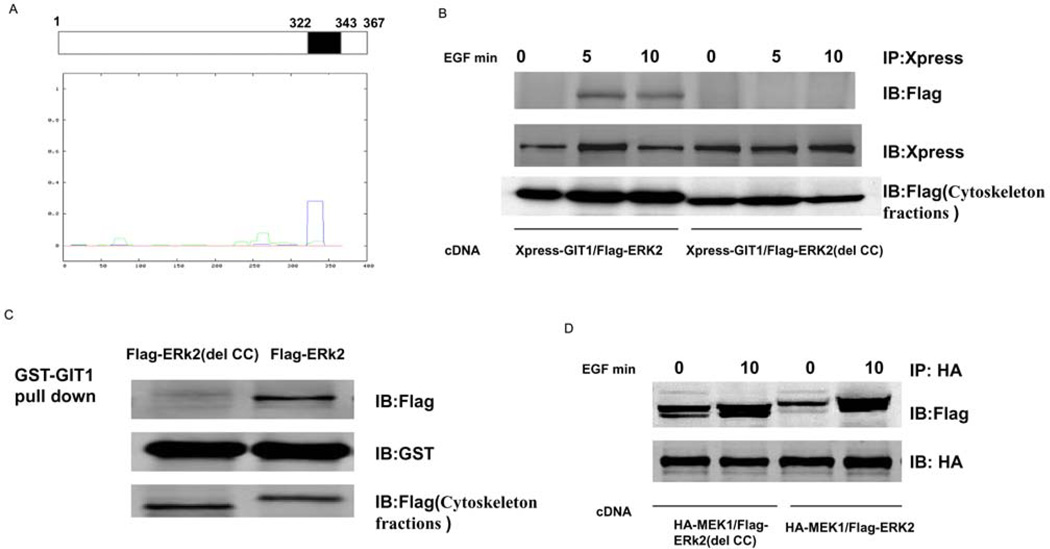
To determine the domains of ERK2 that mediate association with GIT1, we deleted the C- and N-terminal domains of ERK2 and prepared FLAG-tagged constructs termed FLAG–ERK2(1–343) and FLAG-ERK2(del 10–25) respectively. We then co-transfected Xpress–GIT1 with FLAG–ERK2(1–343) or FLAG–ERK2(del 10–25) and measured the protein–protein interaction and subcellular localization. As shown in Figure 2, FLAG–ERK2(del 10–25) and FLAG–ERK2(1–343) bound to GIT1 in cytoskeletal fractions after EGF stimulation. This suggests that the important amino acids in the N- and C-terminal domains of ERK2 are not required for association with GIT1. To examine other regions in ERK2 that may mediate the interaction with GIT1, we analysed ERK2 using the Lupas method and found one major CC domain comprising amino acids 322–343 of ERK2 (Figure 3A). To provide further evidence for the CC domain-mediated interaction between ERK2 and GIT1, we prepared a FLAG-tagged construct termed FLAG–ERK2(del CC) in which amino acids 322–343 of ERK2 were deleted. As expected, FLAG–ERK2(del CC) did not associate with GIT1 in cytoskeletal fractions after EGF stimulation (Figure 3B). To examine the effect of the CC deletion of ERK2, we transfected HeLa cells with HA–MEK1 and FLAG–ERK2 or HA–MEK1 and FLAG–ERK2(del CC) for 24 h, serum-starved them for 6 h, and then stimulated the transfected cells with EGF. As shown in Figure 3(C), the CC deletion in ERK2 did not affect its association with MEK1. To demonstrate direct binding between ERK1/2 and GIT1, we used a GST-pulldown assay. Transfected HeLa cells were treated with EGF for 10 min and then GST–GIT1 was used to co-precipitate ERK2. Wild-type FLAG–ERK2 was pulled down by GST–GIT1, but FLAG–ERK2(del CC) was not pulled down (Figure 3D). Taken together, these results show that the CC domain of ERK2 is essential for the interaction with GIT1.
Figure 2. The N-and C -terminal of ERK2 is not required for association with GIT1 in cytoskeletal fractions.
HeLa cells were co-transfected with Xpress–GIT1 and either FLAG–ERK2(del 10–25) or Xpress–GIT1 with FLAG–ERK2(1–343) for 24 h, serum-starved for 6 h, and then stimulated with EGF as indicated. The cell membrane, cytoplasm and cytoskeleton were separated as described in the Materials and methods section. Cytoskeletal fractions were immunoprecipitated with an anti-Xpress antibody and probed with an anti-FLAG antibody (top panel). To confirm equal protein immunoprecipitation, the blot was reprobed with an anti-Xpress antibody (bottom panel). These results were reproducibly obtained in three independent experiments. IB, immunoblot; IP, immunoprecipitation.
Figure 3. CC domain of ERK2 is required for association with GIT1 in cytoskeletal fractions.
(A) ERK2 sequences were analysed using the Lupas method and one major CC domain (amino acids 322–343) was found to exist in the C-terminus of ERK2. (B) HeLa cells were co-transfected with Xpress–GIT1 and FLAG–ERK2 or Xpress–GIT1 and FLAG–ERK2(del CC) for 24 h, serum-starved for 6 h, and then stimulated with EGF as indicated. The cell cytoskeletons were separated as described in the Materials and methods section. Cell cytoskeleton fractions were immunoprecipitated with an anti-Xpress antibody and probed with an anti-FLAG antibody (top panel). To confirm equal protein loading, the blot was re-probed with an anti-Xpress antibody (middle panel). Protein expression was detected by probing with an anti-FLAG antibody in cytoskeleton fractions (bottom panel). (C) GST–GIT1 was immobilized on glutathione-conjugated beads and incubated with cytoskeleton fractions from FLAG–ERK2- or FLAG–ERK2(del CC)-transfected HeLa cells. Beads were washed extensively and then immunoblotted for FLAG–ERK2 (top panel) and reprobed with an anti-GST antibody to confirm equal loading (middle panel). To confirm equal protein expression, cytoskeleton fractions were blotted with an anti-FLAG antibody (bottom panel). (D) HeLa cells were co-transfected with HA–MEK1 and FLAG–ERK2 or HA–MEK1 and FLAG–ERK2(del CC) for 24 h, serum-starved for 6 h, and then stimulated with EGF as indicated. The cell cytoskeletons were separated as described in the Materials and methods section. Cell cytoskeleton fractions were immunoprecipitated with an anti-HA antibody and probed with an anti-FLAG antibody (top panel). To confirm equal protein loading, the blot was re-probed with an anti-HA antibody (bottom panel). These results were reproducibly obtained in three independent experiments. IB, immunoblot; IP, immunoprecipitation.
3.3. The CC domain is required for ERK1/2 translocation to focal adhesions
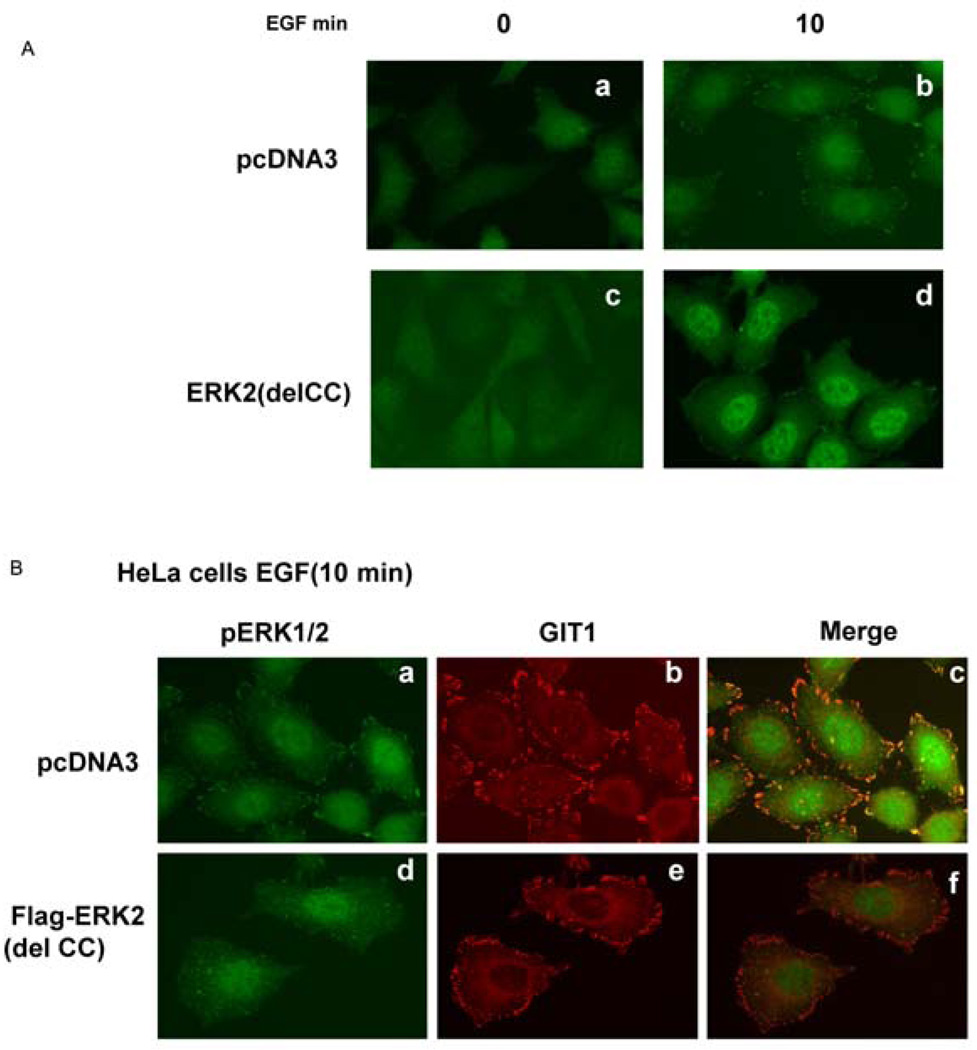
We previously reported that ERK1/2 interacted with GIT1 in focal adhesions and that this association increased after EGF stimulation (Yin et al., 2005). To define the functional role of the ERK2 CC domain in EGF-mediated translocation to focal adhesions, we transfected pcDNA3 and FLAG–ERK2(del CC) into HeLa cells. As shown in Figure 4(A), cell morphology at baseline did not differ beween pcDNA3 and FLAG–ERK2(delCC) cells. However, in response to EGF there was a dramatic increase in pERK1/2 localized to focal adhesions in pcDNA3-transfected cells (Figure 4A, a and b), which was significantly inhibited in FLAG– ERK2(del CC)-transfected cells (Figure 4A, c and d). We believe that ERK2(del CC) acts in a dominant-negative manner by binding paxillin and other proteins, thereby excluding endogenous ERK1/2 from associating with focal adhesions. To show that the effect of ERK2(del CC) was not due to altered GIT1 location, we immuno-stained for GIT1 as well as pERK1/2. In response to EGF, GIT1 translocated to focal adhesions (Figure 4B, b) as previously reported (Yin et al., 2005). There was highly significant co-localization with pERK1/2 (Figure 4B, c). In cells transfected with FLAG–ERK2(del CC), GIT1 still translocated to focal adhesions (Figure 4B, e), but pERK1/2 did not translocate (Figure 4B, d) and did co-localize with GIT1(Figure 4B, f). These findings are consistent with the results shown in Figure 3 and indicate that the CC domain of ERK1/2 is necessary for localization of pERK1/2 in focal adhesions. The fact that GIT1 localization was not altered suggests that GIT1 does not require pERK1/2 for translocation to focal adhesions.
Figure 4. The CC domain of ERK2 is required for pERK1/2 translocation to focal adhesions.
(A) HeLa cells were transfected with pcDNA3 (a and b) or FLAG–ERK2(del CC) (c and d) for 24 h, serum-starved for 6 h, and then stimulated with EGF as indicated. Cells were fixed with 4% formaldehyde and stained with an anti-pERK1/2 antibody. (B) HeLa cells were transfected with pcDNA3 (a, b and c) or FLAG–ERK2(del CC) (d, e and f) for 24 h, serum-starved for 6 h, and then stimulated with EGF for 10 min. Cells were fixed with 4% formaldehyde and stained with an anti-pERK1/2 antibody (a and d) or anti-GIT1 antibody (b and e). Panels c and f are the merged images of a and b, and d and e respectively. These results were reproducibly obtained in three independent experiments.
3.4. Functional effect of the CC domain of ERK2
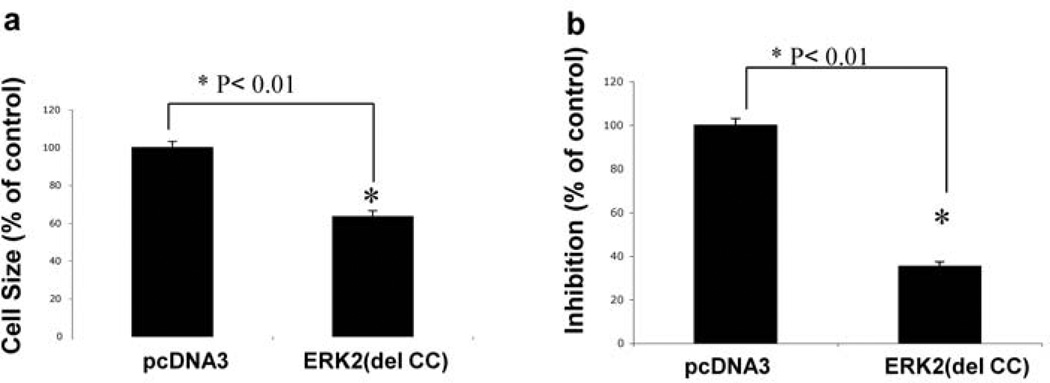
We further studied the functional consequences of preventing the ERK1/2 interaction with GIT1. As an experimental model we transfected HeLa cells with FLAG–ERK2(del CC) and measured cell spreading. Cells transfected with pcDNA3 or FLAG– ERK2(delCC) were seeded in fibronectin-coated dishes for 10 min and cell areas were calculated by ImageJ software. FLAG–ERK2(del CC) significantly inhibited cell spreading by 42% compared with pcDNA3 (Figure 5a). To demonstrate further the functional role of the ERK2 CC domain, we performed a Boyden chamber migration assay. EGF significantly increased cell migration after transfection with pcDNA3. In contrast, cell migration was dramatically inhibited by transfection with FLAG– ERK2(del CC) (Figure 5b). These results suggest an important role for the CC domain of ERK2 in agonist-stimulated cell spreading and migration.
Figure 5. Functional effect of the CC domain of ERK2.
(a) HeLa cells were transfected with pcDNA3 or FLAG–ERK2(del CC) for 24 h and resuspended in DMEM with 0.5 µg/ml trypsin inhibitor. Cells were washed three times and seeded on to fibronectin coated-dishes (10 µg/ml) and cell spreading was measured at 10 min. Cells were fixed with 4% formaldehyde. Then, 100 attached cells were randomly selected to measure the cell area using ImageJ software (*P<0.01; mean±S.E.M.; n=6). The cell area for pcDNA3 was arbitrarily set to 100. (b) HeLa cells transfected with pcDNA3 or FLAG–ERK2(del CC) for 24 h, were serum-starved for 6 h and then seeded in the upper portion of a Boyden chamber on collagen precoated PVP-free polycarbonate membranes. EGF was added to the lower chamber. Cells were incubated for 6 h at 37 °C in a 5% CO2 humidified incubator. The membranes were removed and cells were stained. The relative increases in cell density were determined by quantitative densitometry (*P<0.01; mean±S.E.M.; n=6).
4. Discussion
The major findings of the present study are that ERK1/2, via the C-terminal CC domain, interacts with GIT1, which acts as a scaffold to mediate localization of activated ERK1/2 to focal adhesions. Functionally we show that EGF-mediated ERK1/2 translocation and activation in focal adhesions is required for cell spreading and migration. We previously reported that GIT1 binds MEK1 and ERK1/2 through its CC2 domain and ERK1/2 activation in focal adhesions was Src-dependent (Yin et al., 2004, 2005). In the present study, we provide evidence that the CC domain of ERK2 is required for association with GIT1 in focal adhesions.
Previously, Fincham et al. (2000) showed that activated ERK1/2 co-localized with paxillin after fibronectin treatment, and localization of activated ERK to focal adhesions was c-Src-dependent. Based on this information, we propose a model to explain the role of the ERK1/2 CC domain in EGF-mediated ERK1/2 translocation and activation in focal adhesions. In serum-starved cells, GIT1 exists in a pre-assembled complex with MEK1/2 (Yin et al., 2004). Upon EGF stimulation, c-Src is activated and phosphorylates critical tyrosine residues in GIT1 (Ishida et al., 1998). GIT1 tyrosine phosphorylation induces GIT1 to associate with ERK1/2 via the GIT1 CC2 domain. GIT1 then translocates to focal adhesions via its interactions with paxillin, PAK (p21-activated kinase) and PIX (PAK-interacting exchange protein). Once the GIT1–ERK1/2 complex is present in focal adhesions, MEK1/2 phosphorylates and activates ERK1/2. Our hypothesis is supported by the fact that ERK2(del CC), which cannot bind GIT1, acts in a dominant-negative manner to block activated-ERK1/2 localization to focal adhesions (Figure 4), probably by failing to bind to GIT1 and competing with endogenous ERK1/2 for binding to other proteins such as paxillin, FAK (focal adhesion kinase) and PAK (Klemke et al., 1997; Liu et al., 2002; Webb et al., 2004). The results of the present study suggest that ERK is a key target of GIT1, a protein that localizes to cell adhesions; however, other effector proteins, such as paxillin, may also contribute to this process (Webb et al., 2004). For example, it is possible that GIT1 and paxillin co-regulate cell migration in different phases. It is also possible that ERK2(del CC), a mutation that abolishes the ability of ERK2 to bind GIT1, affects the association between GIT1 and paxillin. Another possiblility is that the ERK2(del CC) mutant competes with the endogenous ERK to bind paxillin.
ERK1/2 phosphorylation of cytoskeletal proteins has been implicated in the migration of numerous cell types. Activated ERK1/2 has many substrates including cytosolic and cytoskeletal components, as well as transcription factors. Among cytoskeletal proteins, MLCK, calpain, FAK and paxillin are most likely to be involved in ERK1/2-mediated cell migration. ERK1/2 phosphorylates m-calpain at Ser50, which is required for focal adhesion turnover and cell migration (Glading et al., 2004). Activated ERK1/2 promotes phosphorylation of MLCK and MLC, which may be involved in focal adhesion turnover and membrane protrusion at the leading edge of migrating cells (Webb et al., 2004). In addition, activated ERK1/2 phosphorylates and modifies the regulatory domain of GRASP 65, a protein that is critical for Golgi polarization and cell polarity in migrating cells (Bisel et al. 2008). Liu et al. (2002) have shown that ERK1/2 phosphorylates paxillin in HGF (hepatocyte growth factor)-stimulated epithelial cells, which enhances the paxillin–FAK association. A recent study by Chen et al. (2008) showed that decreasing phosphorylation of ERK1/2 caused an increase in α5β1 cell-surface expression, which supresses cell motility. When taken together, these results suggest that pERK1/2 has the same function in cell migration, regardless of its subcellular location. These intriguing observations suggest a sophisticated regulation of ERK1/2 in cell migration. We propose that GIT1, specifically at focal adhesions, regulates ERK1/2 phosphorylation of FAK and/or paxillin, and thereby modulates disassembly of the FAK–paxillin complex. A role for JNK (c-Jun N-terminal kinase) in paxillin phosphorylation (and hence focal-adhesion assembly) has also previously been demonstrated (Huang et al., 2003). Based on the present study, it appears likely that pathway-specific scaffold proteins control the relative activation of JNK [possibly by JIP-1 (JNK-interacting protein 1)] and ERK1/2 (via GIT1) in focal adhesions. We speculate that the relative abundance of these scaffolds and the kinases that regulate their conformation and binding properties determines the magnitude of specific MAPK activation in specific locations.
Our findings demonstrate that GIT1 serves as a scaffold for MAPK activation, similar to the yeast protein Ste5p and the mammalian proteins KSR, MP1 and Sef. KSR transports MEK1 from the cytoplasm to the plasma membrane and facilitates activation of ERK1/2 in response to membrane receptor stimulation (Xing et al., 1997; Muller et al., 2001). MP1 is a MAPK scaffold specifically targeted to endosomes by the adaptor protein P14 (Schaeffer et al., 1998). A previous paper has shown that Sef acts as a MEK–ERK1/2 scaffold localized to the Golgi apparatus (Torii et al., 2004). We propose that GIT1 be added to this list as a MEK1–ERK1/2 scaffold that is required for localization of activated ERK1/2 to focal adhesions. Like KSR, GIT1 constitutively binds MEK1, but there is an increased interaction with ERK1/2 following agonist stimulation. Previous studies and the present study show that interfering with the GIT1 scaffold function does not affect EGF-stimulated ERK1/2 activation in the cytoplasm (Yin et al., 2005), ERK1/2 translocation to the nucleus (Yin et al., 2004), or nuclear ERK1/2 activation (Figures 4A and 4B). These findings suggest that the scaffold function of GIT1 is specific for ERK1/2 activation at focal adhesions. Therefore other scaffold proteins (such as KSR, MP1 and Sef) probably regulate ERK1/2 activation at other sites within the cell (Glading et al., 2004). Targeting by these different scaffolds addresses the fundamental question of how a ubiquitous signalling pathway, such as ERK1/2, can direct divergent cellular responses depending on the stimulus and its subcellular location.
Acknowledgments
Funding
This work was supported by the National Natural and Science Foundation [grant number 3067086 (to G.Y.)]; the Jiangsu Natural and Science Foundation [grant number BK2006249 (to G.Y.)]; Talent Person in Medicine Foundation [grant number RC2007059 (to G.Y.)]; the Six Persons with Ability Foundation [grant number 07-B-043 (to G.Y.)]; and the National Institutes of Health [grant number RO1 HL63462 (to B.C.B.)].
Abbreviations
- CC
coiled-coil
- DMEM
Dulbecco’s modified Eagle’s medium
- EGF
epidermal growth factor
- ERK1/2
extracellular-signal-regulated kinase 1/2
- FAK
focal adhesion kinase
- GIT1
G-protein-coupled receptor kinase-interacting protein 1
- GST
glutathione transferase
- HA
haemagglutinin
- JNK
c-Jun N-terminal kinase
- KSR
kinase suppressor of Ras
- MAPK
mitogen-activated protein kinase
- MEK1
MAPK/ERK kinase 1
- MLC
myosin light chain
- MLCK
MLC kinase
- MP1
MEK partner 1
- PAK
p21-activated kinase
- pERK1/2
phosphorylated ERK1/2
Footnotes
REFERENCES
- Bisel B, Wang Y, Wei JH, Xiang Y, Tang D, Miron-Mendoza M, et al. ERK regulates Golgi and centrosome orientation towards the leading edge through GRASP 65. J Cell Biol. 2008;182:837–843. doi: 10.1083/jcb.200805045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Meng LH, Zhu CH, Lin LP, Lu H, Ding J. ADAM15 suppresses cell motility by driving integrin α5β1 cell surface expression via ERK inactivation. Int J Biochem Cell Biol. 2008;40:2164–2173. doi: 10.1016/j.biocel.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Eblen ST, Catling AD, Assacah MC, Weber MJ. Biochemical and biological functions of the N-terminal, noncatalytic domain of extracellular signal-regulated kinase 2. Mol Cell Biol. 2001;21:249–259. doi: 10.1128/MCB.21.1.249-259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham VJ, James M, Frame MC, Winder SJ. Active ERK/MAP kinase is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. EMBO J. 2000;19:2911–2923. doi: 10.1093/emboj/19.12.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glading A, Uberall S, Keyse M, Lauffenburger DA, Wells A. Membrane proximal ERK signaling is required for M-calpain activation downstream of epidermal growth factor receptor signaling. J Biol Chem. 2001;276:23341–23348. doi: 10.1074/jbc.M008847200. [DOI] [PubMed] [Google Scholar]
- Glading A, Bodnar RJ, Reynolds IJ, Shiraha H, Satish L, Potter DA, et al. Epidermal growth factor activates m-calpain (calpain II), at least in part, by extracellular signal-regulated kinase-mediated phosphorylation. Mol Cell Biol. 2004;24:2499–2512. doi: 10.1128/MCB.24.6.2499-2512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Rajfur Z, Borchers C, Schaller MD, Jacobson K. JNK phosphorylates paxillin and regulates cell migration. Nature. 2003;424:219–223. doi: 10.1038/nature01745. [DOI] [PubMed] [Google Scholar]
- Ishida M, Ishida T, Thomas S, Berk BC. Activation of extracellular signal-regulated kinases (ERK1/2) by angiotensin II is dependent on c-Src in vascular smooth muscle cells. Circ Res. 1998;82:7–12. doi: 10.1161/01.res.82.1.7. [DOI] [PubMed] [Google Scholar]
- Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271(41):24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- Liu ZX, Yu CF, Nickel C, Thomas S, Cantley LG. Hepatocyte growth factor induces ERK-dependent paxillin phosphorylation and regulates paxillin-focal adhesion kinase association. J Biol Chem. 2002;277:10452–10458. doi: 10.1074/jbc.M107551200. [DOI] [PubMed] [Google Scholar]
- Muller J, Ory S, Copeland T, Piwnica-Worms H, Morrison DK. C-TAK1 regulates Ras signaling by phosphorylating the MAPK scaffold, KSR1. Mol Cell. 2001;8:983–993. doi: 10.1016/s1097-2765(01)00383-5. [DOI] [PubMed] [Google Scholar]
- Sakagami H, Kuribayashi N, Iida M, Sakagami T, Takeda M, Fukuchi K, et al. Induction of DNA fragmentation by tannin-and lignin-related substances. Anticancer Res. 1995;15(15B):2121–2128. [PubMed] [Google Scholar]
- Schaeffer HJ, Catling AD, Eblen ST, Collier LS, Krauss A, Weber MJ. MP1: a MEK binding partner that enhances enzymatic activation of the MAP kinase cascade. Science. 1998;281:1668–1671. doi: 10.1126/science.281.5383.1668. [DOI] [PubMed] [Google Scholar]
- Shattil SJ, Ginsberg MH. Integrin signaling in vascular biology. J Clin Invest. 1997;100:S91–S95. [PubMed] [Google Scholar]
- Tanoue T, Adachi M, Moriguchi T, Nishida E. A conserved docking motif in MAP kinase common to substrates, activators and regulators. Nat Cell Biol. 2000;2:110–116. doi: 10.1038/35000065. [DOI] [PubMed] [Google Scholar]
- Torii S, Kusakaba T, Yamamoto T, Maekawa M, Nishida E. Sef is a spatial regulator for Ras/MAP kinase signaling. Dev Cell. 2004;7:33–44. doi: 10.1016/j.devcel.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- Xing H, Kornfeld K, Muslin AJ. The protein kinase KSR interacts with 14-3-3 protein and Raf. Curr Biol. 1997;7:294–300. doi: 10.1016/s0960-9822(06)00152-7. [DOI] [PubMed] [Google Scholar]
- Xu B, Stippec S, Robinson FL, Cobb MH. Hydrophobic as well as charged residues in both MEK1 and ERK2 are important for their proper docking. J Biol Chem. 2001;276:26509–26515. doi: 10.1074/jbc.M102769200. [DOI] [PubMed] [Google Scholar]
- Yin G, Haendeler J, Yan C, Berk BC. GIT1 functions as a scaffold for MEK1-extracellular signal-regulated kinase 1 and 2 activation by angiotensin II and epidermal growth factor. Mol Cell Biol. 2004;24:875–885. doi: 10.1128/MCB.24.2.875-885.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin G, Zheng QL, Yan C, Berk BC. GIT1 is a scaffold for ERK1/2 activation in focal adhesion. J Biol Chem. 2005;280:27705–27712. doi: 10.1074/jbc.M502271200. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhou B, Zheng CF, Zhang ZY. A bipartite mechanism for ERK2 recongnition by its cognate regulators and substrate. J Biol Chem. 2003;278:29901–29912. doi: 10.1074/jbc.M303909200. [DOI] [PubMed] [Google Scholar]